Antioxidant and Nutrient Profile of Tomato Processing Waste from the Mixture of Indigenous Croatian Varieties: Influence of Drying and Milling
Abstract
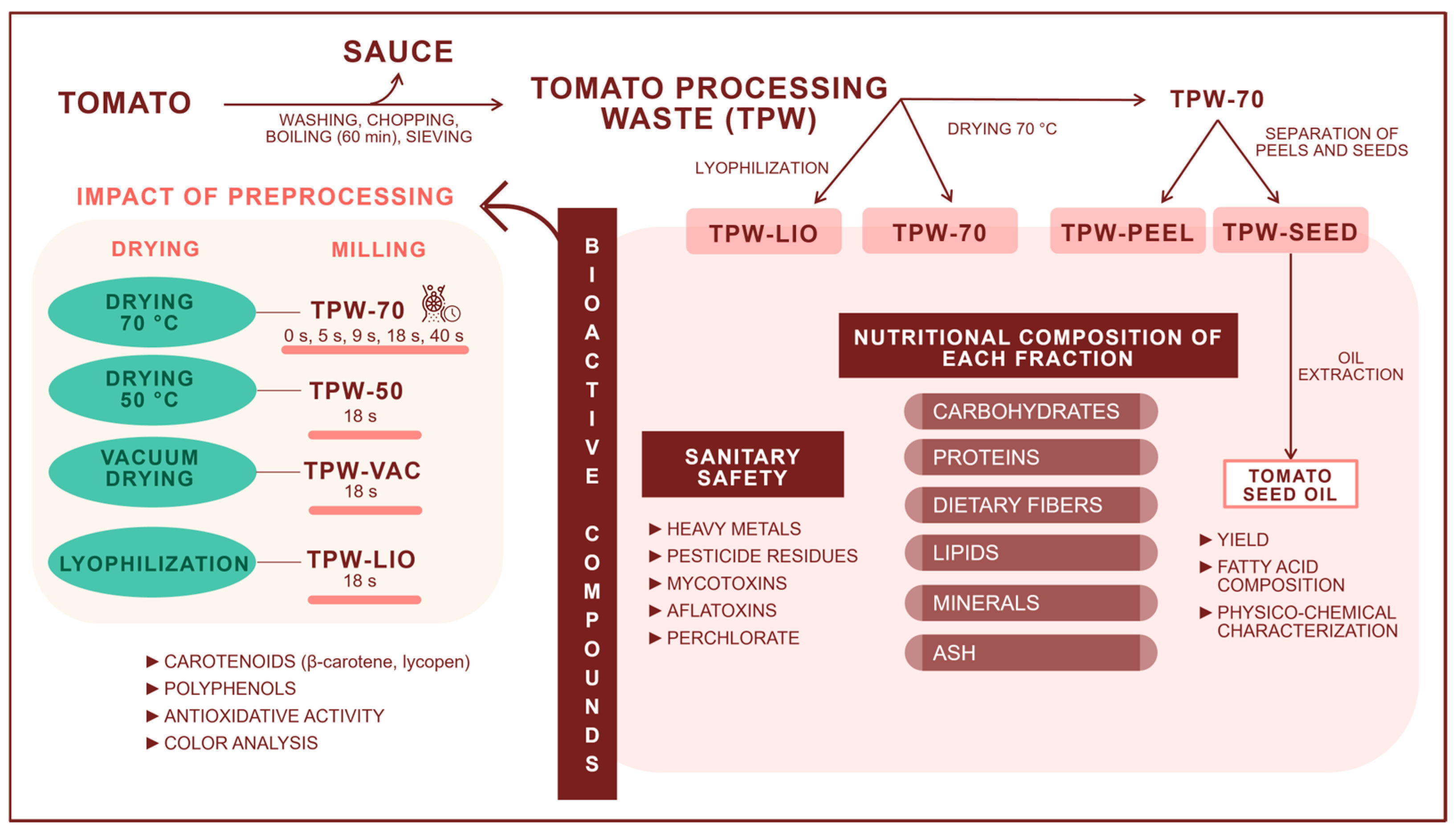
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Particle Size Characterization
2.3. Determination of TPW Sanitary Safety
2.4. Proximate Composition of TPW
2.5. Mineral Composition of TPW
2.6. Extraction and Characterization of TPW-SEED Oil
2.7. Antioxidants in TPW
2.7.1. Determination of Antioxidant Activity
2.7.2. Chromatographic Determination of Individual Polyphenols
2.7.3. Chromatographic Determination of Carotenoids
2.8. Color Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Distribution
3.2. Sanitary Safety of TPW
3.3. Proximate Composition of TPW, TPW-PEEL, and TPW-SEED
3.4. Mineral Composition of TPW, TPW-PEEL, and TPW-SEED
3.5. Total Polyphenols, Carotenoids, and Antioxidant Capacity of TPW, TPW-PEEL, and TPW-SEED
3.6. Characterization of TPW-SEED Oil
3.7. Impact of Drying Method and Grinding Degree on the Content of Total Antioxidants, Polyphenols, β-Carotene, and Lycopene
3.8. Impact of Drying Method and Grinding Degree on Color Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectrometry |
| AV | p-Anisidine value |
| BI | Browning index |
| EFSA | European Food Safety Agency |
| FAME | Fatty acids methyl esters |
| HPLC | High-performance liquid chromatography |
| LC-MS/MS | Liquid chromatography–mass spectrometry |
| MRL | Maximal residue levels |
| ORAC | Oxygen radical absorbance capacity |
| PV | Peroxide value |
| TEAC | Trolox equivalent antioxidant capacity |
| TP | Total phenols |
| TPW | Tomato processing waste |
| TPW-50 | Tomato processing waste air dried at 50 °C |
| TPW-70 | Tomato processing waste air dried at 70 °C |
| TPW-70-M0 | Tomato processing waste air dried at 70 °C and milled for 0 s |
| TPW-70-M5 | Tomato processing waste air dried at 70 °C and milled for 5 s |
| TPW-70-M9 | Tomato processing waste air dried at 70 °C and milled for 9 s |
| TPW-70-M18 | Tomato processing waste air dried at 70 °C and milled for 18 s |
| TPW-70-M40 | Tomato processing waste air dried at 70 °C and milled for 40 s |
| TPW-LIO | Tomato processing waste dried by lyophilization |
| TPW-PEEL | Peel fraction of tomato processing waste |
| TPW-SEED | Tomato seeds obtained from tomato processing waste |
| TPW-VAC | Vacuum-dried tomato processing waste |
References
- Boccia, F.; Di Donato, P.; Covino, D.; Poli, A. Food Waste and Bio-Economy: A Scenario for the Italian Tomato Market. J. Clean. Prod. 2019, 227, 424–433. [Google Scholar] [CrossRef]
- Directive-2008/98-EN-Waste Framework Directive-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2008/98/oj/eng (accessed on 6 July 2025).
- The European Green Deal-European Commission. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 6 July 2025).
- Food Waste: 132 Kg per Inhabitant in the EU in 2022. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20240927-2 (accessed on 6 July 2025).
- Løvdal, T.; Van Droogenbroeck, B.; Eroglu, E.C.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of Tomato Surplus and Waste Fractions: A Case Study Using Norway, Belgium, Poland, and Turkey as Examples. Foods 2019, 8, 229. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing By-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chandran, D.; Tomar, M.; Bhuyan, D.J.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Radha; Dhumal, S.; Singh, S.; et al. Valorization Potential of Tomato (Solanum lycopersicum L.) Seed: Nutraceutical Quality, Food Properties, Safety Aspects, and Application as a Health-Promoting Ingredient in Foods. Horticulturae 2022, 8, 265. [Google Scholar] [CrossRef]
