3.1. Microplastics in Surface Waters
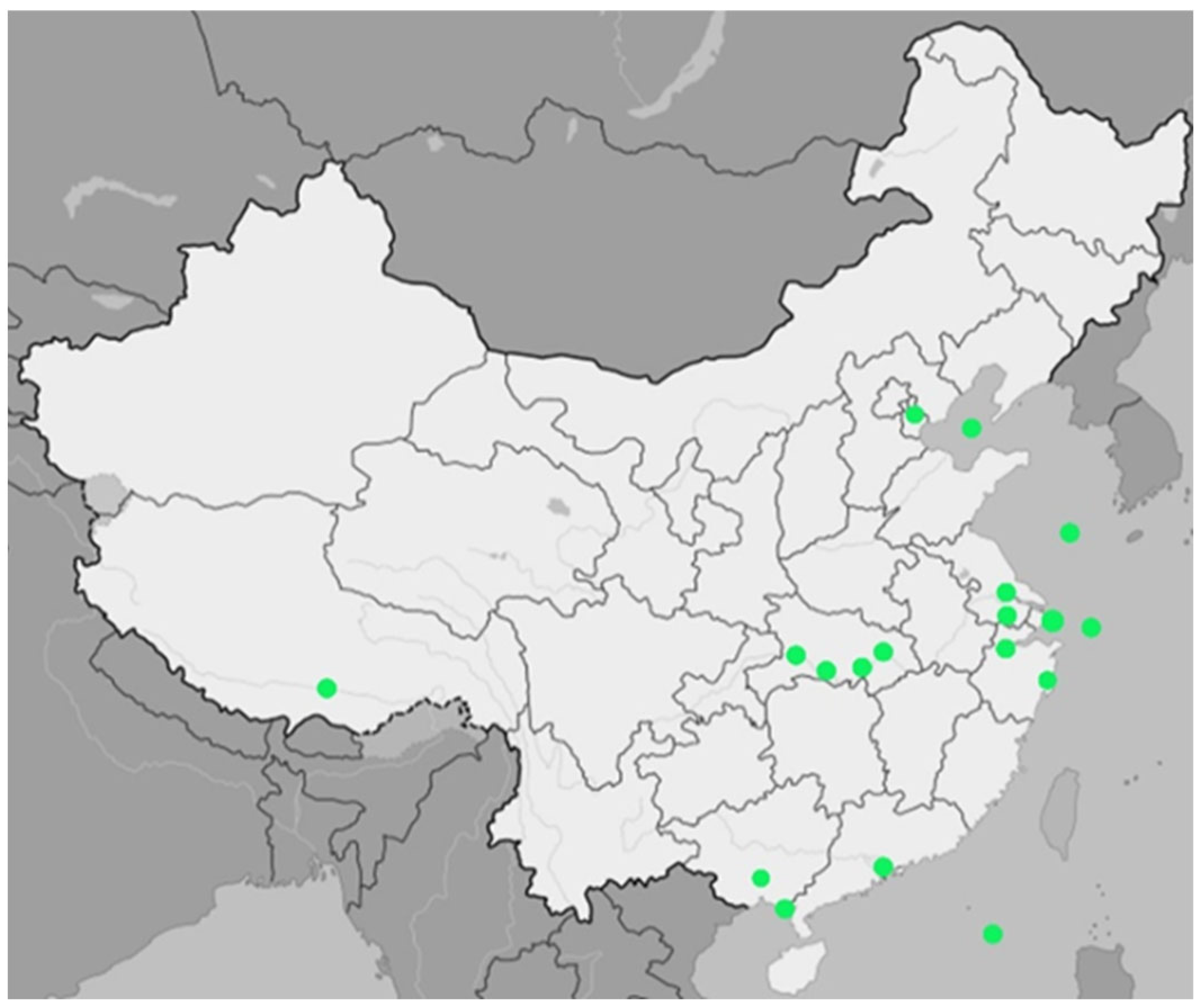
Asia: In the case of Asia, 55 publications regarding the presence of MP in water were selected (
Figure 1). The highest countrywide mean value was reported in Japan at the level of ~28,728 particles/m
3 (
Figure 1,
Supplementary File S1—Asia). The highest levels of MP were found in the rivers Awano (~124,000 particles/m
3), Ayaragi (~117,000 particles/m
3), Asa (~129,500 particles/m
3) and Majime (~580,000 particles/m
3) [
Supplementary File S1—Asia]. Another criterion taken into account during the analysis was water body type. The results of this analysis showed that both rivers and coastal regions have accumulated similar concentrations of MP (~28,827 particles/m
3 and ~27,077 particles/m
3, respectively) (
Supplementary File S1—Asia).
Further studies from 2021 provided additional insights. For instance, a study analyzing surface waters in Japanese rivers reported microplastic concentrations ranging from 300 to 1240 particles/m
3 [
26]. In Southeast Asia, the Chao Phraya River in Thailand exhibited mean concentrations of ~80 particles/m
3 in urban zones and ~48 particles/m
3 in estuary zones [
27]. The Saigon River in Vietnam showed 68–20 particles/m
3 in urban areas and ~42 particles/m
3 near its estuary [
28]. In contrast, the Citarum River in Indonesia had significantly lower concentrations, with ~12 particles/m
3 in urban zones and approximately 0.08 ± 0 particles/m
3 in estuarine areas [
29].
