Impact of Kickxia elatine In Vitro-Derived Stem Cells on the Biophysical Properties of Facial Skin: A Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Suspension Culture
2.2. Preparation of the Cream Containing K. Elatine Cell Suspension Extract
2.3. Cream Preparation
2.4. Study Group and Skin Biophysical Parameter Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| CAS | Chemical Abstract Service |
| CID | Compound ID |
| EI | Erythema index |
| MI | Melanin index |
| TEWL | Transepidermal water loss |
| UV | Ultraviolet |
References
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Evolving Beauty: The Rise of Sustainable Cosmetics. Available online: https://www.cas.org/resources/cas-insights/the-rise-of-natural-ingredients-for-cosmetics (accessed on 31 May 2025).
- Martins, A.M.; Marto, J.M. A Sustainable Life Cycle for Cosmetics: From Design and Development to Post-Use Phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- What Is the Demand for Natural Ingredients for Cosmetics on the European Market? Available online: https://www.cbi.eu/market-information/natural-ingredients-cosmetics/what-demand (accessed on 31 May 2025).
- Europe Natural Cosmetics Market Size & Forecast 2025–2035. Available online: https://www.futuremarketinsights.com/reports/natural-cosmetics-industry-analysis-in-europe (accessed on 24 June 2025).
- Moruś, M.; Baran, M.; Rost-Roszkowska, M.; Skotnicka-Graca, U. Plant Stem Cells as Innovation in Cosmetics. Acta Pol. Pharm. 2014, 71, 701–707. [Google Scholar] [PubMed]
- Budzianowska, A.; Banaś, K.; Budzianowski, J.; Kikowska, M. Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology. Appl. Sci. 2025, 15, 2571. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant Cell Culture as Emerging Technology for Production of Active Cosmetic Ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Gardiki, V.; Pavlou, P.; Siamidi, A.; Papageorgiou, S.; Papadopoulos, A.; Iakovou, K.; Varvaresou, A. Plant Stem Cells in Cosmetic Industry. Plants 2025, 14, 433. [Google Scholar] [CrossRef]
- Marchev, A.S.; Georgiev, M.I. Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules 2020, 25, 2006. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of Pentacyclic Triterpenoids-Rich Callus Extract of Chaenomeles japonica (Thunb.) Lindl. Ex Spach on Viability, Morphology, and Proliferation of Normal Human Skin Fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef]
- Pietrosiuk, A.; Budzianowska, A.; Budzianowski, J.; Ekiert, H.; Jeziorek, M.; Kawiak, A.; Kikowska, M.; Krauze-Baranowska, M.; Królicka, A.; Kuźma, Ł.; et al. Polish Achievements in Bioactive Compound Production From In Vitro Plant Cultures. Acta Soc. Bot. Pol. 2022, 91, 9110. [Google Scholar] [CrossRef]
- Hermosaningtyas, A.A.; Chanaj-Kaczmarek, J.; Kikowska, M.; Gornowicz-Porowska, J.; Budzianowska, A.; Pawlaczyk, M. Potential of Plant Stem Cells as Helpful Agents for Skin Disorders—A Narrative Review. Appl. Sci. 2024, 14, 7402. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B.; Nahorska, A. Potential of Plant Cell Cultures as a Source of Bioactive Compounds for Cosmetic Applications. Pol. J. Cosmetol. 2015, 18, 170–175. (In Polish) [Google Scholar]
- Pieroni, A.; Quave, C.L.; Villanelli, M.L.; Mangino, P.; Sabbatini, G.; Santini, L.; Boccetti, T.; Profili, M.; Ciccioli, T.; Rampa, L.G.; et al. Ethnopharmacognostic Survey on the Natural Ingredients Used in Folk Cosmetics, Cosmeceuticals and Remedies for Healing Skin Diseases in the Inland Marches, Central-Eastern Italy. J. Ethnopharmacol. 2004, 91, 331–344. [Google Scholar] [CrossRef]
