Effect of Virtual-Reality-Based Training, Including Preceding Trunk Stabilization Education, on Postural Control and Balance in Patients with Stroke: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Intervention
2.3.1. Trunk Stabilization Education
2.3.2. VR-Based Training
2.4. Outcome Measures
2.4.1. Postural Control
- K-TIS
- 2.
- Postural Assessment Scale for Stroke (PASS)
2.4.2. Static Balance
- COP
2.4.3. Dynamic Balance
- Berg Balance Scale (BBS)
- 2.
- Limit of Stability (LOS)
2.4.4. Data Analysis
3. Results
3.1. General Characteristics and Homogeneity of Participants
3.2. Pre-Test Outcome Measures and Baseline Homogeneity of Participants
3.3. Postural Control (K-TIS and PASS)
3.4. Dynamic Balance (BBS and LOS)
3.5. Static Balance (COP)
3.5.1. Eyes-Open
3.5.2. Eyes-Closed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, E.J. Rehabilitation in Stroke Syndromes. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 817–853. [Google Scholar] [CrossRef]
- Popkirov, S.; Stone, J.; Buchan, A.M. Functional neurological disorder: A common and treatable stroke mimic. Stroke 2020, 51, 1629–1635. [Google Scholar] [CrossRef]
- Bohannon, R.W. Lateral trunk flexion strength: Impairment, measurement reliability and implications following unilateral brain lesion. Int. J. Rehabil. Res. 1992, 15, 249–251. [Google Scholar] [CrossRef]
- Ikai, T.; Kamikubo, T.; Takehara, I.; Nishi, M.; Miyano, S. Dynamic postural control in patients with hemiparesis. Am. J. Phys. Med. Rehabil. 2003, 82, 463–469. [Google Scholar] [CrossRef]
- Horak, F.; Esselman, P.; Anderson, M.E.; Lynch, M. The effects of movement velocity, mass displaced, and task certainty on associated postural adjustments made by normal and hemiplegic individuals. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Forget, R.; Lamarre, Y. Postural adjustments associated with different unloadings of the forearm: Effects of proprioceptive and cutaneous afferent deprivation. Can. J. Physiol. Pharmacol. 1995, 73, 285–294. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Bagur-Calafat, C.; Girabent-Farrés, M.; Caballero-Gómez, F.M.; Hernández-Valiño, M.; Urrutia Cuchi, G. The effect of additional core stability exercises on improving dynamic sitting balance and trunk control for subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.J.; Tang, P.-F. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert Rev. Neurother. 2007, 7, 1417–1436. [Google Scholar] [CrossRef]
- Chen, L.; Lo, W.L.A.; Mao, Y.R.; Ding, M.H.; Lin, Q.; Li, H.; Zhao, J.L.; Xu, Z.Q.; Bian, R.H.; Huang, D.F. Effect of virtual reality on postural and balance control in patients with stroke: A systematic literature review. BioMed Res. Int. 2016, 2016, 7309272. [Google Scholar] [CrossRef]
