Gallium-Containing Bioactive Glasses: Their Influence on Ion Release and the Bioactivity of Resulting Glass Polyalkenoate Cements
Abstract
1. Introduction
2. Materials and Methods
2.1. Glass Synthesis
2.2. GPC Sample Preparation
2.3. Ion-Release Profiles
2.4. pH Analysis
2.5. Preparation of Ion Release and Cell Culture Extracts
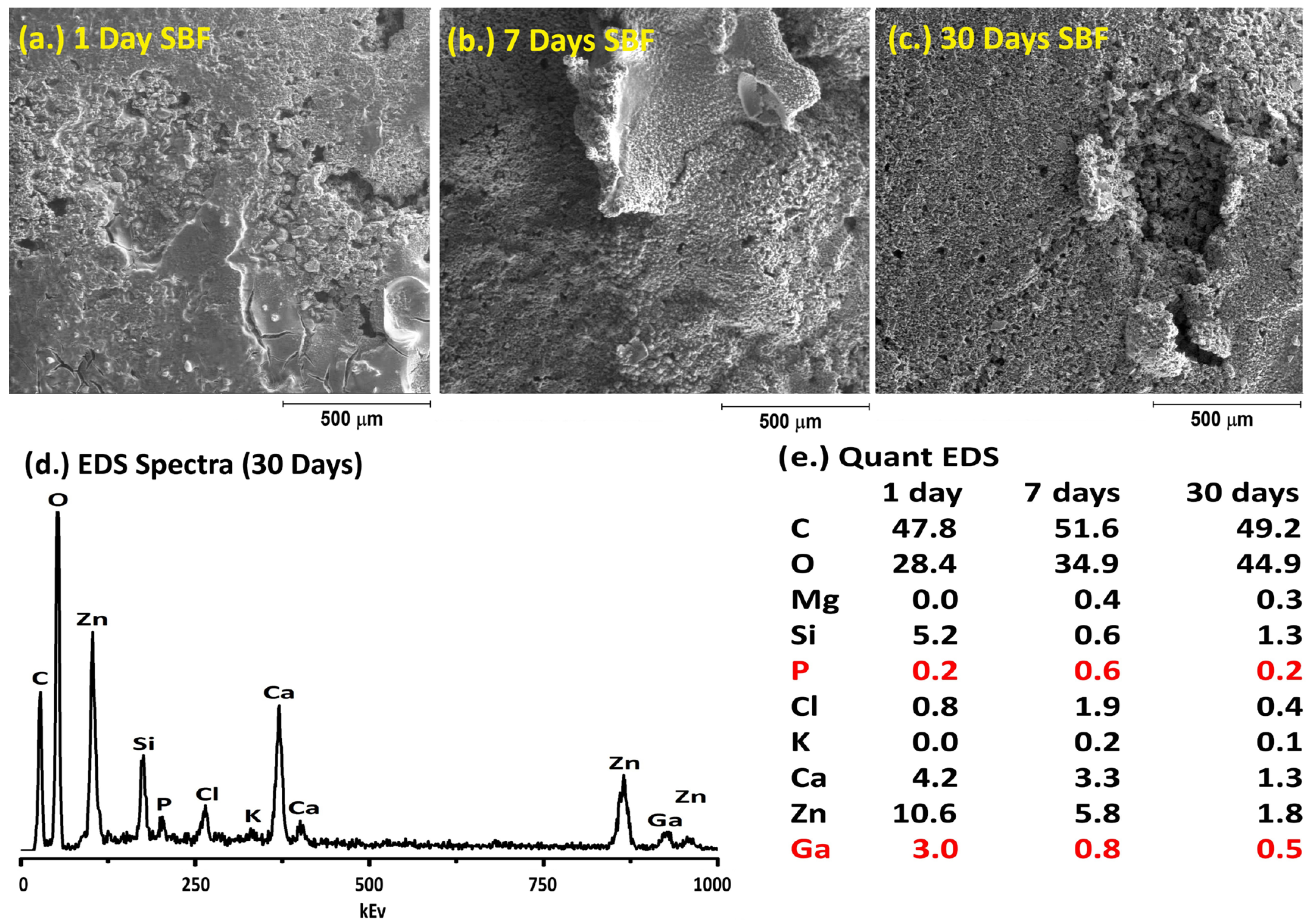
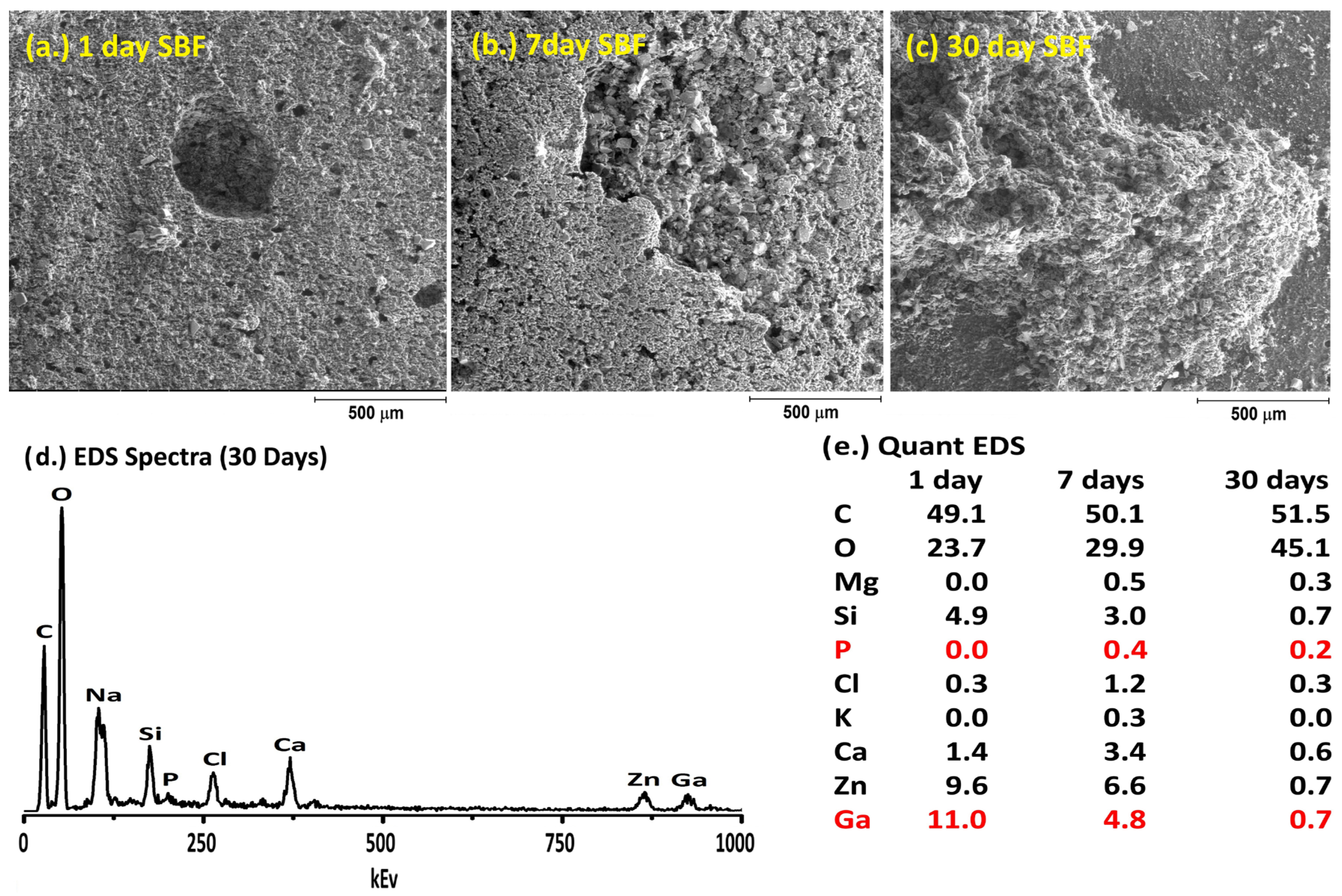
2.6. Simulated Body Fluid Trial
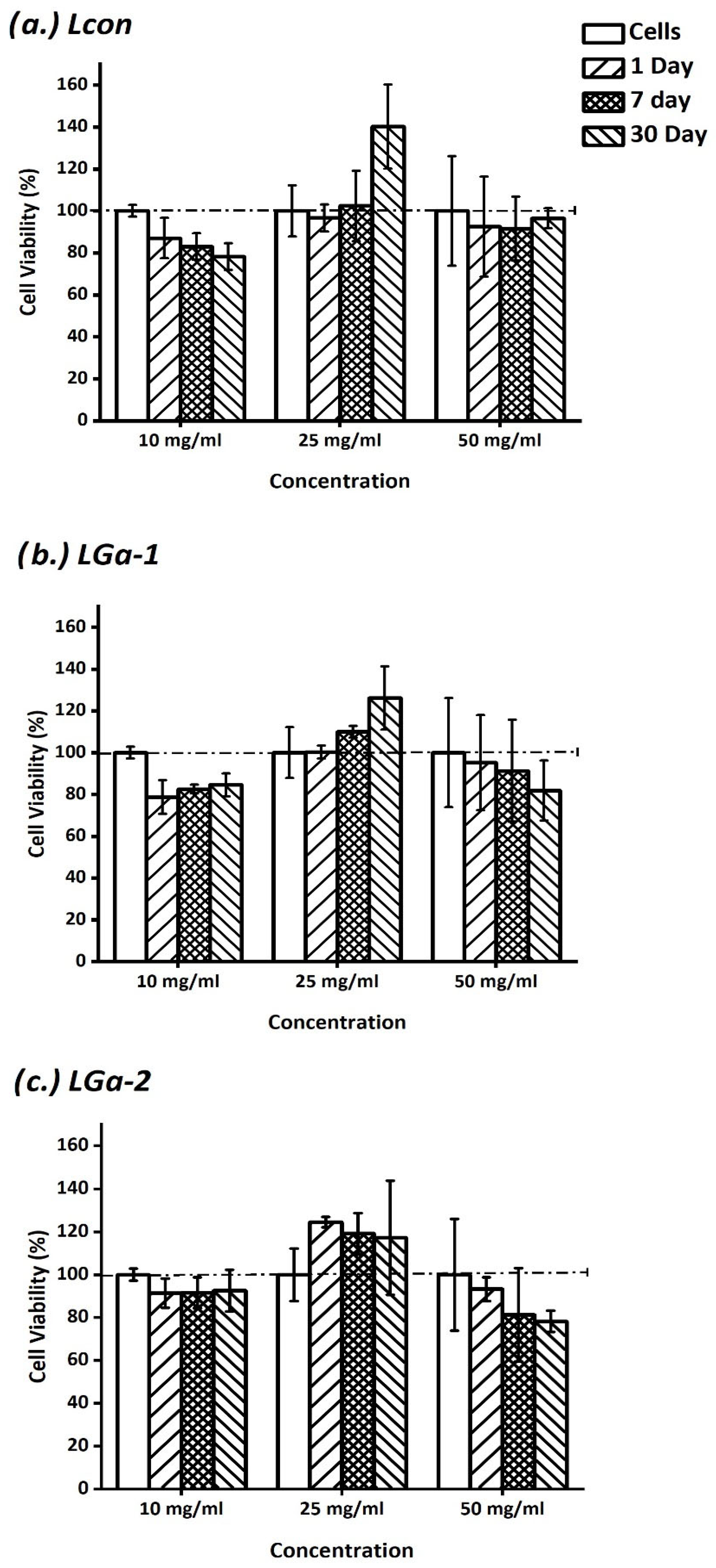
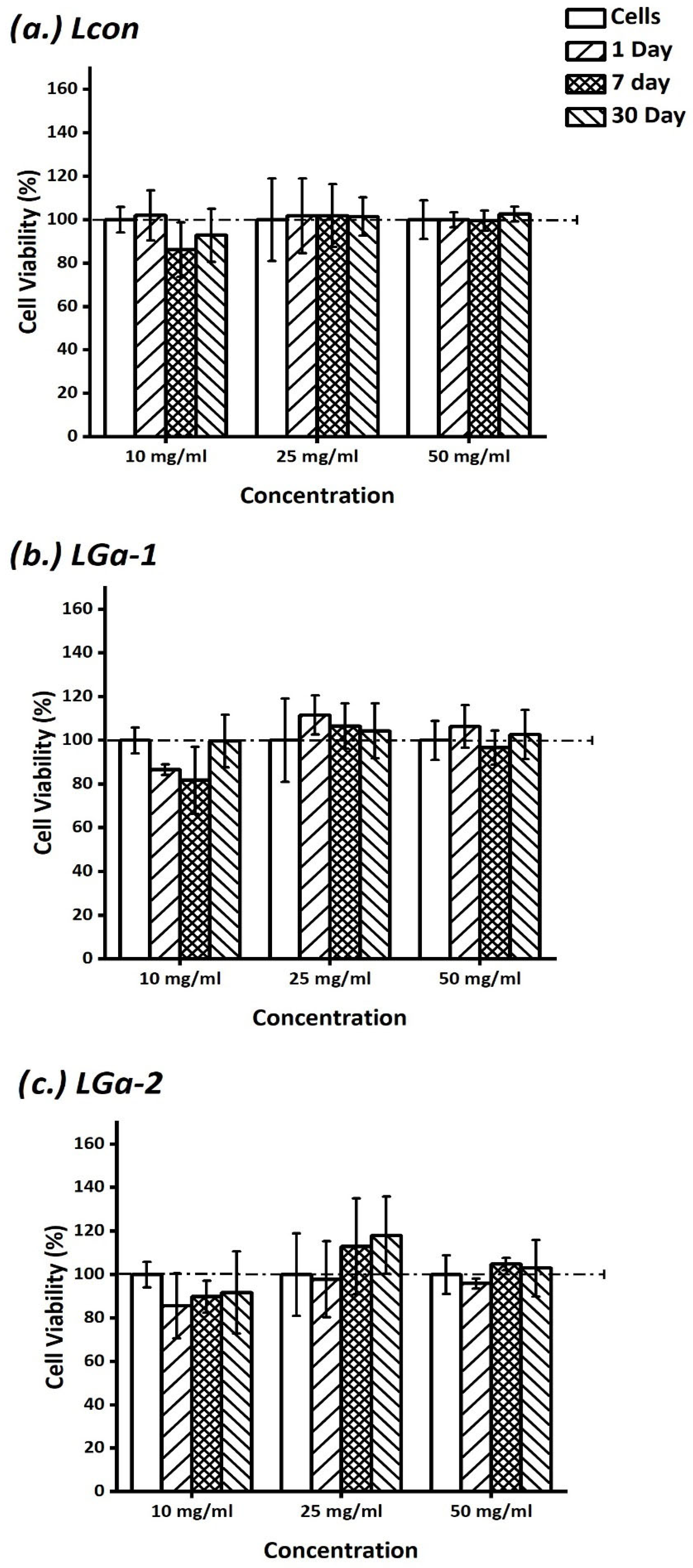
2.7. In Vitro Assessment of Cement Extracts
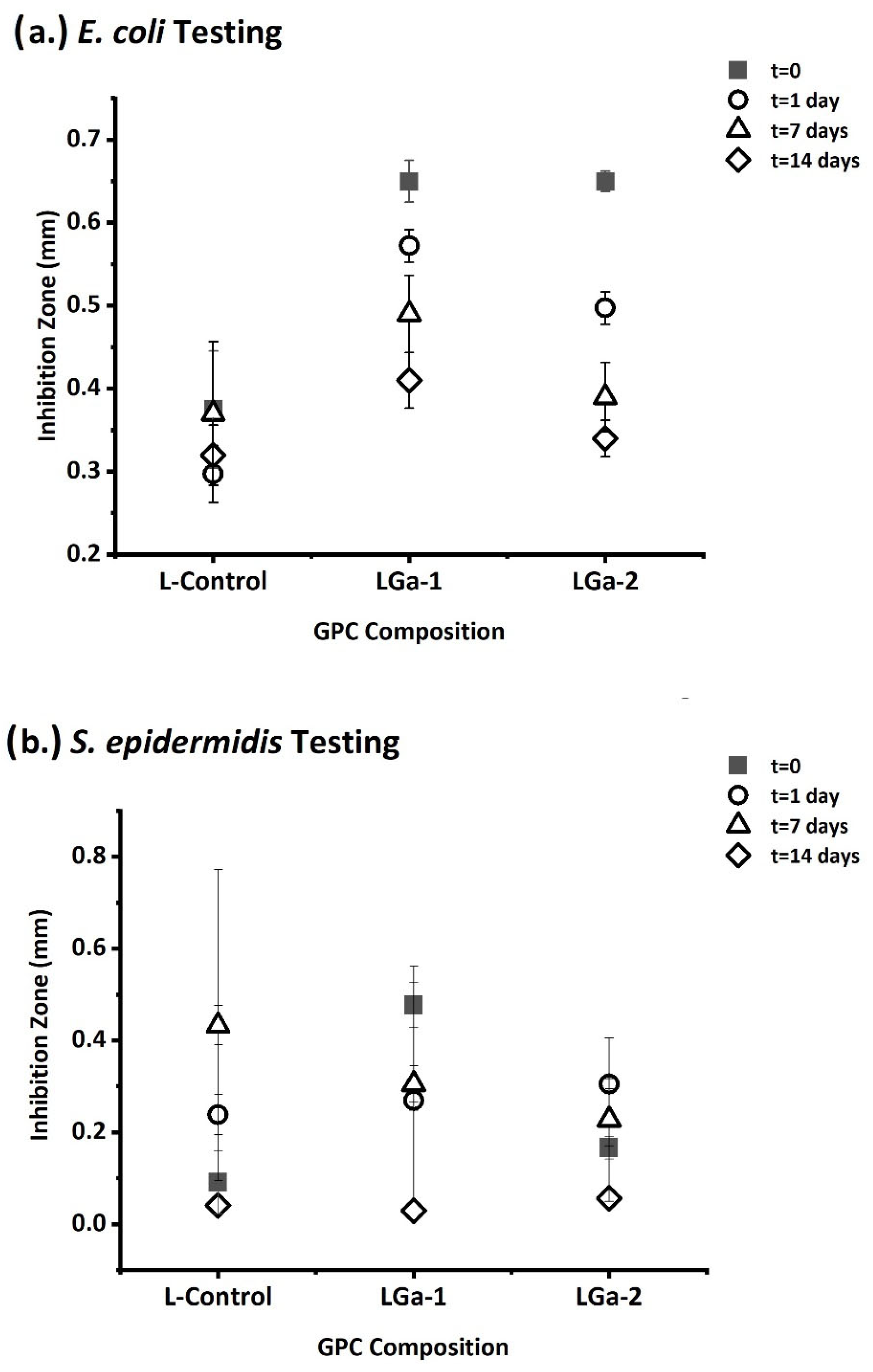
2.8. Antibacterial Evaluation
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GPC | Glass Polyalkenoate Cement |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive Spectroscopy |
| CaP | Calcium Phosphate |
| SBF | Simulated Body Fluid |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectroscopy |
| PAA | Polyacrylic Acid |
| HA | Hydroxyapatite |
| COO- | Carboxylate |
References
- Cho, S.; Cheng, A.C. A review of glass ionomer restorations in the primary dentition. J. Can. Dent. Assoc. 1999, 65, 491–495. [Google Scholar] [PubMed]
- DeBruyne, M.A.A.; DeMoor, R.J.G. The use of glass ionomer cements in both conventional and surgical endodontics. Int. Endod. J. 2004, 37, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Szoradi, G.T.; Feier, A.M.; Zuh, S.G.; Russu, O.M.; Pop, T.S. Polymethyl Methacrylate bone cement polymerization induced thermal necrosis at the cement–bone interface: A narrative review. Appl. Sci. 2024, 14, 11651. [Google Scholar] [CrossRef]
- Hill, R. Glass Ionomer Polyalkenoate Cements and related materials: Past, present and future. Br. Dent. J. 2022, 232, 653–657. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Can glass polyalkenoate (glass ionomer) dental cements be considered bioactive? A review. Heliyon 2024, 10, e25239. [Google Scholar] [CrossRef]
- Makanjuola, J.; Deb, S. Chemically activated glass ionomer cements as bioactive materilas in dentistry: A review. Prosthesis 2023, 5, 327–345. [Google Scholar] [CrossRef]
- Kim, H.-J.; Bae, H.E.; Lee, J.-E.; Park, I.-S.; Kim, H.-G.; Kwon, J.; Kim, D.-S. Effect of bioactive glass incorporation into glass ionomer cement on demineralized dentin. Sci. Rep. 2021, 11, 7016. [Google Scholar] [CrossRef]
- Carter, D.H.; Sloan, P.; Brook, I.M.; Hatton, P.V. Role of exchanged ions in the integration of ionomeric (glass polyalkenoate) bone substitutes. Biomaterials 1997, 18, 459–466. [Google Scholar] [CrossRef]
