Upcycling Luffa cylindrica (Luffa Sponge) Seed Press Cake as a Functional Ingredient for Meat Substitute Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Characterization of Seed Press Cake of L. cylindrica Seeds Powder (LP), Tapioca Flour (TF) and Their Blends
2.2.1. Techno-Functional Characteristics
2.2.2. Reducing Sugars and Antioxidant Characteristics
2.2.3. Microbial Characterization
2.3. Meat Substitute Formulations
2.3.1. Formulations
2.3.2. Physicochemical and Sensory Analyses of Meat Substitute Formulation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Seed Press Cake of L. cylindrica Seeds Powder (LP), Tapioca Flour (TF) and Their Blends
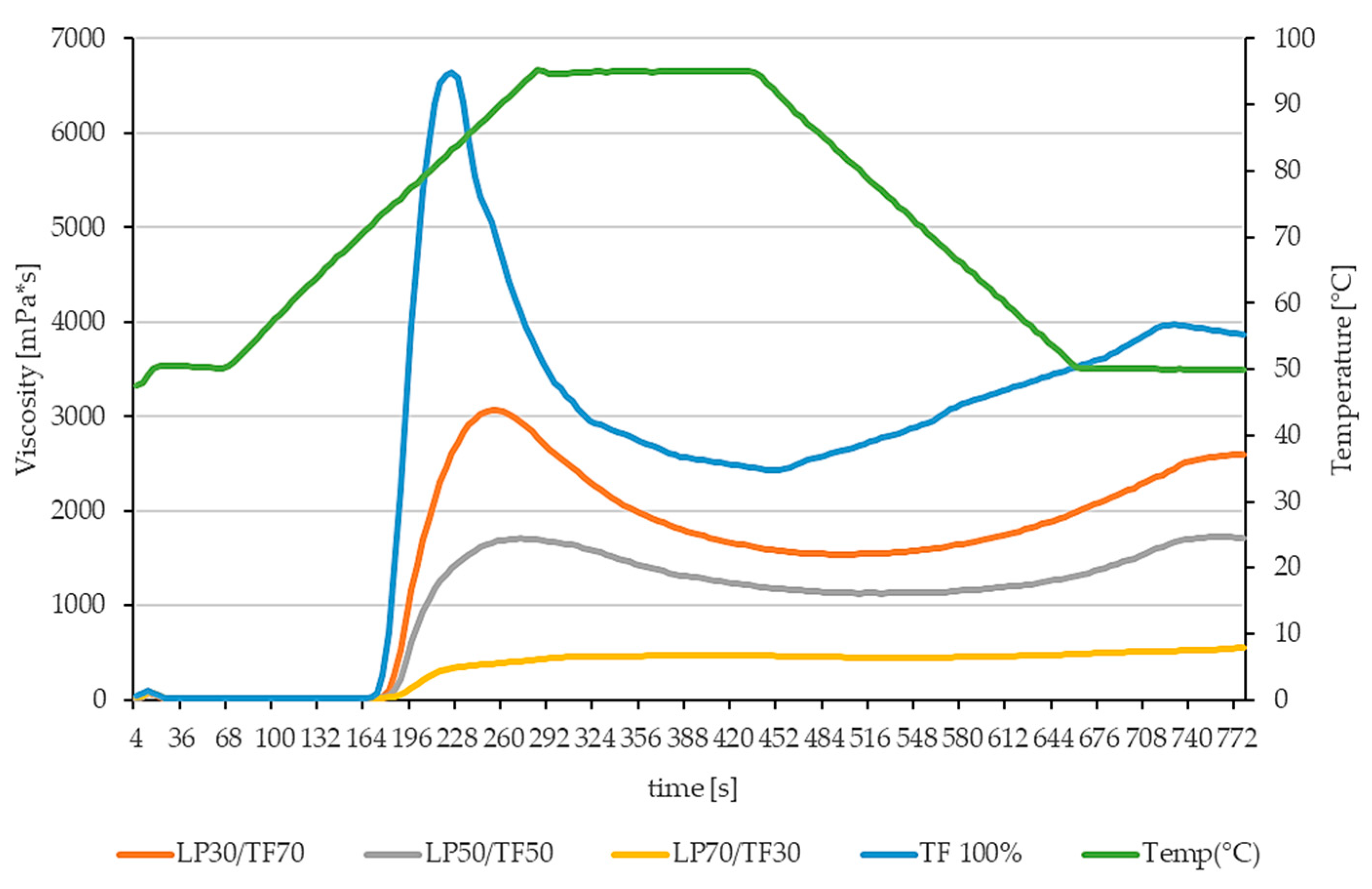
3.1.1. Techno-Functional Characteristics
3.1.2. Reducing Sugars and Antioxidant Characteristics
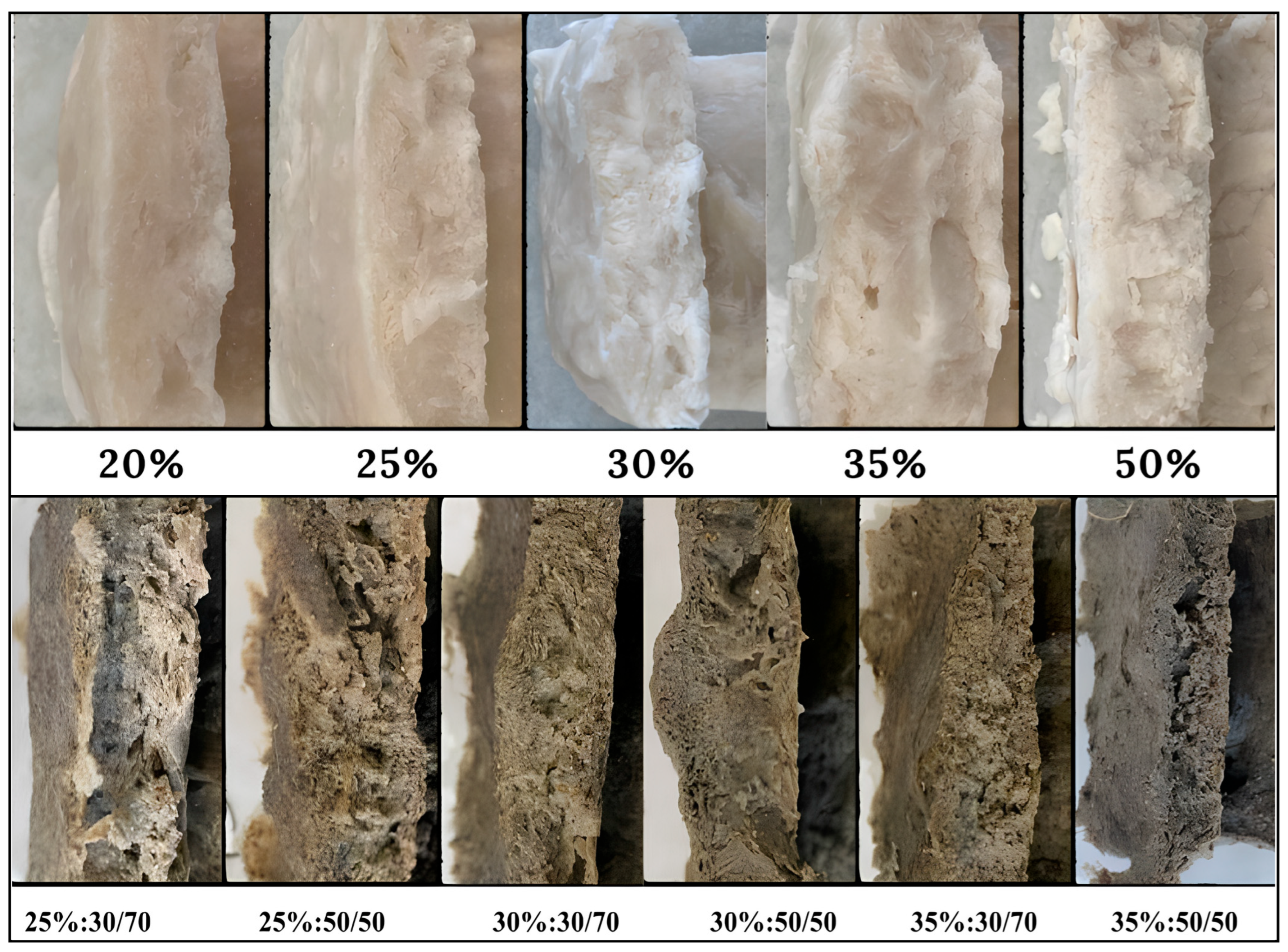
3.2. Meat Substitute Formulations
3.3. Meat Substitute Quality
3.4. Limitations and Future Prospects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Partap, S.; Kumar, A.; Sharma, N.K.; Jha, K.K. Luffa cylindrica: An Important Medicinal Plant. J. Nat. Prod. Plant Resour. 2012, 2, 127–134. [Google Scholar]
- Azeez, M.A.; Bello, O.S.; Adedeji, A.O. Traditional and Medicinal Uses of Luffa cylindrica: A Review. J. Med. Plants Stud. 2013, 1, 102–111. [Google Scholar]
- Ogunyemi, T.C.; Ekuma, C.M.; Egwu, J.E.; Abbey, D.M. Proximate and Mineral Composition of Sponge Gourd (Luffa cylindrica) Seed Grown in South-Western Nigeria. J. Sci. Res. Rep. 2020, 26, 61–67. [Google Scholar] [CrossRef]
- Elemo, G.N.; Elemo, B.O.; Erukainure, O.L. Characterization of Sponge Gourd (Luffa aegyptiaca Mill.) Seed Oil. J. Trop. Agric. 2011, 49, 128–130. [Google Scholar]
- Zhao, G.; Wang, M.; Gan, Y.; Gong, H.; Li, J.; Zheng, X.; Liu, X.; Zhao, S.; Luo, J.; Wu, H. Identification of Suitable Reference Genes for Quantitative Reverse Transcription PCR in Luffa (Luffa cylindrica). Physiol. Mol. Biol. Plants 2022, 28, 737–747. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, G.; Gong, H.; Li, J.; Luo, C.; He, X.; Luo, S.; Zheng, X.; Liu, X.; Guo, J.; et al. A High-Quality Sponge Gourd (Luffa cylindrica) Genome. Hortic. Res. 2020, 7, 128. [Google Scholar] [CrossRef]
- Patel, S.B.; Ghane, S.G. Phyto-Constituents Profiling of Luffa echinata and In Vitro Assessment of Antioxidant, Anti-Diabetic, Anticancer and Anti-Acetylcholine Esterase Activities. Saudi J. Biol. Sci. 2021, 28, 3835–3846. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Constituents and Pharmacology of Luffa cylindrica—A Review. Int. J. Pharm. Rev. Res. 2019, 9, 68–79. [Google Scholar]
- Muthumani, P.; Meera, R.; Mary, S.M.; Mathew, J.; Devi, P.; Kameswari, B.; Eswara, P. Phytochemical Screening and Anti-Inflammatory, Bronchodilator and Antimicrobial Activities of the Seeds of Luffa cylindrica. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 11–22. [Google Scholar]