High levels of MP were also found in Southern Korea, where the average detected concentration in surface waters was ~22,280 particles/m
3. A higher amount was found in Jinhae Bay—~88,000 particles/m
3 [
30]. On the other hand, the lowest concentration was found on the coast of Geoje Island, where the average content was ~1 particles/m
3 [
31]. The mean content of MP, depending on the type of reservoir, showed that coastal areas were characterized by higher concentrations in comparison to rivers (respectively: ~699 and ~32,751 particles/m
3) (
Supplementary File S1—Asia). The most frequently studied country in terms of the occurrence of MP in surface waters was China (n = 20;
Figure 2), where the average was ~7260 particles/m
3. The highest levels were found in the Pearl River Estuary of Guangzhou waters—42,100 particles/m
3 [
32]. In turn, the lowest occurred in the Yellow Sea and East Chinese Sea waters—~130 and ~170 particles/m
3, respectively [
33,
34]. In the case of China, the lowest mean MP levels were found in the coastal areas, and the highest in the lakes (~2328 and ~13,903 particles/m
3, respectively). In the regions of Thailand and India, the average MP level in the waters was similar, at ~715 and ~710 particles/m
3, respectively. In the case of Thailand, the lowest level was found in the Inner Gulf of Thailand—~10 particles/m
3 [
35]. In India, the lowest level was found in the areas of the Ganges/Ballia, Ganges/Patna, Ganges/Bhagalpur, Ganges/Farakka, and Ganges/Diamond Harbor rivers, where it ranged from ~0.38–0.684 particles/m
3 [
36]. Additionally, in the case of India, MPs were absent in some parts of the Munnar and Chenai rivers [
37].
On the other hand, in terms of the water body type (
Table 1), the lowest MP level in India was found in lakes (~27 particles/m
3), while in Thailand, the average level was quite similar in rivers and coasts (about 692 and 605 particles/m
3). Taiwan, on the other hand had the lowest number of available publications regarding the topic (n = 3). The analysis carried out showed that the mean MP content in surface water was about ~16 particles/m
3. The highest level was found in water from the Xindian River, and the lowest was in Kaohsiung Bay (~36.95 and 0.48 particles/m
3, respectively) [
38,
39]. In summary, the average content of MPs in surface waters in Asia oscillated around ~12,918 particles/m
3, where most analyses were performed in the period 2018–2019. In turn, the comparison by reservoir type showed that coastal areas had the highest MP level (
Table 1,
Supplementary File S1—Asia). However, the observed discrepancy between the mean and median values likely reflects site-specific outliers, especially in areas affected by urban discharge or aquaculture.
