Featured Application
This work identifies plant sources of shikonin derivatives with roots rich in these highly pigmented and potently anti-inflammatory, cytoprotective, and antioxidant phytochemicals that have for centuries been utilized as components of dyes, traditional medicines, food, and cosmetics. Many of these plant sources are overexploited in the wild, and we have developed novel scalable, sterile, plant tissue culture methods for producing, concentrating, and extracting these valuable compounds.
Abstract
The naphthazarins shikonin and alkannan are strongly chromogenic, dark red enantiomers, each of which has biological activity, that are found primarily in the plant family Boraginaceae. These compounds and their many chemical metabolites, derivatives, oligomers, and analogs (“shikonoids”) are an important group of phytochemicals, utilized since antiquity as components of dyes, traditional medicines, and food and cosmetics. They are now recognized for their potent anti-inflammatory and regulatory activity on a variety of molecular signaling pathways in humans. Since many Boraginaceae species are overly exploited or endangered, we developed a pilot-scale in vitro shikonoid production system using Plagiobothrys arizonicus (Gray) Greene ex A.Gray, the Arizona popcorn flower, native to the southwestern USA and the Sonoran floristic province in the Madrean region of Mexico. Aseptic root cultures were initiated from fresh leaf tissue and stimulated to continuously produce shikonoids in liquid shake cultures layered under paraffin oil from which the shikonoids were extracted and concentrated. The crude, red extracellular product from these rapidly expanding root masses was also fractionated by Centrifugal Counter-Current Chromatography (CCC) into its component shikonin derivatives. A number of these shikonoids profoundly up-regulated detoxification and antioxidant proteins (phase 2 enzymes) and inhibited inflammation in mammalian cell bioassay systems. This prototype shikonoid production methodology can be readily scaled to either batch or chemostat culture.
1. Introduction
The natural chromogenic naphthoquinone [1] enantiomers shikonin and alkannin and their oligomers, metabolites, analogs, and derivatives (heretofore called “shikonoids”, a few of which are represented in Figure 1) are dark red to purple-colored lipophilic phytochemicals that are found primarily in the roots of plants of over 150 species in the Boraginaceae family, including the well-studied species Lithospermum erythrorhizon, Alkanna tinctoria, Arnebia euchroma, Batschia canensi, and Anchusa officinalis [2]. Shikonin, the R-enantiomer of 5,8-dihydroxy-2[(1R)-1-hydroxy-4-methylpent-3-en-1-yl]naphthalene-1,4-dione, and its derivatives are primarily associated with species found in plant species native to eastern Asia. In contrast, the S-enantiomer, alkannin, and its derivatives are primarily associated with European flora.
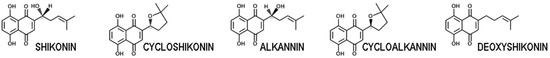
Figure 1.
The naphthoquinones (naphthazarins) alkannin and shikonin, found widely in the Boraginales, and some common derivatives.
Shikonoids have been used for 20 centuries for dyeing silk in Europe, and in traditional Chinese medicine, in which context they are known as Zi Cao or Hong Tiao. Today, they are widely used in food colorings, textile dyes, traditional medicines, and cosmetics. Given their long-standing use in both traditional Chinese and European herbal medicines [3,4], shikonoids have been associated with a broad spectrum of reported biological activities including anti-diabetic [4,5], antibacterial [6,7], cytotoxicity in cancer cell lines [8], anti-inflammatory, antiangiogenic, and anti-cancer properties [4,9,10], as well as antileishmanial activity [11], inhibition of human acyl coenzyme A: cholesterol acyltransferase [12], inhibition of human telomerase [13], larvicidal activity against the West Nile virus vector Culex pipiens [14], radical scavenging properties [15], cyclooxygenase inhibition [16], and anti-HIV effects [4,17]. Commercial Zi Cao preparations are made using a wide range of botanical sources of naphthazarins. While the actual ingredients, potency, and formulations of these preparations are highly variable, their principal bioactive phytochemicals are shikonoids, about which much has been published and from which hundreds of derivatives have been developed [3,4,11,15,18,19,20]. Extensive structure–activity studies on shikonin analogs (both natural and synthetic) have focused on their antitumor properties, rather than their potential for chemoprotection and chronic disease prevention. Although “shikonoids” are referred to as a group herein for convenience of narrative, it should be noted that biological activity of the enantiomers in many cases, where tested, is not congruent.
Because of their widespread utility, the commercial demand for certain species within the Boraginaceae family has led to unsustainable harvesting practices. Lithospermum erythrorhizon, in particular, has been over-exploited for many years as the primary source of shikonin-based “edible dyes” in Korea, China, and Japan [21]. The increasing demand for shikonoids as dyestuffs has driven the indiscriminate harvesting of both L. erythrorhizon and Arnebia euchroma from the wild for both domestic (folk and Western medicine) and pharmaceutical purposes. A critical worldwide shortage of these source plant resources began in the early 1980s and has worsened with the growing interest in these compounds [22]. Concequently, A. euchroma, which is very difficult to propagate, is now critically endangered [23], and a multitude of plants which may not be optimal from either a yield or quality perspective are being substituted as a source of these dyes. In 1984, a company in Japan started producing shikonin in 750 L bioreactors from cultured L. erythrorhizon cells [24]. They and others developed simple non-proprietary methods for stimulating secondary product formation (e.g., shikonin/alkannin) from undifferentiated plant cells in large volume bioreactors, reporting yields of 20–23% shikonin on a dry weight basis after 3 weeks of culture, compared to yields of about 1.5–2% from plants which could only be harvested non-sustainably every 3 to 7 years [25,26]. This ongoing endeavor was the world’s first large-scale secondary metabolite production of a commercial raw material from plant tissue culture, to be followed later by production of taxol from Taxus sp. cell cultures [25]. The shikonin thus produced was used for the highly successful but short-lived consumer product BioLip lipstick, in Japan, in the 1980s [25,27].
Plant cell culture has thus become well-established as a means for producing shikonin [19]. All of the shikonoids can be produced in this manner, and relative quantities can be manipulated by exogenous factors. In liquid culture, concentrations as high as 14 mM shikonin (4 g/L) are discharged into the culture medium [3] from which it can be simply and efficiently extracted and purified. Interestingly, a racemic mixture of R- and S-forms (shikonin and alkannin), and of their derivatives, is produced in the cell cultures of certain shikonoid-producing genera such as Echium [28] in contrast to the predominance of one or the other enantiomer found both in roots and cell cultures of Lithospermum spp., for example [29]. Considering the advantages and flexibility of producing and isolating these compounds from cell cultures, we have chosen to explore the use of root cultures as potential sources of these compounds, which can simplify the processes of harvesting and separation.
Key to our interest in these compounds was our observation that because of their electrophilic Michael reaction centers, they appeared to be excellent candidates to upregulate the critically important Keap1/Nrf2/ARE detoxification pathway in mammals [30,31,32,33]. Whereas some other naphthoquinones have been evaluated for this capacity, the shikonoids have not been previously evaluated [34,35].
