Assessment of Water Quality in the Tributaries of the Rega River (Northwestern Poland) as a Fish Habitat
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Measurement and Analysis of Water Quality
2.3. Statistical Analysis
3. Results and Discussion
3.1. Hydrochemical Conditions of the Studied Rivers
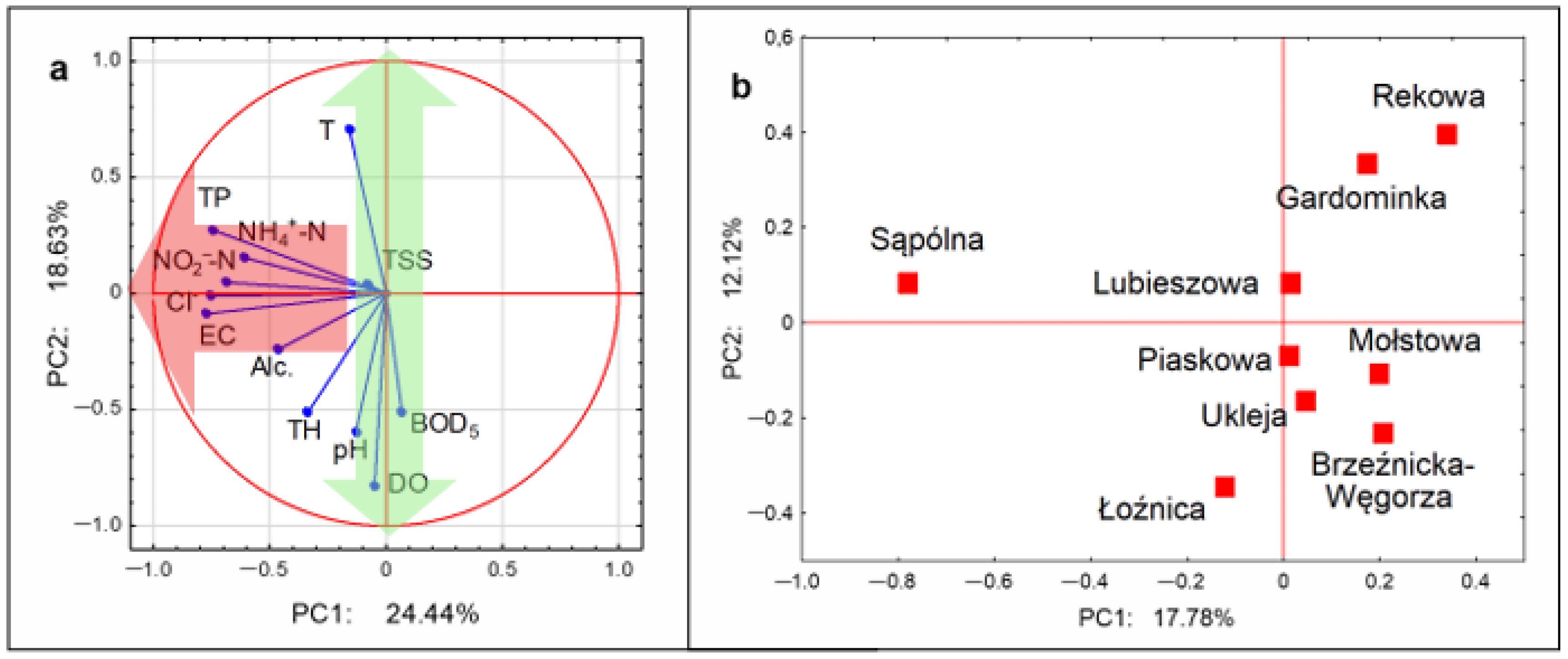
3.2. Multivariate Analysis
4. Conclusions
- Establishing buffer zones to limit human activities that may negatively impact the aquatic environment (e.g., restrictions on construction).
- Investing in water quality and ichthyofauna monitoring through modern measurement systems.
- Restoring fish habitats, including the construction of gravel ridges in tributaries. These structures would serve as natural spawning grounds for fish, especially salmonids, and at the same time act as minor flow barriers that do not impede fish migration but can significantly enhance water retention.
- Protecting fish migration routes and spawning grounds.
- Implementing educational programs for local communities to raise awareness of the importance of water protection.
- Revising quantitative and qualitative wastewater discharge limits in water permits, considering changing flow conditions—especially during summer low-flow periods.
