Abstract
Resistance of various bacterial pathogens to the activity of clinically approved drugs currently leads to serious infections, rapid spread of difficult-to-treat diseases, and even death. Taking the threats for human health in mind, researchers are focused on the isolation and characterization of novel natural products, including plant secondary metabolites. These molecules serve as inspiration and a suitable structural platform in the design and development of novel semi-synthetic and synthetic derivatives. All considered compounds have to be adequately evaluated in silico, in vitro, and in vivo using relevant approaches. The current review paper briefly focuses on the chemical and metabolic properties of resveratrol (1), as well as its oligomeric structures, viniferins, and viniferin-based molecules. The core scaffolds of these compounds contain so-called privileged structures, which are also present in many clinically approved drugs, indicating that those natural, properly substituted semi-synthetic, and synthetic molecules can provide a notably broad spectrum of beneficial pharmacological activities, including very impressive antimicrobial efficiency. Except for spectral verification of their structures, these compounds suffer from the determination or prediction of other structural and physicochemical characteristics. Therefore, the structure–activity relationships for specific dihydrodimeric and dimeric viniferins, their bioisosteres, and derivatives with notable efficacy in vitro, especially against chosen Gram-positive bacterial strains, are summarized. In addition, a set of descriptors related to their structural, physicochemical, pharmacokinetic, and toxicological properties is generated using various computational tools. The obtained values are compared to those of clinically approved drugs. The particular relationships between these in silico parameters are also explored.
1. Introduction
Nowadays, some representatives of bacterial or fungal strains cause very serious pneumonia or various invasive infections that are not easily treatable. The unwanted phenomena occur when pathogens are not sufficiently susceptible to the activity of commonly used therapeutic agents [1]. The increasing number of multidrug resistant bacteria is rather alarming, in fact; such an occurrence notable contributes to the increased spread of severe infections, which can even be a cause of death. Current clinically utilized antibacterial chemotherapeutics can become almost ineffective against these very serious or fatal threats. In this regard, the requirement of continuously searching for new antimicrobially promising molecules [1,2] is logical and de facto mandatory. Scientific efforts to develop effective antibacterial agents with convenient structural, physicochemical, pharmacokinetic (PK), selectivity, and toxicological properties employ various research strategies, including those focused on plant-derived molecules, especially their secondary metabolites.
Polyphenols represent a chemical class of naturally occurring compounds produced by various plants to protect themselves against potential environmental harm [3]. Biologically valuable resveratrol (RSV; 1), chemically 3,4′,5-trihydroxystilbene (Figure 1), belongs to stilbenoids, pharmacologically a very attractive polyphenol family. These natural compounds (also termed natural products, NPs) share a typical structural arrangement of C6–C2–C6 [4,5] employing two hydroxylated six-membered aromatic rings (two so-called C6 motifs) connected with a lipophilic acyclic ethen-1,2-diyl moiety (C2 motif).
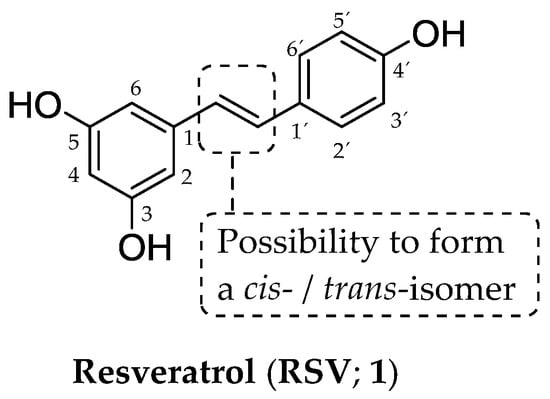
Figure 1.
Chemical structure of resveratrol (RSV; 1), a compound from a stilbenoid group.
The presence of a double bond [4,5] allows the existence of cis- and trans-isomers (Figure 1) [6]; however, a biologically active trans-form is the dominant one with several favorable properties, including higher chemical stability, compared to the characteristics of a cis-isomer [7,8]. The fundamental structural arrangement of stilbenoids differs from the arrangement found in the structures of other NPs, which also contain two aromatic ring systems [9], i.e., xanthones (C6–C1–C6) or flavonoids (C6–C3–C6).
Stilbenoids belong to phytoalexins, a category of low-molecular-weight (MW) compounds produced locally within the structures of higher plants as their self-defense response to ultraviolet light and the harmful activity of various fungi, oomycetes, or bacteria [10,11]. Those molecules can be chemically divided into several groups according to the presence of a phenolic, terpenoid, or indole scaffold. In addition, highly lipophilic S-atoms can also be incorporated in their structure [12]. Stilbenoids are commonly extracted from leaves infected with some fungal species, for example, Plasmopara viticola, Sphaeropsis sapinea, or Botrytis cinerea [13,14].
The plant secondary metabolite (1) was first isolated in 1939 from the roots of Veratrum grandiflorum O. Loes by a Japanese researcher Dr. Michio Takaoka [15]. A few years later, from its isolation and characterization, the biologically active substance (1) was also found in the roots of Polygonum cupsidatum, a plant that has been widely used in traditional Chinese medicine [16]. The RSV (1) molecule is present mainly in Vitis vinifera; other common sources are Vaccinium vitis-idaea, V. myrtillus, or Arachis hypogea [17].
The beneficial biological activity of the non-flavonoid phenolic compound (1) is directly related to the so-called French paradox. In fact, French people have a higher intake of various foods containing saturated fatty acids, but they hardly suffer from diseases that affect the cardiovascular (CV) system. Paradoxically, the death rate within the French population has been lower compared to that observed in other European nations. RSV (1) in wine protects the CV system through different mechanisms, including reduction in oxidative stress, increased synthesis of nitric oxide, and improved metabolic capacity [18,19].
RSV (1) is absorbed by epithelial cells in the small intestine (enterocytes) into the bloodstream [20]. After being absorbed, the plant metabolite involved undergoes very rapid biotransformation, resulting in its rapid conversion to several metabolites formed within phase II of biotransformation. The main metabolites (M), which were detected (Figure 2) and their structures were clearly confirmed spectrally, are resveratrol-4′-O-glucuronide (metabolite M1 (2)), resveratrol-3-O-glucuronide (metabolite M2 (3)) and resveratrol-3-O-sulfate (metabolite M3 (4)), respectively.
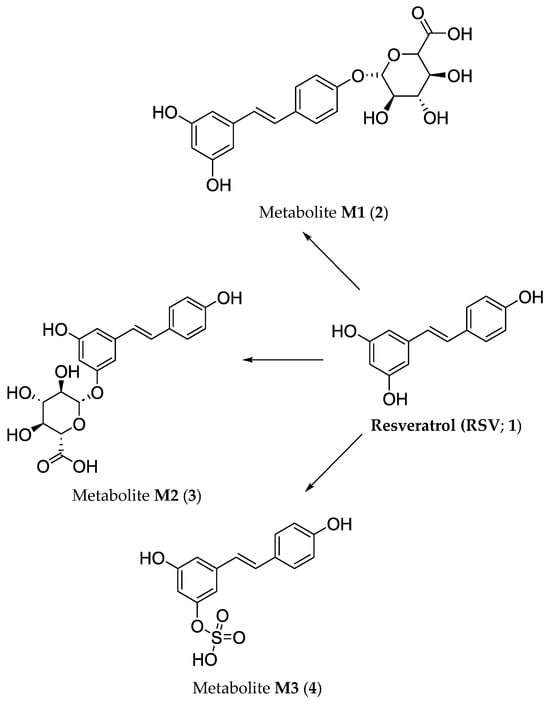
Figure 2.
Biotransformation pathways in vivo of resveratrol (RSV; 1) that provide main hydrophilic glucuronide M1 (2) and M2 (3) as well as sulfate M3 (4) metabolites.
There are two pathways in which all given hydrophilic metabolites continue within the body. They can be transported to the intestinal lumen via the apical membrane or reach the bloodstream by passing through the basolateral membrane [21]. The fact is that RSV (1) does not undergo phase I metabolic reactions, i.e., neither oxidation, reduction, nor hydrolytic reactions are relevant pathways for its ‘biochemical processing’ in vivo [20]. A brief overview of particular metabolic phases is provided in a section related to the biotransformation of viniferins.
The more detailed characterization of RSV (1), i.e., its natural occurrence, anticancer, neuroprotective, antidiabetic, antihyperlipidemic, anti-obesity, anti-atherosclerotic, hepatoprotective, cardioprotective, immunostimulatory, antioxidant, anti-ageing, wound healing, anti-inflammatory, antimicrobial, and antiviral properties, as well as PK features related to absorption, distribution, metabolism, and excretion (ADME) can be found in an excellent review paper of Bejenaru et al. (2024) [22].
One would think that viniferins, oligomeric structures ‘constructed by nature’ on a structural platform of RSV (1), could be slightly ‘in the shadow’ of this biological precursor despite their very beneficial pharmacodynamic (PD) potential. In fact, viniferins, together with their semi-synthetic and synthetic derivatives, deserve extraordinary attention because they might offer a wide spectrum of pharmacological effects that can be prospectively utilized to improve human health. When properly substituted, these compounds can be highly effective antimicrobial [23], antimycobacterial [24], antioxidant [25], antidiabetic [26], anti-obesity [27], antihyperlipidemic [28], anti-inflammatory [29], neuroprotective [30], anti-psoriatic [31], antiplasmodial [32], antiviral [33], antifungal [34] or anticancer [35] agents.
The main goals of a current review paper are to provide a more detailed overview of a set of viniferins and viniferin-based compounds with notable in vitro activity observed against several Gram-positive bacterial strains. The authors of this review follow and progress the research of Fuloria et al. (2022) [13] as well as Bejenaru et al. (2024) [22] in a different way. In fact, one of the essential topics discussed presently is the characterization of the key structural building blocks, the so-called privileged scaffolds, found in these molecules. The introduction of precisely chosen substituents at these structural motifs led to the derivatives with significant antibacterial efficiency. Therefore, the very thorough characterization of these molecules is required for their further development, eventually achieving specific phases of the pre-clinical evaluation. Therefore, the objectives of this review paper are set in five directions as follows:
- introduction of several chemical aspects related to privileged bicyclic structures containing O-atoms
These frameworks are the very essential structural features found not only in the structure of viniferins, their semi-synthetic and synthetic derivatives, but also in a group of clinically approved drugs utilized to effectively treat various medical conditions.
- in silico characterization of naturally occurring viniferins, their semi-synthetic and synthetic derivatives
The pharmacologically promising molecules have to be evaluated in silico, in vitro, and in vivo using relevant approaches to provide a more detailed perspective on possible similarities between their desired characteristics and the properties of clinically approved drugs. Experimental estimation or calculation of these properties is absolutely vital for the pre-clinical and clinical evaluation of the compounds. Therefore, notable attention is paid to the calculation of various structural, physicochemical, PK, biochemical, and toxicological descriptors of viniferins and compounds synthesized on a viniferin structural platform. The eventual relationships between these in silico parameters are also investigated chemometrically.
- indication of the advantages and limitations of the interactive tools employed
The majority of the desired descriptors is generated via different free interactive tools accessible online. The purpose of the review paper is not only to highlight several advantages but also to present some shortcomings related to these applets.
- focusing attention on the advantages and limitations of the analyzed naturally occurring viniferins, their semi-synthetic and synthetic derivatives
Finally, the objective is also to outline the advantages and shortcomings of the considered compounds in light of the computed parameters and to guide possible further development and structural optimization.
2. Some Types of Privileged Bicyclic Structures Present in the Structure of Clinically Approved Drugs and Viniferins
The core (central) scaffold of a ligand, i.e., an endogenous or exogenous molecule (pharmacologically active compound, drug candidate, or drug) capable of interacting with required biological target(s), can be regarded as a privileged structure if the respective derivatives of this ligand can effectively interact with several biomolecule targets, for example, receptors, and show notably higher affinity and selectivity for these desired targets than other structures [36].
The general chemical structures of biologically active compounds of natural origin or semi-synthetic and synthetic agents containing a benzo[b]furan (5) core (previously known as a coumarone moiety, although this term is currently considered incorrect), bicyclic 2,3-dihydrobenzo[b]furan (coumaran; 6) structure and bioisosteric 1,3-dihydroisobenzo[b]furan (phthalane; 7) scaffold are provided in Figure 3.
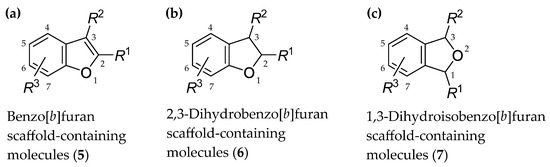
Figure 3.
General chemical structures of biologically active agents containing (a) a benzo[b]furan core (5); (b) a 2,3-dihydrobenzo[b]furan (coumaran) scaffold (6); (c) a 1,3-dihydroisobenzo[b]furan (phthalane) core (7). R1, R2, R3 = properly selected substituents attached to suitable positions of particular bicyclic systems; six-membered aromatic ring can also be multi-substituted or condensed with other aromatic or nonaromatic cycle.
Particular bioisosteric modifications, as highly relevant strategies used in a drug discovery and development process, reflect the requirements to improve potency, optimize various structural and physicochemical properties, increase selectivity toward a desired biological target, reduce off-target effects, and improve toxicological characteristics of pharmacologically active agents [37,38]. Bioisosteric replacement means the exchange of a 2,3-dihydrobenzo[b]furan (6) core for 1,3-dihydroisobenzo[b]furan (7), for example. These scaffolds structurally differ from each other in the position of an O-atom within a five-membered ring as a part of a condensed bicyclic moiety.
Heterocyclic systems with convenient size, steric, and stereochemical properties comprising O-atoms are present in naturally occurring molecules with very promising pharmacological activites, as viniferins show. Furthermore, the biological importance of condensed O-heterocycles can be supported by knowing the structure of several synthetic drugs already clinically approved worldwide by relevant regulatory authorities, for example, the Food and Drug Administration (FDA) [39], the European Medicines Agency [40], or the Chinese National Medical Products Administration [41], for the treatment of a wide range of medical conditions [39,40,41], including bacterial infections [42].
The ‘classic’ benzo[b]furan core is a fundamental building block in the structure of amiodarone (8) used as a treatment modality for life-threatening ventricular arrhythmias [43], vilazodone (9; major depressive disorder) [44], as well as elraglusib (10; orphan drug designated for Ewing sarcoma) [45]. The chemical structures of drugs (8)–(10) are provided in Figure 4. The 2,3-dihydro[b]benzofuran scaffold is present in the structure of tasimelteon (11; non-24 h sleep–wake disorder) [46], ramelteon (12; insomnia) [47], prucalopride (13; constipation) [48] as well as darifenacin (14; urge incontinence and/or increased urinary frequency and urgency) [49]. The chemical structures of these drugs are shown in Figure 5.
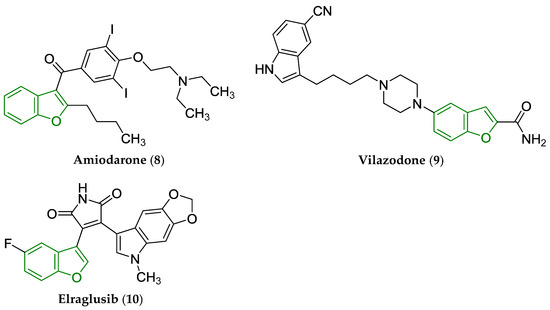
Figure 4.
Chemical structure of clinically approved drugs from various PD groups containing a privileged benzo[b]furan scaffold, i.e., amiodarone (8), vilazodone (9), and elraglusib (10). The privileged benzo[b]furan scaffold is indicated by a green color in their structures.
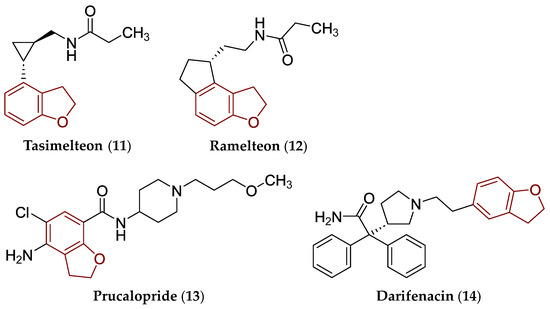
Figure 5.
Chemical structure of clinically approved drugs from various PD groups containing a privileged 2,3-dihydrobenzo[b]furan scaffold, i.e., tasimelteon (11), ramelteon (12), prucalopride (13), and darifenacin (14). The 2,3-dihydrobenzo[b]furan scaffold is indicated by a reddish brown color in their structures.
Escitalopram (15), as an (S)-(+)-enantiomer (Figure 6) of citalopram, contains a bioisosteric 1,3-dihydroisobenzo[b]furan moiety. The compound (15) is officially approved for the treatment of major depressive disorder and anxiety [50].
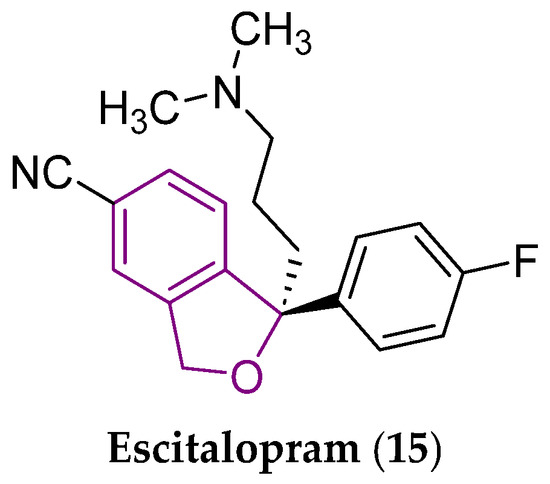
Figure 6.
Chemical structure of escitalopram (15), an antidepressant and anxiolytic drug [50]. The central 1,3-dihydroisobenzo[b]furan scaffold is indicated by a purple color in its structure.
Different arrangement of a five-membered ring within the scaffolds (5)–(7), i.e., a different position of an O-atom, the presence or absence of a double bond between C2 and C3 and, if present, its contribution to the aromatic nature of a given bicyclic system, as well as the number and position of particular stereogenic centers (Figure 3), results in different structural and physicochemical properties of particular compounds.
All given characteristics might notably affect biological activities (PD properties), affinity and selectivity for desired biological targets, PK features, as well as toxicity of the molecules containing those structural motifs.
Particular viniferins contain a privileged bicyclic 2,3-dihydrobenzo[b]furan or benzo[b]furan structure. They can be found naturally as various oligomeric structures of the plant metabolite (1); they are dihydrodimers, dimers, trimers, as well as tetramers [13] structurally properly arranged in a three-dimensional (3D) space. The oligomers, to which attention has been paid within a current review paper, are dihydrodimeric and dimeric molecules. In fact, the most investigated isoforms are the dihydrodimers of RSV (1), i.e., delta-viniferin (δ-viniferin) and epsilon-viniferin (ε-viniferin).
Their chemical structure is rather specific, allowing them to form several isomeric forms. The lipophilic double bond-containing ethen-1,2-diyl moiety between two bulky suitably substituted aromatic systems is the factor according to which viniferins and structurally related molecules can be distinguished into particular cis- and trans-isomers. It should be noted that both the cis- and trans-forms of these compounds can be pharmacologically active; however, the measure of their particular activities can be different [51,52,53].
Viniferins, as the RSV’s (1) dihydrodimers or dimers, can exist as four geometric isomers, which can differ in their 3D arrangement, with respect to the presence of a double bond within a linker, but also possible hydrogenation of a double bond within a five-membered ring has to be taken into consideration (Figure 3 and Figure 7). The particular geometric isomers are cis-delta-viniferin (cis-δ-viniferin; 16), trans-delta-viniferin (trans-δ-viniferin; 17), cis-epsilon-viniferin (cis-ε-viniferin; 18) and trans-epsilon-viniferin (trans-ε-viniferin; 19), respectively. The chemical structures of individual viniferins (16)–(19) are represented in Figure 7.
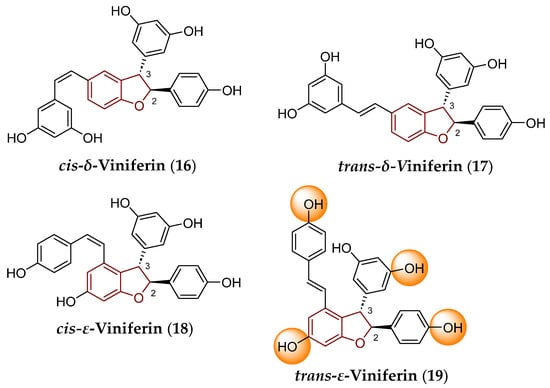
Figure 7.
Chemical structure of individual isomers of δ-viniferin (compounds 16 and 17) and ε-viniferin (18 and 19). The privileged 2,3-dihydrobenzo[b]furan scaffold is indicated by a reddish brown color in their structures. The OH groups, which can be involved in glucuronidation and sulfation as reactions of a phase II of biotransformation, are indicated by an orange color for the molecule of trans-ε-viniferin (19).
Regardless of the absolute configuration, i.e., spatial arrangement of atoms and groups within those chiral compounds, the (+)-cis-δ-viniferin, (–)-cis-δ-viniferin, (+)-trans-δ-viniferin, (–)-trans-δ-viniferin, as well as (+)-cis-ε-viniferin, (–)-cis-ε-viniferin, (+)-trans-ε-viniferin and (–)-trans-ε-viniferin molecules can be distinguished.
Other forms of viniferins that contain a various count of monomeric RSV (1) building blocks also exist. Trimeric structure of alpha-viniferin (α-viniferin), cyclic tetrametic scaffold of beta-viniferin (β-viniferin), tetrameric scaffold of vitisin A (also termed R2-viniferin) as well as vitisin B (R-viniferin), and polymerized oligostructure of gamma-viniferin (γ-viniferin) can be mentioned in this regard [13].
Other notable knowledge about individual viniferins and several of their derivatives have already been summarized and published. Therefore, an excellent review of Fuloria et al. (2022) [13] could be given as an example. Their paper aimed, among others, at biosynthetic pathways, biological sources, detailed spectral characterization (also in connection with proper determination of stereochemical properties) using ultraviolet/visible, infrared, and 1H/13C nuclear magnetic resonance spectral analyses, as well as field-desorption–mass spectrometry or pharmacological properties potentially beneficial for human health of viniferins as well as structurally related viniferin molecules.
3. Biotransformation of Viniferins
In general, biotransformation reactions to which the most structurally different endogenous and exogenous compounds (including drugs) are subjected can be meaningfully divided into two main categories—phases [54,55]. Phase I reactions are connected with the introduction of various polar functional moieties into the structure of these molecules. Therefore, particular oxidations (addition of O-atoms, including oxidative hydroxylation of (hetero)aromatic systems), reductions (for example, transformation of a CO-group to the C(OH) one, splitting of S–S bonds or reduction in unsaturated bonds), and hydrolytic reactions (for example, splitting of ester or amide bonds) are concerned by the activity of appropriate enzymes [54].
Phase II reactions are related to the condensation of the metabolites that rise as products of phase I reactions or condensation reactions of parent compounds with suitable hydrophilic endogenous molecules. In this regard, glucuronic acid, particular amino acids (cysteine, glutamine, or glycine), glutathione (tripeptide consisting of cysteine, glutamic acid, and glycine), activated acetic acid (acetyl-CoA), or sulfuric acid can be mentioned in order to achieve increased polarity or resulting ‘final’ metabolites and make their excretion from the body easier. The less frequent but still possible is the methylation of a substrate, i.e., its condensation with L-methionine that leads to a nonpolar metabolite [54,55].
The group of cytochrome P450 (CYP) enzymes is notably involved in phase I reactions. These hemoproteins facilitate the introduction of suitable polar functional moieties into the structure of particular endogenous and exogenous substrates [56]. The terminology valid for the respective CYP isoenzymes is based on a proper combination of numbers and capital letters. The name of an enzyme begins with an Arabic number related to a family of this enzyme, followed by a capital letter indicating a respective subfamily, and finally another Arabic number is included in connection with the labeling of a particular protein [57]. Examples of such CYP isoenzymes are CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4.