Our hypothesis was that, of the large number of shikonoid-producing plants, primarily in the Boraginaceae family, we ought to be able to identify certain species that responded well to established root culture techniques and continued to produce easily extractible shikonoids and that we should be able to confirm the biological activity of these compounds readily using established mammalian cell culture bioassays. We evaluated more than 40 representatives within the Boraginaceae family for convenience of in vivo cultivation and suitability for in vitro root culture, for shikonoid production, and for ability to induce the key chemoprotective enzyme NQO1 (NAD(P)H:quinone oxidoreductase-1). Furthermore, we hypothesized that some of these shikonoids would simultaneously inhibit the inflammatory marker inducible nitric oxide synthase (iNOS), as well as directly protect against reactive oxygen species (ROS).
The results of the bioassays described herein resulted in the selection of the lesser-known plant Plagiobothrys arizonicus, the Arizona popcorn flower, that to our knowledge has never been utilized for root culture or shikonoid production in vitro. P. arizonicus is native to the southwestern United States and the Sonoran floristic province in the Madrean region of northwestern Mexico, with its range including Arizona, California, Nevada, Utah, and New Mexico. It thrives in mid-elevation desert and woodland or scrub habitats across the southwest US. Economically, P. arizonicus is presently of little commercial value and is not grown as a crop, medicinal plant, or widely used horticulturally. Its value is mostly ecological (as part of native flora) and cultural in some indigenous traditions (for pigments), rather than economic.
The techniques we have described herein could be scaled up as a facile and cost-effective approach for producing a phytochemical that could be of substantial value in the production of dietary supplements, cosmetics, and foods. Furthermore, discovery of the potent cytoprotective, antioxidant, and anti-inflammatory activity of cycloshikonin augurs well for its use directly and as a possible scaffold molecule for pharmaceuticals.
2. Materials and Methods
2.1. Boraginaceae Seed, Live Plant Material, and Culture
Seeds or plant specimens of Boraginaceae species known to produce shikonoid compounds were obtained and screened for availability of suitable quantities of leaf material for facile aseptic culture initiation. Since initiation of successful callus and/or embryogenic cultures can require many “false starts” (e.g., leaf material that is not fully decontaminated, is overly decontaminated and thus killed, or does not respond to the grid of plant growth regulators it is subjected to), some plants with insufficient or insufficiently fast-growing leaves were bypassed. Thus, many species were brought through the initial stages of aseptic tissue culture and assessed for root proliferation prior to our selection of Plagiobothrys arizonicus (Gray) Greene ex A.Gray for the pilot study.
2.1.1. Aseptic Leaf Explant Culture
Mature leaves were excised from soil-grown P. arizonicus plants, gently washed in distilled water, quick-dipped in 70% ethanol, treated with 15% Clorox® bleach with 400 ppm Tween® 20 for 15–20 min, then rinsed three times in sterile, distilled water. The aseptic leaves were cut into strips (5–8 cm) using a scalpel, forceps, and sterile technique in a biological safety cabinet (The Baker Company, Sanford, ME, USA) and explanted on semi-solid MS basal culture medium prepared as initially described by Murashige and Skoog [36], but containing 0.6% Gelzan™ (Caisson Labs, North Logan, UT, USA) as a solidifying agent amended with indole-3-acetic acid (IAA) (10 µM) and kinetin (1 µM), and maintained in the dark in a plant growth chamber (Percival Scientific, Perry, IA, USA) at 25 °C for two weeks. Cultivation in the dark avoids the photooxidation or photoconversion of many plant secondary products, and the presence of light inhibits root formation in many plant species. Plant growth compounds and culture media components were from Sigma-Aldrich (St. Louis, MO, USA) and Caisson Labs (North Logan, UT, USA).
2.1.2. Sustained Root Culture
Sterile, adventitious roots were excised from the in vitro leaf explants on semi-solid MS medium solidified with 0.6% Gelzan™ containing both IAA (10 µM) and kinetin (1 µM) [36]. This medium contains ammonium ions which block the production of shikonoids in culture. For root proliferation, they were then sub-cultured in 30 mL of liquid MS basal medium without Gelzan™ in 125 mL Nalgene sterile disposable polyethylene terephthalate (PETG) flasks (Thermo Fisher Scientific, Waltham, MA, USA). The root cultures were maintained in a temperature-controlled growth chamber (Percival Scientific, Perry, IA, USA) at 25 °C, wrapped in aluminum foil to shield from ambient light, and placed on a rotary shaker at 100 rpm. Removal of ammonium ions as well as addition of nitrate as a nitrogen sources and elevation of copper levels has been shown by others to be effective in stimulating shikonoid biosynthesis; thus, once root growth was prolific, cultures were transferred to RC (Root Culture) medium [37] or M-9 medium [38,39].
2.2. Analytical Methods
Direct assessment of shikonoid production from plant roots was made by HPLC and comparison to authentic standards made in acetonitrile. Roots or other plant parts were homogenized at about 100 mg/mL in a mixture of solvents that we have widely used before in phytochemical exploration (1:1:1:1 dimethyl sulfoxide, acetonitrile, dimethyl formamide, water; abbreviated DADH) [40] and were then diluted accordingly into either bioassay buffer or HPLC mobile phase. Analytical standards of shikonin and its derivatives were from Calbiochem (San Diego, CA, USA), TCI (Portland, OR, USA), Santa Cruz Biochemicals, Inc. (Santa Cruz, CA, USA), Enzo Life Sciences (Farmingdale, NY, USA), and Stanford Materials Corp. (Lake Forest, CA, USA) and were diluted in acetonitrile.
HPLC was performed with a Waters 2690 Alliance system equipped with a 2996 photodiode array (PDA) detector and Empower software (Waters, Milford, MA, USA). The method was adapted from Sharma [41] using a flow rate of 1 mL/min on a Whatman (Millipore Sigma, St. Louis, MO, USA), Partisil 10 ODS-2, C18 column, 250 × 4.6 mm, and monitored at a wavelength of 500 nm. A linear gradient from 80% water and 20% acetonitrile to 100% acetonitrile between 0 and 20 min was followed by isocratic 100% acetonitrile from 20 min to 30 min. All solvent mixtures in the gradient contained 0.1% trifluoroacetic acid (TFA). All reagents were HPLC grade or reagent grade and from Thermo Fisher Scientific (Waltham, MA, USA) or Sigma-Aldrich (St. Louis, MO, USA). High Speed Centrifugal Counter-Current Chromatography (HSCCC) was performed on acetonitrile/ethyl acetate extracts from P. arizonicus root cultures. The instrument was a 127.5 mL preparative coil spectrum (Dynamic Extractions Ltd., Tredegar Blaenau Gwent, UK) run at 1600 rpm in the center-to-periphery flow configuration at 1 mL/min. A 16:14:14:5 heptane–ethyl acetate–ethanol–water phase system was adapted from [42], with the lower phase serving as the mobile phase. Detection was by single wavelength detector (SPD-6A Shimadzu, Columbia, MD, USA) with a preparative flow cell, set to 280 nm, standard response, 0.04 Abs with output to a strip chart recorder (500 µV, 1 mm/min).
