FTIR Characterization of Asphalt SARA Fractions in Response to Rubber Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
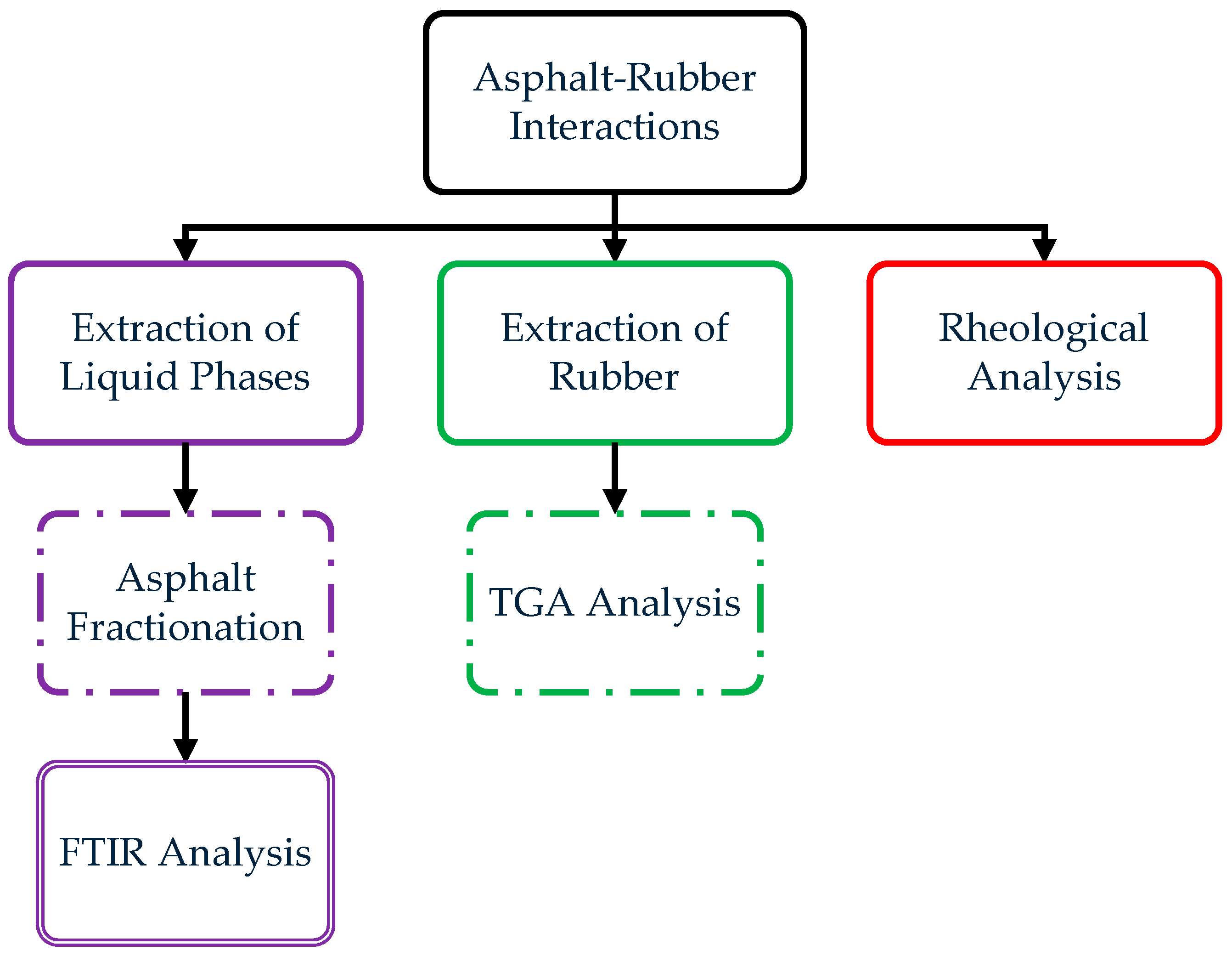
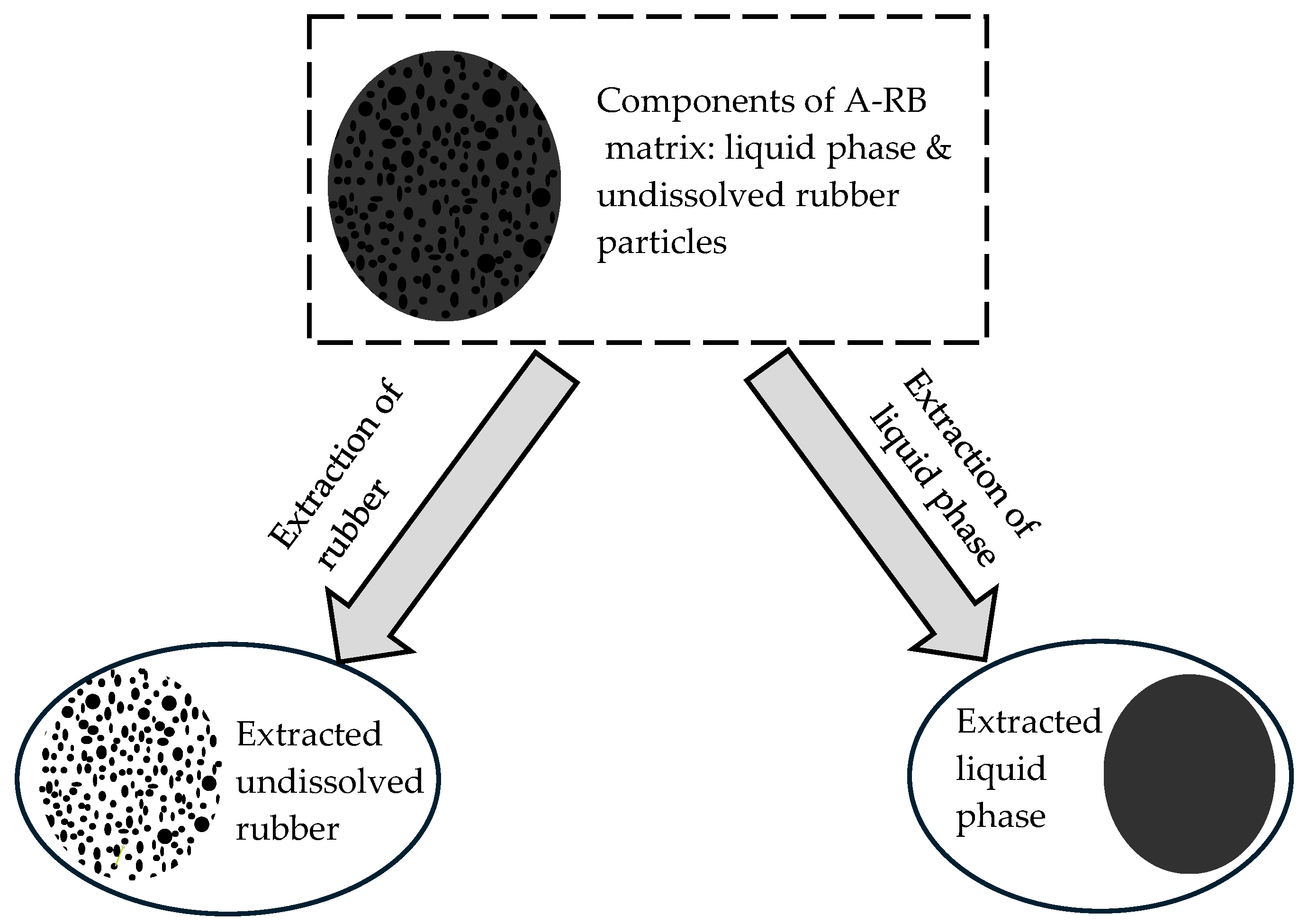
2.2.1. Asphalt–Rubber Interactions