- Laranjeira, T.; Costa, A.; Faria-Silva, C.; Ribeiro, D.; de Oliveira, J.M.P.F.; Simões, S.; Ascenso, A. Sustainable Valorization of Tomato By-Products to Obtain Bioactive Compounds: Their Potential in Inflammation and Cancer Management. Molecules 2022, 27, 1701. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of Tomato Waste as a Source of Carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Radić, K.; Galić, E.; Vinković, T.; Golub, N.; Vitali Čepo, D. Tomato Waste as a Sustainable Source of Antioxidants and Pectins: Processing, Pretreatment and Extraction Challenges. Sustainability 2024, 16, 9158. [Google Scholar] [CrossRef]
- López-Yerena, A.; Domínguez-López, I.; Abuhabib, M.M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Pérez, M. Tomato Wastes and By-Products: Upcoming Sources of Polyphenols and Carotenoids for Food, Nutraceutical, and Pharma Applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 10546–10563. [Google Scholar] [CrossRef]
- Zareie, Z.; Nooshkam, M.; Moayedi, A. Tomato Agro-Industrial Wastes as a Rich Source of Bioactive Compounds. In Food Byproducts Management and Their Utilization; Gómez-García, R., Vilas-Boas, A.A., Campos, D.A., Pintado, M.M., Aguilar, C.N., Eds.; Apple Academic Press: New York, NY, USA, 2024; ISBN 978-1-003-37780-1. [Google Scholar]
- Rajan, A.; Kumar, S.; Sunil, C.K.; Radhakrishnan, M.; Rawson, A. Recent Advances in the Utilization of Industrial Byproducts and Wastes Generated at Different Stages of Tomato Processing: Status Report. Food Process Preserv. 2022, 46, e17063. [Google Scholar] [CrossRef]
- Baker, P.W.; Preskett, D.; Krienke, D.; Runager, K.S.; Hastrup, A.C.S.; Charlton, A. Pre-Processing Waste Tomatoes into Separated Streams with the Intention of Recovering Protein: Towards an Integrated Fruit and Vegetable Biorefinery Approach to Waste Minimization. Waste Biomass Valor. 2022, 13, 3463–3473. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Wu, Y.; Tang, Z.; Zhang, D.; Lyu, J.; Khan, K.S.; Xiao, X.; Yu, J. Evaluation of Nutritional Composition, Biochemical, and Quality Attributes of Different Varieties of Tomato (Solanum lycopersicum L.). J. Food Compos. Anal. 2024, 132, 106384. [Google Scholar] [CrossRef]
- Fibiani, M.; Paolo, D.; Leteo, F.; Campanelli, G.; Picchi, V.; Bianchi, G.; Lo Scalzo, R. Influence of Year, Genotype and Cultivation System on Nutritional Values and Bioactive Compounds in Tomato (Solanum lycopersicum L.). Food Chem. 2022, 389, 133090. [Google Scholar] [CrossRef]
- Flores, I.R.; Vásquez-Murrieta, M.S.; Franco-Hernández, M.O.; Márquez-Herrera, C.E.; Ponce-Mendoza, A.; Del Socorro López-Cortéz, M. Bioactive Compounds in Tomato (Solanum lycopersicum) Variety Saladette and Their Relationship with Soil Mineral Content. Food Chem. 2021, 344, 128608. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Felli, M.; Sulli, M.; Diretto, G.; Savatin, D.V.; Mazzucato, A.; Merendino, N.; Costantini, L. Tomato Pomace Food Waste from Different Variants as a High Antioxidant Potential Resource. Food Chem. 2024, 452, 139509. [Google Scholar] [CrossRef] [PubMed]
- Luksta, I.; Spalvins, K. Methods for Extraction of Bioactive Compounds from Products: A Review. Environ. Clim. Technol. 2023, 27, 422–437. [Google Scholar] [CrossRef]