- Introducing continuous monitoring of strategic locations, particularly tributaries with the highest concentration of spawning grounds (e.g., the Lubieszowa, Mołstowa, and Sąpólna rivers).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fialho, A.P.; Oliveira, L.G.; Tejerina-Garro, F.L.; de Mérona, B. Fish-habitat relationship in a tropical river under anthropogenic influences. Hydrobiologia 2008, 598, 315–324. [Google Scholar] [CrossRef]
- Esselman, P.C.; Infante, D.M.; Wang, L.; Wu, D.; Cooper, A.R.; Taylor, W.W. An index of cumulative disturbance to river fish habitats of the conterminous United States from landscape anthropogenic activities. Ecol. Restor. 2011, 29, 133–151. [Google Scholar] [CrossRef]
- Moraga, D.; Vivancos, A.; Ruiz, V.H.; Rojas, O.; Díaz, G.; Manosalva, A.; Habit, E. A century of anthropogenic river alterations in a highly diverse river coastal basin: Effects on fish assemblages. Front. Environ. Sci. 2022, 10, 943586. [Google Scholar] [CrossRef]
- Ezzati, G.; Kyllmar, K.; Barron, J. Long-term water quality monitoring in agricultural catchments in Sweden: Impact of climatic drivers on diffuse nutrient loads. Sci. Total Environ. 2023, 864, 160978. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.; Tetzlaff, D.; Hinkelmann, R.; Soulsby, C. Effects of 66 years of water management and hydroclimatic change on the urban hydrology and water quality of the Panke catchment, Berlin, Germany. Sci. Total Environ. 2023, 900, 165764. [Google Scholar] [CrossRef]
- Gavrilaș, S.; Burescu, F.L.; Chereji, B.D.; Munteanu, F.D. The Impact of Anthropogenic Activities on the Catchment’s Water Quality Parameters. Water 2025, 17, 1791. [Google Scholar] [CrossRef]
- Mendiguchía, C.; Moreno, C.; García-Vargas, M. Evaluation of natural and anthropogenic influences on the Guadalquivir River (Spain) by dissolved heavy metals and nutrients. Chemosphere 2007, 69, 1509–1517. [Google Scholar] [CrossRef]
- Singh, V.; Ahmed, G.; Vedika, S.; Kumar, P.; Chaturvedi, S.K.; Rai, S.N.; Vamanu, E.; Kumar, A. Toxic heavy metal ions contamination in water and their sustainable reduction by eco-friendly methods: Isotherms, thermodynamics and kinetics study. Sci. Rep. 2024, 14, 7595. [Google Scholar] [CrossRef]
- WWAP 2017. The United Nations World Water Development Report 2017: Wastewater, the Untapped Resource. United Nations World Water Assessment Programme (WWAP). Paris, United Nations Educational, Scientific and Cultural Organization. Available online: https://www.unido.org/sites/default/files/2017-03/UN_World_Water_Development_Report_-_Full_0.pdf (accessed on 25 May 2025).
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H. Water Pollution from Agriculture: A Global Review. Executive Summary [Internet]; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2017; Available online: http://www.fao.org/3/a-i7754e.pdf (accessed on 25 May 2025).
- Piniewski, M.; Tattari, S.; Koskiaho, J.; Olsson, O.F.; Giełczewski, M.; Marcinkowski, P.; Księżniak, M.; Okruszko, T. How effective are River Basin Management Plans in reaching the nutrient load reduction targets? Ambio 2021, 50, 706–722. [Google Scholar] [CrossRef]
- Kundzewicz, Z.; Zaleski, J.; Nachlik, E.; Januchta-Szostak, A. Managing water—Challenges for Poland. Science 2021, 1, 79–102. (In Polish) [Google Scholar] [CrossRef]
- GIOŚ 2022; Chief Inspectorate of Environmental Protection. In State of the Environment in Poland. Report 2022. Water Quality; Environmental Monitoring Library: Warsaw, Poland, 2022; pp. 61–91.
- Radtke, G.; Bernaś, R.; Dębowski, P.; Skóra, M. The ichthyo-fauna of the Rega river system. Sci. Ann. Pol. Angl. Assoc. 2010, 23, 51–78. (In Polish) [Google Scholar]
- Bonisławska, M.; Nędzarek, A.; Rybczyk, A.; Tański, A. The Influence of Anthropogenic Pollution on the Physicochemical Conditions of the Waters of the Lower Section of the Sąpólna River. Water 2024, 16, 35. [Google Scholar] [CrossRef]
- WIOŚ. Surface Water Quality Assessment in the West Pomeranian Voivodeship for 2008; WIOŚ: Szczecin, Poland, 2009; p. 8. (In Polish) [Google Scholar]
- RMI. Regulation of the Ministry of Infrastructure of June 25, 2021 on the classification of ecological status, ecological potential and chemical status, and the method of classification of the status of surface water bodies, as well as environmental quality standards for priority substances. J. Laws 2021, 1475. [Google Scholar]
- Bonisławska, M.; Tański, A.; Nędzarek, A.; Tórz, A.; Formicki, K.; Korzelecka-Orkisz, A. Suitability of Waters of the Rega River Catchment for the Construction and Operation of Artificial Spawning Grounds for Migratory Salmonids. Wylęgarnia 2021; Wydawnictwo Instytutu Rybactwa Śródlądowego: Olsztyn, Poland, 2021; pp. 119–128. (In Polish) [Google Scholar]
- WIOŚ in Szczecin 2018, Provincial Inspectorate of Environmental Protection. In The State of the Environment in the West Pomeranian Voivodeship. Report 2018; WIOŚ: Szczecin, Poland, 2018. (In Polish)
- APHA. Standard Methods for Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1999; p. 1325. ISBN 0875532357. [Google Scholar]
- Directive 2006/44/EC of the European Parliament and of the Council of 6 September 2006 on the quality of fresh waters needing protection or improvement in order to support fish life. Off. J. Eur. Union L 2006, 20, 264.
