Acute Effects of Olive Leaf Tea and Olive Leaf Powder Biscuits on Postprandial Glycemia, Lipid Profile and Inflammatory Markers: A Randomized Controlled Crossover Trial in Healthy Volunteers
Abstract
1. Introduction
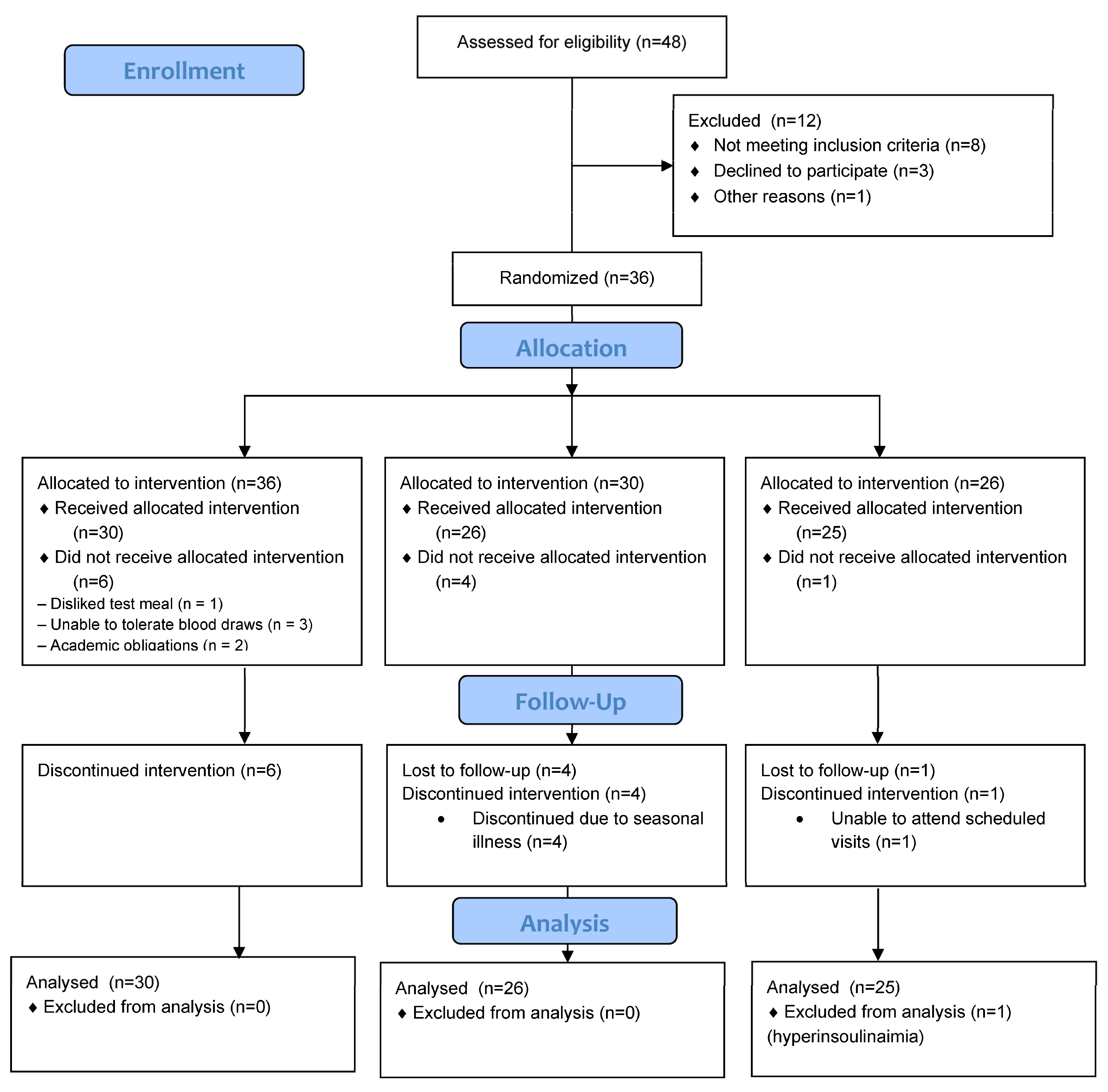
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Pre-Trial Procedures
2.4. Experimental Treatments
2.5. Measurements
2.6. Meal Analysis
2.7. Sample Size Calculations
2.8. Data Analysis
3. Results
3.1. Sensory Evaluation
3.2. Participant Characteristics
3.3. Biomarkers Analysis
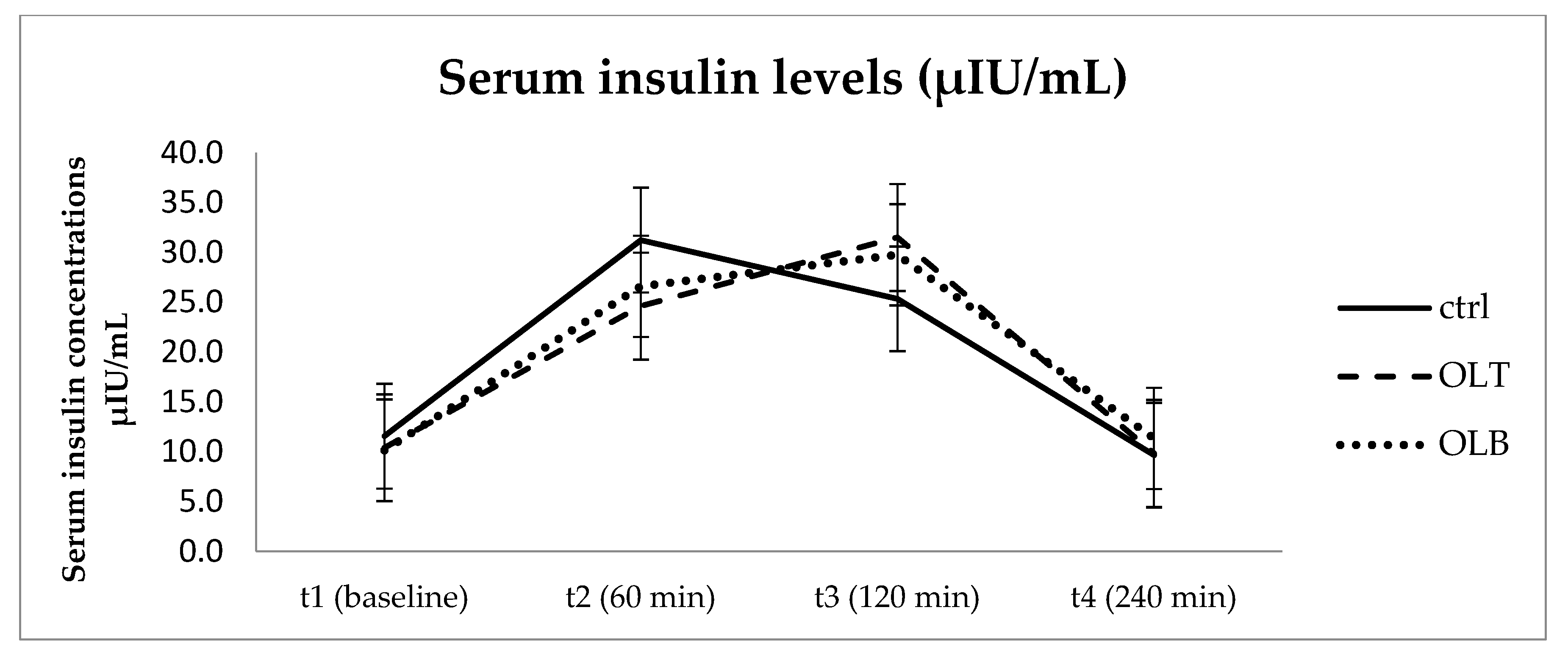
3.3.1. Insulin Levels
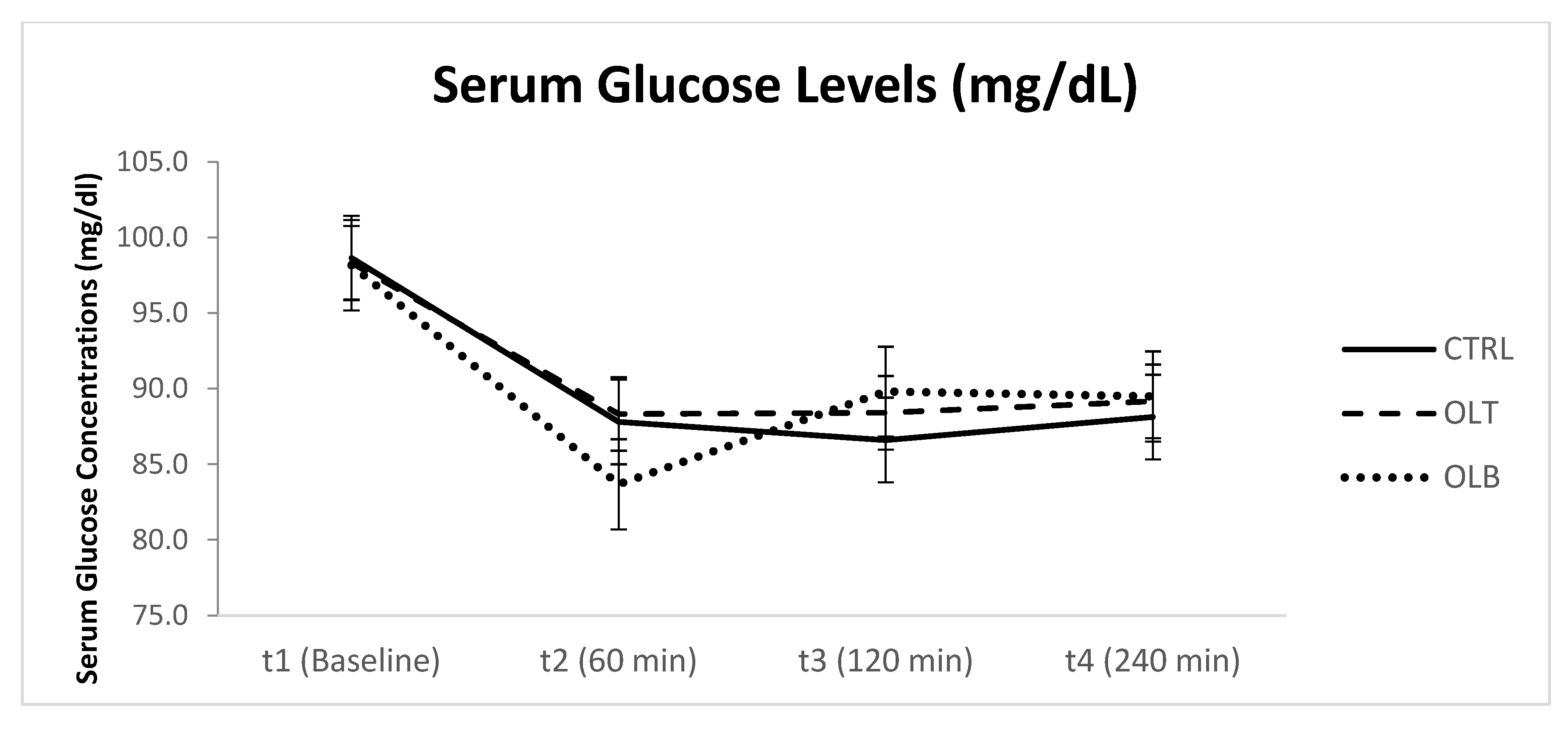
3.3.2. Glucose Levels
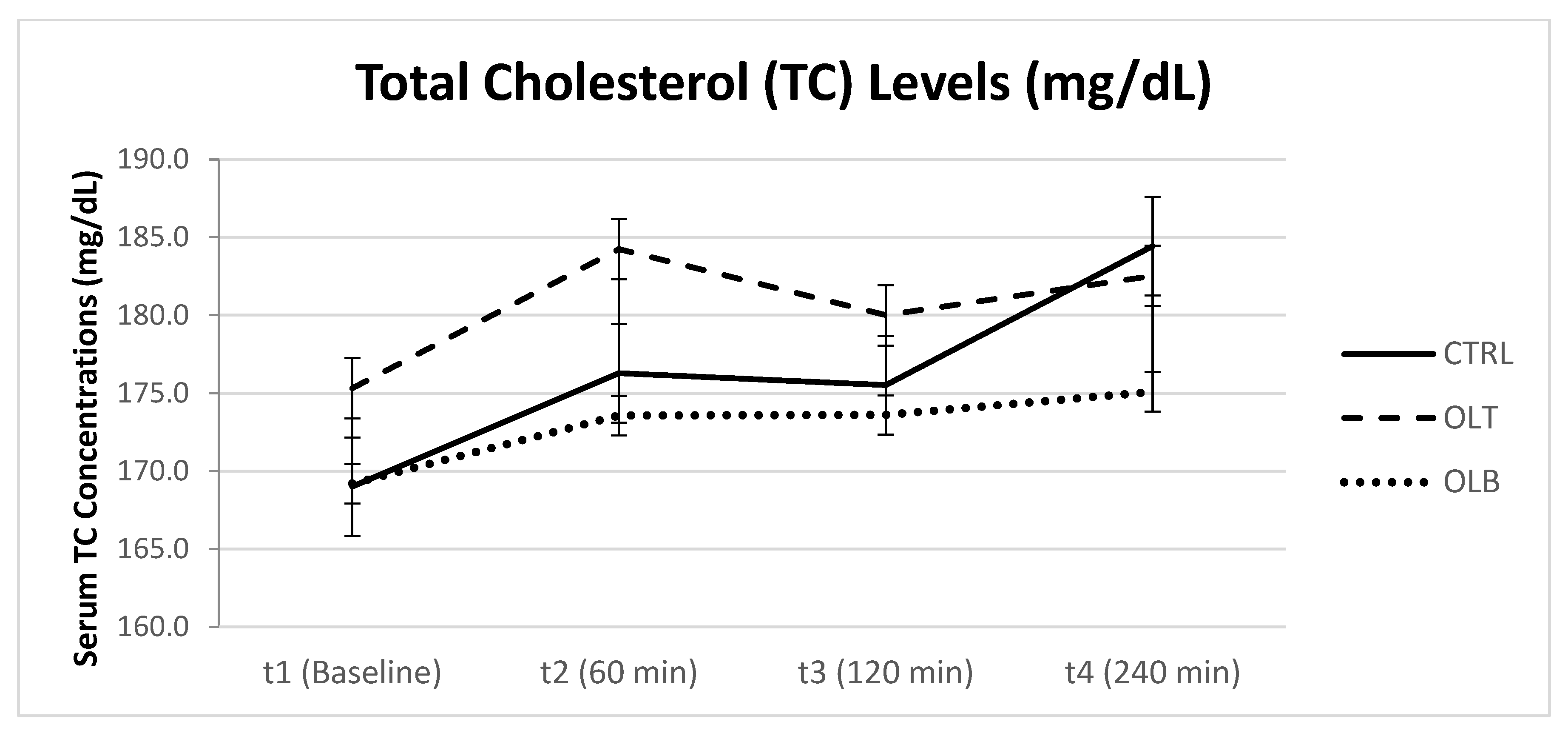
3.3.3. Cholesterol Levels
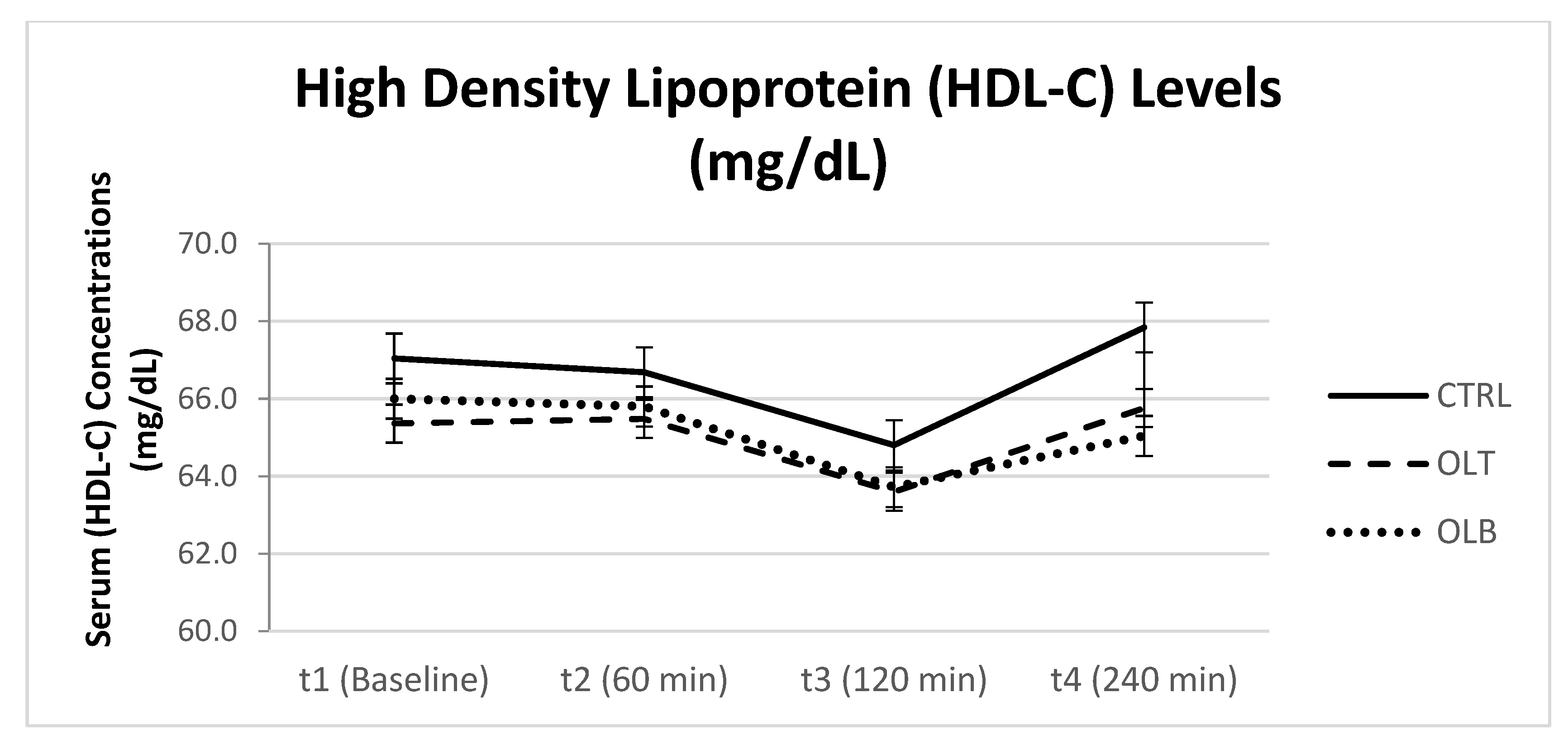
3.3.4. HDL-Cholesterol Levels
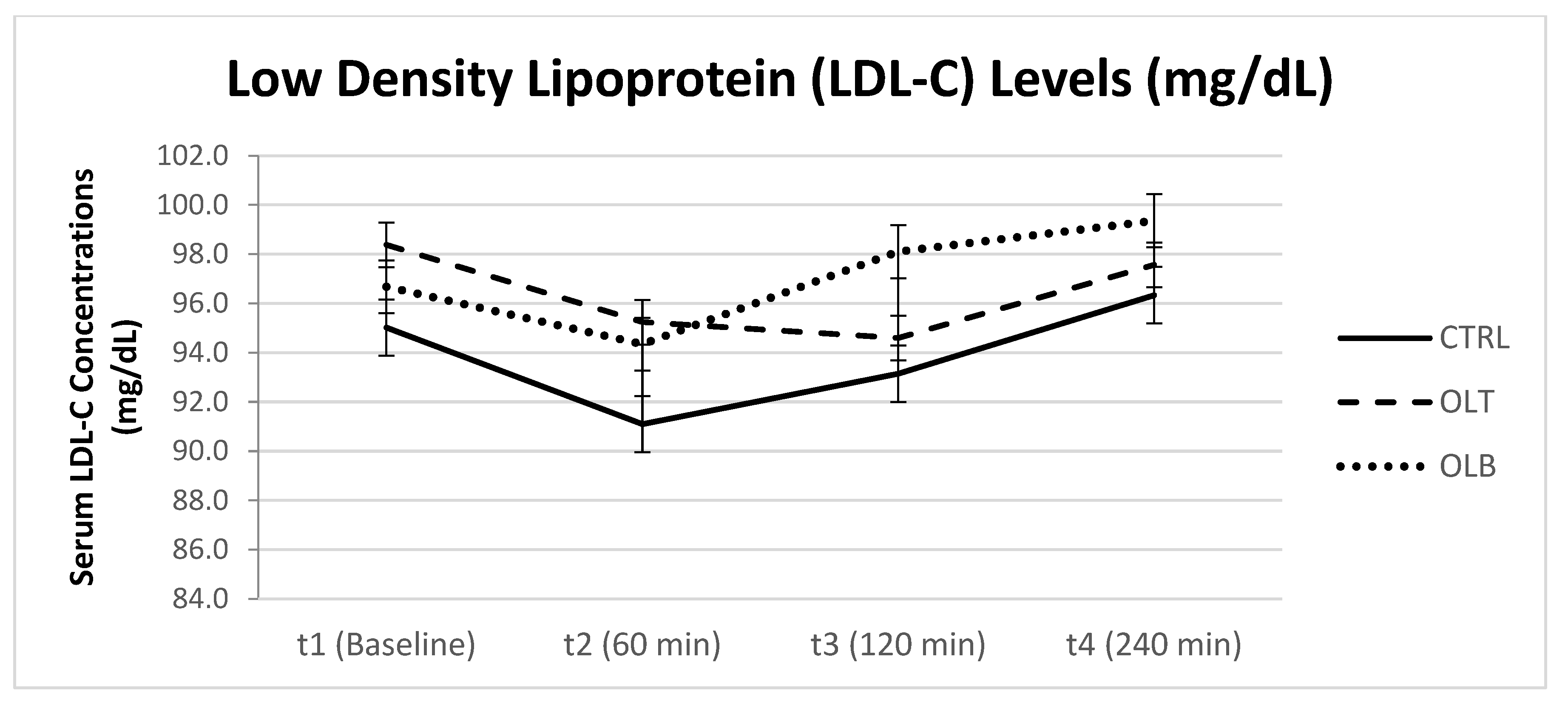
3.3.5. LDL-Cholesterol Levels
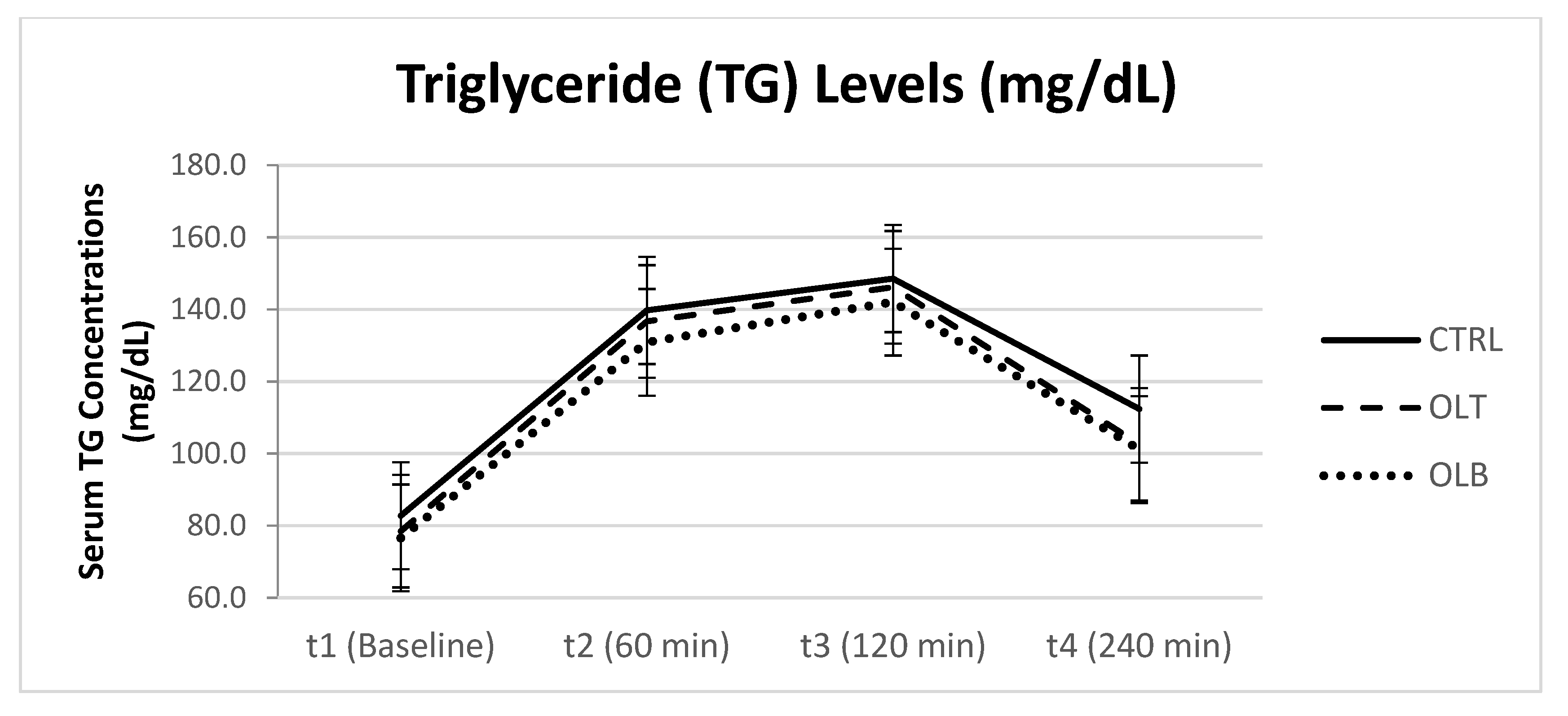
3.3.6. Triglyceride (TG) Levels
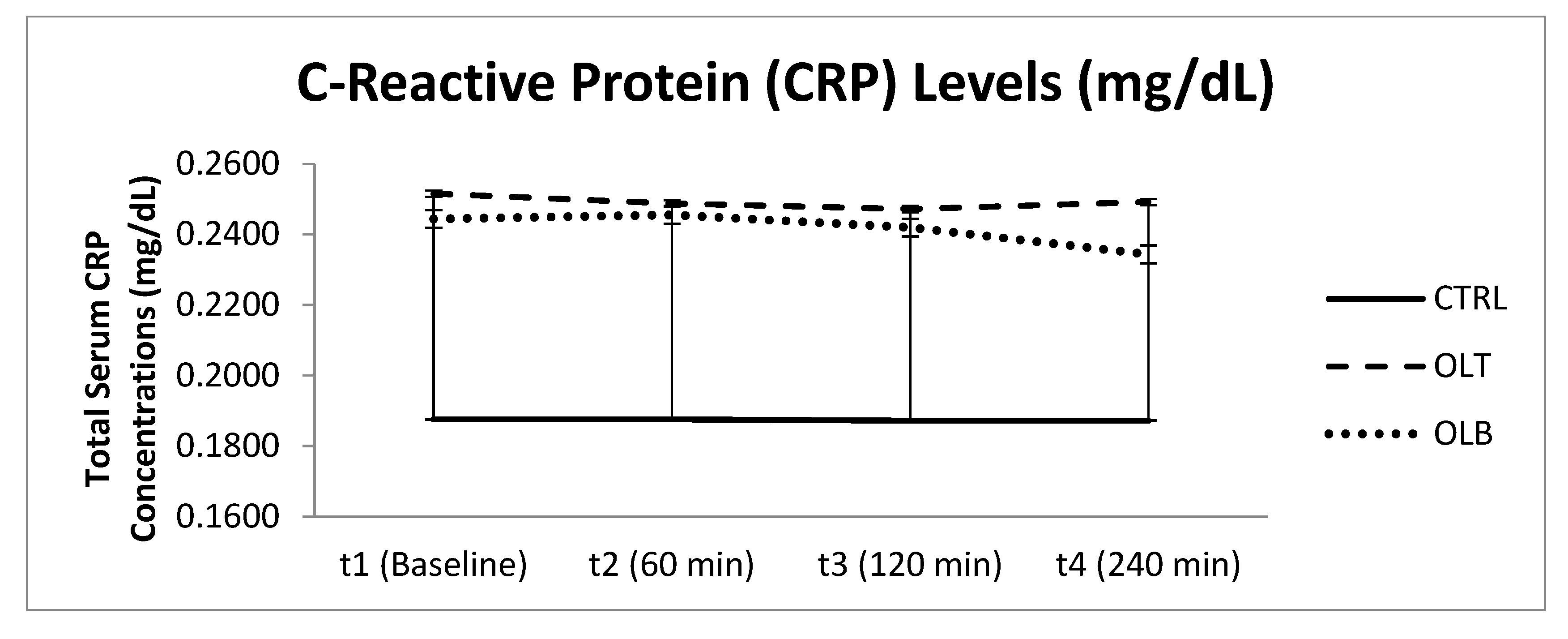
3.3.7. C-Reactive Protein (CRP) Levels
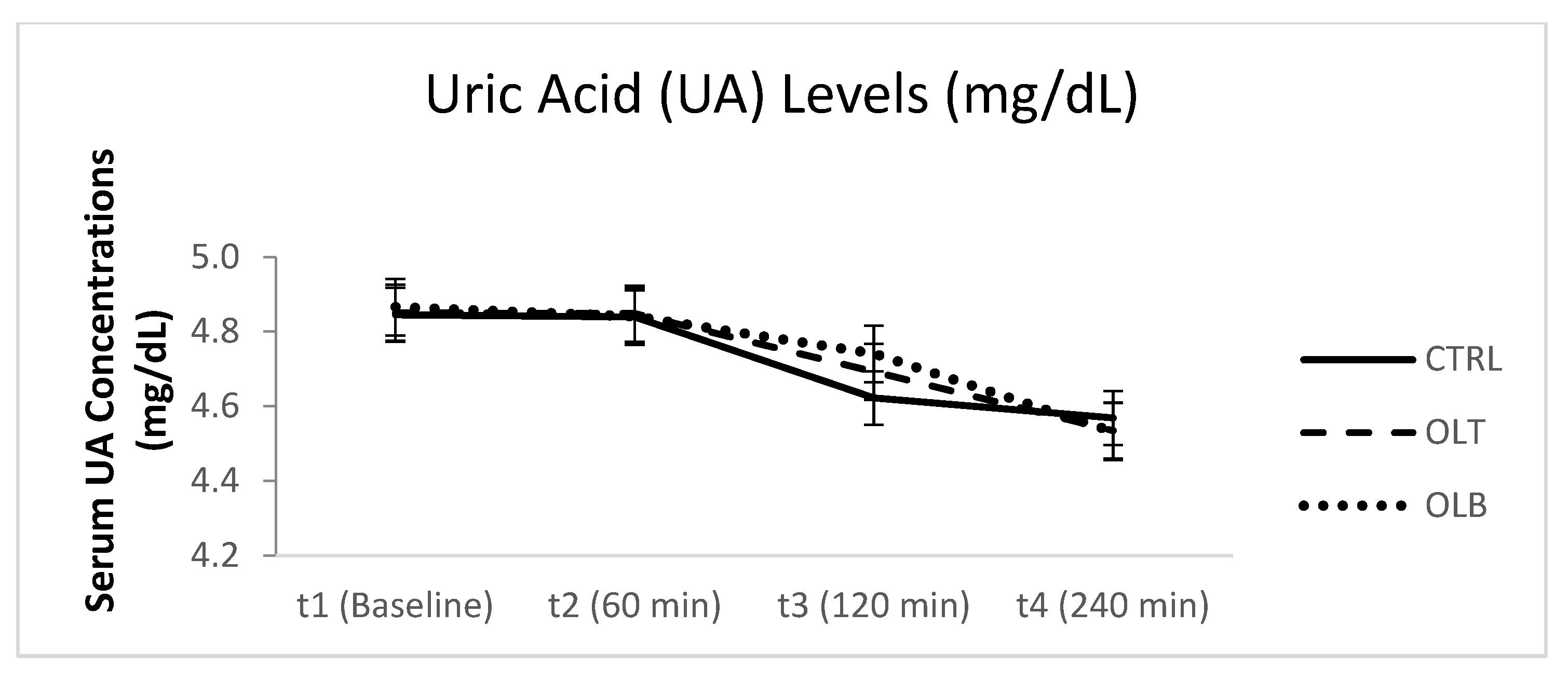
3.3.8. Uric Acid (UA) Levels
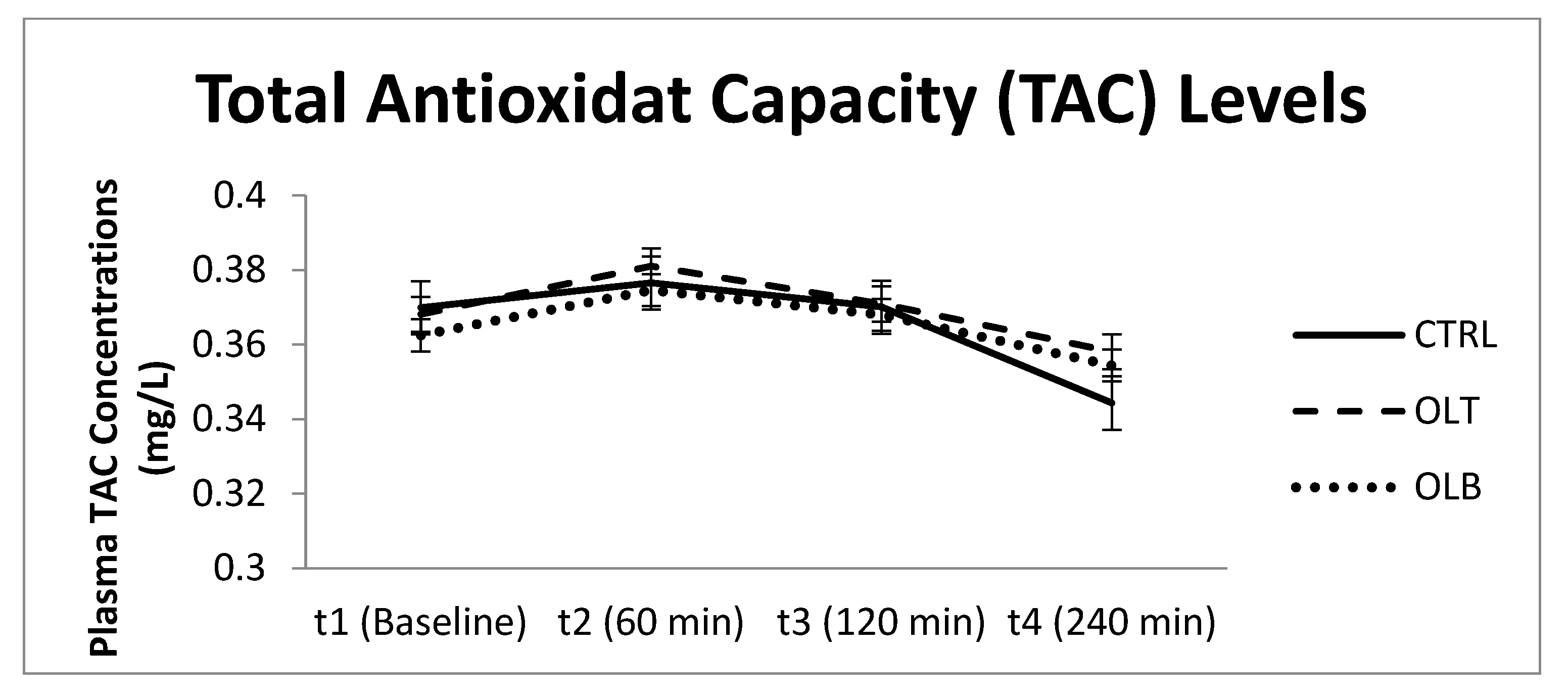
3.3.9. Total Plasma Antioxidant Capacity (TAC) Levels (mg/L)
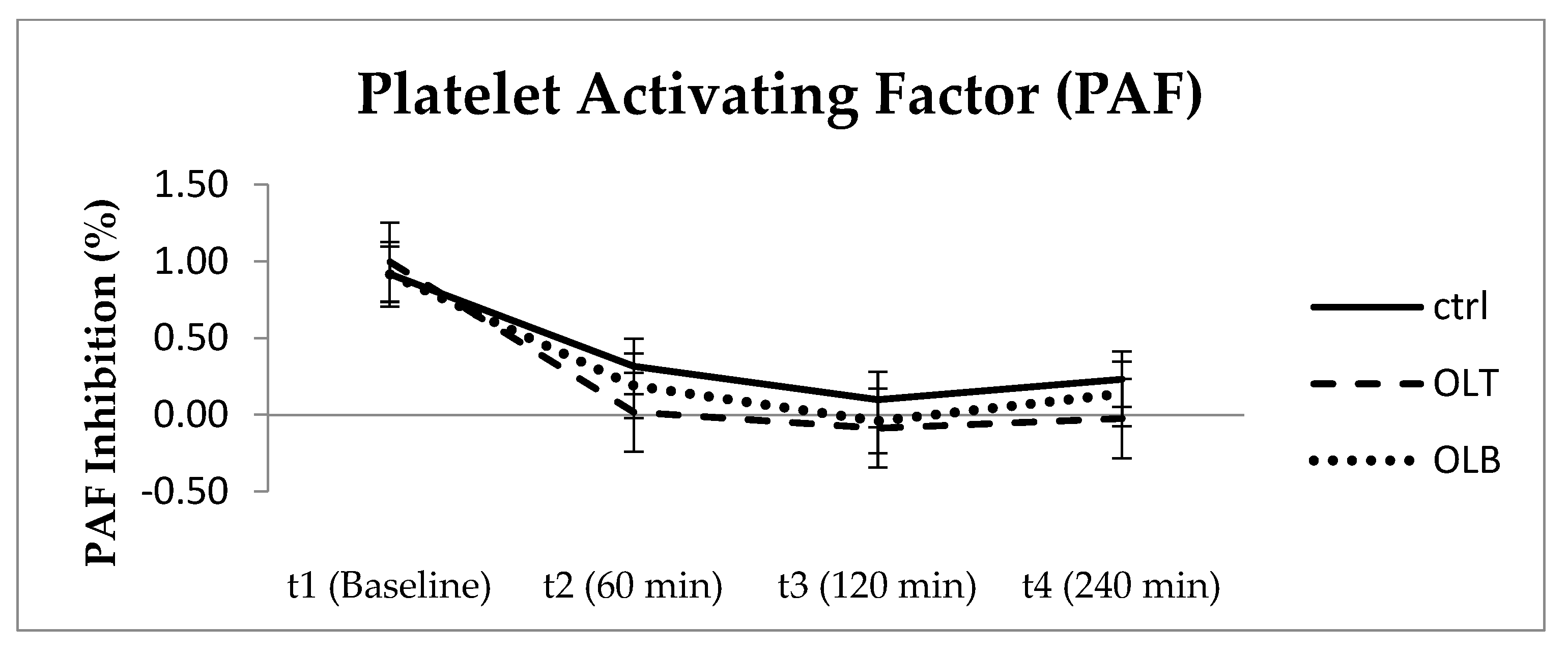
3.3.10. Platelet Aggregation Factor (PAF)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, T.; Fujiwara, M.; Yao, Z. Postprandial hyperglycemia and postprandial hypertriglyceridemia in type 2 diabetes. J. Biomed. Res. 2019, 33, 1–16. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Kraakman, M.J.; Flynn, M.C.; Nagareddy, P.R.; Schalkwijk, C.G.; Murphy, A.J. Postprandial Glucose Spikes, an Important Contributor to Cardiovascular Disease in Diabetes? Front. Cardiovasc. Med. 2020, 7, 570553. [Google Scholar] [CrossRef]
- Sasso, E.; Baticic, L.; Sotosek, V. Postprandial Dysmetabolism and Its Medical Implications. Life 2023, 13, 2317. [Google Scholar] [CrossRef]
