Influence of the Milling Cutter Drill on Implant Placement Accuracy in Partially Guided Surgery: An In Vitro Experimental Study
Abstract
Featured Application
Abstract
1. Introduction
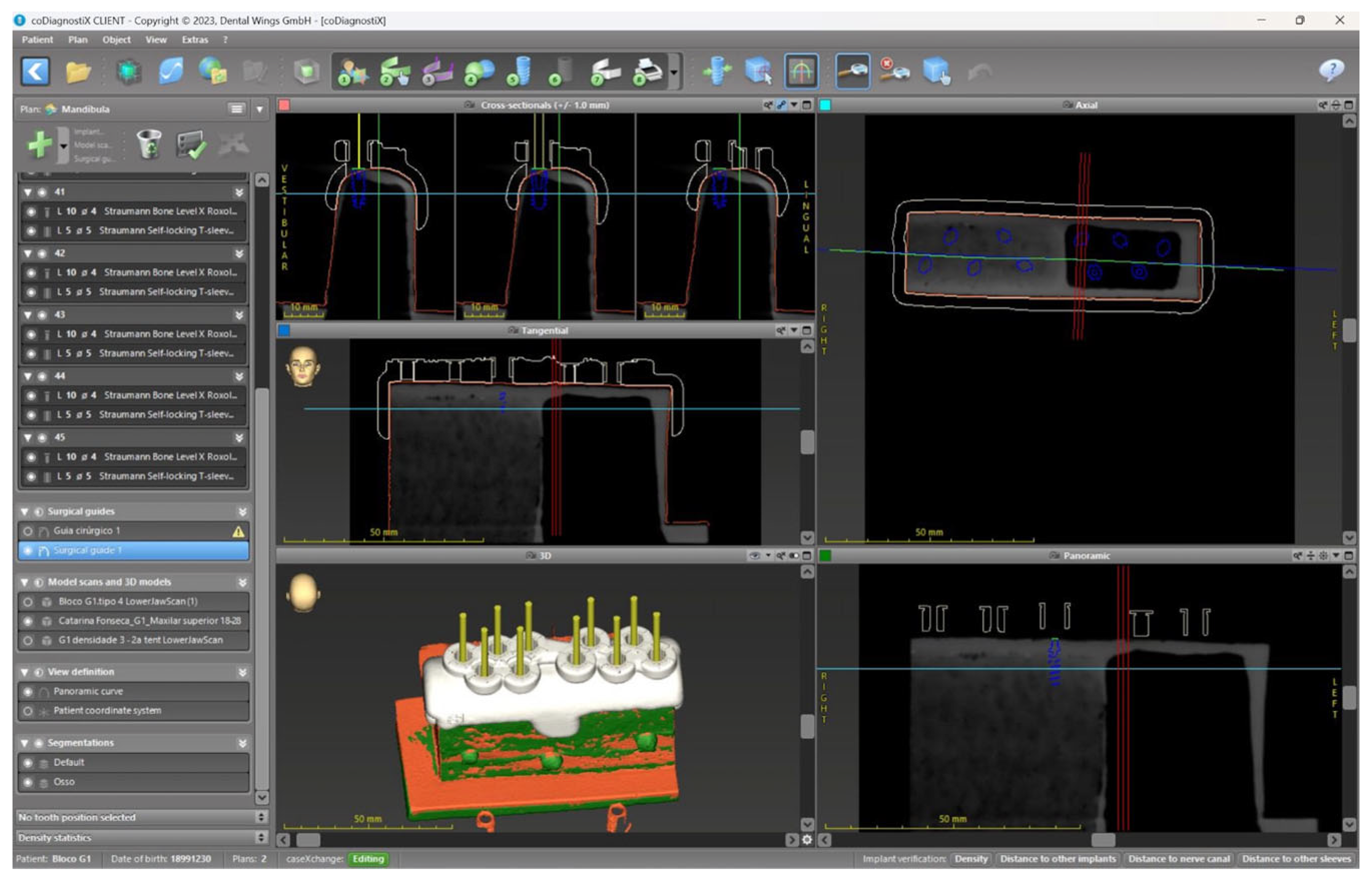
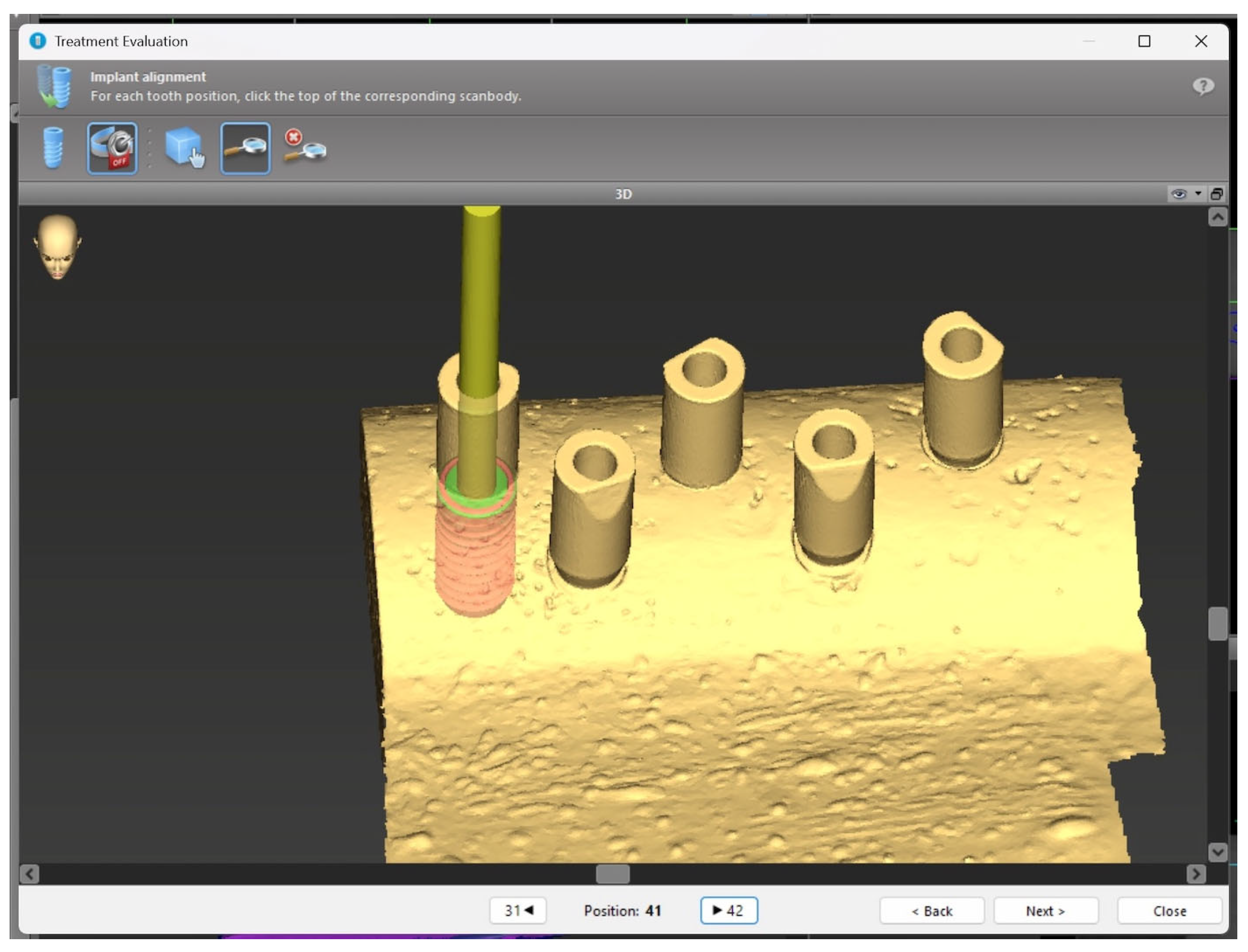
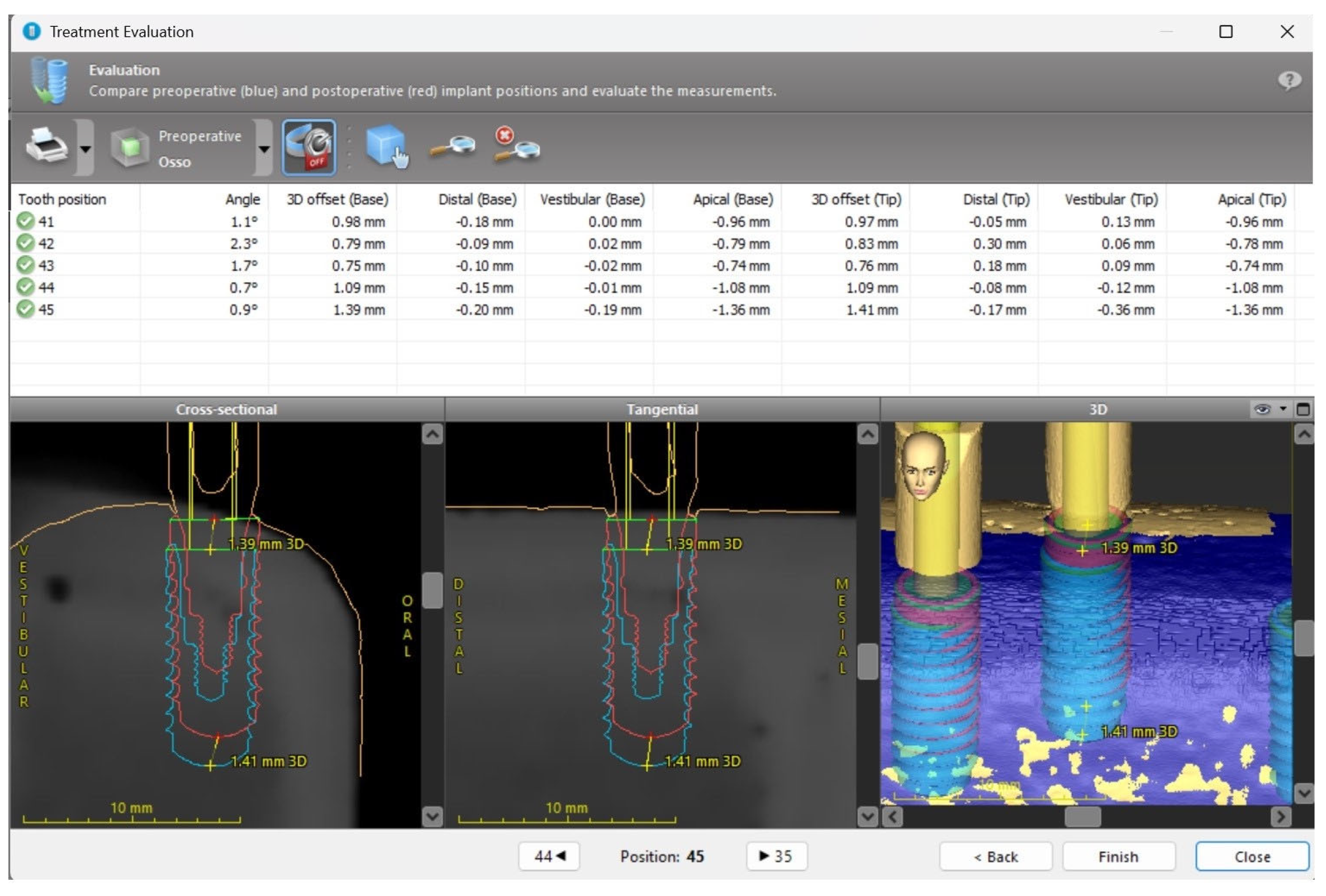
2. Materials and Methods
2.1. Sample
2.2. Surgical Protocol
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Influence of the Milling Cutter Drill
4.2. Influence of Bone Density
4.3. Influence of Implant Type
4.4. Limitations and Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| BL | Bone Level (implant type) |
| BL | Bucco-lingual (in measurement context) |
| BLT | Bone Level Tapered (implant type) |
| BLX | Bone Level X (implant type) |
| CAIS | Computer-Assisted Implant Surgery |
| CAM | Computer-Aided Manufacturing |
| CAD | Computer-Aided Design |
| CBCT | Cone Beam Computed Tomography |
| DD | Digital Dentistry |
| FOV | Field of View |
| PEEK | Polyether ether ketone |
| PGS | Partially Guided Surgery |
| s-CAIS | Static Computer-Assisted Implant Surgery |
| SPSS | Statistical Package for the Social Sciences |
References
- Gawali, N.; Shah, P.P.; Gowdar, I.M.; Bhavsar, K.A.; Giri, D.; Laddha, R. The Evolution of Digital Dentistry: A Comprehensive Review. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S3), S1920–S1922. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Bornstein, M.M.; Jung, R.E.; Ferrari, M.; Waltimo, T.; Zitzmann, N.U. Recent Trends and Future Direction of Dental Research in the Digital Era. Int. J. Environ. Res. Public Health 2020, 17, 1987. [Google Scholar] [CrossRef] [PubMed]
- da Silva Salomão, G.V.; Chun, E.P.; Panegaci, R.D.S.; Santos, F.T. Analysis of Digital Workflow in Implantology. Case Rep. Dent. 2021, 2021, 6655908. [Google Scholar] [CrossRef] [PubMed]