Mass spectroscopy was performed on collected HPLC peaks by direct injection into a single-quad Agilent 1260 Mass Spectroscope (Agilent Technologies, Santa Clara, CA, USA) in positive ion mode. Comparisons were made to authentic standards of the following naphthazarins, and accurate molecular wieghts as well as UV/Vis spectra were used to validate putative identifications: shikonin, deoxyshikonin, 2-methylbutyryl shikonin, β,β-dimethylacrylyl shikonin, isovaleryl shikonin, lawsone, lapachol, menadione, 1,2-naphthoquinone, 1,4-naphthoquinone, naphthazarin, plumbagin, and cycloshikonin.
2.3. Bioassays
2.3.1. NQO1
Hepa 1c1c7 murine hepatoma cells and H9c2 rat myocardiocytes were grown for 24 h in flat-bottomed 96-well plates, followed by exposure to inducing agents for 48 h, and were lysed with digitonin; then, the colorimetric “Prochaska” bioassays of the prototypical cytoprotective enzyme NQO1 were performed as previously described [43,44]. Plates were read with a SpectraMax Plus spectrophotometer-microtiter plate reader (Molecular Devices, San Jose, CA, USA). Concentrations required to double the specific activity of NQO1 (CD values) were used to quantify inducer potency. At least three separate plates were run, and in each plate there were eight separate replicates per concentration of standard compound or plant extract.
2.3.2. iNOS
Shikonoids were evaluated for their inhibitory potencies on LPS-activated iNOS, a marker of inflammation, in RAW264.7 murine macrophage-like cells. After incubation with shikonoids in the presence of LPS in microtiter plates, NO (an index of iNOS induction) was measured as nitrite by the Griess reaction as described by Liu et al. [45]. The nitrite values of cells treated with LPS but without test compounds were used as controls.
2.3.3. Protection Against ROS Toxicity
The protection by shikonoids against ROS generated by the exogenous oxidant (tert-butyl hydroperoxide) in H9c2 cells was analyzed using the fluorescence-generating probe 2′,7′-dichlorodihydro-fluorescein diacetate [46]. The cells treated with tert-butyl hydroperoxide only were used as controls. A hypoxia/reoxygenation-induced cardiomyocyte injury model in H9c2 cells was used to evaluate the protection of shikonoids against IR-caused cell death in vitro as described by Tian et al. [47].
2.4. Statistics
As this was a pilot study, much of what is presented represents screening and is not amenable to statistical analyses. The bioassays reported were performed in two or three independent plates (replications), and each assay (in 96 well plates) included eight replicates per concentration. The results were only utilized if within 5% of the mean value.
3. Results and Discussion
3.1. Plant Screening and Selection
A total of 44 seed and live plant material specimens of a diverse collection of the Boraginaceae family were grown in the laboratory to evaluate and obtain leaf tissues from which to derive axenic tissue cultures (Table 1). Seeds were sown in commercial potting soil in 4 inch pots and germinated under 16 h light/8 h dark lighting (18 inches from a bank of 8 2-ft T8 fluorescent tubes) and ambient temperature in the laboratory. A single plant was maintained in soil as a leaf explant source for adventitious root cultures under aseptic conditions. The tissue culture media formulae were developed from initial MS basal medium [36] and M9 medium [38] components, supplemented with plant auxins and/or cytokinins. All species were explanted to all four medium formulations. Growth medium, plant, and conditions providing the best yields were achieved; they are designated by numbers “1” through “4” in Table 1 and are as follows: (1) M-9 Medium containing elevated CuSO4 for shikonoid production [38,39]; (2) MS-Basal Medium (semi-solid in Petri plates) + Indole-3-Acetic Acid [10 µM]/Kinetin [1 µM]; (3) MS-Basal Medium (semi-solid in Petri plates) + Thidiazuron [5 µM] (to stimulate organogenesis); (4) MS-Basal Medium (semi-solid in Petri plates) + 1-Naphthaleneacetic Acid [10 µM]/Kinetin [1 µM].

Table 1.
Source material # for undifferentiated callus and aseptic roots.
Accessions were selected based on specimen availability and published information suggesting that they might be reasonable sources of shikonoids. In addition, preserved herbarium samples of Cryptantha sp. were provided by the New York Botanical Garden for evaluation of their NQO1 induction potency. Plants in which we have identified shikonoids but have been unsuccessful in establishing axenic tissue cultures from include the following species: Alkanna orientalis (Alkanet), Anchusa officinales (Bugloss), Arnebia sp., Batschia sp., Borage sp., Cryptantha sp., Cynoglossum officinale, Echium italicum, Echium plantagineum, Eritrichium sp., Hackelia floribunda (Begger’s Lice, Stickweed), Heliotropium curassavicum (Salt Heliotrope), Lithospermum sp., (Stary Night), Lithospermum canescens (Hoary Puccoon), Lithospermum erythrorhizon, (Zi Cao, Red Gromwell), Mertensia virginica (Virginia Bluebells), Myosotis sylvatica (Forget-Me-Not), Onosmodium molle (Marbleseed), Onosma sp., Plagiobothrys figuratus, and Symphytum officinalis (Comfrey).
Root culture media formulae and culture conditions originally designed for Lithospermum erythrorhizon [37,38,39,48] were used to guide the initial evaluation of the other 43 Boraginales species. Whereas robust establishment of axenic root cultures was not achieved in a large number of accessions, one of those in particular, Plagiobothrys arizonicus, was highly prolific and produced abundant yield of shikonoids. P. arizonicus, the Arizona popcorn flower, a species native to the southwestern United States, has been previously documented to contain alkannin (Figure 1). Ethnobotanical records indicate that the leaves of P. arizonicus were traditionally used by Native Americans as a source of body and face paint [1,18]. This species was used to further test our hypothesis that we could readily harvest these compounds in high yield and that they would be effective inducers of both antioxidant and anti-inflammatory responses in mammalian systems.
3.2. Plagiobothrys arizonicus Root Culture and Recovery Protocol Development
Protocols were ultimately tailored in order to design a pilot-scale in vitro root culture system for Plagiobothrys arizonicus. P. arizonicus phenotypically presents as a low-profile rosette-forming specimen, approximately 15 inches high at maturity. Its most visually striking characteristic is that the abaxial leaf margins are outlined in bright red, as are the leaf mid-vein and roots (Figure 2), pinpointing the anatomical sites of the highest concentrations of shikonoids. P. arizonicus is not only phenotypically very interesting but it is also highly prolific in in vitro culture and it proved to be the optimal candidate of those screened.

Figure 2.
Plagiobothrys arizonicus. (A) Flowering P. arizonicus (southwestdesertflora.com, 10 June 2024). (B) P. arizonicus dorsal side of leaf with shikonoid-containing margins and mid-vein. (C) Lab-grown P. arizonicus source material.
