Cryogenic System for FTIR Analysis of Hydrocarbon Fuels at Low Temperature and Atmospheric Pressure
Abstract
1. Introduction
2. Materials and Methods
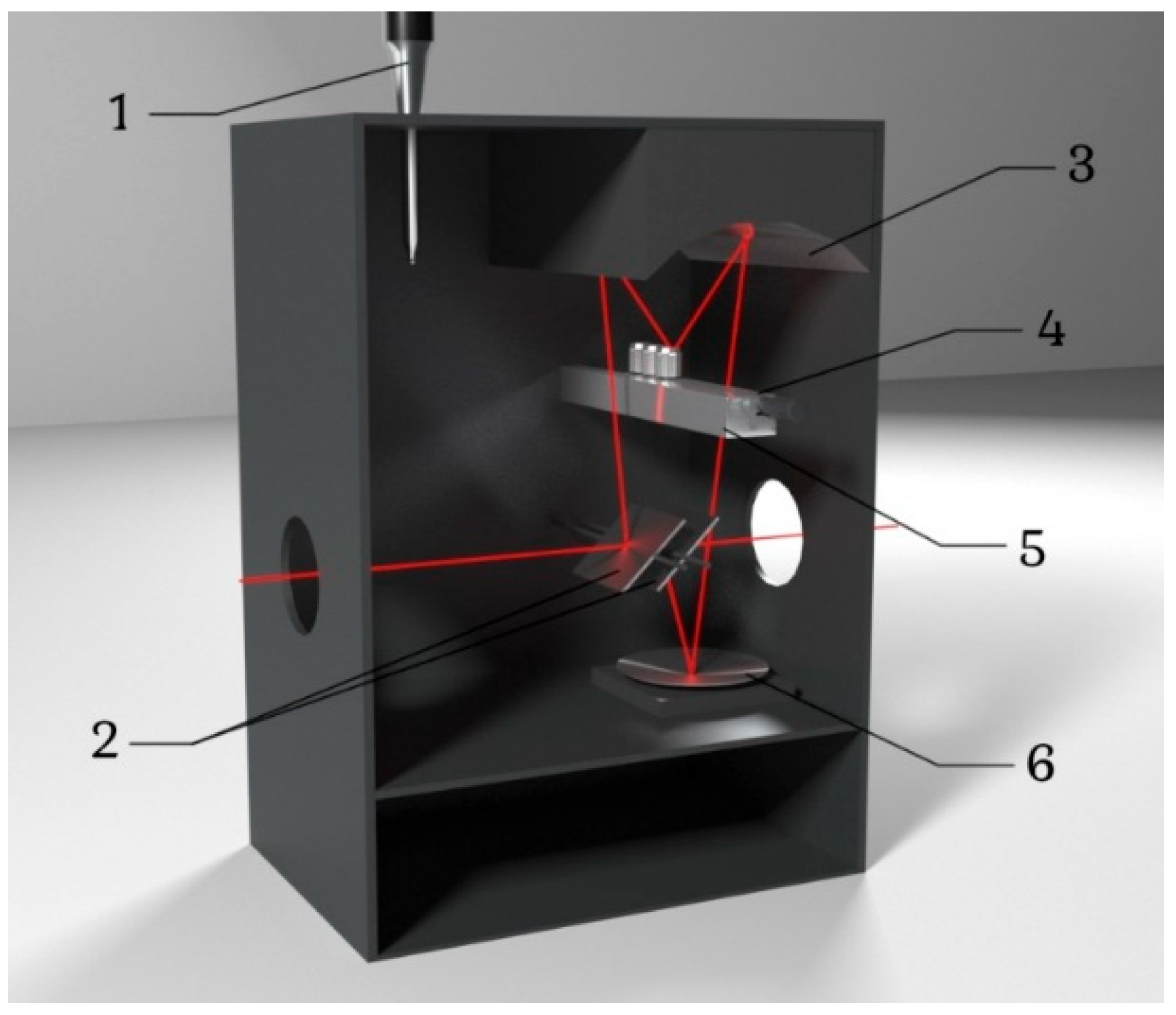
