Green Macroalgae Biomass Upcycling as a Sustainable Resource for Value-Added Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Collection of Green Macroalgae Ulva sp.
2.3. Physicochemical Analysis
2.3.1. Chemical Composition
2.3.2. Fatty Acids Profile
2.4. Pigments Analysis (Chlorophylls and Carotenoids)
2.5. Extraction of Oven-Dried Powder of Ulva sp.
2.6. Polyphenols
2.6.1. Total Phenolic Content (TPC)
2.6.2. Total Flavonoid Content (TFC)
2.7. DPPH Radical Scavenging Activity
2.8. ABTS Radical Scavenging Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis
3.1.1. Chemical Composition
3.1.2. Fatty Acid Profile
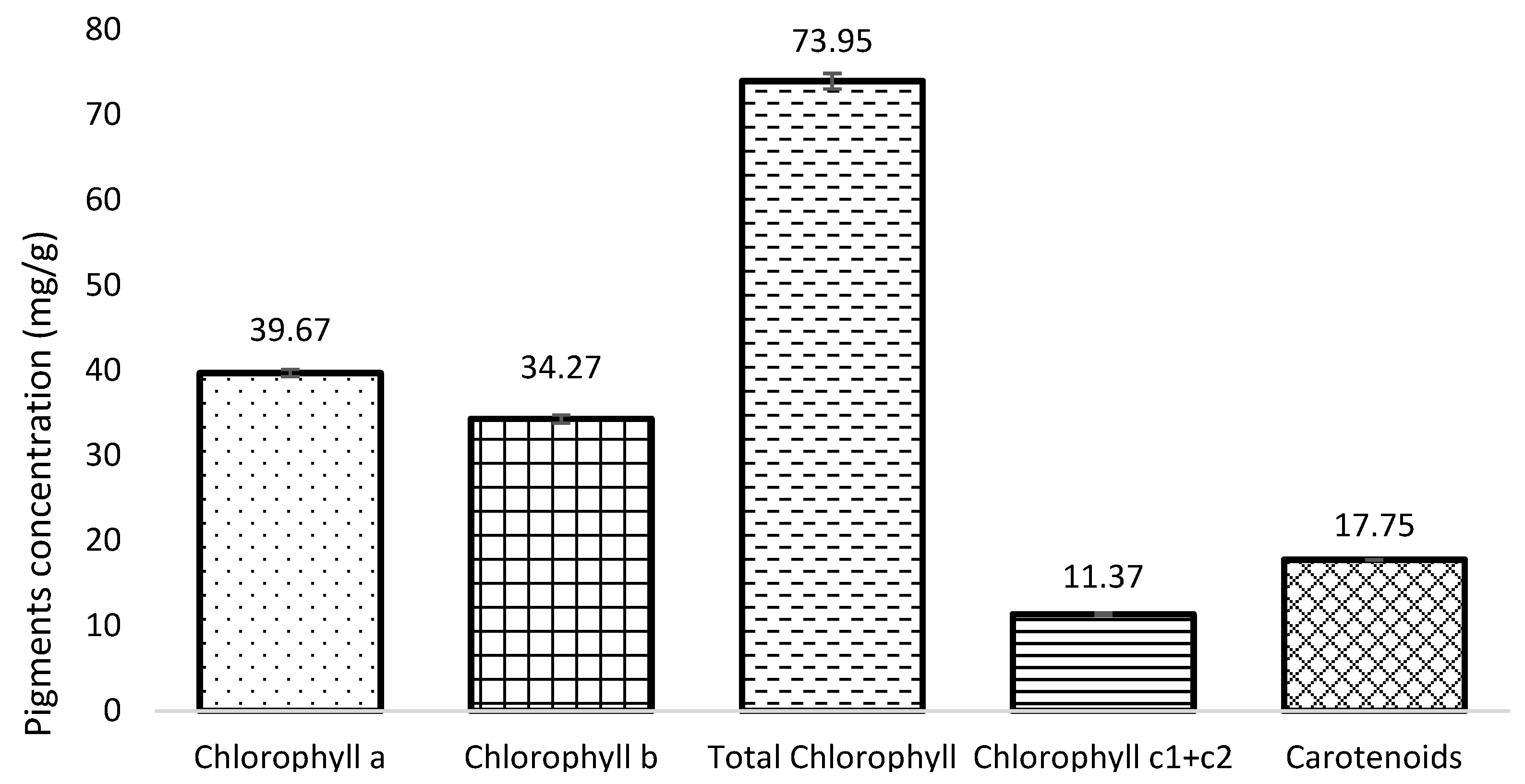
3.2. Pigment Content (Chlorophylls and Carotenoids)
3.3. Phenolic Compounds
3.4. Antioxidant Activity (DPPH and ABTS Assays)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIC | Minimum Inhibitory Concentration |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| PBS | Phosphate-Buffered Saline |
| DOL | Department of Oceanography and Limnology |
| UFRN | Federal University of Rio Grande do Norte |
| UODP | Ulva sp. Oven-Dried Powder |
| AOAC | Association of Official Analytical Chemists |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| NIST | National Institute of Standards and Technology |
| RI | Retention Index |
| TPC | Total Phenolic Content |
| GAE | Gallic acid Equivalents |
| TFC | Total Flavonoids Content |
| QE | Quercetin Equivalents |
| TE | Trolox Equivalents |
| CFU | Colony-Forming Units |
| SD | Standard Deviation |
| DW | Dry Weight |
| RT | Retention Time |
| PUFA | Polyunsaturated Fatty Acids |
| MET | Methanolic Extract |
| ETA | Ethanolic Extract |
| WAT | Aqueous Extract |
| ND | Not Detected |
| CAPES | Coordination for Personal Improvement of Higher Education |
| LEA | Food Engineering Laboratory |
References
- Palaniyappan, S.; Sridhar, A.; Kari, Z.A.; Téllez-Isaías, G.; Ramasamy, T. Evaluation of Phytochemical Screening, Pigment Content, In Vitro Antioxidant, Antibacterial Potential and GC-MS Metabolite Profiling of Green Seaweed Caulerpa racemosa. Mar. Drugs 2023, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, H.; Hu, H.; Liu, Y.; Mao, Y.; Zhou, H.; Xu, X.; Zhang, F. Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of north China. Aquaculture 2006, 252, 264–276. [Google Scholar] [CrossRef]