- Sasounian, R.; Martinez, R.M.; Lopes, A.M.; Giarolla, J.; Rosado, C.; Magalhães, W.V.; Velasco, M.V.R.; Baby, A.R. Innovative Approaches to an Eco-Friendly Cosmetic Industry: A Review of Sustainable Ingredients. Clean Technol. 2024, 6, 176–198. [Google Scholar] [CrossRef]
- Wren, R.C. Potter’s New Cyclopaedia of Botanical Drugs and Preparations, 7th ed.; Sir Isaac Pitman & Sons, Ltd.: London, UK, 1956. [Google Scholar]
- Janaćković, P.; Gavrilović, M.; Miletić, M.; Radulović, M.; Kolašinac, S.; Stevanović, Z.D. Small Regions as Key Sources of Traditional Knowledge: A Quantitative Ethnobotanical Survey in the Central Balkans. J. Ethnobiol. Ethnomed. 2022, 18, 70. [Google Scholar] [CrossRef]
- Yuldashev, M.P.; Malikov, V.M.; Batirov, É.K. Flavonoids of the Epigeal Part of Kickxia elatine. Chem. Nat. Compd. 1996, 32, 30–32. [Google Scholar] [CrossRef]
- Dhivya, S.M.; Kalaichelvi, K. Studies on Ethno-Medicinal Plants Used by the Irulas Tribes of Nellithurai Beat, Karamadai Range of Western Ghats, Tamil Nadu, India. Int. J. Pharm. Chem. Sci. 2015, 3, 2116–2124. [Google Scholar]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Savo, V.; Bonsangue, G.; Letizia Gargano, M.; Venturella, G.; La Bella, S. Ethnobotanical Investigation on Wild Medicinal Plants in the Monti Sicani Regional Park (Sicily, Italy). J. Ethnopharmacol. 2014, 153, 568–586. [Google Scholar] [CrossRef] [PubMed]
- Hermosaningtyas, A.A.; Totoń, E.; Budzianowska, A.; Lisiak, N.; Romaniuk-Drapała, A.; Kruszka, D.; Rewers, M.; Kikowska, M. Biotechnology Production of Cell Biomass from the Endangered Kickxia elatine (L.) Dumort: Its Untargeted Metabolomic Analysis and Cytotoxic Potential Against Melanoma Cells. Biomedicines 2025, 13, 1382. [Google Scholar] [CrossRef] [PubMed]
- Kroma, A.; Pawlaczyk, M.; Feliczak-Guzik, A.; Urbańska, M.; Jenerowicz, D.; Seraszek-Jaros, A.; Kikowska, M.; Gornowicz-Porowska, J. Phytoecdysteroids from Serratula coronata L. for Psoriatic Skincare. Molecules 2022, 27, 3471. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2005. [Google Scholar]
- Posit team. RStudio: Integrated Development Environment for R; Posit Software; PBC: Boston, MA, USA, 2025; Available online: http://www.posit.co/ (accessed on 4 June 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation, Version 1.1.4. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 4 June 2025).
- Wickham, H.; Girlich, R. Tidyr: Tidy Messy Data, Version 1.3.1. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 4 June 2025).
- Wickham, H.; Henry, L. Purrr: Functional Programming Tools, Version 1.1.0. Available online: https://CRAN.R-project.org/package=purrr (accessed on 4 June 2025).
- Robinson, D.; Hayes, A. Couch Broom: Convert Statistical Objects into Tidy Tibbles, Version 1.0.8. Available online: https://CRAN.R-project.org/package=broom (accessed on 4 June 2025).
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Nowak, I. Noninvasive Evaluation of the Influence of Aucubin-containing Cosmetic Macroemulsion on Selected Skin Parameters. J. Cosmet. Dermat. 2021, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.A.; Wahab, R.A.; Rehman, G.U.; Abidin, M.H.Z.; Wong, K.Y. A Novel Water-in-Oil-in-Water Double Nanoemulsion of α-Mangostin and Kojic Acid for Topical Applications. Arab. J. Sci. Eng. 2024, 49, 9291–9305. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, T. Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice. Nutrients 2019, 11, 2948. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Choi, D.; Son, B.; Park, S.; Park, T. Activation of OR10A3 by Suberic Acid Promotes Collagen Synthesis in UVB-Irradiated Dermal Fibroblasts via the cAMP-Akt Pathway. Cells 2022, 11, 3961. [Google Scholar] [CrossRef]
- CosIng-Cosmetics-GROWTH-European Commission. Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 25 June 2025).