- Adewuyi, A.; Oderinde, R.A. Analysis of the Lipids and Molecular Speciation of the Triacylglycerol of the Oils of Luffa cylindrica and Adenopus breviflorus. CyTA J. Food 2012, 10, 313–320. [Google Scholar] [CrossRef]
- Anaun, T.E.; Ogundele, O.D. Physicochemical Analysis and Functional Properties of Sponge Gourd (Luffa cylindrica Linn.) Seed Oil Obtained from Owo, Ondo State, Nigeria. Achiev. J. Sci. Res. 2021, 3, 97–104. [Google Scholar]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of Seed Oil Cakes as Industrial Co-Streams for Production of Innovative Bioplastics: A Review. Trends Food Sci. Technol. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Wang, T.; Masedunskas, A.; Willett, W.; Fontana, L. Vegetarian and Vegan Diets: Benefits and Drawbacks. Eur. Heart J. 2023, 44, 3423–3439. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.T.; Mateus, N.; de Freitas, V.; Fernandes, A. Plant-Based Meat Analogues: Exploring Proteins, Fibers and Polyphenolic Compounds as Functional Ingredients for Future Food Solutions. Foods 2024, 13, 2303. [Google Scholar] [CrossRef]
- Benković, M.; Jurinjak Tušek, A.; Sokač Cvetnić, T.; Jurina, T.; Valinger, D.; Gajdoš Kljusurić, J. An Overview of Ingredients Used for Plant-Based Meat Analogue Production and Their Influence on Structural and Textural Properties of the Final Product. Gels 2023, 9, 921. [Google Scholar] [CrossRef]
- Nedviha, S.; Harasym, J. Functional and Antioxidative Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Flours Binary Blends. Foods 2024, 13, 596. [Google Scholar] [CrossRef]
- Kaushal, P.; Kumar, V.; Sharma, H.K. Comparative Study of Physicochemical, Functional, Antinutritional and Pasting Properties of Taro (Colocasia esculenta), Rice (Oryza sativa) Flour, Pigeonpea (Cajanus cajan) Flour and Their Blends. LWT 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Kiiru, S.; Ng’ang’a, J.; Konyole, S.; Roos, N.; Hetzer, B.; Marel, A.; Kinyuru, J. Physicochemical Characterisation and Impact of Gryllus bimaculatus Addition on Gluten-Free Flour Blends. Int. J. Food Sci. Technol. 2024, 59, 4620–4634. [Google Scholar] [CrossRef]
- Wojciechowicz-Budzisz, A.; Skřivan, P.; Sluková, M.; Švec, I.; Pejcz, E.; Stupák, M.; Czubaszek, A.; Harasym, J. Comprehen-sive Characterization of Micronized Wholemeal Flours: Investigating Technological Properties across Various Grains. Foods 2024, 13, 39. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Bhajan, C.; Soulange, J.G.; Sanmukhiya, V.M.R.; Olędzki, R.; Harasym, J. Phytochemical Composition and Antioxidant Properties of Tambourissa ficus, a Mauritian Endemic Fruit. Appl. Sci. 2023, 13, 10908. [Google Scholar] [CrossRef]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of β-Glucuronidase-Positive Escherichia Coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-chloro-3-indolyl β-D-glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- Xiao, T.; Sun, M.; Cao, S.; Hao, J.; Rao, H.; Zhao, D.; Liu, X. Enhancing Water Retention and Mechanisms of Citrus and Soya Bean Dietary Fibres in Pre-Fermented Frozen Dough. Food Chem. X 2024, 22, 101269. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.N.; Ramachandra Swamy, S.R.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Kumar, S.; Tharanathan, R.N. Grinding Characteristics and Hydration Properties of Coconut Residue: A Source of Dietary Fiber. J. Food Eng. 2006, 72, 281–286. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of Spent Coffee Grounds as Food Ingredient in Bakery Products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Nuwamanya, E.; Baguma, Y.; Emmambux, M.N.; Rubaihayo, P. Crystalline and Pasting Properties of Cassava Starch Are Influenced by Its Molecular Properties. Afr. J. Food Sci. 2010, 4, 008–015. [Google Scholar]
- Abdul-Hamid, A.; Luan, Y.S. Functional Properties of Dietary Fibre Prepared from Defatted Rice Bran. Food Chem. 2000, 68, 15–19. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich By-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Zheng, Q.; Hu, N.; Zheng, M.; Liu, J. Effects of Soluble and Insoluble Dietary Fiber from Corn Bran on Pasting, Thermal, and Structural Properties of Corn Starch. Starch/Stärke 2022, 74, 2100254. [Google Scholar] [CrossRef]
- Kalaydzhiev, H.; Ivanova, P.; Silva, C.L.M.; Chalova, V.I. Functional Properties of Protein Isolate and Acid Soluble Protein-Rich Ingredient Co-Produced from Ethanol-Treated Industrial Rapeseed Meal. Pol. J. Food Nutr. Sci. 2019, 69, 129–136. [Google Scholar] [CrossRef]
- Vingadassalon, A.; Pejcz, E.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Groton, K.; Aurore, G.; Harasym, J. Terminalia catappa Kernel Flour Characterization as a Functional and Bioactive Ingredient for Cookies Formulation. Appl. Sci. 2024, 14, 11201. [Google Scholar] [CrossRef]
- Xu, B.; Chen, X.; Zhao, H.; Zhang, Z.; Liu, H. Preparation of Nano-Pico Droplets Using an Open Fibrous System. Droplet 2023, 2, e27. [Google Scholar] [CrossRef]
- Sefer, O.A.; Ege, G.K.; Saltik, D.; Yuce, H. A Novel Natural Fibers-Based Bio-Composite Prepared from Silk Fibroin and Luffa cylindrica. J. Polym. Environ. 2025, 33, 1459–1468. [Google Scholar] [CrossRef]
- Neves, A.C.C.; Lopes, F.; Simonassi, N.; Vieira, C.; Monteiro, S.N. Properties of Luffa cylindrica Mats Reinforced Castor Oil-Based Polyurethane Composite. Polymers 2022, 14, 5533. [Google Scholar] [CrossRef] [PubMed]