Various clinically approved drugs act as more or less effective inhibitors [58] or serve as inducers [59] of these hemoproteins. However, inconvenient inhibition or induction of particular CYP isoenzymes through the activity in vivo of many already approved drugs belonging to different PD groups was the primary cause of their withdrawal from pharmaceutical market in previous decades. Alpidem (anxiolytics), amodiaquine (antimalarial drug), ibufenac (anti-inflammatory agent), remoxipride (antipsychotics), or tolrestat (antidiabetic drug) could be mentioned in that context [60].
Free OH groups attached to the aromatic systems of particular viniferins are involved in a process of glucuronidation and sulfation in vivo similarly to the case of RSV (1; Figure 2). These hydrophilic moieties are conjugated with glucuronic acid and sulfuric acid via the activity of UDP-glucuronosyltransferase and sulfotransferase [5,61,62,63], respectively. Hydroxyls that interacted with the corresponding condensation agents were indicated by an orange color in a molecule of trans-ε-viniferin (19), which was chosen as an example to illustrate these metabolic modifications in vivo.
The parent vinferins and viniferin-based compounds containing free OH moieties become more hydrophilic after they pass metabolic phase II, allowing them to be more easily excreted from the human body and from experimental animal organisms. Individual metabolites were already isolated and their identity was clearly confirmed by relevant spectral methods [61,62,63]. Furthermore, not all phase II metabolites were pharmacologically ineffective; the example of some O-glucuronide conjugates of trans-ε-viniferin (19) might be given because they provided beneficial anti-steatotic properties [61].
4. Several Aspects of in Silico Evaluation of Some Pharmacologically Notable Natural, Semi-Synthetic, and Synthetic Compounds Containing (Not Only) Various Privileged Scaffolds
4.1. Selected Structural and Physicochemical Properties Which Can Be Effectively Predicted
Particular structural and physicochemical properties of the privileged structures (Figure 3), lead compounds, pharmacologically active molecules, drug candidates, as well as clinically approved drugs can be defined by relevant descriptors [64], including MW (expressed in Daltons (Da) or g/mol units), presence, number, and position or absence of stereocenters (nsc), fraction of sp3-hybridized carbon atoms (Fsp3), molar refractivity (MR; in meters per mole units; m3/mol), size, shape, van der Waals volume (VvdW; in square Å units), number and position of double and triple bonds, rotatable bonds (nrotb), rigid bonds (nrigb) and rings (nr), number of atoms within the biggest ring, number of carbons (nC), number and position of heteroatoms (nhet), flexibility (ratio between nrotb and nrigb), number and position of hydrogen-bond donors (nOHNH) and hydrogen-bond acceptors (nON), lipophilicity, acid-base properties, topological polar surface area (tPSA; in square Å units) or intrinsic solubility (S) in the aqueous environment (in mg/mL or mol/L units as well as calculated in log S units when a logarithmic scale is used).
These parameters can be calculated using various cheminformatics techniques [65] employing a diverse palette of predictor platforms, including freely available SwissADME [66] or ADMETlab ver. 3.0 [67]. These interactive tools allow for a convenient analysis of the chemical structure of an individual compound or a set of structurally defined molecules. Their chemical structures are transformed into an understandable ‘chemical language’—line notation using a well-established attractive Simplified Molecular Input Line Entry System (SMILES) format before required analyses can be performed [68].
These structural and physicochemical descriptors contribute to a so-called drug-like profile of pharmacologically promising natural, semi-synthetic, or synthetic agents or drug candidates [69,70,71]. Drug-likeness qualitatively characterizes similarity between these molecules and drugs that have been approved worldwide by official regulatory authorities and are clinically used. When attention is specifically paid to NPs, a NP-likeness term, described with an NP score index, is also commonly used. The descriptor defines the similarity of the analyzed molecules to the structural area covered by NPs [72]. The NP score index, ranging from −5.000 to 5.000, indicates the probability that a compound is NP [67].
The drug-like features and NP-likeness of pharmacologically active compounds [64] also include, in particular, the following:
- pharmacokinetic and biochemical characteristics, that is, ADME indices
These factors include permeability across various biological barriers, including stratum corneum (SC), or blood–brain barrier (BBB), affinity to various plasma or transport proteins, i.e., plasma protein binding (PPB), or interactions with P-glycoprotein (p-gp), as well as biotransformation (metabolism) with a precise definition of metabophore as a feature responsible for biotransformation, for example. All of these properties are usually included in PK characteristics, i.e., the biochemical aspect is considered to be the integral field covered by metabolism.
- pharmacodynamic properties
The PD characteristics include, among others, a proper characterization of the pharmacophore, as a set of compounds’ structural, electronic, physicochemical, or steric features responsible for the desired pharmacological activity.
- toxicity
The precise definition of toxicophore as a structural feature primarily responsible for toxicity, lethal dose values, or toxic behavior toward particular cell lines, tissues, organs, and organ systems can be mentioned in that regard.
The particular PK, PD, and toxicological properties of the compounds could also be effectively predicted [66,67].
The significance of chosen structural and physicochemical descriptors that characterize natural, semi-synthetic, and synthetic molecules in connection with their PK, PD, or toxicological properties is briefly outlined in the following sections.
4.1.1. Molecular Weight, Stereochemical Properties, Molar Refractivity, Flexibility, Size, Shape, Molecular Volume, and Presence of Heteroatoms in the Structure of Pharmacologically Active Compounds and the Relationships of These Characteristics to PK/PD Properties
Biologically effective NPs generally have higher MW values than those of semi-synthetic or synthetic derivatives. In addition, more oxygens are incorporated into the structure of NPs, but there are fewer nitrogens, halogens, and sulfurs [73]. Passive absorption of NPs can be rather questionable; these molecules can be transported in vivo by employing various active mechanisms because their chemical structure is similar to biosynthetic intermediates or products of biotransformation in vivo [74].
Not a benzo[b]furan scaffold, but both 2,3-dihydrobenzo[b]furan and bioisosteric 1,3-dihydroisobenzo[b]furan moieties potentially contain 1–2 stereogenic centers (stereocenters), i.e., carbon atoms to which four different substituents are attached (Figure 3). These centers could be carbons at positions 2 and 3 for compounds employing the 2,3-dihydrobenzo[b]furan moiety or carbons at positions 1 and 3 for derivatives with the 1,3-dihydroisobenzo[b]furan core. The centers can be formed if the simple requirements concerning the selection of the R1 and R2 substituents (Figure 3) are met as follows: R1 ≠ H and R2 = H (C2 stereocenter within a fundamental structural core is present), R1 = H and R2 ≠ H (C3 stereocenter is present), or R1 ≠ H and R2 ≠ H (both C2 and C3 stereocenters are present).
The stereochemical properties of molecules can notably modulate their biological activities (PD characteristics), affinity, and selectivity for desired biological targets, PK properties as well as toxicity. Pharmacologically effective agents of natural origin contain a higher fraction of Fsp3, as the ratio between a number of sp3-hybridized carbons and total carbon count [75], compared to that of synthetic molecules [76,77]. Thus, the probability of finding the stereocenters within a structure of NPs is higher.
The MR values, that is, a measure of polarizability as a feature of molecules to form a dipole moment [78], affect the extent of their flexibility as a function of rotation in a 3D space. The MR descriptor can contribute to the explanation of a biological effect of (not only) NPs [79].
Previous analysis [80] revealed that the molecular volume of pharmacologically active NPs, which were precisely accommodated in desired protein targets, was found in an interval of 300 Å3 to 800 Å3 and correlated with spatial parameters of binding cavities within those targets.
The stereochemical properties, Fsp3 parameter, MR, shape, molecular volume (or VvdW), number and positions of single bonds, nrotb and nrigb, number and position of multiple bonds, number, position, size, and steric properties of aliphatic chains as well as number, position, size and arrangement in the 3D space of nonaromatic and aromatic rings within the structure of NPs, and semi-synthetic and synthetic molecules affect their flexibility or, on the other hand, can contribute to overall structural rigidity [81,82].
These factors, together with nhet and positions of heteroatoms or groups containing heteroatoms, can notably affect the formation of bioactive conformations of concerned molecules interacting with desired biological targets. In addition, these characteristics are responsible for the nature (type) of noncovalent interactions or the formation of covalent bonds with these targets [83,84].
Properly introduced groups that act as hydrogen-bond donors and/or hydrogen-bond acceptors frequently play pivotal roles in the formation of hydrogen bonds between compounds of (not only) natural origin and relevant biological targets. This type of a noncovalent interaction can considerably modulate PD/PK characteristics, selectivity profile, and toxicity of particular ligands [85,86,87].
4.1.2. Lipohydrophilic Properties, Acid-Base Features, and Solubility of Pharmacologically Active Compounds and the Relationships of These Characteristics to PK/PD Properties
Lipophilicity of a compound refers to its ability to be dissolved in an apolar solvent [88]. This physicochemical property is discussed more or less frequently in connection with the action of pharmacologically active agents, including those of natural origin [89], toward the desired biological target(s), their PK characteristics and toxicity properties as well [90,91]. Lipophilicity can be classically defined with a decadic logarithm of the ratio (P) between an equilibrium concentration of a drug within a lipophilic phase (octan-1-ol medium, for example) and its equilibrium concentration within a hydrophilic phase (water or suitable buffer). The log P can be experimentally determined or can also be effectively predicted using various methods based on a whole-molecule approach, fragment-based or atomic-based procedures. In this regard, the MLOGP [92], CLOGP 4.0 [93], WLOGP [94], ALOGPS [95], XLOGP3 [96], as well as Molinspiration Cheminformatics’ milogP 2.2 [97] methods are used extensively to calculate lipohydrophilic characteristics.
The decadic logarithm of a distribution coefficient D (log D7.4), also termed a decadic logarithm of an apparent partition coefficient, indicates the distribution of a compound between lipophilic and hydrophilic environments at a physiological pH value of 7.4 [98]. This descriptor can also be predicted [67].
The tPSA parameter (in square Å units), defined as the sum of polar surface area of all polar atoms (number of nitrogens (nN) and oxygens (nO) is taken into account) in the structure of a compound [99,100,101], is often considered to optimize the PK features of the biologically active molecules proposed within the process of drug design and development.
In fact, the significance of a calculated tPSA descriptor can be discussed in various directions. Clark [102] concluded that molecules with tPSA > 140.0 Å2 showed poor passive intestinal absorption. Kelder et al. [103] found that passively and transcellularly transported noncentral nervous system (CNS)-active drugs had to be characterized with tPSA ≤ 120.0 Å2, and the molecules that affected CNS had to be defined with tPSA < 60.0–70.0 Å2. In addition, simple calculations (nN + nO) ≤ 5 and [CLOGP 4.0 − (nN + nO)] > 0.00 indicated a high probability that these compounds enter the CNS [104].
Acid-base properties of a biologically efficient compound, prospective drug candidate, or a drug are described with a value of a dissociation constant (pKa) parameter for a particular center of protonation. These features play one of the decisive roles in the fate of the molecule in the body with regard to its aqueous solubility, absorption when being administered per os, permeability via biological barriers, including the BBB, efflux processes involving p-gp, PD properties, ability to selectively interact with desired biological target(s), renal and hepatic clearance, distribution volume, binding to plasma proteins, tissue distribution, and metabolic behavior [105,106,107].
Correct knowledge of compounds’ pKa descriptors contributes to the formation of their drug-like features [108]. The properties can be experimentally measured or predicted [109] employing various commercial software packages or interactive tools that are freely accessible. However, the degree of ionization calculated for these compounds could more or less differ from the experimentally determined pKa values on the basis of their chemical nature.
The aqueous solubility of NPs significantly affects their PD and PK properties [110]. The intrinsic solubility (S) can be calculated on a logarithmic scale using both the topological methods developed by Delaney (log SESOL parameter) [111] and Ali et al. (log SALI) [112] and a fragmental method created by a Belgian company SILICOS-IT (log SS-IT), described in [66] and compared to other solubility predictors in [113]. All of these algorithms were successfully implemented within the engine of the SwissADME predictor platform [66].
The values of several descriptors mentioned in Section 4.1.1 and Section 4.1.2 were also considered in very well-known Lipinski’s Rule of Five (Ro5) and Veber rules to evaluate in silico the possible passive absorption of pharmacologically effective compounds, drug candidates, or drugs. Scientists [114,115] concluded that these small molecules, whose definition is provided in the next part with respect to their MWs, with a poor ability to be passively absorbed in vivo were characterized with the values of individual structural and physicochemical parameters as follows: MW > 500.00 Da, nrotb > 10, nOHNH > 5, nON > 10, the sum of (nOHNH + nON) > 12, CLOGP 4.0 > 5.00 (lipophilicity parameter generated in silico by a CLOGP 4.0 method [93]) or MLOGP > 4.15 (lipophilicity parameter generated in silico by a Moriguchi’s method [92]), and tPSA > 140.00 Å2.
However, analyses [116,117] of the structural and physicochemical characteristics of oral drugs approved by FDA in the period of 2000–2022 showed that the threshold values anchored within Ro5 could not be taken absolutely rigorously to accurately evaluate the ability of these drugs to be effectively absorbed after administration per os. The drugs that did not meet particular Ro5 limit values were termed ‘beyond rule of five’. In fact, most of the analyzed drugs violated none or one of Ro5, and a minority of them violated more than one rule. The violation in two or more requirements might be connected with bioavailability issues [116,117].
The attempts to acceptably predict drug-like features of compounds were mirrored in the research of Egan et al. [118], Muegge et al. [119], Ghose et al. [120], as well as Oprea [121]. The researchers used several crucial descriptors from those briefly characterized in a previous section of a current review paper, and their acceptable intervals (limit values) are listed in Table 1. Egan et al. [118] used the log P values calculated for the set of investigated compounds by a fragmental ALOGP method, which was developed previously by Ghose et al. [122] and was also employed in research [120]. Similarly, Muegge et al. [119] adopted an atomic procedure for the log P calculation developed by Ghose and Crippen [123].

Table 1.
Summation of structural and physicochemical criteria defining drug-likeness of compounds according to Egan et al. [118], Muegge et al. [119], Ghose et al. [120], and Oprea [121].
Passive absorption of compounds after they are administered per os can also be predicted using a parallel artificial membrane permeability assay. This evaluation enables calculation of a relevant logarithm of an effective intestinal membrane permeability (log Peff) parameter in humans [124].
The p-gp biomolecule serves as an effective 170 kDa membrane transport system [125]—an efflux pump present in a variety of cell lines accessible for a wide spectrum of substrates, including xenobiotics (drugs). Overexpression of a given transporter in cancer cells limits an effective concentration of anticancer agents within the intracellular environment, reducing the effect of treatment modalities based on those compounds. Inhibitors of p-gp can potentially improve the biological availability of such anticancer therapeutics to make the treatment more effective [126].
The ability of a compound to act as the p-gp inhibitor or serve as the suitable substrate for p-gp could be experimentally determined or conveniently predicted [67]. The more detailed view on this topic is provided in Section 5.2.3.
Binding of pharmacologically active compounds (drug candidates, drugs) to plasma proteins, for example, human serum albumin, affects their disposition, PD and PK parameters, as well as their therapeutic index [127,128]. Two main approaches could be considered to predict the binding of these compounds to such type of proteins, reflecting quantitative structure–activity relationship (QSAR) techniques. There are ligand-based methods and structure-based methods, the fundamental principles, advantages, and limitations of which are described in [129]. In fact, a detailed knowledge of the extent of PPB of a molecule is important because only its free form (unbound fraction) is capable of providing a pharmacological effect. In addition, decreased PPB could increase the plasma levels of a compound, which might be connected with toxicity issues [130].
The value of a corresponding PPB parameter, generated via ADMETlab ver. 3.0 [67], lower than 90.00% is regarded as the most suitable [127] for promising biologically active compounds, drug candidates, and drugs. However, this threshold could not be taken strictly, because many drugs approved by FDA for clinical use during a previous decade were defined with PPB ≥ 95.00% or even ≥ 99.00% and they are still extremely therapeutically valuable [131].
The steric properties, lipophilicity, as well as degree of ionization (pKa values), which represents one of the electronic parameters of compounds, can be considered crucial descriptors for the development of in silico models that predict the BBB permeation of these molecules, i.e., the ratio between their steady-state concentrations in the brain and in the blood [132,133]. The calculations could be carried out using various interactive applets, for example, SwissADME [66], or Enalos Cloud Platform [132], a user-friendly tool freely accessible online based on the construction of neural networks. The compounds involved in the study can be drawn directly within this applet or their SMILES codes can be entered to calculate the required descriptors.
The ADMETlab ver. 3.0 interactive tool [67] allowed the prediction of the probability that an investigated molecule could be an effective inhibitor or would serve as a suitable substrate for various isoenzymes belonging to the CYP group, i.e., CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4, CYP2B6, and CYP2C8.
The inhibition and/or induction of the particular biomolecule(s) might more or less notably affect a broad palette of the processes in the human body, from the impact on biotransformation of numerous drugs belonging to different PD classes to inhibition/activation of pathways related to cancer. The more detailed view on this topic is provided in Section 5.2.3.
4.2. Selected Toxicological Characteristics Which Can Be Effectively Predicted
Noncytotoxic properties of a pharmacologically active compound, if not being a platform for the design of an effective anticancer agent with a convenient selectivity profile, are essential features of its drug-likeness. The ADMETlab ver. 3.0 applet [67] can be employed as a suitable tool to predict toxicity of molecules, and several toxicity descriptors, which are used in current evaluation, can be characterized in a very brief way.
Drug-induced liver injury (DILI), a reaction of biologically active NPs, semi-synthetic, or synthetic compounds, threatens human health [134]. The ability of a compound to cause DILI could be described with a DILI indicator.
Similarly, human hepatotoxicity (H-HT), drug-induced nephrotoxicity (DINf), hematotoxicity (HeT), ototoxicity (OT), and drug-induced neurotoxicity (DINe) indices, as well as a parameter predicting the impact of compounds on a potassium channel in the heart encoded by a human ether-à-gogo-related gene (hERG indicator) might also be predicted [67]. The more detailed view on this topic is presented in Section 5.2.3.
4.3. Prediction of Some Parameters That Describe Drug-Likeness
The drug-like profile can be predicted using an efficient quantitative estimate of a drug-likeness (QED) parameter as the concept of desirability in connection with the PD characteristics of a compound. The calculation of QED via ADMETlab ver. 3.0 [67] for an individual molecule was based on its MW, descriptors defining stereochemical properties, nOHNH, nON, charge, aromaticity, log P, and solubility. The QED descriptor helped to emphasize the differences between drugs and nondrugs. This approach may be essential in relation to the eventual screening and evaluation of compounds for which no success is probable within next phases of pre-clinical or clinical research [135,136].
The predicted QED index ranged from 0.000 to 1.000 [67]; the closer the QED value to 1.000, the greater the probability that an analyzed compound could be a drug.
Ivanenkov et al. [137] introduced the brand new concept of an MCE-18 (Medicinal Chemistry Evolution-18) descriptor in the investigation of drug-likeness for biologically active molecules. The parameter can effectively evaluate the compounds following their innovativeness from the viewpoint of Medicinal Chemistry and Pharmacology as well as according to their potential to be convenient structural platforms for drug design and development. The calculation of a given descriptor was based on the nature and quality of 3D scaffolds of compounds containing a relatively higher nC in the sp3 hybridization [137]. However, the Fsp3 parameter [75] introduced in previous paragraphs gained less significance in this approach [137]. The value of MCE-18 ≥ 45.000 generated via ADMETlab ver. 3.0 for individual molecules was suitable [67].
5. Several Notes on Structure–Physicochemical Properties–Antimicrobial Activity Relationships of Viniferins and Viniferin-Based Compounds
5.1. Relationships Between the Structure and Activity of Chosen Viniferins and Viniferin-Based Compounds Against Chosen Gram-Positive Bacterial Strains
The research in the field of viniferins, their derivatives, and analogues in relation to antimicrobial screening in vitro is very impressive. In fact, scientists and scientific teams worldwide have investigated hundreds of variously substituted viniferin-derived compounds. In regard to such extensive research, this section is devoted especially to the so-called ‘classical’ (dimeric and dehydrodimeric) viniferins and viniferin-derived molecules with extraordinary efficiency estimated in vitro against chosen Gram-positive bacteria, i.e., selected strains of Staphylococcus aureus, Streptococcus pneumoniae, and Listeria monocytogenes, respectively. The available information connected with the activity in vitro of these molecules against some Gram-negative bacterial strains is also included to support the importance of systematic research aimed at particular naturally occurring viniferins, their semi-synthetic and synthetic derivatives, which could possibly be utilized for improvement in human health.
In addition, the authors of the current review paper include information, if available in the scientific literature, on the predicted interactions between the considered compounds and their desired biological targets.
Mattio et al. [138] synthesized and tested in vitro a set of structurally different stilbenoids, including trans-δ-viniferin (17), trans-ε-viniferin (19), and their more lipophilic derivatives (Figure 8) containing a double bond between C2 and C3 of the benzo[b]furan system, i.e., dehydro-δ-viniferin (20) and dehydro-ε-viniferin (also known as viniferifuran; 21), against a panel of Gram-positive and Gram-negative bacteria, including S. aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 27853 (Gram-negative bacterium), respectively.
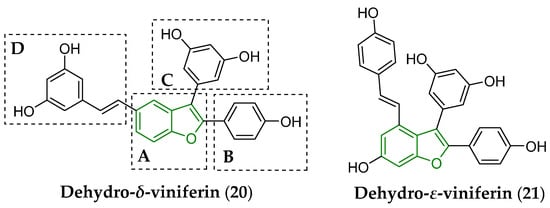
Figure 8.
Chemical structure of dehydrogenated viniferins, i.e., dehydro-δ-viniferin (20) and dehydro-ε-viniferin (21), antibacterially screened in vitro [138]. The fundamental benzo[b]furan scaffold is indicated by a green color in the structure of both compounds. The A–D letters indicate particular structural compartments of the molecule (20).
The considered compounds showed notable efficacy mainly against S. aureus described with estimated values of a minimum inhibitory concentration (MIC) of 16 μg/mL for both compounds (17) and (21), as well as with MIC = 2 μg/mL for molecule (20). Moreover, the dehydrogenated NP (20) induced the death of S. aureus cells at a relatively low minimal bactericidal concentration (MBC) of 16 µg/mL [138]. Anti-staphylococcal efficiency in vitro of both dimers (17) and (19) was also supported by previous conclusions [139,140].
The presence of a lipophilic styryl moiety and even a double bond in an acyclic hydrocarbon chain of the D compartment (Figure 8) of dehydro-δ-viniferin (20) were not the mandatory structural requirements to maintain the activity in vitro [141] of a given molecule against S. aureus ATCC29213.
The proper modifications of both A and D fragments, including the introduction of a triple bond or a short lipophilic hydrocarbon chain, were mirrored in the design and synthesis of antimicrobially efficient molecules (22)–(24), and their structures are also provided (Figure 9).
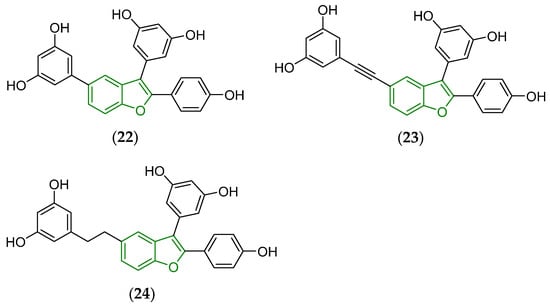
Figure 9.
Chemical structure of antibacterially active derivatives (22)–(24) of dehydro-δ-viniferin (20) containing a modified fragment D [141]. The fundamental benzo[b]furan scaffold is indicated by a green color in their structures.
The estimated MICs and MBCs for particular compounds when tested against S. aureus ATCC29213 were the following: MIC = 4 μg/mL, MBC ≥ 512 μg/mL (22), MIC = 2 μg/mL, MBC = 8 μg/mL (23), and MIC = MBC = 4 μg/mL (24), respectively. The screened derivatives (22)–(24) were more promising than a dehydro-δ-viniferin (20) reference compound showing MIC = 2 μg/mL and MBC ≥ 512 μg/mL, respectively.