- Lewis, G.N.; Rosie, J.A. Virtual reality games for movement rehabilitation in neurological conditions: How do we meet the needs and expectations of the users? Disabil. Rehabil. 2012, 34, 1880–1886. [Google Scholar] [CrossRef]
- McNulty, P.A.; Thompson-Butel, A.G.; Faux, S.G.; Lin, G.; Katrak, P.H.; Harris, L.R.; Shiner, C.T. The efficacy of Wii-based movement therapy for upper limb rehabilitation in the chronic poststroke period: A randomized controlled trial. Int. J. Stroke 2015, 10, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.; Celik, O. Systematic review of Kinect applications in elderly care and stroke rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wingham, J.; Adie, K.; Turner, D.; Schofield, C.; Pritchard, C. Participant and caregiver experience of the Nintendo Wii SportsTM after stroke: Qualitative study of the trial of WiiTM in stroke (TWIST). Clin. Rehabil. 2015, 29, 295–305. [Google Scholar] [CrossRef]
- Lee, D.; Bae, Y. The effectiveness of driving game on trunk control and gait ability in stroke. J. Mot. Behav. 2020, 52, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Alhwoaimel, N.; Hughes, A.-M.; Warner, M.; Burridge, J.; Brown, S.; Verheyden, G.; Thijs, L.; Wee, S.K.; Turk, R. Trunk exercise programme post-stroke using a virtual reality video game-based system: A mixed methods feasibility study. In Proceedings of the 3rd Saudi Physical Therapy Association Conference (SPTA) 2019, Riyadh International Convention & Exhibition Center, Riyadh, Saudi Arabia, 6–8 September 2019. [Google Scholar]
- Foreman, M.H.; Engsberg, J.R. A virtual reality tool for measuring and shaping trunk compensation for persons with stroke: Design and initial feasibility testing. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668318823673. [Google Scholar] [CrossRef]
- Yu, S.-H.; Park, S.-D. The effects of core stability strength exercise on muscle activity and trunk impairment scale in stroke patients. J. Exerc. Rehabil. 2013, 9, 362. [Google Scholar] [CrossRef]
- Magee, D. Instability and Stabilization: Theory and Treatment, 2nd ed.; Seminar Workbook; Orthopaedic Physical Therapy Products: Minneapolis, MN, USA, 1999. [Google Scholar]
- Marshall, P.W.; Murphy, B.A. Core stability exercises on and off a swiss ball. Arch. Phys. Med. Rehabil. 2005, 86, 242–249. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Truijen, S.; Verbruggen, C.; Van de Venis, L.; Saeys, W. The effect of trunk training on muscle thickness and muscle activity: A systematic review. Disabil. Rehabil. 2019, 41, 1751–1759. [Google Scholar] [CrossRef]
- Macedo, L.G.; Maher, C.G.; Latimer, J.; McAuley, J.H. Motor control exercise for persistent, nonspecific low back pain: A systematic review. Phys. Ther. 2009, 89, 9–25. [Google Scholar] [CrossRef]
