Surface Charge and Phosphorus Retention in Metal-Activated Biochars from Different Pyrolysis Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Pyrolysis for Biochar Production
2.2. Preparation of Activated Biochar
2.3. Characterisation of Biochar by ATR-FTIR
2.4. Determination of Cation Exchange Capacity (CEC) and Metals Absorbed by Biochar
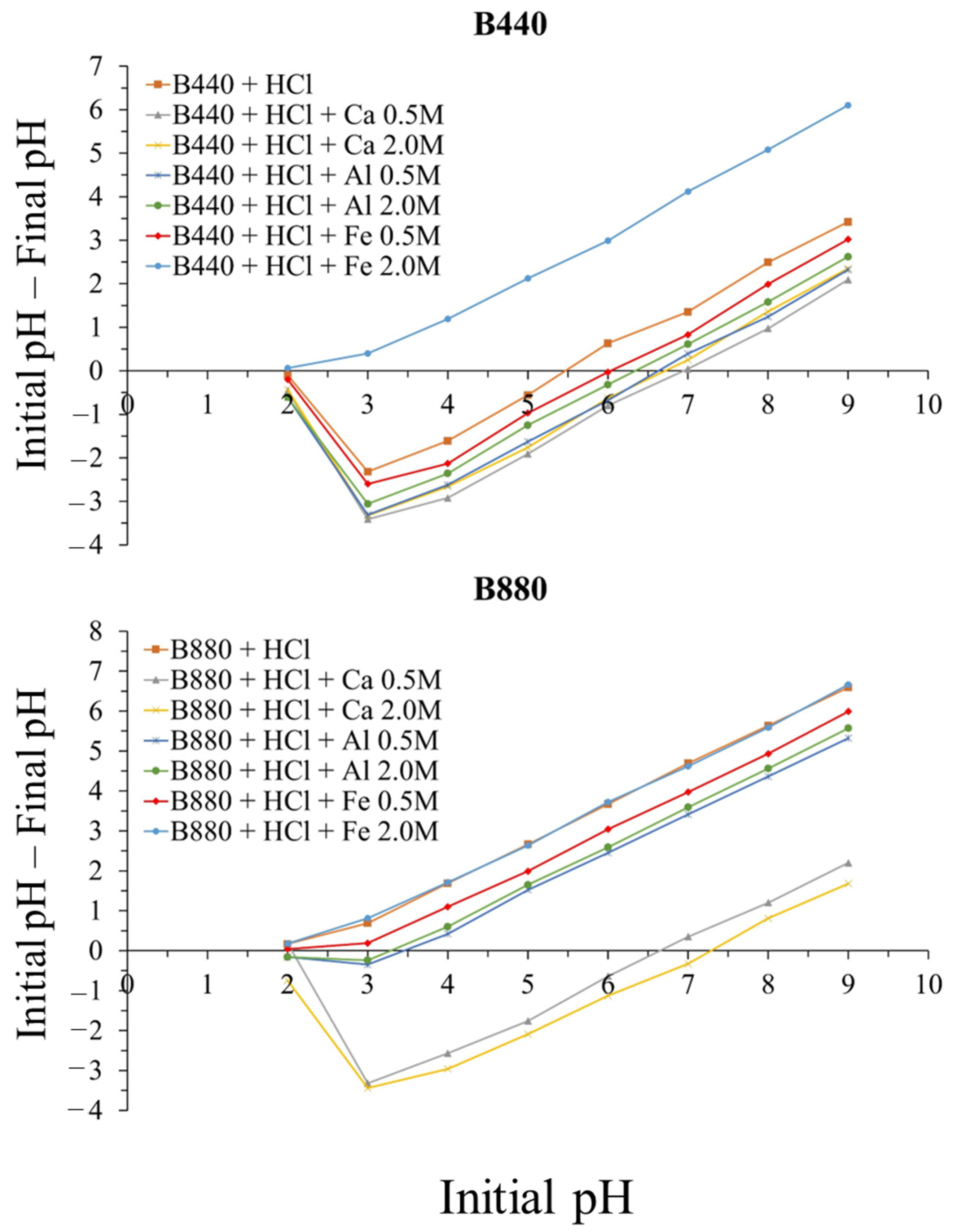
2.5. Determination of pH and pH Value at Zero-Point Charge of Biochars
2.6. Phosphate Absorption by Biochar
2.7. Phosphate Determination
2.8. Phosphate Adsorption Isotherms and Model Fitting
3. Results
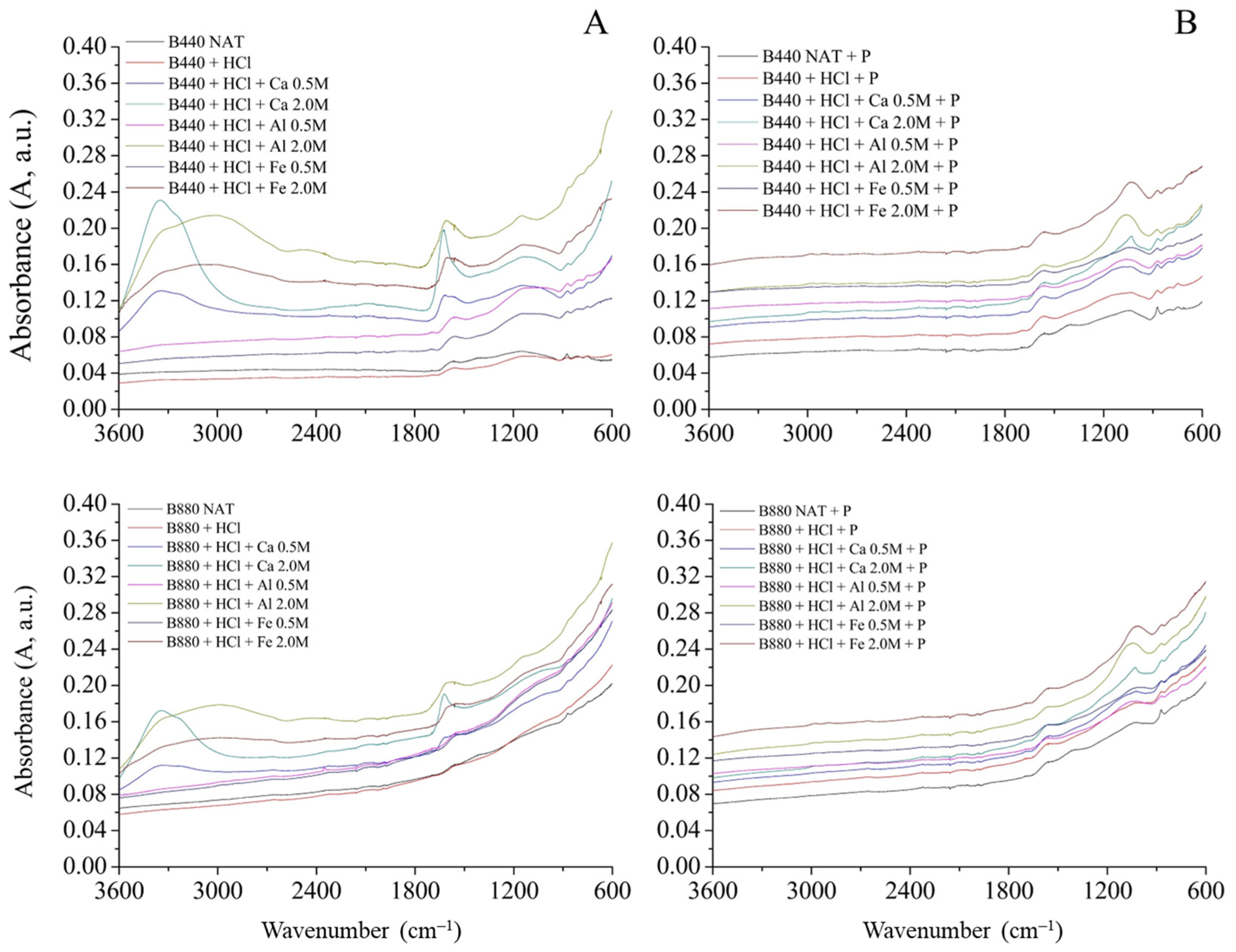
3.1. ATR-FTIR Spectra of B440 and B880 Biochars
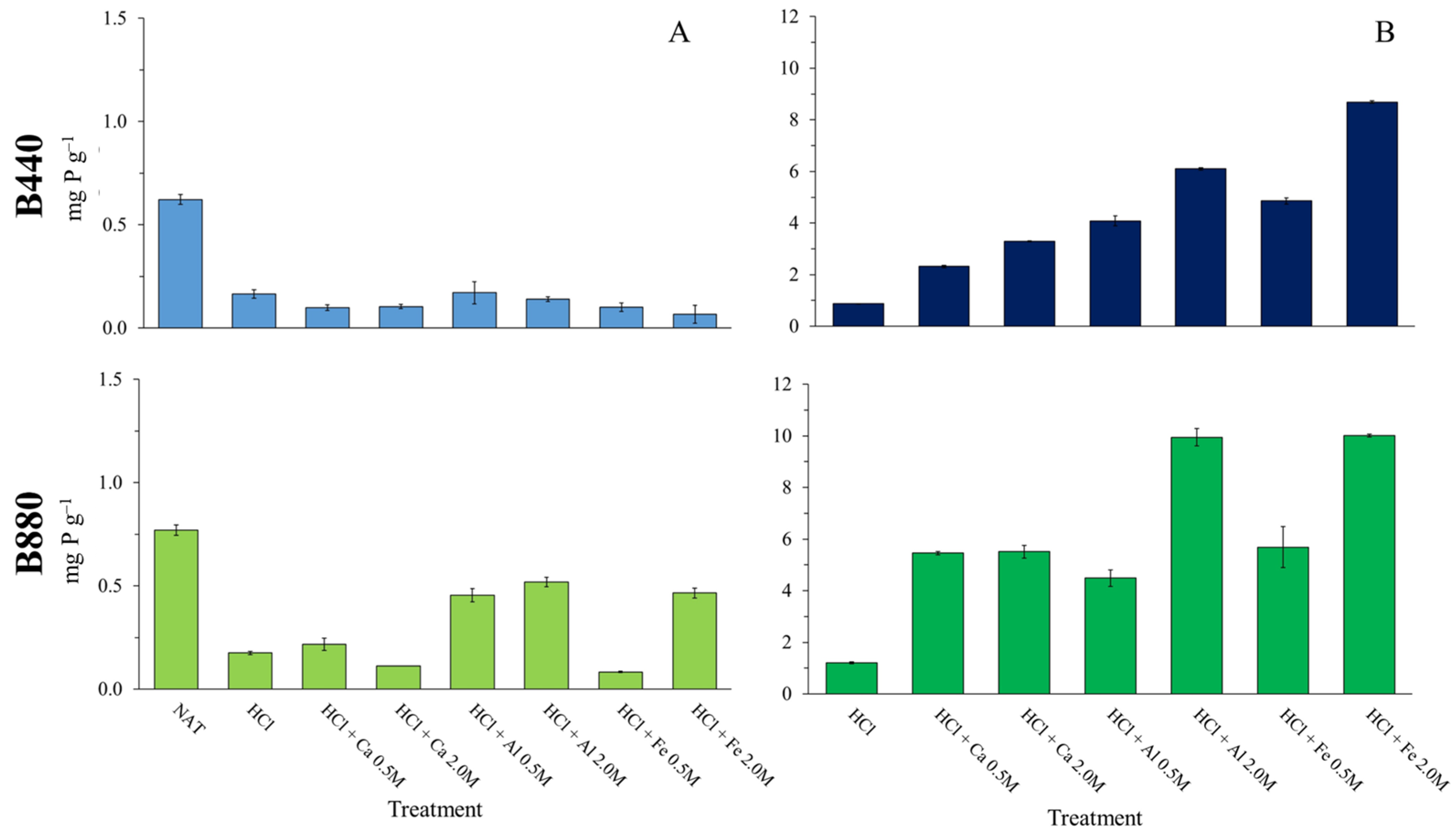
3.2. Metal Absorption by Biochars
3.3. Effect of HCl and Metal Salt Treatments on Phosphorus Adsorption
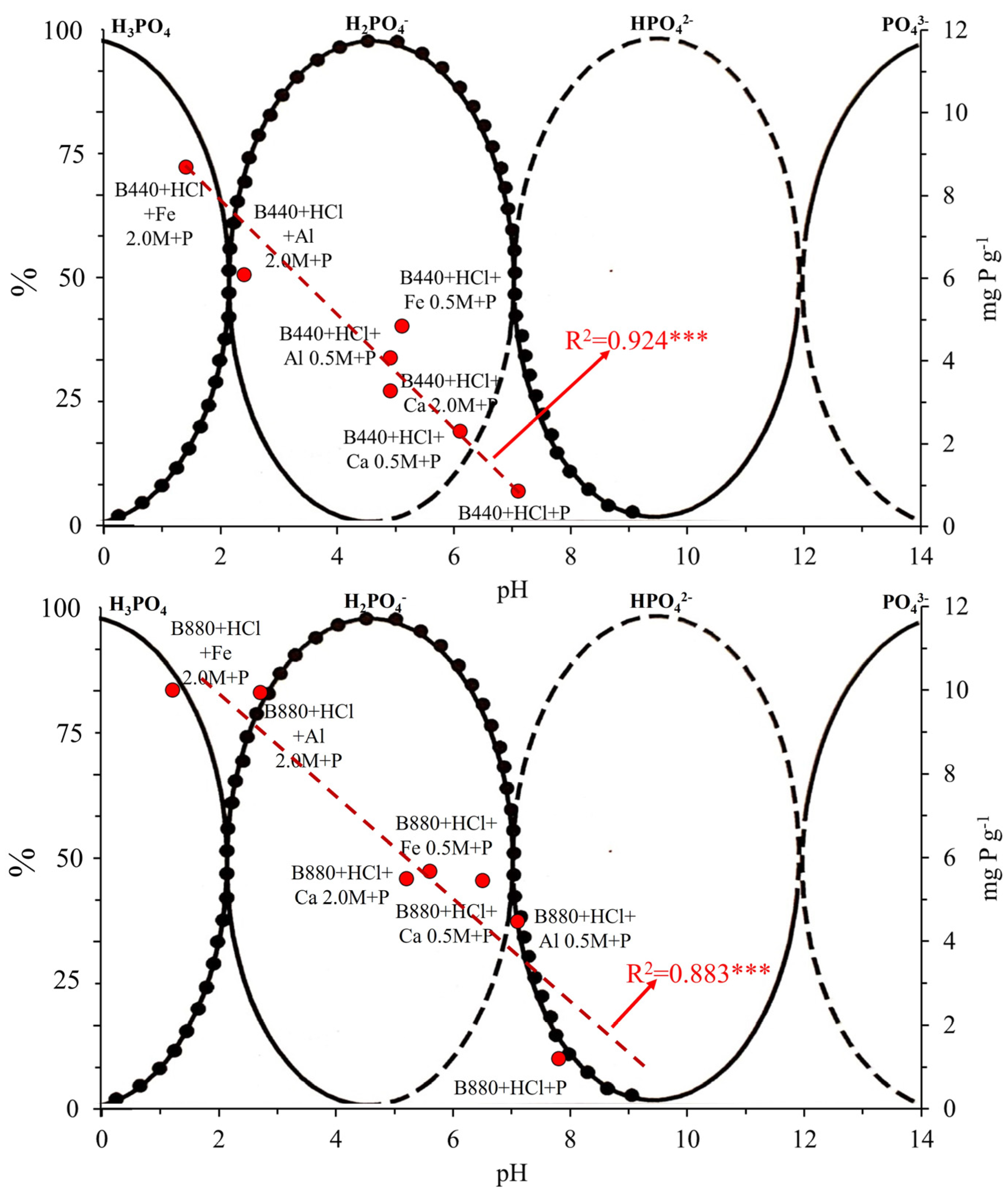
3.4. Influence of Solution pH and Surface Charge on P Adsorption
3.5. Mechanistic Interpretation Based on FTIR Evidence
3.6. Phosphate Adsorption Isotherms and Comparison with Theoretical Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Fertilizer Trends and Outlook to 2022, Summary Report; Food and Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus flows and balances of the European Union Member States. Sci. Total Environ. 2016, 542 Pt B, 1078–1093. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Tracking phosphorus security: Indicators of phosphorus vulnerability in the global food system. Food Secur. 2015, 7, 337–350. [Google Scholar] [CrossRef]
