Abstract
Hydrogen has gradually become one of the indispensable sources of energy for mankind. Since the discovery of hydrogen embrittlement (hydrogen-induced degradation of material properties) more than 100 years ago, fatigue properties in hydrogen environments have been studied. Fatigue crack growth of materials in a hydrogen environment is a complex process involving the interaction of multiple factors. Hydrogen binds to atoms within the material, leading to diffusion and aggregation of hydrogen atoms, which causes an increase in internal stresses. These stresses may concentrate at the crack tip, accelerating the rate of crack expansion and leading to fatigue fracture of the material. The work of current researchers has summarised a number of fatigue models to help understand this phenomenon. This paper firstly summarises the existing hydrogen embrittlement mechanisms as well as hydrogen embrittlement experiments. It then focuses on the mechanism of fatigue crack propagation in hydrogen environments and related literature. It also analyses and summarises a cluster diagram of the literature generated using CiteSpace. The fatigue life prediction methods for materials in hydrogen environment are then summarised in this paper. It aims to provide some guidance for the selection and design of materials in developing fields such as fatigue materials in hydrogen environment. Finally, challenges in the current research on the fatigue properties of materials under hydrogen embrittlement conditions are pointed out and discussed to guide future research efforts.
1. Introduction
Hydrogen has become one of the most popular energy carriers these years. Its high-energy storage and low pollution stand out from various energy carriers. After observing hydrogen embrittlement explosions during hydrogen transport, researchers have turned their research towards hydrogen embrittlement. Hydrogen embrittlement is an important problem that has long plagued the field of metallic materials engineering and refers to the overall degradation of the mechanical properties of metals in a hydrogen environment [1,2,3]. Although most metallic materials exhibit excellent toughness and plasticity at ambient temperatures and pressures, once exposed to hydrogen-enriched environments, their mechanical properties and durability tend to deteriorate drastically and even undergo severe brittle damage. The study of hydrogen embrittlement involves not only the field of materials science and engineering, but also chemistry, physics, and other disciplines, and has attracted much attention because of its wide range of applications and far-reaching impact [4,5,6].
In the past decades, scientists have conducted extensive research on hydrogen embrittlement and achieved some important results [7,8,9,10]. However, the mechanism of hydrogen embrittlement is extremely complex and still not fully understood, and there are some limitations in the methods of preventing and mitigating hydrogen embrittlement. The classical hydrogen embrittlement mechanism also fails to fully express the hydrogen embrittlement phenomenon. Therefore, in-depth understanding of the mechanisms, influencing factors, and preventive measures of hydrogen embrittlement is of great significance for improving the reliability and durability of metallic materials. By revealing the intrinsic mechanism of hydrogen embrittlement, we can design new materials that are more hydrogen-resistant and take targeted measures to mitigate the effects of hydrogen embrittlement [11,12,13]. Meanwhile, by exploring new testing methods and evaluation techniques, we can more accurately assess the performance of materials in hydrogen environments and identify potential safety hazards in a timely manner.
Current research considers hydrogen embrittlement to involve several aspects, such as the interaction between hydrogen atoms and metal atoms, changes in lattice structure, and crack formation and extension. Hydrogen atoms enter the metal through adsorption, diffusion, and absorption and accumulate at defects such as grain boundaries, dislocations, or internal cavities, leading to an increase in material embrittlement [14,15,16,17]. The most noteworthy of the many hydrogen embrittlement phenomena is that the increase in brittleness not only manifests itself in the fracture strength and toughness of the material, but may also lead to a sharp decrease in the fatigue life of the material. Hydrogen embrittlement fatigue is also a material degradation phenomenon involving the fracture and damage of metals or alloys due to fatigue loading in the presence of hydrogen. This phenomenon has a significant impact on the safety and endurance of engineering structures, especially in the oil and gas, aerospace, and nuclear industries [18,19]. In recent years, scientists have developed numerous prediction models in order to predict the fatigue life of materials in hydrogen environment, such as empirical models [20,21,22,23,24,25], physical models [26,27,28,29,30,31], machine learning models [32,33], and finite element models [34,35,36,37,38,39]. These models have solved the problem of fatigue life prediction for some materials to a certain extent, but the hydrogen embrittlement mechanism is still not uniformly explained, which leads to a certain degree of failure to reach a consensus among different models.
In order to further investigate the internal causes of hydrogen embrittlement, scientists have summarised the influencing factors of fatigue crack growth in hydrogen environment. They found that the influencing factors of fatigue crack growth under hydrogen are very complex, including the chemical composition of the material, microstructure, external environmental conditions, and other aspects [40,41]. For example, materials containing alloying elements that readily absorb hydrogen or have a high lattice defect density are more sensitive to hydrogen. In addition, external environmental conditions such as hydrogen pressure, temperature, and humidity also have a significant effect on hydrogen embrittlement properties. Under conditions of high pressure, high temperature and high humidity, hydrogen enters the metal at a higher rate, thus exacerbating hydrogen embrittlement [26,27,28,29]. This further promotes the growth of fatigue cracks in the material, leading to a decrease in the fatigue life of the material. In order to better utilise hydrogen energy and mitigate the hydrogen embrittlement fatigue behaviour, scientists have explored numerous material protection measures. For example, reasonable material selection and stress design, chemical treatment to improve material properties, regular maintenance and inspection by staff, reasonable control of environmental conditions, and so on.
The purpose of this paper is to explore in depth the mechanisms, prediction models, influencing factors, and preventive measures of material fatigue crack growth under hydrogen embrittlement conditions, with a view to providing theoretical support and practical guidance for solving this problem.
2. Hydrogen–Metal Interaction and Fatigue Mechanisms
2.1. Overview of Hydrogen Embrittlement Phenomena
Hydrogen embrittlement is a phenomenon in which metallic materials undergo the degradation of material properties in a hydrogen-containing environment. Although hydrogen embrittlement has been extensively studied, its exact mechanism is still a complex issue, and there is no unified theory that can explain all cases. A number of possible hydrogen embrittlement mechanisms have been proposed, but these may vary depending on the material, environmental conditions, and stress state. The existing hydrogen embrittlement mechanism is mainly modelled in eight aspects: diffusion of hydrogen atoms in the crystal lattice, hydrogen-induced crack growth, chemical reaction between hydrogen and metal, effect of hydrogen on dislocations motion, hydrogen-induced stress corrosion cracking, hydrogen interaction with the electronic structure of the material, hydrogen-promoted formation of crystalline defects, and diffusion and aggregation of hydrogen within the metal.
Four factors of material property degradation can be considered in the existing hydrogen embrittlement mechanism.
- (i)
- Diffusion of hydrogen atoms in the lattice: Diffusion of hydrogen atoms in the lattice, which can penetrate the metal lattice and lead to lattice deformation, grain boundary strengthening and interactions between hydrogen atoms and other atoms in the metal crystal. This may lead to a concentration of stresses within the crystal, which increases the brittleness of the material. The mobility of hydrogen in metal lattices is a large topic. This discussion is limited to the kinetics of hydrogen diffusion, relative to temperature, hydrogen concentration, isotopic mass, and concentration of a third element at and above room temperature [42]. Hydrogen-induced intergranular cracking is the most common type of hydrogen embrittlement. Hydrogen atoms will preferentially diffuse into the grain boundaries and then accumulate in the grain boundaries. Different grain boundaries can significantly affect hydrogen migration. As shown in Figure 1a, the ratio of H diffusion barriers in various grain boundaries to the energy barrier in bulk Fe differs markedly. For the Σ3 and Σ5 grain boundaries, the logarithmic form of this ratio is greater than zero, indicating that these boundaries hinder hydrogen migration. In contrast, for the Σ9 grain boundary, the logarithmic ratio in the y and z directions is less than zero, suggesting that this boundary facilitates hydrogen diffusion. Therefore, it is necessary to reveal the diffusion mechanism of hydrogen atoms at grain boundaries, which contributes to a deeper understanding of hydrogen embrittlement. Notably, He et al. [43] found that the difficulty of hydrogen diffusion along grain boundaries depends mainly on the connectivity of the low electron density regions along the grain boundaries.
- (ii)
- Hydrogen-induced crack propagation: The accumulation of hydrogen atoms within the metal may lead to the formation and extension of cracks. This crack growth can occur in a number of ways, such as the combination of hydrogen atoms with metal atoms to form brittle compounds or the release of hydrogen gas. From scanning electron microscopy observations, the researchers found that in these low-Mn + Si high-purity steels [44], the mode of hydrogen-induced cracking was some type of segregation of interfaces, possibly between martensitic laths, rather than rupture occurring in air. Similar behaviour was found in other high-purity steels. Traidia et al. [45] presented a comprehensive finite element model for numerical simulation of hydrogen-induced cracking (HIC) in steel pipes exposed to sulphur-containing compounds such as hydrogen sulphide (H2S), as illustrated in Figure 1b. The computational results show that in the extension stage, the crack growth behaves as a trap that attracts more hydrogen, and the hydrostatic stress field at the crack tip accelerates the emergence and extension of HIC-related cracks. In addition, HIC decreases with increasing pH and decreasing H2S partial pressure.
- (iii)
- Chemical reactions of hydrogen with metals: Hydrogen forms compounds with metal surfaces that may lead to increased brittleness of the material. These compounds may be brittle and may lead to the formation and extension of surface cracks. The interaction of hydrogen with metals is the cause or basis of many phenomena, ranging from the chemisorption of hydrogen on surfaces, the dissolution of hydrogen in metals, the catalysed reaction of hydrogen as a reactant or stoichiometric constituent, etc., to the formation of metal hydrides. Hydrogen-induced corrosion and hydrogen embrittlement of steel are well known in the chemical processing industry and metallurgy [46,47]. The researchers [48] describe optical observations and x-ray diffraction measurements of the reaction of iron and hydrogen at high pressure to produce iron hydride. These results greatly extend the pressure range characterised by the technically important Fe-hydrogen phase diagram and have implications for issues ranging from hydrogen degradation and ferrous metal embrittlement to the presence of hydrogen in Earth’s metal cores.