Established root cultures were sub-cultured by dividing the “root mat” into thirds using a sterile scalpel and replacing the basal growth medium with a medium tailored for root proliferation and shikonoid-alkannin production (Root Culture-Medium or RC-Medium) [37]. Roots typically required growth for several weeks in RC-Medium with replacement of the liquid medium every 10–14 days prior to releasing red pigmented compounds. Mature root cultures of Plagiobothrys arizonicus formed a “mat” that covered the entire circumference of the 125 mL culture flask bottom and amounted to approximately 18 g fresh weight or 145 mg dry weight when grown in RC-Medium for 2–3 weeks. Monthly sub-culture of the root mat was required for culture maintenance. To limit the number of liquid cultures maintained, the root mat was sub-cultured onto agar containing MS basal medium with no growth hormones (see Figure 3). Shikonoid production was dependent on keeping all of the tissue cultures on plates and in liquid shake flasks away from excessive incident light; therefore, aluminum foil was used to wrap or cover cultures at this stage, and periodic inspections were rapidly made in low light. These findings are consistent with the common wisdom that light is a key environmental factor regulating the synthesis of plant secondary metabolites (phytochemicals), their production, and their accumulation as reported by Wu and others [49], and that plant root cultures can be a route to preferential production of pigmented phytochemicals [50,51].

Figure 3.
Plagiobothrys arizonicus root cultures with production of red colored shikonoids. (A) P. arizonicus roots on solid medium, and (B) in liquid medium/paraffin, all showing clear shikonoid production. (C) Partitioning of shikonoids from liquid paraffin (lower phase) with heptane (upper phase).
3.2.1. Shikonoid Recovery and Analysis
A variety of organic solvents used in initial attempts to harvest the shikonoids produced in liquid root cultures destroyed the roots but provided valuable data for shikonoid production. For example, after 3 days of treatment in liquid RC-Medium (30 mL), a Plagiobothrys arizonicus root culture yielded a total of 0.533 mg shikonoids/gram fresh weight of roots or 0.0533%. The initial extraction of the red pigment in ethyl acetate was followed by an acetone flask rinse which removed large quantities of red pigment that had adhered to the walls of the flask, yielding an additional 3.6 mg for a total yield of 4.1 mg shikonoids from an established root culture maintained in shikonoid-stimulating RC-Medium for 3 days. This production, and others like it, resulted from approximately one gram of root tissue transferred from a Petri plate of semi-solid growth medium after 10–14 days, or root cultures grown in sterile flasks in liquid medium for 10–14 days (Figure 3A), permitting migration of shikonoids into the liquid RC-Medium. The liquid culture medium was harvested and overlain with ≥30 mL of ethyl acetate for bi-phasic shikonoid extraction. The mixture of spent culture medium and ethyl acetate was mixed vigorously by hand and left to fully partition overnight at 4 °C. The red, upper, ethyl acetate phase was collected (approximately 40 mL) and evaporated to dryness by rotary evaporation.
In order to maintain the viability of P. arizonicus root cultures for continuous culture, roots were switched from semi-solid culture to liquid RC-Medium in shake flasks overlain with non-cytotoxic paraffin oil at a 10:1 ratio, medium to paraffin, respectively. This step allowed the lipophilic shikonoids produced by the growing root culture to rapidly migrate to the paraffin oil layer of the biphasic mixture whilst the rotational (agitation) action of the shaker flask supplied vital aeration to the root cultures [52,53]. The upper (paraffin oil) layer turned bright red as the shikonoids moved from the fibrous root mass and were concentrated in the upper layer, leaving other components in the lower, light brown spent medium (Figure 3B). This method has previously been shown to be ≥80% efficient in extracting shikonoids from de-differentiated plant cell cultures [53]. To harvest the shikonoids from the paraffin oil for analysis, a separatory funnel was used to separate the harvests from multiple root culture flasks (Figure 3C).
Once partitioned, the bright red paraffin phase was mixed with two volumes of methanol, discarding the methanol phase upon separation. An equal volume of water was added to the oil, then an equal volume of heptane. Upon shaking, the shikonoids partitioned from the oil/water to the heptane phase. For more complete extraction, the methanol/water/heptane extraction steps were repeated as described previously [54,55]. The paraffin did not inhibit the growth of the root cultures, and once the shikonoids were harvested from the culture medium, the roots were sub-cultured to initiate follow-up cultures and to repeat the shikonoid production cycle. This paraffin oil overlay method permitted continuous cultivation, either for maintenance on semi-solid medium in plates or new flasks. Ultimately, the highly prolific root cultures of P. arizonicus generated a harvestable yield of shikonoids approximately every 3 days once the optimal stage of root maturation was achieved at about 2 weeks.
Optimization of extraction of in vitro generated shikonoids has been examined by others previously, but not from root cultures; these efforts ranged from the use of solvents (oils) to microwaving and ultrasonic cellular disruption to soxhlet-type extractions [56].
3.2.2. Preliminary Characterization
The presence of shikonoids in the shake-liquid root cultures was verified by HPLC with UV-Vis detection. A commercial shikonin standard was used to compare the HPLC retention times and spectra of peaks. The spectral signature of the shikonin standard (characteristic UV absorbance between 480 and 500 nm), was highly similar to the six different HPLC peaks isolated from the root culture (Figure 4A,B). For example, spent RC-Medium from a single flask, partitioned with organic solvent, yielded 1.6 mg of red residue that was re-dissolved in 50 µL of acetonitrile and chromatographed by HPLC. The three most prominent peaks (Figure 4A) were collected separately and assayed for NQO1 phase 2 cytoprotective enzyme induction activity. Two of the three peaks had spectral signatures suggestive of shikonin or a close derivative and all three peaks induced NQO1. All 15 peaks obtained from HPLC separation of similar root cultures were subject to mass spectroscopy and their masses ranged from 197.0814(m/z) to 543.2024 (m/z), including one with a similar mass to that of shikonin (287.0924 (m/z)).
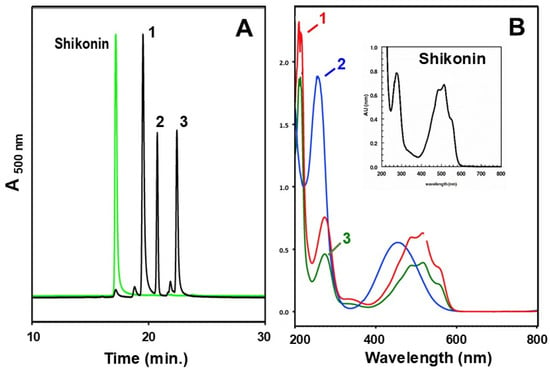
Figure 4.
Compounds extracted from a P. arizonicus root culture grown in liquid medium, overlaid with liquid paraffin, partitioned from that paraffin, and then separated by HPLC (A). The spectra for the three major peaks (labeled 1–3) are shown in (B). Peak 2 (Rt = 20.6 min) does not have the signature spectral characteristics of the other two peaks; however, it does induce NQO1.
Since there are a great many shikonoids, and our primary goal was not to identify all and/or novel compounds from these cultures, we did not attempt to match shikonin derivatives in these cultures with structures, although we did bioassay a selection of pure derivatives (e.g., β,β-dimethylacrylyl shikonin, isovaleryl shikonin, cycloshikonin, and 2-methylbutyryl shikonin) as outlined in Section 3.3. Future, more extensive compound identification work could, at this point, be readily accomplished with the culture systems we have put together.