- Aitken, D.; Bulboa, C.; Godoy-Faundez, A.; Turrion-Gomez, J.L.; Antizar-Ladislao, B. Life cycle assessment of macroalgae cultivation and processing for biofuel production. J. Clean. Prod. 2014, 75, 45–56. [Google Scholar] [CrossRef]
- Pantis, A.; Nikoloudakis, C.; Tsoutsos, T. A Critical Review of Macroalgae Exploitation Pathways Implemented under the Scope of Life Cycle Assessment. ChemEngineering 2024, 8, 74. [Google Scholar] [CrossRef]
- Parsons, S.; Allen, M.J.; Abeln, F.; McManus, M.; Chuck, C.J. Sustainability and life cycle assessment (LCA) of macroalgae-derived single cell oils. J. Clean. Prod. 2019, 232, 1272–1281. [Google Scholar] [CrossRef]
- Hashemi, M.; Mirmohamadsadeghi, S.; Khoshnevisan, B.; Galán-Martín, Á.; Denayer, J.F.M.; Karimi, K. Life cycle assessment of bioenergy and value-added biochemical production from Nizimudinia zanardini brown macroalgae. Sci. Total Environ. 2025, 976, 179225. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Pereira, C.S.G.P.; Silva, A.; Barciela, P.; Jorge, A.O.S.; Perez-Vazquez, A.; Pereira, A.G.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Prieto, M.A. Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds. Mar. Drugs 2024, 22, 478. [Google Scholar] [CrossRef] [PubMed]
- Narayanankutty, A.; Famurewa, A.C.; Oprea, E. Natural Bioactive Compounds and Human Health. Molecules 2024, 29, 3372. [Google Scholar] [CrossRef] [PubMed]
- Minicante, S.A.; Bongiorni, L.; De Lazzari, A. Bio-Based Products from Mediterranean Seaweeds: Italian Opportunities and Challenges for a Sustainable Blue Economy. Sustainability 2022, 14, 5634. [Google Scholar] [CrossRef]
- Francezon, N.; Tremblay, A.; Mouget, J.-L.; Pasetto, P.; Beaulieu, L. Algae as a Source of Natural Flavors in Innovative Foods. J. Agric. Food Chem. 2021, 69, 11753–11772. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Rey, F.; Leal, M.C.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects. Mar. Drugs 2021, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. World cuisine of seaweeds: Science meets gastronomy. Int. J. Gastron. Food Sci. 2018, 14, 55–65. [Google Scholar] [CrossRef]