- (iv)
- Influence of hydrogen on dislocation motion: Hydrogen atoms may influence dislocation motion in metals and thus the plastic behaviour of materials. This effect may cause the material to be more susceptible to brittle fracture upon loading. In 310S stainless steel, Ferreira et al. [49] observed that the presence of hydrogen reduces the elastic interactions between the barrier and both full and partial dislocations, thus improving the mobility of the dislocations. In high-purity aluminium, it was observed that the introduction and removal of hydrogen from the system leads to a reversal of the direction of motion of dislocations stacked on the barrier, consistent with the reduction in elastic interactions by solute hydrogen. These observations provide direct support for a hydrogen shielding mechanism [49]. Gu et al. [50] present a new framework to quantify the effect of hydrogen on dislocations using large-scale three-dimensional (3D) discrete dislocation dynamics (DDD) simulations. In this model, the first-order elastic interaction energy associated with hydrogen-induced volume changes is considered [50,51,52].
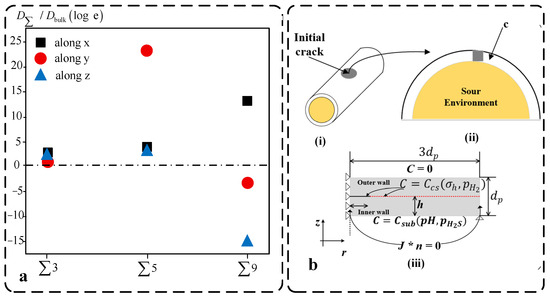
Figure 1.
Experimental database and numerical model. (a) The ratio of the H diffusion barrier at different grain boundaries to the energy barrier in bulk Fe [43]. (b) Hydrogen pipeline modelling [45]; (i–iii) Schematic diagram of the model geometry.
Furthermore, other factors for material property degradation can also be considered in the existing hydrogen embrittlement mechanism.
- (i)
- Hydrogen-induced stress corrosion cracking: This similar failure morphology is shown in Figure 2a. In the presence of stress, hydrogen promotes corrosion of the metal and may lead to crack formation and expansion in areas of stress concentration, which can initiate brittle fracture. This mechanism is particularly significant in specific environments, such as in salt water or in environments containing chloride ions [53,54].
- (ii)
- Hydrogen interacts with the electronic structure of materials: Hydrogen atoms may interact with the electrons in metals, changing the electronic structure and properties of the material, thus affecting its mechanical properties. The molecular structure of this interaction is shown in Figure 2b. This change in electronic structure may lead to an increase in the brittleness of the material [55,56,57].
- (iii)
- Hydrogen-promoted crystal defect formation: Hydrogen atoms may promote the formation of defects in metal crystals, such as vacancies, interstices, dislocations, etc., which may lead to increased brittleness of the material [14,15,16]. Figure 2c shows crystal-plasticity contours for Pd–H alloys: as the hydrogen concentration rises from 0 to 7500 appm, the local fraction of screw dislocations at a logarithmic strain of 0.35 increases markedly, confirming that hydrogen enhances dislocation multiplication and heterogeneity.
- (iv)
- Diffusion and aggregation of hydrogen within metals: Diffusion and aggregation of hydrogen atoms in metals may lead to localised stress concentrations that increase the brittleness of the material. This process of diffusion and aggregation may be influenced by factors such as material structure and temperature [58,59]. As shown in Figure 2d, the mobile hydrogen may be located at different interstitial positions. Depending on the potential energy, the atoms can migrate to various positions.
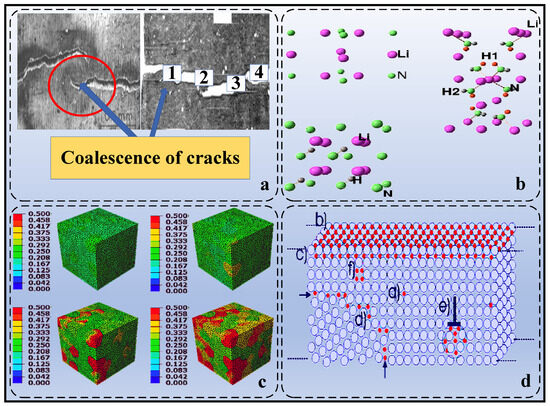
Figure 2.
Multiscale schematic of hydrogen–metal interactions. (a) Stress corrosion cracking morphology of API-X52 steel in low-pH NS4 solution [60]. (b) Full-potential energy calculation cell of the NH–LiH system [57]. (c) 3D simulation of Pd–H alloy showing the distribution of screw dislocations at different hydrogen concentration [15]. (d) Schematic of typical hydrogen traps such as intragranular vacancies, dislocations, and interfaces [54].
Research on hydrogen embrittlement has gradually evolved from macroscopic tensile testing to advanced in situ techniques such as transmission electron microscopy and atomic force microscopy, as illustrated in Figure 3. Currently, many theoretical models have been proposed to describe hydrogen embrittlement phenomena and mechanisms [61,62,63,64,65,66], such as Hydrogen Enhanced Dislocation Emission (HEDE), Hydrogen Enhanced Local Plasticity (HELP), and Adsorption-Induced Dislocation Emission (AIDE) and so on. Several reviews have provided detailed introductions to these topics [67,68,69,70,71,72,73]. A clear summary of these mechanisms is shown in Figure 3. The HEDE model focuses on the interaction of hydrogen atoms with dislocations. In this model, hydrogen atoms adsorb around the dislocations and change the structure and energy of the dislocations, resulting in an increase in the energy required to move the dislocations, which results in a loss of plasticity in the metal. This model is based on the interaction of hydrogen atoms with dislocations and assumes that the hydrogen atoms increase the energy barrier for the movement of the dislocations, thus making the metal more susceptible to fracture. The HELP model focuses on the description of plastic deformations induced by hydrogen atoms in a localised region. In this model, hydrogen atoms adsorb near-local dislocations or grain boundaries and reduce the local yield strength, making the material more susceptible to localised plastic deformation. The HELP model suggests that hydrogen atoms induce plastic deformation in a localised region, which reduces the toughness of the material. The AIDE model focuses on describing the process of dislocation emission caused by the adsorption of hydrogen atoms on the metal surface. In this model, hydrogen atoms adsorbed near the source of dislocation emission weaken the energy barrier to dislocation emission and promote dislocation emission and motion. This model suggests that the adsorption of hydrogen atoms reduces the energy barrier to dislocation emission, which increases the probability of dislocation emission and leads to brittle behaviour of the material. Each of these models has its own focus, but all of them attempt to explain the microscopic changes induced by hydrogen atoms in metals that lead to the loss of toughness and plasticity of the material. Under low to moderate stress, HELP dominates; under high stress, HEDE becomes the primary mechanism. In the transitional region, a mixed-mode behaviour is typically observed. Their study contributes to a deeper understanding of the hydrogen embrittlement phenomenon and provides a theoretical basis for the prevention and control of hydrogen embrittlement.
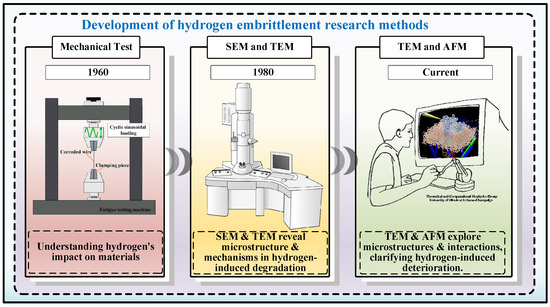
Figure 3.
The development of hydrogen embrittlement research methods [67].
One of the common problems in the current explanation of the hydrogen embrittlement phenomenon is the lack of a comprehensive theoretical framework and a consistent mechanism model. Despite the extensive research work performed by scientists, there are still many unanswered questions about the mechanism of hydrogen embrittlement. This has led to some common problems in explaining the hydrogen embrittlement phenomenon.
2.2. Summarising Experimental Approaches
Experimental methods for hydrogen embrittlement play an important role in providing insights into hydrogen embrittlement phenomena, guiding material design and optimisation, validating theoretical models, and providing guidance for applications [8]. Through these methods, the degree of material embrittlement in hydrogen-containing environments can be quantitatively assessed, the formation mechanism of hydrogen embrittlement can be revealed, material design and optimisation can be informed, the accuracy of theoretical models can be verified, and engineering design and material selection can be guided. Therefore, the experimental method of hydrogen embrittlement is of great significance in solving the hydrogen embrittlement problem. Now the current hydrogen embrittlement experiments are divided into five categories.
Figure 4 clearly shows four classical hydrogen embrittlement experiments, which are briefly described.
The hydrogen embrittlement loading test, as shown in Figure 4a [74,75,76], is designed to assess the embrittlement behaviour of metals in a hydrogen environment. After a metal sample is exposed to a hydrogen-containing environment, the fracture behaviour and changes in mechanical properties of the metal are observed by applying loads such as tension, bending or compression to determine the effect of hydrogen on the properties of the metal. It is generally used to assess the risk of hydrogen embrittlement that a metal may face under actual working conditions and to guide material selection and engineering design. The general steps are to prepare a metal sample, expose it to a hydrogen-containing environment, apply loading and record mechanical property data, and finally analyse the fracture surface.
Hydrogen diffusion testing, as shown in Figure 4b [77,78,79,80], is designed to study the diffusion behaviour of hydrogen in metals, including diffusion rates, diffusion paths and hydrogen aggregation behaviour. It is generally used to understand the mechanism of hydrogen diffusion in metals, to predict the risk of hydrogen embrittlement, and to develop preventive strategies. The general procedure is to introduce hydrogen into a metal sample using techniques such as electrochemical hydrogen charging or hydrogen permeability testing, and to assess the diffusion behaviour of hydrogen by monitoring the hydrogen concentration distribution or permeation rate.
Hydrogen embrittlement mechanism research [79,81,82] is conducted through theoretical simulation and computational chemistry methods, to study the interaction between hydrogen and metal and hydrogen embrittlement mechanism, in order to deeply understand the nature of hydrogen embrittlement phenomenon. Figure 4c schematically summarises this transition: at low hydrogen concentrations and moderate stresses, the dominant mechanism is hydrogen-enhanced local plasticity (HELP/HESIV), whereas at higher concentrations or elevated stresses, it shifts to hydrogen-enhanced decohesion (HEDE/HESIV) [61,83]. Generally, this technique is used to provide theoretical guidance for engineering practice, to guide the material design and processing technology. The general steps are model building, calculations and analyses, verification of the accuracy of the model, and interpretation and extrapolation of the results.