- Scott, L.N.; Fiume, M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Panthenol, Pantothenic Acid, and Derivatives as Used in Cosmetics. Int. J. Toxicol 2022, 41, 77–128. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M. Toxicological Profile of Diethyl Phthalate: A Vehicle for Fragrance and Cosmetic Ingredients. Food Chem. Toxicol. 2001, 39, 97–108. [Google Scholar] [CrossRef]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in Cosmetic and Personal Care Products: Concentrations and Possible Dermal Exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef]
- Siddiqui, A.H.; Stolk, L.L.; Bhaggoe, R.; Hu, R.; Schutgens, R.B.H.; Westerhof, W. L-Phenylalanine and UVA Irradiation in the Treatment of Vitiligo. Dermatology 1994, 188, 215–218. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Underbill, C.B.; Lakkakorpi, J.; Ditre, C.M.; Uitto, J.; Yu, R.J.; Van Scott, E. Citric Acid Increases Viable Epidermal Thickness and Glycosaminoglycan Content of Sundamaged Skin. Dermatol. Surg. 1997, 23, 689–694. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Citric Acid, Inorganic Citrate Salts, and Alkyl Citrate Esters as Used in Cosmetics. Int. J. Toxicol. 2014, 33, 16S–46S. [Google Scholar] [CrossRef]
- Korponyai, C.; Szél, E.; Behány, Z.; Varga, E.; Mohos, G.; Dura, Á.; Dikstein, S.; Kemény, L.; Erős, G. Effects of Locally Applied Glycerol and Xylitol on the Hydration, Barrier Function and Morphological Parameters of the Skin. Acta Derm. Venerol. 2017, 97, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Cho, D.-H.; Bae, J.-E.; Kim, J.; Choi, J.Y.; Min, D.; Na, H.W.; Kim, H.J.; Park, W. Composition for Enhancing Skin Elasticity or Improving Skin Wrinkles. U.S. Patent US20220378676A1, 1 December 2022. [Google Scholar]
- Bissett, D.L.; Robinson, L.R.; Raleigh, P.S.; Miyamoto, K.; Hakozaki, T.; Li, J.; Kelm, G.R. Reduction in the Appearance of Facial Hyperpigmentation by Topical N-Acetyl Glucosamine. J. Cosmet Dermatol 2007, 6, 20–26. [Google Scholar] [CrossRef]
- Ding, X.-J.; Zhang, Z.-Y.; Jin, J.; Han, J.-X.; Wang, Y.; Yang, K.; Yang, Y.-Y.; Wang, H.-Q.; Dai, X.-T.; Yao, C.; et al. Salidroside Can Target Both P4HB-Mediated Inflammation and Melanogenesis of the Skin. Theranostics 2020, 10, 11110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Luo, S.; Zhang, H.; Liu, B.; Pan, Z. Study of Salidroside and Its Inflammation Targeting Emulsion Gel for Wound Repair. Molecules 2023, 28, 5151. [Google Scholar] [CrossRef]
- Thiboutot, D.; Thieroff-Ekerdt, R.; Graupe, K. Efficacy and Safety of Azelaic Acid (15%) Gel as a New Treatment for Papulopustular Rosacea: Results from Two Vehicle-Controlled, Randomized Phase III Studies. J. Am. Acad. Derm. 2003, 48, 836–845. [Google Scholar] [CrossRef]
- Shucheng, H.; Zhou, X.; Du, D.; Li, J.; Yu, C.; Jiang, X. Effects of 15% Azelaic Acid Gel in the Management of Post-Inflammatory Erythema and Post-Inflammatory Hyperpigmentation in Acne Vulgaris. Dermatol. Ther. 2024, 14, 1293–1314. [Google Scholar] [CrossRef]
- Choudhary, V.; Kaddour-Djebbar, I.; Custer, V.E.; Uaratanawong, R.; Chen, X.; Cohen, E.; Yang, R.; Ajebo, E.; Hossack, S.; Bollag, W.B. Glycerol Improves Skin Lesion Development in the Imiquimod Mouse Model of Psoriasis: Experimental Confirmation of Anecdotal Reports from Patients with Psoriasis. Int. J. Mol. Sci. 2021, 22, 8749. [Google Scholar] [CrossRef]