After a preliminary demonstration of the protective effects of reagent-grade shikonin on myocardial infarction (manuscript in preparation), our attention was drawn to the remarkable structural characteristics of shikonin, i.e., the presence of highly electrophilic (Michael reaction acceptor) centers in the naphthazarin substructure. Such reactivity is a universal property of inducers of the classic Keap1/Nrf2/ARE regulatory system for phase 2 cytoprotective genes [57]. Shikonin was found to be a potent inducer of this response. Moreover, many intriguing close structural analogs exist in the same plants. Therefore, to obtain more potent and less cytotoxic structurally related naphthoquinones, we characterized the shikonoid content of a number of commercial Zi Cao preparations. We identified two separate minor components common to many of the commercial Zi Cao and shikonin preparations that possess little cytotoxicity but appear to account for much of their biological activity. One of these compounds with low cytotoxicity and high biological activity was cycloshikonin. We validated its identity by synthesis in accordance with previously published methods [58] and by NMR and mass spectroscopy. Whereas cycloshikonin has anti-cancer activity comparable to shikonin [59], it is not a known natural plant product. Since its formation from shikonin requires mild acid conditions analogous to those found in the stomach, we believe it is an artifact of plant extraction. However, it is also a metabolite of bacteria found in the human gut microbiome (Bacteroides fragilis subsp. thetaotus) [60]. We found that as an inducer of the classic phase 2 enzyme NQO1, cycloshikonin has a 20-fold more favorable Chemoprotective Index (CI) than shikonin (see Figure 5). It is therefore of considerable interest for further exploration.
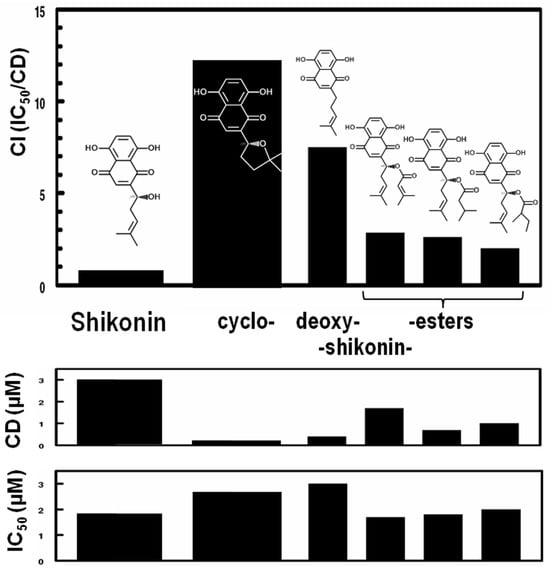
Figure 5.
CI (Chemoprotective Index = IC50/CD), CD (Concentration to Double), and IC50 (Inhibitory Concentration to achieve 50% inhibition of growth) for NQO1 activity in Hepa 1c1c7 cell cultures treated with shikonoin, cycloshikonin, and three esters of shikonin: (left to right) β,β-dimethylacrylyl shikonin, isovaleryl shikonin, and 2-methylbutyryl shikonin.
3.2.3. Fractionation
We developed Centrifugal Counter-Current Chromatography (CCC) methods to fractionate shikonoid-containing preparations to recover specific shikonoids at high yield and purity that were directly bioassayed as further discussed in Section 3.3. This fractionation was at a scale suitable for experiments in animals and expanded upon approaches with which we have been successful for other bioactive phytochemicals [61,62]. Figure 6 presents examples of preparative scale fractionations of a crude root extract containing shikonin (fractions 11–13) and cycloshikonin (fractions 17–19) as well as unidentified shikonin derivative(s) (fractions 5–6) and a quite potent and as-yet unidentified inducer of NQO1 (fraction 14). We can thus move seamlessly from analytical HPLC of plant extracts, to analytical scale CCC, and then to preparative CCC [61,62]. We have extensive experience developing such protocols [63,64,65], and these preliminary results could readily be extended to other target shikonoids.
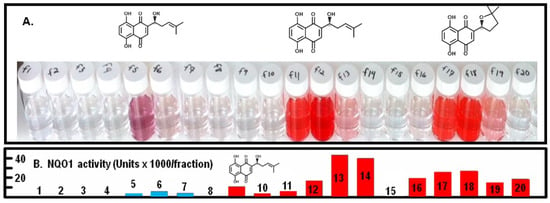
Figure 6.
(A) Preparative CCC separation of a mixture of three shikonoids: fractions 5–6 (unknown), fractions 11–13 (shikonin), fractions 17–19 (cycloshikonin). (B) NQO1 inducer activity of sequential 15 mL fractions obtained from preparative CCC-fractionation of an Arnebia euchroma root extract. Red peaks are shikonoids (fractions 9 and 10 are shikonin/alkannin), and blue peaks are not shikonoids, since they do not show the very characteristic naphthazarin UV–visible absorption spectra.
3.3. Bioactivities of Shikonoids
We evaluated several structurally related shikonoids in mammalian cell biomarker systems targeting three different mechanistic pathways in order to evaluate their chemoprotective potencies:
3.3.1. Induction of Cytoprotective Phase 2 Enzyme by Shikonoids
The most striking structural characteristics of shikonoids are their highly electrophilic (Michael reaction) centers. Therefore, we predicted that shikonin will induce Nrf2-dependent genes that code for cytoprotective phase 2 enzymes like NQO1. Enzymatic activity of NQO1 in cell lysates was measured using our well-established 96-well microtiter plate assay (the Prochaska Assay), with results expressed as the Concentration(s) required for Doubling (CD), the Inhibitory Concentration at 50% (IC50), and the Chemoprotective Index (CI; the IC50/CD) [43,44]. As predicted, shikonin is a potent inducer of NQO1 in Hepa1c1c7 cells (CD = 3.0 µM) but showed some toxicity (IC50 = 1.9 µM) (Figure 5). These results are entirely consistent with the only other report we are aware of: the 3-fold increase in the CD of cells cultured with shikonin aligns nicely with a study in which primary rat hepatocytes showed a 3.0-fold increase in levels of NQO1 protein upon challenge with shikonin [66]. Strikingly, cycloshikonin had a 20-fold higher CI (13.3) than shikonin (CI = 0.7). By way of comparison, the CD of sulforaphane, the most potent known naturally occurring NQO1 inducer in Hepa 1c1c7 cells, is ~0.2 µM [43,45,67], and that of the synthetic inducer β-naphthoflavone, used as a positive control in all Prochaska Assays [43], was 0.04 µM. Several other pure (reagent grade) shikonoids evaluated in the same system had intermediate CIs (Figure 5). The NQO1 inducer potencies of naphthazarin and selected shikonoids in an alternative cell ine (H9c2 rat myocardiocytes) are shown in Table 2.

Table 2.
Activities of selected shikonoids (compared to the positive controls sulforaphane and β-naphthoflavone) in cell culture systems.
3.3.2. Inhibition of LPS-Activated iNOS by Shikonoids
Inducible nitric oxide synthase (iNOS) is widely recognized as an inflammatory marker. The expression of iNOS can be induced by inflammatory mediators, such as bacteria endoxin LPS, through the Iκκ/NF-κB pathway. Induction of iNOS contributes to the process of cell injury in various diseases including myocardial infarction and heart failure, and shikonin-mediated inhibition of iNOS induction has previously been demonstrated in various tissues [68,69,70,71]. Several shikonoids were assayed for their inhibitory effects on LPS-activated iNOS in RAW264.7 cells. All the tested shikonoids inhibited NO (an index of iNOS induction) production dose-dependently, which reflects their anti-inflammatory activities. The IC50 values of the shikonoids we evaluated are shown in Table 2, and they are consistent with those previously reported for shikonin itself [68,69,70,71], but these reports did not evaluate iNOS activity of the derivatives we examined. Furthermore, it should be noted that the inhibition of LPS-activated iNOS shown in Table 2 for cyclocshikonin are comparable to that of shikonin and of the prototypical anti-inflammatory phytochemical used as a positive control, sulforaphane.