- Saliu, T.D.; Oladoja, N.A. Nutrient recovery from wastewater and reuse in agriculture: A review. Environ. Chem. Lett. 2021, 19, 2299–2316. [Google Scholar] [CrossRef]
- Hashem, M.S.; Qi, X. Treated wastewater irrigation—A review. Water 2021, 13, 1527. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Mannina, G.; Alduina, R.; Badalucco, L.; Barbara, L.; Capri, F.C.; Cosenza, A.; Di Trapani, D.; Gallo, G.; Laudicina, V.A.; Muscarella, S.M.; et al. Water resource recovery facilities (WRRFs): The case study of Palermo University (Italy). Water 2021, 13, 3413. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Ping, L.; Jiang, R.; Wang, Y. Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef]
- Wang, Y.; Munir, T.; Wu, X.; Huang, Y.; Li, B. Phosphorus recovery and reuse: Innovating with biochar in the circular economy. Sci. Total Environ. 2025, 973, 179143. [Google Scholar] [CrossRef]
- Nobaharan, K.; Bagheri Novair, S.; Asgari Lajayer, B.; van Hullebusch, E. Phosphorus removal from wastewater: The potential use of biochar and the key controlling factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Di Trapani, D.; Laudicina, V.A.; Mannina, G. Phosphorus recovery from ultrafiltered membrane wastewater by biochar adsorption columns: The effect of loading rates. Heliyon 2024, 10, e34659. [Google Scholar] [CrossRef]