- Reddy, K.S.; Katan, M.B. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Przytuła, A.; Szymańska, M.; Popiołek-Kalisz, J. Dietary strategies for cardiovascular disease risk factors prevention. Curr. Probl. Cardiol. 2024, 49, 102746. [Google Scholar] [CrossRef] [PubMed]
- Chareonrungrueangchai, K.; Wongkawinwoot, K.; Anothaisintawee, T.; Reutrakul, S. Dietary Factors and Risks of Cardiovascular Diseases: An Umbrella Review. Nutrients 2020, 12, 1088. [Google Scholar] [CrossRef]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef]
- Burton-Freeman, B. Postprandial metabolic events and fruit-derived phenolics: A review of the science. Br. J. Nutr. 2010, 104, S1–S14. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Fragopoulou, E.; Antonopoulou, S.; Rementzis, J.; Phenekos, C.; Demopoulos, C.A. Effect of fast-food Mediterranean-type diet on type 2 diabetics and healthy human subjects’ platelet aggregation. Diabetes Res. Clin. Pract. 2006, 72, 33–41. [Google Scholar] [CrossRef]
- Singh, I.; Mok, M.; Christensen, A.-M.; Turner, A.H.; Hawley, J.A. The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 127–132. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S.H. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Agricultural Research Service FoodData Central. Available online: https://fdc.nal.usda.gov (accessed on 10 May 2025).
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- De Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) Leaf Polyphenols Improve Insulin Sensitivity in Middle-Aged Overweight Men: A Randomized, Placebo-Controlled, Crossover Trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef]
- Meireles, M.; Cortez-Ribeiro, A.; Polck, D.; Almeida-de-Souza, J.; Ferro-Lebres, V. Olive Leaf Tea Impact on Postprandial Glycemia: A Randomized Cross-Over Trial. Foods 2023, 12, 528. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Abdollahi, S.; Mousavirad, M.; Clark, C.C.T.; Soltani, S. The effects of olive leaf extract on cardiovascular risk factors in the general adult population: A systematic review and meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2022, 14, 151. [Google Scholar] [CrossRef]