In fact, there was enough room to improve the antimicrobial efficacy of trans-δ-viniferin (17)-derived molecules. Taking this idea in mind, the plant metabolite (17) was used as a suitable ‘starting structural template’ for the design, synthesis, and screening in vitro of several tens of derivatives employing an isomerization, O-methylation, halogenation, and dimerization strategy [142]. The purpose of such modifications was not only to ‘simply’ improve PD but also to optimize structural, physicochemical, and PK properties of proposed molecules.
These derivatives (Figure 10) fought the screened bacteria more effectively than standard drugs, i.e., trans-δ-viniferin (17) and vancomycin, a structurally complex hydrophilic glycopeptide antibiotic that contains a tricyclic system, isolated from Streptomyces orientalis.
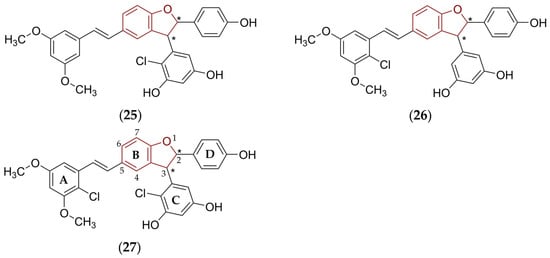
Figure 10.
Beneficial structural modifications of trans-δ-viniferin (17) that led to the compounds (25)–(27) with notably improved anti-staphylococcal activity in vitro [142]. The fundamental 2,3-dihydrobenzo[b]furan scaffold is indicated by a reddish brown color in their structures, asterisk (*) symbols indicate stereogenic centers.
Thus, the prepared structural analogues (Figure 10) of the compound (17) were tested in vitro against two S. aureus strains, i.e., Newman (methicillin-susceptible S. aureus; MSSA) and COL (methicillin-resistant S. aureus; MRSA) [142]. Simultaneous O-methylation of precisely chosen OH group(s) and introduction of halogen atoms into the proper positions of particular aromatic systems were beneficial structural modifications, which improved antibacterial activity of the resulting chlorinated compounds (25)–(27).
The estimated MIC values for (25)–(27) against MSSA were as follows: 0.5–1.0 μg/mL (25), 1 μg/mL (26), and 1–2 μg/mL (27). The efficiency of the most active derivatives against MRSA was described with MICs of 1 μg/mL (26) and 0.6–1 μg/mL (27). The inhibitory effect of all of these molecules against MSSA was higher compared to the activity of trans-δ-viniferin (17) and very comparable to the inhibitory activity of vancomycin [142].
The presence of covalently bonded chlorines, which were chosen as the halogen atoms most commonly employed in Medicinal Chemistry in the process of the drug design and development [143], in the structure of synthesized and biologically evaluated compounds was related to the possible improvement in oxidative stability, that is, less susceptibility to the oxidation process through the activity of the CYP isoenzymes and increased permeation via biological barriers [142]. In addition, electronic and steric properties of chlorines in the structure of these ligands could alter the noncovalent interactions with amino acids close to the desired biological target or via the formation of covalent bonds [144,145]. The replacement of chlorines with bioisosteric Br-atoms in the structure of the compound (27) significantly reduced activity [142].
Additional QSAR analysis for the group of prepared and microbiologically screened derivatives [142], including the compounds (25)–(27), was carried out using the Maestro software tool (Schrödinger Release 2021-1; Schrödinger, LLC, New York, NY, USA). The evaluation revealed that the Cl-atom attached to a 2-position of a ring A, and Cl-atom attached simultaneously to a 2-position of a ring C (Figure 10) increased the anti-staphylococcal activity in vitro. On the other hand, the presence of the Cl-substituent in a 4-position of a ring A decreased the potency. Therefore, the proper selection, steric properties, and orientation (position) of the substituents attached to aromatic systems A–D, as well as proper filling of a binding site of a desired biological target, which was not specified, in fact, within the research [142], were essential requirements for these compounds to be antimicrobially active. In addition, the molecules (26) and (27) inhibited the growth of S. epidermidis at a relatively low MIC of 0.5–1 μg/mL (26) and 1 μg/mL (27), respectively [142].
Interactions between trans-ε-viniferin (19) and vancomycin resulted in bacteriostatic activity against the MRSA ATCC 33591 strain. The synergistic effect of these compounds was defined with a very low MIC value of 0.00625 μg/mL [146].
The molecules (16)–(27) contained various functional groups capable of forming hydrogen bonds and other noncovalent interactions involving π and/or σ electrons (stacked π–π or π–alkyl interactions) as well as electrostatic, hydrophobic, or van der Waals interactions with particular amino acid residues present within the structure of specific enzymes of S. aureus strains, for example, pyruvate kinase.
Gebrehiwot et al. (2024) [147] predicted the formation of hydrogen bonds between the OH group attached to the benzo[b]furan moiety of ε-viniferin and specific residues of individual amino acids (asparagine and lysine were involved) of pyruvate kinase. Furthermore, the π–anion interaction was predicted between the bicyclic scaffold of this biologically active compound and the fragment of aspartic acid present in the structure of the enzyme [147]. The π–alkyl interactions, which were formed between the phenyl ring attached to the 3-position of ε-viniferin as well as its other aromatic system as a part of the substituent attached to the 5-position and residues of leucine and lysine amino acids were generated in silico as well [147].
Another synthetic analogue (28) of trans-ε-viniferin (19) containing a flexible ethan-1,2-diyl chain, which linked two aromatic systems (Figure 11), showed a notable inhibitory impact in vitro on the growth of a Gram-positive Streptococcus pneumoniae strain, and eliminated the bacterium in biofilms [148].
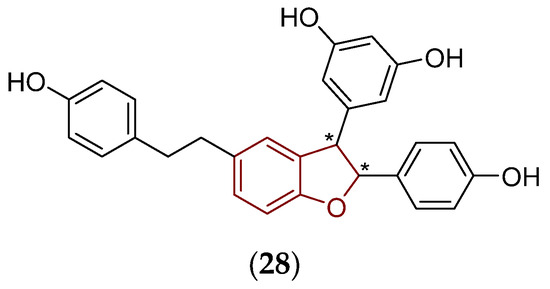
Figure 11.
Chemical structure of the compound (28) containing a structurally flexible ethan-1,2-diyl moiety which was active in vitro against S. pneumoniae [148]. The fundamental 2,3-dihydrobenzo[b]furan scaffold is indicated by a reddish brown color in its structure, asterisk (*) symbols indicate stereogenic centers.
The Gram-positive L. monocytogenes Scott A strain was also chosen to screen the biological potential in vitro of the compounds structurally related to both dehydro-δ-viniferin (20) and dehydro-ε-viniferin (21). In fact, their structural optimization had to be performed with care, because many of the proposed and synthesized derivatives were inefficient in microbiological experiments [149]. The optimization of flexibility, steric, and lipophilic properties, together with decreased number of OH groups and their bonding to proper positions at aromatic systems within such a modified scaffold, resulted in the derivative (29). This compound (Figure 12) fought in vitro the L. monocytogenes Scott A pathogen more effectively (MIC = 8 μg/mL, MBC = 64 μg/mL) than its structural template, dehydro-ε-viniferin (21), showing MIC = 16 μg/mL and MBC > 512 μg/mL [149], respectively.
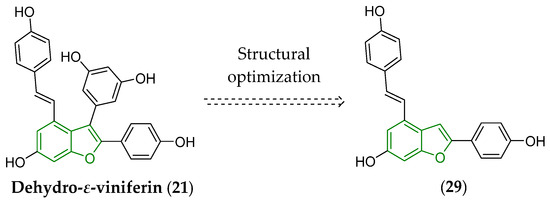
Figure 12.
Simplification of the structure of dehydro-ε-viniferin (21) leading to the compound (29) capable to effectively inhibit in vitro the growth of L. monocytogenes Scott A [149]. The fundamental benzo[b]furan scaffold is indicated by a green color in the structure of both derivatives.
Moreover, such structural simplifications that provided the derivative (29) did not affect its antiproliferative activity in vitro [150] against several cancer cell lines, i.e., A375 (melanoma), H460 (non-small cell lung cancer), and PC3 (prostate cancer), or a healthy (noncancerous) WS1 cell line, i.e., human normal skin fibroblasts, compared to the impact of dehydro-ε-viniferin (21) on all of these lines.
More recent research [151] also confirmed that hydrogenation of a double bond between C2 and C3 within the structure of dehydro-ε-viniferin (21), which provided trans-ε-viniferin (19), did not eliminate the efficiency against L. monocytogenes OEK ATCC35152.
The observations briefly summarized in the previous paragraphs strongly support the idea to utilize the discussed molecules (Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12) as promising template compounds in the design and development of antibacterials effective in vivo that could provide beneficial outcomes in the treatment of various infections caused by Gram-positive pathogens.
In addition, dehydro-δ-viniferin (20) was able to inhibit in vitro a specific virulence system of Yersinia pseudotuberculosis, a Gram-negative nonhemolytic pathogen that causes severe infection in humans. Therefore, the molecule (20) could be considered a suitable structural platform for the design of novel derivatives capable of inhibiting the growth of a given bacterial strain [152].
Cho et al. (2013) investigated several tens of compounds produced by plants and observed that ε-viniferin, at c = 10 μg/mL, markedly inhibited in vitro the formation of biofilm due to the activity of P. aeruginosa PA14 (clinical isolate) and PAO1 (laboratory reference strain) as well as enterohemorrhagic Gram-negative Escherichia coli O157:H7 (ATCC43895) [153]. This compound also inhibited the production of α-hemolysin (known as α-toxin) by the activity of S. aureus [154]. The toxin is involved in the development of sepsis and pneumonia [153,155].
5.2. Relationships Between the Predicted Structural, Physicochemical, Pharmacokinetic, and Toxicological Properties of Chosen Viniferins and Viniferin-Based Compounds That Were Very Effective Against Gram-Positive Bacteria
5.2.1. General Overview
Section 5.2 is devoted to the calculation of particular descriptors that characterize the structural, physicochemical, PK, and toxicological properties of antibacterially effective compounds (16)–(29), the chemical names of which are provided in Table S1 in Supplementary Materials. The respective computational procedures were performed using the SwissADME [66], ADMETlab ver. 3.0 [67], ALOGPS [95], Molinspiration Cheminformatics [97], Enalos Cloud Platform [132], and MolGpKa [156] tools which are freely accessible online as well as a commercially available ChemDraw ver. 22.2.0.3300 software package (CambridgeSoft, Cambridge, MA, USA).
The generated structural and physicochemical parameters (Tables S2–S4) of the investigated molecules were discussed in connection with the properties observed for clinically approved drugs. In addition, the descriptors that defined the (16)–(29) set were also analyzed in relation to the predicted:
- ability to passively permeate via various biological barriers, i.e., SC, intestinal barrier, and the BBB, impact on p-gp (evaluated compounds eventually acting as inhibitors or substrates), and binding to plasma proteins (Table S4);
- inhibitory activity toward respective CYP isoenzymes, i.e., CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and CYP2B6, and the ability to serve as their substrates (Tables S5 and S6);
- toxicological features, i.e., DILI, H-HT, DINf, HeT, OT, DINe, and impact on an hERG channel in the heart (Tables S7 and S8).
The positive or negative linearity of the proposed relationships (models) was evaluated according to a Pearson correlation coefficient (r) calculated via Microsoft Excel as an integral part of a Microsoft 365 software package (Microsoft Corp., Redmont, WA, USA). The respective values of the r parameter are listed in Tables S6 and S8.
The chemical structures of the antibacterially effective compounds (16)–(29) were transformed into relevant SMILES codes [68] using the ADMETlab ver. 3.0 interactive tool [67] before all desired structural, physicochemical, PK, and toxicological descriptors were calculated. These codes were successfully generated for all molecules as their topological representations that contained relevant sequences of correctly ordered characters without spaces (Table S1). The verification of these codes was also carried out using SwissADME [66], and the codes were the same.
Before inspecting particular relationships, several notes about the drawing of analyzed molecules (16)–(29) within the interactive applet [67] could be provided. The stereochemical aspects required for these compounds were considered, as published in research papers [138,139,140,141,142,146,147,148,149,151,152,153]. Pairs of corresponding enantiomers, that is, the compound (16) versus the molecule (17), and (18) versus (19), as well as structural regioisomers differing in positions of OH groups at aromatic rings, i.e., (20) versus (21), showed no differences in calculated formulas, as could be logically expected. Similarly, the generated values of their MW, nsc, Fsp3, MR, VvdW, nrotb, nrigb, nr, nC, nhet, flexibility, nOHNH, nOH, lipophilicity (minimal differences in calculated log D7.4 values were noticed) and solubility descriptors, tPSA, as well as the ability to inhibit the p-gp (p-gp-I) were the same (Tables S2–S4).
A more detailed view on the relationships between (i) in silico structural and physicochemical properties (Tables S2–S4), (ii) chosen in silico structural as well as physicochemical properties (Tables S2–S4) and ability to permeate via selected biological barriers (Table S4), (iii) chosen in silico structural as well as physicochemical properties (Tables S2–S4) and ‘virtual’ impact on selected CYP isoenzymes (Tables S5 and S6), (iv) chosen in silico structural as well as physicochemical descriptors and possible toxicological issues (Table S7) of the analyzed compounds (16)–(29) was provided using a chemometric principal component analysis (PCA) method [157]. The details are offered in the following paragraphs.
The chemometric PCA technique (Table S6) was performed using a trial version of an XLSTAT ver. 2019 2.2 statistical software add-in (Addinsoft, New York, NY, USA) working in the Microsoft Excel environment.
Regarding PCA that considered ‘only’ structural and physicochemical descriptors, all relevant parameters listed in Tables S2–S4 were included in that analysis. The calculated acid-base dissociation constants (pKa) were excluded from the evaluation because all the investigated derivatives (16)–(29) were weak acids with pKas ≥ 8.1 and, in addition, contained a variable count of groups, which theoretically could be deprotonated (Figures S2–S15). The details connected with this evaluation are provided in Section 5.2.2.
The nsc, Fsp3, nrotb, nrigb, nr, nc, nhet, nOHNH, and nOH parameters were not included in PCAs focusing on the exploration of the relationships between structural as well as physicochemical descriptors of the compounds (16)–(29), their ability to permeate via various biological barriers, and the impact on the chosen CYP isoenzymes (Tables S5 and S6), respectively. The reason was the minimum variance of the calculated values. For example, only five different Fsp3 values could be utilized if a given parameter would be implemented (Table S2). Furthermore, the log SESOL, log SALI, and log SS-IT solubility descriptors were not included as well because almost all of these outputs indicated poor aqueous solubility of (16)–(29). Finally, the calculated pKa parameters were not considered. The linearity of the inspected relationships was described with positive or negative r values (Table S6).
The PCA technique was also used to adequately interpret the relationships between the chosen structural as well as physicochemical descriptors and possible toxicological issues (Table S7). The nsc, Fsp3, nrotb, nrigb, nr, nc, nhet, nOHNH, nOH, log SESOL, log SALI, log SS-IT, and pKa parameters were excluded from this chemometric evaluation.
5.2.2. Predicted Structural and Physicochemical Properties of Chosen Viniferins and Viniferin-Based Compounds
The preliminary view on eventual similarities or differences in the in silico structural and physicochemical descriptors, i.e., MW, nsc, Fsp3, MR, VvdW, nrotb, nrigb, nr, nC, nhet, flexibility, nOHNH, nON, MLOGP, CLOGP 4.0, WLOGP, ALOGPS, XLOGP3, miLogP 2.2, log D7.4, tPSA, log SESOL, and log SALI (Tables S2–S4), of the evaluated compounds (16)–(29) was provided through PCA (Figure 13). The entire set of these molecules was visualized in the constructed coordinate system of Principal Component 1 (PC 1) × PC 2. The analyzed derivatives (16)–(29) were grouped or visually ‘isolated’ following similarities or differences in given properties. It should be emphasized that the respective PCs did not prove the existence of ‘real’ structural or physicochemical parameters; they only indicated the mathematical possibility that such descriptors existed [157].
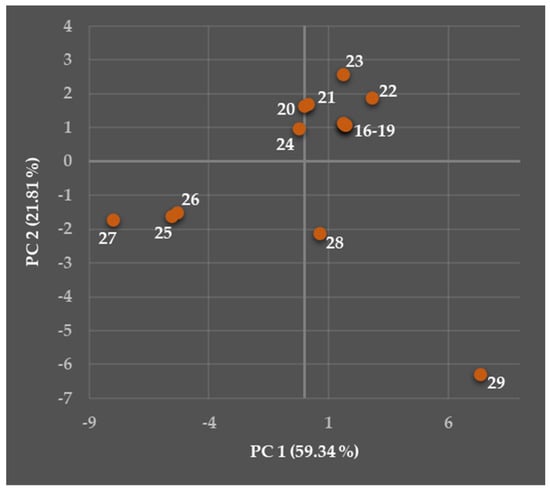
Figure 13.
Two-dimensional observation (mapping) showing both Principal Component 1 (PC 1) and PC 2 scores of the compounds (16)–(29) following their eventual similarities or differences in the calculated structural and physicochemical descriptors.
The interpreted PC 1 and PC 2 accounted for 81.15% of the total variance in the current data (PC 1: 59.34%, PC 2: 21.81%). The calculated eigenvalue (λe) parameters [157] were the following: 14.24 (for PC 1) and 5.24 (PC 2). The details about this PCA with brief definitions of several fundamental terms are provided in Supplementary Materials.
The compounds (25)–(27) were characterized with both negative PC 1 and PC 2, and (27) was visually ‘slightly isolated’. The molecules (20) and (24) were defined with negative PC 1 and positive PC 2, and, in addition, were relatively close to two miniseries (16)–(19) and (21)–(23) defined with both positive PC 1 and PC 2. The compounds (28) and (29) were visually ‘isolated’ and defined with positive PC 1 and negative PC 2.
Therefore, such a distribution of the molecules (16)–(29) indicated differences in the structural and physicochemical parameters generated. The more comprehensive insight into those relationships is provided in the following paragraphs.
- Molecular weight, fraction of sp3 carbon atoms, molecular refractivity, and van der Waals volume
All compounds currently discussed (16)–(29) were so-called small molecules (potential small-molecule drugs, eventually) with MW < 900.00 Da [158]. This threshold value would not be taken so strictly because the cut-off limit to define a pharmacologically active compound as a small-molecule drug could be evaluated from different aspects. For example, Ghose et al. [120] suggested the limit of MW < 480.00 Da and Li et al. [159] used MW < 1500.00 Da as the upper value.
The MW values of evaluated compounds (16)–(29) ranged from 344.10 Da (29) to 550.09 Da (27); most of them had MW ≤ 454.14 Da (Table S2), and an average MW parameter for the entire set of molecules was 458.41 Da. Following the very strict rule [120], only synthetic derivatives (22), (28), and (29) could be potential small-molecule drugs. However, such a rigorous limit may not be taken into account to absolute extent, and MW < 900.00 Da [158] to fulfill a given condition seemed to be more reasonable.
Macielag [160] concluded that the distribution of effective antibacterial agents biologically available per os was bimodal. The first group of these molecules was characterized with MW = 350.00–450.00 Da, and the second group, including several antibiotics, had MW = 700.00–900.00 Da [160].
The MW values around 450.00 Da favored the majority of presently analyzed derivatives as eventual therapeutic agents, which could be administered per os [160] to combat infections caused by Gram-positive bacteria as listed in Section 5.1. Moreover, these molecules were defined with MW > 414.00 Da and log D7.4 ≥ 2.40 (Table S2) so that their improved permeability might be expected. The compounds with MW < 414.00 Da and log D7.4 ≥ 1.30 also showed higher permeability [161].
Other relevant connections between MW and predicted PK, as well as toxicological characteristics, are discussed in the following paragraphs.
The MW parameter might be less relevant to describe a drug-like profile of pharmacologically active molecules or drug candidates compared to the significance of their conformational properties [117]. The compounds (16)–(29) contained only C2 and C3 atoms as possible centers of chirality depending on the presence or absence of a double bond within their five-membered ring and selection of particular substituents (Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12, Table S2). The majority of molecules involved in the present study was not isolated or synthesized and biologically screened in vitro as pure geometric isomers. This unbiased reason did not allow for more detail on the relationships between their stereochemical properties and activity against Gram-positive bacteria.
Relatively high values of the Fsp3 parameter were notably connected with developability of pharmacologically active small-molecule compounds and drug candidates, i.e., probability that they will be successful in all required pre-clinical and clinical phases of development to become officially approved drugs for clinical use by relevant authorities. The correlation of a given descriptor with those defining the aromatic nature was inverse [162]. The synthetic halogenated and methoxylated compounds (25)–(27), as well as the derivative (28), characterized by the highest values of nrotb = 5–6 and Fsp3 = 0.133–0.143 (Table S2), are those with the highest potential to have desired drug-like properties.
Indeed, there is enough room for further optimization of the viniferin-based compounds to develop and reach real small-molecule drug candidates or even drugs from this group of bioactive compounds. Considering the mentioned, their aliphatic Fsp3 parameter might be the subject for such an optimization.
Lovering et al. [163] observed that clinically approved drugs were characterized with more than threefold higher values of this descriptor, depending on the route of administration, compared to the molecules (25)–(28). The more recent analysis [117] pointed to a median Fsp3 value of 0.260 as a structural feature for drugs approved by FDA during the period of 2000–2022. New entities approved by this regulatory authority for clinical use during a longer period 1998–2017 were defined with a calculated value of 90th percentile Fsp3 = 0.750 and median Fsp3 = 0.400 [164], respectively.
The compounds (16)–(28) were described with nrig = 28–29, nr = 5, and nC = 26–30. Only a sterically less bulky synthetic derivative (29) was defined with lower values of these parameters, i.e., nrig = 23, nr = 4, and nC = 22 (Table S2). Relatively lower aromatic ring counts are favorable in terms of drug design and development [117]. Therefore, the molecule (29) containing fewer aromatic systems could be important as a promising template for further more detailed structural optimization. That suggestion corresponded with findings [165] that structurally relative smaller flexible molecules could more effectively combat in vitro several Gram-positive bacteria than the compounds containing a relative higher count of planar (hetero)aromatic ring systems.
The continuous increase in both MW and size of FDA-approved drugs absorbed after being administered per os [117,164] was observed. The MR parameter, as a size descriptor, ranged from 103.09 m3/mol (29) to 533.54 m3/mol (27; Table S2); however, its significance for developability could be relatively low compared to that of nC [163]. It might be hypothesized that the improvement in antibacterial activity could be related to the precise introduction of a nonaromatic lipophilic sterically bulky S-atom within the structure of presently investigated compounds [162,166]. However, the overall developability of such bioisosteric molecules and derivatives might be rather questionable because of the further increase in lipophilicity.
The calculated values of VvdW (Table S2) for (16)–(29) were within an interval from 300 Å3 to 800 Å3, indicating the possibility that these molecules interact with specific enzyme targets of particular bacteria [80]. The lowest VvdW = 344.10 Å3 was assigned to the compound (29; Table S2) that contained a properly substituted condensed bicyclic core and two aromatic rings, which were hydroxylated at 4-positions (Figure 12). The highest value of a given parameter (VvdW = 550.09 Å3) was generated for the derivative (27; Table S2) with a suitably substituted condensed bicyclic scaffold and three aromatic rings at which Cl-atoms, OCH3-groups, and OH-moieties were introduced. Sterically bulky substituents were attached to the proper positions of aromatic systems in the structure of the miniseries (25)–(27) increasing the relevant VvdWs, as expected (Figure 10, Table S2). Such predictions could be very useful because VvdW of molecules generally described the impact of nondirectional forces and was notably related to their activity in vitro against some Gram-positive bacteria, including several strains of S. aureus and L. monocytogenes [167,168,169].