- Bulacio Fischer, P.T.; Di Trapani, D.; Laudicina, V.A.; Muscarella, S.M.; Mannina, G. Nutrient Recovery from Zeolite and Biochar Columns: The Case Study of Marineo (Italy) Wastewater Treatment Plant. Water 2025, 17, 848. [Google Scholar] [CrossRef]
- Mannina, G.; Badalucco, L.; Barbara, L.; Cosenza, A.; Di Trapani, D.; Laudicina, V.A.; Muscarella, S.M.; Presti, D. Roadmapping the transition to water resource recovery facilities: The two demonstration case studies of Corleone and Marineo (Italy). Water 2022, 14, 156. [Google Scholar] [CrossRef]
- Paliaga, S.; Muscarella, S.M.; Alduina, R.; Badalucco, L.; Bulacio Fischer, P.T.; Di Leto, Y.; Gallo, G.; Gaglio, R.; Mineo, A.; Laudicina, V.A.; et al. Resource recovery from wastewater treatment: Effects of water reuse and slow-release fertilizers on faba bean within Palermo University (Italy) case study. J. Environ. Manag. 2025, 373, 123839. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Tao, C.; Tian, Z.; Huang, Z.; Lin, H.; Qi, C.; Yu, Z.; Guo, L. Characterization and mechanism of phosphorus adsorption from wastewater by lanthanum calcium doped sludge/wheat straw biochar. Front. Environ. Sci. 2025, 13, 1604542. [Google Scholar] [CrossRef]