- González-Hedström, D.; García-Villalón, Á.L.; Amor, S.; De La Fuente-Fernández, M.; Almodóvar, P.; Prodanov, M.; Priego, T.; Martín, A.I.; Inarejos-García, A.M.; Granado, M. Olive leaf extract supplementation improves the vascular and metabolic alterations associated with aging in Wistar rats. Sci. Rep. 2021, 11, 8188. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Norhayati, M.N.; Mohamad, N. Olive leaf extract effect on cardiometabolic profile among adults with prehypertension and hypertension: A systematic review and meta-analysis. PeerJ 2021, 9, e11173. [Google Scholar] [CrossRef]
- Álvares, A.A.; Garcêz, A.; Silva, L.T.; Averbuch, N.; Garavaglia, J. Olive leaf extract effect on cardiometabolic risk factors: A systematic review and meta-analysis of randomized clinical trials. Nutr. Rev. 2024, 82, 1710–1725. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Toulabi, T.; Yadegarinia, D.; Yarahmadi, S.; Mohammadi, R.; Keyvanfar, A. Efficacy of olive leaves extract on the outcomes of hospitalized covid-19 patients: A randomized, triple-blinded clinical trial. Explor. 2023, 19, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Stevens, Y.; Winkens, B.; Jonkers, D.; Masclee, A. The effect of olive leaf extract on cardiovascular health markers: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Fujie, K.; Yuine, N.; Watabe, Y.; Nakata, Y.; Suzuki, H.; Isoda, H.; Hashimoto, K. Olive leaf tea is beneficial for lipid metabolism in adults with prediabetes: An exploratory randomized controlled trial. Nutr. Res. 2019, 67, 60–66. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Gavrilaș, L.I.; Vodnar, D.C.; Rohn, S.; Mocan, A. Systemic Health Effects of Oleuropein and Hydroxytyrosol Supplementation: A Systematic Review of Randomized Controlled Trials. Antioxidants 2024, 13, 1040. [Google Scholar] [CrossRef]