- Directive 2000/60/EC of the European Parliament and of 23 October 2000 the Council establishinga framework for Community action in the field of water police. Off. J. Eur. Union L 2000, 327, 1.
- Wang, Y.; Xiao, Q.; He, B.; Razafindrabe, B.H.N. The Characteristics and Source Contribution Analysis of Nutrients in Water Bodies of Small Watersheds in the Pearl River Delta. Water 2025, 17, 1739. [Google Scholar] [CrossRef]
- About the voivodeship. Agriculture. Provincial Inspectorate of Environmental Protection Szczecin. Available online: https://wios.szczecin.pl/chapter_16000.asp (accessed on 22 December 2011).
- Available online: https://bdl.stat.gov.pl/bdl/dane/podgrup/temat (accessed on 26 June 2025).
- Jiru, E.B.; Wegari, H.T. Soil and water conservation practice effects on soil physicochemical properties and crop yield in Ethiopia: Review and synthesis. Ecol. Process. 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Masha, M.; Belayneh, M.; Bojago, E.; Tadiwos, S.; Dessalegn, A. Impacts of land-use and topography on soil physicochemical properties in the Wamancho watershed, Southern Ethiopia. J. Agric. Food Res. 2023, 14, 100854. [Google Scholar] [CrossRef]
- Newcombe, C.P.; Jensen, J.O.T. Channel suspended sediment and fisheries: A synthesis for quantitative assessment of risk and impact. N. Am. J. Fish. Manag. 1996, 16, 693–727. [Google Scholar] [CrossRef]
- Bonisławska, M.; Formicki, K.; Smaruj, I.; Szulc, J. Total suspended solids in surface waters versus embryonic development of pike (Esox lucius L.). EJPAU 2011, 14, 7. Available online: http://www.ejpau.media.pl/volume14/issue1/art-07.html (accessed on 14 September 2023).
- Kemp, P.; Sear, D.; Collins, A.; Naden, P.; Jones, I. The impacts of fine sediment on riverine fish. Hydrol Process. 2011, 25, 1800–1821. [Google Scholar] [CrossRef]
- Curran, J.C.; Cannatelli, K.M. The impact of beaver dams on the morphology of a river in the eastern United States with implications for river restoration. Earth Surf. Process. Landf. 2014, 39, 1236–1244. [Google Scholar] [CrossRef]
- Rozhkova-Timina, O.I.; Popkov, V.K.; Mitchell, P.J.; Kirpotin, S.N. Beavers as ecosystem engineers—A review of their positive and negative effects. IOP Conf. Ser. Earth Environ. Sci. 2018, 201, 012015. [Google Scholar] [CrossRef]
- Meybeck, M. Carbon, nitrogen and phosphorus transport by world rivers. Am. J. Sci. 1982, 282, 401–450. [Google Scholar] [CrossRef]
- Short Statistical Yearbook of the Republic of Poland 2000-2019. Available online: https://stat.gov.pl (accessed on 18 July 2020).
- Lazar, J.G.; Addy, K.; Gold, A.J.; Groffman, P.M.; McKinney, R.A.; Kellogg, D.Q. Beaver Ponds: Resurgent Nitrogen Sinks for Rural Watersheds in the Northeastern United States. J. Environ. Qual. 2015, 44, 1684–1693. [Google Scholar] [CrossRef]
- Bason, C.W.; Kroes, D.E.; Brinson, M.M. The effect of beaver ponds on water quality in rural coastal plain streams. SENA 2017, 16, 584–602. [Google Scholar] [CrossRef]
- Kelso, B.H.L.; Smith, R.V.; Laughlin, R.J. Effects of carbon substrates on nitrite accumulation in freshwater sediments. Appl. Environ. Microbiol. 1999, 65, 61–66. [Google Scholar] [CrossRef]