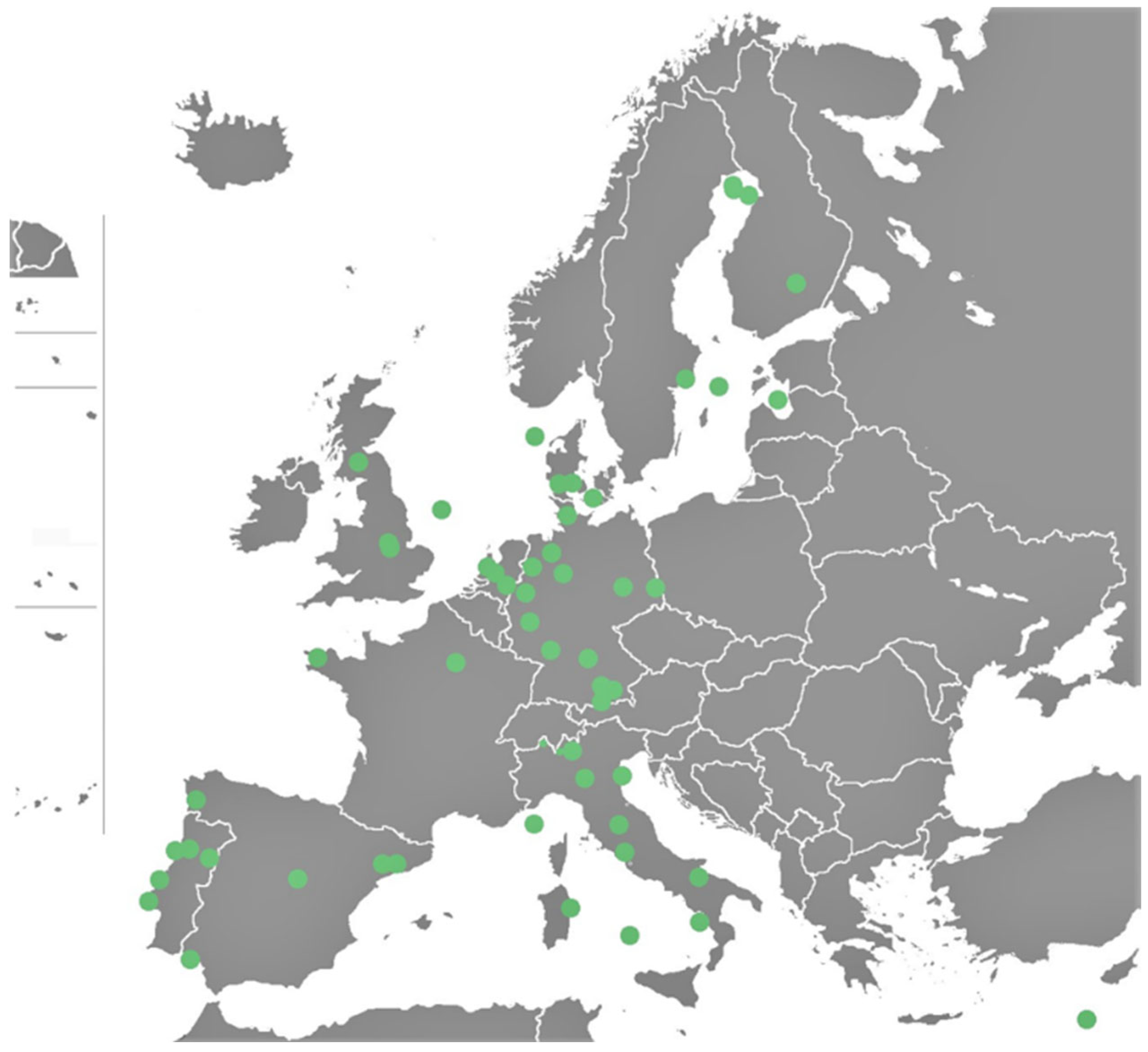
Europe: The MPs in surface water level were analyzed on the basis of 50 available publications (
Figure 3). The analysis showed that the highest average MP level was found in Denmark (~120,300 particles/m
3), while the lowest was in Italy and the Mediterranean Sea (
Supplementary File S2 Europe). In the case of Italy, the lowest levels were found in Lake Bolsena (~0.34–4.08 particles/m
3), and the highest were found in the coastal areas of Goro (~50 particles/m
3) and the Mignone River (~544 particles/m
3) [
40,
41,
42]. The highest MP level in the Mediterranean Sea was 7.68 particles/m
3 in the vicinity of the Aegean-Levantine Sea [
43]. Additionally, a study conducted by Adamopoulou et al. [
44] confirmed the presence of microplastics in the surface waters of the Aegean and Levantine Seas. In this study, 84 samples were collected using a manta net between 2014 and 2020, covering open waters, coastal areas, and enclosed bays (Korfu and Saronikos). Microplastic concentrations varied greatly, ranging from ~0.08 to 10.8 particles/m
3, with the highest levels recorded in samples influenced by surface ocean currents. High concentrations of MP were found in surface water in the Netherlands, Portugal and France (~44,961, 51,860 and 17,500 particles/m
3, respectively). In the remaining countries analyzed, the level did not exceed ~3400 particles/m
3 (
Supplementary File S2 Europe).
When analyzing the MP level depending on the type of reservoir, the highest levels of MPs were detected in the coastal areas (~24,372 particles/m
3), and the lowest were detected in rivers (~8166 particles/m
3) (
Table 2) (
Supplementary File S2—Europe).
Similar to Asia, the difference between the mean and median values observed in Europe likely reflects the impact of extreme values at specific locations, especially in highly urbanized estuaries or coastal zones influenced by anthropogenic discharge.
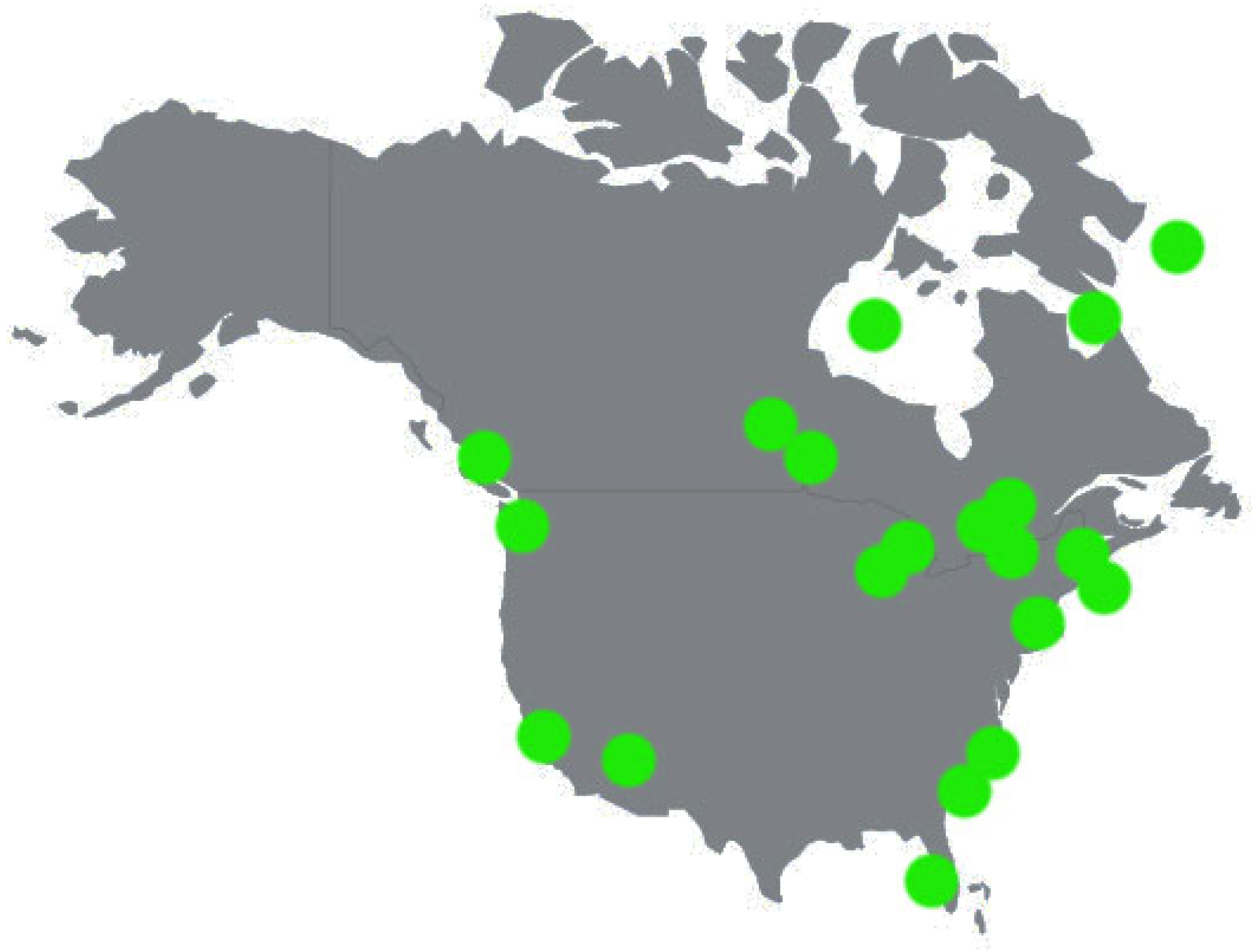
North America: In the case of the USA (n = 9,
Figure 3), the highest level was found in Charleston Harbor Estuary, with MPs at the level of ~19,500 particles/m
3. On the other hand, the lowest was found in Chesapeake Bay, in which the detected concentrations ranged from ~0.007 to ~1.2 particles/m
3 [
45,
46]. The analysis of the MPS level in individual types of water reservoirs showed that in the coastal areas, the MP level was the highest; the average was about 4156 particles/m
3, and the lowest in rivers was ~3.8 particles/m
3 (
Supplementary File S3—North America).