- Della Bona, A.; Cantelli, V.; Britto, V.T.; Collares, K.F.; Stansbury, J.W. 3D Printing Restorative Materials Using a Stereolithographic Technique: A Systematic Review. Dent. Mater. 2021, 37, 336–350. [Google Scholar] [CrossRef]
- Abdelnabi, M.H.; Swelem, A.A. 3D-Printed Complete Dentures: A Review of Clinical and Patient-Based Outcomes. Cureus 2024, 16, e69698. [Google Scholar] [CrossRef]
- Mangano, F.G.; Hauschild, U.; Veronesi, G.; Imburgia, M.; Mangano, C.; Admakin, O. Trueness and Precision of 5 Intraoral Scanners in the Impressions of Single and Multiple Implants: A Comparative In Vitro Study. BMC Oral Health 2019, 19, 101. [Google Scholar] [CrossRef]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone Beam Computed Tomography in Implant Dentistry: Recommendations for Clinical Use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef]
- Bernauer, S.A.; Zitzmann, N.U.; Joda, T. The Complete Digital Workflow in Fixed Prosthodontics Updated: A Systematic Review. Healthcare 2023, 11, 679. [Google Scholar] [CrossRef]
- Van Assche, N.; Quirynen, M.; Jacobs, R.; van Steenberghe, D. Accuracy of implant placement based on pre-surgical planning of three-dimensional cone-beam images: A pilot study. J. Clin. Periodontol. 2007, 34, 816–821. [Google Scholar] [CrossRef]
- D’Haese, J.; Van De Velde, T.; Komiyama, A.; Hultin, M.; De Bruyn, H. Accuracy and complications using computer-designed stereolithographic surgical guides for oral rehabilitation by means of dental implants: A review of the literature. Clin. Implant Dent. Relat. Res. 2012, 14, 321–335. [Google Scholar] [CrossRef]
- Valente, F.; Schiroli, G.; Sbrenna, A. Accuracy of computer-aided oral implant surgery: A clinical and radiographic study. Int. J. Oral Maxillofac. Implants 2009, 24, 234–242. [Google Scholar] [PubMed]