2.2.2. Extraction of Liquid Phases
2.2.3. Asphalt Fractionation
2.2.4. FTIR Analysis
2.2.5. Extraction of Rubber
2.2.6. TGA Analysis
2.2.7. Rheological Analysis
3. Results and Discussion
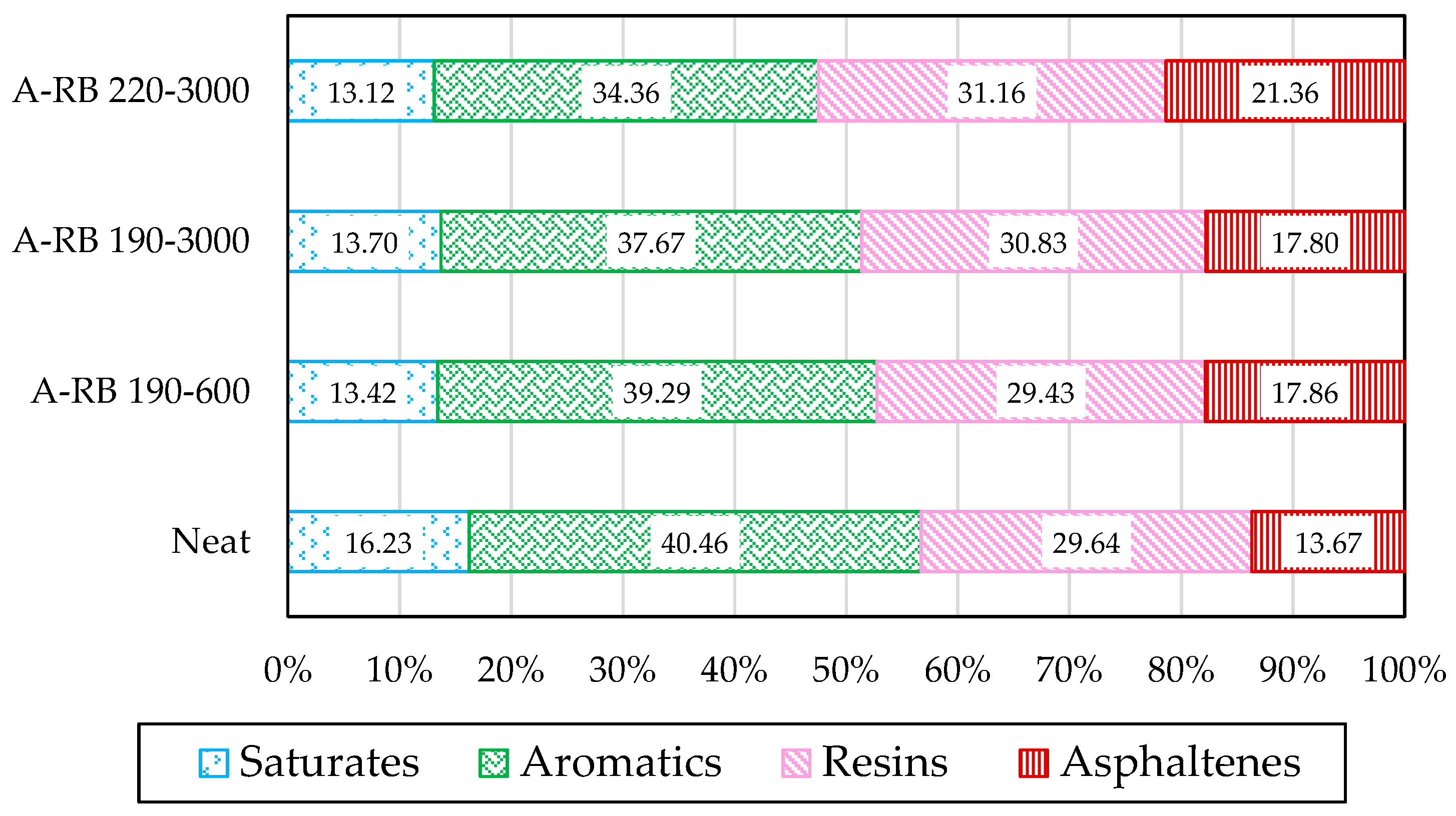