The investigated compounds contained nhet = 4 (29)–8 (27), and their flexibility parameter (Table S3) ranged from 0.069 (23) to 0.207 (derivatives (25), (26) and (27), respectively). The current calculations confirmed the conclusions published in [162,164]. The mentioned research stated that a limitation of the aromatic ring count, especially the moieties containing no heteroatoms, could be beneficial for oral drugs and, in a broader sense, contribute to the developability of the compounds that could become drug candidates or drugs.
- Hydrogen bonding and lipohydrophilic properties
Recent analysis [117] suggested that hydrogen bonding and lipohydrophilic properties appear to be the factors that contribute more significantly to a drug-like profile of pharmacologically effective compounds or drug candidates, which were biologically available after administration per os, than their MW parameters. The compounds (16)–(29) contained nOHNH = 3–5 and nON = 4–6 (Table S3). Subseries (16)–(24) characterized with nOHNH > 4 was very slightly outside of the calculated 90th percentile nOHNH value (90th percentile nOHNH = 4) observed for drugs, which FDA approved during 1998–2017 [164].
The hydrogen bonding and lipophilicity contributed in different ways to the efficiency in vitro of molecules against Gram-positive bacteria [170]. The nOHNH parameter was a rather conservative structural index for highly lipophilic compounds and suitably indicated the extent of passive permeability via biological membranes [171]. The derivative (29), to which attention was drawn in the previous paragraphs, was defined with MW < 414.00 Da, nOHNH = 3, tPSA = 73.83 Å2, and log D7.4 = 3.86 (Tables S2–S4).
If molecules are characterized with nOHNH > nON, the probability that they can form a large portion of hydrate structures is higher and their free forms are in the minority [172]. The opposite situation was observed for all molecules (16)–(29), that is, their nON > nOHNH (Table S3), indicating that they can be found in free forms.
Proper spatial arrangement of particular functional groups within the structure of currently discussed compounds together with balance in lipohydrophilic properties determined their activity in vitro against Gram-positive foodborne pathogens [138]. The cell wall of Gram-positive bacteria contains, among others, lipophilic compartments [173], which facilitate the permeation of lipophilic compounds, and, in addition, they can be suitable biological targets for numerous antimicrobially effective molecules [174].
The lipophilicity log P descriptor was generated for (16)–(29) using a Moriguchi’s method (MLOGP) [92] integrated in SwissADME [66], fragmental CLOGP 4.0 approach (CLOGP 4.0) [93] implemented within a ChemDraw ver. 22.2.0.3300 software package, Wildman and Crippen’s atomic-based principle (WLOGP) [94] integrated in SwissADME [66], whole-molecule-based procedure (ALOGPS) accessible via the Virtual Computational Chemistry Laboratory (VCCLAB) applet [95], atomic/group-based approach (XLOGP3) [96] available through SwissADME [66], and finally a Molinspiration Cheminformatics’ method (miLogP 2.2) based on group contributions [97].
The outputs of almost all employed predictors (CLOGP 4.0, WLOGP, ALOGPS, XLOGP3, and miLogP 2.2) indicated a high lipophilic nature of all analyzed compounds because of the presence of multiple aromatic systems, double or triple bonds, linear hydrocarbon chains or lipophilic atoms. The MLOGP readouts provided lower log P values ranging from 2.39 (22) to 4.16 (27), as listed in Table S3. The evaluation of the most precise predictive method for (16)–(29) was not possible, in fact, due to the lack of their experimentally estimated lipophilicity descriptors.
The introduction of covalently bonded lipophilic chlorines into the structure of (25)–(27) increased their log P values, as expected. The derivative (27), which contained two Cl-atoms and three aromatic rings, was the most lipophilic. Its average log P = 6.70 was calculated according to the log P outputs of all currently employed in silico procedures (Table S3). The lowest lipophilicity (average log P = 4.60) was predicted for both cis-ε-viniferin (18) and trans-ε-viniferin (19). Therefore, the compounds (16)–(29) could be ranked following the decrease in their average log Ps as follows: 6.70 (27), 6.15 (25), 6.03 (26), 5.24 (20), 5.18 (21), 5.03 (24), 4.93 (28), 4.86 (23), 4.72 (29), 4.67 (22), 4.63 (both (16) and (17)), and 4.60 (both (18) and (19)).
The highly lipophilic nature of presently evaluated viniferins and viniferin-based compounds met trends and observations related to the efficiency in vitro of more than 60 flavonoids against chosen Gram-positive bacteria [91]. Relatively higher values of lipophilic descriptors (CLOGP 4.0 was generated roughly within an interval from 4.00 to 7.50) were calculated for the most effective compounds against several strains of S. aureus, including those resistant to methicillin. The relationship between lipophilicity and activity was not linear [91].
The tPSA indicator (Table S4) varied from 73.83 Å2 (29) to 114.29 Å2 (calculated for (20)–(24)). The relevance of the lipophilicity parameters and tPSA to the ability of these compounds to passively permeate biological barriers is provided in Section 5.2.3.
The pH-dependent log D7.4 parameter for the molecules (16)–(29) might be highly relevant for their further development and structural optimization [175,176,177]. Attempts to even replace a log P descriptor with the more meaningful log D7.4 one [178,179,180] within Ro5 were also noticed in respect to the implementation of acid-base properties and ionization (pKa values) of compounds. The acceptance of such an approach for molecules currently analyzed was not necessary because they did not contain the center(s) of protonation [180,181], i.e., basic N-atom(s), and their neutral forms were expected to exist. That statement was also supported by their nOHNH > nON (Table S3).
In general, the most convenient drug-likeness could be predicted for the molecule (29), chemically (E)-2-(4-hydroxyphenyl)-4-(4-hydroxystyryl)benzofuran-6-ol, focusing on its calculated average lipophilicity (log P and log D7.4 values), all the requirements for other structural and physicochemical properties published in [118,119,120,121] and comprehensively summarized (MW, MR, nrotb, nr, nC, nhet, nOHNH, nON, and tPSA) in Table 1, as well as with respect to its QED = 0.436 (Table S7).
- Acid-base properties and solubility
The compounds (16)–(29) were weak acids. That assumption was verified according to the calculations of particular pKa values for all theoretically ionizable groups using a freely available MolGpKa interactive tool [156] working on a graph-convolutional neural network principle. The pKa values generated for the individual theoretical centers of deprotonation ranged from 8.1 to 9.7 as indicated in Figures S2–S15 (Supplementary Materials). The relative lower values of the respective pKas for both compounds (25) and (27) were due to the introduction of Cl-atoms at their aromatic systems.
The adequate solubility of compounds during their experimental evaluation in vitro [25,141] or in aqueous compartments of the body is a very crucial prerequisite to properly evaluate a broad spectrum of biological properties or achieve the desired biological availability. In fact, a possible issue related to antimicrobially active agents (16)–(29) is their insufficient aqueous solubility. The very negative values of particular log S outputs (log SESOL, log SALI, and log SS-IT) from solubility prediction tools [66,111,112] indicated that almost all derivatives were poorly soluble and the least lipophilic molecule (29), as only one exception, was moderately soluble following its log SALI value (Table S4).
In fact, the poor solubility of viniferins, and stilbenes in general, is a well-known obstacle. The alternatives to overcome a given limitation were based on the incorporation of these pharmacologically active agents into suitable liposomal structures of various types [182,183] or decrease in their lipophilicity [142].
5.2.3. Predicted Pharmacokinetic Properties of Chosen Viniferins and Viniferin-Based Compounds
- The passive permeation via stratum corneum
Potts and Guy [184] constructed and developed a predictive model to investigate the capability of compounds to permeate via mammalian SC. The quantitative measure of such a prediction was described with a permeability coefficient (Kp; in cm/s units) or its decadic logarithm (log Kp). This well-known predictive model employed MW, molecular volume, and lipophilicity, i.e., the log P parameter related to an octan-1-ol/water partition system.
The lipophilicity of the molecules (16)–(29) correlated relatively notably with their in silico ability to passively permeate through SC. The r parameters that described the most positive linear relationships between the outputs of particular log P predictors and log Kps, generated through SwissADME [66], were as follows: 0.836 (WLOGP), 0.784 (XLOGP3), 0.779 (CLOGP 4.0), 0.771 (miLogP 2.2), and 0.690 (ALOGPS), respectively. The correlation with a calculated log D7.4 parameter was weaker (r = 0.462) in that regard.
On the other hand, the increased MW, VvdW, or flexibility was not linearly related to the increase in log Kp (Tables S3 and S4). The calculated relatively weak r values characterizing these poorly linear relationships were 0.304, 0.226, and 0.458.
The highest ability to passively permeate via SC was assigned to the compound (27) with log Kp = −4.47, followed by other halogenated compounds (25) and (26), for which identical log Kp = −4.71 were generated. The molecule (29) with the lowest MW and VvdW could be found as fourth in this rank, showing log Kp = −4.72 due to a lower number of polar atoms incorporated in its structure, i.e., because of the lowest tPSA = 73.83 Å2 among all evaluated compounds (Table S4).
Ottaviani et al. [185] found out that compounds with log Kp < −6.00 showed low permeation via SC. Therefore, none of the investigated molecules (16)–(29) should be excluded from the discussion focusing on the determination of the eventual ability to cross via a given biological barrier, as indicated by their log Kps > −6.00 (Table S4).
The reason why a more comprehensive conclusion could not be drawn was that the relationship between log Peff and log Kp was not linear (Figure S1 in Supplementary Materials). Presently, the detailed information about the capability of the membrane to retain the molecules under the study could not be provided without additional experimental procedures.
- The passive permeation via other biological barriers
The suitable MW value together with increased lipophilic surface area of compounds can facilitate their permeation through lipophilic barriers [186]. If a compound, defined with MW = 350.00–400.00 Da, could effectively passively permeate via biological membranes, it should have sufficient lipophilicity, that is, log D7.4 > 1.70. Slightly heavier molecules with MW = 400.00–450.00 Da should be characterized with log D7.4 > 3.40 and finally compounds with MW > 500.00 Da should be the most lipophilic ones, as described with log D7.4 > 4.50 [160], to increase their changes for passive permeation through membranes.
The log D7.4 values for the (16)–(29) set, calculated with respect to the physiological compartment (pH = 7.4), ranged from 3.67 (28) to 4.84 (23) as listed in Table S3. These numerical thresholds indicated that lipophilicity of the investigated molecules was very slightly insufficient to allow them to passively permeate because log D7.4 ranged from 4.26 (27) to 4.33 (25).
The high predicted lipophilicity (log D7.4 roughly within an interval from 4.90 to 7.80) of the flavonoid series was also related to notable efficiency in vitro against various Gram-positive pathogens. This improvement in efficiency was assigned to a precisely chosen number, length, and positions of alkyl chains within the structure of particular flavonoids [91].
In general, compounds with log Peff < 2.00 were considered those with low permeability, and derivatives with log Peff > 2.50 were highly permeable [67,124]. The log Peff values (Table S4), ranging from 0.002 (26) to 0.603 (29), also indicated the limited permeability for the (25)–(27) set because this biopharmaceutically important descriptor was related to the prediction of the extension of absorption of these compounds in all human intestinal parts [187]. The increase in MW, MR, VvdW, and flexibility contributed more or less negatively to log Peff as described with a corresponding r value of −0.862 (MW versus log Peff), −0.893 (MR versus log Peff), −0.919 (VvdW versus log Peff), and −0.480 (flexibility versus log Peff), respectively (Table S6).
Following only ‘isolated’ tPSA values, all discussed molecules could not be characterized by poor intestinal absorption through a passive mechanism because their tPSAs < 140.0 Å2 [102].
In addition, the majority of them would not be able to passively target the CNS following the criteria published in [103,104]. This suggestion was also supported by the results of the Enalos Cloud Platform applet [132] (Table S4). This prediction was confirmed with the calculations via SwissADME [66] based on the generated WLOGP [94] and tPSA [99] parameters. However, these conclusions might not be completely consistent with the real situation, as Caillaud et al. [188] observed. The authors focused on the treatment of Alzheimer’s disease and found that the naturally occurring trans-ε-viniferin (19) molecule passed a given physiological barrier.
On the other hand, high lipophilicity of synthetic halogenated compounds (25)–(27) predicted via CLOGP 4.0 [93] indicated their greater potential to passively enter the CNS [104].
- The impact on P-glycoprotein
The calculated value of a p-gp-I descriptor, ranging from 0.000 to 1.000, provided information about the capability of the investigated molecules to inhibit p-gp: the higher the value, the higher the probability to inhibit p-gp [67]. Similarly, the value of a p-gp-S parameter, varying from 0.000 to 1.000, predicted the ability of the analyzed molecules to serve as a substrate for p-gp: the higher the readout, the higher the probability to serve as the p-gp substrates. These calculations were performed by ADMETlab ver. 3.0 [67].
Experimental data referring to the ability of viniferins and vinferin-based compounds to suitably modulate the activity of p-gp are currently not available. The present in silico evaluations showed (Table S4) that a sterically bulky highly lipophilic compound (27) can theoretically act as a moderate inhibitor of p-gp (p-gp-I = 0.409). Thus, this molecule could possibly contribute to the weakening of the multi-drug resistance of bacteria or could help accumulate anticancer agents in the intracellular environment. This might be the way to eventually contribute to the success of anticancer therapy [189]. Other compounds from the (16)–(29) set theoretically showed a lower ability of the p-gp inhibition.
The high lipophilicity of (27) was not sufficient for its acting as the p-gp inhibitor. The improved inhibitory activity might be reached if at least one center of protonation, i.e., a tertiary N-atom, and/or a longer hydrocarbon chain would be appropriately introduced into the structure of the molecule [190]. However, such modifications would definitely affect antibacterial efficacy of the resulting derivatives.
The in silico analysis also revealed that the compounds (16)–(29) should not serve as suitable substrates for a given type of an ATP-binding cassette transporter. The average log P value around 5.00 could help improve the chances that the investigated molecules would serve as the substrates (Table S4). However, other structural and physicochemical properties were not suitable to be considered for this purpose. The lipophilic properties and MW were factors positively correlated with the ability of compounds to serve as the p-gp substrates. On the other hand, the sum of (nOHNH + nON) negatively contributed to this characteristic [190].
- The binding to plasma proteins
The extent to which small molecules (small-molecule drugs) bind to plasma proteins depends on their MW, lipophilicity (log Ps, log D7.4), ionization state (pKa values), and aqueous solubility [191]. The presently investigated compounds showed ‘virtually’ [67] rather notable PPB (Table S4) ranging from 91.18% (18) to 98.06% (28). These calculations were partially in agreement with research [191] stating that the derivatives with MW = 500.00–700.00 Da were more than 98.00% bound to these proteins. However, the PPB extent for the molecules defined with MW = 300.00–500.00 Da was considerably lower.
Interestingly, if several compounds with the highest PPB and MW < 450.15 Da were excluded from an analysis, i.e., if the molecules (28), (23) and (29) were ‘removed’, the relationship between average log P values of the remaining compounds and their PPBs was rather sufficiently described with a second-order polynomial function, the corresponding Equation (1), and the coefficient of determination (R2). These characteristics were generated using Microsoft Excel and are provided below.
PPB = −0.542 × (average log P)2 + 7.785 × (average log P) + 67.903
R2 = 0.800
R2 = 0.800
The observation was in agreement with the predicted ability of various clinically approved drugs to bind to plasma proteins [192]. However, the main limitation of this analysis was that the compounds, which interacted the most closely with plasma proteins, were excluded from the proposed calculation.
Lipophilicity was a very notable parameter that affected PPB [191,193], but it was not an absolutely decisive factor in the current case. The set of the most lipophilic compounds defined with the average log P values of 6.70 (27), 6.15 (25), and 6.03 (26), respectively, showed 94.51% ≤ PPB ≤ 95.77% (Table S4).
These values were not the highest within all calculated PPBs. For example, less lipophilic derivatives (28) and (23) were characterized with PPB of 98.06% and 97.00% (Table S4), respectively. The most positive r values that described the relationships between lipophilicity and PPB parameters were 0.487 (MLOGP versus PPB) and 0.485 (CLOGP 4.0 versus PPB).
The certain but not decisive meaning of lipophilicity for PPB in the present analysis was indirectly confirmed by the observation that increased tPSA, calculated for the entire (16)–(29) set, was partially related to the decrease in PPB (r = −0.533).
Taking into account primarily acid-base properties, decreased PPB was observed in the following rank [191]: acidic compounds > neutral molecules > zwitterionic compounds > basic molecules. The presently evaluated compounds were neutral in the physiological environment considering their pKas (Figures S2–S15). Therefore, this factor might contribute to relatively high PPB outputs (Table S4).
- The impact on the cytochrome P450 isoenzymes
The CYP hemoprotein isoenzyme superfamily system participates in many key processes required for the maintenance of physiological conditions in the human body [194]. The particular CYP isoenzymes (for example, CYP3A4) are notably involved in the biotransformation of a wide palette of endogenous molecules, many xenobiotics, that is, exogenous compounds, including drugs, as well as in the bioactivation pathways of various entities related to cancer (procarcinogens) [195,196]. The hemoproteins that participate in the activation of procarcinogens via different mechanisms are especially CYP1A1, CYP1A2, CYP1B1, CYP2A6, CYP2A13, and CYP2E1 [196,197].
The significance of knowing about eventual inhibitory activity of the various exogenous ligands, that is, viniferins and their derivatives in this case, toward particular CYP isoenzymes, or about possible induction of these biomolecules could be illustrated by several examples from clinical practice. The biotransformation in vivo of the clinically approved drugs, which were already mentioned, was dependent on the activity of particular CYP isoenzymes. The inhibitors or inducers of these hemoprotein biomolecules could markedly affect the extent of metabolism of respective drugs.
Amiodarone (8) is oxidatively N-deethylated via the activity of CYP3A4 providing two potentially life-threatening hepatotoxic metabolites [198]. Vilazodone (9) is biotransformed primarily by a given isoenzyme and secondarily by CYP2C19 and CYP2D6 [199,200]. The inhibition of CYP3A4 significantly decreases the dosage of this antidepressant [201]. The CYP3A4/5 and CYP1A2 isoenzymes are mainly responsible for extensive biotransformation of tasimelteon (11) [202], and powerful inhibitors or inducers of these isoenzymes notably affect its PK properties [203]. Darifenacin (14) is metabolized through the CYP3A4 and CYP2D6 pathways, and effective inhibitors or inducers of both CYP3A4 and CYP2D6 show a significant impact on darifenacin’s (14) biotransformation [204].
The preliminary view on eventual similarities or differences of the antibacterially effective compounds (16)–(29) in their in silico structural and physicochemical descriptors, i.e., MW, MR, VvdW, flexibility, MLOGP, CLOGP 4.0, WLOGP, ALOGPS, XLOGP3, miLogP 2.2, log D7.4, tPSA (Tables S2–S4), and PK (biochemical) parameters, is provided in Figure 14. The PK (biochemical) characteristics of (16)–(29) are represented by the CYP1A2-I, CYP1A2-S, CYP2C19-I, CYP2C9-I, CYP2C9-S, CYP2D6-I, CYP2D6-S, and CYP2B6-I indices (Tables S5 and S6) related to their in silico inhibitory activity (I letter is listed in the name of a particular parameter) or the ‘virtual’ ability to serve as the substrates for respective enzymes (S letter).
Figure 14.
Two-dimensional observation (mapping) showing both PC 1 and PC 2 scores of the compounds (16)–(29) following their eventual similarities or differences in the calculated structural and physicochemical descriptors, as well as the in silico ability to affect the CYP isoenzymes.
The values of the given parameters might vary from 0.000 to 1.000 (Table S5). The impact of the compounds (16)–(29) on particular isoenzymes was evaluated with respect to the following criteria: no inhibitory activity or no substrate (values of particular descriptors were relatively close to 0.000), moderate inhibitory activity or moderate capability to serve as a substrate (values of individual parameters were around 0.500), and notable inhibitory activity or a very suitable substrate (values of particular descriptors were relatively close to 1.000).
All the compounds (16)–(29) could be found in the constructed coordinate system of PC 1 × PC 2 (Figure 14), and they showed certain groupings or relative ‘isolation’. The PCs accounted for 70.83% of the total variance in the data as follows: PC 1 (46.78%, λe = 9.36) and PC 2 (24.05%, λe = 4.81). The details about this PCA are provided in Supplementary Materials.
The rank of the considered molecules was based on the combination of PC 1 and PC 2 starting with the derivatives with the lowest PC 1. The compounds (29), (17), (16), (18), (19), and (28) were characterized with both negative PC 1 and PC 2, the derivatives (22), (23), (21), and (20) were defined with negative PC 1 and positive PC 2. The molecule (24) was close to the latter set and was characterized with both positive PC 1 and PC 2. The compound (25) was described with both positive PC 1 and PC 2, and finally the derivatives (26) and (27) were defined with positive PC 1 and negative PC 2. In addition, the compounds (27) and (28) were visually ‘slightly isolated’ (Figure 14).
The observed distribution of the analyzed compounds (16)–(29) indicated probable differences in their ‘virtual’ impact on particular CYP isoenzymes. More comprehensive insight into these relationships is provided in the following paragraphs.
The current calculations revealed that all investigated antimicrobially effective molecules (16)–(29) might function as powerful CYP3A inhibitors (Table S5). Despite the absence of a moiety that acted as a center of protonation, i.e., a functional group containing a tertiary N-atom, the chemical structure of the analyzed compounds allowed the consideration of such inhibitory ability. The reason was the presence of a condensed furan ring system and, as in the case of the compound (23), a triple bond [205]. The predictions should be taken with special care because the inhibitory activity in vivo of these compounds would positively or negatively affect the biotransformation of drugs from various PD groups via the activity of CYP3A [206]. The ADMETlab ver. 3.0 predictor [67] also ‘suggested’ that the molecules (16)–(29) would not serve as suitable substrates for CYP3A4, CYP2C19, or CYP2B6 (Table S5).
The calculations carried out for planar cis-δ-viniferin (16), trans-δ-viniferin (17), cis-ε-viniferin (18), trans-ε-viniferin (19), both derivatives (24) and (28) containing a planar bicyclic scaffold together with a flexible hydrocarbon chain, methoxylated and halogenated compounds (26) and (27), as well as for the molecule (29) predicted their ability to effectively inhibit both CYP1A2 and CYP2B6 (Table S5). These predictions allowed for their possible utilization as anticancer agents or eventual structural platforms for the design of compounds showing such type of pharmacological activity [207].
This PCA also showed that the increase in log D7.4 (r = 0.703) and tPSA ≤ 114.29 Å2 were relatively acceptable trends to describe the predicted CYP1A2 inhibitory efficacy of the (16)–(29) set (Tables S4 and S6). The calculations met scientific knowledge published in [208]. On the other hand, inhibition of the CYP1A2 isoenzyme was not only related to anticancer properties of drugs but could be connected with other biological purposes [209].
The planar benzo[b]furan bicyclic system that contained no stereogenic center and a flexible ethan-1,2-diyl chain within the compounds (22) and (24) were convenient structural factors for regarding these derivatives as the substrates for CYP1A2 (Table S5). Both molecules contained hydrogen-bond acceptor groups, were highly lipophilic (Table S3), and were characterized with tPSA = 114.29 Å2 (Table S4). In fact, an increased tPSA parameter was only partially related to a higher probability that an analyzed compound could serve as the CYP1A2 substrate (r = 0.501; Table S6).
The introduction of a double or triple bond into a hydrocarbon chain within fragment D, as indicated for dehydro-δ-viniferin (20) in Figure 8, decreased the capability of a compound to serve as a suitable CYP1A2 substrate. The increased lipophilicity (log D7.4) did not guarantee a higher probability that a molecule would serve as a suitable substrate for CYP1A2 (r = 0.589). The current findings were in agreement with research [208] concluding that the planar arrangement of both ligand and active site of CYP1A2 was convenient for the oxidation of double bonds or oxidative hydroxylation of large molecules containing aromatic systems. Hydrophobic interactions between ligands and CYP1A2 played central roles in these processes.
Polymorphic CYP2C19 isoform biotransforms numerous drugs belonging to various PD classes and is responsible for differences in their PK properties that lead to the formation of pharmacologically active or inactive metabolites [210]. Many different pharmacophore models for effective CYP2C19 inhibitors and substrates have been proposed and more or less extensively described. In that regard, review papers [211,212] can be mentioned as suitable examples.