- Bhatkar, N.S.; Shirkole, S.S.; Mujumdar, A.S.; Thorat, B.N. Drying of Tomatoes and Tomato Processing Waste: A Critical Review of the Quality Aspects. Dry. Technol. 2021, 39, 1720–1744. [Google Scholar] [CrossRef]
- Çetin, N. Comparative Assessment of Energy Analysis, Drying Kinetics, and Biochemical Composition of Tomato Waste under Different Drying Conditions. Sci. Hortic. 2022, 305, 111405. [Google Scholar] [CrossRef]
- Croatian Plant Genetic Resources Database. Available online: https://cpgrd.hapih.hr/ (accessed on 6 July 2025).
- Vidinamo, F.; Fawzia, S.; Karim, M.A. Effect of drying methods and storage with agro-ecological conditions on phytochemicals and antioxidant activity of fruits: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 353–361. [Google Scholar] [CrossRef]
- Regulation-2023/915-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj/eng (accessed on 6 July 2025).
- Regulation-333/2007-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2007/333/oj/eng (accessed on 6 July 2025).
- Croatian Standards Institute. Available online: https://repozitorij.hzn.hr/norm/HRN+EN+15662%3A2018 (accessed on 6 July 2025).
- Regulation-396/2005-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2005/396/oj/eng (accessed on 6 July 2025).
- Andrija Štampar School of Public Health. SOP-450-053 Edition 01. In Method for Determination of Mycotoxins by LC-MS/MS; Department of Environmental Protection and Health Ecology/Andrija Štampar School of Public Health: Zagreb, Croatia, 2016. [Google Scholar]
- Latimer, G.W., Jr. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016; ISBN 978-0-935584-87-5. [Google Scholar]
- Food, Nutrition and Agriculture 24 Carbohydrates in Human Nutrition. Available online: https://www.fao.org/4/x2650t/x2650t02.htm (accessed on 7 July 2025).
- Merrill, A.L.; Watt, B.K. Energy Value of Foods: Basis and Derivation; USDA Handbook No. 74; United States Department of Agriculture: Washington, DC, USA, 1973.
- Sangeetha, K.; Ramyaa, R.B.; Mousavi Khaneghah, A.; Radhakrishnan, M. Extraction, Characterization, and Application of Tomato Seed Oil in the Food Industry: An Updated Review. J. Agric. Food Res. 2023, 11, 100529. [Google Scholar] [CrossRef]
- Republic of Croatia. Ordinance on Edible Oils and Fats. Official Gazette, No. 11/2019. Ministry of Economy, Republic of Croatia. 2019. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/full/2019_01_11_229.html (accessed on 6 July 2025).
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/71268.html (accessed on 7 July 2025).
- UNE EN ISO 660:2021; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity (ISO 660:2020). Standards European: Brussels, Belgium, 2021. Available online: https://www.en-standard.eu/une-en-iso-660-2021-animal-and-vegetable-fats-and-oils-determination-of-acid-value-and-acidity-iso-660-2020/ (accessed on 7 July 2025).
- ISO 3961:2013; Animal and Vegetable Fats and Oils—Determination of Iodine Value. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/61278.html (accessed on 7 July 2025).
- ISO 3657:2013; Animal and Vegetable Fats and Oils—Determination of Saponification Value. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/60526.html (accessed on 7 July 2025).
- ISO 6885:2006; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/40052.html (accessed on 7 July 2025).
- Regulation-796/2002-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2002/796/oj/eng (accessed on 6 July 2025).