- Billington, R.W.; Williams, J.A.; Pearson, G.J. Ion processes in glass ionomer cements. J. Dent. 2006, 34, 544–555. [Google Scholar] [CrossRef]
- Akinmade, A.; Nicholson, J. Glass-ionomer cements as adhesives. J. Mater. Sci. Mater. Med. 1993, 4, 95–101. [Google Scholar] [CrossRef]
- Böker, K.O.; Richter, K.; Jäckle, K.; Taheri, S.; Grunwald, I.; Borcherding, K.; von Byern, J.; Hartwig, A.; Wildemann, B.; Schilling, A.F. Current State of Bone Adhesives—Necessities and Hurdles. Materials 2019, 12, 3975. [Google Scholar] [CrossRef] [PubMed]
- Hatton, P.V.; Kearns, V.R.; Brook, I.M. Bone–cement fixation: Glass–ionomer cements. J. Rep. Tech. 2008, 252–263. [Google Scholar]
- Boyd, D.; Clarkin, O.M.; Wren, A.W.; Towler, M.R. Zinc-based glass polyalkenoate cements with improved setting times and mechanical properties. Acta Biomater. 2008, 4, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.; Watts, S.; Hill, R.; Wren, A.W.; Clarkin, O.M.; Towler, M.R. The role of Sr2+ on the structure and reactivity of SrO-CaO-ZnO-SiO2 ionomer glasses. J. Mater. Sci. Mater. Med. 2008, 19, 953–957. [Google Scholar] [CrossRef]
- Wren, A.W.; Kidari, A.; Cummins, N.M.; Towler, M.R. A Spectroscopic investigation into the setting and mechanical properties of titanium containing glass ionomer cements. J. Mater. Sci. Mater. Med. 2010, 21, 2355–2364. [Google Scholar] [CrossRef]
- Deb, S.; Nicholson, J.W. The effect of Strontium oxide in glass ionomer cements. J. Mater. Sci. Mater. Med. 1999, 10, 471–474. [Google Scholar] [CrossRef]
- Lewis, G.; Towler, M.R.; Boyd, D.; German, M.J.; Wren, A.W.; Clarkin, O.M.; Yates, A. Evaluation of two novel aluminum-free, zinc-based glass polyalkenoate cements as alternatives to PMMA bone cement for use in vertebroplasty and balloon kyphoplasty. J. Mater. Sci. Mater. Med. 2010, 21, 59–66. [Google Scholar] [CrossRef]
- Mokhtari, S.; Skelly, K.D.; Krull, E.A.; Coughlan, A.; Mellott, N.P.; Gong, Y.; Borges, R.; Wren, A.W. Copper-containing glass polyalkenoate cements based on SiO2–ZnO–CaO–SrO–P2O5 glasses: Glass characterization, physical and antibacterial properties. J. Mater. Sci. 2017, 52, 8886–8903. [Google Scholar] [CrossRef]
- Rico, H.; Villa, L.F. Zinc, a new coherent therapy for osteoporosis. Calcif. Tissue Int. 2000, 67, 422–423. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Perrotte, P.; Dubas, F.; Philippon, J.; Poisson, F.M. Myoclonic encephalopathy after exposure to aluminium. Lancet 1996, 34, 910–911. [Google Scholar] [CrossRef]
