Seaweed Pelvetia canaliculata as a Source of Bioactive Compounds for Application in Fried Pre-Coated Mackerel (Scomber scombrus) Fillets: A Functional Food Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Chemicals
2.3. Pelvetia canaliculata Extract Preparation
2.4. Formulation of Edible Coating Solutions
2.5. Evaluation of the Antioxidant Activity of Extracts and Edible Coating Solutions
2.5.1. Total Phenolic Content
2.5.2. Radical 2,2-Diffiphenyl-1-Pylrilhydrazine (DPPH) Scavenging Activity
2.5.3. Ferric Reducing Activity Power (FRAP)
2.6. Application of Coatings on Mackerel Fillets
2.7. Frying Process
2.8. Analysis of Fried Mackerel Fillets
2.8.1. Colour Differences
2.8.2. Evaluation of Textural Attributes
2.8.3. Moisture and Ash Contents
2.8.4. Total Lipid Content
2.8.5. Analysis of the Fatty Acid Profile
2.8.6. Statistical Analysis
3. Results and Discussion
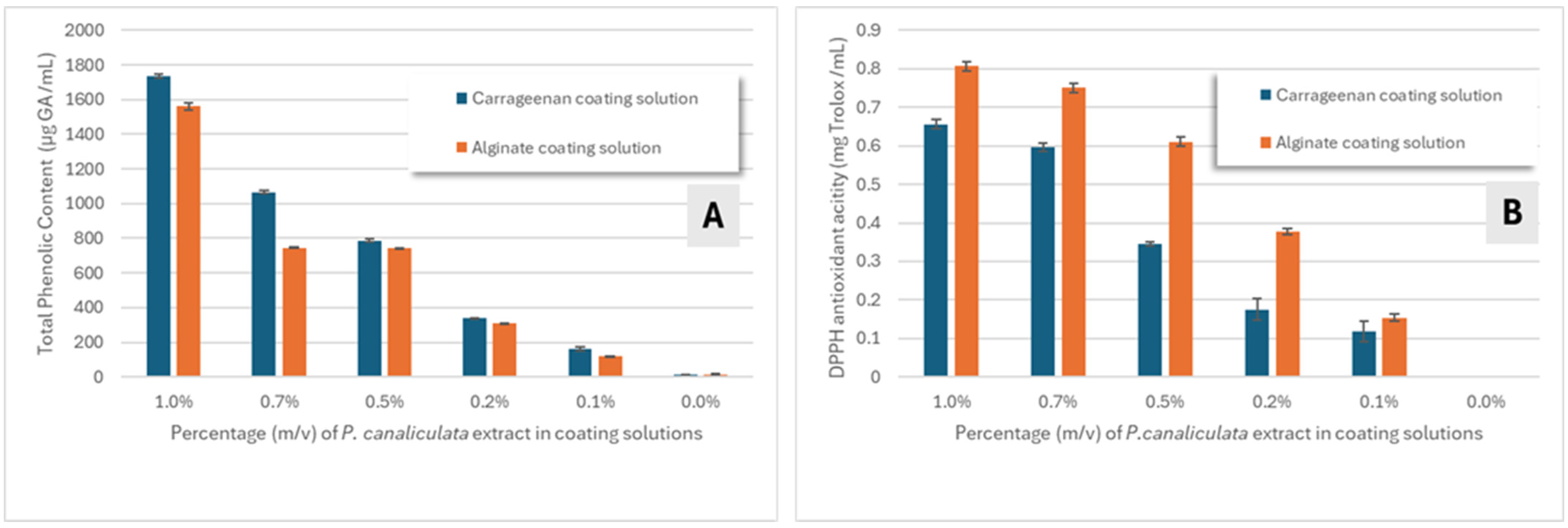
3.1. P. canaliculata Extracts: Total Phenolic Content and Antioxidant Activity Evaluation
3.2. Characterization of Edible Coating Solutions
3.3. Characterization of Fried Mackerel Fillets
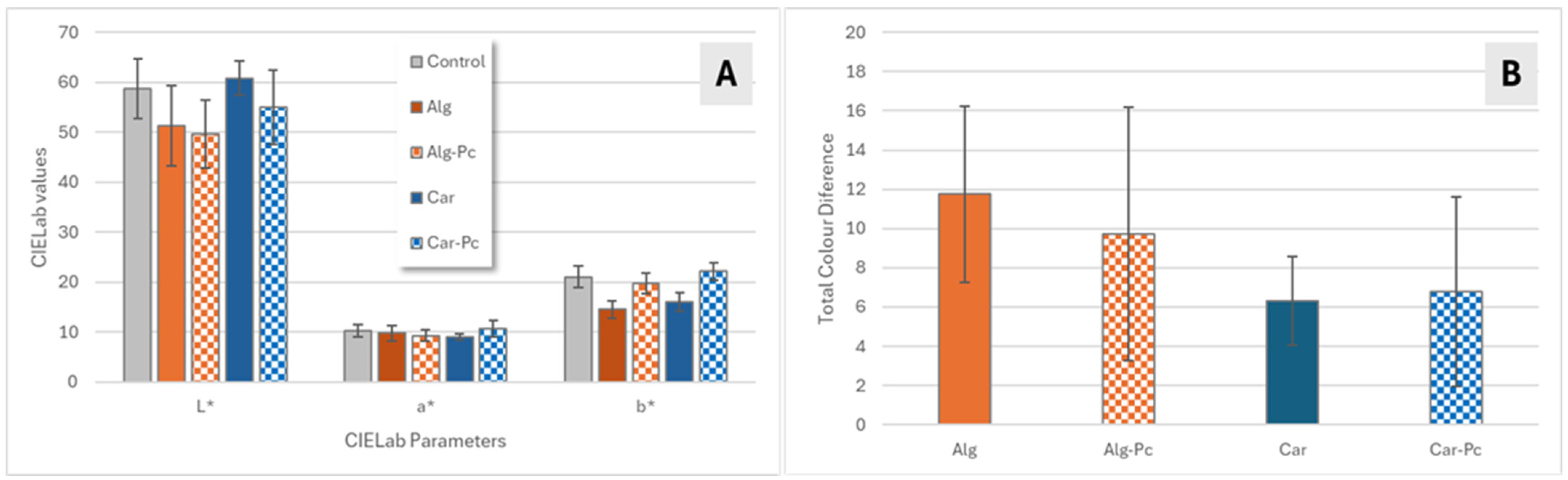
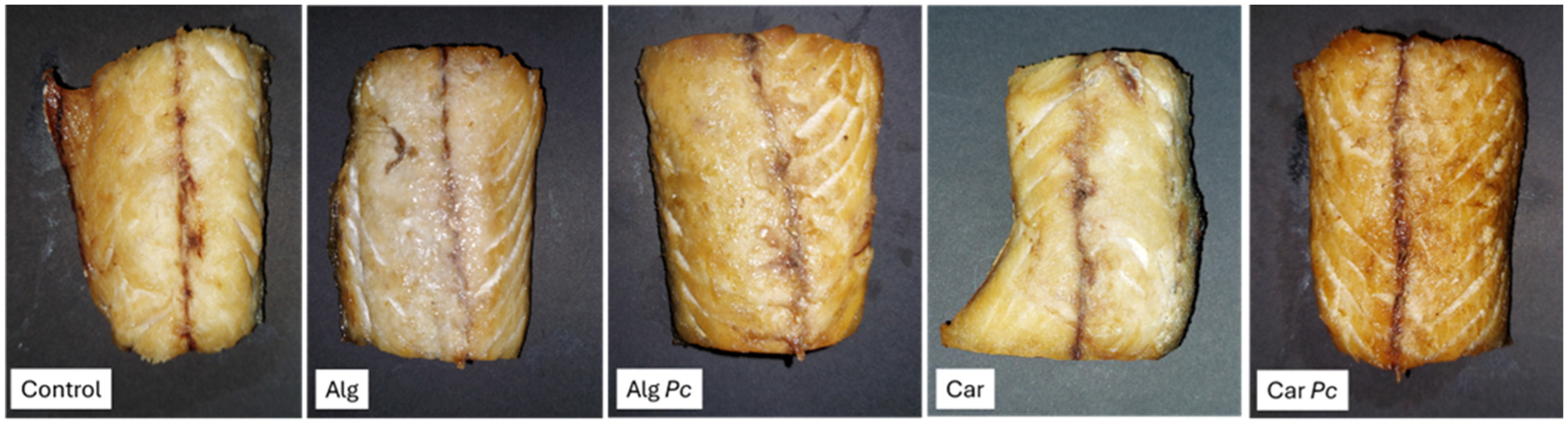
3.3.1. Colour Evaluation
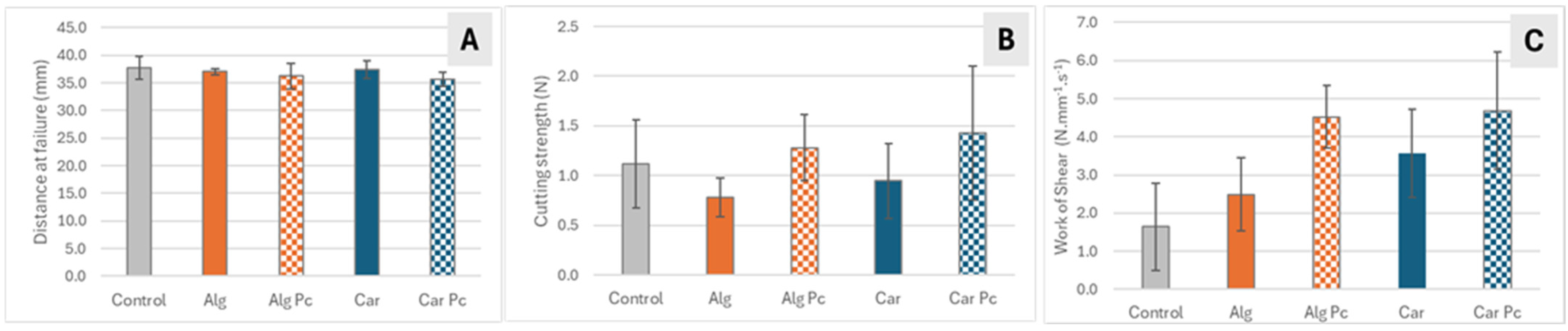
3.3.2. Textural Attributes of Fried Mackerel Fillets
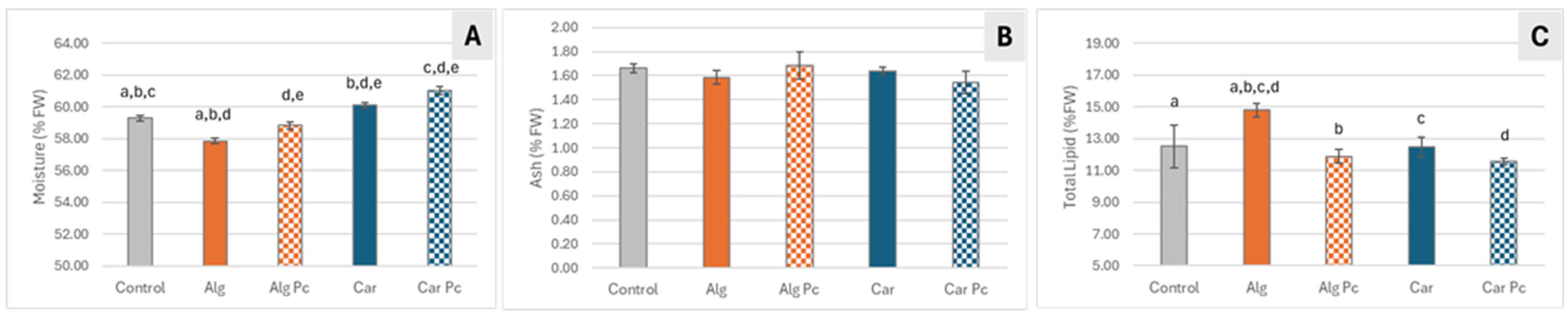
3.3.3. Nutritional Characterization of Fried Mackerel Fillets
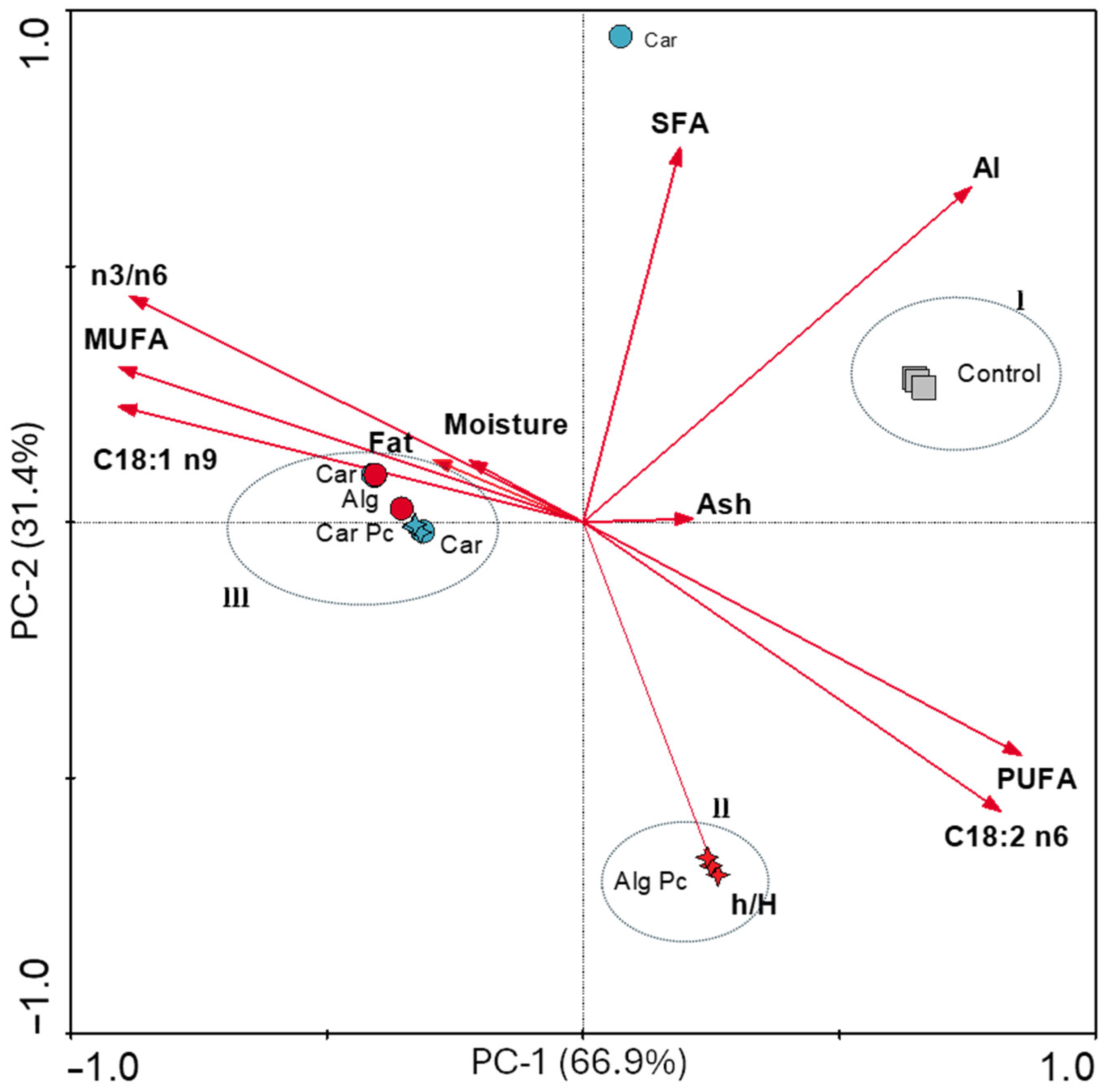