- Cassetta, M.; Stefanelli, L.V.; Giansanti, M.; Di Mambro, A.; Calasso, S. Accuracy of computer-guided implant placement: A prospective clinical study. J. Periodontal Implant Sci. 2013, 43, 68–76. [Google Scholar]
- Flügge, T.; Kramer, J.; Nelson, K.; Nahles, S.; Kernen, F. Digital Implantology—A Review of Virtual Planning Software for Guided Implant Surgery. Part II: Prosthetic Set-Up and Virtual Implant Planning. BMC Oral Health 2022, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Pimkhaokham, A.; Jiaranuchart, S.; Kaboosaya, B.; Arunjaroensuk, S.; Subbalekha, K.; Mattheos, N. Can Computer-Assisted Implant Surgery Improve Clinical Outcomes and Reduce the Frequency and Intensity of Complications in Implant Dentistry? A Critical Review. Periodontology 2000 2022, 90, 197–223. [Google Scholar] [CrossRef]
- Putra, R.H.; Yoda, N.; Astuti, E.R.; Sasaki, K. The Accuracy of Implant Placement with Computer-Guided Surgery in Partially Edentulous Patients and Possible Influencing Factors: A Systematic Review and Meta-Analysis. J. Prosthodont. Res. 2022, 66, 29–39. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, J.H.; Jeong, S.M.; Lee, J.H. Effect of Flat-End Drills on Implant Placement Accuracy in Guided Surgery: A Randomized Controlled Trial. Clin. Oral Implants Res. 2021, 32, 345–352. [Google Scholar]
- Zhang, Y.; Wu, Y.; Li, H.; Wang, C. The Role of Drill Geometry in Implant Osteotomy Precision: An In Vitro Study on Flat versus Tapered Tips. Int. J. Oral Maxillofac. Implants 2023, 38, 55–63. [Google Scholar]
- Oliveira, C.A.; Mendes, R.; Rocha, L.; Rodrigues, F.; Silva, M.; Santos, P.; Gomes, T.; Cunha e Silva, B.; Lima, H.; Barbosa, J.; et al. Effectiveness of Flat Drills in Guided Implant Surgery: A Prospective Multicenter Study. Clin. Implant Dent. Relat. Res. 2023, 25, 102–109. [Google Scholar]
- Saran, R.; Ginjupalli, K.; George, S.D.; Chidangil, S.; Unnikrishnan, V.K. LASER as a Tool for Surface Modification of Dental Biomaterials: A Review. Heliyon 2023, 9, e17457. [Google Scholar] [CrossRef]