- El-Shafei, R.; Hegazy, H.; Acharya, B. A Review of Antiviral and Antioxidant Activity of Bioactive Metabolite of Macroalgae within an Optimized Extraction Method. Energies 2021, 14, 3092. [Google Scholar] [CrossRef]
- Yordi, E.G.; Martínez, A.P.; Radice, M.; Scalvenzi, L.; Abreu-Naranjo, R.; Uriarte, E.; Santana, L.; Matos, M.J. Seaweeds as Source of Bioactive Pigments with Neuroprotective and/or Anti-Neurodegenerative Activities: Astaxanthin and Fucoxanthin. Mar. Drugs 2024, 22, 327. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, Z.; Zhu, Y.; Wen, J.; Wen, Y.; Liu, Y.; Chen, W.; Zhao, C. Green algal polysaccharides and derivatives as potential therapeutics for metabolic diseases. Food Biosci. 2024, 62, 105310. [Google Scholar] [CrossRef]
- Boukid, F.; Castellari, M. Algae as Nutritional and Functional Food Sources. Foods 2023, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Devaprakash, M.; Thirumalaivasan, R.; Sivakumar, N.; Shyam Kumar, R.; Ponmurugan, K. Nutraceuticals and Functional Foods from Algae: Formulation and Health Benefits. In Value Added Products from Bioalgae Based Biorefineries: Opportunities and Challenges; Arya, S.K., Khatri, M., Singh, G., Eds.; Springer Nature: Singapore, 2024; pp. 289–341. ISBN 978-981-97-1662-3. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Mutavski, Z.; Jerković, I.; Nikolić, N.Ć.; Radman, S.; Flanjak, I.; Aladić, K.; Šubarić, D.; Vulić, J.; Jokić, S. Comprehensive Phytochemical Profiling of Ulva lactuca from the Adriatic Sea. Int. J. Mol. Sci. 2024, 25, 11711. [Google Scholar] [CrossRef] [PubMed]
- Esim, N.; Dawar, P.; Arslan, N.P.; Orak, T.; Doymus, M.; Azad, F.; Ortucu, S.; Albayrak, S.; Taskin, M. Natural metabolites with antioxidant activity from micro-and macro-algae. Food Biosci. 2024, 62, 105089. [Google Scholar] [CrossRef]
- Vinosha, M.; Palanisamy, S.; Jeneeta, S.; Rajasekar, P.; Marudhupandi, T.; Karthikeyan, M.; Mohandoss, S.; You, S.; Prabhu, N.M. Antibacterial, anticancer and antioxidant effects of sulfated galactan from Halymenia dilatata: In vitro and in vivo analysis. Food Biosci. 2024, 60, 104420. [Google Scholar] [CrossRef]
