Circular Fertilization Strategy Using Sulphur with Orange Waste Enhances Soil Health and Broccoli Nutritional and Nutraceutical Quality in Mediterranean Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Treatment
2.2. Chemical and Biochemical Soil Analysis
2.3. QBS–ar Index
2.4. Broccoli Nutritional and Nutraceutical Analysis
2.5. Statistical Analysis
3. Results
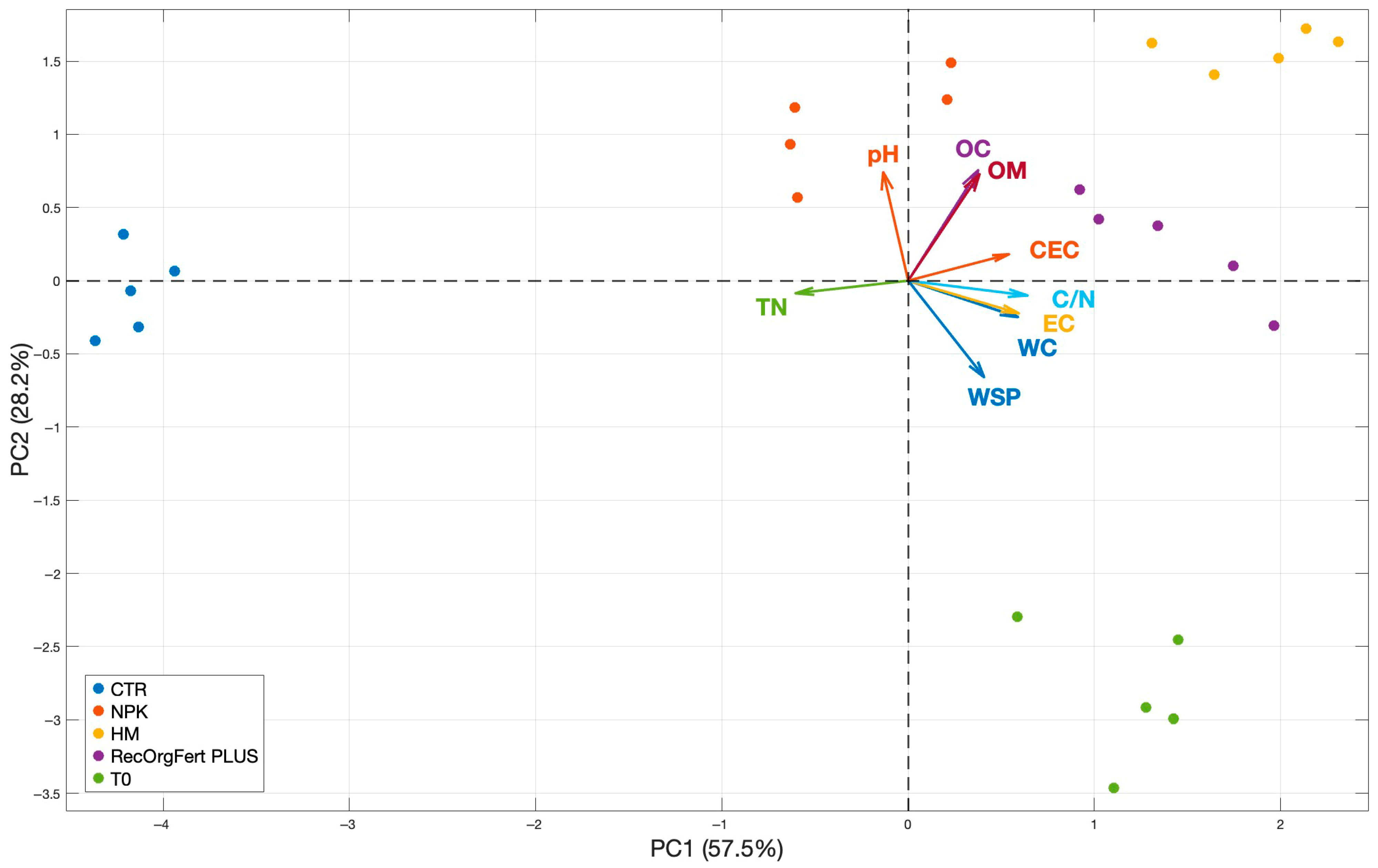
3.1. Soil Physicochemical Properties
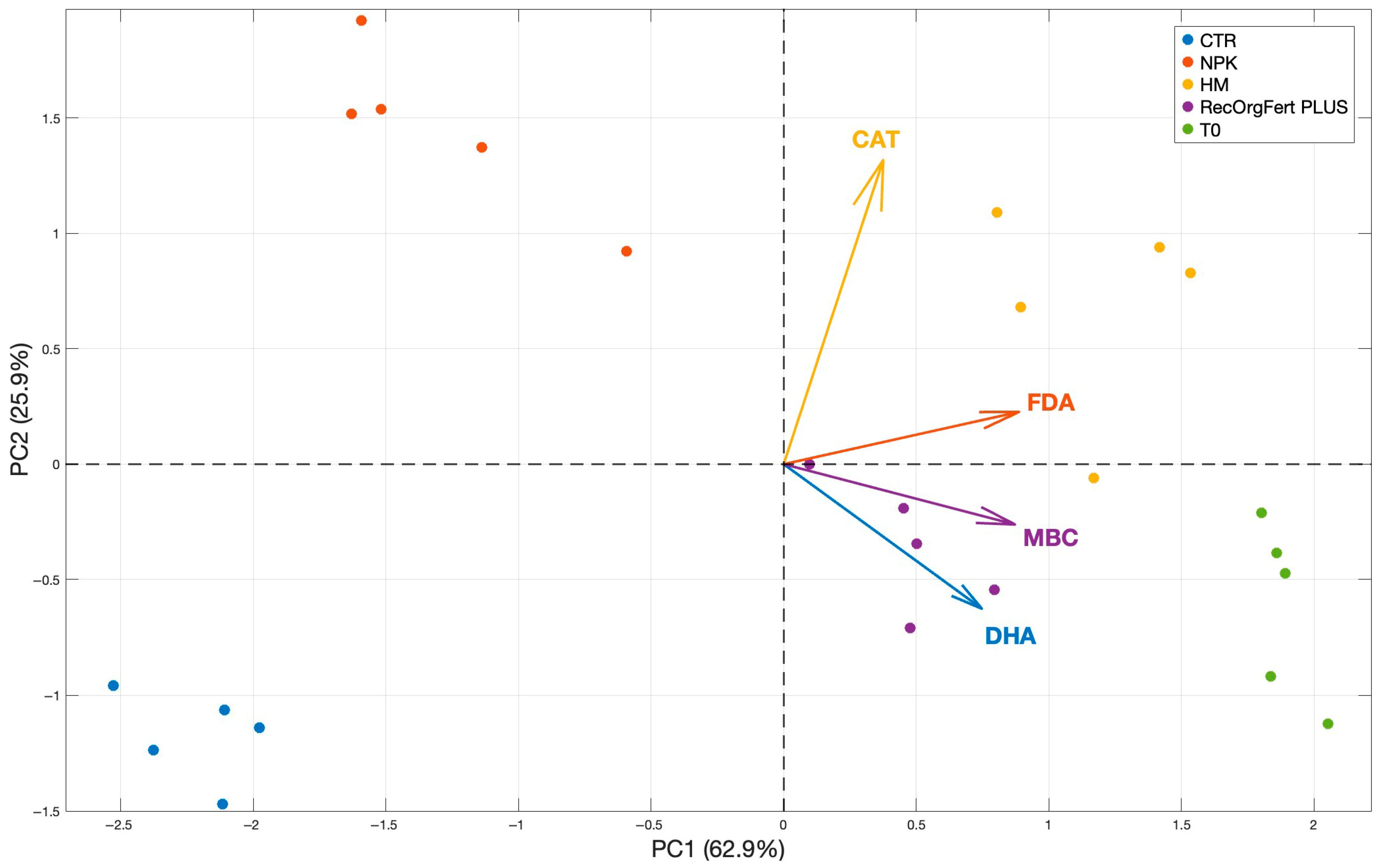
3.2. Biological Properties of the Soil
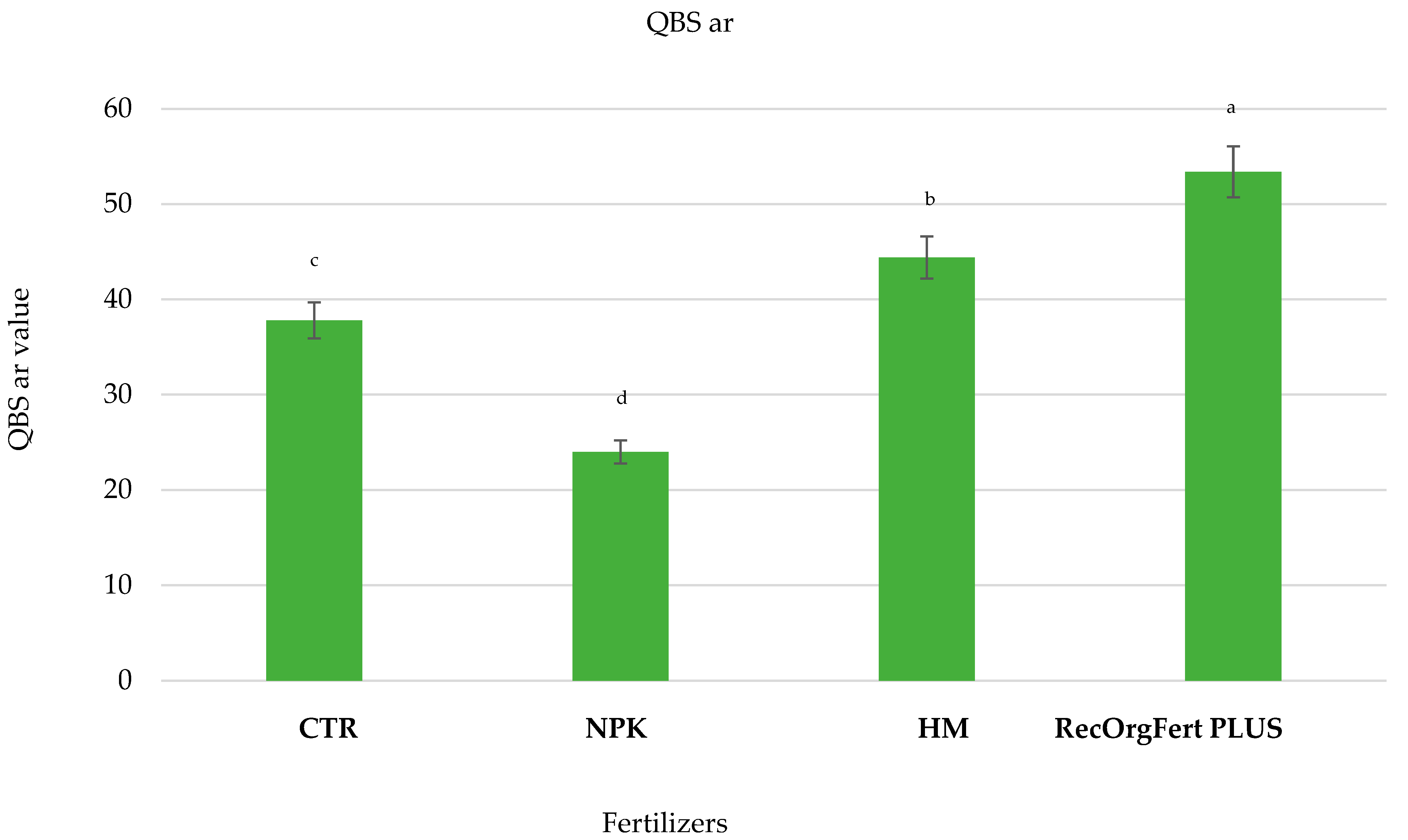
3.3. QBS-ar Index
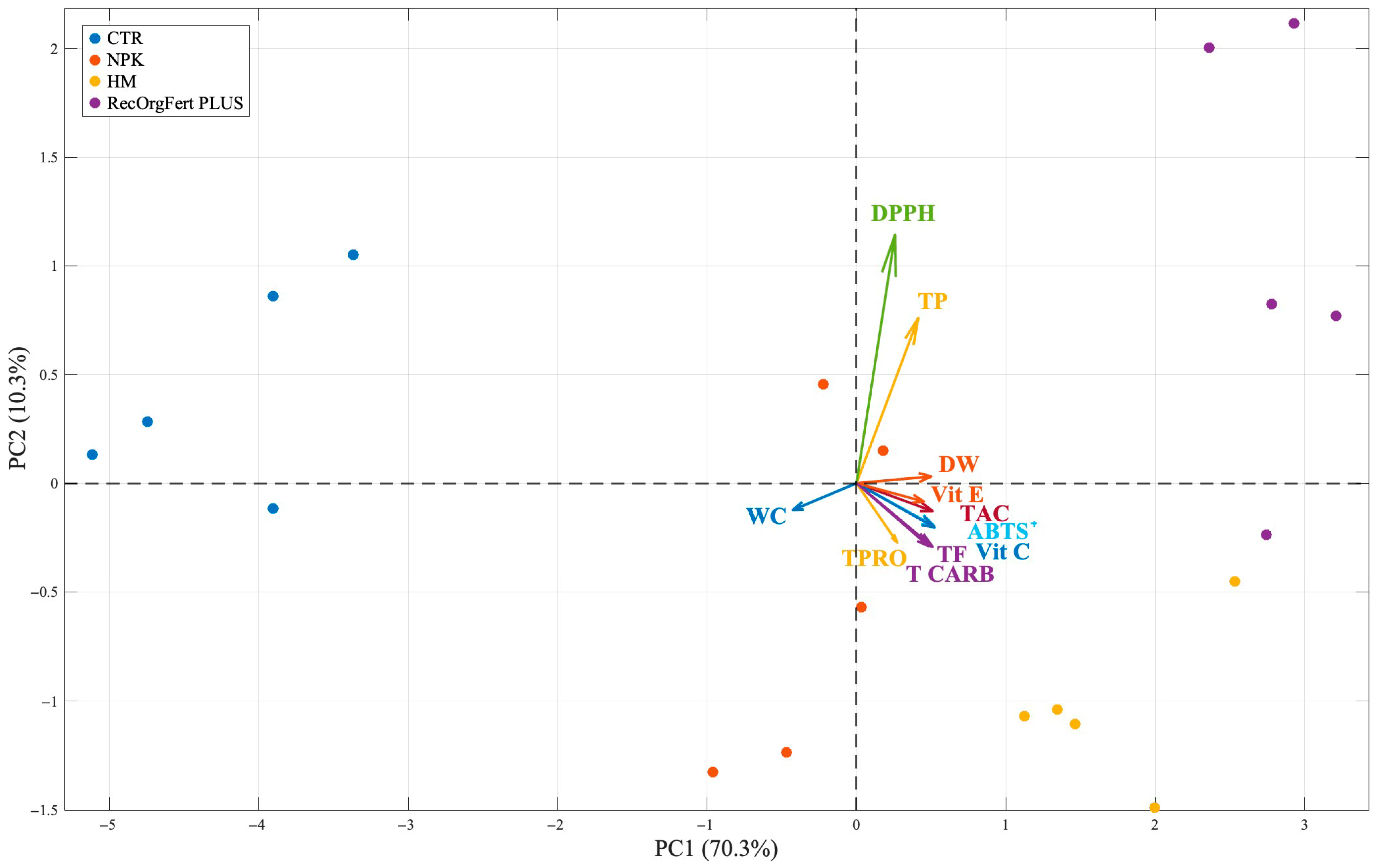
3.4. Broccoli Quality
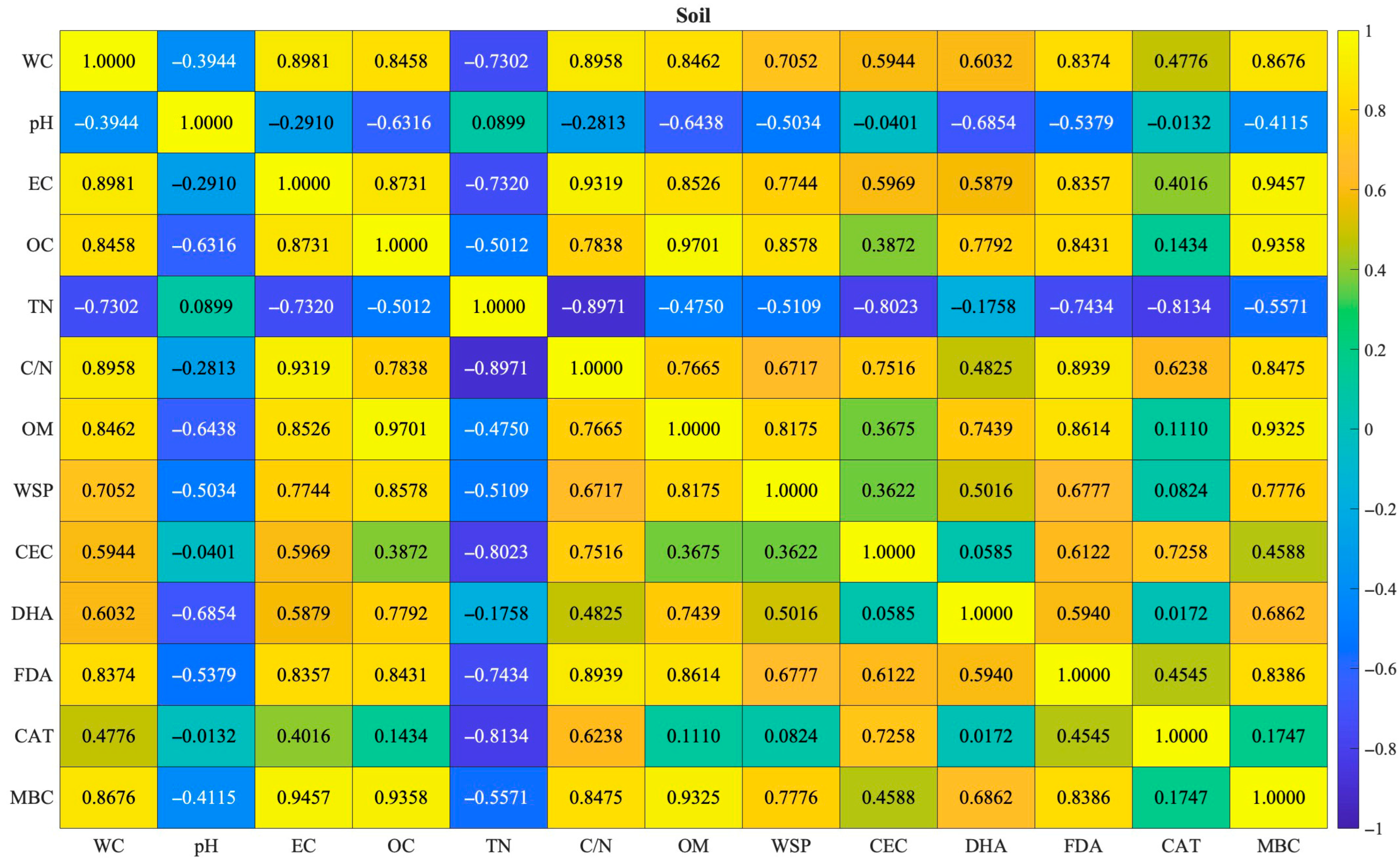
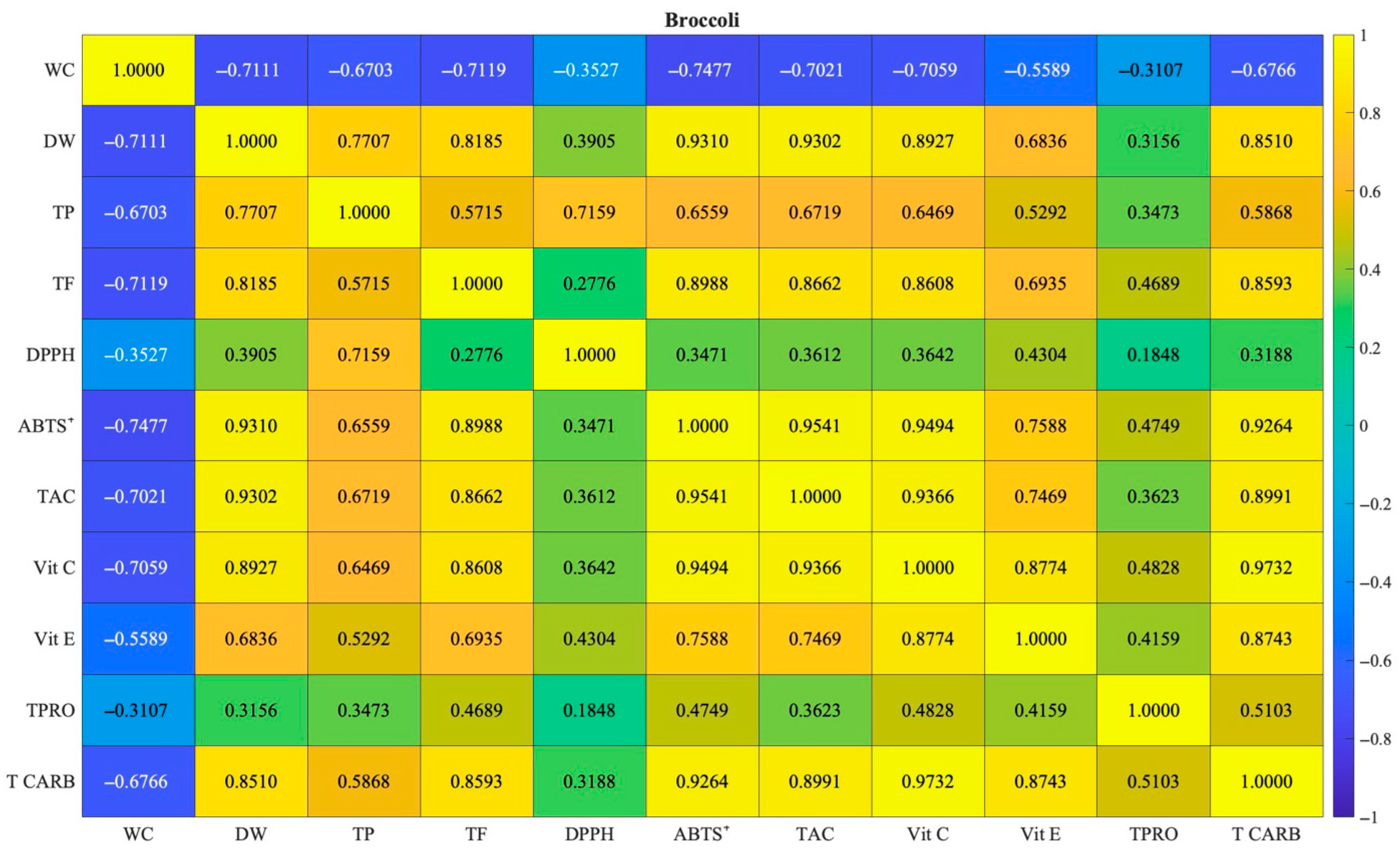
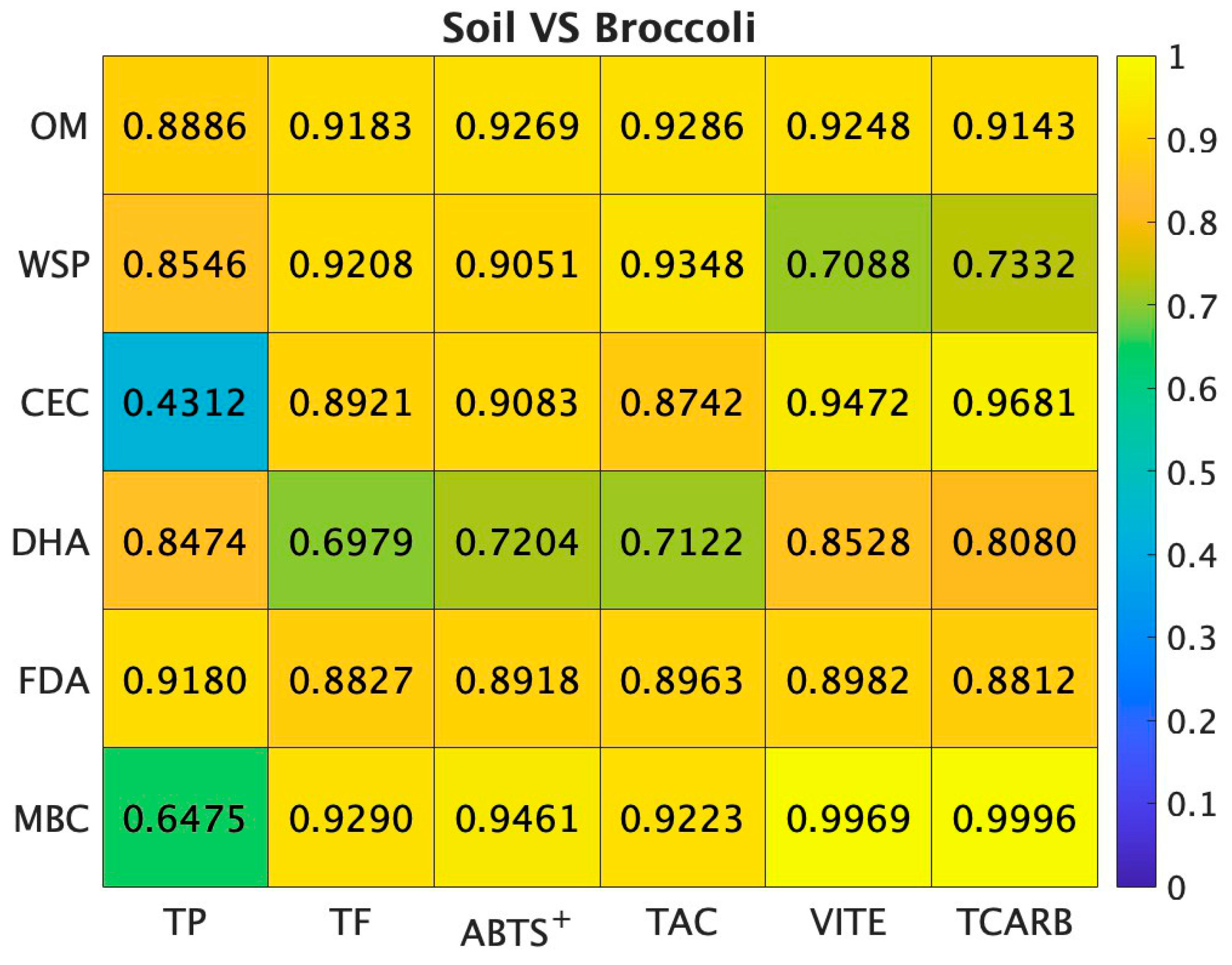
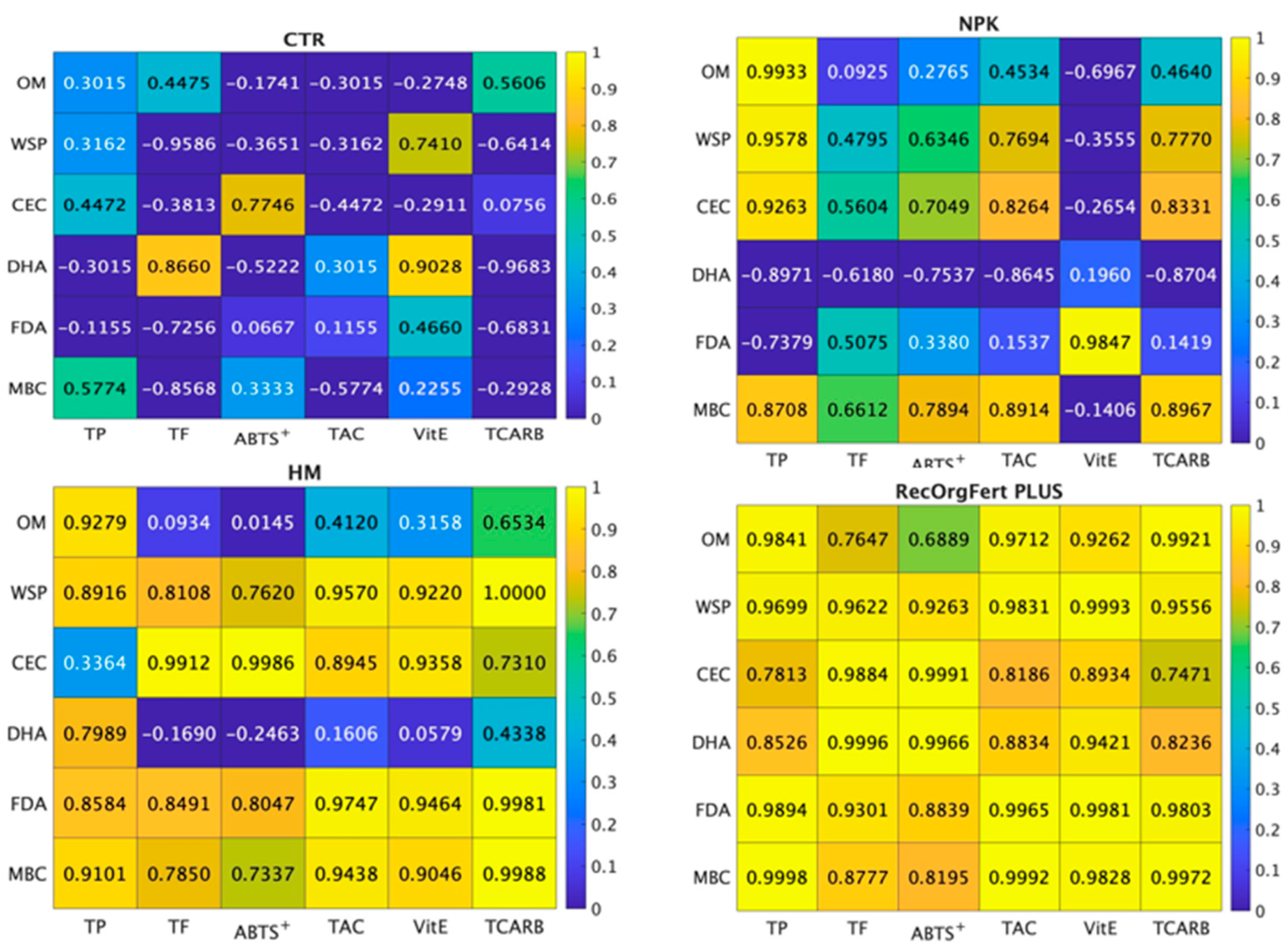
3.5. Correlations of Soil Parameters and Broccoli Quality Traits
4. Discussion
4.1. Soil Physicochemical Properties
4.2. Biological Properties of the Soil
4.3. QBS-ar Index
4.4. Broccoli Quality
4.5. Correlations of Soil Parameters and Broccoli Quality Traits
4.6. General Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| T0 | Time zero |
| CTR | Control, soil without fertilizer |
| NPK | Nitrogen–phosphorus–potassium |
| HM | Horse manure |
| S | Sulphur |
| WC | Water content |
| EC | Electrical conductivity |
| OC | Organic carbon |
| TN | Total nitrogen |
| C/N | Carbon–nitrogen ratio |
| OM | Organic matter |
| WSP | Water-soluble phenols |
| CEC | Cation exchange capacity |
| DHA | Dehydrogenase |
| FDA | Fluorescein diacetate hydrolase |
| CAT | Catalase |
| MBC | Microbial biomass carbon |
| DW | Dry weight |
| TP | Total phenols |
| TF | Total flavonoids |
| DPPH | 2,2-difenil-1-picrilidrazile |
| ABTS+ | 2,2′-azino-bis-3-etilbenzotiazolin-6-solfonato |
| TAC | Total antioxidant capacity |
| Vit C | Vitamin C |
| Vit E | Vitamin E |
| TPRO | Total proteins |
| TCARB | Total carbohydrates |
| PCA | Principal component analysis |
References
- Tahat, M.; Alananbeh, K.; Othman, Y.; Leskovar, D. Soil Health and Sustainable Agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Lazcano, C.; Boyd, E.; Holmes, G.; Hewavitharana, S.; Pasulka, A.; Ivors, K. The Rhizosphere Microbiome Plays a Role in the Resistance to Soil-Borne Pathogens and Nutrient Uptake of Strawberry Cultivars under Field Conditions. Sci. Rep. 2021, 11, 3188. [Google Scholar] [CrossRef]