- Lekholm, U.; Zarb, G.A. Patient Selection and Preparation. In Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Brånemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence Publishing Co., Ltd.: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Suzuki, M.; Calasans-Maia, J.A.; Rocha, L.A.; Mattos, C.M.; Granjeiro, J.M. The Effect of Bone Density on the Primary Stability of Dental Implants: A Critical Review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1031–1037. [Google Scholar]
- Kim, M.J.; Cho, Y.D.; Hwang, C.J. Enhancing Surgical Accuracy with Flat Milling Cutters in Irregular Bone Crests: A Clinical Study. J. Periodontal Implant Sci. 2024, 54, 15–23. [Google Scholar]
- Hossain, M.; Iwasaki, C.; Takechi, M.; Yoshida, K.; Nakamura, T.; Tanaka, H.; Saito, A.; Ono, Y.; Suzuki, N.; Ueda, S.; et al. Influence of Bone Quality on Implant Stability in Immediate Loading Cases. J. Prosthodont. Res. 2023, 67, 73–80. [Google Scholar]
- González-García, R.; Monje, A.; Delgado-Ruiz, R. Preoperative Bone Quality Assessment Using CBCT and Its Correlation with Implant Stability. Int. J. Oral Maxillofac. Implants 2023, 38, 289–296. [Google Scholar]
- Lopes, P.; Neves, M.; Oliveira, H.; Santos, R.; Almeida, S.; Costa, L.; Fernandes, B.; Rodrigues, A.; Melo, C.; Castro, M.; et al. Evaluation of Implant Stability in Different Bone Types Using BL, BLT, and BLX Systems. Clin. Oral Investig. 2022, 26, 6705–6712. [Google Scholar]
- Fischer, K.; Stenberg, T.; Sennerby, L. Influence of Implant Design on Primary Stability: A Randomized Clinical Trial. J. Clin. Periodontol. 2023, 50, 181–189. [Google Scholar]
- Costa, G.; Martins, A.; Dias, R. Implant Design and Bone Preservation: Finding the Balance for Clinical Success. Eur. J. Dent. 2023, 17, 512–520. [Google Scholar]


| Variable | Frequency n (%) | |