3.3.4. Principal Component Analysis of Atlantic Mackerel Fillet Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinto, F.R.; Duarte, A.M.; Silva, F.; Barroso, S.; Mendes, S.; Pinto, E.; Almeida, A.; Sequeira, V.; Vieira, A.R.; Gordo, L.S.; et al. Annual Variations in the Mineral Element Content of Five Fish Species from the Portuguese Coast. Food Res. Int. 2022, 158, 111482. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A Critical Review on the Health Benefits of Fish Consumption and Its Bioactive Constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef]
- Bennett, A.; Basurto, X.; Virdin, J.; Lin, X.; Betances, S.J.; Smith, M.D.; Allison, E.H.; Best, B.A.; Brownell, K.D.; Campbell, L.M.; et al. Recognize Fish as Food in Policy Discourse and Development Funding. Ambio 2021, 50, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Karadzic, V.; Vaz, S. The Seafood Market in Portugal: Driving Forces and Consequences. Mar. Policy 2015, 61, 87–94. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.E.; Elvevoll, E.O. Health Benefits of Marine Foods and Ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef]
- Romotowska, P.E.; Karlsdóttir, M.G.; Gudjónsdóttir, M.; Kristinsson, H.G.; Arason, S. Seasonal and Geographical Variation in Chemical Composition and Lipid Stability of Atlantic Mackerel (Scomber Scombrus) Caught in Icelandic Waters. J. Food Compos. Anal. 2016, 49, 9–18. [Google Scholar] [CrossRef]
- PORDATA. Available online: https://www.pordata.pt/pt/estatisticas/agricultura-floresta-e-pescas/pesca/peixe-capturado (accessed on 27 May 2025).