- Reusche, E.; Pilz, P.; Oberascher, G.; Linder, B.; Egensperger, R.; Gloeckner, K.; Trinka, E.; Iglseder, B. Subacute fatal aluminium encephalopathy after reconstructive otoneurosurgery: A Case Report. Hum. Pathol. 2001, 32, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Bush, A. Aluminium and Alzheimers Disease. Alzheimer’s Aust. 2004, 1–5. [Google Scholar]
- Firling, C.E.; Hill, T.A.; Severson, A.R. Aluminium toxicity perturbs long bone calcification in the embryonic chick. Arch. Toxicol. 1999, 73, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hatton, P.V.; Hurrell-Gillingham, K.; Brook, I.M. Biocompatability of glass ionomer bone cements. J. Dent. 2006, 34, 598–601. [Google Scholar] [CrossRef]
- Smith, D.C. Development of glass-ionomer cement systems. Biomaterials 1998, 19, 467–478. [Google Scholar] [CrossRef]
- Griffin, S.; Hill, R. Influence of poly(acrylic acid) molar mass on the fracture properties of glass polyalkenoate cements. J. Mater. Sci. 1998, 33, 5383–5396. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.; Schricker, S.R. A review of powder modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2011, 21, 1319–1328. [Google Scholar] [CrossRef]
- Brown, R.F.; Day, D.E.; Day, T.E.; Jung, S.; Rahaman, M.N.; Fu, Q. Growth and differentiation of osteoblastic cells on 13-93 bioactive glass fibers and scaffolds. Acta Biomater. 2008, 4, 387–396. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Rezwan, K.; Françon, V.; Armitage, D.; Nazhat, S.N.; Jones, F.H.; Boccaccini, A.R. Surface functionalization of Bioglass®-derived porous scaffolds. Acta Biomater. 2007, 3, 551–562. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.Z.; Rezabeigi, E.; Drew, R.A. Glass ionomer cements with enhanced mechanical and remineralizing properties containing 45S5 bioglass-ceramic particles. J. Mech. Behav. Biomed. Mater. 2019, 97, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Yli-Urpo, H.; Lassila, L.V.; Närhi, T.; Vallittu, P.K. Compressive strength and surface characterization of glass ionomer cements modified by particles of bioactive glass. Dent. Mater. 2005, 21, 201–209. [Google Scholar] [CrossRef]
- Hatton, P.V.; Hurrell-Gillingham, K.; Reaney, I.M.; Miller, C.A.; Crawford, A. Devitrification of ionomer glass and its effect on the in vitro biocompatability of glass ionomer cements. Biomaterials 2003, 24, 3153–3160. [Google Scholar]
- Kovarik, R.E.; Haubenreich, J.E.; Gore, D. Glass ionomer cements: A review of composition, chemistry, and biocompatibility as a dental and medical implant material. J. Long-Term Eff. Med. Implant. 2005, 15, 655–671. [Google Scholar] [CrossRef]