- Minghetti, P.; Sosa, S.; Cilurzo, F.; Casiraghi, A.; Alberti, E.; Tubaro, A.; Loggia, R.; Montanari, L. Evaluation of the Topical Anti-Inflammatory Activity of Ginger Dry Extracts from Solutions and Plasters. Planta Med. 2007, 73, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hagiwara, G.; Abe, M. Effects of Consuming 3.0 g of Ginger Powder Containing High Levels of 6-Shogaol and 6-Gingerol on Skin Temperature. J. Edu. Health Sci. 2016, 62, 346–350. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and Stability Studies of Verbascoside, a Novel Antioxidant, in Dermo-Cosmetic and Pharmaceutical Topical Formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A Review of Its Occurrence, (Bio)Synthesis and Pharmacological Significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, A.N.M. Vitamins, Nutraceuticals, Food Additives, Enzymes, Anesthetic Aids, and Cosmetics. In Therapeutic Use of Medicinal Plants and their Extracts: Volume 2; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2018; Volume 74, pp. 407–534. ISBN 978-3-319-92386-4. [Google Scholar]
- Jabłońska-Trypuć, A.; Pankiewicz, W.; Czerpak, R. Traumatic Acid Reduces Oxidative Stress and Enhances Collagen Biosynthesis in Cultured Human Skin Fibroblasts. Lipids 2016, 51, 1021–1035. [Google Scholar] [CrossRef]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Gugliandolo, E.; Peritore, A.F.; Di Paola, R.; Cuzzocrea, S. Topical Application of Adelmidrol + Trans-Traumatic Acid Enhances Skin Wound Healing in a Streptozotocin-Induced Diabetic Mouse Model. Front. Pharmacol. 2018, 9, 871. [Google Scholar] [CrossRef]
- Ponnurangam, M.; Balaji, S. Tune in to the Terrific Applications of Turanose. Eur. Food Res. Technol. 2024, 250, 375–387. [Google Scholar] [CrossRef]
- Behera, P.; Balaji, S. The Forgotten Sugar: A Review on Multifarious Applications of Melezitose. Carbohydrate Res. 2021, 500, 108248. [Google Scholar] [CrossRef]
- Marion, C.; Clement, F.; Rackelboom, J.; Agnaou, R. Cleansing Compositions with Non-Modified Clay and Polyglycerol Surfactant. Patent WO2022136469A1, 30 June 2022. [Google Scholar]
- Cong, L.; Ma, J.; Zhang, Y.; Zhou, Y.; Cong, X.; Hao, M. Effect of Anti-Skin Disorders of Ginsenosides—A Systematic Review. J. Ginseng Res. 2023, 47, 605–614. [Google Scholar] [CrossRef]
- Shin, H.-S.; Park, S.-Y.; Hwang, E.-S.; Lee, D.-G.; Mavlonov, G.T.; Yi, T.-H. Ginsenoside F2 Reduces Hair Loss by Controlling Apoptosis through the Sterol Regulatory Element-Binding Protein Cleavage Activating Protein and Transforming Growth Factor-β Pathways in a Dihydrotestosterone-Induced Mouse Model. Biol. Pharm. Bull. 2014, 37, 755–763. [Google Scholar] [CrossRef]
- Park, S.-H.; Seo, W.; Eun, H.S.; Kim, S.Y.; Jo, E.; Kim, M.-H.; Choi, W.-M.; Lee, J.-H.; Shim, Y.-R.; Cui, C.; et al. Protective Effects of Ginsenoside F2 on 12-O-Tetradecanoylphorbol-13-Acetate-Induced Skin Inflammation in Mice. Biochem. Biophys. Res. Comm. 2016, 478, 1713–1719. [Google Scholar] [CrossRef]
- Shin, H.-S.; Park, S.-Y.; Hwang, E.-S.; Lee, D.-G.; Song, H.-G.; Mavlonov, G.T.; Yi, T.-H. The Inductive Effect of Ginsenoside F2 on Hair Growth by Altering the WNT Signal Pathway in Telogen Mouse Skin. Eur. J. Pharmacol. 2014, 730, 82–89. [Google Scholar] [CrossRef]
- Yöyen Ermiş, D.; Ermiş, E.; Cansev, S.; Oral, H.B.; Göktalay, G. Effects of Uridine and Uridine Nucleotides on Proliferation and Migration of L929 Murine Fibroblast Cell Line. Uludağ Üniversitesi Tıp Fakültesi Dergisi 2025, 51, 105–110. [Google Scholar] [CrossRef]
- Miyase, T.; Ishino, M.; Akahori, C.; Ueno, A.; Ohkawa, Y.; Tanizawa, H. Phenylethanoid Glycosides from Plantago asiatica. Phytochemistry 1991, 30, 2015–2018. [Google Scholar] [CrossRef]
- Kim, K.H.; Bae, G.-U.; Kim, Y.K. Plantago asiatica Extracts Inhibit UV-Induced Matrix Metalloproteinase-1 in Human Dermal Fibroblasts and Prevent Skin Photoaging in Hairless Mice. Bull. Korean Chem. Soc. 2015, 36, 659–664. [Google Scholar] [CrossRef]
- Feasibility Analysis of ALA-PHE Dipeptide for Cosmetic Applications—Manufacturer’s Perspective. Available online: https://www.nbinno.com/?news/ZVT-feasibility-analysis-of-ala-phe-dipeptide-for-cosmetic-applications-manufacturers-perspective (accessed on 25 June 2025).