The next analyzed country was Canada (n = 9). In this case, the lowest level occurred in the Holland River, with ~0.1 particles/m
3, while the highest occurred in lake Winnipeg and on the west coast of Vancouver Island (~1933 and ~2000 particles/m
3, respectively) [
47,
48,
49,
50].
When analyzing the MPS level according to the waterbody type (
Table 3), the results showed that in rivers in Canada, the average level of MPS was about ~132 particles/m
3 in lakes, at ~5 particles/m
3, and in the sea/bays, at ~715 particles/m
3 (
Supplementary File S3—North America).
As in previous regions, the noticeable difference between the mean and median values is likely driven by a few exceptionally high concentrations recorded in specific coastal or estuarine sites, causing a skewed distribution of MP levels.
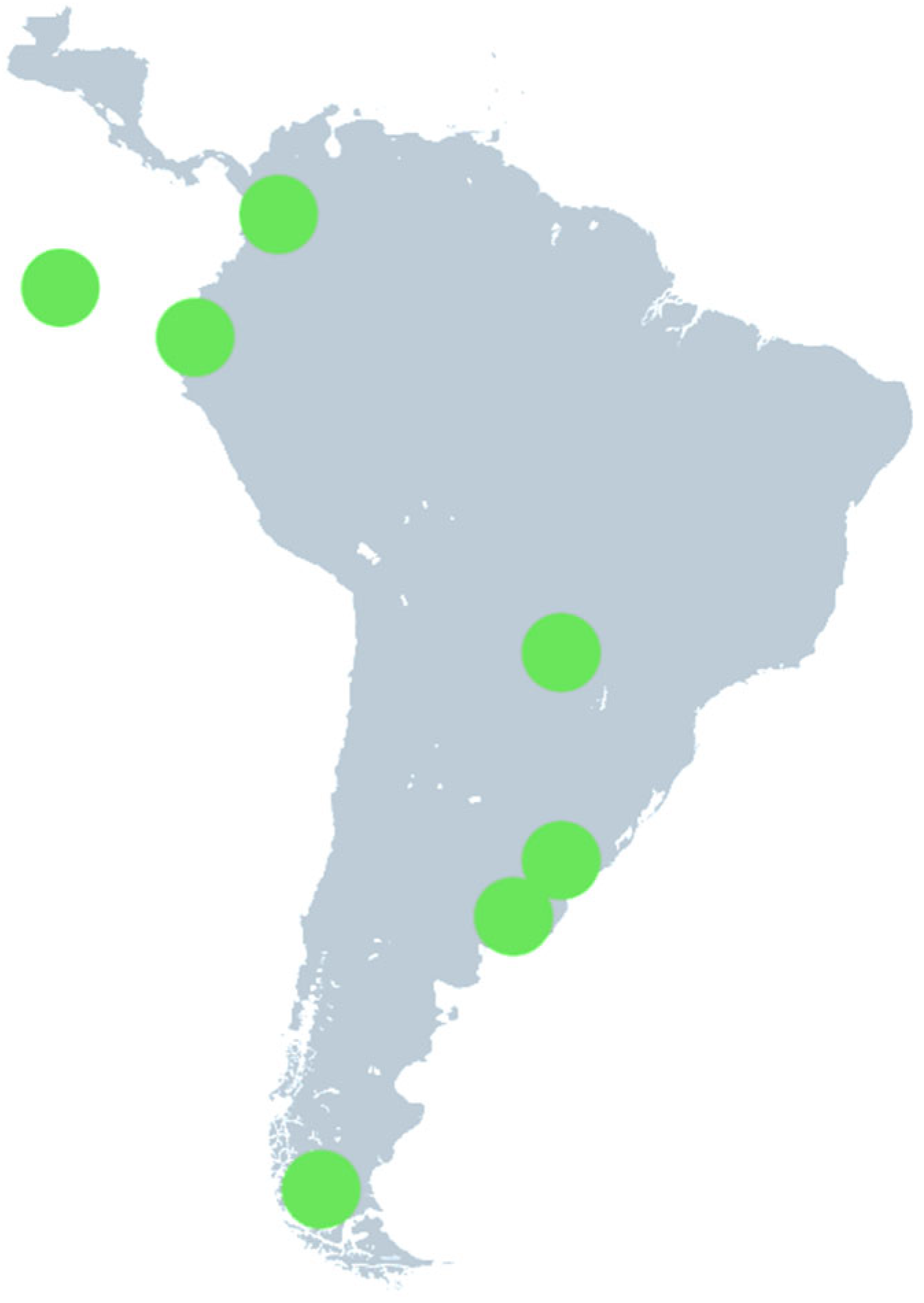
South America: In the case of South America, publications were selected mainly from the areas of Argentina, Ecuador, Paraguay and Colombia (n = 8,
Figure 4), where the highest levels were found in The Bahía Blanca estuary (Argentina) in the medium range from about 596 to 30,893 particles/m
3. In turn, the lowest levels were found in the Tropical Eastern Pacific and Galápagos, where the level did not exceed 1 particles/m
3 [
5,
51]. In the remaining areas, the level ranged from approximately ~0.4 to 3700 particles/m
3; however, most of the values did not exceed 200 particles/m
3 (
Supplementary File S4—South America).