- Korwin-Kossakowski, M.; Myszkowski, L.; Kazuń, K. Acute toxicity of nitrite to tench (Tinca tinca L.) larvae. Pol. Arch. Hydrobiol. 1995, 42, 213–216. [Google Scholar]
- Kroupová, H.; Valentová, O.; Svobodová, Z.; Šauer, P.; Machova, J. Toxic effects of nitrite on freshwater organisms: A review. Rev. Aquac. 2018, 10, 525–542. [Google Scholar] [CrossRef]
- Wolfram, J.; Stehle, S.; Bub, S.; Petschick, L.L.; Schulz, R. Water quality and ecological risks in European surface waters—Monitoring improves while water quality decreases. Environ. Int. 2021, 152, 106479. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, N.P.; Phillips, G.; Poikane, S.; Várbíró, G.; Bouraoui, F.; Malagó, A.; Lilli, M.A. River and lake nutrient targets that support ecological status: European scale gap analysis and strategies for the implementation of the Water Framework Directive. Sci. Total Environ. 2022, 20, 151898. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff-Wrześniewska, A.; Strzelczyk, M.; Helis, M.; Paszkiewicz-Jasińska, A.; Gruss, Ł.; Pulikowski, K.; Skorulski, W. Identification of catchment areas with nitrogen pollution risk for lowland river water quality. Arch. Environ. Protect. 2022, 48, 53–64. [Google Scholar] [CrossRef]
- Yu, J.; Mo, L.; Dai, J.; Bai, K.; Mo, J.; Zhang, S. Nitrogen and Phosphorus Pollution Discharge and Water Quality Evaluation in a Small Basin of the Upper Reaches of Lijiang River. Water 2024, 16, 104. [Google Scholar] [CrossRef]
- Pal, S.; Chakraborty, K. Different aspects of chloride in freshwater: A review. Inter. J. Curr. Trends. Sci. Tech. 2017, 7, 20295–20303. [Google Scholar]
- Panno, S.V.; Hackley, K.C.; Hwang, H.H.; Greenberg, S.E.; Krapac, I.G.; Landsberger, S.; O’Kelly, D.J. Database for the Characterization and Identification of Sources of Sodium and Chloride in Natural Waters of Illinois; Open File Series 2005-1; Illinois State Geological Survey: Champaign, IL, USA, 2005. [Google Scholar]
- Cao, K.F.; Chen, Z.; Wu, Y.H.; Mao, Y.; Shi, Q.; Chen, X.W.; Bai, Y.; Li, K.; Hu, H.Y. The noteworthy chloride ions in reclaimed water: Harmful effects, concentration levels and control strategies. Water Res. 2022, 215, 118271. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Z.; Li, W.; Zhang, Y.; Ren, Z.; Li, X.; Wu, Y.; Li, J.; Zhu, W.; Ma, Z.; et al. Chloride accumulation in inland rivers of China and its toxic impact on cotton. J. Environ. Manag. 2024, 371, 123122. [Google Scholar] [CrossRef]
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef]
- Okwuosa, O.B.; Eyo, J.E.; Omovwohwovie, E.E. Role of fish as bioindicators: A Review. Iconic Res. Eng. J. 2019, 2, 1456–8880. [Google Scholar]
- Formicki, K.; Więcaszek, B.; Czerniejewski, P.; Rybczyk, A.; Tański, A.; Korzelecka-Orkisz, A.; Ostaszewska, T.; Zakęś, Z. Preliminary results of the salmonids monitoring in the river Rega (north-western Poland). Front. Mar. Sci. 2019, 6, 157. [Google Scholar] [CrossRef]