- Ahuja, K.D.K.; Thomas, G.A.; Adams, M.J.; Ball, M.J. Postprandial platelet aggregation: Effects of different meals and glycemic index. Eur. J. Clin. Nutr. 2012, 66, 722–726. [Google Scholar] [CrossRef]
- Silvan, J.M.; Guerrero-Hurtado, E.; Gutiérrez-Docio, A.; Alarcón-Cavero, T.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive-Leaf Extracts Modulate Inflammation and Oxidative Stress Associated with Human, H. pylori Infection. Antioxidants 2021, 10, 2030. [Google Scholar] [CrossRef]
- Munteanu, C.; Kotova, P.; Schwartz, B. Impact of Olive Oil Components on the Expression of Genes Related to Type 2 Diabetes Mellitus. Nutrients 2025, 17, 570. [Google Scholar] [CrossRef]
| Meal | Energy (kcal) | Total Carbohydrates (g) | Dietary Fiber (g) | Protein (g) | Lipids (g) |
|---|---|---|---|---|---|
| CTRL | 652.83 | 78.05 | 2.28 | 8.10 | 34.53 |
| OLT | 652.83 | 78.05 | 2.28 | 8.10 | 34.53 |
| OLB | 652.83 | 78.05 | 2.28 | 8.10 | 34.53 |
| Attribute | 0.5% Concentration | 1% Concentration |
|---|---|---|
| Taste | 7.11 ± 1.33 a | 6.13 ± 1.61 b |
| Aroma | 6.87 ± 1.59 a | 6.37 ± 1.56 a |
| Aftertaste | 7.32 ± 1.45 a | 5.97 ± 1.63 b |
| Color | 5.55 ± 1.75 a | 6.66 ± 1.16 b |
| Overall Liking | 7.53 ± 1.48 a | 6.24 ± 1.86 b |
| General Characteristics | N |
|---|---|
| Participants (total) | 24 |
| Men | 11 |
| Women | 13 |
| Mean ± SD | |
| Age (years) | 31.23 ± 9.8 |
| Anthropometric characteristics | Mean ± SD |
| Weight (kg) | 73.48 ± 19.24 |
| Height (cm) | 171.24 ± 9.45 |
| BMI (kg/m2) | 24.71 ± 4.76 |
| FAT (%) | 25.59 ± 6.08 |
| Muscle Mass (kg) | 51.43 ± 12.28 |
| TBW (%) | 51.86 ± 3.69 |
| BM (kcal/day) | 1633.29 ± 368.37 |
| WHR | 0.80 ± 0.09 |
| General habits | N |
| Smoking | |
| Smokers | 9 |
| Non-smokers | 15 |
| Physical activity | |
| Low | 14 |
| Medium | 6 |
| High | 4 |