- Amin, F.; Jilani, M.I. Environmental, Microbiological and Chemical Implications of Fertilizers Use in Soils: A Review. Int. J. Chem. Biochem. Sci. 2024, 25, 56–73. [Google Scholar]
- Zhang, L.; Zhao, Z.; Jiang, B.; Baoyin, B.; Cui, Z.; Wang, H.; Li, Q.; Cui, J. Effects of Long-Term Application of Nitrogen Fertilizer on Soil Acidification and Biological Properties in China: A Meta-Analysis. Microorganisms 2024, 12, 1683. [Google Scholar] [CrossRef]
- Badagliacca, G.; Testa, G.; La Malfa, S.G.; Cafaro, V.; Lo Presti, E.; Monti, M. Organic Fertilizers and Bio-Waste for Sustainable Soil Management to Support Crops and Control Greenhouse Gas Emissions in Mediterranean Agroecosystems: A Review. Horticulturae 2024, 10, 427. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-Term Effects of Organic Amendments on Soil Fertility. In Sustainable Agriculture Volume 2; Lichtfouse, E., Hamelin, M., Navarrete, M., Debaeke, P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2011; pp. 761–786. ISBN 978-94-007-0394-0. [Google Scholar]
- Ali, A.; Jabeen, N.; Farruhbek, R.; Chachar, Z.; Laghari, A.A.; Chachar, S.; Ahmed, N.; Ahmed, S.; Yang, Z. Enhancing Nitrogen Use Efficiency in Agriculture by Integrating Agronomic Practices and Genetic Advances. Front. Plant Sci. 2025, 16, 1543714. [Google Scholar] [CrossRef]
- Muscolo, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Panuccio, M.R. Sulfur Bentonite-Organic-Based Fertilizers as Tool for Improving Bio-Compounds with Antioxidant Activities in Red Onion. J. Sci. Food Agric. 2020, 100, 785–793. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Z.; Lv, Q.; Zhang, Y.; Tao, S.; Ren, X.; Gao, H.; Gao, Z.; Hu, S. Sulfur Dynamics in Saline Sodic Soils: The Role of Paddy Cultivation and Organic Amendments. Ecol. Indic. 2024, 162, 112014. [Google Scholar] [CrossRef]