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Čulina, P.; Cvitković, D.; Pfeifer, D.; Zorić, Z.; Repajić, M.; Elez Garofulić, I.; Balbino, S.; Pedisić, S. Phenolic Profile and Antioxidant Capacity of Selected Medicinal and Aromatic Plants: Diversity upon Plant Species and Extraction Technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Gas-Pascual, E.; Orchard, C.J.; Sari, E.N.; Riedl, K.M.; Schwartz, S.J.; Francis, D.M.; Cooperstone, J.L. Analysis of Tomato Carotenoids: Comparing Extraction and Chromatographic Methods. J. AOAC Int. 2019, 102, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L. Collaborators Determination of Lutein, β-Carotene, and Lycopene in Infant Formula and Adult Nutritionals by Ultra-High Performance Liquid Chromatography: Collaborative Study, Final Action 2016.13 for β-Carotene and Lycopene Only. J. AOAC Int. 2020, 103, 818–832. [Google Scholar] [CrossRef] [PubMed]
- EU Pesticides Database—European Commission. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 7 July 2025).
- Costa, F.R.S.; Maia, P.L.; da Silva, F.S.; Nobre, C.A.; Silva, R.O.; Milhome, M.A.L. Analysis of Residues of Pesticides in Tomato Processed Foods. J. Braz. Chem. Soc. 2023, 34, 1734–1742. [Google Scholar] [CrossRef]
- Dione, M.M.; Djouaka, R.; Mbokou, S.F.; Ilboudo, G.S.; Ouedraogo, A.A.; Dinede, G.; Roesel, K.; Grace, D.; Knight-Jones, T.J.D. Detection and Quantification of Pesticide Residues in Tomatoes Sold in Urban Markets of Ouagadougou, Burkina Faso. Front. Sustain. Food Syst. 2023, 7, 1213085. [Google Scholar] [CrossRef]
- The 2022 European Union Report on Pesticide Residues in Food|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/8753 (accessed on 7 July 2025).
- Eslami, E.; Carpentieri, S.; Pataro, G.; Ferrari, G. A Comprehensive Overview of Tomato Processing By-Product Valorization by Conventional Methods versus Emerging Technologies. Foods 2022, 12, 166. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. Tomato Waste from Processing Industries as a Feedstock for Biofuel Production. Bioenerg. Res. 2019, 12, 1000–1011. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable Valorisation of Tomato Pomace: A Comprehensive Review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Boobis, A.; Eskola, M.; Fink-Gremmels, J.; Fürst, P.; Heppner, C.; Schlatter, J.; van Leeuwen, R. Risk Assessment of Contaminants in Food and Feed. EFSA J. 2012, 10, s1004. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Ah-Hen, K.; Vega-Gálvez, A.; Honores, C.; Moraga, N.O. Stevia Rebaudiana Leaves: Effect of Drying Process Temperature on Bioactive Components, Antioxidant Capacity and Natural Sweeteners. Plant Foods Hum. Nutr. 2016, 71, 49–56. [Google Scholar] [CrossRef]
- Pazalja, M.; Salihović, M.; Sulejmanović, J. Heavy metals content in ashes of wood pellets and the health risk assessment related to their presence in the environment. Sci. Rep. 2011, 11, 17952. [Google Scholar] [CrossRef]
- Chabi, I.B.; Zannou, O.; Dedehou, E.S.C.A.; Ayegnon, B.P.; Oscar Odouaro, O.B.; Maqsood, S.; Galanakis, C.M.; Pierre Polycarpe Kayodé, A. Tomato Pomace as a Source of Valuable Functional Ingredients for Improving Physicochemical and Sensory Properties and Extending the Shelf Life of Foods: A Review. Heliyon 2024, 10, e25261. [Google Scholar] [CrossRef] [PubMed]
- Nakov, G.; Brandolini, A.; Estivi, L.; Bertuglia, K.; Ivanova, N.; Jukić, M.; Komlenić, D.K.; Lukinac, J.; Hidalgo, A. Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers. Antioxidants 2022, 11, 2087. [Google Scholar] [CrossRef]
- Del Valle, M.; Cámara, M.; Torija, M. Chemical Characterization of Tomato Pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical Profile, Functional and Antioxidant Properties of Tomato Peel Fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Labate, J.A.; Breksa, A.P.; Robertson, L.D.; King, B.A.; King, D.E. Genetic Differences in Macro-Element Mineral Concentrations among 52 Historically Important Tomato Varieties. Plant Genet. Resour. 2018, 16, 343–351. [Google Scholar] [CrossRef]