Stress Corrosion Cracking (SCC) Test [18,84]: simulates the occurrence of hydrogen embrittlement of metals in stressful and corrosive environments and evaluates the stress corrosion susceptibility of metals to hydrogen. It is generally used to assess the durability and safety of metals in environments with corrosive and loaded effects. The key test variables, which includes electrochemical potential, load ratio, solution pH, and dissolved-hydrogen activity, are listed in Figure 4d [85], providing a practical envelope for designing SCC experiments consistent with service conditions. The general procedure is to expose a metal sample to a hydrogen-containing corrosive medium and apply a certain amount of stress to monitor the crack growth and the fracture behaviour of the metal. The microstructure and crystal defects of metal samples are analysed by using various characterisation techniques [18,86], such as SEM, TEM, XRD, etc., to understand the effect of hydrogen on the microscopic properties of metals. They are generally used to reveal the microscopic mechanism of hydrogen–metal interaction and provide theoretical support for the hydrogen embrittlement phenomenon. The general steps are as follows: microstructural images or crystallographic data of the metal samples are collected, analysed and interpreted.
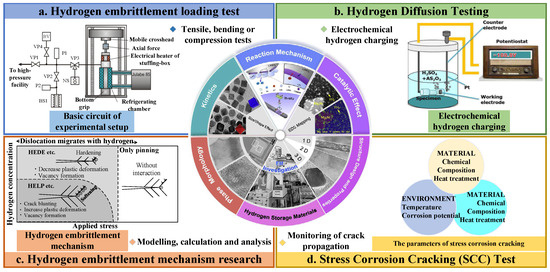
Figure 4.
Four typical test experiments for hydrogen embrittlement: (a) general view of the UTS100K test machine and the high pressure test chamber [75]; (b) schematic diagram of electrochemical treatment system for hydrogen charging of 304 stainless steel [77]; (c) HELP + HEDE model: schematic of the transition of the HE mechanism, HELP (HESIV) → HEDE (HESIV), for different hydrogen concentrations and applied stress conditions [61,83]; (d) the parameters of stress corrosion cracking [85].
Current hydrogen embrittlement experiments suffer from a variety of shortcomings. Firstly, the complexity and high cost of some methods limit the feasibility and popularity of the experiments. Secondly, the lack of a unified standardised method leads to the differentiation of experimental conditions and test methods, making it difficult to compare and verify the reliability of the results. Furthermore, some of the experimental methods cannot adequately simulate the hydrogen embrittlement properties under actual working conditions and are affected by scaling effects, while the lack of dynamic monitoring capability limits the accuracy of the experimental results. Finally, some of the methods can be carried out in the laboratory, but it is difficult to be directly applied to actual engineering, which lacks practicality.
3. Fatigue Crack Growth in Hydrogen Environment
3.1. Mechanisms of Fatigue Crack Initiation and Growth
In the previous section, we have sorted out the classical mechanism of hydrogen embrittlement as well as the experimental methods. Next, this paper will focus on the existing study of fatigue crack growth in hydrogen environment. The relationship between fatigue crack growth and hydrogen embrittlement in hydrogen environment is intricate and significant. As mentioned earlier, in a hydrogen environment, hydrogen atoms penetrate into the metal, leading to hydrogen embrittlement, which makes the material more susceptible to crack growth. This phenomenon is closely related to SCC, in which crack growth is accelerated in a hydrogen environment due to the stress corrosion sensitivity of the material. When hydrogen embrittlement occurs, hydrogen atoms penetrate into the metal lattice, leading to lattice expansion and stress concentration, which reduces the material’s ductility and increases its brittleness. This makes the material more susceptible to crack propagation and fracture, especially in hydrogen environments, where stress concentrations at the crack tip can exacerbate embrittlement. Hydrogen embrittlement encompasses a range of performance degradation phenomena of materials in hydrogen environments, so that fatigue crack propagation in hydrogen environments and hydrogen embrittlement phenomena is in an encompassed relationship.
Figure 5 presents an overview of the articles published about fatigue crack growth in hydrogen environment that can be retrieved on Web of Science Core Collection (SCI-EXPANDED + SSCI) between 2014 and 2024. The CiteSpace software is used to achieve the presentation of the top ten keywords of researchers’ papers in the field of fatigue crack growth in hydrogen environment in the last decade. CiteSpace (version 6.3.R1) was used for bibliometric visualisation. The parameters set are time slicing = 1 year, node type = keywords, and Pathfinder network pruning. Wos was selected in this study, but different databases and other clustering algorithms may have some impact on the output results. It is worth noting that researchers have been enthusiastic about the field of hydrogen embrittlement in the last decade. In particular, the fatigue behaviour in hydrogen environments tops the research list. This equally proves that fatigue cracking in hydrogen environment seriously affects the progress of engineering in practical engineering applications. Included in Figure 5a, it can also be seen that researchers have also explored a lot about the prediction of fatigue life of materials in hydrogen environment.
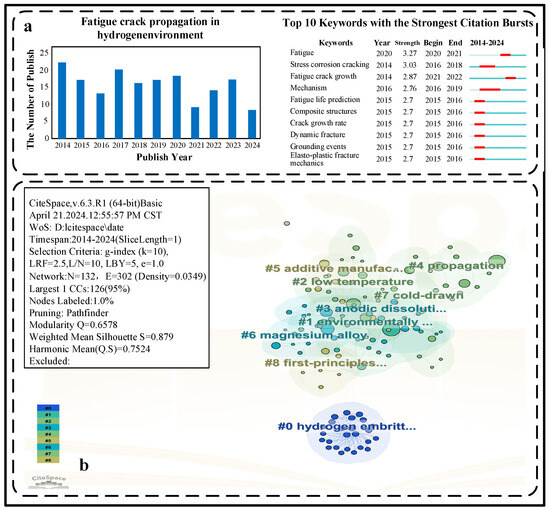
Figure 5.
Visualisation of the literature on fatigue crack growth in hydrogen environments in recent years: (a) trends in the number of publications and keywords on fatigue crack growth in hydrogen environments in Wos (2014–2024) produced via CiteSpace software (version 6.3. R1), by year; (b) keyword time line mapping of fatigue crack growth in hydrogen environments in Wos (2014–2024) produced by CiteSpace software (version 6.3. R1).
In order to further explore the trend of research directions in recent years, keyword clustering on these papers was performed. This helps researchers to catch the hotspots of research. When evaluating the effectiveness of clustering [87], Q and S are two commonly used metrics that provide an assessment of different aspects of the quality of the clustering results. The Q value is usually used to assess the tightness within the clusters or the quality of the clusters, which measures the degree of similarity between the samples within the same cluster, while the S value is used to assess the dissimilarity of the clusters from the overall data, i.e., how much the samples within the clusters are separated from the overall data. Ideally, one would like to have a high Q value and a high S value, which indicates that the samples within the clusters in the clustering result are sufficiently similar and there is less dissimilarity between the clusters and the data as a whole, which helps to explain the structure of the data and extract useful information. The clustering result of the localisation is Q = 0.6578 and S = 0.879. This shows that the clustering structure is relatively clear, and the internal consistency of the clusters is high, proving the reliability of the keyword grouping.
In Figure 5b, the literature keyword timeline spectrogram by using CiteSpace software is presented. This can help researchers to quickly understand the development of this field. It can be seen from the figure that in the last 10 years, researchers have carried out research and prediction of the fatigue life of materials around the hydrogen embrittlement mechanism.
Studies have shown that hydrogen penetration in metals is a key factor in crack growth. Hydrogen atoms can penetrate into the crack tip and cause hydrogenation reactions, which leads to an accelerated rate of crack growth. In addition, stress corrosion cracking (SCC) in a hydrogen environment is also closely related to crack growth. The crack growth rate is affected by various factors such as stress level, material properties and environmental conditions. The mechanism of fatigue crack generation and propagation in metallic materials in a hydrogen environment involves complex physico-chemical processes, mainly related to the adsorption and diffusion of hydrogen atoms and their interaction with the metal crystal structure. The following section will discuss the existing mechanisms of fatigue crack growth in hydrogen environment.
As mentioned earlier, hydrogen atoms can be absorbed on the metal surface and diffuse in the metal crystal structure [11,12,88]. This process can be affected by stress and temperature. In the stress field of a metal, hydrogen atoms are more likely to be absorbed and diffuse into crack tips or high stress regions. At crack tips or high stress regions [13,45], hydrogen atoms may accumulate and cause changes in the local stress field. These stress concentration regions can exacerbate the stress state at the crack tip [19,89], thus promoting further crack growth. Andrew J. Slifka et al. [88] conducted fatigue crack growth tests on two pipeline steel alloys, API 5L X52 and X100. Baseline tests were conducted in air, and these results were compared to tests conducted in pressurised hydrogen. They found that the fatigue crack growth rates of X100 and X52 were significantly higher in the pressurised hydrogen environment than in air. It can be seen from the a-plot of Figure 6 that the rate of crack propagation in the material is also significantly enhanced with the increase of hydrogen concentration. Kelestemur et al. [11] investigated the fatigue crack growth behaviour and its characteristics of AISI-304 stainless steel after tensile overload in three different atmospheres, i.e., dry argon, humid air, and hydrogen. After their experimental study, it was found that hydrogen promotes negative effect on the fatigue life of the material, but the humidity of the air shows little effect on the fatigue life.
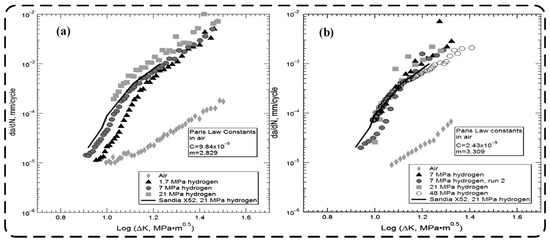
Figure 6.
Fatigue crack growth rate data: (a) fatigue crack growth rate data for X100 steel; (b) fatigue crack growth rate data for X52 [88].