- Park, J.I.; Lee, J.E.; Shin, H.J.; Song, S.; Lee, W.K.; Hwang, J.S. Oral Administration of Glycine and Leucine Dipeptides Improves Skin Hydration and Elasticity in UVB-Irradiated Hairless Mice. Biomol. Ther. 2017, 25, 528–534. [Google Scholar] [CrossRef]
- Milani, M.; Sparavigna, A. The 24-Hour Skin Hydration and Barrier Function Effects of a Hyaluronic 1%, Glycerin 5%, and Centella asiatica Stem Cells Extract Moisturizing Fluid: An Intra-Subject, Randomized, Assessor-Blinded Study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Shahiq-uz-zaman; Khan, B.A.; Haji, M.; Khan, S.; Ahmad, M.; Rasool, F.; Mahmood, T.; Rasul, A. Evaluation of Various Functional Skin Parameters Using Topical Cream of Calendula officinalis Extract. Afr. J. Pharm. Pharmacol. 2011, 5, 199–206. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, H.M.S.; Iqbal, A.; Khan, B.A.; Bashir, S. Glycyrrhiza Glabra Extract Cream: Effects on Skin Pigment “Melanin”. In Proceedings of the IPCBEE; IACSIT Press: Singapore, 2011; Volume 5. [Google Scholar]
- Wanitphakdeedecha, R.; Ng, J.N.C.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of Olive Leaf Extract–Containing Cream for Facial Rejuvenation: A Pilot Study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.W. Anti-Aging Activities of Pyrus pyrifolia var culta Plant Callus Extract. Trop. J. Pharm. Res. 2017, 16, 1579–1588. [Google Scholar] [CrossRef][Green Version]
- Park, D.E.; Adhikari, D.; Pangeni, R.; Panthi, V.K.; Kim, H.J.; Park, J.W. Preparation and Characterization of Callus Extract from Pyrus pyrifolia and Investigation of Its Effects on Skin Regeneration. Cosmetics 2018, 5, 71. [Google Scholar] [CrossRef]
- Adhikari, D.; Panthi, V.; Pangeni, R.; Kim, H.; Park, J. Preparation, Characterization, and Biological Activities of Topical Anti-Aging Ingredients in a Citrus junos Callus Extract. Molecules 2017, 22, 2198. [Google Scholar] [CrossRef] [PubMed]
- Laneri, S.; Dini, I.; Tito, A.; Di Lorenzo, R.; Bimonte, M.; Tortora, A.; Zappelli, C.; Angelillo, M.; Bernardi, A.; Sacchi, A.; et al. Plant Cell Culture Extract of Cirsium eriophorum with Skin Pore Refiner Activity by Modulating Sebum Production and Inflammatory Response. Phytother. Res. 2021, 35, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Kim, H.-I.; Kim, S.-Y.; Seo, H.H.; Song, J.; Kim, J.; Shin, D.S.; Jo, Y.; Choi, H.; Lee, J.H.; et al. Anti-Aging Effects of Leontopodium alpinum (Edelweiss) Callus Culture Extract through Transcriptome Profiling. Genes 2020, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Apone, F.; Colucci, G. Plant Cell Cultures as Source of Cosmetic Active Ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]

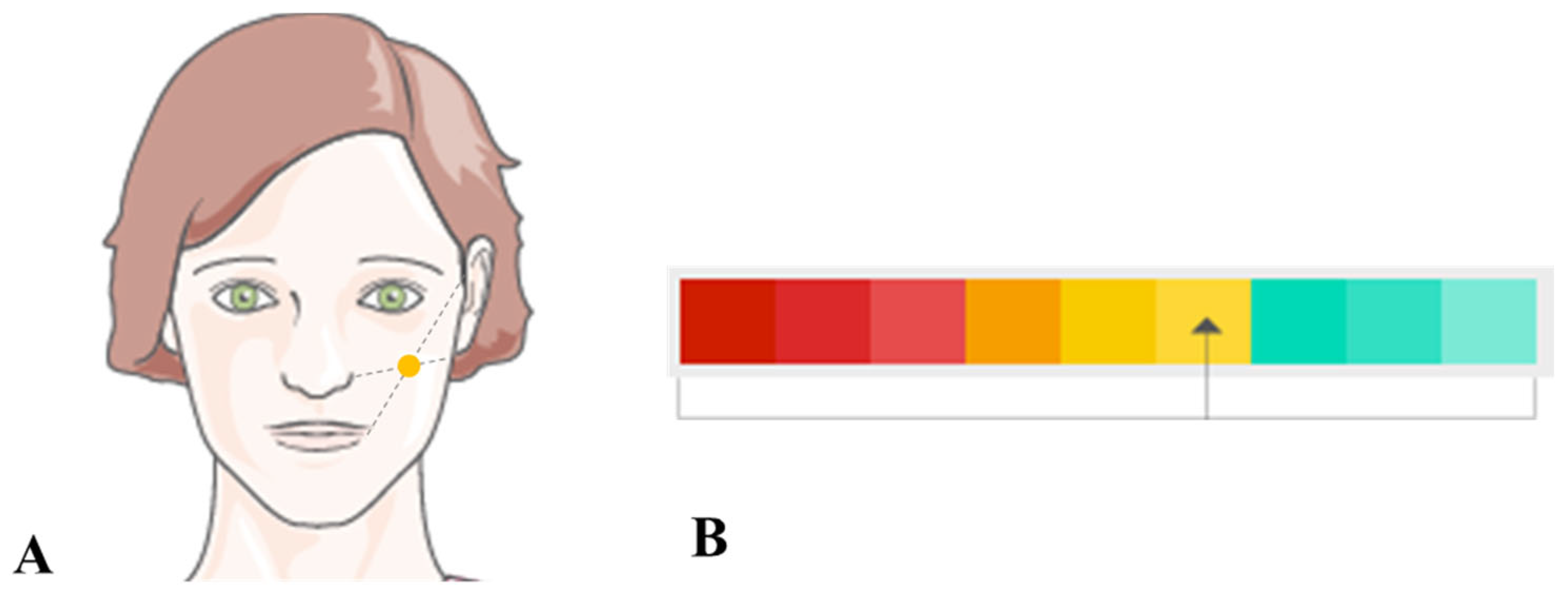

| Colour Spectrum | Skin Texture | Wrinkle Depth (mm) | Vascular Lesions (mm2) | Skin Discolorations (%) |
|---|---|---|---|---|
| Green | 0–8 excellent | 0–0.05 small, shallow | 0–2 well-vascularised skin | 0–10 Within the correct amount and distribution of melanin in the skin |
| Yellow | 8–10 good | 0.06–0.1 medium | 2.01–10 poorly vascularised skin | 11–55 Epidermal hyperpigmentation (yellow, yellow-brown) |
| Red | >10 incorrect | 0.11–2.7 deep | >10 very poorly vascularised skin | 56–100 Skin discolouration—greyish brown colour |