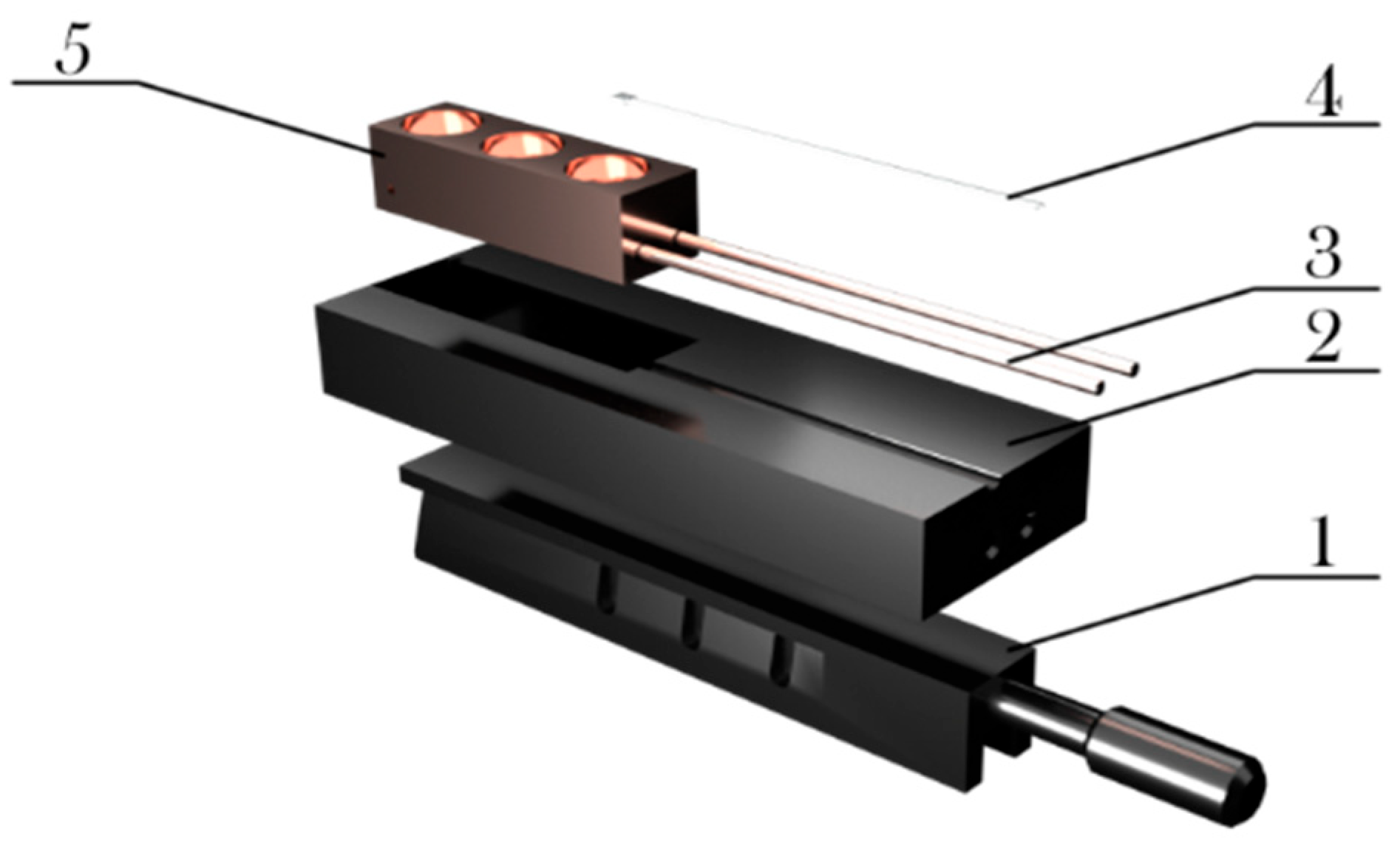
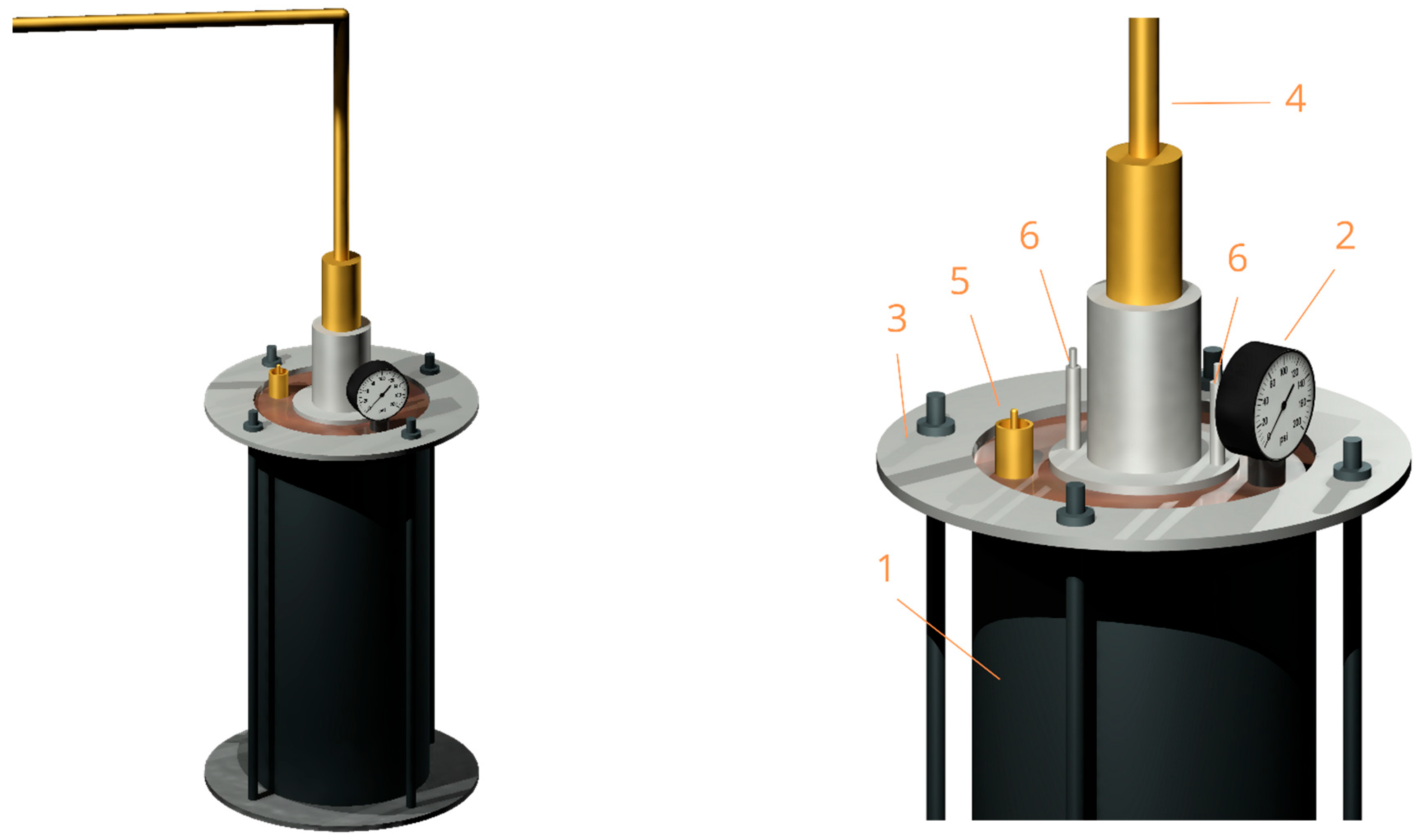
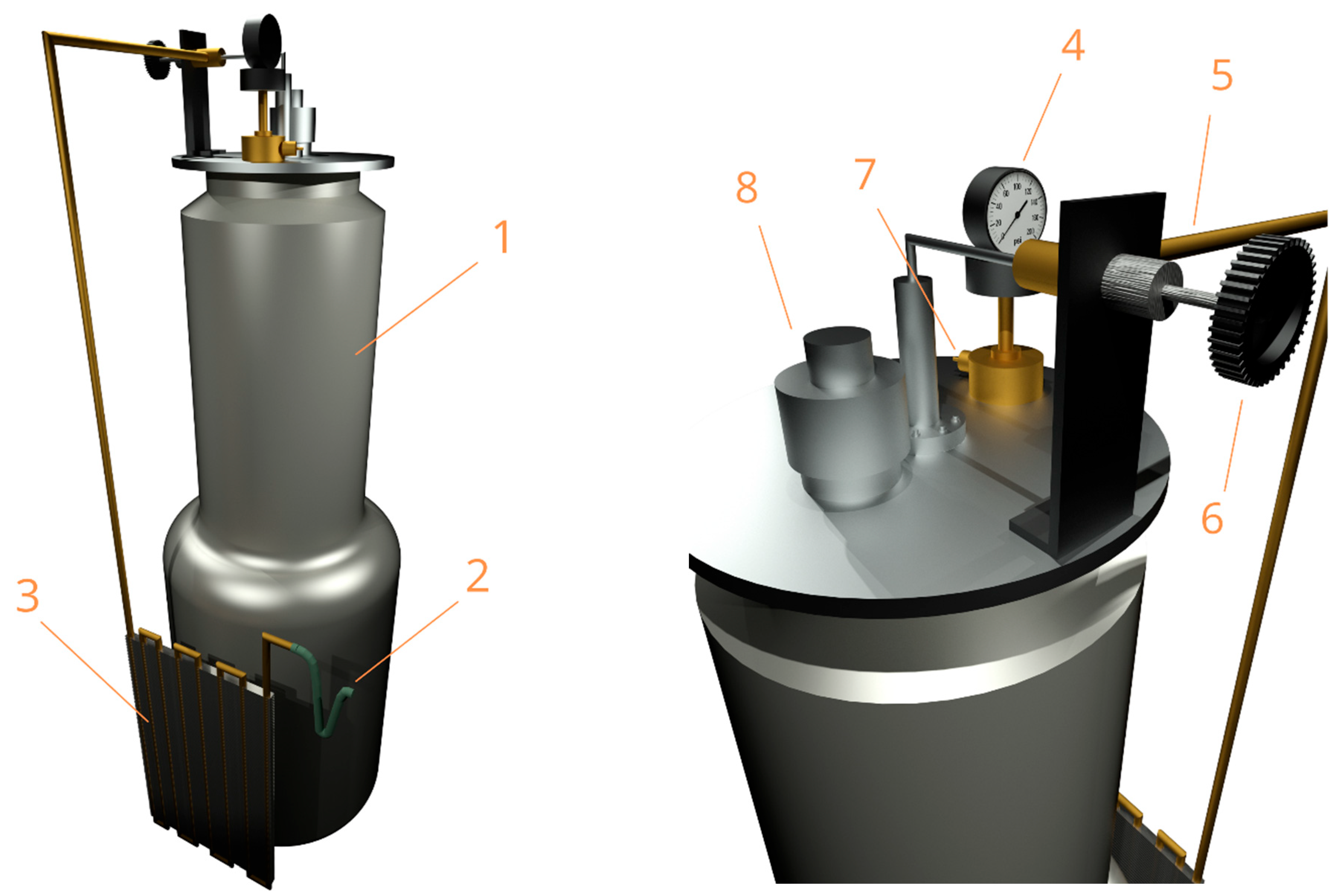
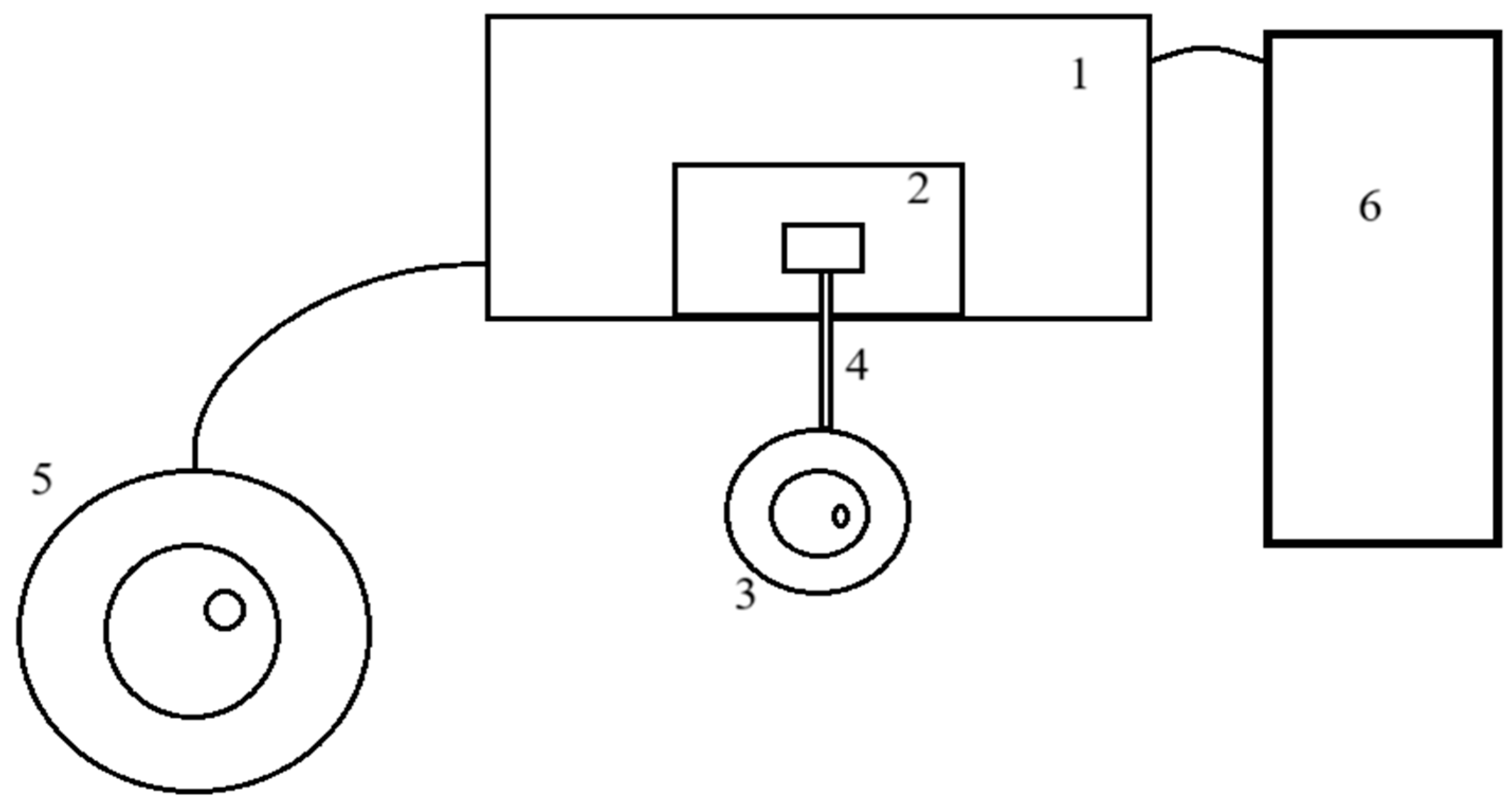
2.1. Spectrometer Configuration: Experimental Design and Modification
- Dewar sealed; check that valve is working; pressure ≥ 6 psi (0.4 atm).
- Heater powers contact element; nitrogen circulates via thermal expansion.
- No stray electrical connections, avoiding short circuits.
- Thermocouple is functional and connected to PID controller.
- Capillaries are insulated for minimal heat loss and stable cooling.