The researchers also analysed the extension mechanism of the material’s microscopic grain boundaries. The diffusion of hydrogen atoms at metal grain boundaries may lead to grain boundary embrittlement and crack formation. Grain boundaries are the conduits for crack propagation, and the presence of hydrogen atoms makes the grain boundaries more susceptible to cracking and propagation. This promotes the propagation of fatigue cracks in metals. The interaction of hydrogen atoms with the lattice structure of the metal may lead to deformation of the crystals or the creation of microscopic defects, thus reducing the ductility and toughness of the metal and making it more susceptible to crack propagation and fracture. For example, Nishikawa et al. [19] conducted two experiments in order to investigate the mechanism of fatigue crack growth along the crystal of mild steel in a low-pressure hydrogen environment. One investigated the effect of cyclic pre-strain on crack growth behaviour. The other was an in situ observation of intergranular fatigue crack growth in a hydrogen environment. They concluded that the credible mechanism for intergranular fatigue crack propagation is due to the increased number of slip repetitions enhanced by hydrogen, which damages the grain boundaries ahead of the crack tip. Therefore, in a hydrogen environment, fatigue cracks are more likely to propagate along the grain boundaries.
In addition to the effects of hydrogen atom diffusion, in many cases, chemical reactions may occur between certain metals and hydrogen atoms [90,91,92], resulting in the formation of hydrides or hydride phases, which can further affect the physical and mechanical properties of the metal. This chemical reaction accelerates the process of crack growth and metal damage. This further affects the fatigue life of the material. Guo et al. [12] investigated the corrosion fatigue crack growth mechanism of high strength steel bar HRB400 in different corrosive environments. Fatigue crack growth tests were also conducted under different fatigue load types, environments and stress ratios. The results show that hydrogen embrittlement plays a dominant role in the corrosion fatigue process of HRB400 bars. The contribution of anodic dissolution to the FCG rate increases with the level of aggressiveness. The contribution of hydrogen embrittlement to the FCG rate increased with increasing stress ratio and stress intensity factor range.
Understanding the mechanisms of these fatigue cracks generation and propagation is crucial to predict and protect against hydrogen embrittlement. Therefore, through experiments and theoretical simulations, scientists have endeavoured to reveal the interactions between hydrogen and metals in order to develop effective protective measures and material design strategies to ensure the safety and reliability of metallic structures in hydrogen environments [18]. There are some important issues and challenges regarding the existing mechanisms of hydrogen embrittlement fatigue crack generation and extension. The current understanding of the mechanism of hydrogen embrittlement assisted fatigue crack generation and extension is not deep and comprehensive enough, especially the mechanism of crack behaviour under complex stress states has not been fully clarified. Secondly, most of the existing studies focus on the macroscopic level, and there is a lack of in-depth research on the effects of hydrogen atom diffusion and aggregation in materials on cracking behaviour at the microscopic scale. In addition, the mechanism of hydrogen embrittlement fatigue crack generation and propagation is affected by a variety of factors, including the nature of the material itself, the stress state at the crack tip, the hydrogen environment, etc., and the complex interactions between these factors have not been fully understood.
3.2. Factors Affecting Fatigue Crack Growth
The effect of hydrogen environment on fatigue crack growth is influenced by a combination of factors. Over the course of many years of research, researchers have identified many factors that affect fatigue crack growth in hydrogen environments. Some of the major factors that have been explored by researchers are listed below [41,93,94,95,96,97,98,99,100]: Firstly, hydrogen concentration is one of the most important factors affecting fatigue crack growth. According to the research by Dadfarnia et al. [101], it is concluded that the higher the hydrogen concentration, the faster the crack propagation. This is because it increases the opportunities for hydrogen–metal interactions. Stress level is another important factor. Higher stress levels promote the diffusion and aggregation of hydrogen and, as mentioned earlier, hydrogen atoms migrate and aggregate at high stresses, leading to faster crack growth. In actual working conditions, the temperature will vary depending on where the material is installed and the environment in which it is used. Temperature also affects the rate of hydrogen diffusion and the plasticity of the metal. Usually, the higher the temperature, the greater the entropy contained in the hydrogen atom, the faster the activity, and the faster the crack expansion rate, so the high temperature will promote the diffusion of hydrogen atoms and the softening of the metal. In addition to this, we still need to consider that different types of metallic materials have different sensitivities to hydrogen. Certain metallic materials may be more sensitive to hydrogen, resulting in faster crack propagation. There is evidence that different materials are not equally sensitive to hydrogen. In actual working conditions, if the metal is exposed to an environment containing corrosive substances, the corrosion effect will work together with the stress to accelerate the crack growth process. This is similar to the SCC effect mentioned earlier, where hydrogen atoms will act as a catalyst-like agent, further affecting the fatigue life of the material. At the same time, according to the research by Dadfarnia et al. [101], changes in loading frequency can also affect the diffusion and concentration of hydrogen in the metal, thereby influencing the crack propagation rate. A lower loading frequency allows more time for hydrogen to diffuse and accumulate at the crack tip, thereby accelerating crack growth, whereas at higher frequencies the limited diffusion time mitigates hydrogen-assisted damage and the crack-growth behaviour approaches that observed in inert environments. Finally, the microstructure of the metal also plays an important role, e.g., grain size and grain boundary properties, which also affect the behaviour of hydrogen diffusion and crack growth. As mentioned in the previous section on hydrogen embrittlement mechanisms, hydrogen atoms can travel through and accumulate at weak grain boundaries to form internal defects in the metal, which in turn affects the fatigue performance of the material.
A review of several studies [88,101,102,103,104,105,106,107] reveals that the factors affecting fatigue crack growth in hydrogen environments are complex and must be considered comprehensively to fully understand the mechanisms of crack propagation and to develop appropriate preventive measures. However, there is still a lack of fundamental understanding of the factors influencing hydrogen embrittlement. A more in-depth exploration of these factors is needed.
4. Fatigue Life Modelling and Prediction in Hydrogen Environment
Fatigue life prediction in hydrogen environments is very important, especially for metallic materials in engineering applications. Materials in hydrogen environments are susceptible to hydrogen embrittlement, which results in reduced mechanical properties, reduced ductility, and significantly shorter fatigue life. Therefore, fatigue life prediction can help engineers and scientists to assess the service life and safety of materials in hydrogen environments.
Existing fatigue life prediction models for hydrogen environments are broadly classified into four categories:
The first one is empirical models [20,21,22,23,24,25], which are usually based on experimental data and statistical analyses, and describe the fatigue life of materials under different conditions by fitting curves or equations. Common empirical models include Bhaskar’s equation, Bharat’s equation, etc. [23,24,25]. These models have the advantage of simplicity and ease of use, but may suffer from low accuracy in predicting complex situations.
The second category is physical models [26,27,28,29,30,31], which are based on the understanding of the microstructure and crack behaviour of the material and use mathematical equations to describe the crack growth process and consider the effect of hydrogen in the material. These models usually require an in-depth understanding of materials science and mechanics and can provide more accurate predictions, but can be more complex to build and solve.
The third category is numerical simulations [28,32,33]. This category encompasses various numerical methods, including the finite element method (FEM) as a specific case. Numerical simulations can model the stress field and cracking behaviour of materials by discretizing the structure into finite elements and solving mechanical equations for each element. In addition to FEM, phase-field modelling can be applied to simulate the evolution of microstructural features like cracks and phase boundaries in a diffuse manner, enabling detailed insights into fracture and phase transformation processes. Other numerical methods, such as molecular dynamics, can also be explored to provide insights into material behaviour at the atomic level. These simulations can handle complex geometries and loading conditions, offering detailed information on stress distribution and phase evolution but requiring significant computational resources and specialised software.
The fourth category is surrogate modelling such as machine learning methods [34,35,36,37,38,39]. Machine learning methods use large amounts of experimental data and features to train models to predict the fatigue life of materials. Common machine learning algorithms include regression analysis, support vector machines and neural networks. These methods can handle complex nonlinear relationships and high-dimensional features, but require sufficient data volume and proper feature selection.
Research works for fatigue life modelling in hydrogen environments have been summarised in Table 1 and Table 2. The current fatigue life prediction models for hydrogen environments still suffer from several major shortcomings: lack of comprehensive consideration of hydrogen-induced damage mechanisms, insufficient accuracy of model parameters, limited applicability, insufficient validation, and difficulty in considering the interaction of multiple factors. To solve these problems, it is essential to gain an in-depth understanding of how hydrogen affects the microstructure and mechanical properties of materials. Additionally, improving model parameter accuracy, enhancing model versatility, validating reliability, and accounting for complex interactions among multiple factors are necessary steps. These efforts aim to provide more reliable support and guidance for applying hydrogen technology in the engineering field.

Table 1.
Table of literature summary.

Table 2.
Comparative summary of fatigue life modelling and prediction for hydrogen environments.
5. Prevention of Fatigue Characteristic Degradation in Hydrogen Environment
Preventing the degradation of fatigue properties in hydrogen environments is a critical engineering issue, as such degradation can lead to the unintentional failure of metallic structures, posing a serious threat to the safety of people and property. In order to deal with this problem effectively, a comprehensive approach is required, involving material selection, design optimisation, environmental management, and monitoring and maintenance. Some common approaches are discussed [43,47,88].
Preventive measures for fatigue crack growth in hydrogen environments can be considered from several perspectives. First, material selection and optimisation [108,109,110,111,112] are essential, as selecting materials with high resistance to hydrogen embrittlement is key to preventing fatigue performance degradation in hydrogen-rich environments. For example, the literature [113] suggests that high-strength pipeline steels are generally more susceptible to hydrogen embrittlement than mild steels, underscoring the importance of careful material selection. Proper alloy design [114] can enhance hydrogen embrittlement resistance by adjusting alloy composition and microstructure, which helps to slow the rate of fatigue crack propagation. For instance, high-strength, corrosion-resistant alloys or composites may be chosen and optimised for enhanced hydrogen resistance.
Surface treatments also play a vital role. Effective surface treatments can slow hydrogen diffusion and aggregation within the material, improving resistance to hydrogen embrittlement and extending fatigue life. Techniques such as surface coatings, electrochemical treatments, or specialised coatings reduce hydrogen diffusion and adsorption, thereby minimising hydrogen-induced fatigue degradation.