- Viaroli, P.; Soana, E.; Pecora, S.; Laini, A.; Naldi, M.; Fano, E.A.; Nizzoli, D. Space and time variations of watershed N and P budgets and their relationships with reactive N and P loadings in a heavily impacted river basin (Po river, Northern Italy). Sci. Total Environ. 2018, 639, 1574–1587. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Li, Y. Spatio-temporal dynamics of nitrogen and phosphorus input budgets in a global hotspot of anthropogenic inputs. Sci. Total Environ. 2019, 656, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, X.; Liu, C.; Wang, Y.; Li, M. Variation of net anthropogenic phosphorus inputs (NAPI) and riverine phosphorus fluxes in seven major river basins in China. Sci. Total Environ. 2020, 742, 140514. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, W.; Liu, Y.; Li, W.; Shao, D. Influence of anthropogenic nitrogen inputs and legacy nitrogen change on riverine nitrogen export in areas with high agricultural activity. J. Environ. Manag. 2023, 338, 117833. [Google Scholar] [CrossRef]
- Szklarek, S.; Górecka, A.; Wojtal-Frankiewicz, A. The effects of road salt on freshwater ecosystems and solutions for mitigating chloride pollution—A review. Sci. Total Environ. 2022, 805, 150289. [Google Scholar] [CrossRef]
- Bonisławska, M.; Tański, A.; Mokrzycka, M.; Brysiewicz, A.; Nędzarek, A.; Tórz, A. The effect of effluents from rainbow trout ponds on water quality in the Gowienica River. J. Water Land Develop. 2013, 19, 3–11. [Google Scholar] [CrossRef]
- Nędzarek, A.; Bonisławska, M.; Tórz, A.; Gajek, A.; Socha, M.; Harasimiuk, F.B. Water quality in the central reach of the Ina River (West Pomerania, Poland). Pol. J. Environ. Stud. 2015, 24, 207–214. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Gao, D.; Yu, H. Source identification of surface water pollution using multivariate statistics combined with physicochemical and socioeconomic parameters. Sci. Total Environ. 2022, 806, 151274. [Google Scholar] [CrossRef]
- Benadela, L.; Bekkoussa, B.; Gaidi, L. Multivariate analysis and geochemical investigations of groundwater in a semi-arid region, case of superficial aquifer in Ghriss Basin, Northwest Algeria. JGSE 2022, 10, 233–249. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Kong, M.; Zhou, J.; Zhao, H.; Tang, Z.; Zhang, M.; Wang, Z. Insights into the long-term pollution trends and sources contributions in Lake Taihu, China using multi-statistic analyses models. Chemosphere 2020, 242, 125272. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Z.; Kong, M.; Chen, X.; Zhou, C.; Huang, K.; Wang, Z. Tracing the potential pollution sources of the coastal water in Hong Kong with statistical models combining APCS-MLR. J. Environ. Manag. 2019, 245, 143–150. [Google Scholar] [CrossRef]
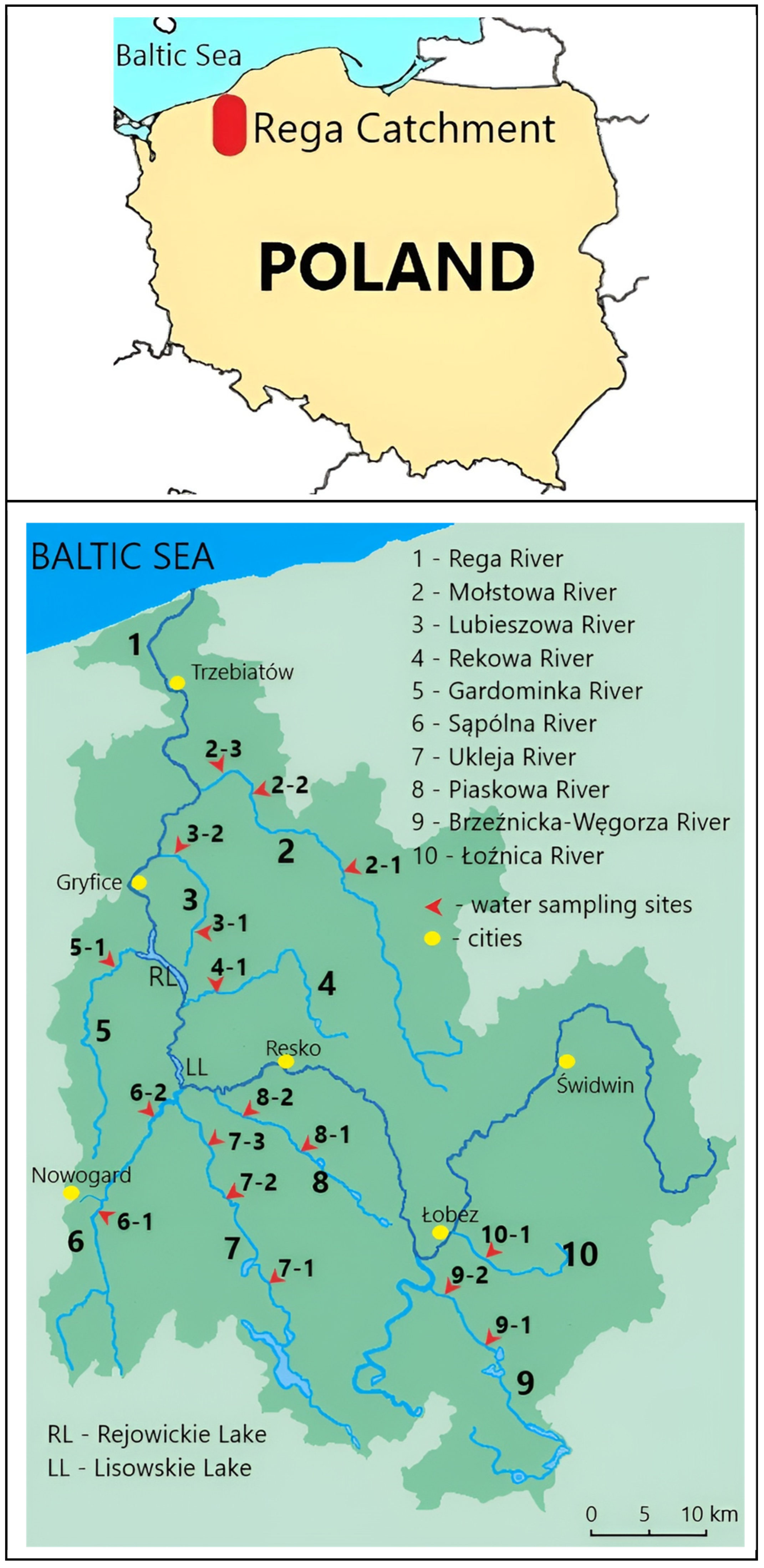
| River | GPS Coordinates | Mean Width (m) | Mean Depth (m) | Guidelines (RMI 2021) |
|---|---|---|---|---|
| River’s first-order tributaries | ||||
| Mołstowa | 53°44′43″ N 15°36′03″ E | 54.4 | 377.0 | R1_poj |
| Ukleja | 53°29′24″ N 15°29′15″ E | 45.4 | 457.0 | R1_poj |
| Gardominka | 53°51′32″ N 15°13′47″ E | 26.6 | 112.6 | R1_poj |
| Rekowa | 53°41′39″ N 15°27′11″ E | 22.0 | 110.4 | P1_poj |
| Piaskowa | 53°41′39″ N 15°27′11″ E | 18.0 | 86.70 | P1_poj |
| Lubieszowa | 53°56′12″ N 15°14′22″ E | 14.3 | 65.94 | P1_poj |
| Łoźnica | 53°38′23″ N 15°37′32″ E | 13.4 | 88.14 | PN |
| River’s second-order tributaries | ||||
| Brzeźnicka-Węgorza | 53°29′25″ N 15°43′30″ E | 37.8 | 276.0 | R1_poj |