- Hu, L.; Ren, S.; Shen, Q.; Chen, J.; Ye, X.; Ling, J. Proteomic Study of the Effect of Different Cooking Methods on Protein Oxidation in Fish Fillets. RSC Adv. 2017, 7, 27496–27505. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Lee, M.-J.; Tirtawijaya, G.; Cho, W.-H.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Application of Deep, Vacuum, and Air Frying Methods to Fry Chub Mackerel (Scomber Japonicus). Processes 2021, 9, 1225. [Google Scholar] [CrossRef]
- Liberty, J.T.; Dehghannya, J.; Ngadi, M.O. Effective Strategies for Reduction of Oil Content in Deep-Fat Fried Foods: A Review. Trends Food Sci. Technol. 2019, 92, 172–183. [Google Scholar] [CrossRef]
- Abrante-Pascual, S.; Nieva-Echevarría, B.; Goicoechea-Oses, E. Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods 2024, 13, 4186. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Archana, G.; Azhagu Saravana Babu, P.; Sudharsan, K.; Sabina, K.; Palpandi Raja, R.; Sivarajan, M.; Sukumar, M. Evaluation of Fat Uptake of Polysaccharide Coatings on Deep-Fat Fried Potato Chips by Confocal Laser Scanning Microscopy. Int. J. Food Prop. 2016, 19, 1583–1592. [Google Scholar] [CrossRef]
- García, M.A.; Ferrero, C.; Bértola, N.; Martino, M.; Zaritzky, N. Edible Coatings from Cellulose Derivatives to Reduce Oil Uptake in Fried Products. Innov. Food Sci. Emerg. Technol. 2002, 3, 391–397. [Google Scholar] [CrossRef]
- Andriani, V.; Abyor Handayani, N. Recent Technology of Edible Coating Production: A Review. Mater. Today Proc. 2023, 87, 200–206. [Google Scholar] [CrossRef]
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible Films and Coatings Containing Bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Pirozzi, A.; Pataro, G.; Donsì, F.; Ferrari, G. Edible Coating and Pulsed Light to Increase the Shelf Life of Food Products. Food Eng. Rev. 2021, 13, 544–569. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food Applications of Emulsion-Based Edible Films and Coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Volpe, M.G.; Siano, F.; Paolucci, M.; Sacco, A.; Sorrentino, A.; Malinconico, M.; Varricchio, E. Active Edible Coating Effectiveness in Shelf-Life Enhancement of Trout (Oncorhynchusmykiss) Fillets. LWT-Food Sci. Technol. 2015, 60, 615–622. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Yu, D.; Regenstein, J.M.; Xia, W. Bio-Based Edible Coatings for the Preservation of Fishery Products: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2481–2493. [Google Scholar] [CrossRef]
- Karbowiak, T.; Hervet, H.; Léger, L.; Champion, D.; Debeaufort, F.; Voilley, A. Effect of Plasticizers (Water and Glycerol) on the Diffusion of a Small Molecule in Iota-Carrageenan Biopolymer Films for Edible Coating Application. Biomacromolecules 2006, 7, 2011–2019. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Barroso, M.F.; Simal-Gandara, J.; et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics 2020, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.P.; Alvarez, C.; Zhao, M.; Tiwari, U.; Curtin, J.; Garcia-Vaquero, M.; Tiwari, B.K. Innovative Processing Strategies and Technologies to Obtain Hydrocolloids from Macroalgae for Food Applications. Carbohydr. Polym. 2020, 248, 116784. [Google Scholar] [CrossRef]