- Kalusuraman, G.; Siva, I.; Munde, Y.; Selvan, C.; Kumar, S.A.; Amico, S.C. Dynamic-Mechanical Properties as a Function of Luffa Fibre Content and Adhesion in a Polyester Composite. Polym. Test. 2020, 89, 106538. [Google Scholar] [CrossRef]
- Chipón, J.; Ramírez, K.; Morales, J.; Díaz-Calderón, P. Rheological and Thermal Study about the Gelatinization of Different Starches (Potato, Wheat and Waxy) in Blend with Cellulose Nanocrystals. Polymers 2022, 14, 1560. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Cao, J.; Ouyang, Z.; Pang, Z. Pasting, Rheological, and Tribological Properties of Rice Starch and Oat Flour Mixtures at Different Proportions. Foods 2022, 11, 2115. [Google Scholar] [CrossRef]
- Tian, Y.; Qu, J.; Zhou, Q.; Ding, L.; Cui, Y.; Blennow, A.; Zhong, Y.; Liu, X. High Pressure/Temperature Pasting and Gelling of Starch Related to Multilevel Structure—Analyzed with RVA 4800. Carbohydr. Polym. 2022, 295, 119858. [Google Scholar] [CrossRef]
- Liu, H.-M.; Miao, W.-B.; Wang, R.; Chen, N.; Ma, S.; Wang, X.-D. Improvement of Functional and Rheological Features of Tigernut Tuber Starch by Using Gum Derived from Chinese Quince Seeds. LWT 2021, 143, 111180. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.-M.; Min, G.; Qiao, D.; Zhang, B.; Niu, M.; Jia, C.; Xu, Y.; Lin, Q. Starch-Protein Interplay Varies the Multi-Scale Structures of Starch Undergoing Thermal Processing. Int. J. Biol. Macromol. 2021, 181, 179–187. [Google Scholar] [CrossRef]
- Riaz, A.; Pasha, I. Correlation Studies of Starch Pasting Properties Determined Through Rapid Visco Analyzer. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2021, 64, 192–197. [Google Scholar] [CrossRef]
- Chen, T.; Wei, C.-K.; Li, T.; Zhang, H.-L.; Ni, Z.-J.; Khan, M.R.; Wei, Z.-J. Effects of Reducing Sugars on the Structural and Flavor Properties of the Maillard Reaction Products of Lycium barbarum Seed Meal. Foods 2023, 12, 4346. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.P. Chemically Reducing Properties of Maillard Reaction Intermediates. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–40. [Google Scholar] [CrossRef]
- Adubiaro, H.; Ogunbusola, M.; Olaleye, A. The Amino Acids Composition of Smooth Loofah (Luffa cylindrica L.), Roselle (Hibiscus sabdariffa) and Sesame (Sesamum indicum) Seeds. FUW Trends Sci. Technol. J. 2017, 2, 285–288. [Google Scholar]
- Pereira, J.; Hu, H.; Xing, L.; Zhang, W.; Zhou, G. Influence of Rice Flour, Glutinous Rice Flour, and Tapioca Starch on the Functional Properties and Quality of an Emulsion-Type Cooked Sausage. Foods 2020, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.R.; Ifesan, B.T.; Ifesan, B.O.T. Nutritional Characteristics and Sensory Evaluation of Tapioca Supplemented with Fermented and Germinated Moringa (Moringa oleifera) Seed Flour. GSC Biol. Pharm. Sci. 2023, 24, 177–186. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, S.; Shang, X.; Xie, X.; Zeng, W.; Lu, L.; Kong, Q.; Yan, H. Metabolomic and Transcriptomic Profiling Reveals Distinct Nutritional Properties of Cassavas with Different Flesh Color. Food Chem. X 2021, 2, 100016. [Google Scholar] [CrossRef]