| Skin Parameter | Placebo (n = 20) | Cream Containing K. elatine Cell Extract (n = 20) | p-Value Between Groups | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Skin hydration [AU] | 47.81 ± 13.57 | 57.68 ± 14.60 | 40.36 ± 10.16 | 63.00± 10.27 | 0.057 c | 0.191 c |
| p-value | 0.001 *,a | <0.001 *,a | ||||
| Transepidermal water loss [g/h/m2] | 11.09 ± 3.12 | 12.12 ± 7.32 | 14.82 ± 3.62 | 11.76 ± 2.42 | 0.001 *,c | 0.289 b |
| p-value | 0.927 b | <0.001 *,a | ||||
| Luminosity | 8.19 ± 1.47 | 8.32 ± 2.31 | 8.32 ± 1.43 | 7.63 ± 1.21 | 0.773 c | 0.295 c |
| p-value | 0.830 a | 0.009 *,a | ||||
| Erythema index [AU] | 255.22 ± 58.83 | 286.30 ± 50.92 | 114.83 ± 19.62 | 113.68 ± 26.12 | 0.042 *,c | 0.051 b |
| p-value | 0.002 *,a | 0.812 b | ||||
| Melanin index [AU] | 127.43 ± 18.27 | 127.33 ± 20.73 | 276.58 ± 77.12 | 295.40 ± 99.01 | 0.331 c | 0.947 b |
| p-value | 0.667 b | 0.210 a | ||||
| Sebum level | 25.15 ± 14.58 | 26.50 ± 28.81 | 12.40 ± 12.65 | 35.85 ± 31.19 | 0.004 *,b | 0.449 b |
| p-value | 0.823 b | <0.001 *,b | ||||
| Skin surface pH | 5.45 ± 0.33 | 5.07 ± 0.51 | 5.53 ± 0.46 | 5.44 ± 0.42 | 0.281 c | 0.019 *,b |
| p-value | 0.001 *,a | 0.522 b | ||||
| Skin Parameter | Placebo (n = 20) | Cream Containing K. elatine Cell Extract (n = 20) | p-Value Between Groups | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Skin structure | 8.67 ± 1.43 | 9.42 ± 1.64 | 9.23 ± 1.24 | 8.50 ± 1.02 | 0.191 c | 0.040 *,c |
| p-value | 0.023 *,a | 0.028 *,a | ||||
| Wrinkle depth [mm] | 0.09 ± 0.02 | 0.14 ± 0.21 | 0.07 ± 0.03 | 0.08 ± 0.03 | 0.011 *,b | 0.516 b |
| p-value | 0.178 b | 0.520 b | ||||
| Vascular lesions [mm2] | 1.78 ± 1.50 | 2.83 ± 0.93 | 2.72 ± 0.93 | 1.54 ± 1.73 | 0.019 *,b | 0.005 *,b |
| p-value | 0.002 *,b | 0.011 *,b | ||||
| Skin discolouration [%] | 18.06 ± 7.46 | 22.71 ± 6.59 | 20.98 ± 3.30 | 14.84 ± 3.54 | 0.029 *,b | <0.001 *,b |
| p-value | <0.001 *,b | <0.001 *,b | ||||
| Nr | Identified Compound | Molecular Formula | PubChem CID | CAS | Information | Ref. |
|---|---|---|---|---|---|---|
| 1 | Octanedioic acid (Suberic acid) | C8H14O4 | 10457 | 505-48-6 | It has not yet been registered in CosIng, but it has been discovered that suberic acid inhibits morphological changes in the skin caused by UV-B radiation (dry skin, wrinkle formation, and epidermal thickness) and induces the expression of genes responsible for the production of collagen and hyaluronic acid. | [35,36,37] |
| 2 | Pantothenate (Pantothenic acid) | C9H17NO5 | 6631 | 79-83-4 | Antistatic, conditioning for hair and skin (CosIng). | [37,38] |
| 3 | Diethyl-phthalate | C12H14O4 | 6781 | 84-66-2 | Denaturant, film formation, hair conditioning, fragrance, plasticiser, solvent (CosIng). No significant toxicity after exposure to the compound. | [37,39,40] |
| 4 | N-Acetyl-L-phenylalanine (Phenylalanine) | C9H11NO2 | 74839 | 63-91-2/150-30-1 | Hair care, fragrance, skin care (CosIng). Precursor of melanin. | [37,41] |
| 5 | Citric acid | C6H8O7 | 311 | 77-92-9/ 5949-29-1 | Buffering, chelating, scent (CosIng). No significant toxicity upon skin contact. It increases the thickness of the viable epidermis and dermal glycosaminoglycans in sun-damaged skin. | [37,42,43] |
| 6 | N-Acetyl-DL-methionine (allantonin acetyl methionine) | C7H13NO3S | 6180 | 4207-40-3 | Antistatic, moisturising, skin-protecting, soothing (CosIng). | [37] |