- Pires, D.; Passos, R.; do Carmo, B.; Tchobanov, C.F.; Forte, S.; Vaz, M.; Antunes, M.; Neves, M.; Tecelão, C.; Baptista, T. Pelvetia Canaliculata as an Aquafeed Supplement for Gilthead Seabream Sparus Aurata: A Biorefinery Approach for Seaweed Biomass Valorisation. Sustainability 2022, 14, 11469. [Google Scholar] [CrossRef]
- Sousa, G.; Trifunovska, M.; Antunes, M.; Miranda, I.; Moldão, M.; Alves, V.; Vidrih, R.; Lopes, P.A.; Aparicio, L.; Neves, M.; et al. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Pelvetia Canaliculata to Sunflower Oil. Foods 2021, 10, 1732. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stengel, D.B. Concise Review of the Macroalgal Species Pelvetia Canaliculata (Linnaeus) Decaisne & Thuret. J. Appl. Phycol. 2022, 34, 2807–2825. [Google Scholar] [CrossRef]
- Connan, S.; Deslandes, E.; Gall, E.A. Influence of Day–Night and Tidal Cycles on Phenol Content and Antioxidant Capacity in Three Temperate Intertidal Brown Seaweeds. J. Exp. Mar. Biol. Ecol. 2007, 349, 359–369. [Google Scholar] [CrossRef]
- Qin, Y. Seaweed Hydrocolloids as Thickening, Gelling, and Emulsifying Agents in Functional Food Products. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–152. [Google Scholar]
- Yang, M.; Shi, J.; Xia, Y. Effect of SiO2, PVA and Glycerol Concentrations on Chemical and Mechanical Properties of Alginate-Based Films. Int. J. Biol. Macromol. 2018, 107, 2686–2694. [Google Scholar] [CrossRef]
- Neves, M.; Miranda, A.; Lemos, M.F.L.; Silva, S.; Tecelão, C. Enhancing Oxidative Stability of Sunflower Oil by Supplementation with Prickled Broom (Pterospartum Tridentatum) Ethanolic Extract. J. Food Sci. 2020, 85, 2812–2821. [Google Scholar] [CrossRef]
- Neves, M.; Antunes, M.; Fernandes, W.; Campos, M.J.; Azevedo, Z.M.; Freitas, V.; Rocha, J.M.; Tecelão, C. Physicochemical and Nutritional Profile of Leaves, Flowers, and Fruits of the Edible Halophyte Chorão-Da-Praia (Carpobrotus Edulis) on Portuguese West Shores. Food Biosci. 2021, 43, 101288. [Google Scholar] [CrossRef]
- DrLange. Colour Review; DrLange Application Report, no8.0e; DrLange: St. Louis, MO, USA, 1994. [Google Scholar]
- Antunes, M.; Neves, M.; Pires, D.; Passos, R.; do Carmo, B.; Tchobanov, C.F.; Forte, S.; Vaz, M.; Baptista, T.; Tecelão, C. Proximate Composition and Fatty Acid Profile of Gilthead Seabream (Sparus Aurata) Fed with Pelvetia Canaliculata-Supplemented Diets: An Insight towards the Valorization of Seaweed Biomass. Foods 2023, 12, 1810. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Queguineur, B.; Hanniffy, D.; Troy, D.J.; Kerry, J.P.; O’Brien, N.M. In Vitro and Cellular Antioxidant Activities of Seaweed Extracts Prepared from Five Brown Seaweeds Harvested in Spring from the West Coast of Ireland. Food Chem. 2011, 126, 1064–1070. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of Pressurised Liquid Extraction and Solid-Liquid Extraction Methods on the Phenolic Content and Antioxidant Activities of Irish Macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Stach, M.; Kolniak-Ostek, J. The Influence of the Use of Different Polysaccharide Coatings on the Stability of Phenolic Compounds and Antioxidant Capacity of Chokeberry Hydrogel Microcapsules Obtained by Indirect Extrusion. Foods 2023, 12, 515. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Yu, G. The Effect of Frying Conditions on the Physical and Chemical Quality Attributes of Clearhead Icefish (Protosalanx Hyalocranius) During Deep Frying and Air Frying. Foods 2025, 14, 920. [Google Scholar] [CrossRef]