3.3.3. Protection Against Oxidative Stress by Shikonoids
H9c2 cells are a rat-derived cardiomyoblast line that exhibits morphological characteristics similar to those of immature embryonic cardiomyocytes. They preserve several elements of the electrical and hormonal signaling pathway found in adult cardiac cells [72]. Reactive oxygen species (ROS) generated with the re-admission of oxygen play a cardinal role in the pathogenesis of IR injury. The potency of each agent in suppressing ROS generation (expressed as IC50 in Table 2) is very closely correlated with its NQO1 inducer potencies in the same cell line, which strongly suggests that primary protection against ROS occurs via the Nrf2 pathway. In a separate experiment, we used a hypoxia/reoxygenation cardiomyocyte injury model in H9c2 cells [47], using cell viability as an indicator of the cardioprotective effects of selected shikonoids in vitro. All tested shikonoids protected against hypoxia/re-oxygenation injury in a dose-dependent manner. Their protective potencies are expressed as ED50 in Table 2. As with the other bioassays reported herein, it is of note that the antioxidant activity (suppression of ROS generation in cultured cells) reported herein for cycloshikonin was superior to that for both shikonin and sulforaphane.
4. Conclusions
The primary objectives of this study were to explore plant species suitable for shikonoid production using small-scale in vitro culture and fractionation systems that are eminently scalable and to validate biological activity of shikonoids and shikonoid-rich fractions derived from these systems. More than 40 representatives in the Boraginaceae family were evaluated, and a single species, Plagiobothrys arizonicus, was selected for further investigation. There was robust cytoprotective, anti-inflammatory, and antioxidant potency of the shikonoids produced by crude root cultures of this species, both from the crude spent medium and from shikonoid fractions taken to HPLC purity from the root culture media. The potencies of these preparations were within one order of magnitude of that of sulforaphane, the most potent known natural inducer of the sentinel chemoprotective enzyme NQO1. NQO1 inducing activity (as a proxy for Nrf2 induction) of cycloshikonin was over 10-fold greater than that of shikonin in the Hepa1c1c7 cell system and over twice as great in H9c2 cells; its iNOS inhibitory activity was comparable to that of shikonin, and its ROS suppression was over twice as potent. Thus, there are strong indications that P. arizonicus would be a viable candidate for further biomedical research and that cycloshikonin likewise merits further consideration. Moreover, the simplicity and reliability of the root culture system offer a practical platform for the sustained production of bioactive compounds for research or potential commercial applications.
Author Contributions
Conceptualization, J.W.F., K.K.S., H.L.; methodology, J.W.F., K.K.S., K.L.W., H.L.; validation, J.W.F., K.K.S., K.L.W., H.L.; investigation, K.K.S., K.L.W., H.L.; writing—original draft preparation, J.W.F.; writing—review and editing, J.W.F., K.K.S., K.L.W., H.L.; visualization, J.W.F., K.K.S., K.L.W.; supervision, J.W.F.; project administration, J.W.F.; funding acquisition, J.W.F. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by The Cullman Chemoprotection Center, The Brassica Foundation for Chemoprotection Research, and The Lewis B. & Dorothy Cullman Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We gratefully acknowledge the early assistance of James Barrow and Jesse Alt on this project, obtaining compound masses and confirming the identity of shikonin and some of its derivatives by mass spectroscopy. We are grateful to Dennis Stevenson (New York Botanical Garden) for providing preserved herbarium samples of Cryptantha sp. We are grateful to Paul Talalay (now deceased) for his early insights into this group of compounds and their electrophilic nature and presumptive capacity to induce the Nrf2/Keap1/ARE cytoprotective pathway. Unrestricted philanthropic funding from the Lewis B. and Dorothy Cullman Foundation is greatly appreciated. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ARE | Antioxidant response element |
| CCC | Centrifugal Counter-Current Chromatography |
| HSCCC | High Speed Centrifugal Counter-Current Chromatography |
| IR | Ischemia/reperfusion |
| IAA | Indole acetic acid |
| iNOS | Inducible nitric oxide synthase |
| Keap1 | Kelch-like ECH-associated protein |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| NQO1 | NAD(P)H:quinone oxidoreductase-1 |
| Nrf2 | NF-E2 p45-related factor 2 |
| ROS | reactive oxygen species |
| TFA | Trifluoroacetic acid |
References
- Hedges, K. Santa Ysabel Ethnobotany; San Diego Museum of Man: San Diego, CA, USA, 1986; Volume 30. [Google Scholar]
- Jin, R. Theoretical study on the antioxidant activity of alkannin and its derivatives. Appl. Mech. Mater. 2011, 138–139, 1056–1062. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products. Angew. Chem. Int. Ed. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Wang, R.; Yin, R.; Zhou, W.; Xu, D.; Li, S. Shikonin and its derivatives: A patent review. Expert Opin. Ther. Pat. 2012, 22, 977–997. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.Y.; Kang, T.H.; Hwang, E.J.; Kang, C.H. Pharmaceutical Composition Comprising Shikonin Derivatives from Lithospermum Erythrorhizon for Treating Diabetes Mellitus and the Use Thereof; WIPO: Geneva, Switzerland, 2010. [Google Scholar]
- Shen, C.C.; Syu, W.J.; Li, S.Y.; Lin, C.H.; Lee, G.H.; Sun, C.M. Antimicrobial activities of naphthazarins from Arnebia euchroma. J. Nat. Prod. 2002, 65, 1857–1862. [Google Scholar] [CrossRef]
- Meselhy, M.R.; Kadota, S.; Tsubono, K.; Kusai, A.; Hattori, M.; Namba, T. Shikometabolins A, B, C and D, novel dimeric naphthoquinone metabolites obtained from shikonin by human intestinal bacteria. Tetrahedron Lett. 1994, 35, 583–586. [Google Scholar] [CrossRef]
- Sevimli-Gur, C.; Akgun, I.H.; Deliloglu-Gurhan, I.; Korkmaz, K.S.; Bedir, E. Cytotoxic naphthoquinones from Alkanna cappadocica (perpendicular). J. Nat. Prod. 2010, 73, 860–864. [Google Scholar] [CrossRef]
- Kundakovic, T.; Fokialakis, N.; Dobric, S.; Pratsinis, H.; Kletsas, D.; Kovacevic, N.; Chinou, I. Evaluation of the anti-inflammatory and cytotoxic activities of naphthazarine derivatives from Onosma leptantha. Phytomedicine 2006, 13, 290–294. [Google Scholar] [CrossRef]