2.2. Materials
2.3. Gasifier for Creating IR-Inactive Environment
2.4. Estimation of Uncertainty and Error
2.5. Calibration of Cryogenic Cell by Temperature
2.6. Experimental Methodology
2.7. Methods of Preliminary Results Processing
3. Results
3.1. Uncertainty Assessment
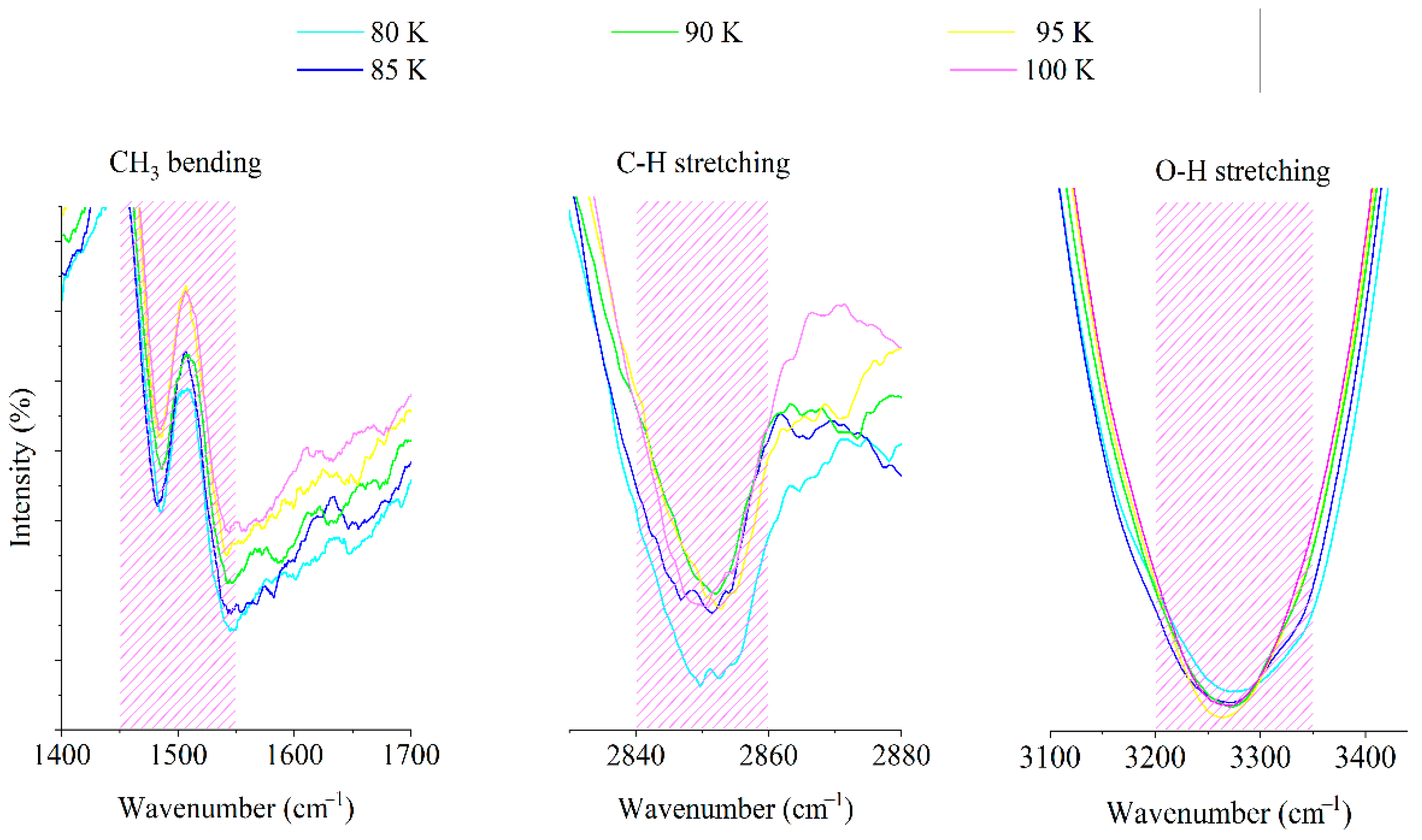
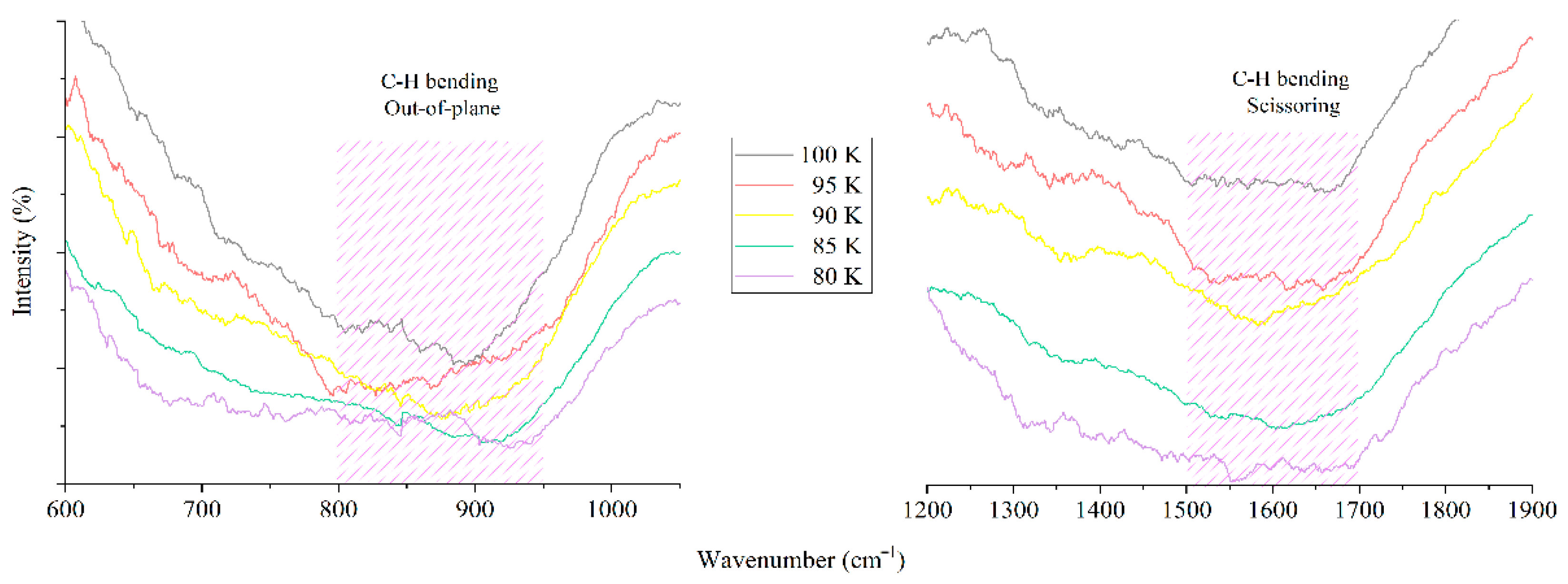
3.2. FTIR Spectroscopy Data of Hydrocarbon Samples Obtained Using the Cryogenic System
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DRIFT | Diffuse Reflectance Infrared Fourier Transform |
| ESA | European Space Agency |
| FTIR | Fourier-transform InfraRed |
| IR | InfraRed |
| NASA | National Aeronautics and Space Administration (American) |
| PID | Proportional-Integral-Derivative |
| RSS | Root Sum Square |
Appendix A

References
- Shaalan, N.M. An approach to fabricate nanomaterials using a closed low-temperature growth system. Mater. Chem. Phys. 2024, 328, 129930. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Arkharova, N.A.; Karimov, D.N. Synthesis of nano-sized solid electrolyte Pr1-ySryF3-y and the effect of heat treatment on the ionic conductivity of fluoride nanoceramics. Crystallogr. Rep. 2024, 69, 561–568. [Google Scholar] [CrossRef]
- Carrascosa, H.; Muñoz Caro, G.M.; Martín-Doménech, R.; Cazaux, S.; Chen, Y.-J.; Fuente, A. Formation and desorption of sulphur chains (H2Sx and Sx ) in cometary ice: Effects of ice composition and temperature. Mon. Not. R. Astron. Soc. 2024, 533, 967–978. [Google Scholar] [CrossRef]
- Kakkenpara Suresh, S.; Dulieu, F.; Vitorino, J.; Caselli, P. Experimental study of the binding energy of NH3 on different types of ice and its impact on the snow line of NH3 and H2O. Astron. Astrophys. 2024, 682, A163. [Google Scholar] [CrossRef]
- Sokolov, D.Y.; Yerezhep, D.; Vorobyova, O.; Golikov, O.; Aldiyarov, A.U. Infrared analysis and effect of nitrogen and nitrous oxide on the glass transition of methanol cryofilms. ACS Omega 2022, 7, 46402–46410. [Google Scholar] [CrossRef]
- Yerezhep, D.; Akylbayeva, A.; Golikov, O.; Sokolov, D.Y.; Shinbayeva, A.; Aldiyarov, A.U. Analysis of vibrational spectra of tetrafluoroethane glasses deposited by physical vapor deposition. ACS Omega 2023, 8, 19567–19574. [Google Scholar] [CrossRef]
- Bhavani, G.; Durga Rao, T.; Niranjan, M.K.; Kumar, K.R.; Sattibabu, B.; Petkov, V.; Kannan, E.S.; Reddy, B.H. Structural, magnetic, optical and electronic properties of Gd2NiIrO6. Phys. B Condens. Matter 2024, 695, 416477. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Q.; Zheng, T.; Xie, M.; Shen, H.; Li, Y.; Guo, F.; Zhang, Q.; Duan, M.; Wu, K.-H. Comparing low-temperature NH3-SCR activity, operating temperature window and kinetic properties of the Mn-Fe-Nb/TiO2 catalysts prepared by different methods. Sep. Purif. Technol. 2025, 356, 129906. [Google Scholar] [CrossRef]