3.1. Analysis of Asphalt Fractions
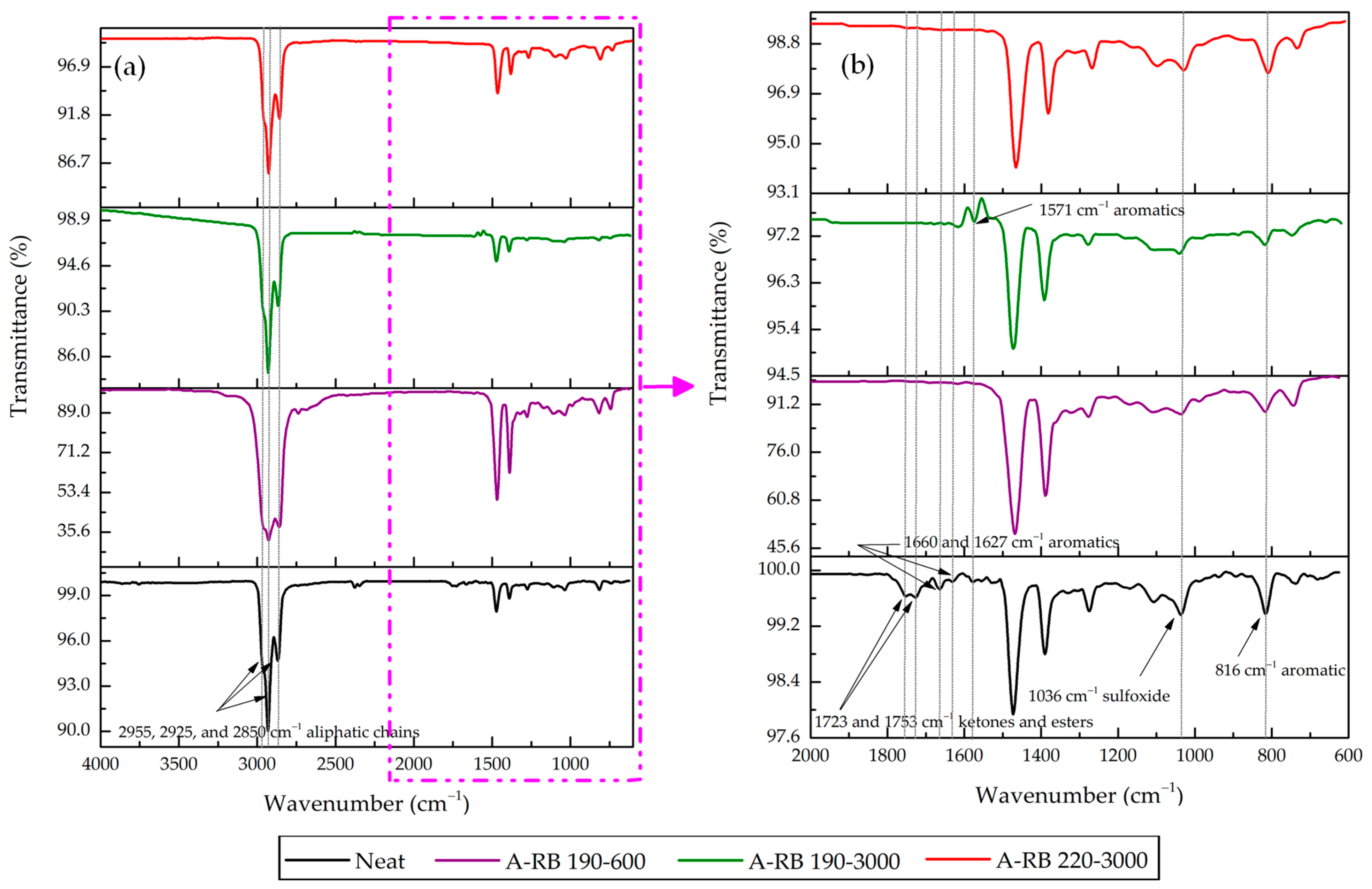
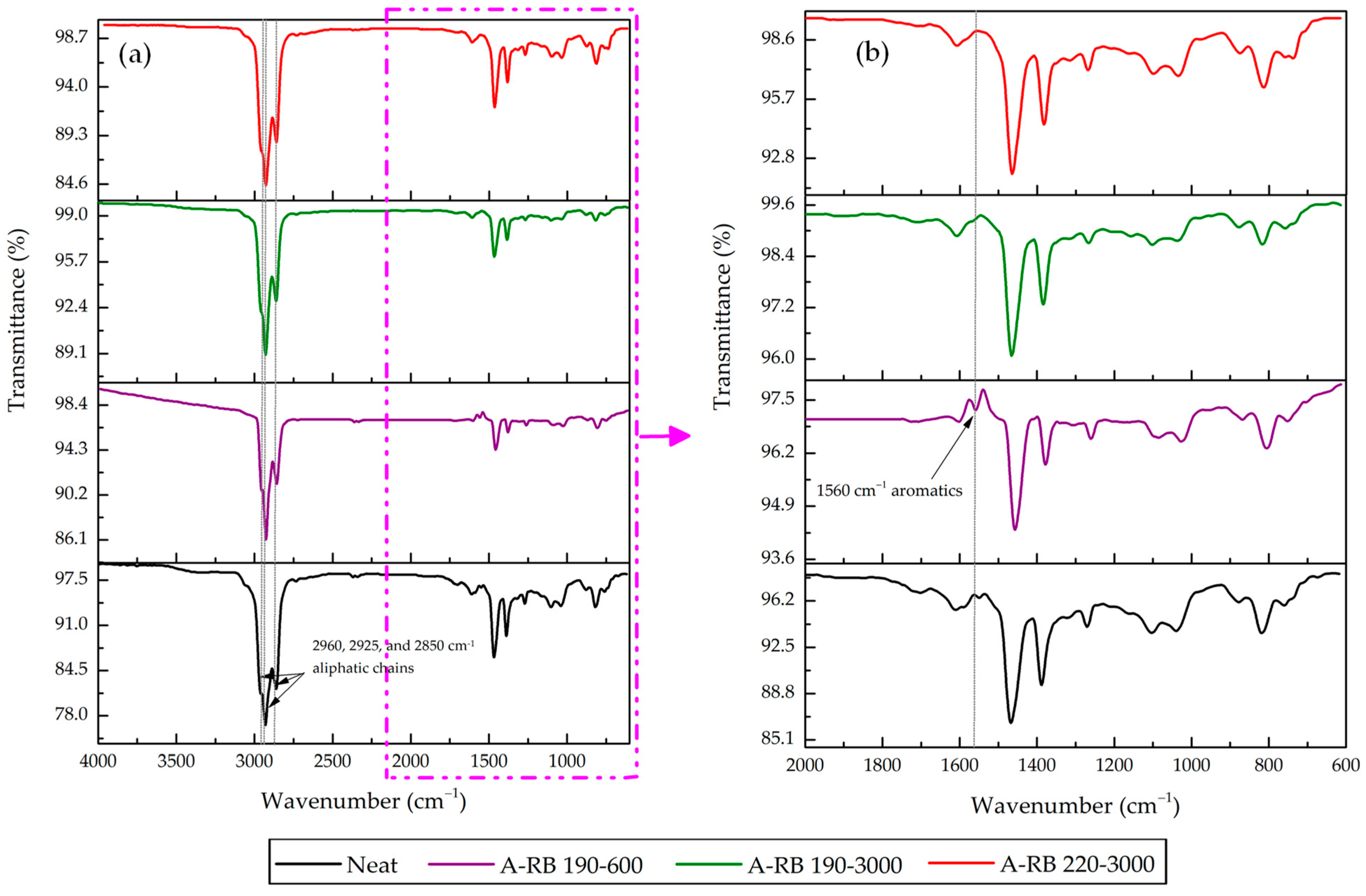
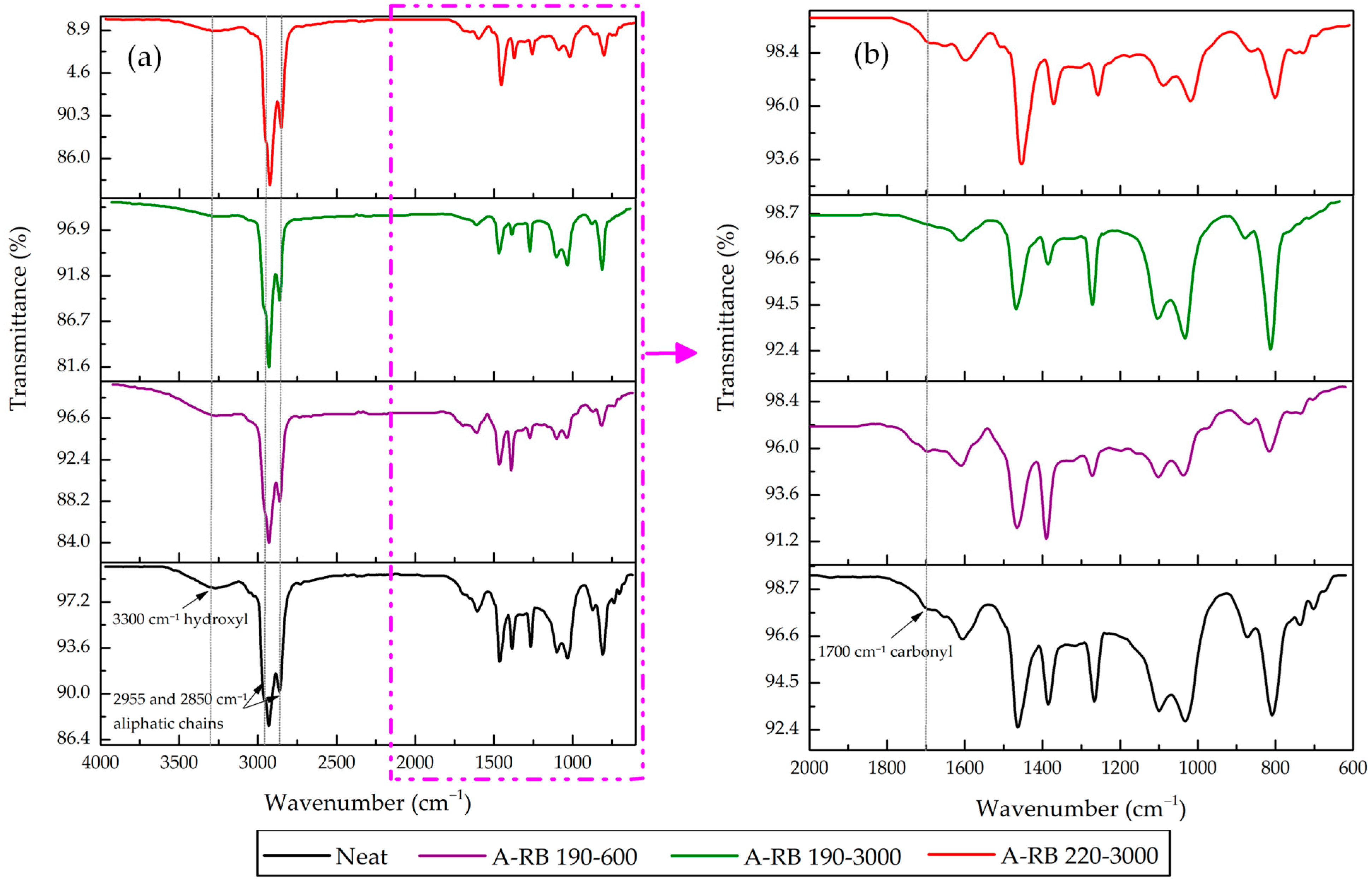
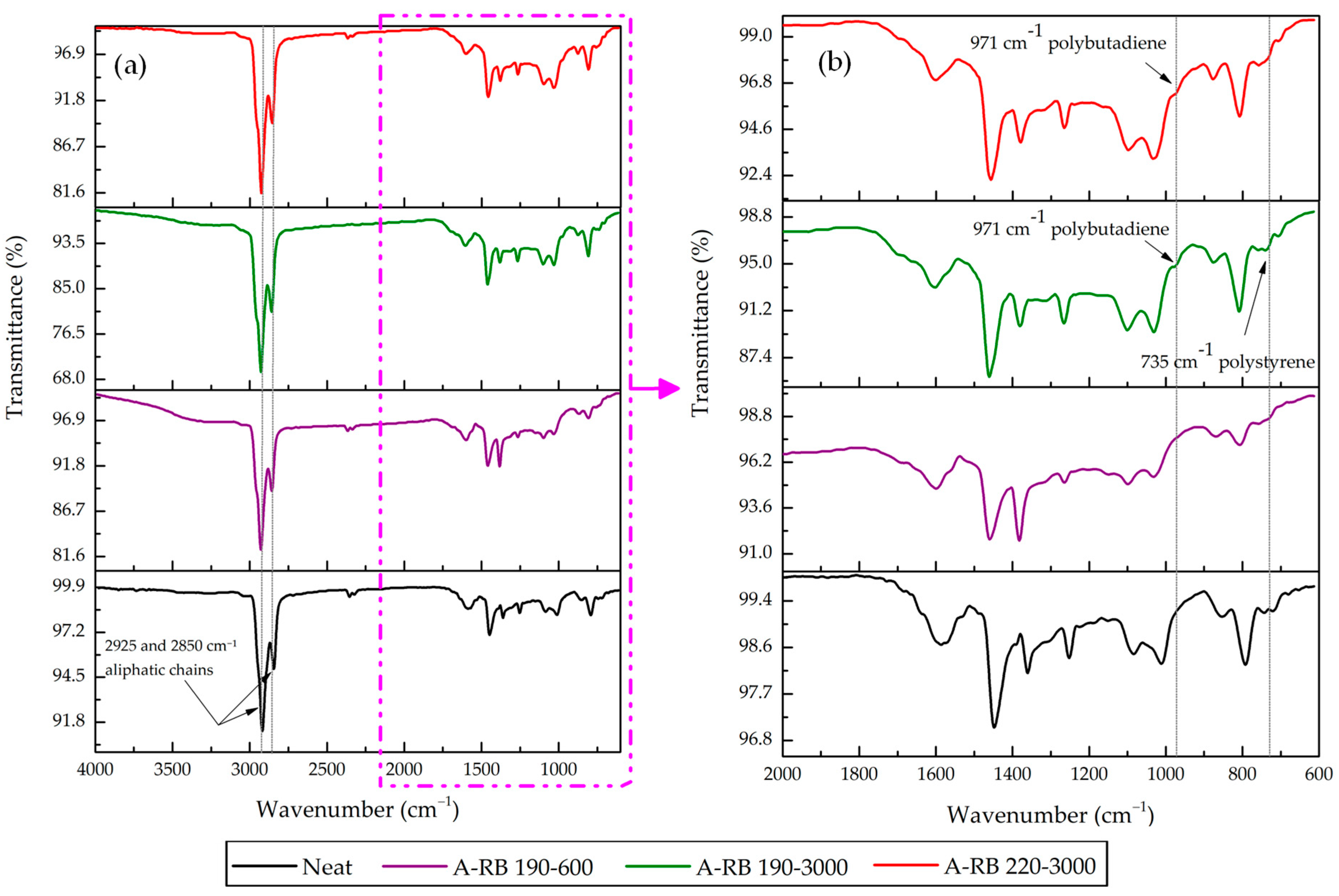