- Khasanah, Y.; Indrianingsih, A.W.; Triwitono, P.; Murdiati, A. Antioxidant, Total Phenolic Content and Physicochemical Properties of Modified Cassava Flour. IOP Conf. Ser. Earth Environ. Sci. 2023, 1241, 012094. [Google Scholar] [CrossRef]
- Zanchi, D.; Vernhet, A.; Poncet-Legrand, C.; Cartalade, D.; Tribet, C.; Schweins, R.; Cabane, B. Colloidal Dispersions of Tannins in Water–Ethanol Solutions. Langmuir 2007, 23, 9949–9959. [Google Scholar] [CrossRef]
- Rai, S.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Characterization of Saponins from the Leaves and Stem Bark of Jatropha curcas L. for Surface-Active Properties. Heliyon 2023, 9, e15807. [Google Scholar] [CrossRef]
- Chavez, A.L.; Bedoya, J.M.; Sánchez, T.; Iglesias, C.; Ceballos, H.; Roca, W. Iron, Carotene, and Ascorbic Acid in Cassava Roots and Leaves. Food Nutr. Bull. 2000, 21, 410–413. [Google Scholar] [CrossRef]
- Widowati, S.; Misgiyarta; Setyawan, N.; Herawati, H.; Widaningrum; Suhirman, S.; Noerwijati, K.; Budiono, R.; Astuti, S.D.; Tjahjohutomo, R.; et al. Physicochemical and Functional Properties of Cassava Flour Produced by Controlled Fermentation Using Mixed Culture from Various Bacteria and Yeast. J. Agric. Food Res. 2025, 19, 101684. [Google Scholar] [CrossRef]
- Kao, T.H.; Huang, C.W.; Chen, B.H. Functional Components in Luffa cylindrica and Their Effects on Anti-Inflammation of Macrophage Cells. Food Chem. 2012, 135, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2004. [Google Scholar]
- Zając, A.; Dymińska, L.; Lorenc, J.; Hanuza, J. Fourier Transform Infrared and Raman Spectroscopy Studies of the Time-Dependent Changes in Chicken Meat as a Tool for Recording Spoilage Processes. Food Anal. Methods 2017, 10, 640–648. [Google Scholar] [CrossRef]
- Hosen, A.; Al-Mamun, A.; Robin, M.A.; Habiba, U.; Sultana, R. Maillard Reaction: Food Processing Aspects. J. Food Nutr. Res. 2021, 4, 44–52. [Google Scholar] [CrossRef]
- Onigemo, M.A.; Dairo, F.A.S.; Oso, Y.A.A. Amino Acids Profile of Loofah Gourd Luffa cylindrica (M.J. Roem) Seeds Subjected to Different Heat Processing Methods. Niger. J. Anim. Prod. 2020, 47, 280–288. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation—Structural and Chromatic Aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Fratianni, A.; Cinquanta, L.; Panfili, G. Degradation of Carotenoids in Orange Juice during Microwave Heating. LWT-Food Sci. Technol. 2010, 43, 867–871. [Google Scholar] [CrossRef]