| Sapólna | 53°31′59″ N 15°09′26″ E | 60.1 | 87.7 | RzN |
| River | Position | Points on the Map | Mean Width m | Mean Depth m | Mean Flow m·s−1 |
|---|---|---|---|---|---|
| Mołstowa | Rzesznikowo Mołstowo Bielikowo | 2-1 2-2 2-3 | 15.0 11.09 9.1 | 1.6 1.2 1.5 | 0.65 0.87 0.45 |
| Ukleja | Mieszewo Troszczyno Miłogoszcz | 7-1 7-2 7-3 | 6.4 17.1 15.7 | 0.3 0.4 1.5 | 0.12 0.19 0.51 |
| Gardominka | Truskolas | 5-1 | 7.5 | 0.6 | 0.23 |
| Rekowa | Wicimie | 4-1 | 5.2 | 0.2 | 0.13 |
| Piaskowa | Świętochowo Maliniec | 8-1 8-2 | 1.5 6.3 | 0.2 0.3 | 0.29 0.65 |
| Lubieszowa | Charnowo Lubieszewo | 3-1 3-2 | 4.5 4.0 | 0.5 0.6 | 0.47 0.45 |
| Łoźnica | Wysiedle | 10-1 | 8.1 | 0.3 | 0.52 |
| Brzeźnicka-Węgorza | Brzeźniak Lesięcin | 9-1 9-2 | 12.2 10.5 | 0.7 0.5 | 0.56 0.93 |
| Sapólna | near Nowogard Żabówko | 6-1 6-2 | 10.1 7.3 | 0.4 0.4 | 0.31 0.24 |
| Parameter | Method | Units |
|---|---|---|
| Temperature | Standard Method 2550 | °C |
| Conductivity | Standard Method 2510 | μS·cm−1 |
| pH | Standard Method 4500-H+ | |
| Total suspended solids (TSSs) | Standard Method 2540D | mg·L−1 |
| Alkalinity | Standard Method 2320 | mg CaCO3·L−1 |
| Total hardness (TH) | Standard Method 2340 | mg CO3·L−1 |
| Chloride by Argentometric Method | Standard Method 4500-B | mg Cl−·L−1 |
| Dissolved oxygen (DO) | Standard Method 4500-O B | mg O2·L−1 |
| Biochemical Oxygen Demand (BOD5) | Standard Method 5210 B | mg O2·dm−3 |
| Nitrite-nitrogen (NO2-N) | Standard Method 4500-NO2− | mg·L−1 (as NO2-N) |
| Total ammonia nitrogen (NH4-N) | Standard Method 4500-NH3 | mg·L−1 (as NH3-N) |
| Total phosphorus (TP) | Standard Method 4500-P | mg·L−1 (as P) |
| Directive 2006/44/EC | |||
|---|---|---|---|
| Indicator name, unit | Salmonid waters | Cyprinid waters | |
| Temperature, °C | <21.5 | <28.0 | |
| pH | 6.0–9.0 | ||
| TSSs mg·L−1 | ≤25 | ||
| DO mgO2·L−1 | 50% ≥ 9.0 100% ≥ 7.0 | 50% ≥ 7.0 100% ≥ 7.0 | |
| BOD5 mgO2·L−1 | ≤3.0 | ≤6.0 | |
| TP mg·L−1 | ≤0.2 | ≤0.4 | |
| N-NO2 mg·L−1 | ≤0.01 | ≤0.03 | |
| N-NH4 mg·L−1 | ≤1.0 | ||
| Regulation of the Ministry of Infrastructure | |||
| Indicator name, unit | The threshold value for water quality class | ||
| I | II | III–V | |
| Indicators characterizing oxygen conditions and organic pollution | |||
| DO mgO2·L−1 | ≥8.5 | ≥7.5 | Not classified—nc |
| BOD5 mgO2·L−1 | ≤3.0 | ≤3.8 | |
| Indicators characterizing salinity | |||
| Conductivity in 20 °C µS·cm−1 | ≤360 | ≤480 | Not classified—nc |
| Indicators characterizing biogenic conditions | |||
| N-NH4 mg·L−1 | ≤0.12 | ≤0.30 | Not classified—nc |
| TP mg·L−1 | ≤0.10 | ≤0.30 | |
| Parameter River | Temp. °C | pH | Conductivity µS·cm−1 | TSSs mg·L−1 | DO | BOD5 |
|---|---|---|---|---|---|---|
| mgO2·L−1 | ||||||
| Mołstowa | 9.1 ± 4.3 a | 7.5 ± 0.6 bcd | 161 ± 28 a I | 57 ± 56 bd | 9.4 ± 1.6 b I | 2.6 ± 1.3 bcd I |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 2.5–17.9 | 6.6–8.1 | 121–234 | 2–318 | 6.3–11.5 | 0.2–5.0 | |
| Ukleja | 9.3 ± 5.8 a | 7.6 ± 0.5 bd | 201 ± 48 b I | 41 ± 25 ae | 9.4 ± 2.1 b I | 2.8 ± 1.0 b I |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.3–21.5 | 6.5–8.1 | 114–381 | 4–104 | 4.9–13.8 | 0.6–5.2 | |