| 7 | D-Tryptophan | C11H12N2O2 | 9060 | 54-12-6/73-22-3 | Antistatic, skin-conditioning, fragrance (CosIng). | [37] |
| 8 | Xylitol | C5H12O5 | 6912 | 87-99-0 | Moisturises the skin and improves barrier function. Antiseborrhoeic, deodorant, humectant, fragrance, skin conditioning (humectant) (CosIng). | [37,44] |
| 9 | 2-Isopropylmalic acid | C7H12O5 | 77 | 3237-44-3 | It has not been registered in CosIng, but studies have shown that this compound improves skin elasticity and reduces wrinkles. | [45] |
| 10 | N-Acetylglucosamine | C8H15NO6 | 24139 | 7512-17-6 | It has not yet been registered in CosIng, but clinical studies have shown that it may reduce facial hyperpigmentation. | [46] |
| 11 | Salidroside (Rhodioloside) | C14H20O7 | 159278 | 10338-51-9 | Skin protection (CosIng). It inhibits inflammation and melanin production in the skin. Speeds up wound healing. | [37,47,48] |
| 12 | Meglutol (3-hydroxy-3-methylglutaric acid) | C6H10O5 | 1662 | 503-49-1 | Known for its cholesterol-lowering effects, glutaric acid is available in its basic form in CosIng. | [37] |
| 13 | Azelaic acid | C9H16O4 | 2266 | 123-99-9 | Buffering, scent (CosIng). Its application to treat rosacea and the management of inflammatory conditions in acne vulgaris was previously studied. | [37,49,50] |
| 14 | N-Acetyl-L-tyrosine | C11H13NO4 | 68310 | 537-55-3 | Moisturising the skin, tanning, styling, and hair care (CosIng). | [37] |
| 15 | PG 34:2 (1-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphoglycerol) | C40H75O10P | 52927246 | 92347-24-5 | Emulsion stabilisation, skin condition, skin protection, surfactant—emulsifying (CosIng). A report from a clinical patient with molecular test results showed that phosphatidylglycerol may improve psoriatic lesions. | [37,51] |
| 16 | 6-Gingerol | C17H26O4 | 442793 | 23513-14- | It has not yet been registered in CosIng, but clinical studies have shown that beverages containing 6-gingerol may affect skin temperature. The possibility of local anti-inflammatory action was shown in an ex vivo study using human skin. | [52,53] |
| 17 | Sodium gluconate | C6H12O7.Na | 23672301 | 527-07-1/14906-97-9 | Moisturising the skin, chelation (CosIng). | [37] |
| 18 | Raffinose | C18H32O16 | 439242 | 512-69-6 | Moisturising the skin (CosIng), | [37] |
| 20 | Acteoside syn. Verbascoside | C29H36O15 | 5281800 | 61276-17-3 | antioxidant, anti-inflammatory, antineoplastic, and photoprotective activities. Chelation, whitening, skin protection (CosIng). | [37,54,55] |
| 24 | Riboflavin | C17H20N4O6 | 493570 | 201-507-1/204-988-6 | Dye, skin conditioning (CosIng). | [37] |
| 22 | Flavin mononucleotide (riboflavin 5′-phosphate) | C17H21N4O9P | 643976 | 146-17-8 | There is no data on the use of flavin mononucleotide as a cosmetic ingredient; however, this compound has been used as a food additive. | [56] |
| 23 | Traumatic Acid | C12H20O4 | 5283028 | 6402-36-4 | There are no records regarding the use of traumatic acid as a cosmetic ingredient. Nevertheless, the results show the potential of traumatic acid as a means to support collagen biosynthesis and wound healing. | [57,58] |
| 24 | D-Turanose | C12H22O11 | 5460935 | 547-25-1 | There is no information on the use of Turanose in cosmetics, but this compound has been used in several applications, such as acting as a protein stabiliser in medicines, an anti-inflammatory agent, and a sweetener in beverages. | [59] |