In addition, efforts have been made to control hydrogen diffusion and adsorption [115,116,117]. Controlling these processes can reduce hydrogen accumulation and damage within the material, achieved by altering the microstructure, optimising hydrogen loading conditions, or adjusting the hydrogen concentration in the environment. If hydrogen atoms are already present within the material, hydrogen removal techniques, such as heat treatment, electrochemical methods, or gas-phase treatment, can be applied during the manufacturing process. These techniques reduce hydrogen content in metals, mitigating hydrogen’s impact on mechanical properties and slowing fatigue crack growth.
Stress control and design improvements [118,119,120] also prove effective. Sound structural design can reduce stress levels in hydrogen environments, slowing fatigue property degradation. Methods may include reducing stress concentration areas [121,122], optimising structural shape [123,124], or altering loading methods to minimise embrittlement risks [125,126].
Finally, regular monitoring and maintenance are crucial for timely detection and management of hydrogen-induced damage. Non-destructive testing, surface inspection, and real-time monitoring techniques can help identify damage early, enabling necessary maintenance. Additionally, environmental control during usage—adjusting parameters such as temperature, pressure, and humidity—can further mitigate hydrogen’s effects on material properties, extending the material’s lifespan. Protective measures, working environment adjustments, or process optimizations may all contribute to this goal.
A combination of these approaches and the selection of appropriate preventive measures for each case can effectively reduce the risk of fatigue property degradation in hydrogen environments and improve the reliability and service life of materials. In summary, preventing the degradation of fatigue properties in hydrogen environment requires comprehensive consideration of multiple factors such as materials, design, environment and monitoring. Only through reasonable material selection, design optimisation, environmental management, and monitoring and maintenance can the rate of fatigue crack expansion be effectively slowed down to ensure the safe and reliable operation of engineering structures.
6. Challenges and Future Directions
6.1. Limitations and Challenges
The study of the multiple challenges and difficulties of fatigue crack growth in metals in hydrogen embrittlement environments is of great significance in improving the safety, reliability, and durability of engineering structures. This is not only related to the safety of metallic materials in engineering applications, but also has a direct role in guiding the design of more durable materials and the development of suitable engineering applications, as well as promoting scientific research and technological innovation in related fields.
Some challenges are summarised in the section.
Firstly, the mechanism of influence in this field has not been fully revealed, and there are knowledge gaps and deficiencies. There is still a lack of comprehensive understanding of the specific mechanisms of fatigue crack growth of metals in hydrogen embrittlement environments, which hinders our in-depth understanding and effective control of the phenomenon. As mentioned earlier, although there are now several recognised mechanisms for hydrogen embrittlement, such as the HEDE, AIDE, HELP, etc., these mechanisms are not contradictory to each other, but only the direction and angle of the consideration is different. People still need to combine them to form a mechanism more in line with human cognition.
Secondly, the complexity of modelling and controlling experimental conditions is also a major challenge. This involves a combination of multiple factors, such as the effects of pressure, temperature, hydrogen concentration, and other environmental factors. The interaction of these factors increases the difficulty of the study and requires careful design and strict control of the experimental conditions in order to obtain reliable and accurate data. Section 3 provides a systematic description of the factors affecting fatigue crack growth in hydrogen environments. It can be seen that different factors have different effects on the final fatigue crack growth rate, and considering these factors more completely is crucial for future research. In addition, long-term performance and stability studies require significant time and resources. In order to validate and assess the fatigue crack growth behaviour of metals in hydrogen embrittlement environments, continuous monitoring and validation is required to ensure the reliability and reproducibility of experimental results. One can only deal with problems quickly once complete surveillance has been carried out. The complexity of data acquisition and analysis is also an issue that should not be underestimated. An in-depth understanding of the mechanisms of fatigue crack growth in metallic materials in hydrogen embrittlement environments requires a large amount of experimental data and advanced data analysis techniques, which take a lot of time and effort to acquire and process.
Finally, effectively predicting and managing the fatigue performance of metallic materials in hydrogen embrittlement environments is equally challenging. This requires close integration of theoretical research with engineering practice to develop reliable and practical prediction models and management strategies to cope with complex and variable environmental conditions and material properties.
In summary, the study of fatigue crack growth of metals in hydrogen embrittlement environments faces many complex challenges and requires interdisciplinary co-operation and the integrated use of advanced technological tools and methodologies in order to deepen the understanding of the laws and mechanisms and to provide better support and guidance for scientific research and engineering applications in related fields.
6.2. Future Directions
Based on the above discussion, future fatigue research in hydrogen environments can be focus on the following aspects:
- (1)
- Real-time monitoring and model-driven closed-loop integration: AI is being actively applied in industrial scenarios. To leverage its rapid prediction capabilities, real-time measurement data should be integrated with physics-informed machine learning models for real-time crack growth prediction. An intelligent monitoring and early-warning system should be established to identify and mitigate crack propagation risks through continuous data acquisition and analysis. This is essential for maximising component performance and ensuring structural safety.
- (2)
- Multiscale digital twin modelling: microscale properties have a significant impact on fatigue behaviour. Advanced techniques such as density functional theory, crystal plasticity finite element method, and phase-field modelling should be incorporated to capture hydrogen transport and cyclic slip localization effects. This will enable the development of efficient multiscale fatigue simulation frameworks under hydrogen environments, facilitating accurate prediction of crack growth behaviour and the influence of various microstructural mechanisms.
- (3)
- Standardised database for high-temperature and high-pressure hydrogen environments: The establishment of standardised databases is critical for the development and deployment of hydrogen-related equipment. A unified dataset on hydrogen-assisted fatigue under elevated temperature and pressure conditions should be developed, along with practical guidelines and empirical correlations. Such databases and standards will support material selection, component design, and lifecycle management.
- (4)
- Uncertainty quantification and structural integrity assessment: Throughout the full lifecycle of hydrogen energy infrastructure, uncertainties exist in material properties, geometric dimensions, and external loading conditions. Understanding how these uncertainties impact structural integrity is crucial. Moreover, in pipelines, storage tanks, and wind–hydrogen integrated systems, risk-based classification and maintenance optimisation can provide decision support for safe and efficient hydrogen infrastructure deployment.
7. Conclusions
Green hydrogen energy has emerged as one of the key solutions to the severe environmental challenges posed by conventional fossil fuels. With the rapid development of large-scale hydrogen infrastructure, fatigue behaviour under hydrogen environments has become a critical bottleneck affecting the safe and reliable operation of hydrogen-related equipment. In this review, recent progress on the fatigue behaviour of metallic materials in hydrogen environments is thoroughly discussed. The following conclusions can be drawn:
- (1)
- Classic micro-mechanisms such as HELP, HEDE, and AIDE have been developed to describe hydrogen embrittlement in metals. Multiscale interactions between hydrogen and microstructural features, such as dislocations and grain boundaries, are key contributors to the deterioration of strength–toughness synergy. While the transitions between different mechanisms have been explored, a unified mechanistic framework that can quantitatively distinguish dominant and secondary mechanisms across different regimes is still lacking.
- (2)
- Advancements in experimental techniques, including high-pressure hydrogen loading-fracture systems, permeation testing, and in situ TEM/AFM, have enabled real-time observation of hydrogen accumulation at crack tips, grain boundary decohesion, and hydride formation. Most studies confirm that hydrogen significantly accelerates fatigue crack growth in steels, leading to a pronounced reduction in fatigue life.
- (3)
- Various modelling approaches have been developed to predict fatigue life in hydrogen environments, including empirical formulas, physics-based models, finite element simulations, and machine learning techniques. Each method has its own advantages and limitations: empirical models enable quick estimation but lack mechanistic insight; physics-based and numerical models offer better physical fidelity but rely heavily on microstructural parameters and computational resources; machine learning shows great potential in high-throughput prediction but is constrained by data quality and interpretability.
- (4)
- Multiple mitigation strategies have been proposed to extend the service life of components in hydrogen environments, including the development of low hydrogen-sensitivity alloys, surface coatings/diffusion layers, electrochemical protection, hydrogen de-trapping via heat treatment, stress-relief structural design, and real-time monitoring. Nevertheless, the development of novel hydrogen-resistant materials and protective systems remains a major challenge.
Looking forward, further research is needed in the following key directions: (1) Establishing a unified multiscale mechanistic framework linking hydrogen embrittlement and fatigue. (2) Building standardised databases that cover high-temperature, high-pressure, and cyclic loading conditions. (3) Developing AI-driven digital twin frameworks for real-time monitoring and closed-loop prediction. (4) Creating risk-based life assessment and maintenance optimisation strategies. Advancing these areas will be essential to ensuring the long-term safe operation of hydrogen infrastructure and equipment.
Author Contributions
Conceptualization, S.Y., D.M., A.M.P.D.J. and P.N.; Methodology, S.Y., P.N. and A.M.P.D.J.; Validation, S.Y., D.M. and Y.S.; Formal analysis, S.Y., P.N. and Y.S.; Resources, S.Y., D.M., P.N., A.M.P.D.J. and Y.S.; Writing—original draft, S.Y. and P.N.; Writing—review and editing, S.Y., D.M., A.M.P.D.J. and P.N.; Funding acquisition, S.Y., D.M. and A.M.P.D.J. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the fundings from Project ATE: Agenda para a Transição Energética (02/C05-i01.02/2022.PC644914747-00000023), cofinanced by Plano de Recuperação e Resiliência (PRR), República Portuguesa, through NextGeneration EU. The authors acknowledge Fundação para a Ciência e a Tecnologia (FCT) for its financial support via the projects LAETA Base Funding (DOI: 10.54499/UIDB/50022/2020) and LAETA Programatic Funding (DOI: 10.54499/UIDP/50022/2020); the Guangdong Basic and Applied Basic Research Foundation (No. 2024A1515240025); and the China Scholarship Council (No. 202406070025 and 202406070043).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Yang, S.; Meng, D.; Díaz, A.; Yang, H.; Su, X.; Jesus, A.M.D. Probabilistic modeling of uncertainties in reliability analysis of mid-and high-strength steel pipelines under hydrogen-induced damage. Int. J. Struct. Integr. 2025, 16, 39–59. [Google Scholar] [CrossRef]
- Lee, J.A.; Woods, S. Hydrogen Embrittlement; NASA: Washington, DC, USA, 2016.