3.2. FTIR Spectral Comparisons of Neat Binder and A-RB Fractions
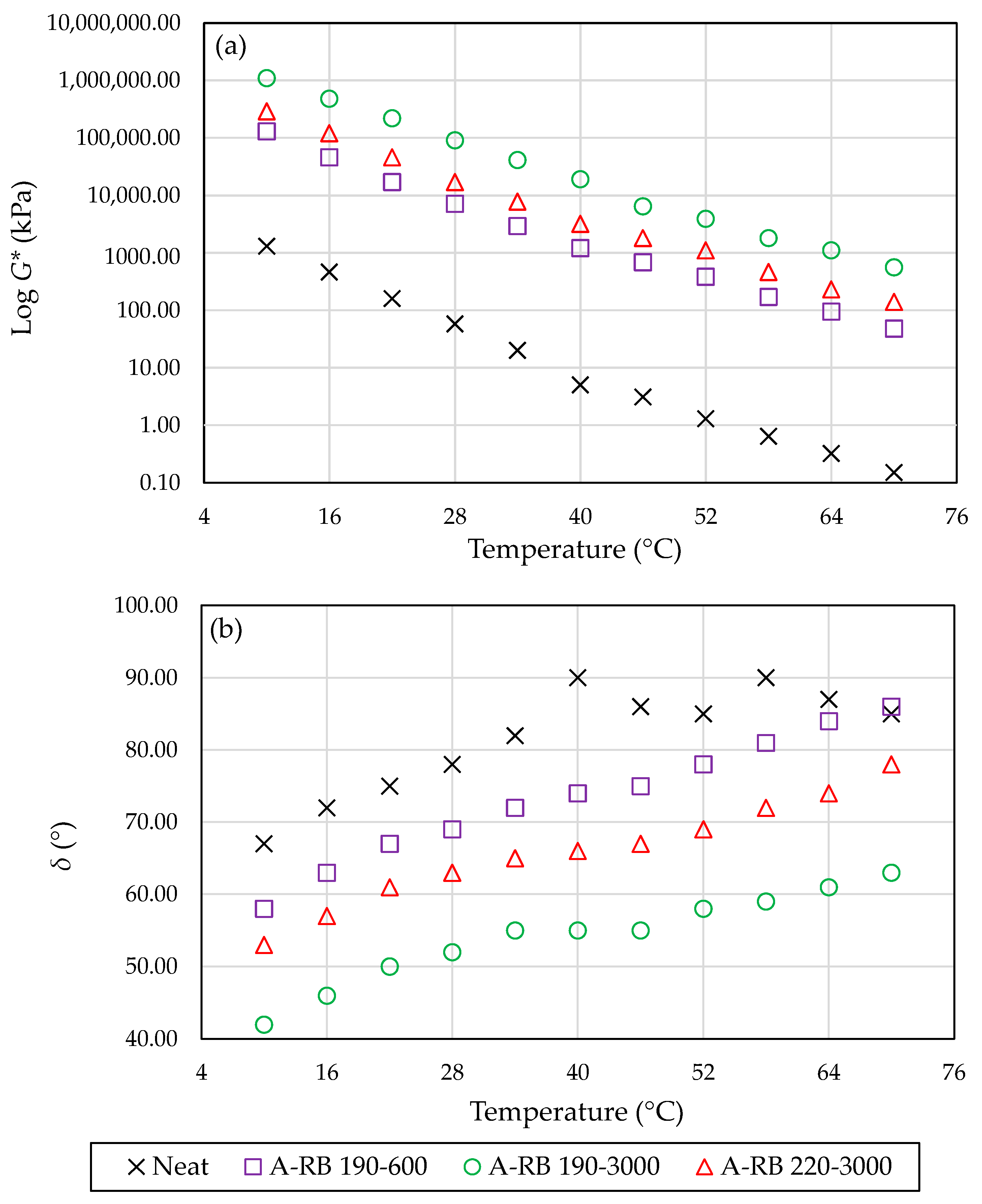
3.3. Rheological Analysis of Asphalt Matrix
3.4. Compositional Changes of Rubber
4. Conclusions
- Asphalt–Rubber Interactions Influenced the Distribution of SARA Fractions: In A-RB 190–3000, saturates and aromatics decreased by 9.38%, while asphaltenes and resins increased by 12.28%, indicating that rubber absorbed light fractions and promoted swelling.