Higher structural flexibility of the compounds in the current study contributed to more effective in silico inhibition of CYP2C19 (r = 0.753). The significance of lipophilicity, described with the outputs of particular computational methods, was lower. In addition, decreased tPSA values of the molecules (16)–(29) were partially favorable (r = −0.655) for the inhibition of CYPC19 (Table S6).
The CYP2C9 isoenzyme is also notably involved in particular biotransformation pathways in humans [213]. The presence and proper position of single bonds within the structure of the evaluated molecules, i.e., increased flexibility, was convenient for their CYP2C9 inhibitory activity (r = 0.746). The increase in MW (r = 0.518) or VvdW (r = 0.527) was less significant for such a predicted biological property (Table S6).
The compounds (24) and (26)–(28) were computationally indicated as the most powerful CYP2C9 inhibitors (Table S5). Higher lipophilicity, connected with a higher count of aromatic systems, slightly favored these derivatives as the CYP2C9 inhibitors compared to their CYP2C19 inhibitory efficiency (Table S5). None of the calculated structural or physicochemical descriptors were significantly positively or negatively connected with the capability of the molecules (16)–(29) to serve as suitable CYP2C9 substrates (|r| < 0.390; Table S6).
Another polymorphic CYP2D6 isoenzyme also participates in the biotransformation of drugs from various PD groups, for example, antihypertensive agents, antidysrhythmics, antipsychotic drugs, antidepressants belonging to different subclasses, or analgesics [214]. The highest potential to inhibit CYP2D6 was calculated for (22)–(24) containing a modified hydrocarbon chain. In general, the lipophilicity predicted for physiological conditions (log D7.4) positively contributed to the increase in this inhibitory property to a relatively notable extent (r = 0.668).
The center(s) of protonation that would be ionized at a physiological pH value, i.e., basic N-atom(s), should be present in a structure of suitable CYP2D6 substrates [215]. The majority of analyzed compounds (16)–(29) might be prospective CYP2D6 substrates, excluding (16), (17), and (28), despite the fact that this structural requirement was missing (Table S5). It might be assumed that aromatic moieties, lipophilic atoms, and hydrogen-bond acceptor groups were sufficient for noncovalent interactions with particular amino acid residues within CYP2D6 to form the required hydrophobic and π–π interactions, as well as hydrogen bonds. This proposal was in agreement with conclusions published in [212]. The lipophilic properties predicted via WLOGP (r = 0.503) and log D7.4 (r = 0.453) only partially positively contributed to the ability of evaluated compounds to serve as the substrates for CYP2D6.
Polymorphic CYP2B6 isoenzyme also contributes to the biotransformation of various drugs belonging to antidepressants, analgesics, antiviral agents, or anticancer compounds [216]. The structural flexibility of the investigated compounds was partially beneficial (r = 0.510) for the increased potential to be effective CYP2B6 inhibitors. The increase in lipophilicity, expressed with log D7.4 (r = −0.817), relatively notably decreased this capability. Moreover, the increased tPSA parameters also partially reduced this property (r = −0.407; Table S6). The calculations revealed that approximately two thirds of the evaluated molecules could act as prospective CYP2B6 inhibitors (Table S5).
5.2.4. Predicted Toxicological Properties of Chosen Viniferins and Viniferin-Based Compounds
The SwissADME tool [66] indicated that the presently considered derivatives (16)–(29) contained no electrophilic group(s), which theoretically would be responsible for the nonselective interactions of these compounds with several pharmacological targets. The class of molecules that showed such properties was termed pan-assay interference compounds [217]. The predictor [66] also contained a database compiled from the series of fragments, previously identified by Brenk et al. [218], which could cause toxicity, chemical reactivity, or metabolic instability. The fragments could also be responsible for inconvenient PK properties. The compounds (16)–(21), (23), (25)–(27), and (29) contained one alert, a stilbene moiety. One alert, the triple bond, was also present in the structure of the compound (23).
Therefore, the next step in the evaluation of the molecules (16)–(29) was the in silico analysis of their toxicological properties, also in relation to structural and physicochemical characteristics. The preliminary view on the eventual similarities or differences in their selected in silico structural and physicochemical descriptors, i.e., MW, MR, VvdW, flexibility, MLOGP, CLOGP 4.0, WLOGP, ALOGPS, XLOGP3, miLogP 2.2, log D7.4, tPSA (Tables S2–S4), and the impact on the ‘virtual’ DILI, H-HT, DINf, OT, DINe, and hERG properties (Tables S7 and S8) showed a certain grouping of the compounds (16)–(29).
The positive information was that the entire set of these derivatives was visualized in the constructed coordinate system of PC 1 and × PC 2 (Figure 15). The PCs accounted for 81.61% of the total variance in the data as follows: PC 1 (54.53%, λe = 9.82) and PC 2 (27.08%, λe = 4.87). The details about this PCA are provided in Supplementary Materials.
Figure 15.
Two-dimensional observation (mapping) showing both PC 1 and PC 2 scores of the compounds (16)–(29) following their eventual similarities or differences in the calculated structural and physicochemical descriptors, as well as the in silico toxicological characteristics.
The compounds (16)–(19) were characterized with both negative PC 1 and PC 2, the derivatives (20)–(24) and (29) were defined with negative PC 1 and positive PC 2. The molecules (28) and (26) were described with positive PC 1 and negative PC 2, and finally, the compounds (25) and (27) were defined by both positive PC 1 and PC 2. The derivative (27) seemed to be ‘slightly isolated’. More comprehensive insights into these relationships are provided in the following paragraphs.
DILI, a form of idiosyncratic toxicity that occurs very frequently in humans, could seriously damage patients’ health and might be the main cause for withdrawal of already clinically approved drugs [219,220]. The in silico DILI indicator, generated within an interval from 0.000 to 1.000 [67], showed a compound without risk of DILI (DILI relatively close to 0.000), medium risk of DILI (DILI around 0.500), or with a high risk of DILI (DILI relatively close to 1.000). The possible threat was also color-coded [67]; a green color indicated no risk, an orange color predicted a medium risk, and finally a red color was the indicator for a highly risky molecule (Table S7).
The values of a calculated DILI parameter (Table S7) did not show a risk of DILI for the compounds (16)–(19), (24), and (28), and suggested a medium DILI risk for the molecules (20)–(23), (25)–(27), and (29). The results of this PCA also indicated that an increased adverse DILI effect of the evaluated compounds relatively notably positively correlated with the increase in their lipophilicity (Table S8). The lipophilicity descriptors, for which attention should be primarily paid in that regard, were WLOGP (r = 0.708), log D7.4 (r = 0.686), and miLogP 2.2 (r = 0.611). These observations supported the findings and conclusions already published in [221,222].
This paper also focused on the exploration of eventual medium or even high risk of hepatotoxicity, described with the H-HT parameter, for the compounds (16)–(29). Their H-HT descriptor might be found in the interval from 0.000 to 1.000, and the rules for the appropriate color-coding of the safe versus risky behavior [67] were the same as for DILI (Table S7). The compounds (16)–(29) were defined with H-HT ≥ 0.595 (Table S8). The risky behavior was partially negatively connected with their log D7.4 values (r = −0.652) and also partially negatively correlated with the DILI effect (r = −0.729). The impact of VvdW on H-HT was only minor (r = 0.388). Therefore, no DILI risk but high risk of H-HT was predicted for relatively less lipophilic molecules (16)–(19) and (28).
Although the Fsp3 parameter was not involved in the analysis, the Fsp3 threshold ≤ 0.280 published in [220] indicated possible hepato- and nephrotoxicity issues related to the set (16)–(29). Their Fsp3 values (Table S2) were found in an interval from 0.000 (calculated for several molecules, for example, (20) or (21)) to 0.143 (28).
The primary pharmacological activity of some clinically approved drugs belonging to analgesics, non-steroidal anti-inflammatory drugs, antivirals, antiparasitic agents, various chemical classes of antihypertensives, diuretics, vasodilating agents, different chemical classes of antimicrobials, or disease-modifying antirheumatic drugs was accompanied by their toxic effects on kidneys [223,224,225,226,227].
The DINf descriptor calculated for (16)–(29), which was associated with possible nephrotoxicity, might be found in the interval from 0.000 to 1.000. The rules for the appropriate color-coding of these compounds concerning their safe versus risky behavior [67] were the same as for DILI (Table S7).
The compounds (16)–(29) ‘virtually’ showed a low to high risk of DINf (Table S7). The relatively notable linear relationship was found between this adverse property and MLOGP (r = 0.907), ALOGPS (r = 0.849), CLOGP 4.0 (r = 0.787), flexibility (r = 0.779), MW (r = 0.734), miLogP 2.2 (r = 0.723), as well as VvdW (r = 0.705).
On the other hand, the increased tPSA partially contributed to the decrease in DINf (r = −0.579; Table S8). If only the compounds, which achieved a minimal threshold value of tPSA ≥ 88.38 Å2, were evaluated, a more favorable linear correlation would be observed as described with Equation (2), R2, and absolute value of the r parameter. These characteristics were calculated using Microsoft Excel and are listed below.
DINf = −0.019 × tPSA + 2.244
R2 = 0.845, |r| = 0.919
R2 = 0.845, |r| = 0.919
Chlorinated compounds (25)–(27) were the most dangerous from that point of view theoretically providing a medium to high DINf risk. In contrast, the lowest calculated DINf risk was a beneficial feature of a natural dehydro-ε-viniferin (21) molecule (Table S7). The current findings were in agreement with conclusions [228]. The research indicated lipophilicity, MW, and tPSA as parameters suitable to distinguish between nephrotoxic and nonnephrotoxic compounds.
The negative impact of antimicrobially active drugs on the integrity and function of erythrocyte membranes is unfortunately a fairly common adverse effect [229]. The HeT descriptor calculated for (16)–(29), which predicted possible hematotoxicity, might be found in the interval from 0.000 to 1.000. The rules for the appropriate color-coding of their safe versus risky behavior were the same as for DILI (Table S7). The values of the HeT index, ranging from 0.003 (two naturally occurring viniferins) to 0.060 (27), allowed one to consider none of the compounds (16)–(29) hematotoxic (Table S7).
Some effective antibacterial drugs belonging to an aminoglycoside antibiotic class or nonaminoglycoside antibiotics, i.e., vancomycin, which was already mentioned, negatively affect cochlea or vestibular apparatus located within the inner ear. The consequence may be a very serious health issue, loss of hearing [230,231].
Although RSV (1) has been reported to alleviate the causes of cis-platin-induced ototoxic effects in experiments under conditions in vitro through various protective mechanisms [232] and 2,3,4′,5-tetrahydroxystilbene-2-O-β-D-glucoside attenuated ototoxic manifestations triggered by gentamicin [233], the prediction of the values of OT, which was related to possible ototoxicity, provided more diverse conclusions.
The OT descriptor calculated for (16)–(29) might be found in the interval from 0.000 to 1.000. The rules for the appropriate color-coding of their safe versus risky behavior were the same as for DILI (Table S7). The present evaluation indicated that only six compounds from the (16)–(29) set ‘virtually’ showed no risk of ototoxicity, i.e., the molecules (20)–(24) and (29) defined with OT ≤ 0.282. On the other hand, the most ototoxic derivatives were characterized with OT ≥ 0.744 (Table S7).
The ototoxic properties of the evaluated compounds (16)–(29) partially positively correlated with MLOGP (r = 0.708), flexibility (r = 0.635), and VvdW (r = 0.617). The tPSA parameter had only a lower significance (r = −0.335). However, the contribution of lipophilicity to ototoxicity was not absolutely clear because a negative correlation with log D7.4 was generated (r = −0.509; Table S7).
Possible neurotoxic effects of various antibacterial chemotherapeutics, antimycobacterial and antileprosy drugs, or antiparasitic agents have been known and reported in the past decades of the previous century [234], and research on the neurotoxic features of these compounds continues [235]. Paradoxically, attention is also focused on the utilization of selected antibacterial chemotherapeutics (for example, minocycline, an antibiotic molecule chemically belonging to a tetracycline class) as neuroprotective agents [236,237].
The DINe parameter related to possible neurotoxicity was another feature calculated for (16)–(29). This descriptor might be found in the interval from 0.000 to 1.000. The rules for the appropriate color-coding of the compounds following their safe versus risky behavior were the same as for DILI (Table S7). The highest risk of neurotoxicity was theoretically related to naturally occurring cis-δ-viniferin (16), cis-ε-viniferin (18), as well as synthetic derivatives (25)–(27). These molecules were characterized with DINe ≥ 0.734. The lowest potential to induce neurotoxic processes was predicted for the compound (23) with DINe = 0.052 (Table S7). The relationships between the generated structural as well as physicochemical descriptors and DINe would not be possible to define as linear due to the relatively low r values (Table S8).
The non-CV drug-induced blockade of a potassium channel, which is encoded by hERG, caused prolongation of a QT interval, resulting in even fatal sudden cardiac death. Therefore, the regulatory authorities decided to withdraw already approved drugs showing such undesirable properties, for example, terfenadine acting as an antihistamine drug or antibacterially effective grepafloxacin, from pharmaceutical markets worldwide as a very reasonable response to concerns about cardiac safety and the occurrence of such fatal cases [238,239,240]. The ability to induce long-QT syndrome (LQTS) was also observed for amiodarone (8) [241]. On the other hand, RSV (1) showed beneficial antidysrhythmic activity in acquired LQTS [242].
The survey of available scientific papers carried out by the authors of this review paper provided no relevant information on whether particular viniferins practically caused the prolongation of the QT interval. The current calculations, however, pointed out the possibility of such toxic behavior (Table S7).
The hERG descriptor calculated for (16)–(29) might be found in the interval from 0.000 to 1.000. The descriptor informed on possible impact on the hERG potassium channel. The rules for the appropriate color-coding of their safe versus risky behavior were the same as for DILI (Table S7). The generated hERG index ranged from 0.319 (29; medium risk of the hERG channel blockade) to 0.869 (28; high risk of the hERG channel blockade).
Many different pharmacophore models have already been proposed to define key requirements for a small molecule (small-molecule drug) to show or not to show notable affinity for hERG, as well as to be or not to be an effective blocker of this potassium channel [243]. Basically, these predictive models can be essentially divided into two categories following the presence or absence of the center(s) of protonation within the structure of the investigated compounds [243,244,245].
For example, Du-Cunny et al. [245] suggested the presence of hydrogen-bond acceptors, aromatic systems to form appropriate π (ligand)–π (aromatic amino acid target) interactions, a hydrophobic moiety to form hydrophobic interactions and appropriate distances between these components as key prerequisites for the structure of molecules (containing no center of protonation), which might block hERG. Regarding such a model, all evaluated compounds (16)–(29) could effectively block a given potassium channel.
The effective hERG inhibitors should be characterized with 110.00 m3/mol ≤ MR (in m3/mol units) < 176.00 m3/mol, calculated log P ≥ 3.70 (in this case, an average log P could be considered) and pKa ≥ 7.3 [246]. These trends were confirmed by more recent research of Yu et al. [247]. The relationship between structural nrotb and nON descriptors and hERG inhibitory activity was not apparent as published in [248].
6. Pro et Contra Connected with Presently Employed in Silico Tools
The computational applets, which were chosen for the majority of current computational analyses, that is, SwissADME [66], ADMETlab ver. 3.0 [67], ALOGPS/VCCLAB [95], Molinspiration Cheminformatics [97], Enalos Cloud Platform [132], and MolGpKa [156], provided some details on the required characteristics that play decisive roles from the viewpoint of drug-likeness and NP-likeness of (not only) antimicrobially highly active viniferins and viniferin-based compounds. The CLOGP 4.0 outputs were also suitably added to the considered evaluations using a commercially available ChemDraw ver. 22.2.0.3300 software, which was also employed to draw chemical structures of the analyzed compounds.
In fact, accessibility (including availability of the respective software/applet, and the financial aspect related to the purchase of the specific software, or software packages), complexity, graphic organization, and clarity of the interactive interface, count, and types of the descriptors which could be generated, speed of the required calculations, relevance of the data obtained, eventual installation of additional software, hardware requirements, support of developers of the employed computational tools, security of the calculated data, and last but not least the validity of such generated parameters from the viewpoint of real biological and biochemical processes in vivo might be the main factors for selection of particular in silico tools. The reasons for which the computational platforms [66,67,95,97,132,156] were chosen are briefly summarized in the following paragraphs.
These web-based tools are currently fully accessible to students, academics, lecturers, researchers, or the broad non-academic community without the need for previous registration. These platforms have been frequently utilized, and the generated data have been generally accepted by the scientific community. The graphical interfaces of all of the applets were relatively intuitive, visually very acceptable, and user-friendly. In addition, brief information connected with the significance of particular descriptors was provided.
Another advantage is that a rather simple generation of a relatively broad palette of relevant structural, physicochemical, PK, biochemical, PD, or toxicological characteristics could be achieved through these interactive tools. In that sense, the complexity, together with the user-friendly interface of SwissADME [66] or ADMETlab ver. 3.0 [67] might especially be mentioned.
Most of the current calculations were ‘only’ based on the correct representation of the chemical structure of investigated compounds using an appropriate sketcher, which was already integrated within the respective applets. For example, the Marvin JS drawing tool was utilized for SwissADME [66], and the molecules’ SMILES codes [68] were automatically generated via JChem Web Services [66]. The proper functioning of ALOGPS/VCCLAB [95], Molinspiration Cheminformatics [97], Enalos Cloud Platform [132], and MolGpKa [156] was connected with the simple installation of the Java SE Platform software (for example, Java SE ver. 24.0.2; Oracle Corp., Austin, TX, USA). In fact, no additional ‘specific’ software was needed for the correct functioning of these applications.
The given predictors also allowed for the effective analyses of numerous compounds, for example, promising pharmacologically active small molecules, drug candidates, or small-molecule drugs in real (relatively short) time. This factor might be regarded as highly favorable for researchers who are not primarily concerned with particular methods of computer-aided ligand-based or structure-based drug design and discovery [249,250]. These approaches eventually require more robust and rather specific commercially available software packages, and the accessibility of high-performance computers, supercomputers, or cloud-based computing systems could be assumed.
Indeed, there was definitely a broad room for the use of other open-source computational platforms such as Mordred [251] or PaDEL-DDPredictor [252]. The significance of generated 2D and 3D descriptors through Mordred [251], a Python-based code, was generally well accepted, its performance was reported to be rather extraordinary; Mordred might calculate an entire molecular descriptor twice as fast as PaDEL-DDPredictor. The installation of Mordred could be achieved using only a single command. However, the proper functioning of such a software tool might require the installation of an additional environment [251], for example, Anaconda or RDKit. In fact, this process might sometimes be considered ‘an obstacle’ for students, lecturers, or researchers who prefer a more straightforward way to generate the desired descriptors and whose computer skills might be not so advanced.
Similar conclusions are drawn considering the PaDEL-DDPredictor tool [252]. The interface might be regarded as convenient and relatively simple to use. However, the calculations require additional procedures, that is, the installation of the Java Runtime Environment (as in the case of predictors preferred in the current research), proper manual creation of relevant output file(s), as well as proper setup of required directories and files. This predictor allows the calculation of a broad palette of ‘classic’ descriptors, in fact, including MW, MR, VvdW, nrotb, nr, nOHNH, nON, several lipophilicity parameters, or tPSA. However, it does not serve as a platform for generating the PK, biochemical, or toxicological characteristics.
The web-based SMARTCyp tool [253] allows free prediction of biotransformation sites in the structure of numerous compounds. On the other hand, only the isoenzymes CYP3A4, CYP2C9, and CYP2D6 are currently considered in this applet.
Specific web-based platforms, which previously offered a wide palette of parameters to be calculated for free [254,255], were notably modified, and comprehensive calculations or even the servers themselves were no longer available to be utilized. The possibility of calculating several absolutely crucial descriptors very frequently employed in Medicinal Chemistry, including the acid-base pKa values or different biochemical constants, was markedly restricted in freely available computational applets in recent years. In addition, these free tools might focus on a single specific property or model. The opportunity to generate and obtain the desired values was mainly connected with commercial software [256], and that factor is very limited (not only) in the academic sphere. In addition, the freely available MolGpKa web server [156] offers the possibility to conveniently predict the pKa descriptor.
Therefore, the combination of the SwissADME [66], ADMETlab ver. 3.0 [67], ALOGPS/VCCLAB [95], Molinspiration Cheminformatics [97], Enalos Cloud Platform [132], and MolGpKa [156] prediction tools for the majority of the current calculations seems to be reasonable and maybe ‘more convenient’ for the wider scientific community or the community of academic and university lecturers. Moreover, the ADMETlab ver. 3.0 tool [67] is regarded as the one providing the best accuracy and precision in the predictions [256]. Several details on accessibility, calculated descriptors, and particular prediction principles of particular computational tools are listed in Supplementary Materials.
The use of the given web-based interactive predictors [66,67,95,97,132,156] was not connected only with benefits, but several issues could also be mentioned. In general, the confidentiality of the calculated data could not always be guaranteed for such a type of computational tools, and the risk of unauthorized use of investigated compounds could be significant.
The SwissADME applet [66] was one of the exceptions; the development company of this web-based software, the Swiss Institute of Bioinformatics, provided the information that it was committed to ensuring the privacy and confidentiality of user personal data. The obstacle was not directly related to the current research because the structure of all of the analyzed compounds was already published in scientific papers, as listed in Section 5.1.
The web-based SMARTCyp tool [253] also allowed free prediction of biotransformation sites in the structure of numerous compounds. However, only the limited number of isoenzymes were considered in this applet, as mentioned.
Numerous predictors offered the calculation of the 1D or 2D descriptors that could not be completely relevant for the evaluation considering the complexity of a 3D space, i.e., stereochemical properties of investigated compounds, experiments under in vitro or in vivo conditions, as well as a very complex biological environment, with all their specificities. However, appropriately chosen in silico approaches could be rather beneficial, especially in the initial stages of the complex evaluation of pharmacologically effective compounds or prospective drug candidates, because they might considerably limit the number of animal studies [257,258].
Some predictor tools provided a broad spectrum of PD, structural, physicochemical, PK, or ‘biological’ data, the adequate interpretation of which might not be trivial and efficient translation of the knowledge obtained to Medicinal Chemistry would not be enabled. In addition, several descriptors could not be satisfactorily predicted via given computational tools because of their complexity. The aqueous solubility, as one of the strategic parameters in Medicinal Chemistry, is mentioned as a suitable example in this regard [259].
There might be a general lack of experimental values for all considered types of descriptors related to investigated molecules, including viniferins and viniferin-based compounds. Therefore, it is not possible to correlate the experimentally estimated data and calculated parameters (for example, particular lipophilicity descriptors, several biochemical or toxicological ‘values’) and to absolutely precisely evaluate the significance of predicted outputs.
The threshold value of MW for the calculations related to the small molecules had to be taken into consideration if they would be evaluated via these web-based in silico tools. The fundamental condition for such an investigation was the generation of a correct SMILES code. For example, the structurally more complex oligomeric molecules [260,261] based on the molecular framework of RSV (1) even with MW > 1500.00 Da, pauciflorol D (MW = 1586.47 Da; heptamer), for example, might be successfully analyzed [66,67] because their correct SMILES codes were generated.
On the other hand, the use of the given type of predictor tools for the evaluation of structurally more complex molecules, inorganic compounds, or highly reactive molecules [66] would not be possible.
The in silico and experimental techniques employed today provide highly valuable information on particular biological and biochemical processes at the molecular level [262,263], including protein(s)–protein(s), drug–(desired) biological target(s), or drug–drug interactions. However, considering the frequent immersion complexity of natural systems, the coherent comprehension of such processes via the in silico and/or in vitro evaluations could not be entirely sufficient.
In general, the appropriate modeling of heterogeneous and highly structured biological environments is still a fairly challenging task. Simulations in silico have been accepted by the scientific community mainly from the perspective of complementing clinical studies in real clinical conditions. In fact, the most essential step is to constantly improve the robustness and reliability of proposed predictive models to align with real clinical data as closely as possible [264].