| Gardominka | 9.1 ± 4.9 a | 7.2 ± 0.5 a | 187 ± 42 b I | 18 ± 14 a | 6.8 ± 2.5 ac nc | 2.0 ± 0.8 ac I |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 1.3–17.9 | 6.1–7.7 | 143–301 | 2–58 | 1.9–10.6 | 0.7–3.7 | |
| Rekowa | 8.4 ± 3.7 a | 7.3 ± 0.5 ac | 153 ± 22 a I | 26 ± 35 ac | 6.0 ± 1.9 a nc | 1.8 ± 0.9 a I |
| min–max | min–max | in-max | min–max | min–max | min–max | |
| 2.3–13.8 | 6.4–7.7 | 112–189 | 2–159 | 3.1–8.9 | 0.6–3.8 | |
| Piaskowa | 9.4 ± 4.1 a | 7.6 ± 0.4 bd | 205 ± 23 b I | 67 ± 50 bd | 8.8 ± 1.8 b I | 2.3 ± 0.9 ac I |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 1.1–15.5 | 6.4–8.0 | 163–236 | 8–224 | 5.2–12.0 | 0.7–4.6 | |
| Lubieszowa | 8.4 ± 4.5 a | 7.3 ± 0.5 ad | 190 ± 30 bc I | 41 ± 44 ad | 7.6 ± 2.0 cd II | 2.0 ± 1.1 ad I |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 1.8–16.9 | 6.1–8.0 | 141–248 | 2–230 | 2.9–11.1 | 0.7–3.9 | |
| Łoźnica | 8.3 ± 3.5 a | 7.7 ± 0.5 d | 210 ± 28 b I | 51 ± 28 bcde | 9.6 ± 1.2 b I | 2.1 ± 0.8 ad I |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 2.2–14.5 | 6.6–8.1 | 169–249 | 6–112 | 8.1–11.6 | 0.8–3.5 | |
| Brzeźnicka-Węgorza | 10.4 ± 6.7 a | 7.7 ± 0.5 d | 170 ± 23 ac I | 55 ± 36 bd | 9.1 ± 1.9 b I | 3.1 ± 1.4 b II |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.4–22.9 | 6.5–8.3 | 120–215 | 14–178 | 5.4–12.9 | 0.7–5.6 | |
| Sąpólna | 8.6 ± 4.3 a | 7.5 ± 0.5 bcde | 269 ± 64 d I | 54 ± 53 bd | 9.1 ± 1.6 b I | 2.4 ± 1.3 ab II |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 2.0–16.9 | 6.5–8.0 | 146–409 | 4–208 | 5.4–11.6 | 0.7–5.6 | |
| p | 0.798 | 0.008 | 0 | 0.004 | 0 | 0.001 |
| Parameter River | TP | NO2-N | NH4-N | Alkalinity mgCaCO3·L−1 | Total Hardness mgCO32−·L−1 | Chlorides mgCl−·L−1 |
|---|---|---|---|---|---|---|
| mg·L−1 | ||||||
| Mołstowa | 0.089 ± 0.036 abc I | 0.020 ± 0.013 bc | 0.064 ± 0.042 a I | 161.2 ± 27.9 a | 229.1 ± 28.8 ab | 25.8 ± 6.8 a |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.047–0.246 | 0.005–0.065 | 0.017–0.193 | 110.0–195.0 | 165.0–285.0 | 14.2–46.2 | |
| Ukleja | 0.092 ± 0.050 abc I | 0.015 ± 0.007 ac | 0.049 ± 0.041 a I | 181.7 ± 22.5 b | 225.5 ± 41.0 ab | 33.7 ± 9.7 ab |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.021–0.297 | 0.005–0.032 | 0.007–0.229 | 111.5–230.0 | 87.5–276.0 | 14.2–56.8 | |
| Gardominka | 0.058 ± 0.017 a I | 0.015 ± 0.009 ac | 0.054 ± 0.065 a I | 167.6 ± 19.9 a | 231.7 ± 27.1 ab | 35.3 ± 6.1 b |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.033–0.087 | 0.005–0.040 | 0.012–0.252 | 122.0–225.0 | 185.0–295.0 | 28.4–49.7 | |
| Rekowa | 0.083 ± 0.025 abc I | 0.012 ± 0.012 a | 0.064 ± 0.029 a I | 161.0 ± 27.8 a | 227.7 ± 27.8 ab | 25.8 ± 5.9 a |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.050–0.144 | 0.003–0.049 | 0.020–0.123 | 109.0–179.5 | 192.5–275.0 | 17.8–39.1 | |
| Piaskowa | 0.081 ± 0.041 abc I | 0.013 ± 0.005 a | 0.077 ± 0.052 ab I | 185.7 ± 14.6 c | 238.5 ± 31.9 ab | 32.1 ± 13.7 ab |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.025–0.198 | 0.004–0.023 | 0.02–0.166 | 170.0–230.0 | 120.0–257.5 | 17.8–95.9 | |
| Lubieszowa | 0.109 ± 0.056 c II | 0.013 ± 0.005 a | 0.050 ± 0.045 a I | 198.2 ± 19.9 c | 244.5 ± 18.5 b | 32.2 ± 13.4 ab |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.053–0.313 | 0.005–0.029 | 0.024–0.269 | 154.0–235.0 | 217.5–286.3 | 17.8–71.0 | |