- Watanabe, M.; Ohta, Y.; Licang, S.; Motoyama, N.; Kikuchi, J. Profiling Contents of Water-Soluble Metabolites and Mineral Nutrients to Evaluate the Effects of Pesticides and Organic and Chemical Fertilizers on Tomato Fruit Quality. Food Chem. 2015, 169, 387–395. [Google Scholar] [CrossRef]
- Agbemafle, R.; Owusu-Sekyere, J.D.; Bart-Plange, A. Effect of Deficit Irrigation and Storage on the Nutritional Composition of Tomato (Lycopersicon esculentum Mill. Cv. Pectomech). Croat. J. Food Sci. Technol. 2015, 10, 59–65. [Google Scholar]
- Ozyigit, I.I.; Can, H.; Uyanik, O.L.; Yalcin, I.E.; Demir, G. Fruit Mineral Nutrient Contents of Field and Greenhouse Grown Tomatoes and Comparison with Standard Values. Not. Bot. Horti Agrobo 2024, 52, 13479. [Google Scholar] [CrossRef]
- Ramesh, K.V.; Paul, V.; Pandey, R. Dynamics of Mineral Nutrients in Tomato (Solanum lycopersicum L.) Fruits during Ripening: Part I—On the Plant. Plant Physiol. Rep. 2020, 26, 23–37. [Google Scholar] [CrossRef]
- Mehta, D.; Prasad, P.; Sangwan, R.S.; Yadav, S.K. Tomato Processing cold·pressing Valorization in Bread and Muffin: Improvement in Physicochemical Properties and Shelf Life Stability. J. Food Sci. Technol. 2018, 55, 2560–2568. [Google Scholar] [CrossRef]
- Isik, F.; Topkaya, C. Effects of Tomato Pomace Supplementation on Chemical and Nutritional Properties of Crackers. Ital. J. Food Sci. 2016, 28, 525–535. [Google Scholar]
- Isik, F.; Yapar, A. Effect of Tomato Seed Supplementation on Chemical and Nutritional Properties of Tarhana. Food Meas. 2017, 11, 667–674. [Google Scholar] [CrossRef]
- Sissaoui, A. Valorization of Tomato Processing By-Products in Livestock Feed: Nutritional Value and Inclusion Rates. Pak. J. Life Soc. Sci. 2025, 23, 8819–8829. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Sarkar, A.; Kaul, P. Evaluation of Tomato Processing By-Products: A Comparative Study in a Pilot Scale Setup. J. Food Process Eng. 2014, 37, 299–307. [Google Scholar] [CrossRef]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in Polyphenols Contents and Antioxidant Capacities of Organically and Conventionally Cultivated Tomato (Solanum lycopersicum L.) Fruits during Ripening. Int. J. Anal. Chem. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Chamorro, I.; Santos-Sánchez, G.; Martín, F.; Fernández-Pachón, M.-S.; Hornero-Méndez, D.; Cerrillo, I. Evaluation of the Impact of the Ripening Stage on the Composition and Antioxidant Properties of Fruits from Organically Grown Tomato (Solanum lycopersicum L.) Spanish Varieties. Foods 2024, 13, 2337. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Carle, R.; Astudillo, L.; Guzmán, L.; Gutiérrez, M.; Carrasco, G.; Palomo, I. Antioxidant and antiplatelet activities in extracts from green and fully ripe tomato fruits (Solanum lycopersicum) and pomace from industrial tomato processing. Evid. Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Vardanian, I.; Sargsyan, G.; Martirosayn, G.; Pahlevanyan, A.; Tsereteli, I.; Martorosyan, H.; Khachatryan, L.; Zurabyan, A.; Harutyunyan, Z. Lycopene in Tomatoes: Genetic Regulation, Agronomic Practices, and Environmental Influence. Funct. Food Sci. 2025, 5, 127–145. [Google Scholar] [CrossRef]
- Jacob, K.; García-Alonso, F.J.; Periago, G.R.J. Stability of Carotenoids, Phenolic Compounds, Ascorbic Acid and Antioxidant Capacity of Tomatoes during Thermal Processing. Arch. Latinoam. Nutr. 2010, 60, 192–198. [Google Scholar] [PubMed]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C. Tomato Seed Oil for Edible Use: Cold Break, Hot Break, and Harvest Year Effects. J. Food Process Pres. 2017, 41, e13309. [Google Scholar] [CrossRef]