- Rubber Released Its Components Partially in SARA Fractions: Rubber partially dissolved and released C=C aromatics at 1560 and 1571 cm−1 into aromatics and saturates. Aliphatic C–H peaks in saturates were intensified, highlighting the migration of rubber aliphatics. Rubber PB and PS components emerged in asphaltenes at 971 and 735 cm−1, respectively, for A-RB 190–3000.
- Partial Dissolution of Rubber Enhanced Rheological Performance: Optimal interactions (190–3000–8) enhanced the rheological performance (higher G* and lower δ). Higher temperature and lower speed led to lower rheological performance due to excessive depolymerized or incomplete swelling, respectively.
- Thermal Analyses of Rubber Confirmed its Partial Dissolution: Rubber dissolution reached 82.0% under optimal conditions, with OL, NR, SR, and FR dissolution exceeding 77%. At 220 °C, dissolution exceeded 90%; however, excessive devulcanization and depolymerization diminished performance merits.
5. Future Work
- Examine how different sources of asphalt and sizes of rubber impact chemical and rheological changes in A-RB components.
- Identify the short- and long-term aging processes that influence the interaction between asphalt and rubber using SARA-based chemical analysis.
- Characterize the thermal stability and degradation characteristics of the SARA fractions in A-RBs using TGA.
- Investigate the microstructural development of SARA fractions in A-RBs using atomic force microscopy to validate morphology–performance relationships.
- Perform life cycle cost analysis of A-RBs to evaluate the economic feasibility of high-temperature and long-duration mixing processes.
- Apply rheological modeling to quantify the viscoelastic behavior of A-RBs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-RB | Asphalt–Rubber Binder |
| DTG | Derivative of Thermograph |
| FR | Filler |
| FTIR | Fourier Transform Infrared Spectroscopy |
| NR | Natural Rubber |
| OL | Oil |
| PB | Polybutadiene |
| PS | Polystyrene |
| SARA | Saturates, Aromatics, Resins, and Asphaltenes |
| SR | Synthetic Rubber |
| TGA | Thermogravimetric Analyzer |
References
- Lamontagne, J. Comparison by Fourier transform infrared (FTIR) spectroscopy of different ageing techniques: Application to road bitumens. Fuel 2001, 80, 483–488. [Google Scholar] [CrossRef]
- Li, Z.; Cao, X.; Li, J.; Yang, X. Research on the correlation between the chemical components and the macroscopic properties of asphalt binder. Materials 2025, 18, 610. [Google Scholar] [CrossRef] [PubMed]
- Claine Petersen, J. Chemical composition of asphalt as related to asphalt durability: State of the art. Transp. Res. Rec. 1984, 99, 13–30. [Google Scholar]
- Martínez-Estrada, A.; Chávez-Castellanos, A.E.; Herrera-Alonso, M.; Herrera-Nájera, R. Comparative study of the effect of sulfur on the morphology and rheological properties of SB- and SBS-modified asphalt. J. Appl. Polym. Sci. 2010, 115, 3409–3422. [Google Scholar] [CrossRef]
- Loise, V.; Caputo, P.; Porto, M.; Calandra, P.; Angelico, R.; Oliviero Rossi, C. A review on bitumen rejuvenation: Mechanisms, materials, methods and perspectives. Appl. Sci. 2019, 9, 4316. [Google Scholar] [CrossRef]
- Zhang, B.; Xi, M.; Zhang, D.; Zhang, H.; Zhang, B. The Effect of styrene–butadiene–rubber/montmorillonite modification on the characteristics and properties of asphalt. Constr. Build. Mater. 2009, 23, 3112–3117. [Google Scholar] [CrossRef]
- Adhikari, B. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Song, P.; Wu, X.; Wang, S. Effect of styrene butadiene rubber on the light pyrolysis of the natural rubber. Polym. Degrad. Stab. 2018, 147, 168–176. [Google Scholar] [CrossRef]
- Mark, J.E.; Erman, B.; Roland, M. The Science and Technology of Rubber, 4th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Heitzman, M. State of the Practice: Design and Construction of Asphalt Paving Materials with Crumb-Rubber Modifier; Federal Highway Administration, Office of Engineering: Washington, DC, USA, 1992. [Google Scholar]
- Abdelrahman, M. Controlling performance of crumb rubber–modified binders through addition of polymer modifiers. Transp. Res. Rec. 2006, 1962, 64–70. [Google Scholar] [CrossRef]
- Pstrowska, K.; Gunka, V.; Prysiazhnyi, Y.; Demchuk, Y.; Hrynchuk, Y.; Sidun, I.; Kułażyński, M.; Bratychak, M. Obtaining of formaldehyde modified tars and road materials on their basis. Materials 2022, 15, 5693. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Apostolidis, P.; van de Ven, M.; Erkens, S.; Skarpas, A. Effect of laboratory aging on chemistry and rheology of crumb rubber modified bitumen. Mater. Struct. 2020, 53, 26. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, Z.; Feng, L.; He, P.; Li, S. Chemical and rheological evaluation of the ageing behaviour of high-content crumb rubber asphalt binder. Polymers 2024, 16, 3088. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pei, J.; Wang, T.; Amirkhanian, S. High temperature rheological characteristics of activated crumb rubber modified asphalts. Constr. Build. Mater. 2019, 194, 122–131. [Google Scholar] [CrossRef]
- Deef-Allah, E.; Abdelrahman, M. Effect of used motor oil as a rejuvenator on crumb rubber modifier’s released components to asphalt binder. Prog. Rubber Plast. Recycl. Technol. 2021, 37, 87–114. [Google Scholar] [CrossRef]