| Łoźnica | 0.104 ± 0.022 c I | 0.016 ± 0.004 ac | 0.043 ± 0.024 a I | 222.6 ± 23.8 d | 260.4 ± 41.3 cd | 26.4 ± 7.4 a |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.06–0.129 | 0.011–0.025 | 0.009–0.094 | 254.0–184.0 | 270.0–322.5 | 14.2–39.1 | |
| Brzeźnicka-Węgorza | 0.065 ± 0.038 ab I | 0.014 ± 0.008 ac | 0.075 ± 0.046 ab I | 191.3 ± 18.1 bc | 220.8 ± 36.5 a | 24.7 ± 7.3 a |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.022–0.220 | 0.001–0.041 | 0.016–0.203 | 161.0–235.0 | 102.5–252.5 | 14.2–42.6 | |
| Sąpólna | 0.173 ± 0.150 d II | 0.026 ± 0.018 d | 0.111 ± 0.133 b I | 197.8 ± 24.7 c | 259.3 ± 23.6 cd | 59.5 ± 32.1 c |
| min–max | min–max | min–max | min–max | min–max | min–max | |
| 0.044–0.676 | 0.016–0.101 | 0.014–0.699 | 152.5–245.0 | 220.0–300.0 | 24.9–127.8 | |
| p | 0 | 0 | 0.001 | 0 | 0 | 0 |
| Indicator | Loading Values | |
|---|---|---|
| PC1 | PC2 | |
| T | −0.16 | 0.71 |
| pH | −0.13 | −0.60 |
| TSSs | −0.08 | 0.04 |
| DO | −0.05 | −0.83 |
| BOD5 | 0.07 | −0.51 |
| NH4+-N | −0.61 | 0.15 |
| NO2−-N | −0.69 | 0.05 |
| TP | −0.75 | 0.27 |
| EC | −0.77 | −0.09 |
| Alc. | −0.46 | −0.24 |
| TH | −0.34 | −0.52 |
| Cl− | −0.75 | −0.01 |
| T | pH | TSSs | DO | BOD5 | NH4+-N | NO2−-N | TP | EC | Alc. | TH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | −0.17 * | ||||||||||
| TSS | −0.01 | −0.05 | |||||||||
| DO | −0.58 * | 0.41 * | 0.17 * | ||||||||
| BOD5 | −0.18 * | 0.14 | 0.03 | 0.45 * | |||||||
| NH4+-N | 0.03 | −0.04 | 0.17 * | −0.04 | −0.03 | ||||||
| NO2−-N | 0.15 | 0.15 | 0.13 | 0.05 | −0.05 | 0.51 * | |||||
| TP | 0.28 * | −0.02 | 0.19 * | −0.07 | −0.13 | 0.43 * | 0.40 * | ||||
| EC | 0.04 | −0.04 | 0.03 | 0.14 | −0.02 | 0.29 * | 0.38 * | 0.47 * | |||
| Alc. | 0.15 | 0.29 | −0.22 * | 0.09 | −0.01 | −0.00 | 0.07 | 0.25 * | 0.41 * | ||
| TH | −0.29 * | 0.26 | −0.10 | 0.19 * | 0.01 | 0.05 | 0.15 | −0.04 | 0.27 * | 0.36 * | |
| Cl− | 0.02 | 0.02 | −0.08 | -0.03 | 0.02 | 0.34 * | 0.38 * | 0.51 * | 0.54 * | 0.22 * | 0.25 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonisławska, M.; Nędzarek, A.; Tański, A.; Tórz, A.; Formicki, K. Assessment of Water Quality in the Tributaries of the Rega River (Northwestern Poland) as a Fish Habitat. Appl. Sci. 2025, 15, 7846. https://doi.org/10.3390/app15147846
Bonisławska M, Nędzarek A, Tański A, Tórz A, Formicki K. Assessment of Water Quality in the Tributaries of the Rega River (Northwestern Poland) as a Fish Habitat. Applied Sciences. 2025; 15(14):7846. https://doi.org/10.3390/app15147846
Chicago/Turabian StyleBonisławska, Małgorzata, Arkadiusz Nędzarek, Adam Tański, Agnieszka Tórz, and Krzysztof Formicki. 2025. "Assessment of Water Quality in the Tributaries of the Rega River (Northwestern Poland) as a Fish Habitat" Applied Sciences 15, no. 14: 7846. https://doi.org/10.3390/app15147846
APA StyleBonisławska, M., Nędzarek, A., Tański, A., Tórz, A., & Formicki, K. (2025). Assessment of Water Quality in the Tributaries of the Rega River (Northwestern Poland) as a Fish Habitat. Applied Sciences, 15(14), 7846. https://doi.org/10.3390/app15147846