- Singh, K.; Singh, R.; Nwosu, N.J.; Omara, P.; Sharma, L.; Dunn, B.L.; Singh, H. Optimizing Nutrient Delivery in Agronomic Crops: A Review of Enhanced Efficiency Fertilizers. J. Plant Nutr. Soil Sci. 2025. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Snapp, S.S. Advancing the Science and Practice of Ecological Nutrient Management for Smallholder Farmers. Front. Sustain. Food Syst. 2022, 6. [Google Scholar] [CrossRef]
- Mahn, A.; Reyes, A. An Overview of Health-Promoting Compounds of Broccoli (Brassica Oleracea Var. Italica) and the Effect of Processing. Food Sci. Technol. Int. 2012, 18, 503–514. [Google Scholar] [CrossRef]
- Shinali, T.S.; Zhang, Y.; Altaf, M.; Nsabiyeze, A.; Han, Z.; Shi, S.; Shang, N. The Valorization of Wastes and Byproducts from Cruciferous Vegetables: A Review on the Potential Utilization of Cabbage, Cauliflower, and Broccoli Byproducts. Foods 2024, 13, 1163. [Google Scholar] [CrossRef]
- Naguib, A.E.-M.M.; El-Baz, F.K.; Salama, Z.A.; Abd El Baky Hanaa, H.; Ali, H.F.; Gaafar, A.A. Enhancement of Phenolics, Flavonoids and Glucosinolates of Broccoli (Brassica Olaracea, Var. Italica) as Antioxidants in Response to Organic and Bio-Organic Fertilizers. J. Saudi Soc. Agric. Sci. 2012, 11, 135–142. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Settineri, G.; Papalia, T.; Mallamaci, C.; Attinà, E. Influence of Soil Properties on Bioactive Compounds and Antioxidant Capacity of Brassica Rupestris Raf. J. Soil Sci. Plant Nutr. 2019, 19, 808–815. [Google Scholar] [CrossRef]
- FAO. Agricultural Biodiversity: FAO Multifunctional Character of Agriculture and Land; FAO: Rome, Italy, 1999. [Google Scholar]
- Muscolo, A.; Mallamaci, C.; Settineri, G.; Calamarà, G. Increasing Soil and Crop Productivity by Using Agricultural Wastes Pelletized with Elemental Sulfur and Bentonite. Agron. J. 2017, 109, 1900–1910. [Google Scholar] [CrossRef]
- Muscolo, A.; Romeo, F.; Marra, F.; Mallamaci, C. Recycling Agricultural, Municipal and Industrial Pollutant Wastes into Fertilizers for a Sustainable Healthy Food Production. J. Environ. Manag. 2021, 300, 113771. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists (AOAC) International: Rockville, MA, USA, 2005. [Google Scholar]
- Mehlich, A. Rapid Determination of Cation and Anion Exchange Properties and pHe of Soils. J. Assoc. Off. Agric. Chem. 1953, 36, 445–457. [Google Scholar] [CrossRef][Green Version]