- Wang, F.; Yao, X.; Zhang, Y.; Tang, J. Synthesis, biological function and evaluation of Shikonin in cancer therapy. Fitoterapia 2019, 134, 329–339. [Google Scholar] [CrossRef]
- Ali, A.; Assimopoulou, A.N.; Papageorgiou, V.P.; Kolodziej, H. Structure/antileishmanial activity relationship study of naphthoquinones and dependency of the mode of action on the substitution patterns. Planta Med. 2011, 77, 2003–2012. [Google Scholar] [CrossRef]
- An, S.; Park, Y.D.; Paik, Y.K.; Jeong, T.S.; Lee, W.S. Human ACAT inhibitory effects of shikonin derivatives from Lithospermum erythrorhizon. Bioorg. Med. Chem. Lett. 2007, 17, 1112–1116. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, W.; Ding, J.; Cai, J.; Duan, W. Shikonin derivatives: Synthesis and inhibition of human telomerase. Bioorg. Med. Chem. Lett. 2002, 12, 1375–1378. [Google Scholar] [CrossRef]
- Michaelakis, A.; Strongilos, A.T.; Bouzas, E.A.; Koliopoulos, G.; Couladouros, E.A. Larvicidal activity of naturally occurring naphthoquinones and derivatives against the West Nile virus vector Culex pipiens. Parasitol. Res. 2009, 104, 657–662. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Tsermentseli, S.K.; Nenadis, N.; Assimopoulou, A.N.; Tsimidou, M.Z.; Papageorgiou, V.P. Structure-radical scavenging activity relationship of alkannin/shikonin derivatives. Food Chem. 2011, 124, 171–176. [Google Scholar] [CrossRef]
- Landa, P.; Kutil, Z.; Temml, V.; Vuorinen, A.; Malik, J.; Dvorakova, M.; Marsik, P.; Kokoska, L.; Pribylova, M.; Schuster, D.; et al. Redox and non-redox mechanism of in vitro cyclooxygenase inhibition by natural quinones. Planta Med. 2012, 78, 326–333. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Zhang, N.; Turpin, J.A.; Buckheit, R.W.; Osterling, C.; Oppenheim, J.J.; Howard, O.M. Shikonin, a component of chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2003, 47, 2810–2816. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Hortic. Res. 2016, 3, 16046. [Google Scholar] [CrossRef]
- Yazaki, K. Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnol. 2017, 34, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M.; et al. Review of Shikonin and Derivatives: Isolation, Chemistry, Biosynthesis, Pharmacology and Toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Seo, Y.C.; No, R.H.; Lee, H.Y. Improved cosmetic activity by optimizing the Lithospermum erythrorhizon extraction process. Cytotechnology 2015, 67, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Albreht, A.; Vovk, I.; Simonovska, B.; Srbinoska, M. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. J. Chromatogr. A 2009, 1216, 3156–3162. [Google Scholar] [CrossRef]
- Manjkhola, S.; Dhar, U.; Joshi, M. Organogenesis, embryogenesis, and synthetic seed production in Arnebia euchroma—A critically endangered medicinal plant of the Himalaya. In Vitro Cell. Dev. Biol. Plant 2005, 41, 244–248. [Google Scholar] [CrossRef]
- Malik, S.; Bhushan, S.; Sharma, M.; Singh Ahuja, P. Physico-chemical factors influencing the shikonin derivatives production in cell suspension cultures of Arnebia euchroma (Royle) Johnston, a medicinally important plant species. Cell Biol. Int. 2011, 35, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Renneberg, R.; Berkling, V.; Loroch, V.; Demain, A.L. Biotechnology for Beginners, 2nd ed.; Elsevier/Academic Press: Amsterdam, The Netherland, 2017. [Google Scholar]
- Ruffoni, B.; Pistelli, L.; Bertoli, A.; Pistelli, L. Plant cell cultures: Bioreactors for industrial production. Adv. Exp. Med. Biol. 2010, 698, 203–221. [Google Scholar] [CrossRef]
- Shimamoto, M. R&D strategy and knowledge creation in Japanese chemical firms, 1980–2010. In Proceedings of the Business History Conference, Business and Economic History On-line: Papers Presented at the BHC Annual Meeting, St. Louis, MO, USA, 31 March–2 April 2011. [Google Scholar]
- Fukui, H.; Tsukada, M.; Mizukami, H.; Tabata, M. Formation of stereoisomeric mixtures of naphthoquinone derivatives in Echium lycopsis callus cultures. Phytochemistry 1983, 22, 453–456. [Google Scholar] [CrossRef]
- Tabata, M. The mechanism of shikonin biosynthesis in Lithospermum cell cultures. Plant Tissue Cult. Lett. 1996, 13, 117–125. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef]
- Yagishita, Y.; Chartoumpekis, D.V.; Kensler, T.W.; Wakabayashi, N. NRF2 and the Moirai: Life and Death Decisions on Cell Fates. Antioxid. Redox Signal 2023, 38, 684–708. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, P.L. The role of NAD(P)H oxidoreductase (DT-Diaphorase) in the bioactivation of quinone-containing antitumor agents: A review. Free Radic. Biol. Med. 2000, 29, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Talalay, P. Chemical Structures of Inducers of Nicotinamide Quinone Oxidoreductase 1 (NQO1). Methods Enzymol. 2004, 382, 423–448. [Google Scholar] [CrossRef]
- Murashigi, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Thomas, E.; Davey, M.R. The Use of Ti Plasmid as a Cloning Vector for Genetic Engineering in Plants; EMBO Press: Heidelberg, Germany, 1982. [Google Scholar]
- Fujita, Y.; Hara, Y.; Suga, C.; Morimoto, T. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon II. A new Medium for the production of shikonin derivatives. Plant Cell Rep. 1981, 1, 61–63. [Google Scholar] [CrossRef]
- Fujita, Y.; Tabata, M.; Nishi, A.; Yamada, Y. New Medium and Production of Secondary Compounds with the Two-Staged Culture Method. In Plant Tissue Culture: Methods and Applications in Agriculture; Thorpe, T.A., Ed.; Maruzen Co.: Tokyo, Japan, 1982; pp. 399–400. [Google Scholar]
- Lee, L.S.; Stephenson, K.K.; Fahey, J.W.; Parsons, T.L.; Lietman, P.S.; Andrade, A.S.; Lei, X.; Yun, H.; Soon, G.H.; Shen, P.; et al. Induction of chemoprotective phase 2 enzymes by ginseng and its components. Planta Med. 2009, 75, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Ghosh, P.; Sharma, U.; Sood, S.; Sinha, A.; Gulati, A. Microwave-Assisted Efficient Extraction and Stability of Juglone in Different Solvents from Juglans regia: Quantification of Six Phenolic Constituents by Validated RP-HPLC and Evaluation of Antimicrobial Activity. Anal. Lett. 2009, 42, 2592–2609. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Sturm, S.; Stuppner, H.; Papageorgiou, V.P. Preparative isolation and purification of alkannin/shikonin derivatives from natural products by high-speed counter-current chromatography. Biomed. Chromatogr. 2009, 23, 182–198. [Google Scholar] [CrossRef]