- Nishikino, T.; Sugimoto, T.; Kandori, H. Low-temperature FTIR spectroscopy of the L/Q switch of proteorhodopsin. Phys. Chem. Chem. Phys. 2024, 26, 22959–22967. [Google Scholar] [CrossRef]
- Yang, Z.; Su, B.; Ding, H.; Qiu, Y.; Zhong, D. Prediction of asphalt low-temperature performance by FTIR spectra using comparative modelling strategy. Road Mater. Pavement Des. 2024, 26, 1–16. [Google Scholar] [CrossRef]
- Moszczyńska, J.; Liu, X.; Wiśniewski, M. Non-thermal ammonia decomposition for hydrogen production over carbon films under low-temperature plasma—In-situ ftir studies. Int. J. Mol. Sci. 2022, 23, 9638. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.; Kavir Dass, C.; Hao, K.; Chen, C.-H.; Li, L.-J.; Singh, A.; Tran, K.; Clark, G.; Xu, X.; Berghäuser, G.; et al. Intrinsic homogeneous linewidth and broadening mechanisms of excitons in monolayer transition metal dichalcogenides. Nat. Commun. 2015, 6, 8315. [Google Scholar] [CrossRef]
- Dillinger, S.; Mohrbach, J.; Hewer, J.; Gaffga, M.; Niedner-Schatteburg, G. Infrared spectroscopy of N2 adsorption on size selected cobalt cluster cations in isolation. Phys. Chem. Chem. Phys. 2015, 17, 10358–10362. [Google Scholar] [CrossRef] [PubMed]
- Fortes, P.R.; Da Silveira Petruci, J.F.; Wilk, A.; Cardoso, A.A.; Raimundo, I.M., Jr.; Mizaikoff, B. Optimized design of substrate-integrated hollow waveguides for mid-infrared gas analyzers. J. Opt. 2014, 16, 094006. [Google Scholar] [CrossRef]
- Serdyukov, V.I.; Sinitsa, L.N.; Lugovskoi, A.A.; Emel’yanov, N.M. Liquid-nitrogen-cooled optical cell for the study of absorption spectra in a fourier spectrometer. Atmos. Ocean. Opt. 2020, 33, 393–399. [Google Scholar] [CrossRef]
- Serdyukov, V.I.; Sinitsa, L.N.; Lugovskoi, A.A.; Emelyanov, N.M. Low-temperature cell for studying absorption spectra of greenhouse gases. Atmos. Ocean. Opt. 2019, 32, 220–226. [Google Scholar] [CrossRef]
- Sung, K.; Mantz, A.W.; Smith, M.A.H.; Brown, L.R.; Crawford, T.J.; Devi, V.M.; Benner, D.C. Cryogenic absorption cells operating inside a Bruker IFS-125HR: First results for 13CH4 at 7μm. J. Mol. Spectrosc. 2010, 262, 122–134. [Google Scholar] [CrossRef]
- Mantz, A.W.; Sung, K.; Brown, L.R.; Crawford, T.J.; Smith, M.A.H.; Malathy Devi, V.; Chris Benner, D. A cryogenic Herriott cell vacuum-coupled to a Bruker IFS-125HR. J. Mol. Spectrosc. 2014, 304, 12–24. [Google Scholar] [CrossRef]