Despite all the shortcomings mentioned, the in silico predictions could serve as a very suitable dynamic basis to consider the strengths and weaknesses of compounds in advance following the proper characterization of their drug-like or NP-like profiles.
7. Conclusions and Future Directions for Viniferins and Viniferin-Based Derivatives
Previous research aiming at viniferins, their semi-synthetic as well as synthetic analogues and derivatives proved that they represented an extraordinarily promising class of compounds capable of fighting in vitro very efficiently several Gram-positive bacterial strains. This knowledge was extremely important in the overall context of the effective treatment of infections caused by these pathogens. However, the path of a compound that shows promising pharmacological activity for a prospective drug candidate, or even a drug approved for clinical use, is rather complicated and not always straightforward. The fundamental step in the process of drug design and development is to characterize such a biologically prospective molecule very comprehensively with respect to its PD, selectivity, structural, physicochemical, PK, and toxicological properties.
The indisputable advantage of the presently investigated viniferins and viniferin-based compounds was the presence of two types of privileged structures, i.e., a benzo[b]furan or 2,3-dihydrobenzo[b]furan core, which formed their fundamental structural framework. The arrangement gave hope that a desired type of pharmacological activity could be achieved and eventually utilized in the future to improve human health. The intention of a current review paper was to advance the knowledge in the field of antibacterially very active viniferins and their derivatives with respect to further phases of their development and optimization.
The presently employed in silico methods provided at least a primary view on the structural, physicochemical, PK, and even toxicological characteristics of these compounds to tentatively indicate their developability and drug-likeness. The proposed structural modifications of naturally occurring viniferins (16)–(21) led to the synthesis of the compounds (22)–(29) with relatively improved antibacterial efficiency. These changes were reflected in the decreased NP score index, informing about a measure by which these structurally modified compounds were still NPs (Table S7). In this regard, 5-{[3-(3,5-dihydroxyphenyl)-2-(4-hydroxyphenyl)benzofuran-5-yl]ethynyl}benzene-1,3-diol (23) was that with the lowest ‘natural character’.
The QED parameter [135,136] indicated the probability that an analyzed compound can be a therapeutic agent. The calculated QED value of 0.436 was related to (E)-2-(4-hydroxyphenyl)-4-(4-hydroxystyryl)benzofuran-6-ol (29) and the output was the highest among the QEDs generated for the entire (16)–(29) set. However, halogenated compounds (25)–(27) were computationally considered to have the most promising potential to be convenient structural platforms that could be utilized in drug design and development following another calculated criterion, the MCE-18 descriptor (Table S7). In fact, their MCE-18s ranged from 90.412 (both (25) and (26)) to 93.529 (27), reliably exceeding the threshold MCE-18 value of ≥ 45.00. Paradoxically, the perspectives of the compound (29) were the lowest in this regard.
These observations could also be visualized globally using the PCA technique (Figure 16). All investigated compounds were distributed in four different areas following their properties within the constructed PC 1 × PC 2 coordinate system. The areas were indicated with one ring and three ellipses. The derivatives were properly defined with positive or negative PCs. These PCs accounted for 66.39% of the total variance in the data as follows: PC 1 (42.10%, λe = 19.37) and PC 2 (24.29%, λe = 11.17). The details about this PCA, including the palette of the descriptors aimed at the given evaluation, are provided in Supplementary Materials.
Figure 16.
Two-dimensional observation (mapping) showing both PC 1 and PC 2 scores of the compounds (16)–(29) following their eventual similarities or differences in the calculated structural, physicochemical, PK, and toxicological characteristics, as well as descriptors defining drug-likeness and NP-likeness (QED, MCE-18 and NP score) of these molecules.
Focusing especially on the most prospective derivatives, the compound (29) was ‘slightly isolated’ on the left, and described with negative PC 1 and positive PC 2. On the other hand, the molecules (25)–(27) were placed in the right part of Figure 16, and characterized with both positive PC 1 and PC 2.
Such conclusions strongly supported the suggestion that there was a broad room for optimization of the structure of the investigated molecules to improve their characteristics. For example, current predictions proposed to optimize lipohydrophilic features, i.e., the design strategies that aim to decrease the lipophilicity of modified compounds, might be preferred. Therefore, a given optimization would be positively reflected in the improved aqueous solubility of the resulting derivatives, and this improvement would be associated with higher biological availability.
The molecules (16)–(29) could act as relatively strong inhibitors or inducers of the chosen CYP isoenzymes. Several of these compounds ‘virtually’ very efficiently inhibited CYP1A2, CYP2C1, CYP2C9, CYP2D6, CYP3A4, and CYP2B6, or served as suitable substrates for CYP1A2, CYP2C9, and CYP2D6 (Table S5) as well. The consequences of such a behavior might be rather broad, from a more or less notable impact on biotransformation in vivo of drugs belonging to various PD classes to inhibition/activation of pathways connected with cancer.
Special attention should also be paid to the eventual toxic properties of the compounds (16)–(29) concerning their undesirable effects on the liver, nerves, system of the inner ear, and CV system as well.
All of these parameters could be modulated via proper and accurate modifications in the structure of the analyzed compounds, which would result in more convenient properties of the resulting derivatives and analogues with respect to the requirement to maintain or even improve their antibacterial potential. In addition, some limitations related to (16)–(29) could potentially be improved with the correct selection of appropriate dosage forms or delivery systems. The research in the field of drug delivery systems is of particular importance (not only) in Medicinal Chemistry. This topic is very comprehensive and requires special attention.
In conclusion, this paper offered a more detailed and (maybe) more critical view on a set of antibacterially notably effective viniferins and viniferin-based compounds with respect to their structural, physicochemical, PK, and toxicological characteristics. The properties can play decisive roles in the next phases of the eventual pre-clinical and clinical evaluation. In addition, several advantages and limitations of the used predictor tools were also provided, and these findings could help develop more accurate web-based alternatives in the future. The notable advances in the design and development of antibacterially effective compounds with favorable PK and toxicological profiles might be achieved via effective interdisciplinary cooperation, continuous innovation, and strict data validation. In other words, the progress achieved has to be dynamically reflected in relevant in silico, in vitro, and in vivo techniques, and these procedures should be integrated into the research and complement each other.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15158350/s1, Table S1: The formulas, chemical names, and Simplified Molecular Input Line Entry System (SMILES) codes of the in silico evaluated compounds (16)–(29). The codes were generated via the ADMETlab ver. 3.0 interactive tool [67]; Table S2: The structural and physicochemical descriptors (MW, nsc, Fsp3, MR, VvdW, nrotb, nrigb, nr and nC) generated via the SwissADME [66] and ADMETlab ver. 3.0 [67] interactive tools for the compounds (16)–(29); Table S3: The structural and physicochemical descriptors (nhet, flexibility, nOHNH, nON, MLOGP, CLOGP 4.0, WLOGP, ALOGPS, XLOGP3, miLogP 2.2, and log D7.4) generated via the SwissADME [66], ADMETlab ver. 3.0 [67], ALOGPS 2.1 [95], and Molinspiration Cheminformatics [97] interactive tools as well as ChemDraw ver. 22.2.0.3300 software package (CambridgeSoft, Cambridge, MA, USA) for the compounds (16)–(29); Table S4: The structural, physicochemical, and pharmacokinetic descriptors (tPSA, log SESOL, log SALI, log Peff, log SS-IT, p-gp-I, p-gp-S, PPB, as well as permeability through skin and blood–brain barrier (BBB)) generated via the SwissADME [66], ADMETlab ver. 3.0 [67] and Enalos Cloud Platform [132] interactive tools for the compounds (16)–(29); Table S5: The prediction for analyzed compounds (16)–(29) to act as inhibitors and/or substrates for several enzymes, i.e., CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and CYP2B6, present within a cytochrome C450 isoenzyme system using the interactive ADMETlab ver. 3.0 tool [67]. Particular descriptors, listed in brackets, characterizing the inhibitory activity of these compounds toward chosen isoenzymes are indicated with an I letter, particular descriptors, listed in brackets, defining the capability of these compounds to serve as the substrates for respective enzymes are indicated with an S letter; Table S6: The readouts from a principal component analysis (PCA) technique exploring the relationships between structural as well as physicochemical properties of the analyzed compounds (16)–(29) and their impact on chosen CYP isoenzymes (capability to act as the inhibitors of particular CYPs, i.e., CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP2B6, indicated with an I letter, capability to serve as the substrates for given isoenzymes indicated with an S letter) via an XLSTAT ver. 2019 2.2 statistical software add-in (Addinsoft, New York, NY, USA) for Microsoft Excel (Microsoft 365; Microsoft Corp., Redmont, WA, USA). The relationships are described with positive or negative values of a Pearson correlation coefficient (r); Table S7: The prediction of toxicological parameters (DILI, H-HT, DINf, HeT, OT, DINe, and hERG), drug-likeness (QED and MCE-18) and natural product-likeness (NP score) generated via the ADMETlab ver. 3.0 interactive tool [67] for the compounds (16)–(29); Table S8: The readouts from a principal component analysis (PCA) technique exploring the relationships between structural as well as physicochemical properties of the analyzed compounds (16)–(29) and their toxicity profiles (human hepatotoxicity, drug-induced nephrotoxicity, hematotoxicity, ototoxicity, drug-induced neurotoxicity, and impact on a hERG channel in the heart) via an XLSTAT ver. 2019 2.2 statistical software add-in (Addinsoft, New York, NY, USA) for Microsoft Excel (Microsoft 365; Microsoft Corp., Redmont, WA, USA). The relationships are described with positive or negative values of a Pearson correlation coefficient (r); Figure S1: The relationship between effective intestinal membrane permeability (log Peff) generated through the ADMETlab ver. 3.0 tool [67] and logarithm of a permeability coefficient (Kp; in cm/s units) predicted via the SwissADME interactive applet [66]. The relationship between log Peff and log Kp was not linear (trend line was indicated by an orange color, a linear relationship was described by an equation as follows: log Kp = 0.1126 × log Peff − 4.9184; R2 = 0.005); Figure S2: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for cis-δ-viniferin (16) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S3: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for trans-δ-viniferin (17) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S4: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for cis-ε-viniferin (18) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S5: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for trans-ε-viniferin (19) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S6: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for dehydro-δ-viniferin (20) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S7: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for dehydro-ε-viniferin (21) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S8: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for the compound (22) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S9: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for the compound (23) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S10: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa5) values for the compound (24) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S11: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa3) values for the compound (25) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S12: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa3) values for the compound (26) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S13: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa3) values for the compound (27) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S14: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa4) values for the compound (28) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Figure S15: The prediction of particular acid-base dissociation constant (pKa; pKa1–pKa3) values for the compound (29) using the MolGpKa interactive tool [156]. The respective groups, which are considered deprotonated, are indicated by full red circles; Note S1. The numbering of references; Note S2: The details considering particular evaluations via principal component analysis; Note S3: Several details considering particular in silico tools.
Author Contributions
Conceptualization, D.N. and I.M.; methodology, D.N. and I.M.; software, D.N. and I.M.; validation, D.N. and I.M.; formal analysis, D.N. and I.M.; investigation, D.N. and I.M.; resources, D.N. and I.M.; data curation, D.N. and I.M.; writing—original draft preparation, D.N. and I.M.; writing—review and editing, D.N. and I.M.; visualization, D.N. and I.M.; supervision, I.M.; project administration, D.N. and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors very gratefully acknowledge the financial support received from the Comenius University Bratislava, Faculty of Pharmacy (Slovakia), the Slovak Research and Development Agency under the Contract No. APVV-22-0133, as well as by the Grant of the Faculty of Pharmacy, Comenius University Bratislava, Grant No. FaF/18/2025.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADME | Absorption, distribution, metabolism, and excretion |
| ALOGPS | Lipophilicity parameter (log P) calculated via a whole-molecule-based ALOGPS method |
| BBB | Blood–brain barrier |
| CLOGP 4.0 | Lipophilicity parameter (log P) calculated via a fragmental CLOGP 4.0 method |
| CNS | Central nervous system |
| CV | Cardiovascular |
| CYP | Cytochrome P450 |
| DILI | Drug-induced liver injury |
| DINe | Drug-induced neurotoxicity |
| DINf | Drug-induced nephrotoxicity |
| FDA | Food and drug administration |
| Fsp3 | Fraction of sp3-hybridized carbon atoms |
| H-HT | Human hepatotoxicity |
| hERG | Human ether-à-gogo-related gene |
| HeT | Hematotoxicity |
| Kp | Permeability coefficient (in cm/s units) |
| log D7.4 | Calculated decadic logarithm of a distribution coefficient (D) at pH = 7.4 |
| log Kp | Calculated decadic logarithm of a permeability coefficient (Kp) |
| log Peff | Effective intestinal membrane permeability (parameter) |
| MCE-18 | Medicinal Chemistry Evolution-18 (parameter) |
| miLogP 2.2 | Lipophilicity parameter (log P) calculated via a Molinspiration Cheminformatics’ method based on group contributions |
| MLOGP | Lipophilicity parameter (log P) calculated via a Moriguchi’s method |
| MR | Molar refractivity (in m3/mol units) |
| MW | Molecular weight (in Da units) |
| NP(s) | Natural product(s) |
| NP score | Natural product score (parameter) |
| nC | Number of carbon atoms |
| nhet | Number of heteroatoms |
| nOHNH | Number of hydrogen-bond donors |
| nON | Number of hydrogen-bond acceptors |
| nr | Number of rings |
| nrigb | Number of rigid bonds |
| nrotb | Number of rotatable bonds |
| nsc | Number of stereogenic centers |
| OT | Ototoxicity |
| p-gp | P-glycoprotein |
| p-gp-I | Capability to inhibit a P-glycoprotein (parameter) |
| p-gp-S | Capability to serve as a substrate for a P-glycoprotein (parameter) |
| PC | Principal component |
| PCA | Principal component analysis |
| PD | Pharmacodynamic(s) |
| PK | Pharmacokinetic(s) |
| pKa | Acid-base dissociation constant |
| PPB | Plasma protein binding (parameter) |
| QED | Quantitative estimate of drug-likeness (parameter) |
| RSV | Resveratrol |
| SC | Stratum corneum |
| SMILES | Simplified molecular input line entry system |
| tPSA | Topological polar surface area (in A2 units) |
| VCCLAB | Virtual computational chemistry laboratory |
| VvdW | van der Waals volume (in Å3 units) |
| WLOGP | Lipophilicity parameter (log P) calculated via a Wildman and Crippen’s atomic-based method |
| XLOGP3 | Lipophilicity parameter (log P) calculated via an atomic/group-based XLOGP3 method |
References
- Moghnieh, R.; Alothman, A.F.; Althaqafi, A.O.; Matar, M.J.; Alenazi, T.H.; Farahat, F.; Corman, S.L.; Solem, C.T.; Raghubir, N.; Macahilig, C.; et al. Epidemiology and outcome of invasive fungal infections and methicillin-resistant Staphylococcus aureus (MRSA) pneumonia and complicated skin and soft tissue infections (cSSTI) in Lebanon and Saudi Arabia. J. Infect. Public Health 2017, 10, 849–854. [Google Scholar] [CrossRef]
- Devi, N.S.; Mythili, R.; Cherian, T.; Dineshkumar, R.; Sivaraman, G.K.; Jayakumar, R.; Prathaban, M.; Duraimurugan, M.; Chandrasekar, V.; Peijnenburg, W.J.G.M. Overview of antimicrobial resistance and mechanisms: The relative status of the past and current. Microbe 2024, 3, 100083. [Google Scholar] [CrossRef]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A.-S. Stilbenes, a versatile class of natural metabolites for inflammation—An overview. Molecules 2023, 28, 3786. [Google Scholar] [CrossRef]
- Beaumont, P.; Courtois, A.; Atgié, C.; Richard, T.; Krisa, S. In the shadow of resveratrol: Biological activities of epsilon-viniferin. J. Physiol. Biochem. 2022, 78, 465–484. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Rius, C.; Abu-Taha, M.; Hermenegildo, C.; Piqueras, L.; Cerda-Nicolas, J.M.; Issekutz, A.C.; Estañ, L.; Cortijo, J.; Morcillo, E.J.; Orallo, F.; et al. Trans- but not cis-resveratrol impairs angiotensin-II-mediated vascular inflammation through inhibition of NF-κB activation and peroxisome proliferator-activated receptor-gamma upregulation. J. Immunol. 2010, 185, 3718–3727. [Google Scholar] [CrossRef]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazué, F.; Ghiringhelli, F.; Latruffe, N. Transport, stability, and biological activity of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Tiku, A.R. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 845–868. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant. Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Bizuneh, G.K. The chemical diversity and biological activities of phytoalexins. Adv. Tradit. Med. 2021, 21, 31–43. [Google Scholar] [CrossRef]
- Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, biosynthesis and pharmacology of viniferin: Potential resveratrol-derived molecules for new drug discovery, development and therapy. Molecules 2022, 27, 5072. [Google Scholar] [CrossRef]
- Pezet, R.; Perret, C.; Jean-Denis, J.B.; Tabacchi, R.; Gindro, K.; Viret, O. δ-Viniferin, a resveratrol dehydrodimer: One of the major stilbenes synthesized by stressed grapevine leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. J. Chem. Soc. Japan (Nippon Kwagaku Kwaishi) 1939, 60, 1090–1100. (In Japanese) [Google Scholar]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Kupe, M.; Karatas, N.; Unal, M.S.; Ercisli, S.; Baron, M.; Sochor, J. Nutraceutical and functional properties of peel, pulp, and seed extracts of six ‘Köhnü’ grape clones. Horticulturae 2021, 7, 346. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and its effects on the vascular system. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and its human metabolites—Effects on metabolic health and obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Bejenaru, L.E.; Biţă, A.; Belu, I.; Segneanu, A.-E.; Radu, A.; Dumitru, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bejenaru, C. Resveratrol: A review on the biological activity and applications. Appl. Sci. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Yim, N.H.; Ha, D.T.; Trung, T.N.; Kim, J.P.; Lee, S.M.; Na, M.K.; Jung, H.J.; Kim, H.S.; Kim, Y.H.; Bae, K.H. The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 1165–1168. [Google Scholar] [CrossRef]
- Seo, H.; Kim, M.; Kim, S.; Al Mahmud, H.; Islam, M.I.; Nam, K.W.; Cho, M.L.; Kwon, H.S.; Song, H.Y. In vitro activity of alpha-viniferin isolated from the roots of Carex humilis against Mycobacterium tuberculosis. Pulm. Pharmacol. Ther. 2017, 46, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sy, B.; Krisa, S.; Richard, T.; Courtois, A. Resveratrol, ε-viniferin, and vitisin B from vine: Comparison of their in vitro antioxidant activities and study of their interactions. Molecules 2023, 28, 7521. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Lin, Y.-S.; Sie, Y.-Y.; Wang, C.-C.; Chang, C.-I.; Hou, W.-C. Vitisin B, a resveratrol tetramer from Vitis thunbergii var. taiwaniana, ameliorates impaired glucose regulations in nicotinamide/treptozotocin-induced type 2 diabetic mice. J. Tradit. Complement. Med. 2023, 13, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Milton-Laskibar, I.; Eseberri, I.; Beaumont, P.; Courtois, A.; Krisa, S.; Portillo, M.P. Beneficial effects of ε-viniferin on obesity and related health alterations. Nutrients 2023, 15, 928. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Yao, X.; Wu, Q.; Wei, M.; Yan, Z. ε-Viniferin, a promising natural oligostilbene, ameliorates hyperglycemia and hyperlipidemia by activating AMPK in vivo. Food Funct. 2020, 11, 10084–10093. [Google Scholar] [CrossRef]
- Buffeteau, G.; Hornedo-Ortega, R.; Gabaston, J.; Daugey, N.; Palos-Pinto, A.; Thienpont, A.; Brotin, T.; Mérillon, J.-M.; Buffeteau, T.; Waffo-Teguo, P. Chiroptical and potential in vitro anti-inflammatory properties of viniferin stereoisomers from grapevine (Vitis vinifera L.). Food Chem. 2022, 393, 133359. [Google Scholar] [CrossRef]
- Pislyagin, E.A.; Tarbeeva, D.V.; Yurchenko, E.A.; Menchinskaya, E.S.; Gorpenchenko, T.Y.; Pokhilo, N.D.; Kalinovskiy, A.I.; Aminin, D.L.; Fedoreyev, S.A. Neuroprotective activity of oligomeric stilbenes from alpha grape stems in in vitro models of Parkinson’s disease. Int. J. Mol. Sci. 2025, 26, 2411. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Lin, Y.-K.; Yang, S.-C.; Alalaiwe, A.; Lin, C.-J.; Fang, J.-Y.; Lin, C.-F. Percutaneous absorption of resveratrol and its oligomers to relieve psoriasiform lesions: In silico, in vitro and in vivo evaluations. Int. J. Pharm. 2020, 585, 119507. [Google Scholar] [CrossRef]
- Lulan, T.Y.K.; Fatmawati, S.; Santoso, M.; Ersam, T. α-Viniferin as a potential antidiabetic and antiplasmodial extracted from Dipterocarpus littoralis. Heliyon 2020, 6, e04102. [Google Scholar] [CrossRef]
- Zwygart, A.C.-A.; Medaglia, C.; Huber, R.; Poli, R.; Marcourt, L.; Schnee, S.; Michellod, E.; Mazel-Sanchez, B.; Constant, S.; Huang, S.; et al. Antiviral properties of trans-δ-viniferin derivatives against enveloped viruses. Biomed. Pharmacother. 2023, 163, 114825. [Google Scholar] [CrossRef] [PubMed]
- Taillis, D.; Becissa, O.; Pébarthé-Courrouilh, A.; Renouf, E.; Palos-Pinto, A.; Richard, T.; Cluzet, S. Antifungal activities of a grapevine byproduct extract enriched in complex stilbenes and stilbenes metabolization by Botrytis cinerea. J. Agric. Food Chem. 2023, 71, 4488–4497. [Google Scholar] [CrossRef]
- Huang, C.; Lin, Z.-Y.; Lee, C.-J.; Lai, W.-H.; Chen, J.-C.; Huang, H.-C. ε-Viniferin and α-viniferin alone or in combination induced apoptosis and necrosis in osteosarcoma and non-small cell lung cancer cells. Food Chem. Toxicol. 2021, 158, 112617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dietrich, J. Privileged scaffolds in lead generation. Expert Opin. Drug Discov. 2015, 10, 781–790. [Google Scholar] [CrossRef]
- Jayashree, B.S.; Nikhil, P.S.; Paul, S. Bioisosterism in drug discovery and development—An overview. Med. Chem. 2022, 18, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Applications of bioisosteres in the design of biologically active compounds. J. Agric. Food. Chem. 2023, 71, 18087–18122. [Google Scholar] [CrossRef]
- Masand, V.H.; Patil, M.K.; Al-Hussain, S.A.; Samad, A.; Rastija, V.; Jawarkar, R.D.; Masand, G.S.; Gawali, R.G.; Zaki, M.E.A. Analyzing oxygen atom distribution in FDA-approved drugs to enhance drug discovery strategies. Chem. Biol. Drug Des. 2025, 105, e70060. [Google Scholar] [CrossRef]
- McVicker, R.U.; O’Boyle, N.M. Chirality of new drug approvals (2013–2022): Trends and perspectives. J. Med. Chem. 2024, 67, 2305–2320. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, L. The clinical application of fruquintinib on colorectal cancer. Expert Rev. Clin. Pharmacol. 2019, 12, 713–721. [Google Scholar] [CrossRef]
- Rusu, A.; Moga, I.-M.; Uncu, L.; Hancu, G. The role of five-membered heterocycles in the molecular structure of antibacterial drugs used in therapy. Pharmaceutics 2023, 15, 2554. [Google Scholar] [CrossRef]
- Mason, J.W. Amiodarone. N. Engl. J. Med. 1987, 316, 455–466. [Google Scholar] [CrossRef]
- Laughren, T.P.; Gobburu, J.; Temple, R.J.; Unger, E.F.; Bhattaram, A.; Dinh, P.V.; Fossom, L.; Hung, H.M.J.; Klimek, V.; Lee, J.E.; et al. Vilazodone: Clinical basis for the US Food and Drug Administration’s approval of a new antidepressant. J. Clin. Psychiatry 2011, 72, 1166–1173. [Google Scholar] [CrossRef]
- Actuate Receives FDA Orphan Drug Designation for Elraglusib for Treatment of Soft Tissue Sarcomas. News Release. Actuate Therapeutics, Inc. 11 September 2024. Available online: https://tinyurl.com/ns69enfa (accessed on 28 April 2025).