|---|---|---|
| Use of Milling cutter drill | Yes | 60 (50%) |
| No | 60 (50%) | |
| Bone Density | D1 | 30 (25%) |
| D2 | 30 (25%) | |
| D3 | 30 (25%) | |
| D4 | 30 (25%) | |
| Implant Type | BL | 40 (33.3%) |
| BLT | 40 (33.3%) | |
| BLX | 40 (33.3%) | |
| Deviation | Milling Cutter Drill | |||
|---|---|---|---|---|
| No | Yes | U | p-Value | |
| Angular (°) | 3.40 (1.90) | 2.45 (2.55) | 1284.000 | 0.007 |
| Neck of implant (mm) | ||||
| 3D | 0.56 (0.69) | 0.73 (0.69) | 1469.000 | 0.082 |
| MD | 0.10 (0.27) | 0.10 (0.10) | 1751.000 | 0.797 |
| BL | 0.20 (0.42) | 0.12 (0.37) | 1672.000 | 0.501 |
| AC | 0.50 (0.57) | 0.65 (0.75) | 1629.000 | 0.369 |
| Apex of the implant (mm) | ||||
| 3D | 0.88 (0.58) | 0.87 (0.67) | 1747.500 | 0.783 |
| MD | 0.25 (0.27) | 0.22 (0.24) | 1621.500 | 0.349 |
| BL | 0.47 (0.41) | 0.38 (0.49) | 1479.500 | 0.092 |
| AC | 0.56 (0.59) | 0.67 (0.80) | 1754.000 | 0.809 |
| Deviation | Type of Bone, Median (IQR) | Kruskal–Wallis | ||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | H | p-Value | |
| Angular (°) | 2.90 (2.30) | 2.50 (1.80) | 3.55 (2.30) | 3.60 (1.70) | 6.747 | 0.080 |
| Neck of the implant (mm) | ||||||
| 3D | 0.99 (0.45) | 0.61 (0.62) | 0.40 (0.53) | 0.55 (0.45) | 9.140 | 0.027 |
| MD | 0.12 (0.30) | 0.08 (0.39) | 0.08 (0.05) | 0.10 (0.10) | 8.559 | 0.036 |
| BL | 0.58 (0.40) | 0.16 (0.37) | 0.06 (0.06) | 0.12 (0.21) | 40.183 | <0.001 |
| AC | 0.74 (0.69) | 0.69 (0.83) | 0.41 (0.43) | 0.51 (0.41) | 3.201 | 0.362 |
| Apex of implant (mm) | ||||||
| 3D | 0.90 (0.54) | 0.84 (0.81) | 0.73 (0.55) | 0.95 (0.49) | 6.064 | 0.109 |
| MD | 0.25 (0.25) | 0.25 (0.25) | 0.19 (0.21) | 0.26 (0.28) | 6.649 | 0.084 |
| BL | 0.39 (0.17) | 0.38 (0.32) | 0.47 (0.41) | 0.67 (0.68) | 6.888 | 0.076 |
| AC | 0.84 (0.71) | 0.69 (0.84) | 0.46 (0.43) | 0.50 (0.36) | 13.047 | 0.005 |
| Deviation | Implant, Median (IQR) | Kruskal–Wallis | |||