- Fahey, J.W.; Dinkova-Kostova, A.T.; Stephenson, K.K.; Talalay, P. The “Prochaska” Microtiter Plate Bioassay for Inducers of NQO1. Methods Enzymol. 2004, 382, 243–258. [Google Scholar] [CrossRef]
- Prochaska, H.J.; Santamaria, A.B.; Talalay, P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc. Natl. Acad. Sci. USA 1992, 89, 2394–2398. [Google Scholar] [CrossRef]
- Liu, H.; Dinkova-Kostova, A.T.; Talalay, P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc. Natl. Acad. Sci. USA 2008, 105, 15926–15931. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Tian, Y.; Daoud, A.; Shang, J. Effects of bpV(pic) and bpV(phen) on H9c2 cardiomyoblasts during both hypoxia/reoxygenation and H2O2-induced injuries. Mol. Med. Rep. 2012, 5, 852–858. [Google Scholar] [CrossRef][Green Version]
- Tabata, M.; Mizukami, H.; Hiraoka, N.; Konoshima, M. Pigment formation in callus cultures of Lithospermum erythrorhizon. Phytochemistry 1974, 13, 927–932. [Google Scholar] [CrossRef]
- Wu, W.; Wu, H.; Liang, R.; Huang, S.; Meng, L.; Zhang, M.; Xie, F.; Zhu, H. Light regulates the synthesis and accumulation of plant secondary metabolites. Front. Plant Sci. 2025, 16, 1644472. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dalawai, D.; Bhat, M.A.; Dandin, V.S.; Paek, K.Y.; Park, S.Y. Biotechnological Production of Useful Phytochemicals from Adventitious Root Cultures. Ref. Ser. Phytochem. 2019, 1–17. [Google Scholar] [CrossRef]
- Subramanian, M.; Gantait, S.; Jaafar, J.N.; Ismail, M.F.; Sinniah, U.R. Micropropagation of white turmeric (Curcuma zedoaria (Christm.) Roscoe) and establishment of adventitious root culture for the production of phytochemicals. Ind. Crops Prod. 2025, 223, 120101. [Google Scholar] [CrossRef]
- Deno, H.; Suga, C.; Morimoto, T.; Fujita, Y. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon VI. Production of shikonin derivatives by a two-layer culture containing an organic solvent. Plant Cell Rep. 1987, 6, 197–199. [Google Scholar] [CrossRef]
- Shimomura, K.; Sudo, H.; Saga, H.; Kamada, H. Shikonin production and secretion by hairy root cultures of Lithospermum erythrorhizon. Plant Cell Rep. 1991, 10, 282–285. [Google Scholar] [CrossRef]
- Boehm, R.; Sommer, S.; Li, S.M.; Heide, L. Genetic engineering on shikonin biosynthesis: Expression of the bacterial ubiA gene in Lithospermum erythrorhizon. Plant Cell Physiol. 2000, 41, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Zare, K.; Nazemiyeh, H.; Movafeghi, A.; Khosrowshahli, M.; Motallebi-Azar, A.; Dadpour, M.; Omidi, Y. Bioprocess engineering of Echium italicum L.: Induction of shikonin and alkannin derivatives by two-liquid-phase suspension cultures. Plant Cell Tissue Organ Cult. 2010, 100, 157–164. [Google Scholar] [CrossRef]
- Liu, T.; Ma, C.; Yang, L.; Wang, W.; Sui, X.; Zhao, C.; Zu, Y. Optimization of shikonin homogenate extraction from Arnebia euchroma using response surface methodology. Molecules 2013, 18, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; De Long, M.J.; Prochaska, H.J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad. Sci. USA 1988, 85, 8261–8265. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, Y.; Terada, A.; Sugyo, Y. Synthesis of naphthoquinone derivatives. 3. Cycloshikonin and its derivatives. A synthetic route to shikonin. J. Org. Chem. 1987, 52, 1437–1439. [Google Scholar] [CrossRef]
- Efferth, T.; Greten, H.J. Microarray-based determination of response of tumor cells to cycloshikonin. Forum Immunopathol. Dis. Ther. 2011, 2, 315–322. [Google Scholar] [CrossRef]
- Meselhy, M.R.; Kadota, S.; Tsubono, K.; Hattori, M.; Namba, T. Biotransformation of shikonin by human intestinal bacteria. Tetrahedron 1994, 50, 3081–3098. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Chou, F.E. Separation and purification of glucosinolates from crude plant homogenates by high-speed counter-current chromatography. J. Chromatogr. A 2003, 996, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.; Garrard, I.J.; Heuvel, R.v.D.; Sutherland, I.A.; Chou, F.E.; Fahey, J.W. Technology Transfer and Scale Up of a Potential Cancer-Preventive Plant Dynamic Extraction of Glucoraphanin. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1913–1922. [Google Scholar] [CrossRef]
- Fahey, J.W.; Reed, J.N.; Readdy, T.L.; Pace, G.M. Somatic embryogenesis from three commercially important inbreds of Zea mays. Plant Cell Rep. 1986, 5, 35–38. [Google Scholar] [CrossRef]
- Farnham, M.W.; Wilson, P.E.; Stephenson, K.K.; Fahey, J.W. Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breed. 2004, 123, 60–65. [Google Scholar] [CrossRef]
- Stephenson, K.K.; Fahey, J.W. Development of Tissue Culture Methods for the Rescue and Propagation of Endangered Moringa spp. Germplasm. Econ. Bot. 2004, 58, S116–S124. [Google Scholar] [CrossRef]
- Huang, C.-S.; Chen, H.-W.; Lin, T.-Y.; Lin, A.-H.; Lii, C.-K. Shikonin upregulates the expression of drug-metabolizing enzymes and drug transporters in primary rat hepatocytes. J. Ethnopharmacol. 2018, 216, 18–25. [Google Scholar] [CrossRef]
- Posner, G.H.; Cho, C.-G.; Green, J.V.; Zhang, Y.; Talalay, P. Design and Synthesis of Bifunctional Isothiocyanate Analogs of Sulforaphane: Correlation between Structure and Potency as Inducers of Anticarcinogenic Detoxication Enzymes. J. Med. Chem. 1994, 37, 170–176. [Google Scholar] [CrossRef]
- Gedara Prasad, R.; Hyun Choi, Y.; Kim, G.Y. Shikonin isolated from Lithospermum erythrorhizon downregulates proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglial cells by suppressing crosstalk between reactive oxygen species and NF-κB. Biomol. Ther. 2015, 23, 110–118. [Google Scholar] [CrossRef]
- Hosseini, S.; Sabouni, F.; Fereidoni, M.; Moghimi, A. Anti-inflammatory effect of shikonin on cultured astrocytes derived from rat brain. Physiol. Pharmacol. 2012, 16, 107–120. [Google Scholar]
- Liao, P.L.; Lin, C.H.; Li, C.H.; Tsai, C.H.; Ho, J.D.; Chiou, G.C.Y.; Kang, J.J.; Cheng, Y.W. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic retinopathy. Sci. Rep. 2017, 7, 44985. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, Y.; Li, W.; Qiu, J.; Du, J.; Wang, L.; Zhang, T. Shikonin ameliorates oxidative stress and neuroinflammation via the Akt/ERK/JNK/NF-κB signalling pathways in a model of Parkinson’s disease. Clin. Exp. Pharmacol. Physiol. 2022, 49, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Hescheler, J.; Meyer, R.; Plant, S.; Krautwurst, D.; Rosenthal, W.; Schultz, G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ. Res. 1991, 69, 1476–1486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).