| INS (Mean ± SE) at Baseline (t1) | INS (Mean ± SE) at 1 h (t2) | INS (Mean ± SE) at 2 h (t3) | INS (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 11.570 ± 1.084 | 31.250 ± 3.311 | 25.353 ± 3.936 | 9.654 ± 0.907 | t1 t2 t3 t4 | 9.328 24.401 17.211 7.778 | 13.812 38.099 33.495 11.530 | p = 0.054 |
| OLT | 10.387 ± 0.642 | 24.614 ± 2.418 | 31.513 ± 3.383 | 9.840 ± 0.879 | t1 t2 t3 t4 | 9.058 19.612 24.515 8.020 | 11.715 29.616 38.510 11.659 | |
| OLB | 10.158 ± 0.757 | 26.607 ± 3.356 | 29.773 ± 4.471 | 11.330 ± 1.529 | t1 t2 t3 t4 | 8.593 19.665 20.524 8.167 | 11.724 33.550 39.021 14.493 | |
| GLU (Mean ± SE) at Baseline (t1) | GLU (Mean ± SE) at 1 h (t2) | GLU (Mean ± SE) at 2 h (t3) | GLU (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 98.125 ± 1.282 | 86.458 ± 3.074 | 85.958 ± 2.958 | 88.167 ± 1.890 | t1 t2 t3 t4 | 95.474 80.099 79.840 84.257 | 100.78 92.817 92.077 92.076 | p = 0.546 |
| OLT | 97.833 ± 1.333 | 87.208 ± 3.753 | 86.875 ± 2.445 | 88.667 ± 1.538 | t1 t2 t3 t4 | 95.075 79.444 81.817 85.486 | 100.60 94.973 91.933 91.848 | |
| OLB | 97.167 ± 1.434 | 82.833 ± 2.983 | 88.500 ± 3.058 | 89.042 ± 1.993 | t1 t2 t3 t4 | 94.200 76.662 82.174 84.918 | 100.13 89.005 94.826 93.165 | |
| TC (Mean ± SE) at Baseline (t1) | TC (Mean ± SE) at 1 h (t2) | TC (Mean ± SE) at 2 h (t3) | TC (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 169.208 ± 6.623 | 176.375 ± 8.189 | 172.167 ± 7.679 | 178.667 ± 7.829 | t1 t2 t3 t4 | 155.51 159.44 156.28 162.47 | 182.91 193.32 188.05 194.86 | p = 0.755 |
| OLT | 173.333 ± 8.247 | 182.458 ± 8.801 | 178.125 ± 9.249 | 180.333 ± 8.579 | t1 t2 t3 t4 | 156.27 164.25 158.99 162.59 | 190.39 200.67 197.26 198.08 | |
| OLB | 167.042 ± 7.377 | 171.375 ± 6.167 | 171.083 ± 6.895 | 172.458 ± 6.201 | t1 t2 t3 t4 | 151.78 158.61 156.82 159.63 | 182.30 184.13 185.35 185.29 | |
| HDL-C (Mean ± SE) at Baseline (t1) | HDL-C (Mean ± SE) at 1 h (t2) | HDL-C (Mean ± SE) at 2 h (t3) | HDL-C (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 67.875 ± 3.464 | 67.458 ± 3.482 | 65.625 ± 3.491 | 68.375 ± 3.487 | t1 t2 t3 t4 | 60.709 60.255 58.404 61.162 | 72.428 74.662 72.846 75.588 | p = 0.637 |