- Dwivedi, S.K.; Vishwakarma, M. Effect of hydrogen in advanced high strength steel materials. Int. J. Hydrogen Energy 2019, 44, 28007–28030. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Sergeev, N.N.; Sergeev, A.N.; Kutepov, S.N.; Kolmakov, A.G.; Gvozdev, A.E. Mechanism of the hydrogen cracking of metals and alloys, part II. Inorg. Mater. Appl. Res. 2019, 10, 32–41. [Google Scholar] [CrossRef]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Metall. Mater. Trans. B 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Pressouyre, G.M.; Bernstein, I.M. An example of the effect of hydrogen trapping on hydrogen embrittlement. Metall. Trans. A 1981, 12, 835–844. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. Atomic mechanism and prediction of hydrogen embrittlement in iron. Nat. Mater. 2013, 12, 145–151. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Lee, Y.K.; Raabe, D.; Tsuzaki, K. Overview of hydrogen embrittlement in high-Mn steels. Int. J. Hydrogen Energy 2017, 42, 12706–12723. [Google Scholar] [CrossRef]
- Kelestemur, M.H.; Chaki, T.K. The effect of overload on the fatigue crack growth behaviour of 304 stainless steel in hydrogen. Fatigue Fract. Eng. Mater. Struct. 2001, 24, 15–22. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Wang, L.; Zhang, J.; Harik, I.E. Corrosion fatigue crack propagation mechanism of high-strength steel bar in various environments. J. Mater. Civ. Eng. 2020, 32, 04020115. [Google Scholar] [CrossRef]
- Sofronis, P.; McMeeking, R.M. Numerical analysis of hydrogen transport near a blunting crack tip. J. Mech. Phys. Solids 1989, 37, 317–350. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Zhou, G.H.; Huang, Y.Z.; Chu, W.Y. Hydrogen-inducing nanovoids in thin crystals of 310 stainless steel. J. Mater. Sci. 1998, 33, 4813–4819. [Google Scholar] [CrossRef]
- Yuan, S.; Zhu, Y.; Huang, M.; Liang, S.; Li, Z. Dislocation-density based crystal plasticity model with hydrogen-enhanced localized plasticity in polycrystalline face-centered cubic metals. Mech. Mater. 2020, 148, 103472. [Google Scholar] [CrossRef]
- Deconinck, L.; Lu, X.; Wang, D.; Johnsen, R.; Verbeken, K.; Depover, T. Hydrogen enhanced localised plasticity of single grain α titanium verified by in-situ hydrogen microcantilever bending. Int. J. Hydrogen Energy 2024, 136, 902–913. [Google Scholar] [CrossRef]
- Singh, M.P.; Shukla, D.K.; Kumar, R.; Arora, K.S. The structural integrity of high-strength welded pipeline steels: A review. Int. J. Struct. Integr. 2021, 12, 470–496. [Google Scholar] [CrossRef]
- Cendales, E.D.; Orjuela, F.A.; Chamarraví, O. Computational modeling of the mechanism of hydrogen embrittlement (HE) and stress corrosion cracking (SCC) in metals. J. Phys. Conf. Ser. 2016, 687, 012067. [Google Scholar] [CrossRef]
- Nishikawa, H.A.; Oda, Y.; Noguchi, H. Investigation of mechanism for intergranular fatigue crack propagation of low carbon steel JIS S10C in hydrogen gas environment. J. Solid Mech. Mater. Eng. 2011, 5, 263–278. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Chen, W.; Yu, M.; Chevil, K.; Eadie, R.; Been, J.; Van Boven, G.; Kania, R.; Keane, S. Crack growth modeling and life prediction of pipeline steels exposed to near-neutral pH environments: Stage II crack growth and overall life prediction. Metall. Mater. Trans. A 2017, 48, 1641–1652. [Google Scholar] [CrossRef]
- Mansor, N.I.I.; Abdullah, S.; Ariffin, A.K.; Syarif, J. A review of the fatigue failure mechanism of metallic materials under a corroded environment. Eng. Fail. Anal. 2014, 42, 353–365. [Google Scholar] [CrossRef]
- Huang, S.; Hui, H. Predictive environmental hydrogen embrittlement on fracture toughness of commercial ferritic steels with hydrogen-modified fracture strain model. Int. J. Hydrogen Energy 2022, 47, 10777–10787. [Google Scholar] [CrossRef]
- Ma, K.; Zheng, J.; Hua, Z.; Gu, C.; Zhang, R.; Liu, Y. Hydrogen assisted fatigue life of Cr–Mo steel pressure vessel with coplanar cracks based on fatigue crack growth analysis. Int. J. Hydrogen Energy 2020, 45, 20132–20141. [Google Scholar] [CrossRef]
- McGaw, M.A.; Kalluri, S.; Moore, D.; Heine, J. The cumulative fatigue damage behavior of MAR-M 247 in air and high-pressure hydrogen. In Advances in Fatigue Lifetime Predictive Techniques; ASTM International: West Conshohocken, PA, USA, 1993; Volume 2. [Google Scholar]
- Guan, Y.B.; Wang, Q.Y.; He, C.; Fu, L.; Lin, L.; Zhang, Y.Q.; Luo, Y.R.; Wang, Z.G.; Wu, X. Low-cycle fatigue mechanical behavior of 30CrMo steel under hydrogen environment and numerical verification of chaboche model. Mater. Res. Express 2024, 11, 016522. [Google Scholar] [CrossRef]
- Kim, S.S.; Choe, S.J.; Shin, K.S. Quantitative models on corrosion fatigue crack growth rates in metals: Part I. Overview of quantitative crack growth models. Met. Mater. 1998, 4, 1–13. [Google Scholar] [CrossRef]
- Gangloff, R.P. Crack tip modeling of hydrogen environement embrittlement: Application to fracture mechanics life prediction. Mater. Sci. Eng. A 1988, 103, 157–166. [Google Scholar] [CrossRef]
- Traidia, A.; Chatzidouros, E.; Jouiad, M. Review of hydrogen-assisted cracking models for application to service lifetime prediction and challenges in the oil and gas industry. Corros. Rev. 2018, 36, 323–347. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wei, S.; Hong, Y.; Zheng, C. Prediction of long-term fatigue life of CFRP composite hydrogen storage vessel based on micromechanics of failure. Compos. Part B Eng. 2016, 97, 274–281. [Google Scholar] [CrossRef]
- Golahmar, A.; Kristensen, P.K.; Niordson, C.F.; Martínez-Pañeda, E. A phase field model for hydrogen-assisted fatigue. Int. J. Fatigue 2022, 154, 106521. [Google Scholar] [CrossRef]
- Lee, H.W. Modeling Very High Cycle Fatigue Life of Low Carbon Structural Steel Subjected to Corrosion and Hydrogen Embrittlement Damage with Unified Mechanics Theory. Doctoral Dissertation, State University of New York at Buffalo, Buffalo, NY, USA, 2023. [Google Scholar]
- Wu, E.; Zhao, Y.; Zhao, B.; Xu, W. Fatigue life prediction and verification of high-pressure hydrogen storage vessel. Int. J. Hydrogen Energy 2021, 46, 30412–30422. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, S.-Y.; Baek, U.B.; Choi, B.-H. Evaluation of the residual fatigue lifetime of a semi-elliptical crack of a Low-Alloy steel pressure vessel under High-Pressure gaseous hydrogen. Int. J. Fatigue 2023, 176, 107875. [Google Scholar] [CrossRef]
- Guo, C.; Liu, S.; Zou, Y.; Zhao, D.; Cheng, M.; Gu, T.; Liu, Y.; Zhang, Q.; Wang, Q.; Feng, Z. Fatigue properties and life prediction of GS80A steel under the effect of hydrogen-rich environment. JOM 2023, 75, 1306–1318. [Google Scholar] [CrossRef]
- Ahmed, N.; Aldaw, M.; Ahmed, R.; Teodoriu, C. Modeling of necking area reduction of carbon steel in hydrogen environment using machine learning approach. Eng. Fail. Anal. 2024, 156, 107864. [Google Scholar] [CrossRef]
- Kim, S.G.; Shin, S.H.; Hwang, B. Machine learning approach for prediction of hydrogen environment embrittlement in austenitic steels. J. Mater. Res. Technol. 2022, 19, 2794–2798. [Google Scholar] [CrossRef]
- Miao, Y.; Li, Y.; Zhang, X.; Xu, J.; Wu, D.; Sun, L.; Liu, H. An intelligent schedule maintenance method for hydrogen fuel cell vehicles based on deep reinforcement learning considering dynamic reliability. Int. J. Hydrogen Energy 2024, 64, 455–467. [Google Scholar] [CrossRef]
- Ismail, M.F.H.; May, Z.; Asirvadam, V.S.; Nayan, N.A. Machine-learning-based classification for pipeline corrosion with monte carlo probabilistic analysis. Energies 2023, 16, 3589. [Google Scholar] [CrossRef]
- Fangnon, E.; Malitckii, E.; Latypova, R.; Vilaça, P. Prediction of hydrogen concentration responsible for hydrogen-induced mechanical failure in martensitic high-strength steels. Int. J. Hydrogen Energy 2023, 48, 5718–5730. [Google Scholar] [CrossRef]
- Gangloff, R.P. Corrosion fatigue crack propagation in metals. In International Conference on Environment Induced Cracking of Metals; NASA-CR-4301; NASA: Washington, DC, USA, 1990. [Google Scholar]
- Somerday, B.P.; Sofronis, P.; Nibur, K.A.; San Marchi, C.; Kirchheim, R. Elucidating the variables affecting accelerated fatigue crack growth of steels in hydrogen gas with low oxygen concentrations. Acta Mater. 2013, 61, 6153–6170. [Google Scholar] [CrossRef]
- Oriani, R.A. The physical and metallurgical aspects of hydrogen in metals. Fusion Technol. 1994, 26, 235–266. [Google Scholar]
- He, Y.; Su, Y.; Yu, H.; Chen, C. First-principles study of hydrogen trapping and diffusion at grain boundaries in γ-Fe. Int. J. Hydrogen Energy 2021, 46, 7589–7600. [Google Scholar] [CrossRef]