- Thirathumthavorn, D.; Trisuth, T. Gelatinization and Retrogradation Properties of Native and Hydroxypropylated Crosslinked Tapioca Starches with Added Sucrose and Sodium Chloride. Int. J. Food Prop. 2008, 11, 858–864. [Google Scholar] [CrossRef][Green Version]
- Barbut, S. The Science of Poultry and Meat Processing; University of Guelph: Guelph, ON, Canada, 2015; Chapter 18. [Google Scholar]
- Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Çubukçu, H.C.; Kılıçaslan, N.S.D.; Durak, İ. Different Effects of Heating and Freezing Treatments on the Antioxidant Properties of Broccoli, Cauliflower, Garlic and Onion: An Experimental In Vitro Study. Sao Paulo Med. J. 2019, 137, 407–413. [Google Scholar] [CrossRef]
- Muramatsu, D.; Uchiyama, H.; Higashi, H.; Kida, H.; Iwai, A. Effects of Heat Degradation of Betanin in Red Beetroot (Beta vulgaris L.) on Biological Activity and Antioxidant Capacity. PLoS ONE 2023, 18, e0286255. [Google Scholar] [CrossRef]
- Morales, F.J.; Jiménez-Pérez, S. Free Radical Scavenging Capacity of Maillard Reaction Products as Related to Colour and Fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef]
- Alshikh, N.; Costa de Camargo, A.; Shahidi, F. Phenolics of Selected Lentil Cultivars: Antioxidant Activities and Inhibition of Low-Density Lipoprotein and DNA Damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Fatty Acid, Carotenoid and Tocopherol Compositions of 20 Canadian Lentil Cultivars and Synergistic Contribution to Antioxidant Activities. Food Chem. 2014, 161, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzyńska, K. Old-Fashioned, but Still a Superfood—Red Beets as a Rich Source of Bioactive Compounds. Appl. Sci. 2023, 13, 7445. [Google Scholar] [CrossRef]
- Appiani, M.; Cattaneo, C.; Laureati, M. Sensory properties and consumer acceptance of plant-based meat, dairy, fish and eggs analogs: A systematic review. Front. Sustain. Food Syst. 2023, 7, 1268068. [Google Scholar] [CrossRef]
- Manippa, V.; Padulo, C.; van der Laan, L.N.; Brancucci, A. Gender differences in food choice: Effects of superior temporal sulcus stimulation. Front. Hum. Neurosci. 2017, 11, 597. [Google Scholar] [CrossRef]
- Feraco, A.; Armani, A.; Amoah, I.; Guseva, E.; Camajani, E.; Gorini, S.; Strollo, R.; Padua, E.; Caprio, M.; Lombardo, M. Assessing gender differences in food preferences and physical activity: A population-based survey. Front. Nutr. 2024, 11, 1348456. [Google Scholar] [CrossRef]
- Fiorentini, M.; Kinchla, A.J.; Nolden, A.A. Role of sensory evaluation in consumer acceptance of plant-based meat analogs and meat extenders: A scoping review. Foods 2020, 9, 1334. [Google Scholar] [CrossRef]
- Szenderák, J.; Fróna, D.; Rákos, M. Consumer Acceptance of Plant-Based Meat Substitutes: A Narrative Review. Foods 2022, 11, 1274. [Google Scholar] [CrossRef]