- Almanassra, I.W.; McKay, G.; Kochkodan, V.; Atieh, M.A.; Al-Ansari, T. A state of the art review on phosphate removal from water by biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Conte, P.; Bertani, R.; Sgarbossa, P.; Bambina, P.; Schmidt, H.P.; Raga, R.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P. Recent developments in understanding biochar’s physical–chemistry. Agronomy 2021, 11, 615. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.P.; Hu, M.F. Magnesium ammonium phosphate crystallization: A possible way for recovery of phosphorus from wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 032032. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.P.; Purakayastha, T.J. Characterization of biochar and their influence on microbial activities and potassium availability in an acid soil. Arch. Agron. Soil Sci. 2019, 65, 1302–1315. [Google Scholar] [CrossRef]
- Pan, F.; Wei, H.; Huang, Y.; Guo, W.; Song, J.; Zhang, Z.; Teng, R.; Gao, M.; Jing, S.; Shi, B. Biochar-Enabled Phosphorus Recovery from Aqueous: Advances, Applications and Challenges. Environ. Res. 2025, 285, 122329. [Google Scholar] [CrossRef]
- Wu, X.; Quan, W.; Chen, Q.; Gong, W.; Wang, A. Efficient Adsorption of Nitrogen and Phosphorus in Wastewater by Biochar. Molecules 2024, 29, 1005. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Wang, X.; Zhang, S.; Chen, H. Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: Influence of temperature. Bioresour. Technol. 2012, 107, 411–418. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus absorption by functionalized biochar: A review. Environ. Chem. Lett. 2022, 21, 497–524. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Hu, X.; Wan, Y.; Wang, S.; Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Pan, J.; Jiang, Y.; Zou, X.; Wang, Y. Low-cost Ca/Mg co-modified biochar for effective phosphorus recovery: Adsorption mechanisms, resourceful utilization, and life cycle assessment. Chem. Eng. J. 2024, 502, 157993. [Google Scholar] [CrossRef]
- Zhao, Q.G.; Li, B.; Zhou, X. Enhanced phosphate removal in water by magnesium-modified biochar/yttrium alginate hybrid biogel polymer. J. Rare Earths 2025, in press. [CrossRef]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Henriksen, S.W.; Andersen, M.N. Mechanism of orthophosphate (PO4–P) absorption onto different biochars. Environ. Technol. Innov. 2020, 17, 100572. [Google Scholar] [CrossRef]
- Chemerys, V.; Baltrėnaitė, E. Modified Biochar: A review on modifications of biochar towards its enhanced absorptive properties. Ann. Agric. Environ. Sci. 2016. Available online: https://etalpykla.vilniustech.lt/handle/123456789/117258 (accessed on 8 August 2025).