- NurSyahirah, S.; Rozzamri, A. Effects of Frying on Fish, Fish Products and Frying Oil—A Review. Food Res. 2022, 6, 14–32. [Google Scholar] [CrossRef]
- Augusto, A.; Marques, S.; Félix, R.; Dias, J.; Alves, N.; Shiels, K.; Murray, P.; Novais, S.C.; Lemos, M.F.L.; Silva, S.F.J. A Novel Seaweed-Based Biodegradable and Active Food Film to Reduce Freezer Burn in Frozen Salmon. Food Hydrocoll. 2024, 156, 110332. [Google Scholar] [CrossRef]
- Chang, L.; Lin, S.; Zou, B.; Zheng, X.; Zhang, S.; Tang, Y. Effect of Frying Conditions on Self-Heating Fried Spanish Mackerel Quality Attributes and Flavor Characteristics. Foods 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Dana, D.; Saguy, I.S. Review: Mechanism of Oil Uptake during Deep-Fat Frying and the Surfactant Effect-Theory and Myth. Adv. Colloid. Interface Sci. 2006, 128–130, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pineda, M.; Yagüe-Ruiz, C.; Vercet, A. Frying Conditions, Methyl Cellulose, and K-Carrageenan Edible Coatings: Useful Strategies to Reduce Oil Uptake in Fried Mushrooms. Foods 2021, 10, 1694. [Google Scholar] [CrossRef] [PubMed]
- El Oudiani, S.; Chetoui, I.; Darej, C. Moujahed N Sex and seasonal variation in proximate composition and fatty acid profile of Scomber scombrus (L. 1758) fillets from the Middle East Coast of Tunisia. Grasas Y Aceites 2019, 70, 1. [Google Scholar] [CrossRef]
- Guizani, S.E.O.; Moujahed, N. Seasonal Variation of Chemical and Fatty Acids Composition in Atlantic Mackerel from the Tunisian Northern-East Coast. J. Food Process. Technol. 2015, 6. [Google Scholar] [CrossRef]
| Coating Solution | Polysaccharide (% m/v) | Glycerol (% m/v) | Tween 80 (% m/v) | P. canaliculata EtOH Extract (% m/v) |
|---|---|---|---|---|
| Carrageenan (Car) | 1 | 0.75 | 0.02 | 0 |
| Carrageenan with P. canaliculata extract (Car Pc) | 1 | 0.75 | 0.02 | 1 |
| Alginate (Alg) | 2 | 0.6 | 0 | 0 |
| Alginate with P. canaliculata extract (Alg Pc) | 2 | 0.6 | 0 | 0.5 |
| P. canaliculata Extract | H2O | EtOH:H2O | EtOH |
|---|---|---|---|
| TPC (µg GA/mg) | 48.7 ± 9.1 a | 55.4 ± 3.7 b | 203 ± 32 a,b |
| DPPH (µg Trolox/mg) | 38.3 ± 3.8 a | 39.9 ± 2.9 b | 180.7 ± 7.4 a,b |
| FRAP (µg Trolox/mg) | 15.8 ± 2.5 a | 16.2 ± 2.1 b | 55.3 ± 4.7 a,b |
| FA | Control | Alg | Alg Pc | Car | Car Pc |
|---|---|---|---|---|---|
| C12:0 | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.01 |
| C13:0 | 0.10 ± 0.01 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.09 ± 0.01 |
| C14.0 | 4.26 ± 0.07 * | 3.90 ± 0.09 | 3.28 ± 0.23 * | 4.25 ± 0.13 | 3.42 ± 0.12 * |
| C15:0 | 0.84 ± 0.03 | 0.84 ± 0.03 | 0.69 ± 0.06 | 0.97 ± 0.04 | 0.73 ± 0.06 |
| C16:0 | 18.30 ± 0.35 | 17.52 ± 0.11 | 15.7 ± 1.5 | 17.02 ± 0.06 | 16.09 ± 0.12 |
| C17:0 | 1.21 ± 0.03 | 1.13 ± 0.07 | 0.91 ± 0.24 | 1.58 ± 0.07 | 0.95 ± 0.21 |
| C18:0 | 6.28 ± 0.09 | 6.06 ± 0.12 | 5.40 ± 0.16 * | 5.88 ± 0.03 | 5.96 ± 0.04 |
| C20:0 | 0.29 ± 0.10 | 0.34 ± 0.07 | 0.29 ± 0.10 | 0.30 ± 0.01 | 0.32 ± 0.04 |
| C21:0 | nd | 0.07 ± 0.02 | 0.02 ± 0.03 | 0.06 ± 0.00 | 0.07 ± 0.00 |
| C22:0 | 0.20 ± 0.04 | 0.23 ± 0.01 | 0.28 ± 0.02 | 0.25 ± 0.02 | 0.27 ± 0.03 |
| C24:0 | 0.13 ± 0.07 | 0.13 ± 0.04 | 0.13 ± 0.11 | 0.12 ± 0.01 | 0.12 ± 0.03 |
| C16:1 n-7 | 2.80 ± 0.26 | 2.91 ± 0.11 | 2.29 ± 0.26 | 3.16 ± 0.17 | 2.34 ± 0.30 |