| OLT | 66.208 ± 3.436 | 66.292 ± 3.509 | 64.458 ± 3.438 | 66.542 ± 3.340 | t1 t2 t3 t4 | 59.100 59.034 57.346 59.632 | 73.317 73.550 71.571 73.451 | |
| OLB | 66.792 ± 2.725 | 66.583 ± 2.957 | 64.417 ± 2.866 | 65.750 ± 2.891 | t1 t2 t3 t4 | 60.155 60.466 58.488 59.770 | 72.428 72.701 70.345 71.730 | |
| LDL-C (Mean ± SE) at Baseline (t1) | LDL-C (Mean ± SE) at 1 h (t2) | LDL-C (Mean ± SE) at 2 h (t3) | LDL-C (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 93.463 ± 5.566 | 89.662 ± 5.960 | 91.787 ± 5.816 | 95.152 ± 5.482 | t1 t2 t3 t4 | 81.949 77.333 79.755 83.812 | 104.74 101.99 103.82 106.49 | p = 0.325 |
| OLT | 95.379 ± 5.360 | 92.216 ± 5.696 | 91.583 ± 4.880 | 94.414 ± 5.093 | t1 t2 t3 t4 | 84.290 80.434 81.487 83.879 | 106.47 104.00 101.68 104.95 | |
| OLB | 94.458 ± 4.973 | 91.805 ± 5.147 | 95.540 ± 5.096 | 96.782 ± 5.107 | t1 t2 t3 t4 | 84.171 81.157 84.999 86.218 | 104.74 102.45 106.08 107.35 | |
| TG (Mean ± SE) at Baseline (t1) | TG (Mean ± SE) at 1 h (t2) | TG (Mean ± SE) at 2 h (t3) | TG (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 82.083 ± 8.442 | 139.197 ± 10.365 | 147.042 ± 15.595 | 110.583 ± 13.913 | t1 t2 t3 t4 | 64.619 117.73 114.78 81.803 | 99.547 160.61 179.30 139.36 | p = 0.975 |
| OLT | 78.000 ± 7.576 | 136.333 ± 11.982 | 145.208 ± 16.805 | 102.042 ± 11.991 | t1 t2 t3 t4 | 62.328 111.55 110.44 77.237 | 93.672 161.12 179.97 126.85 | |
| OLB | 75.083 ± 6.034 | 129.000 ± 8.327 | 139.000 ± 12.866 | 97.667 ± 9.833 | t1 t2 t3 t4 | 62.601 111.78 112.39 77.326 | 87.566 146.23 165.62 118.00 | |
| CRP (Mean ± SE) at Baseline (t1) | CRP (Mean ± SE) at 1 h (t2) | CRP (Mean ± SE) at 2 h (t3) | CRP (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 0.192 ± 0.045 | 0.192 ± 0.042 | 0.190 ± 0.041 | 0.190 ± 0.039 | t1 t2 t3 t4 | 0.099 0.104 0.106 0.110 | 0.284 0.280 0.275 0.270 | p = 0.854 |