- Choi, Y.; Jang, H.M.; Kan, E.; Wallace, A.R.; Sun, W. Absorption of phosphate in water on a novel calcium hydroxide-coated dairy manure-derived biochar. J. Environ. Eng. 2019, 24, 434–442. [Google Scholar]
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of biochar for resource recovery from water: A review. Chem. Eng. J. 2020, 397, 125502. [Google Scholar] [CrossRef]
- Fu, W.; Li, M.; Chen, H.; Qu, J.; Zhang, L.; Qiu, S.; Feng, M.; Yuan, M.; Guo, C.; Zhou, J.; et al. Novel utilization exploration for the dephosphorization waste of Ca–modified biochar: Enhanced removal of heavy metal ions from water. Biochar 2024, 6, 77. [Google Scholar] [CrossRef]
- Liu, L.; He, N.; Borham, A.; Zhang, S.; Xie, R.; Zhao, C.; Hu, J.; Wang, J. The Effect of Iron-Modified Biochar on Phosphorus Adsorption and the Prospect of Synergistic Adsorption between Biochar and Iron-Oxidizing Bacteria: A Review. Water 2023, 15, 3315. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, B.; Wester, A.E.; Chen, J.; He, F.; Chen, H.; Gao, B. Reclaiming phosphorus from secondary treated municipal wastewater with engineered biochar. Chem. Eng. J. 2019, 362, 460–468. [Google Scholar] [CrossRef]
- Zhong, Z.; Yu, G.; Mo, W.; Zhang, C.; Huang, H.; Li, S.; Gao, M.; Lu, X.; Zhang, B.; Zhu, H. Enhanced phosphate sequestration by Fe(III) modified biochar derived from coconut shell. RSC Adv. 2019, 9, 10425–10436. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate absorption on Al-modified biochar: Influence of Al content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification for the removal of inorganics from water. Bioresour. Technol. 2017, 246, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Chan, W.G.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Chinese medicinal herbal residues as a bulking agent for food waste composting. Bioresour. Technol. 2018, 249, 182–188. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef]
- Jones, J.B., Jr.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. Soil Test. Plant Anal. 1990, 3, 389–427. [Google Scholar]
- Nasiruddin Khan, M.; Sarwar, A. Determination of points of zero charge of natural and treated absorbents. Surf. Rev. Lett. 2007, 14, 461–469. [Google Scholar] [CrossRef]
- Vaičiukyniene, D.; Vaitkevičius, V.; Kantautas, A.; Sasnauskas, V. Utilization of by-product waste silica in concrete-based materials. Mater. Res. 2012, 15, 561–567. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Canellas, J.; Soares, A.; Jefferson, B. Removing Ammonia From Wastewater Using Natural and Synthetic Zeolites: A Batch Experiment. ChemRxiv 2019. [Google Scholar] [CrossRef]
- Sarioglu, M. Removal of ammonium from municipal wastewater using natural Turkish (Dogantepe) zeolite. Sep. Purif. Technol. 2005, 41, 1–11. [Google Scholar] [CrossRef]
- Moshoeshoe, M.; Silas Nadiye-Tabbiruka, M.; Obuseng, V. A review of the chemistry, structure, properties and applications of zeolites. Am. J. Mater. Sci. 2017, 7, 196–221. [Google Scholar]
- Muscarella, S.M.; Laudicina, V.A.; Cano, B.; Badalucco, L.; Conte, P.; Mannina, G. Recovering ammonium by treated and untreated zeolitic mixtures: A comprehensive experimental and modelling study. Microporous Mesoporous Mater. 2023, 349, 112434. [Google Scholar] [CrossRef]