- Bandyopadhyay, N.; Kameda, J.; McMahon, C.J. Hydrogen-induced cracking in 4340-type steel: Effects of composition, yield strength, and H2 pressure. Metall. Trans. A 1983, 14, 881–888. [Google Scholar] [CrossRef]
- Traidia, A.; Alfano, M.; Lubineau, G.; Duval, S.; Sherik, A. An effective finite element model for the prediction of hydrogen induced cracking in steel pipelines. Int. J. Hydrogen Energy 2012, 37, 16214–16230. [Google Scholar] [CrossRef]
- Paal, Z.; Menon, P.G. Hydrogen effects in metal catalysts. Catal. Rev. Sci. Eng. 1983, 25, 229–324. [Google Scholar] [CrossRef]
- Wiswall, R. Hydrogen storage in metals. In Hydrogen in Metals II: Application-Oriented Properties; Springer: Berlin/Heidelberg, Germany, 2005; pp. 201–242. [Google Scholar]
- Badding, J.V.; Hemley, R.J.; Mao, H.K. High-pressure chemistry of hydrogen in metals: In situ study of iron hydride. Science 1991, 253, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.J.; Robertson, I.M.; Birnbaum, H.K. Hydrogen effects on the interaction between dislocations. Acta Mater. 1998, 46, 1749–1757. [Google Scholar] [CrossRef]
- Gu, Y.; El-Awady, J.A. Quantifying the effect of hydrogen on dislocation dynamics: A three-dimensional discrete dislocation dynamics framework. J. Mech. Phys. Solids 2018, 112, 491–507. [Google Scholar] [CrossRef]
- Miura, T.; Fujii, K.; Nishioka, H.; Fukuya, K. Effects of hydrogen on interaction between dislocations and radiation-induced defects in austenitic stainless steels. J. Nucl. Mater. 2013, 442, S735–S739. [Google Scholar] [CrossRef]
- Delafosse, D.; Magnin, T. Hydrogen induced plasticity in stress corrosion cracking of engineering systems. Eng. Fract. Mech. 2001, 68, 693–729. [Google Scholar] [CrossRef]
- Del-Pozo, A.; Villalobos, J.C.; Serna, S. A general overview of hydrogen embrittlement. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 139–168. [Google Scholar]
- Laadel, N.E.; El Mansori, M.; Kang, N.; Marlin, S.; Boussant-Roux, Y. Permeation barriers for hydrogen embrittlement prevention in metals—A review on mechanisms, materials suitability and efficiency. Int. J. Hydrogen Energy 2022, 47, 32707–32731. [Google Scholar] [CrossRef]
- Sque, S.J.; Jones, R.; Briddon, P.R. Structure, electronics, and interaction of hydrogen and oxygen on diamond surfaces. Phys. Rev. B 2006, 73, 085313. [Google Scholar] [CrossRef]
- Jena, P.; Singwi, K.S. Electronic structure of hydrogen in simple metals. Phys. Rev. B 1978, 17, 3518–3524. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.X. Electronic structure, stability and bonding of the Li-NH hydrogen storage system. Phys. Rev. B 2006, 74, 195120. [Google Scholar] [CrossRef]
- Pundt, A.; Kirchheim, R. Hydrogen in metals: Microstructural aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Legrand, E.; Bouhattate, J.; Feaugas, X.; Touzain, S.; Garmestani, H.; Khaleel, M.; Li, D.S. Numerical analysis of the influence of scale effects and microstructure on hydrogen diffusion in polycrystalline aggregates. Comput. Mater. Sci. 2013, 71, 1–9. [Google Scholar] [CrossRef]
- Elboujdaini, M.; Revie, R.W. Metallurgical factors in stress corrosion cracking (SCC) and hydrogen-induced cracking (HIC). J. Solid State Electrochem. 2009, 13, 1091–1099. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Wang, H.; Tong, Z.; Zhou, G.; Zhang, C.; Zhou, H.; Wang, Y.; Zheng, W. Research and demonstration on hydrogen compatibility of pipelines: A review of current status and challenges. Int. J. Hydrogen Energy 2022, 47, 28585–28604. [Google Scholar] [CrossRef]
- Jia, G.; Lei, M.; Li, M.; Xu, W.; Li, R.; Lu, Y.; Cai, M. Hydrogen embrittlement in hydrogen-blended natural gas transportation systems: A review. Int. J. Hydrogen Energy 2023, 48, 32137–32157. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Y.F. Hydrogen-induced degradation of high-strength steel pipeline welds: A critical review. Eng. Fail. Anal. 2022, 133, 105985. [Google Scholar] [CrossRef]
- Martin, M.L.; Sofronis, P. Hydrogen-induced cracking and blistering in steels: A review. J. Nat. Gas Sci. Eng. 2022, 101, 104547. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. External corrosion of oil and gas pipelines: A review of failure mechanisms and predictive preventions. J. Nat. Gas Sci. Eng. 2022, 100, 104467. [Google Scholar] [CrossRef]
- Yang, S.; De Jesus, A.M.; Meng, D.; Nie, P.; Darabi, R.; Azinpour, E.; Zhu, S.-P.; Wang, Q. Very high-cycle fatigue behavior of steel in hydrogen environment: State of the art review and challenges. Eng. Fail. Anal. 2024, 166, 108898. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Cheng, A.; Chen, N.Z. Fatigue crack growth modelling for pipeline carbon steels under gaseous hydrogen conditions. Int. J. Fatigue 2017, 96, 152–161. [Google Scholar] [CrossRef]
- Amaro, R.L.; Rustagi, N.; Findley, K.O.; Drexler, E.S.; Slifka, A.J. Modeling the fatigue crack growth of X100 pipeline steel in gaseous hydrogen. Int. J. Fatigue 2014, 59, 262–271. [Google Scholar] [CrossRef]
- Liang, S.; Huang, M.; Zhao, L.; Zhu, Y.; Li, Z. Effect of multiple hydrogen embrittlement mechanisms on crack propagation behavior of FCC metals: Competition vs. synergy. Int. J. Plast. 2021, 143, 103023. [Google Scholar] [CrossRef]
- Lynch, S.P. Towards understanding mechanisms and kinetics of environmentally assisted cracking. Environ.-Induc. Crack. Mater. 2008, 1, 167–177. [Google Scholar] [CrossRef]
- Huang, S.; Hui, H.; Peng, J. Prediction of hydrogen-assisted fracture under coexistence of hydrogen-enhanced plasticity and decohesion. Int. J. Hydrogen Energy 2023, 48, 36987–37000. [Google Scholar] [CrossRef]
- Lovicu, G.; Bottazzi, M.; D’aiuto, F.; De Sanctis, M.; Dimatteo, A.; Santus, C.; Valentini, R. Hydrogen embrittlement of automotive advanced high-strength steels. Metall. Mater. Trans. A 2012, 43, 4075–4087. [Google Scholar] [CrossRef]
- Michler, T.; Yukhimchuk, A.A.; Naumann, J. Hydrogen environment embrittlement testing at low temperatures and high pressures. Corros. Sci. 2008, 50, 3519–3526. [Google Scholar] [CrossRef]
- Briottet, L.; Moro, I.; Lemoine, P. Quantifying the hydrogen embrittlement of pipeline steels for safety considerations. Int. J. Hydrogen Energy 2012, 37, 17616–17623. [Google Scholar] [CrossRef]
- Bae, D.; Lee, J.; Lee, S.; Son, I.; Baek, U.; Nahm, S.; Lee, J. Evaluation on hydrogen embrittlement of material using nondestructive test. Int. J. Precis. Eng. Manuf. 2014, 15, 989–993. [Google Scholar] [CrossRef]
- Tiwari, G.P.; Bose, A.; Chakravartty, J.K.; Wadekar, S.L.; Totlani, M.K.; Arya, R.N.; Fotedar, R.K. A study of internal hydrogen embrittlement of steels. Mater. Sci. Eng. A 2000, 286, 269–281. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y. Hydrogen effect against hydrogen embrittlement. Metall. Mater. Trans. A 2010, 41, 2548–2562. [Google Scholar] [CrossRef]
- Moro, I.; Briottet, L.; Lemoine, P.; Andrieu, E.; Blanc, C.; Odemer, G. Hydrogen embrittlement susceptibility of a high strength steel X80. Mater. Sci. Eng. A 2010, 527, 7252–7260. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. A nanoscale mechanism of hydrogen embrittlement in metals. Acta Mater. 2011, 59, 1557–1569. [Google Scholar] [CrossRef]
- Matsumoto, R.; Taketomi, S. Molecular dynamics simulation of Surface-Adsorbed-Hydrogen-Induced Dislocation Motion in a thin film. Comput. Mater. Sci. 2020, 171, 109240. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of industrial components: Prediction, prevention, and models. Corrosion 2016, 72, 943–961. [Google Scholar] [CrossRef] [PubMed]
- Putri, E.D.W.S.; Triyono, T.; Prabowo, A.R. Estimating failure mechanism of steel specimens using stress corrosion-cracking (SCC) testing methods: State and development. Procedia Struct. Integr. 2022, 41, 266–273. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, T.; He, W.; Luo, Q.; Li, Z.; Zhang, W.; He, J.; Li, Q. Electron microscope investigation on hydrogen storage materials: A review. Int. J. Hydrogen Energy 2020, 45, 12048–12070. [Google Scholar] [CrossRef]
- Xu, R.; Wunsch, D. Survey of clustering algorithms. IEEE Trans. Neural Netw. 2005, 16, 645–678. [Google Scholar] [CrossRef] [PubMed]
- Slifka, A.J.; Drexler, E.S.; Nanninga, N.E.; Levy, Y.S.; McColskey, J.D.; Amaro, R.L.; Stevenson, A.E. Fatigue crack growth of two pipeline steels in a pressurized hydrogen environment. Corros. Sci. 2014, 78, 313–321. [Google Scholar] [CrossRef]
- Elapolu, M.S.; Tabarraei, A. Mechanical and fracture properties of polycrystalline graphene with hydrogenated grain boundaries. J. Phys. Chem. C 2021, 125, 11147–11158. [Google Scholar] [CrossRef]
- Satyapal, S. 2011 Annual Progress Report: DOE Hydrogen and Fuel Cells Program; No. DOE/GO-102011-3422; Office of Energy Efficiency and Renewable Energy (EERE): Washington, DC, USA, 2011.