- Box, J.D. Investigation of the Folin-Ciocalteau Phenol Reagent for the Determination of Polyphenolic Substances in Natural Waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- von Mersi, W.; Schinner, F. An Improved and Accurate Method for Determining the Dehydrogenase Activity of Soils with Iodonitrotetrazolium Chloride. Biol. Fertil. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- Beck, T. Die Messung Der Katalaseaktivität von Böden. Z. Für Pflanzenernährung Und Bodenkd. 1971, 130, 68–81. [Google Scholar] [CrossRef]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil Enzyme Activities and Biodiversity Measurements as Integrative Microbiological Indicators. In Methods for Assessing Soil Quality; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1997; pp. 247–271. ISBN 978-0-89118-944-2. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bano, R.; Roy, S. Extraction of Soil Microarthropods: A Low Cost Berlese-Tullgren Funnels Extractor. Int. J. Fauna Biol. Stud. 2016, 2, 14–17. [Google Scholar]
- Angelini, P.; Fenoglio, S.; Isaia, M.; Jacomini, C.; Migliorini, M.; Morisi, A. Biomonitoraggio. In Tecniche di Biomonitoraggio del Suolo; cedam: Torino, Italy, 2002. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid Composition and Antioxidant Activity of Juices from Chinotto (Citrus × Myrtifolia Raf.) Fruits at Different Ripening Stages. J. Agric. Food Chem. 2010, 58, 3031–3036. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Davies, S.H.R.; Masten, S.J. Spectrophotometric Method for Ascorbic Acid Using Dichlorophenolindophenol: Elimination of the Interference Due to Iron. Anal. Chim. Acta 1991, 248, 225–227. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method For Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. ISBN 978-1-59745-198-7. [Google Scholar]
- Hedge, J.E.; Hofreiter, B.T.; Whistler, R.L. Carbohydrate Chemistry; Academic Press: New York, NY, USA, 1962; Volume 17, pp. 71–80. [Google Scholar]
- Țopa, D.-C.; Căpșună, S.; Calistru, A.-E.; Ailincăi, C. Sustainable Practices for Enhancing Soil Health and Crop Quality in Modern Agriculture: A Review. Agriculture 2025, 15, 998. [Google Scholar] [CrossRef]