- Dhillon, S.; Clarke, M. Tasimelteon: First global approval. Drugs 2014, 74, 505–511. [Google Scholar] [CrossRef]
- Borja, N.L.; Daniel, K.L. Ramelteon for the treatment of insomnia. Clin. Ther. 2006, 28, 1540–1555. [Google Scholar] [CrossRef]
- Bassotti, G.; Usai Satta, P.; Bellini, M. Prucalopride for the treatment of constipation: A view from 2015 and beyond. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 257–262. [Google Scholar] [CrossRef]
- Kershen, R.T.; Hsieh, M. Preview of new drugs for overactive bladder and incontinence: Darifenacin, solifenacin, trospium, and duloxetine. Curr. Urol. Rep. 2004, 5, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kirino, E. Escitalopram for the management of major depressive disorder: A review of its efficacy, safety, and patient acceptability. Patient Prefer. Adherence 2012, 6, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Ha, S.-C.; Park, S.-W. Inhibition of tyrosinase and lipoxygenase activities by resveratrol and its derivatives from seeds of Paeonia lactiflora. Prev. Nutr. Food Sci. 2002, 7, 447–450. [Google Scholar] [CrossRef]
- Mikulski, D.; Molski, M. Quantitative structure–antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-beta-D-glucopyranoside. Eur. J. Med. Chem. 2010, 45, 2366–2380. [Google Scholar] [CrossRef]
- Pébarthé-Courrouilh, A.; Jaa, A.; Valls-Fonayet, J.; Da Costa, G.; Palos-Pinto, A.; Richard, T.; Cluzet, S. UV-exposure decreases antimicrobial activities of a grapevine cane extract against Plasmopara viticola and Botrytis cinerea as a consequence of stilbene modifications—A kinetic study. Pest Manag. Sci. 2024, 80, 6389–6399. [Google Scholar] [CrossRef]
- Guengerich, F.P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001, 14, 611–650. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Desai, U.R. Chemical sulfation of small molecules—Advances and challenges. Tetrahedron 2010, 66, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Makris, T.M.; Sligar, S.G.; Schlichting, I. Structure and chemistry of cytochrome P450. Chem. Rev. 2005, 105, 2253–2277. [Google Scholar] [CrossRef]
- Glue, P.; Clement, R.P. Cytochrome P450 enzymes and drug metabolism—Basic concepts and methods of assessment. Cell. Mol. Neurobiol. 1999, 19, 309–323. [Google Scholar] [CrossRef]
- Guengerich, F.P. Inhibition of cytochrome P450 enzymes by drugs—Molecular basis and practical applications. Biomol. Ther. 2022, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dickins, M. Induction of cytochromes P450. Curr. Top. Med. Chem. 2004, 4, 1745–1766. [Google Scholar] [CrossRef]
- Guengerich, F.P. A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol. Res. 2021, 37, 1–23. [Google Scholar] [CrossRef]
- Beaumont, P.; Amintas, S.; Krisa, S.; Courtois, A.; Richard, T.; Eseberri, I.; Portillo, M.P. Glucuronide metabolites of trans-ε-viniferin decrease triglycerides accumulation in an in vitro model of hepatic steatosis. J. Physiol. Biochem. 2024, 80, 685–696. [Google Scholar] [CrossRef]
- Courtois, A.; Jourdes, M.; Dupin, A.; Lapèze, C.; Renouf, E.; Biais, B.; Teissedre, P.-L.; Mérillon, J.-M.; Richard, T.; Krisa, S. In vitro glucuronidation and sulfation of ε-viniferin, a resveratrol dimer, in humans and rats. Molecules 2017, 22, 733. [Google Scholar] [CrossRef]
- Courtois, A.; Atgié, C.; Marchal, A.; Hornedo-Ortega, R.; Lapèze, C.; Faure, C.; Richard, T.; Krisa, S. Tissular distribution and metabolism of trans-ε-viniferin after intraperitoneal injection in rat. Nutrients 2018, 10, 1660. [Google Scholar] [CrossRef]
- Biala, G.; Kedzierska, E.; Kruk-Slomka, M.; Orzelska-Gorka, J.; Hmaidan, S.; Skrok, A.; Kaminski, J.; Havrankova, E.; Nadaska, D.; Malik, I. Research in the field of drug design and development. Pharmaceuticals 2023, 16, 1283. [Google Scholar] [CrossRef]
- Chen, Y.; Kirchmair, J. Cheminformatics in natural product-based drug discovery. Mol. Inform. 2020, 39, e2000171. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M. Towards a universal SMILES representation—A standard method to generate canonical SMILES based on the InChI. J. Cheminform. 2012, 4, 22. [Google Scholar] [CrossRef]
- Ahire, E.D.; Sonawane, V.N.; Surana, K.R.; Talele, S.G. Drug discovery, drug-likeness screening, and bioavailability: Development of drug-likeness rule for natural products. In Applied Pharmaceutical Practice and Nutraceuticals. Natural Product Development, 1st ed.; Mahapatra, D.K., Aguilar, C.N., Haghi, A.K., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2021; pp. 191–208. [Google Scholar]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gangwal, A.; Lavecchia, A. Artificial intelligence in natural product drug discovery: Current applications and future perspectives. J. Med. Chem. 2025, 68, 3948–3969. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Roggo, S.; Schuffenhauer, A. Natural product-likeness score and its application for prioritization of compound libraries. J. Chem. Inf. Model. 2008, 48, 68–74. [Google Scholar] [CrossRef]
- Camp, D.; Garavelas, A.; Campitelli, M. Analysis of physicochemical properties for drugs of natural origin. J. Nat. Prod. 2015, 78, 1370–1382. [Google Scholar] [CrossRef]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Wei, W.; Cherukupalli, S.; Jing, L.; Liu, X.; Zhan, P. Fsp3: A new parameter for drug-likeness. Drug Discov. Today 2020, 25, 1839–1845. [Google Scholar] [CrossRef]
- Jia, C.-Y.; Li, J.-Y.; Hao, G.-F.; Yang, G.-F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. Today 2020, 25, 248–258. [Google Scholar] [CrossRef]
- Pereda-Miranda, R.; Bautista, E.; Martínez-Fructuoso, L. From relative to absolute stereochemistry of secondary metabolites: Applications in plant chemistry. Rev. Bras. Farmacogn. 2023, 33, 1–48. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. A comparison between two polarizability parameters in chemical–biological interactions. Bioorg. Med. Chem. 2005, 13, 2355–2372. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zygadlo, J.A.; Rubinstein, H.R. Antifumonisin activity of natural phenolic compounds: A structure–property–activity relationship study. Int. J. Food. Microbiol. 2011, 145, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Q.; Wilkinson, B. Drug discovery beyond the ‘rule-of-five’. Curr. Opin. Biotechnol. 2007, 18, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xue, X. Medicinal chemistry strategies for the modification of bioactive natural products. Molecules 2024, 29, 689. [Google Scholar] [CrossRef]
- She, S.-M.; Appendino, M.; Guo, Y.-W. Pitfalls in the structural elucidation of small molecules. A critical analysis of a decade of structural misassignments of marine natural products. Nat. Prod. Rep. 2022, 39, 1803–1832. [Google Scholar] [CrossRef]
- Larsen, E.M.; Wilson, M.R.; Taylor, R.E. Conformation–activity relationships of polyketide natural products. Nat. Prod. Rep. 2015, 32, 1183–1206. [Google Scholar] [CrossRef]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More than resveratrol: New insights into stilbene-based compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Nayik, G.A.; Gupta, D.; Areche, F.O.; Jagdale, Y.D.; Ansari, M.J.; Hemeg, H.A.; Al-Farga, Z.A.; Alotaibi, S.S. Chemical aspects of polyphenol–protein interactions and their antibacterial activity. Crit. Rev. Food Sci. Nutr. 2023, 63, 9482–9505. [Google Scholar] [CrossRef]
- Nandhini, P.; Gupta, P.K.; Mahapatra, A.K.; Das, A.P.; Agarwal, S.M.; Mickymaray, S.; Alothaim, A.S.; Rajan, M. In-silico molecular screening of natural compounds as a potential therapeutic inhibitor for methicillin-resistant Staphylococcus aureus inhibition. Chem. Biol. Interact. 2023, 374, 110383. [Google Scholar] [CrossRef]
- Ngoc, T.D.; Le, T.N.; Nguyen, T.V.A.; Mechler, A.; Hoa, N.T.; Nam, N.L.; Vo, Q.V. Mechanistic and kinetic studies of the radical scavenging activity of 5-O-methylnorbergenin: Theoretical and experimental insights. J. Phys. Chem. B 2022, 126, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Kempińska, D.; Chmiel, T.; Kot-Wasik, A.; Mróz, A.; Mazerska, Z.; Namieśnik, J. State of the art and prospects of methods for determination of lipophilicity of chemical compounds. Trac.-Trends Anal. Chem. 2019, 113, 54–73. [Google Scholar] [CrossRef]
- Bębenek, E.; Rzepka, Z.; Hermanowicz, J.M.; Chrobak, E.; Surażyński, A.; Beberok, A.; Wrześniok, D. Synthesis, pharmacokinetic profile, anticancer activity and toxicity of the new amides of betulonic acid—In silico and in vitro study. Int. J. Mol. Sci. 2024, 25, 4517. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.G.; Chinchilla, N.; Molinillo, J.M.; Macías, F.A. Influence of lipophilicity in O-acyl and O-alkyl derivatives of juglone and lawsone: A structure–activity relationship study in the search for natural herbicide models. Pest. Manag. Sci. 2018, 74, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to Gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple method of calculating octanol/water partition coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Fiese, E.F.; Korst, R.J. pKa, log P and MedChem CLOGP fragment values of acidic heterocyclic potential bioisosteres. QSAR 1991, 10, 109–117. [Google Scholar] [CrossRef]
- Wildman, S.A.; Crippen, G.M. Prediction of physicochemical parameters by atomic contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Tetko, I.V.; Tanchuk, V.Y. Application of associative neural networks for prediction of lipophilicity in ALOGPS 2.1 program. J. Chem. Inf. Comput. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, Y.; Li, Y.; Wang, R.; Lai, L. Computation of octanol–water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics. Available online: https://www.molinspiration.com/about.html (accessed on 15 May 2025).
- Wang, Y.; Xiong, J.; Xiao, F.; Zhang, W.; Cheng, K.; Rao, J.; Niu, B.; Tong, X.; Qu, N.; Zhang, R.; et al. LogD7.4 prediction enhanced by transferring knowledge from chromatographic retention time, microscopic pKa and logP. J. Cheminform. 2023, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.; Doerksen, R.J. Topological polar surface area: A useful descriptor in 2D-QSAR. Curr. Med. Chem. 2009, 16, 21–41. [Google Scholar] [CrossRef]
- Maraf, M.B.; Mountessou, B.Y.G.; Merlin, T.F.H.; Ariane, P.; Fekoua, J.N.N.; Yves, T.B.J.; Raoul, T.T.D.; Zintchem, A.A.A.; Bebga, G.; Mbouombouo, N.I.; et al. Virtual screening, MMGBSA, and molecular dynamics approaches for identification of natural products from South African biodiversity as potential Onchocerca volvulus pi-class glutathione S-transferase inhibitors. Heliyon 2024, 10, e29560. [Google Scholar] [CrossRef]
- Ansari, W.A.; Khan, M.A.; Rizvi, F.; Ali, K.; Hussain, M.K.; Saquib, M.; Khan, M.F. Computational screening of plant-derived natural products against SARS-CoV-2 variants. Future Pharmacol. 2022, 2, 558–578. [Google Scholar] [CrossRef]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of intestinal absorption. J. Pharm. Sci. 1999, 88, 807–814. [Google Scholar] [CrossRef]
- Kelder, J.; Grootenhuis, P.D.J.; Bayada, D.M.; Delbressine, L.P.C.; Ploemen, J.-P. Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm. Res. 1999, 16, 1514–1519. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Charifson, P.S.; Walters, W.P. Acidic and basic drugs in medicinal chemistry: A perspective. J. Med. Chem. 2014, 57, 9701–9717. [Google Scholar] [CrossRef]
- Santibáñez-Morán, M.G.; Medina-Franco, J.L. Analysis of the acid/base profile of natural products from different sources. Mol. Inform. 2020, 39, e1900099. [Google Scholar] [CrossRef]
- Manallack, D.T.; Prankerd, R.J.; Yuriev, E.; Oprea, T.I.; Chalmers, D.K. The significance of acid/base properties in drug discovery. Chem. Soc. Rev. 2013, 42, 485–496. [Google Scholar] [CrossRef]
- Santibáñez-Morán, M.G.; Medina-Franco, J.L. The acid/base characterization of molecules with epigenetic activity. ChemMedChem. 2021, 16, 1744–1753. [Google Scholar] [CrossRef]
- Settimo, L.; Bellman, K.; Knegtel, R.M.A. Comparison of the accuracy of experimental and predicted pKa values of basic and acidic compounds. Pharm. Res. 2014, 31, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, K.; He, D.; Gu, J.; Xu, J.; Xie, J.; Zhang, M.; Liu, Y.; Tan, Q.; Zhang, J. Toward a better understanding of metabolic and pharmacokinetic characteristics of low-solubility, low-permeability natural medicines. Drug Metab. Rev. 2020, 52, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.S. ESOL: Estimating aqueous solubility directly from molecular structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. Revisiting the general solubility equation: In silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J. Chem. Inf. Model. 2012, 52, 420–428. [Google Scholar] [CrossRef]
- Di Stefano, M.; Galati, S.; Lonzi, C.; Granchi, C.; Poli, G.; Tuccinardi, T.; Macchia, M. WaSPred: A reliable AI-based water solubility predictor for small molecules. Int. J. Pharm. 2024, 666, 124817. [Google Scholar] [CrossRef]
- Lipinski, C.A. Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 2016, 101, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Wobst, H.J. A decade of FDA-approved drugs (2010–2019): Trends and future directions. J. Med. Chem. 2021, 64, 2312–2338. [Google Scholar] [CrossRef] [PubMed]
- Pirie, R.; Stanway-Gordon, H.A.; Stewart, H.L.; Wilson, K.L.; Patton, S.P.; Tyerman, J.T.; Cole, D.J.; Fowler, K.; Waring, M.J. An analysis of the physicochemical properties of oral drugs from 2000 to 2022. RSC Med. Chem. 2024, 15, 3125–3132. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Oprea, T.I. Property distribution of drug-related chemical databases. J. Comput. Aided Mol. Des. 2000, 14, 251–264. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J. Prediction of hydrophobic properties of small organic molecules using fragmental methods: An analysis of ALOGP and CLOGP methods. J. Phys. Chem. A 1998, 102, 3762–3772. [Google Scholar] [CrossRef]
- Ghose, A.K.; Crippen, G.M. Atomic physicochemical parameters for three-dimensional-structure-directed quantitative structure–activity relationships. 2. Modeling dispersive and hydrophobic interactions. J. Chem. Inf. Comput. Sci. 1987, 27, 21–35. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C.; Selassie, C.D. Comparative QSAR studies on PAMPA/modified PAMPA for high throughput profiling of drug absorption potential with respect to Caco-2 cells and human intestinal absorption. J. Comput. Aided Mol. Des. 2007, 21, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Juvale, I.I.A.; Hamid, A.A.A.; Halim, K.B.A.; Has, A.T.C. P-glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Al-Abd, A.M.; El-Dine, R.S.; El-Halawany, A.M. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review. J. Adv. Res. 2015, 6, 45–62. [Google Scholar] [CrossRef]
- Kratochwil, N.A.; Huber, W.; Müller, F.; Kansy, M.; Gerber, P.R. Predicting plasma protein binding of drugs: A new approach. Biochem. Pharmacol. 2002, 64, 1355–1374. [Google Scholar] [CrossRef]
- Bohnert, T.; Gan, L.-S. Plasma protein binding: From discovery to development. J. Pharm. Sci. 2013, 102, 2953–2994. [Google Scholar] [CrossRef]
- Lambrinidis, G.; Vallianatou, T.; Tsantili-Kakoulidou, A. In vitro, in silico and integrated strategies for the estimation of plasma protein binding. A review. Adv. Drug. Deliv. Rev. 2015, 86, 27–45. [Google Scholar] [CrossRef]
- Gleeson, M.P. Plasma protein binding affinity and its relationship to molecular structure: An in-silico analysis. J. Med. Chem. 2007, 50, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wright, M.; Hop, C.E.C.A. Rational use of plasma protein and tissue binding data in drug design. J. Med. Chem. 2014, 57, 8238–8248. [Google Scholar] [CrossRef]
- Koutroumpa, N.M.; Tsoumanis, A.; Sarimveis, H.; Lynch, I.; Melagraki, G.; Afantitis, A. Prediction of blood–brain barrier and Caco-2 permeability through the Enalos Cloud Platform: Combining contrastive learning and atom-attention message passing neural networks. J. Cheminform. 2025, 17, 68. [Google Scholar] [CrossRef]
- Shaker, B.; Yu, M.-S.; Song, J.S.; Ahn, S.; Ryu, J.Y.; Oh, K.-S.; Na, D. LightBBB: Computational prediction model of blood–brain-barrier penetration based on LightGBM. Bioinformatics 2021, 37, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Zhang, D.; Liu, J.; Wu, Q.; Chen, J.; Tan, P.; Xing, B.; Han, Y.; Zhang, P.; et al. Mechanism of drug-induced liver injury and hepatoprotective effects of natural drugs. Chin. Med. 2021, 16, 135. [Google Scholar] [CrossRef]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Z.; Liu, Z.; Tao, Y.; Sha, C.; He, M.; Li, X. DrugMetric: Quantitative drug-likeness scoring based on chemical space distance. Brief. Bioinform. 2024, 25, bbae321. [Google Scholar] [CrossRef] [PubMed]
- Ivanenkov, Y.A.; Zagribelnyy, B.A.; Aladinskiy, V.A. Are we opening the door to a new era of medicinal chemistry or being collapsed to a chemical singularity? J. Med. Chem. 2019, 62, 10026–10043. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Rossella, F.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef]
- Sahidin, I.; Wahyuni, W.; Malaka, M.H.; Imran, I. Antibacterial and cytotoxic potencies of stilbene oligomers from stem barks of baoti (Dryobalanops lanceolata) growing in Kendari, Indonesia. Asian J. Pharm. Clin. Res. 2017, 10, 139–143. [Google Scholar] [CrossRef]
- Li, H.; Yin, W.; Liu, P.; Liu, H. Oligostilbenoids investigation of structure–activity relationship with chemical biology information. In Proceedings of the 2015 Seventh International Conference on Measuring Technology and Mechatronics Automation (ICMTMA), Nanchang, China, 13–14 June 2015; Yang, Z., Ed.; Conference Publishing Services: Los Alamitos, CA, USA, 2015; pp. 716–718. [Google Scholar] [CrossRef]
- Mattio, L.M.; Pinna, C.; Catinella, G.; Musso, L.; Pedersen, K.J.; Krogfelt, K.A.; Dallavalle, S.; Pinto, A. Synthesis and antimicrobial activity of δ-viniferin analogues and isosteres. Molecules 2021, 26, 7594. [Google Scholar] [CrossRef]
- Huber, R.; Marcourt, L.; Héritier, M.; Luscher, A.; Guebey, L.; Schnee, S.; Michellod, E.; Guerrier, S.; Wolfender, J.-L.; Scapozza, L.; et al. Generation of potent antibacterial compounds through enzymatic and chemical modifications of the trans-δ-viniferin scaffold. Sci. Rep. 2023, 13, 15986. [Google Scholar] [CrossRef]
- Fang, W.-Y.; Ravindar, L.; Rakesh, K.P.; Manukumar, H.M.; Shantharam, C.S.; Alharbi, N.S.; Qin, H.-L. Synthetic approaches and pharmaceutical applications of chloro-containing molecules for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 173, 117–153. [Google Scholar] [CrossRef]
- Tiz, D.B.; Bagnoli, L.; Rosati, O.; Marini, F.; Sancineto, L.; Santi, C. New halogen-containing drugs approved by FDA in 2021: An overview on their syntheses and pharmaceutical use. Molecules 2022, 27, 1643. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Basri, D.F.; Xian, L.W.; Shukor, N.I.A.; Latip, J. Bacteriostatic antimicrobial combination: Antagonistic interaction between epsilon-viniferin and vancomycin against methicillin-resistant Staphylococcus aureus. Biomed. Res. Int. 2014, 2014, 461756. [Google Scholar] [CrossRef] [PubMed]
- Gebrehiwot, H.; Melaku, Y.; Aliye, M.; Ensermu, U.; Dekebo, A.; Endale, M.; Rentsch, D.; Hunsen, M. Antibacterial and antioxidant efficacies of secondary metabolites from the roots of Cyphostemma adenocaule: A combined in vitro and in silico study. J. Trop. Med. 2024, 2024, 1679695. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Mailar, K.; Masagalli, J.N.; Chae, S.-W.; Song, J.-J.; Choi, W.J. Ruthenium chloride-induced oxidative cyclization of trans-resveratrol to (±)-ε-viniferin and antimicrobial and antibiofilm activity against Streptococcus pneumoniae. Front. Pharmacol. 2019, 10, 890. [Google Scholar] [CrossRef]
- Catinella, G.; Mattio, L.M.; Musso, L.; Arioli, S.; Mora, D.; Beretta, G.L.; Zaffaroni, N.; Pinto, A.; Dallavalle, S. Structural requirements of benzofuran derivatives dehydro-δ- and dehydro-ε-viniferin for antimicrobial activity against the foodborne pathogen Listeria monocytogenes. Int. J. Mol. Sci. 2020, 21, 2168. [Google Scholar] [CrossRef]
- Princiotto, S.; Pinna, C.; Mattio, L.M.; Annunziata, F.; Beretta, G.L.; Pinto, A.; Dallavalle, S. Cytotoxicity of benzofuran-containing simplified viniferin analogues. Pharmaceuticals 2024, 17, 1012. [Google Scholar] [CrossRef]
- Hatem, O.; Steinbach, A.; Schneider, G.; Röckel, F.; Kőrösi, L. Wild Vitis species as stilbenes sources: Cane extracts and their antibacterial activity against Listeria monocytogenes. Molecules 2024, 29, 3518. [Google Scholar] [CrossRef]
- Sundin, C.; Zetterström, C.E.; Vo, D.D.; Brkljača, R.; Urban, S.; Elofsson, M. Exploring resveratrol dimers as virulence blocking agents—Attenuation of type III secretion in Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Sci. Rep. 2020, 10, 2103. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, J.H.; Ryu, S.Y.; Joo, S.W.; Cho, M.H.; Lee, J. Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 biofilm formation by plant metabolite ε-viniferin. J. Agric. Food. Chem. 2013, 61, 7120–7126. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.-H.; Ryu, S.Y.; Cho, M.H.; Lee, J. Stilbenes reduce Staphylococcus aureus hemolysis, biofilm formation, and virulence. Foodborne Pathog. Dis. 2014, 11, 710–717. [Google Scholar] [CrossRef]
- Gouaux, E. α-Hemolysin from Staphylococcus aureus: An archetype of β-barrel, channel-forming toxins. J. Struct. Biol. 1998, 121, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Li, C.; Zhang, J.Z.H.; Ji, C. MolGpka: A web server for small molecule pKa prediction using a graph-convolutional neural network. J. Chem. Inf. Model. 2021, 61, 3159–3165. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Arkin, M.; Wells, J. Small-molecule inhibitors of protein–protein interactions: Progressing towards the dream. Nat. Rev. Drug Discov. 2004, 3, 301–317. [Google Scholar] [CrossRef]