- Haruyama, K.; Kawakami, M.; Otsuka, T. Effect of core stability training on trunk function, standing balance, and mobility in stroke patients: A randomized controlled trial. Neurorehabilit. Neural Repair 2017, 31, 240–249. [Google Scholar] [CrossRef]
- McGalliard, M.K.; Dedrick, G.S.; Brismée, J.M.; Cook, C.E.; Apte, G.G.; Sizer, P.S., Jr. Changes in transversus abdominis thickness with use of the abdominal drawing-in maneuver during a functional task. Pm&r 2010, 2, 187–194. [Google Scholar]
- Jeon, I.-C.; Hwang, U.-J.; Jung, S.-H.; Kwon, O.-Y. Comparison of gluteus maximus and hamstring electromyographic activity and lumbopelvic motion during three different prone hip extension exercises in healthy volunteers. Phys. Ther. Sport 2016, 22, 35–40. [Google Scholar] [CrossRef]
- Jung, S.; Lee, K.; Kim, M.; Song, C. Audiovisual Biofeedback-Based Trunk Stabilization Training Using a Pressure Biofeedback System in Stroke Patients: A Randomized, Single-Blinded Study. Stroke Res. Treat. 2017, 2017, 6190593. [Google Scholar] [CrossRef]
- Verheyden, G.; Vereeck, L.; Truijen, S.; Troch, M.; LaFosse, C.; Saeys, W.; Leenaerts, E.; Palinckx, A.; De Weerdt, W. Additional exercises improve trunk performance after stroke: A pilot randomized controlled trial. Neurorehabilit. Neural Repair 2009, 23, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Delbroek, T.; Hansen, D.; Lemmens, R.J.; Deschuytere, J.; Gielen, E.; Vanrumste, B.; Feys, H. The effect of cognitive-motor dual task training with the biorescue force platform on cognition, balance and dual task performance in institutionalized older adults: A randomized controlled trial. J. Neuroeng. Rehabil. 2017, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; De Weerdt, W. The Trunk Impairment Scale: A new tool to measure motor impairment of the trunk after stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [CrossRef]
- Ko, J.; You, Y. Reliability and responsiveness of the Korean version of the trunk impairment scale for stroke patients. J. Korean Phys. Ther. 2015, 27, 175–182. [Google Scholar] [CrossRef]
- Benaim, C.; Pérennou, D.A.; Villy, J.; Rousseaux, M.; Pelissier, J.Y. Validation of a standardized assessment of postural control in stroke patients: The Postural Assessment Scale for Stroke Patients (PASS). Stroke 1999, 30, 1862–1868. [Google Scholar] [CrossRef]
- Mao, H.-F.; Hsueh, I.-P.; Tang, P.-F.; Sheu, C.-F.; Hsieh, C.-L. Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke 2002, 33, 1022–1027. [Google Scholar] [CrossRef]
- Seo, K.; Kim, J.; Wi, G. The effects of stair gait exercise on static balance ability of stroke patients. J. Phys. Ther. Sci. 2014, 26, 1835–1838. [Google Scholar] [CrossRef]