- Delegated Regulation-2022/2104-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg_del/2022/2104/oj/eng (accessed on 7 July 2025).
- Implementing Regulation-2021/670-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/670/oj/eng (accessed on 7 July 2025).
- Regulation, H. Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; EU: Brussels, Belgium, 1991; Volume 248. [Google Scholar]
- Kandasamy, S.; Naveen, R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Regier, M.; Mayer-Miebach, E.; Behsnilian, D.; Neff, E.; Schuchmann, H.P. Influences of drying and storage of lycopene-rich carrots on the carotenoid content. Dry. Technol. 2005, 23, 989–998. [Google Scholar] [CrossRef]
- Zhang, W.P.; Chen, C.; Ju, H.Y.; Okaiyeto, S.A.; Sutar, P.P.; Yang, L.Y.; Xiao, H.W. Pulsed vacuum drying of fruits, vegetables, and herbs: Principles, applications and future trends. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13430. [Google Scholar] [CrossRef]
- El-Gamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Maqsood, S.; Omer, I.; Eldin, A.K. Quality attributes, moisture sorption isotherm, phenolic content and antioxidative activities of tomato (Lycopersicon esculentum L.) as influenced by method of drying. J. Food Sci. Technol. 2015, 52, 7059–7069. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martinez, P.; Martin-Belloso, O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J. Agric. Food Chem. 2012, 60, 9667–9672. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862. [Google Scholar] [CrossRef]
- Toro-Uribe, S.; Godoy-Chivatá, J.; Villamizar-Jaimes, A.R.; Perea-Flores, M.D.J.; López-Giraldo, L.J. Insight of polyphenol oxidase enzyme inhibition and total polyphenol recovery from cocoa beans. Antioxidants 2020, 9, 458. [Google Scholar] [CrossRef]
- Nishizono, M.; Issasi, C.S.C.; Agutaya, J.K.C.N.; Sasaki, M.; Mizukami, H. Production of Dried Tomato Powder with a High Concentration of Functional Components and Nutrients. J. Antioxid. Act. 2023, 2, 1–21. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Adani, F.; Pepé Sciarria, T.; Squillace, P.; Scarafoni, A.; Iametti, S.; Scaglia, B. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Raw and Fermented Tomato Pomace and Their Correlations with Aglycate-Polyphenols. Antioxidants 2020, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, A.; Aguilera, J.M. Size reduction and particle size influence the quantification of phenolic-type compounds and antioxidant activity in plant food matrices. Trends Food Sci. Technol. 2025, 162, 105081. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Wu, Z.; Li, S.; Eun, J.B. Impact of ball-milling time on the physical properties, bioactive compounds, and structural characteristics of onion peel powder. Food Biosci. 2020, 36, 100630. [Google Scholar] [CrossRef]
- Nain, C.W.; Mignolet, E.; Herent, M.F.; Quetin-Leclercq, J.; Debier, C.; Page, M.M.; Larondelle, Y. The catechins profile of green tea extracts affects the antioxidant activity and degradation of catechins in DHA-rich oil. Antioxidants 2022, 11, 1844. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, A.N.; Harinarayanan, N.C.; Rahman, W.U.; Park, C.E.; Yoon, H.S.; Choi, S.G. Impact of Grinding Under Different Vacuum Levels on Browning, Antioxidant Activity, and Phenolic Compounds of Asian Pears (Pyrus pyrifolia Niitaka). Food Bioprocess. Technol. 2025, 18, 3405–3415. [Google Scholar] [CrossRef]
- Cernîşev, S. Effects of Conventional and Multistage Drying Processing on Non-Enzymatic Browning in Tomato. J. Food Eng. 2010, 96, 114–118. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of Air-Drying Temperature on Physico-Chemical Properties, Antioxidant Capacity, Colour and Total Phenolic Content of Red Pepper (Capsicum annuum, L. Var. Hungarian). Food Chem. 2009, 117, 647–653. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef] [PubMed]