- Du, R.L.; Chang, J.; Ni, S.Y.; Zhai, W.Y.; Wang, J.Y. Characterization and in vitro bioactivity of zinc-containing bioactive glass and glass-ceramics. J. Biomater. Appl. 2006, 20, 341–360. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ma, Z.J. Role of endogenous zinc in the enhancement of bone protein synthesis associated with bone growth of newborn rats. J. Bone Miner. Metab. 2001, 19, 38–44. [Google Scholar]
- Coleman, M.; Kuskie, K.; Liu, M.; Chaffin, K.; Libal, M.; Giguère, S.; Bernstein, L.; Cohen, N. In vitro antimicrobial activity of gallium maltolate against virulent Rhodococcus equi. Vet. Microbiol. 2010, 146, 175–178. [Google Scholar] [CrossRef]
- da Silva, J.G.; Azzolini, L.S.; Wardell, S.M.S.V.; Wardell, J.L.; Beraldo, H. Increasing the antibacterial activity of gallium(III) against Pseudomonas aeruginosa upon coordination to pyridine-derived thiosemicarbazones. Polyhedron 2009, 28, 2301–2305. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Ready, D.; Abou Neel, E.A.; Pickup, D.M.; O’Dell, L.A.; Chrzanowski, W.; Pratten, J.; Newport, R.J.; Smith, M.E.; Wilson, M.; et al. Controlled delivery of antimicrobial gallium ions from phosphate-based glasses. Acta Biomater. 2009, 5, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.; Hellman, S.; Rosenberg, S. Important advances in oncology. Anticancer Drugs 1991, 2, 419. [Google Scholar] [CrossRef]
- Collery, P.; Keppler, B.; Madoulet, C.; Desoize, B. Gallium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 283–296. [Google Scholar] [CrossRef]
- Ortega, R.; Suda, A.; Devès, G. Nuclear microprobe imaging of gallium nitrate in cancer cells. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 210, 364–367. [Google Scholar] [CrossRef]
- de Oliveira Bastos, T.; Soares, B.M.; Cisalpino, P.S.; Mendes, I.C.; dos Santos, R.G.; Beraldo, H. Coordination to gallium(III) strongly enhances the potency of 2-pyridineformamide thiosemicarbazones against Cryptococcus opportunistic fungi. Microbiol. Res. 2010, 165, 573–577. [Google Scholar] [CrossRef]
- Olakanmi, O.; Britigan, B.E.; Schlesinger, L.S. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect. Immun. 2000, 68, 5619–5627. [Google Scholar] [CrossRef]
- Bockman, R.S.; Boskey, A.L.; Blumenthal, N.C.; Alcock, N.W.; Warrell, R.P. Gallium increases bone calcium and crystallite perfection of hydroxyapatite. Calcif. Tissue Int. 1986, 39, 376–381. [Google Scholar] [CrossRef]