- Kumari, S.; Alam, A.N.; Hossain, M.J.; Lee, E.Y.; Hwang, Y.H.; Joo, S.T. Sensory Evaluation of Plant-Based Meat: Bridging the Gap with Animal Meat, Challenges and Future Prospects. Foods 2023, 13, 108. [Google Scholar] [CrossRef]
- Pointke, M.; Ohlau, M.; Risius, A.; Pawelzik, E. Plant-Based Only: Investigating Consumers’ Sensory Perception, Motivation, and Knowledge of Different Plant-Based Alternative Products on the Market. Foods 2022, 11, 2339. [Google Scholar] [CrossRef]
- Sogari, G.; Caputo, V.; Petterson, A.J.; Mora, C.; Boukid, F. A sensory study on consumer valuation for plant-based meat alternatives: What is liked and disliked the most? Food Res. Int. 2023, 169, 112813. [Google Scholar] [CrossRef]

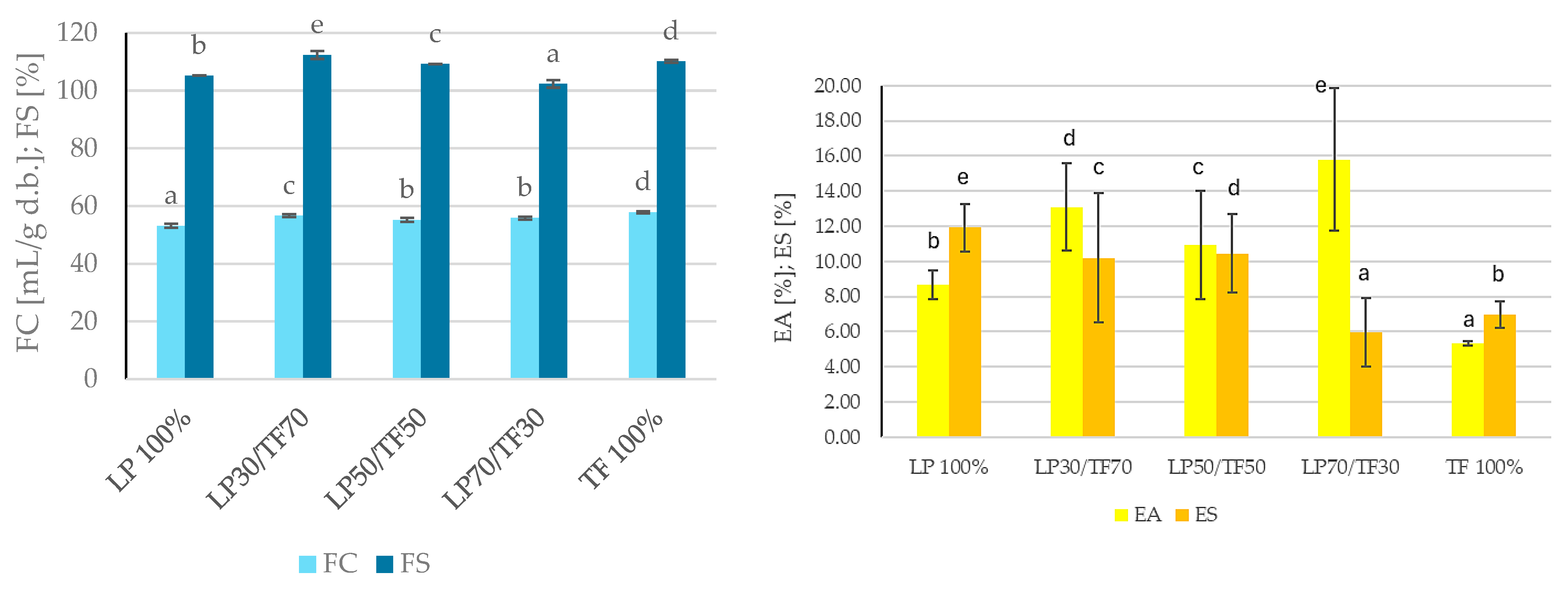
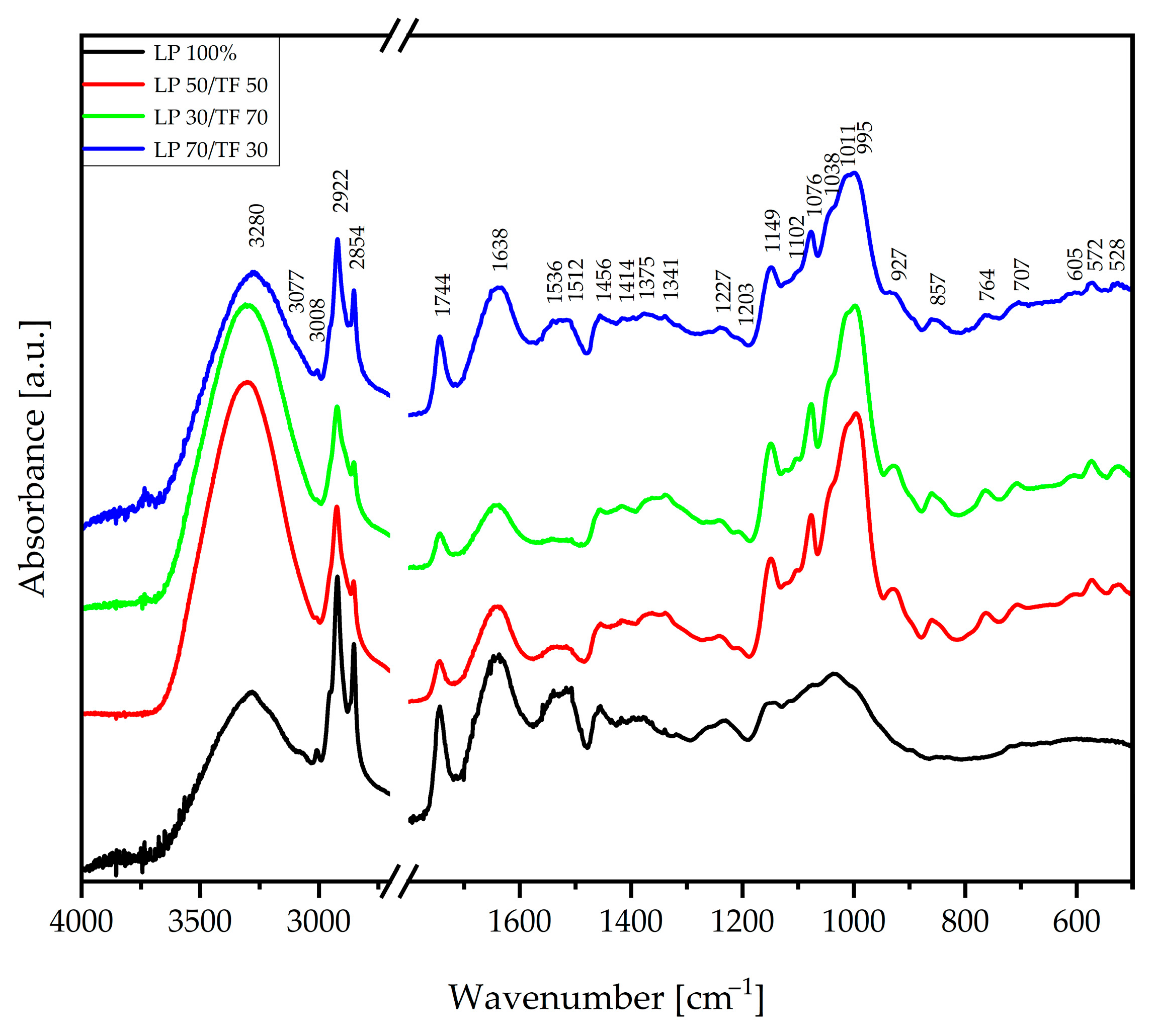
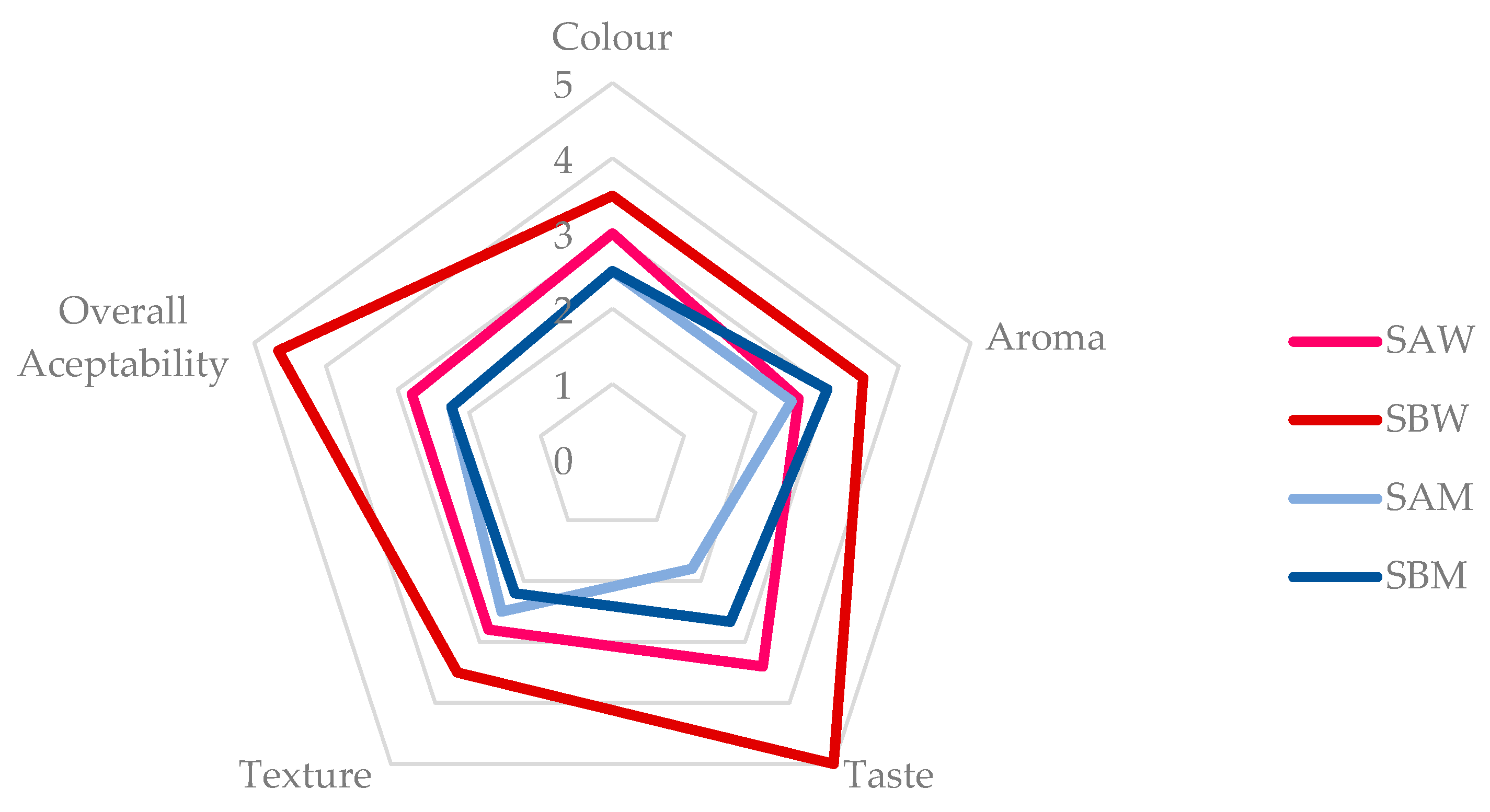
| Sample | WHC [g H2O/g d.b.] | WAC [g H2O/g d.b.] | OAC [g oil/g d.b.] | HLI [g H2O/g oil] | WAI [g H2O/g d.b.] | WSI [g/100 g d.b.] | SP [g H2O/g d.b.] |
|---|---|---|---|---|---|---|---|
| LP 100% | 3.62 ± 0.44 d | 2.88 ± 0.02 e | 1.69 ± 0.02 a | 1.75 ± 0.03 e | 3.59 ± 0.09 a | 7.45 ± 0.64 d | 3.25 ± 0.08 a |
| LP30/TF70 | 3.21 ± 0.31 d | 2.10 ± 0.01 b | 1.81 ± 0.08 b | 1.22 ± 0.01 b | 7.83 ± 0.23 d | 3.37 ± 0.30 b | 7.08 ± 0.28 d |
| LP50/TF50 | 2.59 ± 0.20 c | 2.18 ± 0.02 c | 1.82 ± 0.05 b | 1.29 ± 0.04 c | 6.33 ± 0.45 c | 5.49 ± 0.44 c | 6.24 ± 4.68 d |
| LP70/TF30 | 2.21 ± 0.11 b | 2.47 ± 0.13 d | 1.74 ± 0.03 b | 1.51 ± 0.09 d | 5.05 ± 0.10 b | 6.07 ± 0.27 c | 4.70 ± 0.35 b |
| TF 100% | 1.76 ± 0.12 a | 1.79 ± 0.03 a | 2.08 ± 0.06 c | 0.97 ± 0.01 a | 10.90 ± 0.15 e | 0.88 ± 0.38 a | 9.74 ± 0.19 e |
| Sample | Peak Viscosity | Trough Viscosity | Breakdown Viscosity | Final Viscosity | Setback Viscosity | Peak Time | Pasting Temperature |
|---|---|---|---|---|---|---|---|
| [mPa·s] | [min] | [°C] | |||||
| LP 100% | — a | — a | — a | — a | — a | — a | — a |
| LP30/TF70 | 3073 ± 98 d | 1540 ± 65 d | 1534 ± 34 d | 2594 ± 85 d | 1055 ± 150 d | 4.2 ± 0.00 b | 74.33 ± 0.11 c |
| LP50/TF50 | 1415 ± 294 c | 987 ± 142 c | 428 ± 152 c | 1516 ± 200 c | 528 ± 60 c | 4.7 ± 0.17 c | 73.92 ± 0.60 c |
| LP70/TF30 | 475 ± 20 b | 447 ± 12 b | 28 ± 8 b | 555 ± 19 b | 108 ± 7 b | 6.6 ± 0.13 d | 75.90 ± 0.07 d |
| TF 100% | 6644 ± 45 e | 2431 ± 28 e | 4213 ± 72 e | 3870 ± 234 e | 1439 ± 206 e | 3.7 ± 0.00 b | 72.65 ± 0.07 b |
| Sample | Reducing Sugars [mg GLE/g DM] | DPPH [mg TE/g DM] | ABTS [mg TE/g DM] | FRAP [mg FeSO4/g DM] | |||
|---|---|---|---|---|---|---|---|
| EtOH | H2O | EtOH | H2O | EtOH | H2O | ||
| LP 100% | 8.05 ± 0.68 d | 4.58 ± 0.18 a | 4.25 ± 0.25 a | 4.56 ± 0.04 a | 3.67 ± 0.70 a | 0.101 ± 0.007 b | 0.126 ± 0.016 c |
| LP30/TF70 | 5.32 ± 1.31 b | 5.68 ± 0.07 c | 4.55 ± 0.51 a | 5.26 ± 0.02 d | 5.72 ± 0.33 d | 0.046 ± 0.006 a | 0.090 ± 0.023 b |