- Lazcano, C.; Domínguez, J. The Use of Vermicompost in Sustainable Agriculture: Impact on Plant Growth and Soil Fertility. Soil Nutr. 2011, 10, 187. [Google Scholar]
- Liu, X.; Yang, Y.; Gao, B.; Li, Y.; Wan, Y. Environmentally Friendly Slow-Release Urea Fertilizers Based on Waste Frying Oil for Sustained Nutrient Release. ACS Sustain. Chem. Eng. 2017, 5, 6036–6045. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Li, Y.; Han, X.; Li, B.; Zhang, X.; Li, Q.; Liang, W. Organic Substitutions Improve Soil Quality and Maize Yield through Increasing Soil Microbial Diversity. J. Clean. Prod. 2022, 347, 131323. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; Deyn, G.D.; Gattinger, A. Organic Farming Enhances Soil Microbial Abundance and Activity—A Meta-Analysis and Meta-Regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef] [PubMed]
- Sritongon, N.; Sarin, P.; Theerakulpisut, P.; Riddech, N. The Effect of Salinity on Soil Chemical Characteristics, Enzyme Activity and Bacterial Community Composition in Rice Rhizospheres in Northeastern Thailand. Sci. Rep. 2022, 12, 20360. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Vashisht, B.B.; Singh, P.; Singh, Y. Changes in Soil Aggregate-Associated Organic Carbon, Enzymatic Activity, and Biological Pools under Conservation Agriculture Based Practices in Rice–Wheat System. Biomass Convers. Biorefinery 2023, 13, 13977–13994. [Google Scholar] [CrossRef]
- Song, R.; Zhu, W.Z.; Li, H.; Wang, H. Impact of Wine-Grape Continuous Cropping on Soil Enzyme Activity and the Composition and Function of the Soil Microbial Community in Arid Areas. Front. Microbiol. 2024, 15, 1348259. [Google Scholar] [CrossRef]
- Mulatu, G.; Bayata, A. Vermicompost as Organic Amendment: Effects on Some Soil Physical, Biological Properties and Crops Performance on Acidic Soil: A Review. Finite Elem. Method 2024, 10, 66–73. [Google Scholar] [CrossRef]
- Rodríguez-Pajares, C.; Muñoz-Adalia, E.J.; Fernández-Fernández, M. Microarthropods Communities as Indicators of Soil Quality in a Mediterranean Periurban Forest Using the QBS-Ar Index. For. Syst. 2025, 34, 20906. [Google Scholar] [CrossRef]