- Sparks, D. Environmental Soil Chemistry, 2nd ed.; Academic Press: Oxford, UK, 2003. [Google Scholar]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of biochar formation from various agricultural by-products using FTIR spectroscopy. Mod. Appl. Sci. 2015, 9, 246–253. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J.; Locke, D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 2001, 39, 1971–1979. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, S.; Pang, Y. Rice straw biochar modified by aluminum chloride enhances the dewatering of the sludge from municipal sewage treatment plant. Sci. Total Environ. 2019, 654, 338–344. [Google Scholar] [CrossRef]
- Li, X.; Qin, Y.; Jia, Y.; Li, Y.; Zhao, Y.; Pan, Y.; Sun, J. Preparation and application of Fe/biochar (Fe-BC) catalysts in wastewater treatment: A review. Chemosphere 2021, 274, 129766. [Google Scholar] [CrossRef] [PubMed]
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. Sustainable use of Ca(OH)2 modified biochar for phosphorus recovery and tetracycline removal from water. Sci. Total Environ. 2022, 839, 156159. [Google Scholar] [CrossRef] [PubMed]
- Myglovets, M.; Poddubnaya, O.I.; Sevastyanova, O.; Lindström, M.E.; Gawdzik, B.; Sobiesiak, M.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Preparation of carbon absorbents from lignosulfonate by phosphoric acid activation for the absorption of metal ions. Carbon 2014, 80, 771–783. [Google Scholar] [CrossRef]
- Li, J.H.; Lv, G.H.; Bai, W.B.; Liu, Q.; Zhang, Y.C.; Song, J.Q. Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water. Desalin. Water Treat. 2016, 57, 4681–4693. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, Y.; Ruiz, G.; Sun, W.; Ma, X. Enhanced phosphorus removal and recovery by metallic nanoparticles-modified biochar. Nanotechnol. Environ. Eng. 2020, 5, 26. [Google Scholar] [CrossRef]
- Antunes, E.; Jacob, M.V.; Brodie, G.; Schneider, P.A. Isotherms, kinetics and mechanism analysis of phosphorus recovery from aqueous solution by calcium-rich biochar produced from biosolids via microwave pyrolysis. J. Environ. Chem. Eng. 2018, 6, 395–403. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, D.-Q.; Zheng, X.; Ye, X.; Niu, X.; Lin, Z.; Fu, M.; Zhou, S. Absorption recovery of phosphate from waste streams by Ca/Mg-biochar synthesis from marble waste, calcium-rich sepiolite and bagasse. J. Clean. Prod. 2020, 288, 125638. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, Y.; He, Q.; Zhao, D.; Zhang, K.; Shen, S.; Wang, F. Performance and mechanism of a biochar-based Ca–La composite for the absorption of phosphate from water. J. Environ. Chem. Eng. 2021, 9, 105267. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef] [PubMed]
- Giménez, J.; Martínez, M.; de Pablo, J.; Rovira, M.; Duro, L. Arsenic sorption onto natural hematite, magnetite, and goethite. J. Hazard. Mater. 2007, 141, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, N.; Li, L.; An, J.K.; Zhao, L.; Ren, N.Q. Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresour. Technol. 2015, 178, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and mechanisms of phosphate absorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]

| Parameters | B440 | B880 |
|---|---|---|
| Bulk density (g L−1) | 180 ± 14 | 126 ± 11 |
| Surface area (m2 g−1) | 194 ± 12 | 247 ± 15 |
| Cation exchange capacity (×10−2 mol kg−1) | 14 ± 1 | 12 ± 1 |
| Total pore volume (cm3 g−1) | 38 ± 4 | 51 ± 3 |
| Maximum water retention (%) | 62 ± 11 | 400 ± 32 |
| Reaction (pH) | 9 ± 1 | 10 ± 1 |