| C17:1 n-7 | 0.74 ± 0.03 | 0.64 ± 0.12 | 0.47 ± 0.11 | 0.85 ± 0.04 | 0.57 ± 0.13 |
| trans C18:1 n-9 | 0.20 ± 0.07 | 0.15 ± 0.09 | 0.03 ± 0.04 | 0.27 ± 0.08 | 0.30 ± 0.17 |
| C18:1 n-9 | 24.56 ± 0.10 * | 39.93 ± 0.54 * | 27.14 ± 0.66 | 37.49 ± 0.36 * | 44.94 ± 0.95 * |
| C20:1 n-9 | 1.40 ± 0.01 | 1.35 ± 0.02 | 0.97 ± 0.14 | 1.31 ± 0.05 | 1.27 ± 0.09 |
| C22:1 n-9 | 0.73 ± 0.27 | 0.52 ± 0.05 | 0.37 ± 0.11 | 0.64 ± 0.16 | 0.44 ± 0.06 |
| C24:1 n-9 | 0.77 ± 0.06 | 0.78 ± 0.02 | 0.84 ± 0.11 | 0.83 ± 0.13 | 0.72 ± 0.04 |
| C18:2 n-6 | 15.34 ± 0.55 * | 2.39 ± 0.16 * | 21.4 ± 1.8 * | 2.55 ± 0.19 * | 2.65 ± 0.02 * |
| C18:3 n-6 | 0.08 ± 0.00 | 0.07 ± 0.02 | 0.02 ± 0.04 | 0.08 ± 0.01 | 0.05 ± 0.03 |
| C18:3 n-3 (ALA) | 0.70 ± 0.14 | 0.65 ± 0.03 | 0.58 ± 0.18 | 0.78 ± 0.03 | 0.59 ± 0.07 |
| C18:4 n-3 | 1.14 ± 0.10 | 1.03 ± 0.09 | 0.99 ± 0.07 | 1.14 ± 0.11 | 0.92 ± 0.11 |
| C20:2 n-6 | 0.36 ± 0.05 | 0.26 ± 0.02 | 0.23 ± 0.01 | 0.33 ± 0.05 | 0.26 ± 0.05 |
| C20:4 n-6 | 1.15 ± 0.08 | 1.13 ± 0.07 | 1.24 ± 0.02 | 1.44 ± 0.07 | 1.08 ± 0.12 |
| C20:5 n-3 (EPA) | 5.43 ± 0.20 * | 4.88 ± 0.17 | 4.52 ± 0.06 * | 4.77 ± 0.14 | 3.99 ± 0.45 * |
| C22:5 n-3 | 1.37 ± 0.07 | 1.41 ± 0.19 | 1.14 ± 0.01 | 1.37 ± 0.02 | 1.15 ± 0.03 |
| C22:6 n-3 (DHA) | 11.68 ± 0.52 | 11.48 ± 0.61 | 10.93 ± 0.56 | 12.35 ± 0.13 | 10.66 ± 0.29 |
| SFA | 31.59 ± 0.23 * | 30.39 ± 0.29 | 26.9 ± 2.2 * | 30.61 ± 0.17 | 28.07 ± 0.27 |
| MUFA | 31.21 ± 0.13 * | 46.28 ± 0.50 * | 32.11 ± 0.12 | 44.54 ± 0.19 * | 50.57 ± 0.54 * |
| PUFA | 37.20 ± 0.36 * | 23.32 ± 0.74 * | 41.0 ± 2.1 | 24.85 ± 0.17* | 21.35 ± 0.53 * |
| n3 | 20.33 ± 0.24 | 19.44 ± 0.86 | 18.16 ± 0.37 | 20.42 ± 0.18 | 17.31 ± 0.57 |
| n6 | 16.88 ± 0.51 | 3.88 ± 0.14 | 22.87 ± 1.76 | 4.43 ± 0.16 | 4.06 ± 0.06 |
| n3/n6 | 1.21 ± 0.05 | 5.02 ± 0.38 * | 0.80 ± 0.04 * | 4.61 ± 0.19 * | 4.27 ± 0.19 * |
| h/H | 2.67 ± 0.05 * | 2.89 ± 0.05 | 3.55 ± 0.46 * | 2.86 ± 0.04 | 3.34 ± 0.05 * |
| AI | 0.52 ± 0.01 * | 0.48 ± 0.01 | 0.40 ± 0.04 * | 0.49 ± 0.01 | 0.41 ± 0.01 * |
| TI | 0.33 ± 0.00 | 0.31 ± 0.01 | 0.30 ± 0.03 | 0.29 ± 0.00 | 0.31 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, C.D.; Antunes, M.; Silva, S.F.J.; Neves, M.; Tecelão, C. Seaweed Pelvetia canaliculata as a Source of Bioactive Compounds for Application in Fried Pre-Coated Mackerel (Scomber scombrus) Fillets: A Functional Food Approach. Appl. Sci. 2025, 15, 7623. https://doi.org/10.3390/app15137623
Freire CD, Antunes M, Silva SFJ, Neves M, Tecelão C. Seaweed Pelvetia canaliculata as a Source of Bioactive Compounds for Application in Fried Pre-Coated Mackerel (Scomber scombrus) Fillets: A Functional Food Approach. Applied Sciences. 2025; 15(13):7623. https://doi.org/10.3390/app15137623
Chicago/Turabian StyleFreire, Catarina D., Madalena Antunes, Susana F. J. Silva, Marta Neves, and Carla Tecelão. 2025. "Seaweed Pelvetia canaliculata as a Source of Bioactive Compounds for Application in Fried Pre-Coated Mackerel (Scomber scombrus) Fillets: A Functional Food Approach" Applied Sciences 15, no. 13: 7623. https://doi.org/10.3390/app15137623
APA StyleFreire, C. D., Antunes, M., Silva, S. F. J., Neves, M., & Tecelão, C. (2025). Seaweed Pelvetia canaliculata as a Source of Bioactive Compounds for Application in Fried Pre-Coated Mackerel (Scomber scombrus) Fillets: A Functional Food Approach. Applied Sciences, 15(13), 7623. https://doi.org/10.3390/app15137623