| OLT | 0.259 ± 0.057 | 0.256 ± 0.056 | 0.255 ± 0.055 | 0.257 ± 0.055 | t1 t2 t3 t4 | 0.140 0.141 0.140 0.142 | 0.378 0.371 0.369 0.371 | |
| OLB | 0.250 ± 0.045 | 0.252 ± 0.043 | 0.249 ± 0.044 | 0.241 ± 0.042 | t1 t2 t3 t4 | 0.158 0.163 0.158 0.155 | 0.343 0.341 0.340 0.327 | |
| UA (Mean ± SE) at Baseline (t1) | UA (Mean ± SE) at 1 h (t2) | UA (Mean ± SE) at 2 h (t3) | UA (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 4.731 ± 0.220 | 4.713 ± 0.210 | 4.488 ± 0.211 | 4.429 ± 0.216 | t1 t2 t3 t4 | 4.276 4.279 4.053 3.983 | 5.186 5.147 4.924 4.875 | p = 0.145 |
| OLT | 4.735 ± 0.232 | 4.735 ± 0.228 | 4.583 ± 0.231 | 4.430 ± 0.236 | t1 t2 t3 t4 | 4.255 4.263 4.104 3.941 | 5.214 5.206 5.062 4.918 | |
| OLB | 4.768 ± 0.225 | 4.743 ± 0.221 | 4.649 ± 0.219 | 4.437 ± 0.224 | t1 t2 t3 t4 | 4.303 4.285 4.195 3.974 | 5.234 5.201 5.103 4.900 | |
| TAC (Mean ± SE) at Baseline (t1) | TAC (Mean ± SE) at 1 h (t2) | TAC (Mean ± SE) at 2 h (t3) | TAC (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 0.370 ± 0.017 | 0.377 ± 0.018 | 0.370 ± 0.016 | 0.344 ± 0.017 | t1 t2 t3 t4 | 0.335 0.340 0.337 0.310 | 0.405 0.413 0.403 0.379 | p = 0.629 |
| OLT | 0.368 ± 0.015 | 0.381 ± 0.015 | 0.371 ± 0.014 | 0.358 ± 0. 016 | t1 t2 t3 t4 | 0.338 0.350 0.343 0.325 | 0.398 0.412 0.399 0.391 | |
| OLB | 0.362 ± 0.012 | 0.375 ± 0.013 | 0.368 ± 0.014 | 0.354 ± 0.016 | t1 t2 t3 t4 | 0.337 0.349 0.339 0.321 | 0.388 0.400 0.397 0.387 | |
| PAF (Mean ± SE) at Baseline (t1) | PAF (Mean ± SE) at 1 h (t2) | PAF (Mean ± SE) at 2 h (t3) | PAF (Mean ± SE) at 4 h (t4) | 95% Confidence Interval | p Value (Group × Time) | |||
|---|---|---|---|---|---|---|---|---|
| Time | Lower Bound | Upper Bound | ||||||
| CTRL | 0.916 ± 0.064 | 0.316 ± 0.288 | 0.099 ± 0.124 | 0.231 ± 0.178 | t1 t2 t3 t4 | 0.785 −0.280 −0.158 −0.137 | 1.048 0.913 0.356 0.599 | p = 0.713 |
| OLT | 0.996 ± 0.047 | 0.018 ± 0.059 | −0.086 ± 0.073 | −0.024 ± 0.066 | t1 t2 t3 t4 | 0.898 −0.104 −0.238 −0.161 | 1.094 0.139 0.065 0.113 | |