- Maskan, M. Drying, Shrinkage and Rehydration Characteristics of Kiwifruits during Hot Air and Microwave Drying. J. Food Eng. 2001, 48, 177–182. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Dehydration of Red Bell-Pepper (Capsicum annuum L.): Change in Drying Behavior, Colour and Antioxidant Content. Food Bioprod. Process 2011, 89, 504–513. [Google Scholar] [CrossRef]



| Contaminant | Content | MRL * | Technique | Category * |
|---|---|---|---|---|
| Patulin (µg/kg) | <5.00 | 25 | HPLC | Solid apple products placed on the market for the final consumer, except products listed in 1.3.4 and 1.3.5 |
| Ochratoxin (µg/kg) | <0.01 | 2.0 | LC-MS/MS | Other dried fruits * |
| Aflatoxin B1 (µg/kg) | <0.01 | 2.0 | LC-MS/MS | Ingredients or processed products from dried fruits, placed on the market for the final consumer or used as an ingredient in food, except products listed in 1.1.3 |
| Aflatoxin B1, G1, B2, G2 (µg/kg) | <0.01 | 4.0 | LC-MS/MS | |
| Pb (mg/kg) | ND | 0.05 | AAS | Fruiting vegetables, except products listed in 3.1.4.2 |
| Cd (mg/kg) | ND | 0.02 | AAS | Fruiting vegetables, except products listed in 3.2.4.2 |
| As (mg/kg) | ND | 0.15 | AAS | Non-parboiled milled rice (polished or white rice) |
| Perchlorate (mg/kg) | <0.05 | 0.05 | LC-MS/MS | Fruits and vegetables, except products listed in 6.3.1.1 and 6.3.1.2 |
| g/100 g Dry Matter | TPW-70 | TPW-LIO | TPW-PEEL | TPW-SEED |
|---|---|---|---|---|
| ash | 4.81 ± 0.06 c | 4.16 ± 0.04 b | 5.09 ± 0.08 d | 3.08 ± 0.11 a |
| fat | 7.84 ± 0.02 b | 9.21 ± 0.37 c | 3.89 ± 0.20 a | 25.87 ± 0.18 d |
| protein | 18.00 ± 0.93 b | 17.46 ± 0.02 b | 14.34 ± 0.29 a | 27.12 ± 0.11 c |
| dietary fiber | ||||
| total | 40.10 ± 1.14 a | 41.16 ± 1.37 a | 52.06 ± 1.32 b | 41.30 ± 1.89 a |
| soluble | 7.53 ± 0.81 a | 7.57 ± 0.88 a | 9.09 ± 1.46 a | 5.40 ± 2.20 a |
| insoluble | 32.57 ± 0.44 a | 33.59 ± 0.50 b | 42.97 ± 0.17 d | 35.90 ± 0.31 c |
| available carbohydrates | 29.25 ± 2.04 c,d | 28.01 ± 1.66 b,c | 24.62 ± 1.67 b | 2.62 ± 0.00 a |
| energy (kcal/100 g) | 339.74 b | 347.09 c | 294.99 a | 434.44 d |
| Element (mg/kg) | TPW-70 | TPW-LIO | TPW-PEEL | TPW-SEED |
|---|---|---|---|---|
| Cr | 1.24 ± 0.65 a | 1.06 ± 0.08 a | 1.17 ± 0.75 a | 0.54 ± 0.01 a |
| Mn | 15.3 ± 2.06 b | 18.17 ± 1.38 b | 25.97 ± 4.01 c | 1.0 ± 0.0 a |
| Ni | 2.03 ± 1.01 a | 1.66 ± 0.22 a | 1.42 ± 0.39 a | 2.15 ± 0.23 a |
| Br | 3.07 ± 0.27 b | 3.23 ± 0.21 b | 5.09 ± 0.35 c | 2.11 ± 0.18 a |
| Rb | 3.73 ± 0.09 b | 3.56 ± 0.09 b | 8.98 ± 0.83 c | 0.24 ± 0.01 a |
| Sr | 2.29 ± 0.09 b | 2.85 ± 0.32 b | 4.85 ± 0.92 c | 0.0 ± 0.0 a |
| Fe | 117.5 ± 34.65 b | 131.36 ± 0.68 b | 202.91 ± 12.57 c | 21.6 ± 0.7 a |
| K (g/kg) | 10.79 ± 0.73 b | 11.77 ± 3.36 b | 19.21 ± 1.28 c | 0.92 ± 0.6 a |
| TPW-SEED Oil | Literature Data | EU Regulation | |
|---|---|---|---|
| Yield (%) | 17.4 ± 0.7 | 17–18 [76] | |
| Density (g/cm3) | 0.92 ± 0.01 | 0.81–0.89 [32] | |
| Peroxide value (mmol O2/kg) | 0.00 ± 0.00 | 2.5–2.6 [32] | <20.0 [77] |
| p-anisidine value | 0.62 ± 0.02 | <10.0 [78] | |
| Total oxidation value | 0.62 ± 0.02 | ||
| Free fatty acid (%) | 0.44 ± 0.01 | 1.4–1.5 [32] | <0.80 [77] |
| Saponification number (g KOH/kg) | 204.3 ± 5.1 | 192–193 [32] | |
| Iodine number (g I2/kg) | 118.3 ± 2.1 | 121–131 [32] |
| Compound | TPW-LIO | TPW-VAC | TPW-50 | TPW-70 |
|---|---|---|---|---|
| β-carotene (mg/100 g) | 12.8 ± 0.1 b | 14.1 ± 0.2 c | 11.9 ± 0.3 a | 12.8 ± 0.3 b |
| Lycopene (mg/100 g) | 99.9 ± 0.0 b | 164.8 ± 3.5 c | 83.1 ± 3.1 a | 79.4 ± 0.8 a |
| TP (mg GAE/100 g) | 194.4 ± 8.7 a,b | 204.9 ± 10.7 b,c | 181.2 ± 8.6 a | 221.4 ± 7.0 c |
| TEAC (mg TE/100 g) | 72.8 ± 9.6 b | 58.5 ± 6.6 a,b | 51.8 ± 2.4 a | 63.4 ± 8.4 a,b |
| Rutin (mg/kg) | 27.3 ± 0.9 b | 15.1 ± 6.6 a | 13.7 ± 2.5 a | 16.7 ± 2.2 a |
| Kaempferol-3-rutinoside (mg/kg) | 2.4 ± 0.2 a | 1.9 ± 0.0 a | 2.4 ± 0.5 a | 0.9 ± 0.5 a |
| Catechin (mg/kg) | 15.2 ± 1.4 b | 7.4 ± 9.4 a,b | 5.0 ± 4.4 a,b | 2.4 ± 0.2 a |