- Deef-Allah, E.; Abdelrahman, M.; Hemida, A. Improving asphalt binder’s elasticity through controlling the interaction parameters between CRM and asphalt binder. Adv. Civ. Eng. Mater. 2020, 9, 20190204. [Google Scholar] [CrossRef]
- Li, J.; Xing, X.; Hou, X.; Wang, T.; Wang, J.; Xiao, F. Determination of SARA fractions in asphalts by mid-infrared spectroscopy and multivariate calibration. Measurement 2022, 198, 111361. [Google Scholar] [CrossRef]
- Deef-Allah, E.; Abdelrahman, M.; Ragab, M. Components’ exchanges between recycled materials and asphalt binders in asphalt mixes. Adv. Civ. Eng. Mater. 2022, 11, 20210105. [Google Scholar] [CrossRef]
- Deef Allah, E.; Abdelrahman, M.; Ragab, M. Thermal and chemical validation of the compatibility of virgin and recycled asphalt binders. Curr. Trends Civ. Struct. Eng. 2024, 11, 000756. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, G.; Wang, S.; Wang, X.; Wang, Z.; Zhang, J.; Falchetto, A.C. Variation of SARA fractions of crumb rubber modified asphalt binder-aggregate interface system and its correlation with adhesion properties. Constr. Build. Mater. 2024, 435, 136902. [Google Scholar] [CrossRef]
- Ma, X.; Ma, X.; Wang, Z.; Song, S.; Sheng, Y. Investigation of changing SARA and fatigue properties of asphalt bitumen under ageing and analysis of their relation based upon the BP neural network. Constr. Build. Mater. 2023, 394, 132163. [Google Scholar] [CrossRef]
- Guo, M.; Huang, Y.; Wang, L.; Yu, J.; Hou, Y. Using atomic force microscopy and molecular dynamics simulation to investigate the asphalt micro properties. Int. J. Pavement Res. Technol. 2018, 11, 321–326. [Google Scholar] [CrossRef]
- Chang, Q.; O’Rear, E.A.; Ghos, S.; Zaman, M.; Huang, L.; Wu, X. An atomistic model of aged asphalt guided by the oxidation chemistry of benzylic carbon with application to asphalt rejuvenated with a triglyceride. Constr. Build. Mater. 2023, 400, 132743. [Google Scholar] [CrossRef]
- Han, Z.; Cong, P. Understanding relationships between aging induced variation of asphaltene aggregation morphology and asphalt properties through molecular dynamics simulation. Constr. Build. Mater. 2024, 420, 135610. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, L.; Fini, E.H.; Li, L.; Liu, Z.; Dong, Z. Behaviors of asphalt under certain aging levels and effects of rejuvenation. Constr. Build. Mater. 2020, 249, 118748. [Google Scholar] [CrossRef]
- Fini, E.H.; Buabeng, F.S.; Abu-Lebdeh, T.; Awadallah, F. Effect of introduction of furfural on asphalt binder ageing characteristics. Road Mater. Pavement Des. 2016, 17, 638–657. [Google Scholar] [CrossRef]
- Wang, T.; Weng, Y.; Cai, X.; Li, J.; Xiao, F.; Sun, G.; Zhang, F. Statistical modeling of low-temperature properties and FTIR spectra of crumb rubber modified asphalts considering SARA fractions. J. Clean. Prod. 2022, 374, 134016. [Google Scholar] [CrossRef]
- ASTM D4124-09; Standard Test Method for Separation of Asphalt into Four Fractions. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM E1131-20; Standard Test Method for Compositional Analysis by Thermogravimetry. ASTM International: West Conshohocken, PA, USA, 2020.
- Ghavibazoo, A.; Abdelrahman, M.; Ragab, M. Mechanism of crumb rubber modifier dissolution into asphalt matrix and its effect on final physical properties of crumb rubber-modified binder. Transp. Res. Rec. 2013, 2370, 92–101. [Google Scholar] [CrossRef]
- Deef-Allah, E.; Ragab, M.; Attia, M.; Abdelrahman, M. Microstructural evolution and rheological enhancement of asphalt–rubber binders: Unveiling the role of morphology in performance. Buildings 2025, 15, 1963. [Google Scholar] [CrossRef]
- Ragab, M. Enhancing the Performance of Crumb Rubber Modified Asphalts through Controlling the Internal Network Structure Developed. Ph.D. Thesis, NDSU, Fargo, ND, USA, 2016. [Google Scholar]
- Gaestel, C.; Smadja, R.; Lamminan, K. Contribution à La Connaissance Des Propriétés Des Bitumes Routiers. Rev. Générale Routes Aérodromes 1971, 466, 85–92. [Google Scholar]
- Oliver, J.W.H. Changes in the chemical composition of Australian bitumens. Road Mater. Pavement Des. 2009, 10, 569–586. [Google Scholar] [CrossRef]
- Jones, D.; Harvey, J.; Al-Qadi, I.L.; Mateos, A. (Eds.) Advances in Pavement Design through Full-Scale Accelerated Pavement Testing; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Huang, S.-C.; Glaser, R.; Turner, F. Impact of water on asphalt aging. Transp. Res. Rec. 2012, 2293, 63–72. [Google Scholar] [CrossRef]
- Glaser, R.R.; Schabron, J.F.; Turner, T.F.; Planche, J.-P.; Salmans, S.L.; Loveridge, J.L. Low-temperature oxidation kinetics of asphalt binders. Transp. Res. Rec. 2013, 2370, 63–68. [Google Scholar] [CrossRef]
- Van den Bergh, W. The Effect of Ageing on the Fatigue and Healing Properties of Bituminous Mortars. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2011. [Google Scholar]
- Senneca, O.; Salatino, P.; Chirone, R. A fast heating-rate thermogravimetric study of the pyrolysis of scrap tyres. Fuel 1999, 78, 1575–1581. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. Pyrolysis-thermogravimetric analysis of tyres and tyre components. Fuel 1995, 74, 1277–1283. [Google Scholar] [CrossRef]
- Chen, F.; Qian, J. Studies of the thermal degradation of waste rubber. Waste Manag. 2003, 23, 463–467. [Google Scholar] [CrossRef] [PubMed]
- ASTM D7175-15; Test Method for Determining the Rheological Properties of Asphalt Binder Using a Dynamic Shear Rheometer. ASTM International: West Conshohocken, PA, USA, 2023.