- Zammuto, V.; Rizzo, M.G.; Spanò, A.; Spagnuolo, D.; Di Martino, A.; Morabito, M.; Manghisi, A.; Genovese, G.; Guglielmino, S.; Calabrese, G.; et al. Effects of crude polysaccharides from marine macroalgae on the adhesion and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus. Algal Res. 2022, 63, 102646. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Donn, P.; Prieto, M.A.; Mejuto, J.C.; Cao, H.; Simal-Gandara, J. Functional foods based on the recovery of bioactive ingredients from food and algae by-products by emerging extraction technologies and 3D printing. Food Biosci. 2022, 49, 101853. [Google Scholar] [CrossRef]
- Monroy-García, I.N.; Torres-Romero, S.; Castro-Ochoa, L.D.; Mendoza-Acosta, A.; Viveros-Valdez, E.; Ayala-Zavala, F. Bioactive Compounds from Marine Macroalgae: A Natural Defense Against Oxidative Stress-Related Diseases. Stresses 2025, 5, 22. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Golshany, H.; Yu, Q.; Fan, L. Comparative extraction and antioxidant potential of bioactive compounds from Fucus vesiculosus: Kinetic modeling and UPLC-Q-TOF-MS phenolic profiling. Food Biosci. 2024, 57, 103575. [Google Scholar] [CrossRef]
- Carvalho, A.; Müller, L.; Rosas, V.; Tesser, M.B.; Ventura-Lima, J.; Turan, G.; Pias, M.; Poersch, L.H. Evaluation of the Inclusion of the Seaweed Ulva lactuca Produced in an Integrated System with Biofloc in the Diet of Juvenile Tilapia Oreochromis niloticus. Appl. Sci. 2025, 15, 6410. [Google Scholar] [CrossRef]
- Marques, M.L.M.; Presa, F.B.; Viana, R.L.S.; Costa, M.S.S.P.; Amorim, M.O.R.; Bellan, D.L.; Alves, M.G.C.F.; Costa, L.S.; Trindade, E.S.; Rocha, H.A.O. Anti-Thrombin, Anti-Adhesive, Anti-Migratory, and Anti-Proliferative Activities of Sulfated Galactans from the Tropical Green Seaweed, Udotea flabellum. Mar. Drugs 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, V.A.R.; Martins, N.T.; da Silva, S.L.A.; de Barros-Barreto, M.B.; Pereira, S.B.; Cassano, V. Revealing the diversity of the genus Ulva (Ulvales, Chlorophyta) in southeastern Brazil, with a description of Ulva kanagawae sp. nov. Phycologia 2023, 62, 407–420. [Google Scholar] [CrossRef]
- Barata, D. Clorofíceas Marinhas Bentônicas do Estado do Espírito Santo. Master’s Thesis, Instituto de Botânica da Secretaria de Estado do Meio Ambiente, São Paulo, Brazil, 2004; 210p. Available online: https://smastr16.blob.core.windows.net/pgibt/2013/09/Diogina_Barata_MS.pdf (accessed on 13 June 2025).