The highest level, in terms of the waterbody type, was typical for rivers, with an average of ~1278 particles/m
3; the lowest levels were in sea/coastal regions, with an average of 0.48 (
Table 4,
Supplementary File S4—South America). The discrepancy between the mean and median values in South American waters likely results from a few extreme values, especially in estuarine sites like Bahía Blanca, which strongly influence the average and skew the overall distribution.
Africa: In the case of Africa (
Figure 5), 12 publications were analyzed; these showed the lowest MPs level in the surface water of Lake Victoria, at ~0.14 particles/m
3 [
52], and the highest level in Lake Yenagoa, where the detected concentrations ranged from about 201 to 8369 particles/m
3 [
53]. In turn, the average MP level in the analyzed publications was about 814 particles/m
3 (
Supplementary File S5—Africa and Australia).
The analysis of the MP level due to the type of water reservoir showed that the highest level was found in water from lakes, with an average level of 946 particles/m
3, compared to rivers or coastal regions (457 and 368 particles/m
3, respectively) (
Table 5,
Supplementary File S5—Africa and Australia). As in other regions, the observed discrepancy between average and median values may reflect the influence of isolated hotspots with elevated MP levels, particularly in heavily polluted inland waterbodies.
Australia: In the case of Australia, the smallest number of papers was evaluated (n = 3,
Figure 6). In the analyzed studies, the highest MP level was found in northern central Victoria in the ponds holding shrimps, with this ranging from 170 to 720 particles/m
3, and in the Goulburn River, with the level ranging from ~110 to 650 particles/m
3 [
54]. In turn, the lowest level was found in the Rib reef area, at 0.02 particles/m
3 [
55]. In the other analyzed places, the MP level did not exceed 2 particles/m
3 (
Supplementary File S5—Africa and Australia).
The comparison of MP levels depending on the water body type showed that the highest level was found in the surface water of rivers and lakes. On the other hand, the levels were the lowest in coastal regions, where the average was estimated to be ~0.08 particles/m
3 (
Table 6,
Supplementary File S5—Africa and Australia).
As observed in other regions, the discrepancy between the average and median values may result from high outlier concentrations in specific locations (e.g., aquaculture zones), skewing the overall dataset.
Taken together, the reviewed data reveal substantial regional differences in microplastic concentrations, shaped by local anthropogenic pressures, waterbody types, and research intensity. It should be noted that current knowledge is heavily skewed toward studies from Asia and Europe, while surface waters in South America, Africa, and Australia remain largely understudied. This geographical imbalance limits the global generalizability of conclusions and underscores the urgent need for standardized, large-scale monitoring programs in underrepresented regions to provide a more comprehensive understanding of microplastic pollution patterns worldwide.