- Verron, E.; Masson, M.; Khoshniat, S.; Duplomb, L.; Wittrant, Y.; Baudhuin, M.; Badran, Z.; Bujoli, B.; Janvier, P.; Scimeca, J.-C.; et al. Gallium modulates osteoclastic bone resorption in vitro without affecting osteoblasts. Br. J. Pharmacol. 2010, 159, 1681–1692. [Google Scholar] [CrossRef]
- Bockman, R.; Guidon, P.; Pan, L.; Salvatori, R.; Kawaguchi, A. Gallium nitrate increases type I collagen and fibronectin mRNA and collagen protein levels in bone and Fibroblast cells. J. Cell. Biochem. 1993, 52, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Warrell, R.P.; Isaacs, M.; Coonley, C.J.; Alcook, N.W.; Bockman, R.S. Metabolic effects of gallium nitrate administration by prolonged infusion. Can. Treat. Rep. 1985, 69, 653–655. [Google Scholar]
- Hart, M.M.; Adamson, R.H. Antitumor activity and toxicity of salts of inorganic group IIIa metals: Aluminum, Gallium, Indium, and Thallium. Proc. Natl. Acad. Sci. USA 1971, 68, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Pro, B.; Bociek, R.G.; Chitambar, C.R.; Gregory, S.A.; Leonard, J.P.; Smith, S.; Novick, S. Phase 2 Multicenter trial of gallium nitrate in patients with advanced non-Hodgkin’s Lymphoma. Blood 2004, 104, 2487. [Google Scholar] [CrossRef]
- Crawford, E.D.; Saiers, J.H.; Baker, L.H.; Costanzi, J.H.; Bukowski, R.M. Gallium nitrate in advanced bladdercarcinoma: Southwest oncology group study. Urology 1991, 38, 355–357. [Google Scholar] [CrossRef]
- Warrell, R.P., Jr.; Lovett, D.; Dilmanian, F.A.; Schneider, R.; Heelan, R.T. Low-dose gallium ntrate for prevention of osteolysis in myeloma: Results of a pilot randomized study. J. Clin. Oncol. 1993, 11, 2443–2450. [Google Scholar] [CrossRef]
- Einhorn, L.H.; Roth, B.J.; Ansari, R.; Dreicer, R.; Gonin, R.; Loehrer, P.J. Phase II trial of vinblastine, Ifosfamide, and gallium combination chemotherapy in metastatic urothelial carcinoma. J. Clin. Oncol. 1994, 12, 2271–2276. [Google Scholar] [CrossRef]
- Warrell, R.P., Jr.; Murphy, W.K.; Schulman, P.; O’Dwyer, P.J.; Heller, G. A randomized double-blind study of gallium nitrate compared with Etidronate for acute control of cancer-related hypercalcemia. J. Clin. Oncol. 1991, 9, 1467–1475. [Google Scholar] [CrossRef]
- Cvitkovic, F.; Armand, J.P.; Tubiana-Hulin, M.; Rossi, J.F.; Warrell, R.P., Jr. Randomized, double-blind, phase II trial of gallium nitrate compared with Pamidronate for acute control of cancer-related hypercalcemia. Can. Treat. Rep. 2006, 12, 47–53. [Google Scholar] [CrossRef]
- ISO10993; Biological Evaluation of Medical Devices. British Standards Institution: London, UK, 2003.