- Kenbay, A.A.; Yu Golikov, O.; Aldiyarov, A.U.; Yerezhep, D.E. Low-temperature cell for IR Fourier spectrometric investigation of hydrocarbon substances. Sci. Tech. J. Inf. Technol. Mech. Opt. 2023, 23, 696–702. [Google Scholar] [CrossRef]
- Hudson, R.L.; Gerakines, P.A.; Yarnall, Y.Y. Infrared spectroscopic and physical properties of methanol ices—Reconciling the conflicting published band strengths of an important interstellar solid. Astrophys. J. 2024, 970, 108. [Google Scholar] [CrossRef]
- Carrascosa, H.; Satorre, M.Á.; Escribano, B.; Martín-Doménech, R.; Muñoz Caro, G.M. Physical properties of methanol (CH3OH) ice as a function of temperature: Density, infrared band strengths, and crystallization. Mon. Not. R. Astron. Soc. 2023, 525, 2690–2700. [Google Scholar] [CrossRef]
- Luna, R.; Molpeceres, G.; Ortigoso, J.; Satorre, M.A.; Domingo, M.; Maté, B. Densities, infrared band strengths, and optical constants of solid methanol. Astron. Astrophys. 2018, 617, A116. [Google Scholar] [CrossRef]
- Dollah, A.; Zainol Rashid, Z.; Hidayati Othman, N.; Nurliyana Che Mohamed Hussein, S.; Mat Yusuf, S.; Shuhadah Japperi, N. Effects of ultrasonic waves during waterflooding for enhanced oil recovery. Int. J. Eng. Technol. 2018, 7, 232. [Google Scholar] [CrossRef]
- Mirea, R.; Cican, G. Theoretical assessment of different aviation fuel blends based on their physical-chemical properties. Eng. Technol. Appl. Sci. Res. 2024, 14, 14134–14140. [Google Scholar] [CrossRef]
- Biaktluanga, L.; Lalhruaitluanga, J.; Lalramnghaka, J.; Thanga, H.H. Analysis of gasoline quality by ATR-FTIR spectroscopy with multivariate techniques. Results Chem. 2024, 8, 101575. [Google Scholar] [CrossRef]
- Aldiyarov, A.; Yerezhep, D.; Sokolov, D.; Korshikov, E.; Nurmukan, A.; Tasmukhanova, A.; Ramos, M.A. Multiple phase transitions in ethanol thin films obtained by physical vapor deposition. Appl. Surf. Sci. 2025, 687, 162259. [Google Scholar] [CrossRef]
- Hudson, R.L. An IR investigation of solid amorphous ethanol—Spectra, properties, and phase changes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 187, 82–86. [Google Scholar] [CrossRef]
- Golub, P.; Doroshenko, I.; Pogorelov, V.; Sablinskas, V.; Balevicius, V.; Ceponkus, J. Temperature evolution of cluster structures in ethanol. Dataset Pap. Sci. 2013, 2013, 473294. [Google Scholar] [CrossRef]
- GOST 18499-73; Kerosene for Technical Use. USSR National Committee on Standards: Moscow, Union of Soviet Socialist Republics, 1973.