- Li, Y.; Gu, C.; Gruenhagen, J.; Yehl, P.; Chetwyn, N.P.; Medley, C.D. An enzymatic deconjugation method for the analysis of small molecule active drugs on antibody–drug conjugates. MAbs 2016, 8, 698–705. [Google Scholar] [CrossRef]
- Macielag, M.J. Chemical properties of antimicrobials and their uniqueness. In Antibiotic Discovery and Development; Dougherty, T., Pucci, M., Eds.; Springer Science: Boston, MA, USA, 2021; pp. 793–820. [Google Scholar] [CrossRef]
- Waring, M.J. Defining optimum lipophilicity and molecular weight ranges for drug candidates—Molecular weight dependent lower logD limits based on permeability. Bioorg. Med. Chem. Lett. 2009, 19, 2844–2851. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.J.; Macdonald, S.J.F.; Peace, S.; Pickett, S.D.; Luscombe, C.N. Increasing small molecule drug developability in sub-optimal chemical space. Med. Chem. Commun. 2013, 4, 673–680. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Shultz, M.D. Two decades under the influence of the rule of five and the changing properties of approved oral drugs. J. Med. Chem. 2019, 62, 1701–1714. [Google Scholar] [CrossRef]
- Araya-Cloutier, C.; Vincken, J.-P.; van de Schans, M.G.M.; Hageman, J.; Schaftenaar, G.; den Besten, H.M.W.; Gruppen, H. QSAR-based molecular signatures of prenylated (iso)flavonoids underlying antimicrobial potency against and membrane-disruption in Gram positive and Gram negative bacteria. Sci. Rep. 2018, 8, 9267. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Badura, A.; Krysiński, J.; Nowaczyk, A.; Buciński, A. Prediction of the antimicrobial activity of quaternary ammonium salts against Staphylococcus aureus using artificial neural networks. Arab. J. Chem. 2021, 14, 103233. [Google Scholar] [CrossRef]
- Cherdtrakulkiat, R.; Worachartcheewan, A.; Tantimavanich, S.; Lawung, R.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Discovery of novel halogenated 8-hydroxyquinoline-based anti-MRSA agents: In vitro and QSAR studies. Drug Dev. Res. 2020, 81, 127–135. [Google Scholar] [CrossRef]
- Ramos-Nino, M.E.; Clifford, M.N.; Adams, M.R. Quantitative structure activity relationship for the effect of benzoic acids, cinnamic acids and benzaldehydes on Listeria monocytogenes. J. Appl. Bacteriol. 1996, 80, 303–310. [Google Scholar] [CrossRef]
- Rijo, P.; Duarte, A.; Francisco, A.P.; Semedo-Lemsaddek, T.; Simões, M.F. In vitro antimicrobial activity of royleanone derivatives against Gram-positive bacterial pathogens. Phytother. Res. 2014, 28, 76–81. [Google Scholar] [CrossRef]
- Winiwarter, S.; Bonham, N.M.; Ax, F.; Hallberg, A.; Lennernäs, H.; Karlén, A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J. Med. Chem. 1998, 41, 4939–4949. [Google Scholar] [CrossRef]
- Bryant, M.J.; Black, S.N.; Blade, H.; Docherty, R.; Maloney, A.G.P.; Taylor, S.C. The CSD drug subset: The changing chemistry and crystallography of small molecule pharmaceuticals. J. Pharm. Sci. 2019, 108, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Zhydzetski, A.; Głowacka-Grzyb, Z.; Bukowski, M.; Żądło, T.; Bonar, E.; Władyka, B. Agents targeting the bacterial cell wall as tools to combat Gram-positive pathogens. Molecules 2024, 29, 4065. [Google Scholar] [CrossRef]
- Bitew, M.; Desalegn, T.; Demissie, T.B.; Belayneh, A.; Endale, M.; Eswaramoorthy, R. Pharmacokinetics and drug-likeness of antidiabetic flavonoids: Molecular docking and DFT study. PLoS ONE 2021, 16, e0260853. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.-J.; Fu, L.; Zhang, X.-C.; Long, T.-Z.; He, Y.-H.; Liu, Z.-Q.; Lu, A.-P.; Deng, Y.-F.; Hsieh, C.-Y.; Hou, T.-J.; et al. Improved GNNs for log D7.4 prediction by transferring knowledge from low-fidelity data. J. Chem. Inf. Model. 2023, 63, 2345–2359. [Google Scholar] [CrossRef]
- Win, Z.-M.; Cheong, A.M.Y.; Hopkins, W.S. Using machine learning to predict partition coefficient (log P) and distribution coefficient (log D) with molecular descriptors and liquid chromatography retention time. J. Chem. Inf. Model. 2023, 63, 1906–1913. [Google Scholar] [CrossRef]
- Tinworth, C.P.; Young, R.J. Facts, patterns, and principles in drug discovery: Appraising the Rule of 5 with measured physicochemical data. J. Med. Chem. 2020, 63, 10091–10108. [Google Scholar] [CrossRef] [PubMed]
- Ermondi, G.; Vallaro, M.; Goetz, G.; Shalaeva, M.; Caron, G. Experimental lipophilicity for beyond Rule of 5 compounds. Future Drug Discov. 2019, 1, FDD10. [Google Scholar] [CrossRef]
- Bhal, S.K.; Kassam, K.; Peirson, I.G.; Pearl, G.M. The Rule of Five revisited: Applying log D in place of log P in drug-likeness filters. Mol. Pharm. 2007, 4, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef]
- Courtois, A.; Garcia, M.; Krisa, S.; Atgié, C.; Sauvant, P.; Richard, T.; Faure, C. Encapsulation of viniferin in onion-type multi-lamellar liposomes increases its solubility, its photo-stability and decreases its cytotoxicity on Caco-2 intestinal cells. Food Funct. 2019, 10, 2573–2582. [Google Scholar] [CrossRef]
- Brezani, V.; Blondeau, N.; Kotouček, J.; Klásková, E.; Šmejkal, K.; Hošek, J.; Mašková, E.; Kulich, P.; Prachyawarakorn, V.; Heurteaux, C.; et al. Enhancing solubility and bioefficacy of stilbenes by liposomal encapsulation–the case of macasiamenene F. ACS Omega 2024, 9, 9027–9039. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Ottaviani, G.; Martel, S.; Carrupt, P.-A. Parallel artificial membrane permeability assay: A new membrane for the fast prediction of passive human skin permeability. J. Med. Chem. 2006, 49, 3948–3954. [Google Scholar] [CrossRef]
- Fenart, L.; Casanova, A.; Dehouck, B.; Duhem, C.; Slupek, S.; Cecchelli, R.; Betbeder, D. Evaluation of the effect of charge and lipid coating on ability of 60 nm nanoparticles to cross an in vitro model of the blood–brain barrier. J. Pharmacol. Exp. Ther. 1999, 291, 1017–1022. [Google Scholar] [CrossRef]
- Dahlgren, D.; Lennernäs, H. Intestinal permeability and drug absorption: Predictive experimental, computational and in vivo approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Guillard, J.; Richard, D.; Milin, S.; Chassaing, D.; Paccalin, M.; Page, G.; Rioux Bilan, A. Trans ε viniferin decreases amyloid deposits and inflammation in a mouse transgenic Alzheimer model. PLoS ONE 2019, 14, e0212663. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; De Feo, V.; Zia-Ul-Haq, M. Natural products as alternative choices for P-glycoprotein (P-gp) inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.B.; Kuo, C.L.; Lien, L.L.; Lien, E.J. Structure–activity relationship: Analyses of p-glycoprotein substrates and inhibitors. J. Clin. Pharm. Ther. 2003, 28, 203–228. [Google Scholar] [CrossRef]
- Gleeson, M.P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kanaoka, M. Computational prediction of the plasma protein-binding percent of diverse pharmaceutical compounds. J. Pharm. Sci. 2004, 93, 1480–1494. [Google Scholar] [CrossRef]
- Lobell, M.; Sivarajah, V. In silico prediction of aqueous solubility, human plasma protein binding and volume of distribution of compounds from calculated pKa and AlogP98 values. Mol. Divers. 2003, 7, 69–87. [Google Scholar] [CrossRef]
- Chang, G.W.; Kam, P.C. The physiological and pharmacological roles of cytochrome P450 isoenzymes. Anaesthesia 1999, 54, 42–50. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The central role of cytochrome P450 in xenobiotic metabolism—A brief review on a fascinating enzyme family. J. Xenobiot. 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Abu-Bakar, A.; Tan, B.H.; Halim, H.; Ramli, S.; Pan, Y.; Ong, C.E. Cytochromes P450: Role in carcinogenesis and relevance to cancers. Curr. Drug Metab. 2022, 23, 355–373. [Google Scholar] [CrossRef]
- He, X.; Feng, S. Role of metabolic enzymes P450 (CYP) on activating procarcinogen and their polymorphisms on the risk of cancers. Curr. Drug Metab. 2015, 16, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Zahno, A.; Brecht, K.; Morand, R.; Maseneni, S.; Török, M.; Lindinger, P.W.; Krähenbühl, S. The role of CYP3A4 in amiodarone-associated toxicity on HepG2 cells. Biochem. Pharmacol. 2011, 81, 432–441. [Google Scholar] [CrossRef]
- Boinpally, R.; Gad, N.; Gupta, S.; Periclou, A. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin. Ther. 2014, 36, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Owen, R. Vilazodone: A new treatment option for major depressive disorder. Drugs Today 2011, 47, 531–537. [Google Scholar] [CrossRef]
- Vachharajani, N.N.; Yeleswaram, K.; Boulton, D.W. Preclinical pharmacokinetics and metabolism of BMS-214778, a novel melatonin receptor agonist. J. Pharm. Sci. 2003, 92, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, B.W.; Torres, R.; Dressman, M.A.; Kramer, W.G.; Baroldi, P. Clinical assessment of drug–drug interactions of tasimelteon, a novel dual melatonin receptor agonist. J. Clin. Pharmacol. 2015, 55, 1004–1011. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, Z.; Tay-Sontheimer, J.; Levy, R.H.; Ragueneau-Majlessi, I. Risk of clinically relevant pharmacokinetic-based drug–drug interactions with drugs approved by the U.S. Food and Drug Administration between 2013 and 2016. Drug Metab. Dispos. 2018, 46, 835–845. [Google Scholar] [CrossRef]
- Skerjanec, A. The clinical pharmacokinetics of darifenacin. Clin. Pharmacokinet. 2006, 45, 325–350. [Google Scholar] [CrossRef]
- Zhou, S.; Chan, S.Y.; Goh, B.C.; Chan, E.; Duan, W.; Huang, M.; McLeod, H.L. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin. Pharmacokinet. 2005, 44, 279–304. [Google Scholar] [CrossRef]
- Dresser, G.K.; Spence, J.D.; Bailey, D.G. Pharmacokinetic–pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin. Pharmacokinet. 2000, 38, 41–57. [Google Scholar] [CrossRef]
- Piver, B.; Berthou, F.; Dreano, Y.; Lucas, D. Differential inhibition of human cytochrome P450 enzymes by epsilon-viniferin, the dimer of resveratrol: Comparison with resveratrol and polyphenols from alcoholized beverages. Life Sci. 2003, 73, 1199–1213. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Yang, L.-P.; Zhou, Z.-W.; Liu, Y.-H.; Chan, E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009, 11, 481–494. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Y.; Xiong, Y.; Wu, J.; Wang, M.; Sun, X.; Ding, X.; Yang, L.; Sun, X.; Ge, G. CYP1A inhibitors: Recent progress, current challenges, and future perspectives. Med. Res. Rev. 2024, 44, 169–234. [Google Scholar] [CrossRef]
- Rosemary, J.; Adithan, C. The pharmacogenetics of CYP2C9 and CYP2C19: Ethnic variation and clinical significance. Curr. Clin. Pharmacol. 2007, 2, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F.; Zhou, Z.-W.; Yang, L.-P.; Cai, J.-P. Substrates, inducers, inhibitors and structure–activity relationships of human cytochrome P450 2C9 and implications in drug development. Curr. Med. Chem. 2009, 16, 3480–3675. [Google Scholar] [CrossRef]
- Güner, O.F.; Bowen, J.P. Pharmacophore modeling for ADME. Curr. Top. Med. Chem. 2013, 13, 1327–1342. [Google Scholar] [CrossRef]
- Miners, J.O.; Birkett, D.J. Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol. 1998, 45, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, L.; Dahl, M.-L.; Dalén, P.; Al-Shurbaji, A. Molecular genetics of CYP2D6: Clinical relevance with focus on psychotropic drugs. Br. J. Clin. Pharmacol. 2002, 53, 111–122. [Google Scholar] [CrossRef]
- Smith, D.A.; Ackland, M.J.; Jones, B.C. Properties of cytochrome P450 isoenzymes and their substrates part 2: Properties of cytochrome P450 substrates. Drug Disc. Tech. 1997, 2, 479–486. [Google Scholar] [CrossRef]
- Zanger, U.M.; Klein, K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): Advances on polymorphisms, mechanisms, and clinical relevance. Front. Genet. 2013, 4, 24. [Google Scholar] [CrossRef]
- Baell, J.B.; Nissink, J.W.M. Seven year itch: Pan-assay interference compounds (PAINS) in 2017—Utility and limitations. ACS Chem. Biol. 2018, 13, 36–44. [Google Scholar] [CrossRef]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem. 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Teschke, R. Molecular idiosyncratic toxicology of drugs in the human liver compared with animals: Basic considerations. Int. J. Mol. Sci. 2023, 24, 6663. [Google Scholar] [CrossRef]
- Roth, R.A.; Kana, O.; Filipovic, D.; Ganey, P.E. Pharmacokinetic and toxicodynamic concepts in idiosyncratic, drug-induced liver injury. Expert Opin. Drug Metab. Toxicol. 2022, 18, 469–481. [Google Scholar] [CrossRef]
- Leeson, P.D. Impact of physicochemical properties on dose and hepatotoxicity of oral drugs. Chem. Res. Toxicol. 2018, 31, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Norman, B.H. Drug induced liver injury (DILI). Mechanisms and medicinal chemistry avoidance/mitigation strategies. J. Med. Chem. 2020, 63, 11397–11419. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Drug-induced renal failure: Update on new medications and unique mechanisms of nephrotoxicity. Am. J. Med. Sci. 2003, 325, 349–362. [Google Scholar] [CrossRef]
- Choudhury, D.; Ahmed, Z. Drug-associated renal dysfunction and injury. Nat. Clin. Pract. Nephrol. 2006, 2, 80–91. [Google Scholar] [CrossRef]
- Min, S.-Y.; Ha, D.-S.; Ha, T.-S. Puromycin aminonucleoside triggers apoptosis in podocytes by inducing endoplasmic reticulum stress. Kidney Res. Clin. Prac. 2018, 37, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Gui, T.; Kullak-Ublick, G.A.; Li, Y.; Visentin, M. The role of mitochondria in drug-induced kidney injury. Front. Physiol. 2020, 11, 1079. [Google Scholar] [CrossRef]
- Mody, H.; Ramakrishnan, V.; Chaar, M.; Lezeau, J.; Rump, A.; Taha, K.; Lesko, L.; Ait-Oudhia, S. A review on drug-induced nephrotoxicity: Pathophysiological mechanisms, drug classes, clinical management, and recent advances in mathematical modeling and simulation approaches. Clin. Pharmacol. Drug Dev. 2020, 9, 896–909. [Google Scholar] [CrossRef]
- Shi, Y.; Hua, Y.; Wang, B.; Zhang, R.; Li, X. In silico prediction and insights into the structural basis of drug induced nephrotoxicity. Front. Pharmacol. 2022, 12, 793332. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, G.; Alexander, A.; Saraf, S.; Saraf, S.; Qureshi, A.; Ajazuddin. Recent approaches for reducing hemolytic activity of chemotherapeutic agents. J. Control. Release 2015, 211, 10–21. [Google Scholar] [CrossRef]
- Rybak, L.P.; Whitworth, C.A. Ototoxicity: Therapeutic opportunities. Drug Discov. Today 2005, 10, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Ramkumar, V.; Mukherjea, D. Ototoxicity of non-aminoglycoside antibiotics. Front. Neurol. 2021, 12, 652674. [Google Scholar] [CrossRef] [PubMed]
- Alsaikhan, F.; Jasim, S.A.; Margiana, R.; Catalan Opulencia, M.J.; Yasin, G.; Thaeer Hammid, A.; Tahsinovna Nasretdinova, M.; Mahdi, A.B.; Farhood, B.; Abedi-Firouzjah, R.; et al. Recent update on the protective potentials of resveratrol against cisplatin-induced ototoxicity: A systematic review. Curr. Med. Chem. 2024, 31, 4850–4866. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Lin, H.-Y.; Lin, J.-N.; Tseng, G.-F.; Hwang, C.-F.; Lin, C.-C.; Hsu, C.-J.; Wu, H.-P. 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-glucoside ameliorates gentamicin-induced ototoxicity by modulating autophagy via Sesn2/AMPK/mTOR signaling. Int. J. Mol. Med. 2022, 49, 71. [Google Scholar] [CrossRef]
- Snavely, S.R.; Hodges, G.R. The neurotoxicity of antibacterial agents. Ann. Intern. Med. 1984, 101, 92–104. [Google Scholar] [CrossRef]
- Rusu, A.; Munteanu, A.-C.; Arbănași, E.-M.; Uivarosi, V. Overview of side-effects of antibacterial fluoroquinolones: New drugs versus old drugs, a step forward in the safety profile? Pharmaceutics 2023, 15, 804. [Google Scholar] [CrossRef]
- Hurkacz, M.; Dobrek, L.; Wiela-Hojeńska, A. Antibiotics and the nervous system—Which face of antibiotic therapy is real, Dr. Jekyll (neurotoxicity) or Mr. Hyde (neuroprotection)? Molecules 2021, 26, 7456. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.; Somnuke, P.; Hu, L.; Griemert, E.-V.; Schäfer, M.K.E. Current state of neuroprotective therapy using antibiotics in human traumatic brain injury and animal models. BMC Neurosci. 2024, 5, 10. [Google Scholar] [CrossRef]
- Fermini, B.; Fossa, A.A. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat. Rev. Drug Discov. 2003, 2, 439–447. [Google Scholar] [CrossRef]
- Gottlieb, S. Antihistamine drug withdrawn by manufacturer. BMJ 1999, 319, 7. [Google Scholar] [CrossRef]
- Rubinstein, E. History of quinolones and their side effects. Chemotherapy 2001, 473 (Suppl. S3), S3–S8. [Google Scholar] [CrossRef]
- Taira, C.A.; Opezzo, J.A.W.; Mayer, M.A.; Höcht, C. Cardiovascular drugs inducing QT prolongation: Facts and evidence. Curr. Drug Saf. 2010, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, C.; Wolfes, J.; Kochhäuser, S.; Dechering, D.G.; Reinke, F.; Wasmer, K.; Eckardt, L.; Frommeyer, G. Divergent antiarrhythmic effects of resveratrol and piceatannol in a whole-heart model of long QT syndrome. Int. J. Cardiol. 2017, 243, 233–238. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Barakat, K.H. Development of safe drugs: The hERG challenge. Med. Res. Rev. 2018, 38, 525–555. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Lepailleur, A.; Mignani, S.M.; Dallemagne, P.; Rochais, C. hERG toxicity assessment: Useful guidelines for drug design. Eur. J. Med. Chem. 2020, 195, 112290. [Google Scholar] [CrossRef]
- Du-Cuny, L.; Chen, L.; Zhang, S. A critical assessment of combined ligand- and structure-based approaches to hERG channel blocker modeling. J. Chem. Inf. Model. 2011, 51, 2948–2960. [Google Scholar] [CrossRef] [PubMed]
- Buyck, C.; Tollenaere, J.; Engels, M.; De Clerck, F. An in silico model for detecting potential hERG blocking. In Proceedings of the the 14th European Symposium on Quantitative Structure–Activity Relationships: Designing Drugs—Problems and Solutions (Euro QSAR 2002), Bournemouth International Conference Centre, Bournemouth, UK, 8–13 September 2002. [Google Scholar]
- Yu, H.-B.; Zou, B.-Y.; Wang, X.-L.; Li, M. Investigation of miscellaneous hERG inhibition in large diverse compound collection using automated patch-clamp assay. Acta Pharmacol. Sin. 2016, 37, 111–123. [Google Scholar] [CrossRef]
- Czodrowski, P. hERG me out. J. Chem. Inf. Model. 2013, 53, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Chanana, A.; Kulkarni, Y.R.; Narayan, A.; Shanker, O.; Mahato, B.; Singh, D.; Patel, A.; Havelikar, U.; Singh, R.P.; et al. Computer-aided drug design: Innovation and its application in reshaping modern medicine. Curr. Art. Intel. 2024, 2, e29503752321279. [Google Scholar] [CrossRef]
- Singh, K.; Bhushan, B.; Singh, B. Advances in drug discovery and design using computer-aided molecular modeling. Curr. Comput. Aided Drug Des. 2024, 20, 697–710. [Google Scholar] [CrossRef]
- Moriwaki, H.; Tian, Y.-S.; Kawashita, N.; Takagi, T. Mordred: A molecular descriptor calculator. J. Cheminform. 2018, 10, 4. [Google Scholar] [CrossRef]
- He, Y.; Liew, C.Y.; Sharma, N.; Woo, S.K.; Chau, Y.T.; Yap, C.W. PaDEL-DDPredictor: Open-source software for PD-PK-T prediction. J. Comput. Chem. 2013, 34, 604–610. [Google Scholar] [CrossRef]
- Rydberg, P.; Gloriam, D.E.; Zaretzki, J.; Breneman, C.; Olsen, L. SMARTCyp: A 2D method for prediction of cytochrome P450-mediated drug metabolism. ACS Med. Chem. Lett. 2010, 1, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—Design and description. J. Comput. Aid. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.-Y.; Su, B.-H.; Tu, Y.-S.; Lin, C.; Lin, O.A.; Tseng, Y.J. CypRules: A rule-based P450 inhibition prediction server. Bioinformatics 2015, 31, 1869–1871. [Google Scholar] [CrossRef]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of free online ADMET tools for academic or small biotech environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of animal studies for predicting toxicity in clinical trials: Is it time to rethink our current approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Vashishat, A.; Patel, P.; Gupta, G.D.; Kurmi, B.D. Alternatives of animal models for biomedical research: A comprehensive review of modern approaches. Stem Cell Rev. Rep. 2024, 20, 881–899. [Google Scholar] [CrossRef] [PubMed]
- Llompart, P.; Minoletti, C.; Baybekov, S.; Horvath, D.; Marcou, G.; Varnek, A. Will we ever be able to accurately predict solubility? Sci. Data 2024, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, R.H.; Kouzi, S.A. Resveratrol oligomers: Structure, chemistry, and biological activity. Stud. Nat. Prod. Chem. 2002, 26, 507–579. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Q.; Li, P.; Wang, Z.; Liu, S.; He, C.; Zhang, C.; Xiao, P. Update on phytochemistry and pharmacology of naturally occurring resveratrol oligomers. Molecules 2017, 22, 2050. [Google Scholar] [CrossRef]
- Di Ventura, B.; Lemerle, C.; Michalodimitrakis, K.; Serrano, L. From in vivo to in silico biology and back. Nature 2006, 443, 527–533. [Google Scholar] [CrossRef]
- Grassmann, G.; Miotto, M.; Desantis, F.; Di Rienzo, L.; Tartaglia, G.G.; Pastore, A.; Ruocco, G.; Monti, M.; Milanetti, E. Computational approaches to predict protein–protein interactions in crowded cellular environments. Chem. Rev. 2024, 124, 3932–3977. [Google Scholar] [CrossRef]
- Paliwal, A.; Jain, S.; Kumar, S.; Wal, P.; Khandai, M.; Khandige, P.S.; Sadananda, V.; Anwer, M.K.; Gulati, M.; Behl, T.; et al. Predictive modelling in pharmacokinetics: From in-silico simulations to personalized medicine. Expert Opin. Drug Metab. Toxicol. 2024, 20, 181–195. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).