| 25 | Manginferin | C19H18O11 | 5281647 | 4773-96-0 | Skin protection (CosIng). | [37] |
| 26 | Maltitol | C12H24O11 | 493591 | 585-88-6 | Humectant, fragrance, moisturising, skin conditioning (CosIng). | [37] |
| 27 | Melezitose | C18H32O16 | 92817 | 597-12-6 | It is not listed in CosIng, but it is mentioned as the preferred oligosaccharide for the polymer in the cleaning composition (patent). | [60,61] |
| 28 | Ginsenoside F2 | C42H72O13 | 9918692 | 62025-49-4 | The specific Ginsenoside F2 is not listed in the CosIng database. However, the Ginsenoside library is available and contains notes on skin conditioning. Moreover, in cell and animal studies, Ginsenoside F2 has shown anti-ageing, hair anagen-inducing, and anti-inflammatory properties. | [62,63,64,65] |
| 29 | NADH | C21H29N7O14P2 | 439153 | 606-68-8 | Moisturising the skin, emollient moisturising the skin (CosIng). | [37] |
| 30 | Trehalose | C12H22O11 | 7427 | 99-20-7 | Humectant and moisturising (CosIng). | [37] |
| 31 | Uridine 5′-monophosphate | C9H13N2O9P | 6030 | 58-97-9/27416-86-0 | Not listed in the CosIng database, but the in vivo assay suggested the benefits of UMP as a wound healing agent. | [66] |
| 30 | Plantainoside C | C30H38O15 | 45359577 | - | Not listed in the CosIng database, but the compound was previously identified in Plantago asiatica. The extract from this species showed positive results for its anti-wrinkle and anti-ageing effects (in vitro). | [67,68] |
| 31 | Ala-Phe | C12H16N2O3 | 96814 | 3061-90-3 | Not listed in the CosIng database, but antioxidant and anti-inflammatory properties make this dipeptide a potential candidate for cosmetical formulation. Moreover, it has shown a moisturising effect to help skin hydration. | [69] |
| 32 | Gly-Leu | C8H16N2O3 | 92843 | 869-19-2 | Not listed in the CosIng database, but in vitro and in vivo studies suggested that oral administration of Gly-Leu improves skin hydration and elasticity in UVB-irradiated hairless mice. | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermosaningtyas, A.A.; Kroma-Szal, A.; Gornowicz-Porowska, J.; Urbanska, M.; Budzianowska, A.; Kikowska, M. Impact of Kickxia elatine In Vitro-Derived Stem Cells on the Biophysical Properties of Facial Skin: A Placebo-Controlled Trial. Appl. Sci. 2025, 15, 8625. https://doi.org/10.3390/app15158625
Hermosaningtyas AA, Kroma-Szal A, Gornowicz-Porowska J, Urbanska M, Budzianowska A, Kikowska M. Impact of Kickxia elatine In Vitro-Derived Stem Cells on the Biophysical Properties of Facial Skin: A Placebo-Controlled Trial. Applied Sciences. 2025; 15(15):8625. https://doi.org/10.3390/app15158625
Chicago/Turabian StyleHermosaningtyas, Anastasia Aliesa, Anna Kroma-Szal, Justyna Gornowicz-Porowska, Maria Urbanska, Anna Budzianowska, and Małgorzata Kikowska. 2025. "Impact of Kickxia elatine In Vitro-Derived Stem Cells on the Biophysical Properties of Facial Skin: A Placebo-Controlled Trial" Applied Sciences 15, no. 15: 8625. https://doi.org/10.3390/app15158625
APA StyleHermosaningtyas, A. A., Kroma-Szal, A., Gornowicz-Porowska, J., Urbanska, M., Budzianowska, A., & Kikowska, M. (2025). Impact of Kickxia elatine In Vitro-Derived Stem Cells on the Biophysical Properties of Facial Skin: A Placebo-Controlled Trial. Applied Sciences, 15(15), 8625. https://doi.org/10.3390/app15158625