| Chlorogenic acid (mg/kg) | 11.2 ± 0.2 c | 10.7 ± 1.5 b,c | 9.3 ± 0.7 b | 6.7 ± 0.2 a |
| Caffeic acid (mg/kg) | 12.3 ± 2.6 b | 7.2 ± 0.2 a | 6.5 ± 0.3 a | 6.5 ± 0.8 a |
| Compound Dx 50 | TPW-70-M0 732 µm | TPW-70-M5 542 µm | TPW-70-M9 431 µm | TPW-70-M18 388 µm | TPW-70-M40 210 µm |
|---|---|---|---|---|---|
| β-Carotene (mg/100 g) | 11.1 ± 0.6 a | 11.3 ± 0.4 a | 11.9 ± 0.4 a | 12.8 ± 0.3 b | 11.9 ± 0.1 a |
| Lycopene (mg/100 g) | 75.9 ± 2.5 a,b | 72.5 ± 0.5 a | 73.2 ± 3.3 a | 79.4 ± 0.8 b | 74.1 ± 0.3 a |
| TP (mg GAE/100 g) | 174.9 ± 2.8 a | 211.8 ± 12.8 b | 209.9 ± 0.8 b | 221.4 ± 7.0 b | 230.2 ± 17.7 b |
| TEAC (mg TE/100 g) | 58.4 ± 5.3 a | 54.2 ± 0.7 a | 49.8 ± 2.6 a | 63.4 ± 8.4 a | 61.7 ± 9.7 a |
| Rutin (mg/kg) | 14.7 ± 2.5 a | 15.0 ± 0.2 a | 13.8 ± 0.8 a | 18.2 ± 0.1 b | 18.1 ± 1.3 b |
| Kaempferol-3-rutinoside (mg/kg) | 0.81 ± 0.41 a | 0.81 ± 0.34 a | 1.26 ± 0.29 a | 1.36 ± 0.20 a | 1.06 ± 0.13 a |
| Catechin (mg/kg) | 6.46 ± 0.39 d | 3.50 ± 0.67 c | 3.83 ± 0.12 c | 2.41 ± 0.16 b | 0.91 ± 0.28 a |
| Chlorogenic acid (mg/kg) | 8.85 ± 1.15 b | 7.17 ± 0.47 a | 6.63 ± 0.44 a | 6.67 ± 0.21 a | 7.30 ± 0.03 a |
| Caffeic acid (mg/kg) | 7.03 ± 0.84 a | 7.49 ± 0.44 a | 8.83 ± 0.23 b | 7.96 ± 0.04 a,b | 7.22 ± 0.33 a |
| Sample | L* | a* | b* | C* | h° | BI |
|---|---|---|---|---|---|---|
| Impact of drying technique | ||||||
| TPW-LIO | 56.0 ± 0.1 b,c | 28.2 ± 0.0 c | 52.1 ± 0.1 d | 59.2 ± 0.1 b | 61.6 ± 0.0 d | 0.76 ± 0.00 a |
| TPW-VAC | 48.6 ± 0.0 a | 31.2 ± 0.0 d | 50.6 ± 0.1 c | 59.4 ± 0.1 b | 58.3 ± 0.1 a | 0.74 ± 0.01 a |
| TPW-50 | 57.8 ± 1.8 c | 25.4 ± 0.1 a | 45.4 ± 0.1 b | 52.0 ± 0.2 a | 60.8 ± 0.0 c | 0.71 ± 0.06 a |
| TPW-70 | 55.4 ± 0.0 b | 26.0 ± 0.1 b | 44.8 ± 0.2 a | 51.8 ± 0.2 a | 59.8 ± 0.0 b | 0.73 ± 0.01 a |
| Impact of grinding degree | ||||||
| TPW-70-M0 | 49.3 ± 0.1 a | 21.9 ± 0.2 a | 32.5 ± 0.1 a | 39.2 ± 0.2 a | 56.0 ± 0.1 a | 0.56 ± 0.00 a |
| TPW-70-M5 | 54.1 ± 0.4 c | 26.4 ± 0.2 c | 45.2 ± 0.1 c,d | 52.3 ± 0.3 b | 59.7 ± 0.1 c | 0.71 ± 0.02 b |
| TPW-70-M9 | 52.6 ± 0.1 b | 28.0 ± 0.0 e | 45.5 ± 0.2 d | 53.4 ± 0.2 c | 58.4 ± 0.1 b | 0.73 ± 0.01 b |
| TPW-70-M18 | 55.4 ± 0.0 d | 26.0 ± 0.1 d | 44.8 ± 0.2 b,c | 51.8 ± 0.2 b | 59.8 ± 0.0 c | 0.73 ± 0.01 b |
| TPW-70-M40 | 61.2 ± 0.0 e | 23.5 ± 0.0 b | 49.0 ± 0.2 e | 54.4 ± 0.1 d | 64.4 ± 0.0 d | 0.72 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petković, T.; Galić, E.; Radić, K.; Golub, N.; Jablan, J.; Bival Štefan, M.; Moslavac, T.; Grudenić, K.; Rumora Samarin, I.; Vinković, T.; et al. Antioxidant and Nutrient Profile of Tomato Processing Waste from the Mixture of Indigenous Croatian Varieties: Influence of Drying and Milling. Appl. Sci. 2025, 15, 8447. https://doi.org/10.3390/app15158447
Petković T, Galić E, Radić K, Golub N, Jablan J, Bival Štefan M, Moslavac T, Grudenić K, Rumora Samarin I, Vinković T, et al. Antioxidant and Nutrient Profile of Tomato Processing Waste from the Mixture of Indigenous Croatian Varieties: Influence of Drying and Milling. Applied Sciences. 2025; 15(15):8447. https://doi.org/10.3390/app15158447
Chicago/Turabian StylePetković, Tea, Emerik Galić, Kristina Radić, Nikolina Golub, Jasna Jablan, Maja Bival Štefan, Tihomir Moslavac, Karla Grudenić, Ivana Rumora Samarin, Tomislav Vinković, and et al. 2025. "Antioxidant and Nutrient Profile of Tomato Processing Waste from the Mixture of Indigenous Croatian Varieties: Influence of Drying and Milling" Applied Sciences 15, no. 15: 8447. https://doi.org/10.3390/app15158447
APA StylePetković, T., Galić, E., Radić, K., Golub, N., Jablan, J., Bival Štefan, M., Moslavac, T., Grudenić, K., Rumora Samarin, I., Vinković, T., & Vitali Čepo, D. (2025). Antioxidant and Nutrient Profile of Tomato Processing Waste from the Mixture of Indigenous Croatian Varieties: Influence of Drying and Milling. Applied Sciences, 15(15), 8447. https://doi.org/10.3390/app15158447