- Churchland, C.; Grayston, S.J. Specificity of Plant-Microbe Interactions in the Tree Mycorrhizosphere Biome and Consequences for Soil C Cycling. Front. Microbiol. 2014, 5, 261. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Lee, J.-H.; Seo, H.-R.; Kim, J.-W.; Cho, Y.-S.; Lee, D.; Kim, B.-H.; Yoon, J.-H.; Choe, H.; Lee, Y.B.; et al. Co-Responses of Soil Organic Carbon Pool and Biogeochemistry to Different Long-Term Fertilization Practices in Paddy Fields. Plants 2022, 11, 3195. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic Agriculture in the Twenty-First Century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.C.; Geisen, S.; Wall, D.H. Chapter 5-Soil Fauna: Occurrence, Biodiversity, and Roles in Ecosystem Function. In Soil Microbiology, Ecology and Biochemistry, 5th ed.; Paul, E.A., Frey, S.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 131–159. ISBN 978-0-12-822941-5. [Google Scholar]
- Muscolo, A.; Marra, F.; Canino, F.; Maffia, A.; Mallamaci, C.; Russo, M. Growth, Nutritional Quality and Antioxidant Capacity of Lettuce Grown on Two Different Soils with S-Based Fertilizer, Organic and Chemical Fertilizers. Sci. Hortic. 2022, 305, 111421. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, X.; Hou, H.; Ji, J.; Liu, X.; Lv, Z. Multifaceted Ability of Organic Fertilizers to Improve Crop Productivity and Abiotic Stress Tolerance: Review and Perspectives. Agronomy 2024, 14, 1141. [Google Scholar] [CrossRef]
- Laing, W.A.; Martínez-Sánchez, M.; Wright, M.A.; Bulley, S.M.; Brewster, D.; Dare, A.P.; Rassam, M.; Wang, D.; Storey, R.; Macknight, R.C.; et al. An Upstream Open Reading Frame Is Essential for Feedback Regulation of Ascorbate Biosynthesis in Arabidopsis. Plant Cell 2015, 27, 772–786. [Google Scholar] [CrossRef]
- Howe, J.A.; McDonald, M.D.; Burke, J.; Robertson, I.; Coker, H.; Gentry, T.J.; Lewis, K.L. Influence of Fertilizer and Manure Inputs on Soil Health: A Review. Soil Secur. 2024, 16, 100155. [Google Scholar] [CrossRef]
- Ollio, I.; Santás-Miguel, V.; Gómez, D.S.; Lloret, E.; Sánchez-Navarro, V.; Martínez-Martínez, S.; Egea-Gilabert, C.; Fernández, J.A.; Calviño, D.F.; Zornoza, R. Effect of Biofertilizers on Broccoli Yield and Soil Quality Indicators. Horticulturae 2023, 10, 42. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.; Singh, K.; Katoch, K.; Zaeen, A.A.; Birhan, D.A.; Singh, A.; Sandhu, H.S.; Singh, H.; Sahrma, L.K. Smart Fertilizer Technologies: An Environmental Impact Assessment for Sustainable Agriculture. Smart Agric. Technol. 2024, 8, 100504. [Google Scholar] [CrossRef]