- Khan, A.; Kian, L.K.; Jawaid, M.; Khan, A.A.P.; Alotaibi, M.M.; Asiri, A.M.; Marwani, H.M. Preparation of styrene-butadiene rubber (SBR) composite incorporated with collagen-functionalized graphene oxide for green tire application. Gels 2022, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Gao, Y.; Dong, Z.; Guo, Q.; Li, L.; Li, Y. Effects of commercial antioxidants on aging resistances of asphalt binders. Adv. Mate. Sci. Eng. 2022, 2022, 7725148. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Feng, Z. Infrared spectrum analysis of aged asphalt regenerated by recycled engine oil bottom. Math. Probl. Eng. 2022, 2022, 2224094. [Google Scholar] [CrossRef]
- Ali, A.S.B.; Al Allam, A.M.; Ali, S.I.A.; Isleem, H.F.; Babalghaith, A.M.; Shaffie, E.; Khishe, M. Chemical properties of peat micro particles modified asphalt. Sci. Rep. 2024, 14, 26873. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.S. An Introduction to Spectroscopy for Pavement Engineers; FHWA-HRT-20-033; Federal Highway Administration, Turner-Fairbank Highway Research Center: McLean, VA, USA, 2020. [Google Scholar]
- Zhang, F.; Cannone Falchetto, A.; Yuan, D.; Wang, W.; Wang, D.; Sun, Y. Research on performance variations of different asphalt binders results from microwave heating during freeze-thaw cycles. Constr. Build. Mater. 2024, 448, 138280. [Google Scholar] [CrossRef]
- Petersen, J.C. A Review of the Fundamentals of Asphalt Oxidation: Chemical, Physicochemical, Physical Property, and Durability Relationships; Transportation Research Board: Washington, DC, USA, 2009. [Google Scholar]
- Mullins, O.C. The modified Yen model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, M.; Hao, Y.; Zhou, Z.; Sun, D.; Yu, P.; Wu, Y. Novel polybutadiene rubber with long Cis-1,4 and syndiotactic vinyl segments (CVBR) for high performance sidewall of all-steel giant off-the-road tire. Compos. B Eng. 2024, 275, 111349. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhao, H.; Cao, R.; Huang, B.; Xu, L. Preparation and characterization of microencapsulated phase change materials with enhanced thermal performance for cold storage. Materials 2025, 18, 2074. [Google Scholar] [CrossRef] [PubMed]
- Afanasjeva, N.; González-Córdoba, A.; Palencia, M. Mechanistic approach to thermal production of new materials from asphaltenes of castilla crude oil. Processes 2020, 8, 1644. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Tankov, I.; Pavlova, A. Challenges in characterization of residual oils. a review. J. Pet. Sci. Eng. 2019, 178, 227–250. [Google Scholar] [CrossRef]
- Airey, G. Rheological properties of styrene butadiene styrene polymer modified road bitumens. Fuel 2003, 82, 1709–1719. [Google Scholar] [CrossRef]
- Lu, X.; Isacsson, U. Modification of road bitumens with thermoplastic polymers. Polym. Test. 2000, 20, 77–86. [Google Scholar] [CrossRef]
- Pei, Y.; Jiang, S.; Ding, Z.; Cheng, L.; Li, P.; Jiang, X. Preparation and performance analysis of high-viscosity asphalt containing high-content SBS and crumb rubber by oxygen-free high-temperature treatment. Constr. Build. Mater. 2023, 402, 132763. [Google Scholar] [CrossRef]


| Rubber Percentage a (%) | Mixing Temperature (°C) | Mixing Speed (min−1) | Mixing Time (h) | Code |
|---|---|---|---|---|
| 10 | 190 | 600 | 8 | A-RB 190–600 |
| 3000 | A-RB 190–3000 | |||
| 220 | 3000 | A-RB 220–3000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragab, M.; Deef-Allah, E.; Abdelrahman, M. FTIR Characterization of Asphalt SARA Fractions in Response to Rubber Modification. Appl. Sci. 2025, 15, 8062. https://doi.org/10.3390/app15148062
Ragab M, Deef-Allah E, Abdelrahman M. FTIR Characterization of Asphalt SARA Fractions in Response to Rubber Modification. Applied Sciences. 2025; 15(14):8062. https://doi.org/10.3390/app15148062
Chicago/Turabian StyleRagab, Mohyeldin, Eslam Deef-Allah, and Magdy Abdelrahman. 2025. "FTIR Characterization of Asphalt SARA Fractions in Response to Rubber Modification" Applied Sciences 15, no. 14: 8062. https://doi.org/10.3390/app15148062
APA StyleRagab, M., Deef-Allah, E., & Abdelrahman, M. (2025). FTIR Characterization of Asphalt SARA Fractions in Response to Rubber Modification. Applied Sciences, 15(14), 8062. https://doi.org/10.3390/app15148062