| OLB | 0.916 ± 0.059 | 0.190 ± 0.197 | −0.040 ± 0.200 | 0.138 ± 0.179 | t1 t2 t3 t4 | 0.794 −0.218 −0.454 −0.233 | 1.037 0.599 0.374 0.509 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potsaki, P.; Papagianni, O.I.; Almpounioti, K.; Soulakellis, C.; Voutsa, A.; Katira, O.; Bountziouka, V.; Karantonis, C.; Koutelidakis, A.E. Acute Effects of Olive Leaf Tea and Olive Leaf Powder Biscuits on Postprandial Glycemia, Lipid Profile and Inflammatory Markers: A Randomized Controlled Crossover Trial in Healthy Volunteers. Appl. Sci. 2025, 15, 7857. https://doi.org/10.3390/app15147857
Potsaki P, Papagianni OI, Almpounioti K, Soulakellis C, Voutsa A, Katira O, Bountziouka V, Karantonis C, Koutelidakis AE. Acute Effects of Olive Leaf Tea and Olive Leaf Powder Biscuits on Postprandial Glycemia, Lipid Profile and Inflammatory Markers: A Randomized Controlled Crossover Trial in Healthy Volunteers. Applied Sciences. 2025; 15(14):7857. https://doi.org/10.3390/app15147857
Chicago/Turabian StylePotsaki, Panagiota, Olga I. Papagianni, Kalliopi Almpounioti, Charalampos Soulakellis, Angeliki Voutsa, Olga Katira, Vasiliki Bountziouka, Charalampos Karantonis, and Antonios E. Koutelidakis. 2025. "Acute Effects of Olive Leaf Tea and Olive Leaf Powder Biscuits on Postprandial Glycemia, Lipid Profile and Inflammatory Markers: A Randomized Controlled Crossover Trial in Healthy Volunteers" Applied Sciences 15, no. 14: 7857. https://doi.org/10.3390/app15147857
APA StylePotsaki, P., Papagianni, O. I., Almpounioti, K., Soulakellis, C., Voutsa, A., Katira, O., Bountziouka, V., Karantonis, C., & Koutelidakis, A. E. (2025). Acute Effects of Olive Leaf Tea and Olive Leaf Powder Biscuits on Postprandial Glycemia, Lipid Profile and Inflammatory Markers: A Randomized Controlled Crossover Trial in Healthy Volunteers. Applied Sciences, 15(14), 7857. https://doi.org/10.3390/app15147857