- Mandalka, A.; Cavalcanti, M.I.L.G.; Harb, T.B.; Fujii, M.T.; Eisner, P.; Schweiggert-Weisz, U.; Chow, F. Nutritional Composition of Beach-Cast Marine Algae from the Brazilian Coast: Added Value for Algal Biomass Considered as Waste. Foods 2022, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Pappou, S.; Dardavila, M.M.; Savvidou, M.G.; Louli, V.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from Ulva lactuca. Appl. Sci. 2022, 12, 2117. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000; ISBN 978-0-935584-67-7. [Google Scholar]
- Correia, R.; Grace, M.H.; Esposito, D.; Lila, M.A. Wild blueberry polyphenol-protein food ingredients produced by three drying methods: Comparative physico-chemical properties, phytochemical content, and stability during storage. Food Chem. 2017, 235, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: New York, NY, USA, 1999; Volume 299, pp. 152–178. ISBN 978-0-12-182200-2. [Google Scholar]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.; Alves, R.; Brito, E.; Morais, S.; Sampaio, C.; Pérez-Jiménez, J. Metodologia Científica: Determinacão da atividade antioxidante total em frutas pela captura do radical ABTS+. In Embrapa Agroindústria Tropical Comunicado Técnico #128; Embrapa: Brasília, Brazil, 2007. [Google Scholar]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An overview on the nutritional and bioactive components of green seaweeds. Food Prod. Process. Nutr. 2023, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Limiñana, V.A.; Benoist, T.; Sempere, S.A.; Pérez, S.E.M.; Moya, M.S.P. Chemical composition of sustainable Mediterranean macroalgae obtained from land-based and sea-based aquaculture systems. Food Biosci. 2023, 54, 102902. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Otávio, L.; Pereira, S.; Marquez, U.M.L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Rostad, C.E.; Pereira, W.E. Kovats and lee retention indices determined by gas chromatography/mass spectrometry for organic compounds of environmental interest. J. High Resolut. Chromatogr. 1986, 9, 328–334. [Google Scholar] [CrossRef]
- Kotb, S.S.; Ayoub, I.M.; El-Moghazy, S.A.; Singab, A.N.B. Profiling the Lipophilic Fractions of Pithecellobium dulce Bark and Leaves Using GC/MS and Evaluation of Their Antioxidant, Antimicrobial and Cytotoxic Activities. Chem. Biodivers. 2020, 17, e2000048. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.L.; Silva, S.G.; Costa, J.M.; Da Costa, W.A.; Maia, A.A.B.; De Oliveira, M.S.; De Andrade, E.H.A. Chemical profile of manually extracted andiroba oil (Carapa guianensis Aubl., Meliaceae) from Mamangal community, located in Igarapé-Miri, Pará, Brazil. Sci. Plena 2021, 17, 127201. [Google Scholar] [CrossRef]
- Daneshvand, B.; Ara, K.M.; Raofie, F. Comparison of supercritical fluid extraction and ultrasound-assisted extraction of fatty acids from quince (Cydonia oblonga Miller) seed using response surface methodology and central composite design. J. Chromatogr. A 2012, 1252, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Riley, T.M.; Sapp, P.A.; Kris-Etherton, P.M.; Petersen, K.S. Effects of saturated fatty acid consumption on lipoprotein (a): A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2024, 120, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; West, J.A.; Koulman, A. A Review of Odd-Chain Fatty Acid Metabolism and the Role of Pentadecanoic Acid (C15:0) and Heptadecanoic Acid (C17:0) in Health and Disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am. J. Clin. Nutr. 1994, 60, 986S–990S. [Google Scholar] [CrossRef] [PubMed]
- Rodeiro, I.; Olguín, S.; Santes, R.; Herrera, J.A.; Pérez, C.L.; Mangas, R.; Hernández, Y.; Fernández, G.; Hernández, I.; Hernández-Ojeda, S.; et al. Gas Chromatography-Mass Spectrometry Analysis of Ulva fasciata (Green Seaweed) Extract and Evaluation of Its Cytoprotective and Antigenotoxic Effects. Evid. Based Complement. Alternat. Med. 2015, 2015, 520598. [Google Scholar] [CrossRef] [PubMed]
- Caamaño, E.; Loperena, L.; Hinzpeter, I.; Pradel, P.; Gordillo, F.; Corsini, G.; Tello, M.; Lavín, P.; González, A.R. Isolation and molecular characterization of Thraustochytrium strain isolated from Antarctic Peninsula and its biotechnological potential in the production of fatty acids. Braz. J. Microbiol. 2017, 48, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-T.; Park, D.-H. Optimization of lipid extraction from marine green macro-algae as biofuel resources. Korean J. Chem. Eng. 2015, 32, 2463–2467. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, Y.; Wu, M.; Li, X.; Mai, K.; Ai, Q. ω-6 Polyunsaturated fatty acids (linoleic acid) activate both autophagy and antioxidation in a synergistic feedback loop via TOR-dependent and TOR-independent signaling pathways. Cell Death Dis. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of Valuable Compounds and Bioactive Metabolites from By-Products of Fish Discards Using Chemical Processing, Enzymatic Hydrolysis, and Bacterial Fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef] [PubMed]
- Tjandrawinata, R.R.; Nurkolis, F. A Comparative Analysis on Impact of Extraction Methods on Carotenoids Composition, Antioxidants, Antidiabetes, and Antiobesity Properties in Seagrass Enhalus acoroides: In Silico and In Vitro Study. Mar. Drugs 2024, 22, 365. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.; Ferraz, S.; Valente, S.; Barreto, M.C.; Pinheiro de Carvalho, M.A.A. Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from the Madeira Archipelago. J. Appl. Phycol. 2017, 29, 2427–2437. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Ahmad, N.; Mounsef, J.R.; Lteif, R. Pigment production by Scenedesmus dimorphus using different low-cost and alternative culture media. J. Chem. Technol. Biotechnol. 2021, 97, 287–294. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Gateau, H.; Solymosi, K.; Marchand, J.; Schoefs, B. Carotenoids of Microalgae Used in Food Industry and Medicine. Available online: https://core.ac.uk/reader/95356110?utm_source=linkout (accessed on 1 July 2025).