- Parkins, R.N.; Greenwell, B.S. The interface between corrosion fatigue and stress-corrosion cracking. Met. Sci. 1977, 11, 405–413. [Google Scholar] [CrossRef]
- Jones, D.A. A unified mechanism of stress corrosion and corrosion fatigue cracking. Metall. Trans. A 1985, 16, 1133–1141. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A. Effect of different parameters on hydrogen affected fatigue failure in pipeline steels. Eng. Fail. Anal. 2022, 137, 106262. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Heo, H.M.; Park, J.; Nahm, S.H.; Beak, U.B. Fracture properties and fatigue life assessment of API X70 pipeline steel under the effect of an environment containing hydrogen. J. Mech. Sci. Technol. 2021, 35, 1445–1455. [Google Scholar] [CrossRef]
- Laureys, A.; Depraetere, R.; Cauwels, M.; Depover, T.; Hertelé, S.; Verbeken, K. Use of existing steel pipeline infrastructure for gaseous hydrogen storage and transport: A review of factors affecting hydrogen induced degradation. J. Nat. Gas Sci. Eng. 2022, 101, 104534. [Google Scholar] [CrossRef]
- Li, L.; Li, C.Q.; Mahmoodian, M.; Shi, W. Corrosion induced degradation of fatigue strength of steel in service for 128 years. Structures 2020, 23, 415–424. [Google Scholar] [CrossRef]
- Guy, P.; Laszlo, T.; Julien, C. Effects of hydrogen addition on design, maintenance and surveillance of gas networks. Processes 2021, 9, 1219. [Google Scholar] [CrossRef]
- Ramírez, F.M.; de Moura, M.F.; Moreira, R.D.; Silva, F.G. A review on the environmental degradation effects on fatigue behaviour of adhesively bonded joints. Fatigue Fract. Eng. Mater. Struct. 2020, 43, 1307–1326. [Google Scholar] [CrossRef]
- Pradhan, A.; Vishwakarma, M.; Dwivedi, S.K. A review: The impact of hydrogen embrittlement on the fatigue strength of high strength steel. Mater. Today Proc. 2020, 26, 3015–3019. [Google Scholar] [CrossRef]
- Lee, H.W.; Djukic, M.B.; Basaran, C. Modeling fatigue life and hydrogen embrittlement of bcc steel with unified mechanics theory. Int. J. Hydrogen Energy 2023, 48, 20773–20803. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Sofronis, P.; Brouwer, J.; Sosa, S. Assessment of resistance to fatigue crack growth of natural gas line pipe steels carrying gas mixed with hydrogen. Int. J. Hydrogen Energy 2019, 44, 10808–10822. [Google Scholar] [CrossRef]
- Cialone, H.J.; Holbrook, J.H. Effects of gaseous hydrogen on fatigue crack growth in pipeline steel. Metall. Trans. A 1985, 16, 115–122. [Google Scholar] [CrossRef]
- San Marchi, C.; Somerday, B.P.; Nibur, K.A.; Stalheim, D.G.; Boggess, T.; Jansto, S. Fracture and fatigue of commercial grade API pipeline steels in gaseous hydrogen. In Proceedings of the ASME 2010 Pressure Vessels and Piping Division/K-PVP Conference, Bellevue, WA, USA, 18–22 July 2010; pp. 939–948, ISBN 978-0-7918-49255. [Google Scholar] [CrossRef]
- Cialone, H.; Holbrook, J. Sensitivity of steels to degradation in gaseous hydrogen. In Hydrogen Embrittlement: Prevention and Control; ASTM International: West Conshohocken, PA, USA, 1988. [Google Scholar]
- Amaro, R.L.; Drexler, E.S.; Rustagi, N.E.H.A.; Nanninga, N.E.; Levy, Y.S.; Slifka, A.J. Fatigue crack growth of pipeline steels in gaseous hydrogen-predictive model calibrated to API-5L X52. In Proceedings of the 2012 International Hydrogen Conference, Moran, WY, USA, 9–12 September 2012. [Google Scholar]
- Slifka, A.J.; Drexler, E.S.; Stalheim, D.G.; Amaro, R.L.; Lauria, D.S.; Stevenson, A.E.; Hayden, L.E. The effect of microstructure on the hydrogen-assisted fatigue of pipeline steels. In Proceedings of the ASME 2013 Pressure Vessels and Piping Conference, Paris, France, 14–18 July 2013; p. V06BT06A009, ISBN 978-0-7918-5571-3. [Google Scholar] [CrossRef]
- Drexler, E.S.; Slifka, A.J.; Amaro, R.L.; Barbosa, N.; Lauria, D.S.; Hayden, L.E.; Stalheim, D.G. Fatigue crack growth rates of API X70 pipeline steel in a pressurized hydrogen gas environment. Fatigue Fract. Eng. Mater. Struct. 2014, 37, 517–525. [Google Scholar] [CrossRef]
- Campari, A.; Ustolin, F.; Alvaro, A.; Paltrinieri, N. A review on hydrogen embrittlement and risk-based inspection of hydrogen technologies. Int. J. Hydrogen Energy 2023, 48, 35316–35346. [Google Scholar] [CrossRef]
- Nykyforchyn, H.; Zvirko, O.; Dzioba, I.; Krechkovska, H.; Hredil, M.; Tsyrulnyk, O.; Student, O.; Lipiec, S.; Pala, R. Assessment of operational degradation of pipeline steels. Materials 2021, 14, 3247. [Google Scholar] [CrossRef]
- Hojo, T.; Nagasaka, A.; Kobayashi, J.; Shibayama, Y.; Akiyama, E. Effect of Hydrogen on Fatigue Life and Fracture Morphologies of TRIP-Aided Martensitic Steels with Added Nitrogen. Metals 2024, 14, 346. [Google Scholar] [CrossRef]
- Gong, W.; Wang, X.Y.; Wang, X.; Wang, W.; Yang, Y.L. Comparison of inelasticity creep failure evaluation codes for elevated-temperature non-nuclear pressure equipment. Int. J. Struct. Integr. 2024, 15, 1153–1168. [Google Scholar] [CrossRef]
- Meng, D.; Yang, S.; Yang, H.; De Jesus, A.M.; Correia, J.; Zhu, S.P. Intelligent-inspired framework for fatigue reliability evaluation of offshore wind turbine support structures under hybrid uncertainty. Ocean Eng. 2024, 307, 118213. [Google Scholar] [CrossRef]
- Hoschke, J.; Chowdhury, M.F.W.; Venezuela, J.; Atrens, A. A review of hydrogen embrittlement in gas transmission pipeline steels. Corros. Rev. 2023, 41, 277–317. [Google Scholar] [CrossRef]
- Setoyama, A.; Ogawa, Y.; Nakamura, M.; Tanaka, Y.; Chen, T.; Koyama, M.; Matsunaga, H. Transition mechanism of cycle- to time-dependent acceleration of fatigue crack-growth in 0.4%C Cr-Mo steel in a pressurized gaseous hydrogen environment. Int. J. Fatigue 2022, 163, 107039. [Google Scholar] [CrossRef]
- Ronevich, J.A.; San Marchi, C. Materials compatibility concerns for hydrogen blended into natural gas. In Proceedings of the ASME 2021 Pressure Vessels & Piping Conference, Virtual, 13–15 July 2021; p. V004T06A052, ISBN 978-0-7918-8534-5. [Google Scholar] [CrossRef]
- Wang, B.J.; Wang, S.D.; Xu, D.K.; Han, E.H. Recent progress in fatigue behavior of Mg alloys in air and aqueous media: A review. J. Mater. Sci. Technol. 2017, 33, 1075–1086. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, H.; Kang, H.; Zhou, W.; Zhang, C. A literature review of failure prediction and analysis methods for composite high-pressure hydrogen storage tanks. Int. J. Hydrogen Energy 2019, 44, 25777–25799. [Google Scholar] [CrossRef]
- Gu, K.H.; Lee, K.S.; Lee, G.H.; Nam, K.W. Evaluation of Fatigue Life of Ultra-High-Strength Steel under Stress Corrosion Environment. Appl. Mech. Mater. 2022, 907, 1–7. [Google Scholar] [CrossRef]
- Jafari, S.; Harandi, S.E.; Singh Raman, R.K. A review of stress-corrosion cracking and corrosion fatigue of magnesium alloys for biodegradable implant applications. JOM 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Halama, R.; Kourousis, K. Ratcheting performance of maraging steel fabricated via laser powder bed fusion: Experimental evaluation and numerical prediction. Int. J. Struct. Integr. 2025, 16, 403–429. [Google Scholar] [CrossRef]
- Xu, J.; Du, D.; Song, J.; Li, D.; Li, Y. Fatigue performance analysis of steel joints with high frequency mechanical impact (HFMI) based on the notched stress method. Int. J. Struct. Integr. 2025, 16, 311–326. [Google Scholar] [CrossRef]
- Hussein, A.; Krom, A.H.; Dey, P.; Sunnardianto, G.K.; Moultos, O.A.; Walters, C.L. The effect of hydrogen content and yield strength on the distribution of hydrogen in steel: A diffusion coupled micromechanical FEM study. Acta Mater. 2021, 209, 116799. [Google Scholar] [CrossRef]
- Yang, S.; Meng, D.; Guo, Y.; Nie, P.; de Jesus, A.M. A reliability-based design and optimization strategy using a novel MPP searching method for maritime engineering structures. Int. J. Struct. Integr. 2023, 14, 809–826. [Google Scholar] [CrossRef]
- Arya, A.K.; Katiyar, R.; Kumar, P.S.; Kapoor, A.; Pal, D.B.; Rangasamy, G. A multi-objective model for optimizing hydrogen injected-high pressure natural gas pipeline networks. Int. J. Hydrogen Energy 2023, 48, 29699–29723. [Google Scholar] [CrossRef]
- Galvis-Silva, H.; Okoroafor, E.R. Investigating rock alterations in underground hydrogen storage: A geochemical and geomechanical baseline study. J. Energy Storage 2024, 101, 113939. [Google Scholar] [CrossRef]
- Houari, A.; Kouider, M.; Polat, A.; Amroune, S.; Mohamad, B.A.; Chellil, A.; Campilho, R. Effect of repair patch nature on J-integral reduction in notched plates. Int. J. Struct. Integr. 2024, 15, 1100–1131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).