- Wren, A.W.; Coughlan, A.; Placek, L.; Towler, M.R. Gallium containing glass polyalkenoate anti-cancerous bone cements: Glass characterization and physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 1823–1833. [Google Scholar] [CrossRef]
- Rahimnejad Yazdi, A.; Torkan, L.; Stone, W.; Towler, M.R. The impact of gallium content on degradation, bioactivity, and antibacterial potency of zinc borate bioactive glass. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 367–376. [Google Scholar] [CrossRef]
- Gao, Z.; Song, M.; Liu, R.L.; Shen, Y.; Ward, L.; Cole, I.; Chen, X.B.; Liu, X. Improving in vitro and in vivo antibacterial functionality of Mg alloys through micro-alloying with Sr and Ga. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109926. [Google Scholar] [CrossRef]
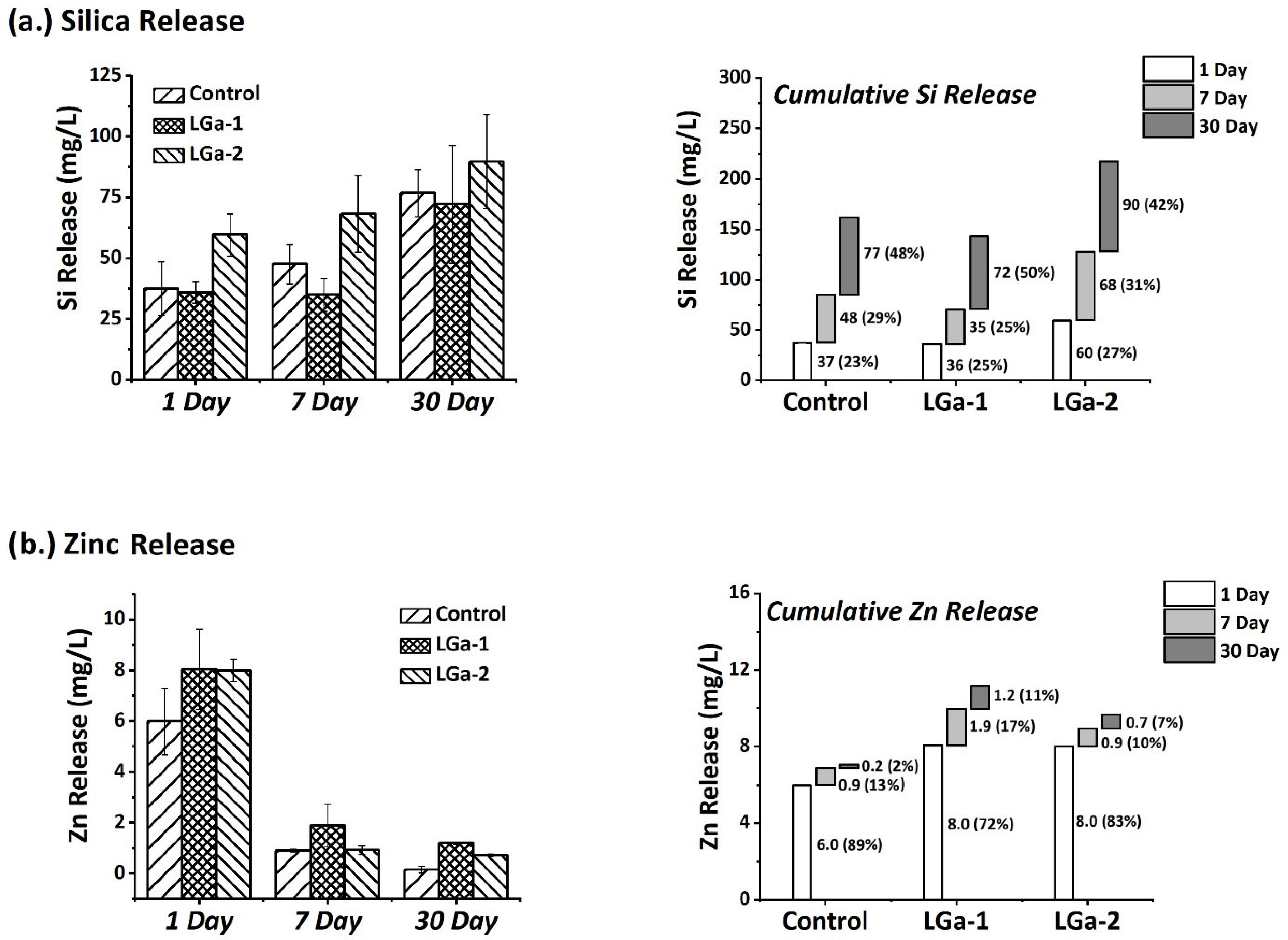
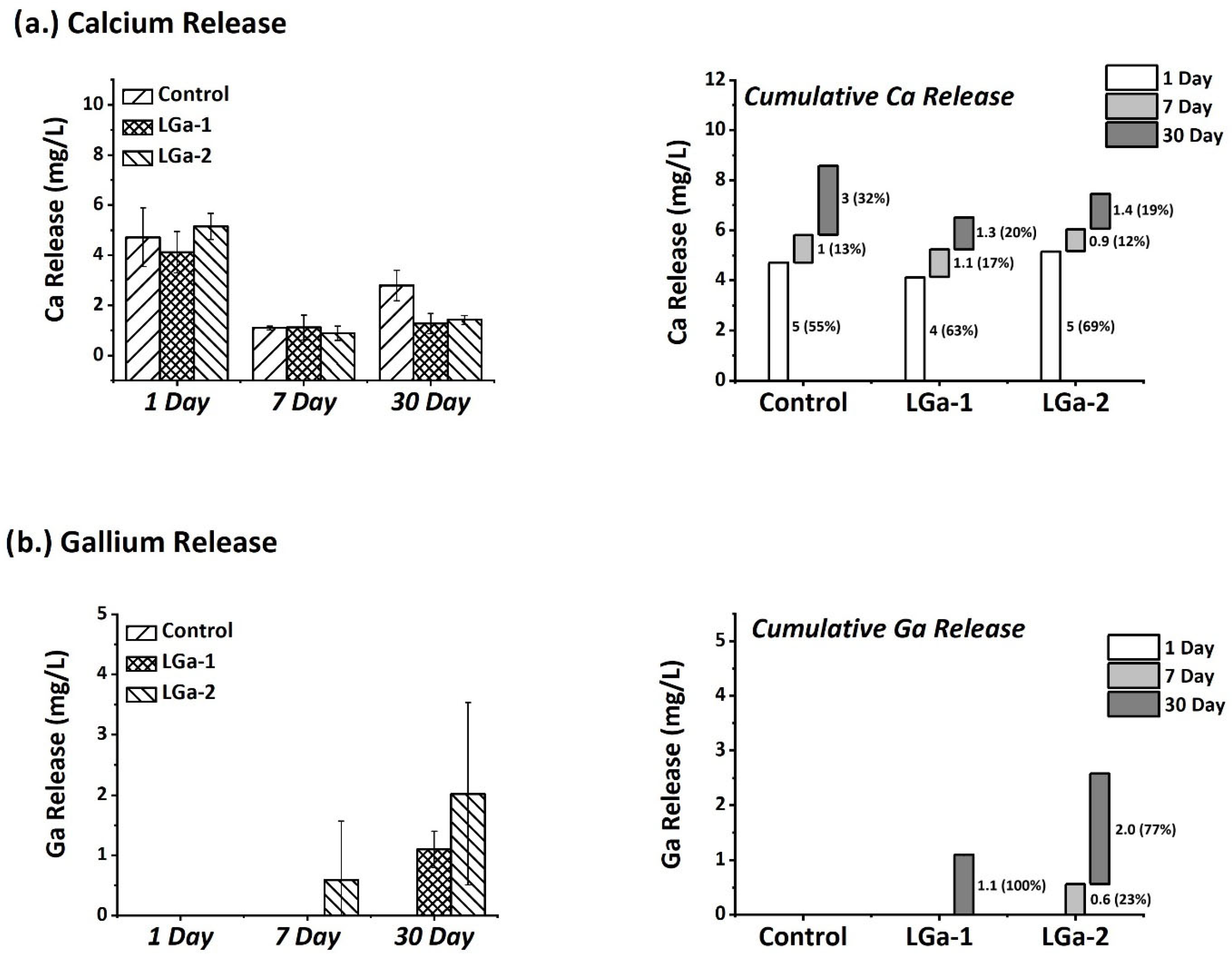
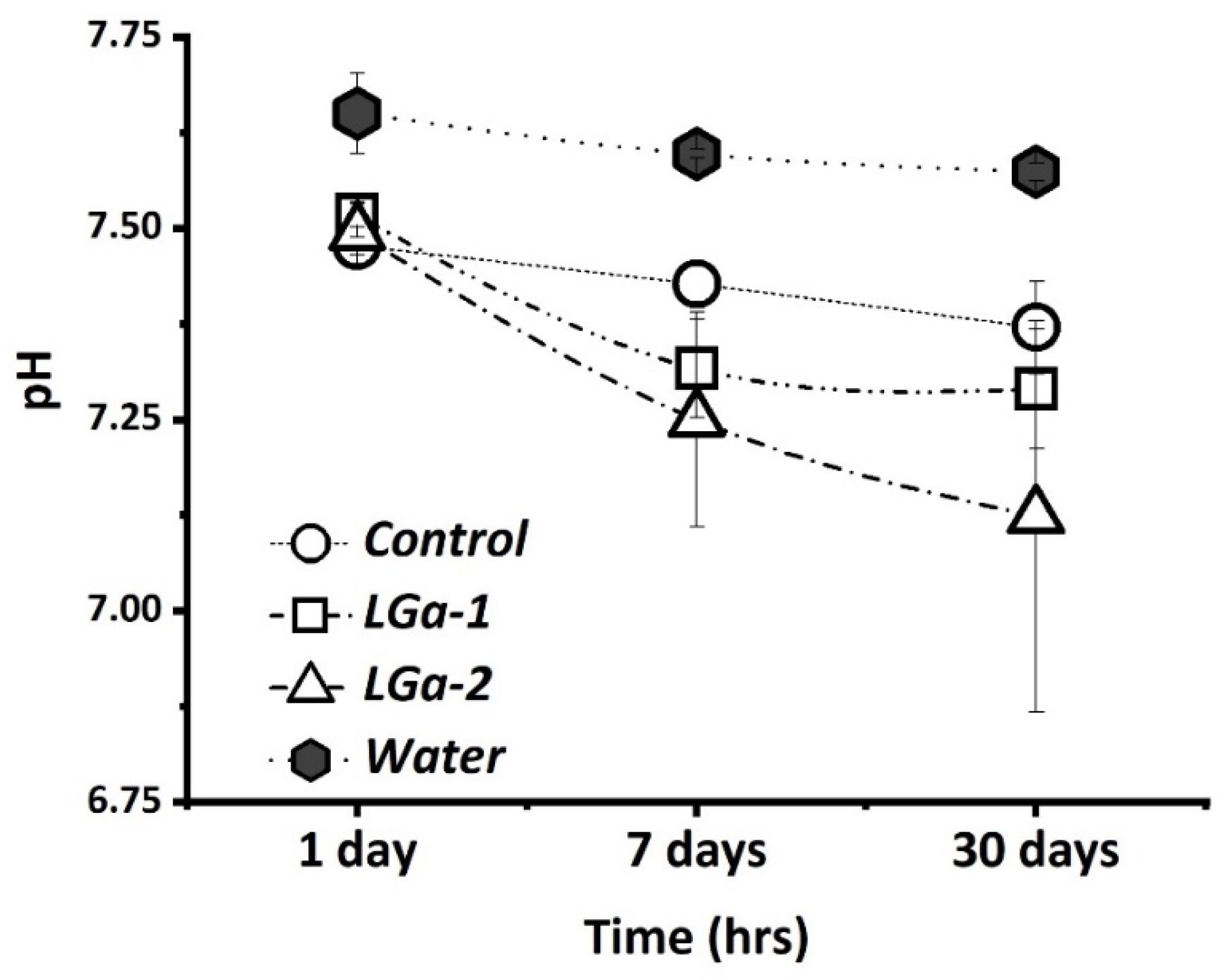
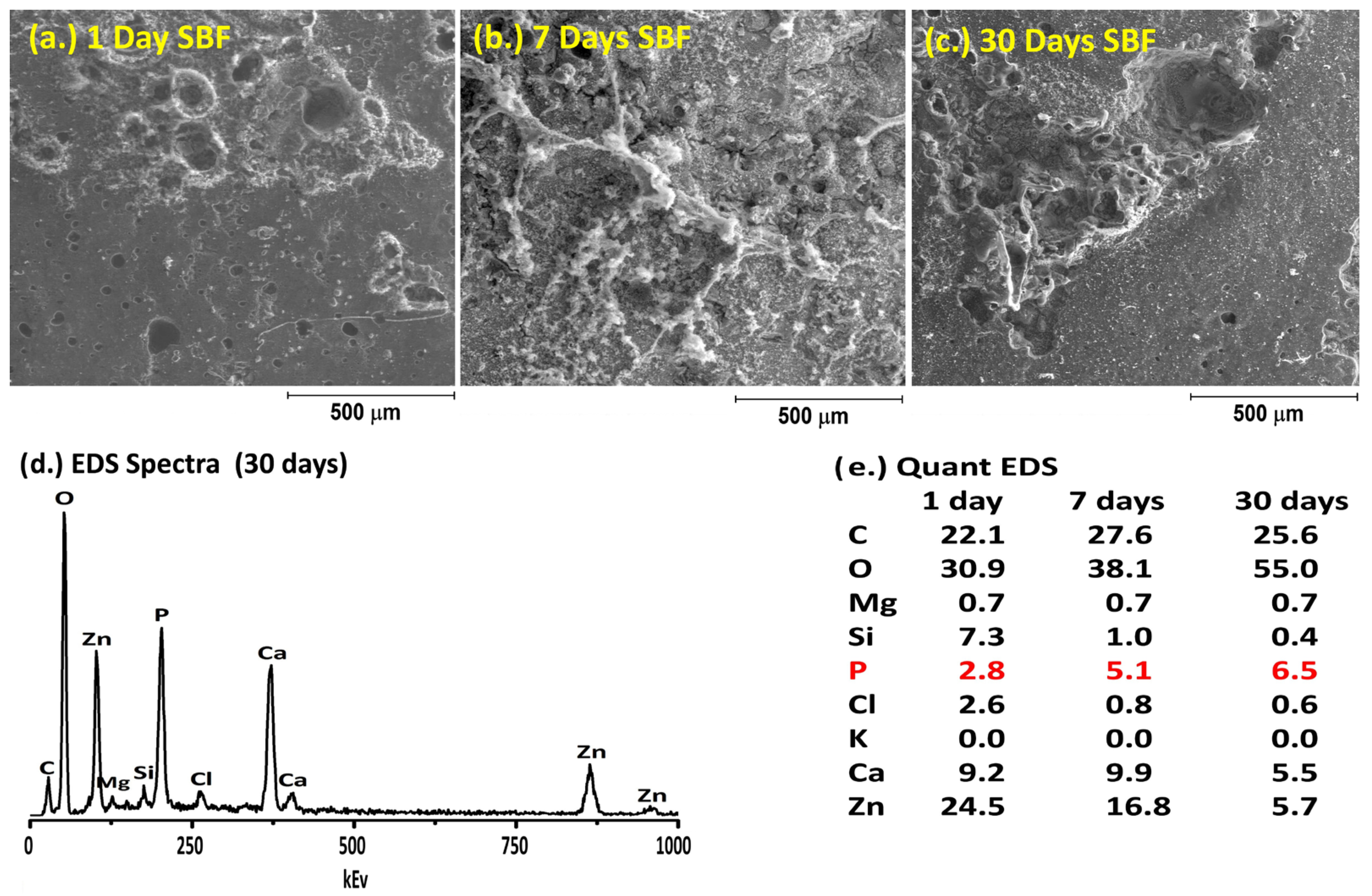
| Control | LGa-1 | LGa-2 | |
|---|---|---|---|
| SiO2 | 0.48 | 0.48 | 0.48 |
| Ga2O3 | 0.00 | 0.08 | 0.16 |
| ZnO | 0.40 | 0.32 | 0.24 |
| CaO | 0.12 | 0.12 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Placek, L.M.; Perry, D.L.; Towler, M.R.; Wren, A.W. Gallium-Containing Bioactive Glasses: Their Influence on Ion Release and the Bioactivity of Resulting Glass Polyalkenoate Cements. Appl. Sci. 2025, 15, 7756. https://doi.org/10.3390/app15147756
Placek LM, Perry DL, Towler MR, Wren AW. Gallium-Containing Bioactive Glasses: Their Influence on Ion Release and the Bioactivity of Resulting Glass Polyalkenoate Cements. Applied Sciences. 2025; 15(14):7756. https://doi.org/10.3390/app15147756
Chicago/Turabian StylePlacek, Lana Margaret, Danielle Lee Perry, Mark Robert Towler, and Anthony William Wren. 2025. "Gallium-Containing Bioactive Glasses: Their Influence on Ion Release and the Bioactivity of Resulting Glass Polyalkenoate Cements" Applied Sciences 15, no. 14: 7756. https://doi.org/10.3390/app15147756
APA StylePlacek, L. M., Perry, D. L., Towler, M. R., & Wren, A. W. (2025). Gallium-Containing Bioactive Glasses: Their Influence on Ion Release and the Bioactivity of Resulting Glass Polyalkenoate Cements. Applied Sciences, 15(14), 7756. https://doi.org/10.3390/app15147756