- Saddiqa, A.; Faisal, Z.; Akram, N.; Afzaal, M.; Saeed, F.; Ahmed, A.; Almudaihim, A.; Touqeer, M.; Ahmed, F.; Asghar, A.; et al. Algal pigments: Therapeutic potential and food applications. Food Sci. Nutr. 2024, 12, 6956–6969. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Environmental Impact on Seaweed Phenolic Production and Activity: An Important Step for Compound Exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Kharaziha, M.; Nemati, S.; Kalateh, A. Nanocomposite hydrogel based on carrageenan-coated starch/cellulose nanofibers as a hemorrhage control material. Carbohydr. Polym. 2021, 251, 117013. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; García, V.; Pastén, A.; López, J.; Goñi, G. Effect of different drying methods on phytochemical content and amino acid and fatty acid profiles of the green seaweed, Ulva spp. J. Appl. Phycol. 2019, 31, 1967–1979. [Google Scholar] [CrossRef]
- Yap, W.-F.; Tay, V.; Tan, S.-H.; Yow, Y.-Y.; Chew, J. Decoding Antioxidant and Antibacterial Potentials of Malaysian Green Seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Vinuganesh, A.; Kumar, A.; Korany, S.M.; Alsherif, E.A.; Selim, S.; Prakash, S.; Beemster, G.T.S.; AbdElgawad, H. Seasonal Changes in the Biochemical Constituents of Green Seaweed Chaetomorpha antennina from Covelong, India. Biomolecules 2022, 12, 1475. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ueng, J.-P.; Tsai, G.-J. Proximate Composition, Total Phenolic Content, and Antioxidant Activity of Seagrape (Caulerpa lentillifera). J. Food Sci. 2011, 76, C950–C958. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Haq, M.; Rahmadi, P.; Chun, B.-S. Nutritional Value and Biofunctionalities of Two Edible Green Seaweeds (Ulva lactuca and Caulerpa racemosa) from Indonesia by Subcritical Water Hydrolysis. Mar. Drugs 2021, 19, 578. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Nazarudin, M.F.; Yasin, I.S.M.; Mazli, N.A.I.N.; Saadi, A.R.; Azizee, M.H.S.; Nooraini, M.A.; Saad, N.; Ferdous, U.T.; Fakhrulddin, I.M. Preliminary screening of antioxidant and cytotoxic potential of green seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Dawood, D.H.; Elmongy, M.S.; Negm, A.; Taher, M.A. Extraction and chemical characterization of novel water-soluble polysaccharides from two palm species and their antioxidant and antitumor activities. Egypt. J. Basic Appl. Sci. 2020, 7, 141–158. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Volobuff, C.R.F.; Santiago, M.; Cardoso, C.A.L.; Vieira, M.D.C.; Pereira, Z.V. Evaluation of Antioxidant Activity, Total Flavonoids, Tannins and Phenolic Compounds in Psychotria Leaf Extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT—Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]