3.3. Type of Microplastic in the Transitional Waters
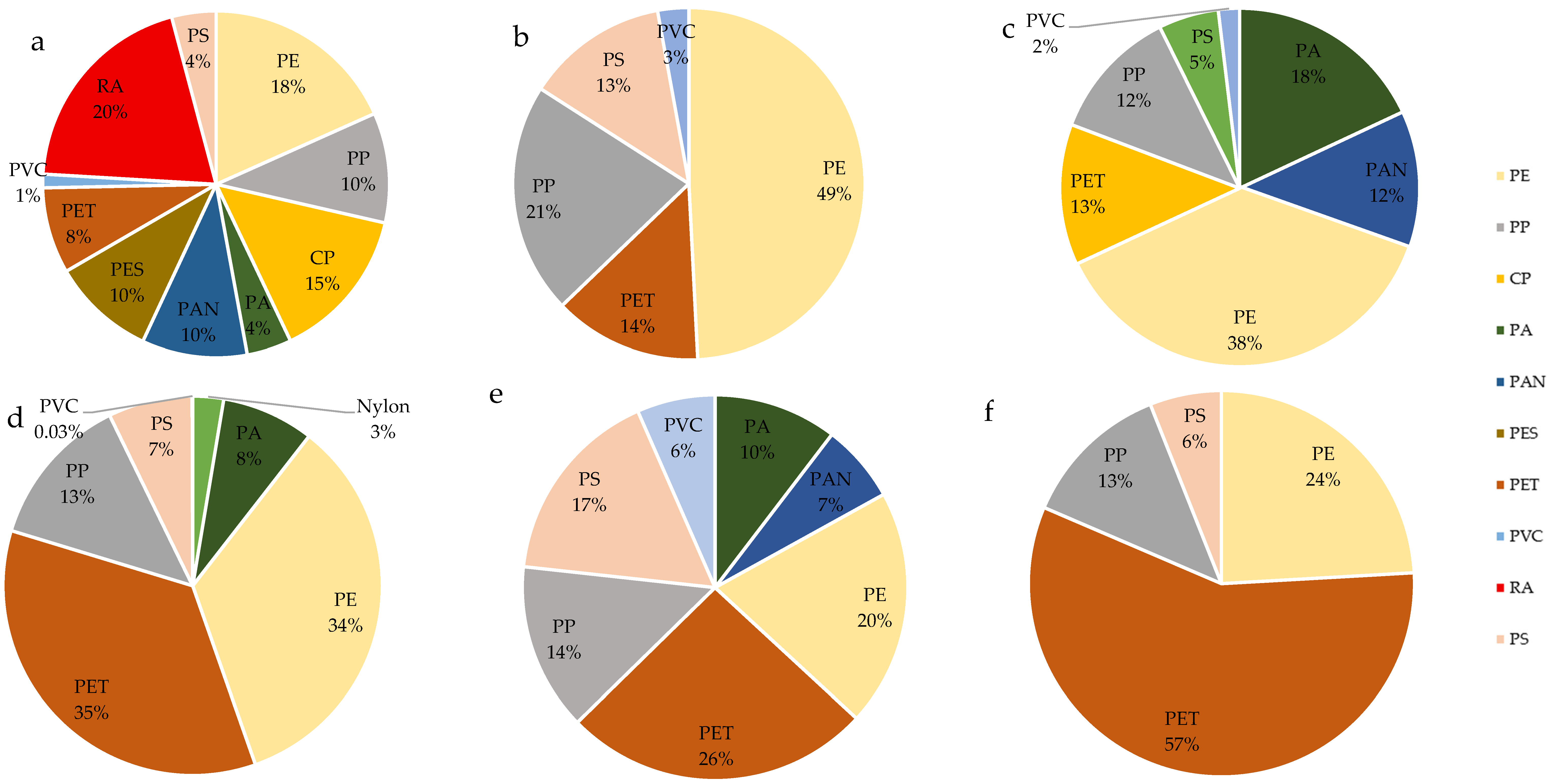
Asia: In an analysis of the literature in terms of the occurrence of types of MPs in surface waters in Asia, the largest share was represented by MPs from rayon (RA), at 40% on average; Polyethylene (PE), Cellophane (CP), Polypropylene (PP) and Polyester were also frequently reported, alongside terephthalic acid (PES) (
Figure 8a,
Supplementary File S1—Asia). In the case of China, a significant share was made up of PE and PP, which on average accounted for 20–30% of the total MP particles. In some places, RA was also found at the level of 31%, and CP at 29.2% [
32,
57,
58,
59,
60,
61]. As in the samples taken from China, in Thailand, a significant share comprised PP and PE, where the percentage for PP ranged from 17 to 60% and for PE ranged from 20 to 73% [
62,
63,
64]. The MP samples from Japan, in turn, were characterized by a significant share of PE—the range was from 45 to 80% [
65,
66,
67]. The MP particles found in Taiwan surface waters mainly comprised PE, PP, and polystyrene (PS) (respectively: 50, 30, 10%) [
68]. The samples from India mainly showed RA, Polyacrylonitrile (PAN), PP and PE, the share of which was up to 50% [
37,
69].
Europe: The MP samples analyzed in Europe mostly showed the participation of PP and PE (
Figure 8b,
Supplementary File S2—Europe). In Italy, the share of PE (about 45%) was dominant, followed by PS (18–24%) and PP (15–17%) [
70,
71]. In turn, in the Netherlands, PA and Polyvinyl chloride (PVC) were at the level of 30% [
72]. In studies on MPs in Germany, the main share comprised PE, from 34 up to 100%. Another type was PP (2.94–68.89%) [
73,
74]. MP samples from Spain, France and the UK were characterized by a significant share of PE, ranging from 4 to 67.1%, and PP, from 16.5 to 23%. Additionally, in the MP samples from the UK and France, publications showed a significant share of PS, ranging from 16.1 to 30% [
6,
75,
76,
77]. MP tests performed in water reservoirs in Portugal were also characterized by a significant share of PP and PE (ranging from 15 to 29.4% and from 29.4 to 33%, respectively) [
78,
79,
80].
North America: An analysis of MP types in the literature (
Figure 8c,
Supplementary File S3—North America) showed that in the USA, the majority was PE (32%) and PP (13%) [
36]. Similarly, in Canada, the range was 27–41.2%. Additionally, significant shares comprised PP (5–21.9%) and polyethylene terephthalate (PET) (8–10%). PA was also detected in one of the studies at the level of 16%, and PVC was detected at 1.7% [
81,
82,
83].
South America: These surface waters (
Figure 8d) were characterized by a significant share of PE, PET, and PP (respectively: 11.8–40%, 15–38.3% and 2.9–17%). Additionally, the presence of PS (2.9–8%), PA (6%), Nylon (2%) and PVC (0.03%) was also observed [
84,
85].
Africa: The most common types of MPs in the waters of Africa were PET, PE, PP and PS (
Figure 8e,
Supplementary File S5—Africa and Australia). The PET range was 6–72.6%, PE: 0.7–60.5%. PP: 7–38%, PS: 10–56%. In some cases, there was a significant proportion was RA (70.4%) or PA, at 43% [
52,
53,
86,
87,
88,
89,
90,
91].