| LP50/TF50 | 4.13 ± 0.37 a | 5.15 ± 0.05 b | 4.91 ± 0.02 a | 5.19 ± 0.07 c | 5.11 ± 0.01 c | 0.053 ± 0.015 a | 0.050 ± 0.000 a |
| LP70/TF30 | 5.10 ± 0.92 b | 5.04 ± 0.13 b | 4.41 ± 0.15 a | 5.06 ± 0.07 b | 4.66 ± 0.12 b | 0.070 ± 0.001 ab | 0.109 ± 0.010 bc |
| TF 100% | 6.90 ± 0.20 c | 5.66 ± 0.13 c | 6.27 ± 0.06 b | 6.11 ± 0.18 e | 6.33 ± 0.06 e | 0.072 ± 0.038 ab | 0.041 ± 0.005 a |
| sample | *** | *** | * | ||||
| solvent | ** | ns | ns | ||||
| sample * solvent | ** | * | ns | ||||
| 2 | 7 | 12 | ||||
|---|---|---|---|---|---|---|
 |  |  | ||||
| Sample | L* | a* | b* | Cutting Force [N] | ||
| Meat Substitute | ||||||
| 2 days storage | 40.36 ± 3.22 b | 11.53 ± 1.19 b | 10.55 ± 1.49 a | 5.62 ± 1.05 a | ||
| 7 days storage | 32.20 ± 1.67 a | 10.70 ± 1.00 b | 10.59 ± 1.15 a | 11.82 ± 4.41 b | ||
| 12 days storage | 39.88 ± 4.45 b | 8.79 ± 0.83 a | 12.87 ± 0.78 b | 20.03 ± 2.40 c | ||
| Controls | ||||||
| LP30/TF70 | 51.23 ± 0.21 b | −1.47 ± 0.08 a | 12.17 ± 0.00 a | --- | ||
| Pork | 46.73 ± 0.17 a | 10.79 ± 0.21 b | 19.81 ± 0.00 b | ---- | ||
| Reducing Sugars [mg GLE/g DM] | DPPH [mg TE/g DM] | ABTS [mg TE/g DM] | FRAP [mg FeSO4/g DM] | |||
| LP30/TF70 | 5.32 ± 1.31 b | 5.68 ± 0.07 b(E) 4.55 ± 0.51 a (W) | 5.26 ± 0.02 a (E) 5.72 ± 0.33 a (W) | 0.05 ± 0.02 a (E) 0.10 ± 0.02 b (W) | ||
| Meat Substitute | 1.34 ± 0.09 a | 0.99 ± 0.03 b (E) 0.63 ± 0.02 a (W) | 0.82 ± 0.01 b (E) 0.54 ± 0.06 a (W) | 0.58 ± 0.02 b (E) 0.14 ± 0.00 a (W) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, G.; Josy, T.; Pejcz, E.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Górska, K.; Zając, A.; Aurore, G.; Harasym, J. Upcycling Luffa cylindrica (Luffa Sponge) Seed Press Cake as a Functional Ingredient for Meat Substitute Formulations. Appl. Sci. 2025, 15, 7753. https://doi.org/10.3390/app15147753
Lawrence G, Josy T, Pejcz E, Wojciechowicz-Budzisz A, Olędzki R, Górska K, Zając A, Aurore G, Harasym J. Upcycling Luffa cylindrica (Luffa Sponge) Seed Press Cake as a Functional Ingredient for Meat Substitute Formulations. Applied Sciences. 2025; 15(14):7753. https://doi.org/10.3390/app15147753
Chicago/Turabian StyleLawrence, Génica, Thaïna Josy, Ewa Pejcz, Agata Wojciechowicz-Budzisz, Remigiusz Olędzki, Katarzyna Górska, Adam Zając, Guylène Aurore, and Joanna Harasym. 2025. "Upcycling Luffa cylindrica (Luffa Sponge) Seed Press Cake as a Functional Ingredient for Meat Substitute Formulations" Applied Sciences 15, no. 14: 7753. https://doi.org/10.3390/app15147753
APA StyleLawrence, G., Josy, T., Pejcz, E., Wojciechowicz-Budzisz, A., Olędzki, R., Górska, K., Zając, A., Aurore, G., & Harasym, J. (2025). Upcycling Luffa cylindrica (Luffa Sponge) Seed Press Cake as a Functional Ingredient for Meat Substitute Formulations. Applied Sciences, 15(14), 7753. https://doi.org/10.3390/app15147753