| Compounds | Results (% DW) |
|---|---|
| Moisture | 14.77 ± 0.20 |
| Crude protein | 3.39 ± 0.31 |
| Crude fiber | 37.63 ± 6.84 |
| Fat | 1.74 ± 0.11 |
| Ash | 30.45 ± 1.19 |
| Peak | Compound Name | RT (min) | Area (%) | RI | Reference |
|---|---|---|---|---|---|
| 1 | Palmitic acid (C16:0) methyl ester | 26.60 | 71.62 | 1920 | 1926 1 |
| 2 | Heptadecanoic acid (C17:0) methyl ester | 28.21 | 1.29 | 2020 | 2026 2 |
| 3 | Undec-10-enoic acid tetradecyl ester | 28.85 | 1.53 | 2061 | - |
| 4 | Linoleic acid (C18:2) methyl ester | 29.37 | 15.54 | 2095 | 2096 3 |
| 5 | Stearic acid (C18:0) methyl ester | 29.75 | 10.01 | 2121 | 2126 4 |
| Samples | TPC (mg GAE/100 g) | TFC (mg QE/100 g) |
|---|---|---|
| WAT | 41.50 ± 0.06 b | 1.77 ± 0.002 a |
| ETA | 6.14 ± 0.03 a | 31.50 ± 0.03 b |
| MET | 7.83 ± 0.02 a | 59.33 ± 0.10 c |
| Samples | DPPH (% of Inhibition) | ABTS (μmol TE/g) |
|---|---|---|
| WAT | 1.66 ± 0.001 b | 7.00 ± 1.50 a |
| ETA | 16.42 ± 1.52 a | 9.39 ± 1.57 b |
| MET | 21.24 ± 2.35 a | 3.57 ± 0.73 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felipe, A.T.d.M.; de Lima, A.S.L.; Paiva, E.M.d.O.; da Cunha, R.B.L.; de Almeida, A.R.; Pinheiro, F.A.S.D.; Ferreira, L.D.S.; Pedrini, M.R.d.S.; Matsui, K.N.; Hoskin, R.T. Green Macroalgae Biomass Upcycling as a Sustainable Resource for Value-Added Applications. Appl. Sci. 2025, 15, 7927. https://doi.org/10.3390/app15147927
Felipe ATdM, de Lima ASL, Paiva EMdO, da Cunha RBL, de Almeida AR, Pinheiro FASD, Ferreira LDS, Pedrini MRdS, Matsui KN, Hoskin RT. Green Macroalgae Biomass Upcycling as a Sustainable Resource for Value-Added Applications. Applied Sciences. 2025; 15(14):7927. https://doi.org/10.3390/app15147927
Chicago/Turabian StyleFelipe, Ana Terra de Medeiros, Alliny Samara Lopes de Lima, Emanuelle Maria de Oliveira Paiva, Roberto Bruno Lucena da Cunha, Addison Ribeiro de Almeida, Francisco Ayrton Senna Domingos Pinheiro, Leandro De Santis Ferreira, Marcia Regina da Silva Pedrini, Katia Nicolau Matsui, and Roberta Targino Hoskin. 2025. "Green Macroalgae Biomass Upcycling as a Sustainable Resource for Value-Added Applications" Applied Sciences 15, no. 14: 7927. https://doi.org/10.3390/app15147927
APA StyleFelipe, A. T. d. M., de Lima, A. S. L., Paiva, E. M. d. O., da Cunha, R. B. L., de Almeida, A. R., Pinheiro, F. A. S. D., Ferreira, L. D. S., Pedrini, M. R. d. S., Matsui, K. N., & Hoskin, R. T. (2025). Green Macroalgae Biomass Upcycling as a Sustainable Resource for Value-Added Applications. Applied Sciences, 15(14), 7927. https://doi.org/10.3390/app15147927