Australia: In the case of literature analyzing the type of MPs present in waters in Australia (
Figure 8f), PET was the most frequently found, ranging from 35 to 77.7%. Then, the PE ranged from 11.1 to 42%. The presence of PS (4–8%), PA (12.9%), Nylon (7.41%) and RA (8.1%) was also shown. Additionally, one of the publications showed a significant share of Polyester (PES), at 30.6% [
54,
55,
92].
3.4. Most Common Microplastic-Associated Taxa and Their Relevance
Biofilms on microplastics (“plastisphere”—a term coined to describe the microbial biofilm that specifically forms on plastic surfaces, often enriched with unique taxa, including potential pathogens and antibiotic resistance gene carriers [
93]) are largely dominated by Proteobacteria, often exceeding ~50% of microbial communities. Numerous studies, such as those conducted by Davidov et al. [
94], Gökdağ et al. [
95], and Jiao et al. [
96], have reported a prevalence of Alphaproteobacteria (e.g., order Rhodobacterales) and Gammaproteobacteria on plastic surfaces. Alphaproteobacteria—especially
Rhodobacteraceae—are typical marine biofilm pioneers, contributing to biofilm matrix formation and organic matter degradation. Gammaproteobacteria include both environmentally important taxa (e.g.,
Alteromonas,
Alcanivorax) and potential pathogens (
Vibrio,
Pseudomonas,
Acinetobacter) [
94,
95,
96,
97].
Alcanivorax (Proteobacteria) is a well-known hydrocarbon-degrading genus that is frequently found in marine plastispheres due to its ability to degrade aliphatic hydrocarbons and possibly plastic-derived compounds [
94,
95].
Alteromonas and related taxa (order Alteromonadales) are common in marine biofilms and help utilize dissolved organic carbon, facilitating further microbial colonization (
Table 7) [
94,
96].
The second most abundant plastisphere members are typically Bacteroidetes (e.g.,
Flavobacteriaceae, Chitinophagaceae). These Gram-negative bacteria specialize in polymer and polysaccharide degradation, helping process organic matter that settles on plastics [
94,
97]. On polyethylene surfaces in the Mediterranean Sea,
Bacteroidetes accounted for ~22–25% of the plastisphere—much higher than in surrounding seawater [
97]. Cyanobacteria also colonize microplastics in sunlit surface layers, but their abundance on plastic is often lower than in open water (e.g., ~5% on MPs vs. ~20% in ambient plankton), likely due to competition within the biofilm or light limitation under biofilm layers [
94,
98]. Actinobacteria generally comprise a smaller fraction of the biofilm (often just a few percent), but some strains contribute to the degradation of recalcitrant compounds and may help break down plastic additives [
97,
99,
100]. In freshwater, Betaproteobacteria (e.g., Burkholderiales) are often dominant, especially in nutrient-rich rivers [
101].
Microplastics serve as a novel niche promoting unique microbial communities. Many dominant bacteria—such as
Alcanivorax,
Alteromonas,
Sphingomonas—are known for degrading complex organic matter or even plastic components [
94,
95].
Alcanivorax, for instance, is often enriched on marine plastics due to its ability to metabolize hydrocarbons [
82].
Pseudomonas and
Sphingomonas, frequently reported on MPs, are metabolically versatile and capable of degrading polyurethane, PET, and other polymers [
97,
99]. Some biofilm members (e.g.,
Nitrospira,
Methylophilaceae) may also play roles in nitrogen and carbon cycling, turning the plastisphere into a site of nutrient transformation [
100,
101].
One major concern is the presence of pathogenic or opportunistic bacteria on plastic surfaces. In marine biofilms,
Vibrio spp. is frequently detected—including
V. parahaemolyticus and strains related to
V. cholerae. Studies show that
Vibrio spp. are often more abundant on floating plastic than in surrounding seawater, raising concerns that microplastics could act as vectors for human and fish pathogens [
99]. In freshwater systems, genera like
Aeromonas,
Pseudomonas, and
Acinetobacter pose similar risks.
Acinetobacter (including
A. baumannii) is a known hospital-associated pathogen that was found to be enriched on riverine and estuarine plastics [
72,
100]. Likewise,
Arcobacter (Campylobacterota) was found on riverine MPs, suggesting the potential transport of faecal indicators or wastewater-derived pathogens even in non-wastewater sites [
101].
A notable example is
Corynebacterium variabile, identified as the dominant taxon on polystyrene MPs in a semi-enclosed harbour in Hong Kong. Although typically associated with soil and water environments, this species can act as an opportunistic pathogen. Its dominance over MPs suggests that stagnant, low-exchange environments may promote rare and potentially harmful taxa on plastic debris [
99,
102].
Table 7.
Dominant bacterial taxa isolated from biofilms on microplastics in freshwater and marine environments (including their average relative abundance, sampling location, and source).
Table 7.
Dominant bacterial taxa isolated from biofilms on microplastics in freshwater and marine environments (including their average relative abundance, sampling location, and source).
| Taxonomy (Phylum—Family—Genus) | Abundance (%) | Sampling Location | Water Type | Reference |
|---|
| Proteobacteria—Pseudomonadaceae—Pseudomonas | ~12% (most frequent genus) | Poyang Lake (China) | Fresh | [101] |
| Proteobacteria—Moraxellaceae—Acinetobacter | ~10% (enriched on MPs) | Pearl River Estuary (China) | Fresh (Estuarine) | [72] |
| Campylobacterota—Arcobacteraceae—Arcobacter | ~3–4% (MP enrichment) | Rhine River (Netherlands) | Fresh | [99] |
| Proteobacteria—Alcanivoracaceae—Alcanivorax | (present; MP-specific) | Eastern Mediterranean Sea | Marine | [94] |
| Proteobacteria—Vibrionaceae—Vibrio | (present; dominant order) | NW Mediterranean Sea | Marine | [99] |
| Actinobacteriota—Corynebacteriaceae—Corynebacterium variabile | (dominant on PS MPs) | Hong Kong coastal waters | Marine | [102] |
On the other hand, recent studies confirm that microplastics (MPs) floating in natural waters harbour a variety of antibiotic resistance genes (ARGs), suggesting that MPs may act as fomites not only for pathogenic bacteria but also for genetic elements associated with antimicrobial resistance. The most commonly detected ARGs include:
Sulphonamide resistance genes—especially
sul1 (frequently linked to the integron gene
intI1) and
sul2, both widely observed in MPs biofilms [
103].