- Xie, M.; Xu, Q.; Xie, L.; Li, Y.; Han, B. Establishment and optimization of the three-band fluorometric indices for oil species identification: Implications on the optimal excitation wavelengths and the detection band combinations. Anal. Chim. Acta 2023, 1280, 341871. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Li, J. Waveconv-slstm-ket: A novel framework for the multi-task analysis of oil spill fluorescence spectra. Appl. Sci. 2025, 15, 3177. [Google Scholar] [CrossRef]
| № | Uncertainty Source | Type | Units | Standard Deviation or Error | Distribution | Standard Uncertainty | Sensitivity Coefficient | Uncertainty Contribution, cm−1 |
|---|---|---|---|---|---|---|---|---|
| 1. | Spectral resolution | B | cm−1 | 0.125 | Rectangular | 1 | 0.072 | |
| 2. | Instrument error | B | cm−1 | 0.500 | Rectangular | 1 | 0.289 | |
| 3. | Digital noise/baseline instability | A | cm−1 | 0.200 | Normal | 0.2 | 1 | 0.200 |
| 4. | Deviation of peak position approximation | A | cm−1 | 0.300 | Normal | 0.3 | 1 | 0.300 |
| Total standard uncertainty | 0.468 | |||||||
| Expanded uncertainty | 0.936 | |||||||
| № | Uncertainty Source | Type | Units | Standard Deviation or Error | Distribution | Standard Uncertainty | Sensitivity Coefficient | Uncertainty Contribution, K |
|---|---|---|---|---|---|---|---|---|
| 1. | Thermocouple voltage | B | mV | 0.01 | Rectangular | dT/dV ≈ 40 K/mV | 0.231 | |
| 2. | Contact thermal resistance | B | K | 0.35 | Rectangular | 1 | 0.202 | |
| 3. | Temperature gradient in bar | A | K | 0.27 | Rectangular | 1 | 0.156 | |
| 4. | PID controller fluctuation | B | K | 0.10 | Normal | 0.1 | 1 | 0.100 |
| Total standard uncertainty | 0.358 | |||||||
| Expanded uncertainty | 0.716 | |||||||
| № | Authors | Temperature, Type | Pressure, Type | CH3 Bending, cm−1 | C-H Stretching, cm−1 | O-H Stretching, cm−1 |
|---|---|---|---|---|---|---|
| 1. | Hudson, Gerakines, and Yarnall [20] | Low (10 K) | Vacuum | Peak at 1459 (occurs with O-H bending as well) | Peak at 2952 | Peak at 3259 |
| 2. | Carrascosa et al. [21] | Low (30–135 K) | Vacuum | 1445–1472 | 2828–2985 | Centered near 3250 |
| 3. | Luna et al. [22] | Low (20–60 K) | Vacuum | Peak at 1460 (occurs with O-H bending as well) | Peak at 2833 | Centered near 3250 |
| 4. | Our results | Low (80–100 K) | Atmospheric | 1450–1550 | 2840–2860 | 3200–3350 |
| № | Authors | Temperature, Type | Pressure, Type | C-H Bending (Rocking and Out-of-Plane), cm−1 | C-H Bending (Scissoring), cm−1 |
|---|---|---|---|---|---|
| 1. | Dollah, A., Zainol Rashid, Z. et al. [23] | Room | Vacuum | 800 | 1300–1500 |
| 2. | Mirea, R., and Cican, G. [24] | Room | Vacuum | 650–800 | 1300–1500 |
| 3. | Biaktluanga, L., Lalhruaitluanga, J. et al. [25] | Room | Vacuum | 800 | 1500 |
| 4. | Our results | Low (80–100 K) | Atmospheric | 800–950 | 1500–1700 |
| № | Authors | Temperature, Type | Pressure, Type | C-H Stretching, cm−1 | O-H Stretching, cm−1 |
|---|---|---|---|---|---|
| 1. | Aldiyarov A., et al. [26] | Low (13–151 K) | Vacuum | 2800–3000 | 3000–3500 |
| 2. | Reggie L. Hudson [27] | Low (10–155 K) | Vacuum | 2970–2986 | 3177–3377 |
| 3. | P. Golub, et al. [28] | Low (40–180 K) | Vacuum | 2800–3000 | 3000–3500 |
| 4. | Our results | Low (80–100 K) | Atmospheric | 2950–3050 | 3350–3400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turlybekova, G.; Kenbay, A.; Aldiyarov, A.; Korshikov, Y.; Lesbayev, A.; Nurmukan, A.; Yerezhep, D. Cryogenic System for FTIR Analysis of Hydrocarbon Fuels at Low Temperature and Atmospheric Pressure. Appl. Sci. 2025, 15, 7944. https://doi.org/10.3390/app15147944
Turlybekova G, Kenbay A, Aldiyarov A, Korshikov Y, Lesbayev A, Nurmukan A, Yerezhep D. Cryogenic System for FTIR Analysis of Hydrocarbon Fuels at Low Temperature and Atmospheric Pressure. Applied Sciences. 2025; 15(14):7944. https://doi.org/10.3390/app15147944
Chicago/Turabian StyleTurlybekova, Gulzhan, Alisher Kenbay, Abdurakhman Aldiyarov, Yevgeniy Korshikov, Aidos Lesbayev, Assel Nurmukan, and Darkhan Yerezhep. 2025. "Cryogenic System for FTIR Analysis of Hydrocarbon Fuels at Low Temperature and Atmospheric Pressure" Applied Sciences 15, no. 14: 7944. https://doi.org/10.3390/app15147944
APA StyleTurlybekova, G., Kenbay, A., Aldiyarov, A., Korshikov, Y., Lesbayev, A., Nurmukan, A., & Yerezhep, D. (2025). Cryogenic System for FTIR Analysis of Hydrocarbon Fuels at Low Temperature and Atmospheric Pressure. Applied Sciences, 15(14), 7944. https://doi.org/10.3390/app15147944