| T0 | CTR | NPK | HM | RecOrgFert PLUS | |
|---|---|---|---|---|---|
| WC | 8.25 c ± 0.91 | 10.1 b ± 0.98 | 14.2 a ± 1.1 | 13.4 a ± 1.1 | 14.5 a ± 0.97 |
| pH | 8.16 a ± 0.09 | 8.30 a ± 0.1 | 8.04 b ± 0.1 | 8.25 a ± 0.09 | 7.5 c ± 0.1 |
| EC | 0.10 c ± 0.09 | 0.40 b ± 0.09 | 1.08 a ± 0.1 | 1.22 a ± 0.08 | 1.16 a ± 0.08 |
| OC | 1.13 d ± 0.18 | 1.17 d ± 0.18 | 2.3 c ± 0.2 | 2.54 b ± 0.18 | 3.37 a ± 0.17 |
| TN | 1.09 a ± 0.02 | 0.11 d ± 0.02 | 0.14 c ± 0.01 | 0.162 b ± 0.01 | 0.194 b ± 0.01 |
| C/N | 1.04 c ± 1 | 10.6 b ± 0.9 | 16.5 a ± 1 | 15.7 a ± 0.9 | 17.4 a ± 1 |
| OM | 1.95 d ± 0.28 | 2.02 d ± 0.35 | 3.99 c ± 0.3 | 4.39 b ± 0.26 | 5.8 a ± 0.32 |
| WSP | 23.3 c ± 1.7 | 26.7 b ± 2.0 | 27.5 b ± 1.6 | 33.4 a ± 1.7 | 35.9 a ± 2.0 |
| CEC | 15.0 b ± 1 | 16.5 a ± 1 | 17.1 a ± 0.98 | 17.3 a ± 0.97 | 17.1 a ± 0.85 |
| DHA | 1.4 b ± 0.28 | 1.1 b ± 0.29 | 2.0 a ± 0.3 | 1.6 b ± 0.3 | 2.4 a ± 0.25 |
| FDA | 13.3 b ± 0.51 | 14.3 b ± 0.48 | 15.3 a ± 0.46 | 15.4 a ± 0.53 | 16.3 a ± 0.55 |
| CAT | 1.3 c ± 0.33 | 3.3 a ± 0.27 | 3.2 a ± 0.29 | 2.1 b ± 0.31 | 2.2 b ± 0.33 |
| MBC | 205 c ± 17.6 | 218 c ± 19.1 | 684 b ± 14.5 | 764 a ± 14.2 | 791 a ± 21.1 |
| Cations and Anions | T0 | CTR | NPK | HM | RecOrgFert PLUS |
|---|---|---|---|---|---|
| Na+ | 0.012 b ± 0.0003 | 0.016 b ± 0.0003 | 0.025 a ± 0.0003 | 0.024 a ± 0.0002 | 0.026 a ± 0.0003 |
| K+ | 0.013 b ± 0.0003 | 0.012 b ± 0.0003 | 0.027 a ± 0.0003 | 0.025 a ± 0.0002 | 0.030 a ± 0.0003 |
| Ca2+ | 0.02 a ± 0.005 | 0.03 a ± 0.005 | 0.03 a ± 0.005 | 0.03 a ± 0.004 | 0.03 a ± 0.005 |
| Mg2+ | 7.6 b ± 0.82 | 9.3 a ± 0.79 | 9.3 a ± 0.85 | 9.3 a ± 0.83 | 9.7 a ± 0.78 |
| Cl− | 0.55 b ± 0.14 | 0.68 a ± 0.16 | 0.66 a ± 0.15 | 0.67 a ± 0.13 | 0.66 a ± 0.14 |
| NO2− | nd | 0.001 b ± 0.0001 | 0.007 a ± 0.0001 | 0.001 b ± 0.0001 | 0.007 a ± 0.0001 |
| NO3− | nd | nd | nd | 0.026 a ± 0.0105 | nd |
| PO43− | nd | nd | 0.002 b ± 0.0001 | 0.006 a ± 0.0001 | 0.002 b ± 0.0001 |
| SO42− | 0.001 c ± 0.0003 | 0.001 c ± 0.0003 | 0.002 b ± 0.0003 | 0.002 b ± 0.0002 | 0.003 a ± 0.0001 |