Tetracycline resistance genes—various
tet genes such as
tetA and
tetW were repeatedly found [
104].
Macrolide resistance genes—particularly
ermF, which encodes an rRNA methyltransferase, conferring resistance to macrolides and lincosamides [
105].
Other ARGs—such as chloramphenicol resistance genes (
cat), aminoglycoside resistance genes (
aad,
aac), trimethoprim resistance genes (
dfr), and fluoroquinolone resistance genes (
qnr). Notably, clinically important β-lactamase genes (
blaCTX-M,
blaTEM,
blaSHV) were detected in
Acinetobacter,
E. coli, and other resistant strains isolated from MP surfaces [
106].
Due to the differences in MP composition, studies have shown that there are differences in the type of ARGs detected [
103,
104]. The most common types of plastics in aquatic environments—PE and PP, as we mentioned before—were also the most frequently colonized by ARG-bearing bacteria [
103,
105]. Studies in the Yangtze River estuary revealed that PE and PP fragments with visible biofilms had higher ARG concentrations than the surrounding water or sediments [
103,
106].
Other polymer types, including PS, polyethylene terephthalate PET, and polyvinyl chloride PVC, also carried ARGs. Bacteria like
Bacillus,
Mycobacterium, and
Pseudomonas isolated from these surfaces contained sul1, tet, and other ARGs. While surface chemistry may influence colonization, exposure time and environmental availability are likely stronger factors—older, photo-oxidized, or porous plastics become hotspots for ARG accumulation [
103].
The occurrence of antibiotic resistance genes (ARGs) on microplastics has been investigated across various regions of the world—from coastal waters and estuaries in Asia, to European seas, and inland rivers and lakes throughout the Americas. A particularly substantial body of data originates from East Asia (notably China), where rapid industrial development and extensive antibiotic usage contribute to significant environmental contamination. For instance, in the Yangtze River estuary, numerous plastic samples were collected, revealing biofilms enriched with diverse ARGs; the average ARG abundances on these microplastics exceeded those found in the surrounding seawater and sediments [
103]. Marine aquaculture regions, such as mariculture ponds in China, are also characterized by high concentrations of ARGs on microplastics, attributed to frequent antibiotic application. One study from such an area identified up to 174 subtypes of resistance genes in the collected samples [
105].
In Europe, the problem is also recognized: microplastics from the Mediterranean and Baltic Seas, as well as rivers, have been studied, with ARGs from similar classes (tetracyclines, sulphonamides, beta-lactams) detected, although typically at lower concentrations, likely due to reduced antibiotic pollution pressure [
107,
108,
109]. The highest ARG concentrations are usually recorded where microplastics come into contact with intense sources of contamination: near municipal and hospital wastewater outfalls, river mouths, or fish and shrimp farms, where antibacterial agents enter the water [
107,
109]. These geographic hotspots—often found along the coasts of Southeast Asia and densely populated river deltas—show the greatest diversity and density of ARGs carried by microplastics [
109]. In contrast, remote waters far from pollution sources (e.g., central ocean regions, mountain lakes) also contain microplastics, but generally with a much lower ARG abundance. Nonetheless, even in these areas, the plastisphere may act as a reservoir of ARG-bearing microbes, transported over long distances by water currents [
110].
Recent studies have confirmed that microplastics also act as hotspots for antibiotic resistance genes (ARGs), harbouring sulphonamide (sul1, sul2), tetracycline (tetA, tetW), macrolide (ermF), chloramphenicol (cat), aminoglycoside (aad, aac), trimethoprim (dfr), fluoroquinolone (qnr), and clinically relevant β-lactamase genes (blaCTX-M, blaTEM, blaSHV) [
109,
111,
112,
113]. Notably, plastic types like PE, PP, PS, PET, and PVC exhibit differential ARG enrichment due to surface chemistry and ageing [
113,
114].
A recent study by Cholewińska et al. [
109] demonstrated that MPs incubated in Oder River water led to a significantly increased pathogen abundance and ARG prevalence in both water and sediment samples, confirming MPs as vectors for antimicrobial resistance in situ.
Advances in nanoplastics research are revealing that particles <1 µm can cross cell membranes, generate reactive oxygen species, and enhance horizontal gene transfer (HGT) within biofilms. For example, a review in 2024 documented increased ARG carriage on aged MPs due to enhanced surface roughness and chemical reactivity [
115,
116]. In soil-based microcosms, the increased diversity of micro-/nanoplastics correlated with elevated ARG and mobile genetic element (MGE) abundance—highlighting emerging eco-toxicological pathways [
117,
118,
119].
Although our work does not focus on nanoplastics or extreme settings, these recent developments—especially the spread of ARGs in remote or highly polluted environments—underscore the urgent need for integrated studies that combine MP characterization, ARG monitoring, and the evaluation of environmental risk in diverse ecosystems.