| Electrical conductivity (dS m−1) | 1.3 ± 0.2 | 2.1 ± 0.3 |
| Moisture (%) | 3.1 ± 0.3 | 7 ± 1 |
| Total limestone (%) | 5.0 ± 0.8 | 2.7 ± 0.3 |
| Total carbon (%) | 65 ± 4 | 72 ± 5 |
| Ashes to 550 °C (%) | 3.4 ± 0.3 | 6.4 ± 0.6 |
| Molar ratio H:C | 0.7 ± 0.2 | 0.2 ± 0.1 |
| Treatment | Al | Ca | Fe |
|---|---|---|---|
| mg g−1 | mg g−1 | mg g−1 | |
| B440 NAT | 0.6 ± 0.2 | 8 ± 1 | 5 ± 1 |
| B440 + HCl | 0.3 ± 0.2 | 4 ± 1 | 1.5 ± 0.1 |
| B440 + HCl + Al 0.5 M | 0.8 ± 0.2 | n.d. | n.d. |
| B440 + HCl + Ca 0.5 M | n.d. | 6 ± 1 | n.d. |
| B440 + HCl + Fe 0.5 M | n.d. | n.d. | 1.2 ± 0.2 |
| B440 + HCl + Al 2.0 M | 8 ± 1 | n.d. | n.d. |
| B440 + HCl + Ca 2.0 M | n.d. | 28 ± 3 | n.d. |
| B440 + HCl + Fe 2.0 M | n.d. | n.d. | 13 ± 1 |
| B880 NAT | 0.3 ± 0.1 | 6 ± 1 | 2.7 ± 0.5 |
| B880 + HCl | 0.2 ± 0.1 | 2.2 ± 0.4 | 1.2 ± 0.3 |
| B880 + HCl + Al 0.5 M | 1.3 ± 0.4 | n.d. | n.d. |
| B880 + HCl + Ca 0.5 M | n.d. | 8 ± 1 | n.d. |
| B880 + HCl + Fe 0.5 M | n.d. | n.d. | 3.9 ± 0.4 |
| B880 + HCl + Al 2.0 M | 14 ± 2 | n.d. | n.d. |
| B880 + HCl + Ca 2.0 M | n.d. | 29 ± 3 | n.d. |
| B880 + HCl + Fe 2.0 M | n.d. | n.d. | 17 ± 2 |
| Sample | pH |
|---|---|
| B440 NAT | 9.1 ± 0.1 |
| B440 + HCl | 7.1 ± 0.1 |
| B440 + HCl + Ca 0.5 M | 6.1 ± 0.3 |
| B440 + HCl + Ca 2.0 M | 4.9 ± 0.2 |
| B440 + HCl + Al 0.5 M | 4.9 ± 0.3 |
| B440 + HCl + Al 2.0 M | 2.4 ± 0.1 |
| B440 + HCl + Fe 0.5 M | 5.1 ± 0.4 |
| B440 + HCl + Fe 2.0 M | 1.4 ± 0.1 |
| B880 NAT | 10.1 ± 0.2 |
| B880 + HCl | 7.8 ± 0.1 |
| B880 + HCl + Ca 0.5 M | 6.5 ± 0.2 |
| B880 + HCl + Ca 2.0 M | 5.2 ± 0.1 |
| B880 + HCl + Al 0.5 M | 7.1 ± 0.3 |
| B880 + HCl + Al 2.0 M | 2.7 ± 0.2 |
| B880 + HCl + Fe 0.5 M | 5.6 ± 0.1 |
| B880 + HCl+ Fe 2.0 M | 1.2 ± 0.4 |
| Freundlich | |||||
| R2 | χ2 | KF (mg L−1) | - | n | |
| B440 + HCl + Fe 2.0 M | 0.968 | 0.5 | 0.3 ± 0.1 | - | 1.9 ± 0.2 |
| B880 + HCl + Fe 2.0 M | 0.939 | 1.4 | 0.5 ± 0.2 | - | 2.1 ± 0.3 |
| Langmuir | |||||
| R2 | χ2 | KL (mg L−1) | qmax (mg g−1) | - | |
| B440 + HCl + Fe 2.0 M | 0.999 | 0.02 | 0.0034 ± 0.0002 | 12.9 ± 0.3 | - |
| B880 + HCl + Fe 2.0 M | 0.994 | 0.1 | 0.0046 ± 0.0005 | 14.9 ± 0.5 | - |
| Langmuir Sips | |||||
| R2 | χ2 | Ks (mg L−1) | qmax (mg g−1) | n | |
| B440 + HCl + Fe 2.0 M | 0.999 | 0.02 | 0.0027 ± 0.0006 | 12.3 ± 0.5 | 1.06 ± 0.05 |
| B880 + HCl + Fe 2.0 M | 0.999 | 0.03 | 0.0017 ± 0.0004 | 13.1 ± 0.3 | 1.26 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscarella, S.M.; Badalucco, L.; Laudicina, V.A.; Conte, P. Surface Charge and Phosphorus Retention in Metal-Activated Biochars from Different Pyrolysis Temperatures. Appl. Sci. 2025, 15, 8855. https://doi.org/10.3390/app15168855
Muscarella SM, Badalucco L, Laudicina VA, Conte P. Surface Charge and Phosphorus Retention in Metal-Activated Biochars from Different Pyrolysis Temperatures. Applied Sciences. 2025; 15(16):8855. https://doi.org/10.3390/app15168855
Chicago/Turabian StyleMuscarella, Sofia Maria, Luigi Badalucco, Vito Armando Laudicina, and Pellegrino Conte. 2025. "Surface Charge and Phosphorus Retention in Metal-Activated Biochars from Different Pyrolysis Temperatures" Applied Sciences 15, no. 16: 8855. https://doi.org/10.3390/app15168855
APA StyleMuscarella, S. M., Badalucco, L., Laudicina, V. A., & Conte, P. (2025). Surface Charge and Phosphorus Retention in Metal-Activated Biochars from Different Pyrolysis Temperatures. Applied Sciences, 15(16), 8855. https://doi.org/10.3390/app15168855