| CTR | NPK | HM | RecOrgFert PLUS | |
|---|---|---|---|---|
| WC | 90.65 a ± 2.1 | 89.14 a ± 2.5 | 87.24 a ± 2.4 | 86.32 a ± 2.6 |
| DW | 9.35 a ± 0.9 | 10.86 b ± 0.5 | 12.76 b ± 0.6 | 13.68 c ± 0.4 |
| TP | 42.09 b ± 1.8 | 43.35 b ± 1.9 | 43.86 b ± 1.9 | 48.87 a ± 2.1 |
| TF | 5.29 b ± 0.3 | 5.76 b ± 0.3 | 6.28 a ± 0.4 | 6.29 a ± 0.4 |
| DPPH | 23.25 a ± 1.2 | 23.54 a ± 1.2 | 23.37 a ± 1.2 | 24.85 a ± 1.3 |
| ABTS+ | 3.39 c ± 0.2 | 4.37 b ± 0.2 | 5.26 a ± 0.3 | 5.29 a ± 0.3 |
| TAC | 4.43 c ± 0.3 | 5.62 b ± 0.3 | 6.98 a ± 0.4 | 7.13 a ± 0.4 |
| Vit C | 18.98 c ± 1.1 | 42.17 b ± 2.3 | 50.97 a ± 2.8 | 51.93 a ± 2.9 |
| Vit E | 1.52 b ± 0.1 | 1.87 a ± 0.1 | 1.89 a ± 0.1 | 1.93 a ± 0.1 |
| TPRO | 80.21 a ± 3.2 | 83.05 a ± 3.3 | 82.42 a ± 3.3 | 82.45 a ± 3.3 |
| TCARB | 124.07 c ± 12.4 | 220.65 b ± 12.1 | 237.93 a ± 12.8 | 239.93 a ± 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, M.; Marra, F.; Santoro, L.; Battaglia, S.; Mallamaci, C.; Muscolo, A. Circular Fertilization Strategy Using Sulphur with Orange Waste Enhances Soil Health and Broccoli Nutritional and Nutraceutical Quality in Mediterranean Systems. Appl. Sci. 2025, 15, 9010. https://doi.org/10.3390/app15169010
Oliva M, Marra F, Santoro L, Battaglia S, Mallamaci C, Muscolo A. Circular Fertilization Strategy Using Sulphur with Orange Waste Enhances Soil Health and Broccoli Nutritional and Nutraceutical Quality in Mediterranean Systems. Applied Sciences. 2025; 15(16):9010. https://doi.org/10.3390/app15169010
Chicago/Turabian StyleOliva, Mariateresa, Federica Marra, Ludovica Santoro, Santo Battaglia, Carmelo Mallamaci, and Adele Muscolo. 2025. "Circular Fertilization Strategy Using Sulphur with Orange Waste Enhances Soil Health and Broccoli Nutritional and Nutraceutical Quality in Mediterranean Systems" Applied Sciences 15, no. 16: 9010. https://doi.org/10.3390/app15169010
APA StyleOliva, M., Marra, F., Santoro, L., Battaglia, S., Mallamaci, C., & Muscolo, A. (2025). Circular Fertilization Strategy Using Sulphur with Orange Waste Enhances Soil Health and Broccoli Nutritional and Nutraceutical Quality in Mediterranean Systems. Applied Sciences, 15(16), 9010. https://doi.org/10.3390/app15169010