|---|---|---|---|---|---|
| BL | BLT | BLX | H | p-Value | |
| Angular (°) | 3.25 (2.15) | 3.20 (2.20) | 2.80 (2.45) | 1.020 | 0.601 |
| Neck of the implant (mm) | |||||
| 3D | 0.45 (0.50) | 0.47 (0.78) | 0.91 (0.54) | 15.860 | <0.001 |
| MD | 0.09 (0.21) | 0.11 (0.36) | 0.08 (0.08) | 3.034 | 0.219 |
| BL | 0.10 (0.22) | 0.20 (0.56) | 0.25 (0.66) | 7.818 | 0.020 |
| AC | 0.45 (0.47) | 0.56 (0.77) | 0.70 (0.79) | 6.903 | 0.032 |
| Apex of the implant (mm) | |||||
| 3D | 0.84 (0.42) | 1.05 (0.66) | 0.90 (0.67) | 4.352 | 0.113 |
| MD | 0.25 (0.19) | 0.25 (0.44) | 0.19 (0.21) | 3.500 | 0.174 |
| BL | 0.43 (0.40) | 0.49 (1.00) | 0.37 (0.38) | 6.201 | 0.045 |
| AC | 0.53 (0.42) | 0.72 (0.95) | 0.68 (0.83) | 4.905 | 0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.R.; Fonseca, C.M.; Correia, A.; Fonseca, P. Influence of the Milling Cutter Drill on Implant Placement Accuracy in Partially Guided Surgery: An In Vitro Experimental Study. Appl. Sci. 2025, 15, 7826. https://doi.org/10.3390/app15147826
Ferreira AR, Fonseca CM, Correia A, Fonseca P. Influence of the Milling Cutter Drill on Implant Placement Accuracy in Partially Guided Surgery: An In Vitro Experimental Study. Applied Sciences. 2025; 15(14):7826. https://doi.org/10.3390/app15147826
Chicago/Turabian StyleFerreira, Ana Raquel, Catarina Mendes Fonseca, André Correia, and Patrícia Fonseca. 2025. "Influence of the Milling Cutter Drill on Implant Placement Accuracy in Partially Guided Surgery: An In Vitro Experimental Study" Applied Sciences 15, no. 14: 7826. https://doi.org/10.3390/app15147826
APA StyleFerreira, A. R., Fonseca, C. M., Correia, A., & Fonseca, P. (2025). Influence of the Milling Cutter Drill on Implant Placement Accuracy in Partially Guided Surgery: An In Vitro Experimental Study. Applied Sciences, 15(14), 7826. https://doi.org/10.3390/app15147826