- Song, G.-B.; Park, E.-C. Comparison of the effects of task-oriented training and virtual reality training on upper extremity function, balance ability, and depression in stroke patients. J. Korean Soc. Phys. Med. 2016, 11, 115–125. [Google Scholar] [CrossRef]
- Berg, K.O.; Maki, B.E.; Williams, J.I.; Holliday, P.J.; Wood-Dauphinee, S.L. Clinical and laboratory measures of postural balance in an elderly population. Arch. Phys. Med. Rehabil. 1992, 73, 1073–1080. [Google Scholar] [PubMed]
- Bogle Thorbahn, L.D.; Newton, R.A. Use of the Berg Balance Test to predict falls in elderly persons. Phys. Ther. 1996, 76, 576–583. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Bagur-Calafat, C.; Girabent-Farrés, M.; Caballero-Gómez, F.M.; du Port de Pontcharra-Serra, H.; German-Romero, A.; Urrútia, G. Long-term follow-up of a randomized controlled trial on additional core stability exercises training for improving dynamic sitting balance and trunk control in stroke patients. Clin. Rehabil. 2017, 31, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.K.; Kwon, O.S.; Kim, J.H.; Lee, D.Y. The effect of trunk stabilization exercise on the thickness of the deep abdominal muscles and balance in patients with chronic stroke. J. Phys. Ther. Sci. 2012, 24, 181–185. [Google Scholar] [CrossRef]
- Yang, D.J. Influence of biofeedback weight bearing training in sit to stand to sit and the limits of stability on stroke patients. J. Phys. Ther. Sci. 2016, 28, 3011–3014. [Google Scholar] [CrossRef] [PubMed]
- Massion, J. Postural control system. Curr. Opin. Neurobiol. 1994, 4, 877–887. [Google Scholar] [CrossRef]
- de Haart, M.; Geurts, A.C.; Huidekoper, S.C.; Fasotti, L.; van Limbeek, J. Recovery of standing balance in postacute stroke patients: A rehabilitation cohort study. Arch. Phys. Med. Rehabil. 2004, 85, 886–895. [Google Scholar] [CrossRef]
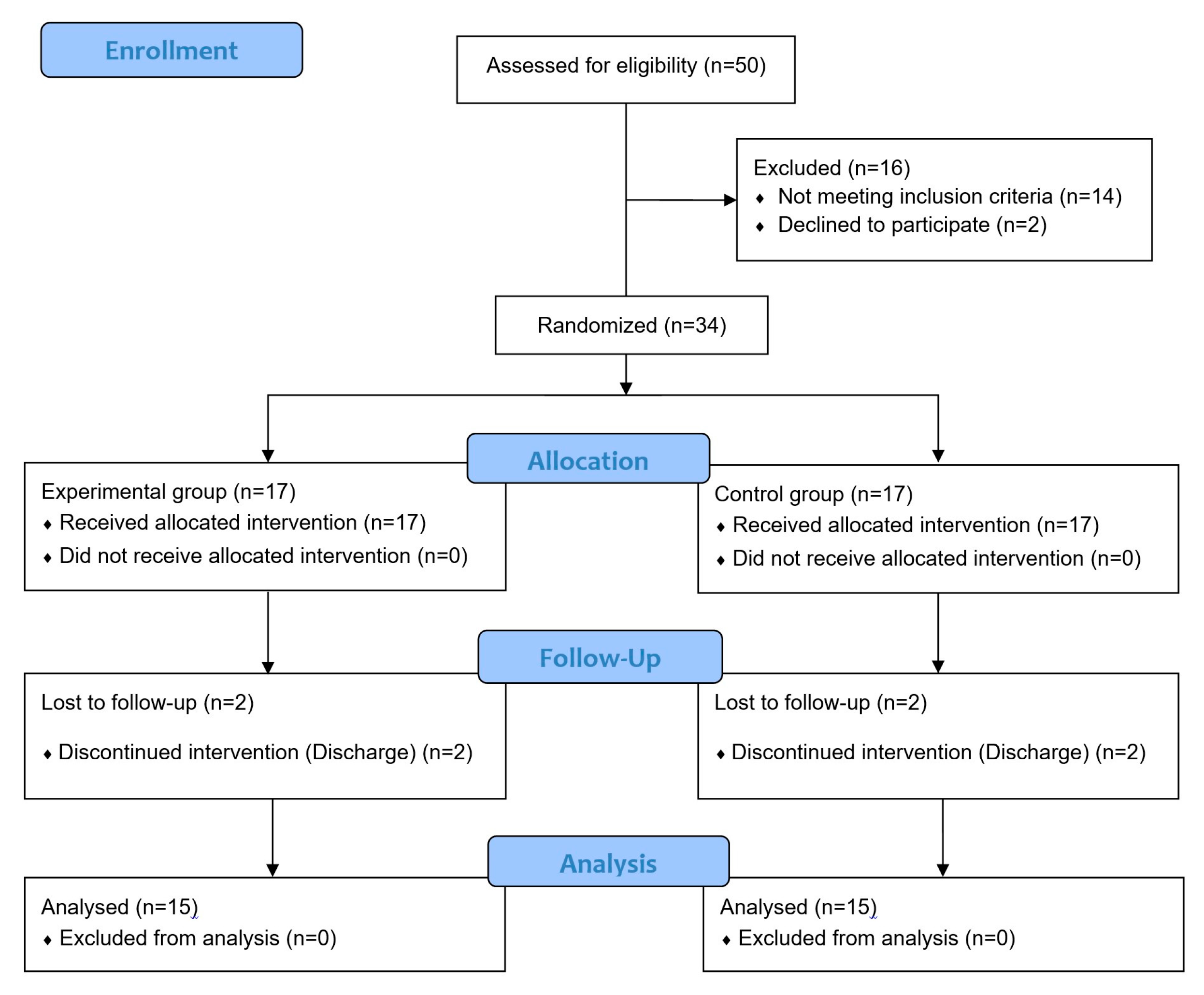
| Variable | Experimental Group (n = 15) | Control Group (n = 15) | t (p) |
|---|---|---|---|
| Age (years) | 62.73 ± 10.83 | 65.67 ± 13.39 | −0.659 (0.515) |
| Height (cm) | 164.07 ± 7.90 | 163.80 ± 8.33 | 0.090 (0.929) |
| Weight (kg) | 65.93 ± 9.93 | 62.73 ± 10.49 | 0.857 (0.399) |
| MMSE-K score | 24.33 ± 3.22 | 25.60 ± 3.48 | 1.034 (0.310) |
| Onset (months) | 2.73 ± 1.22 | 2.47 ± 1.30 | 0.578 (0.568) |
| Variable | Experimental Group (n = 15) | Control Group (n = 15) | t (p) |
|---|---|---|---|
| K-TIS (score) | 12.67 ± 3.57 | 14.87 ± 3.87 | −1.616 (0.117) |
| PASS (score) | 25.00 ± 4.69 | 27.00 ± 4.29 | −1.218 (0.233) |
| BBS (score) | 23.07 ± 13.06 | 30.93 ± 13.03 | −1.651 (0.110) |
| LOS (mm2) | 6673.71 ± 4430.90 | 6749.42 ± 4393.63 | −0.047 (0.963) |
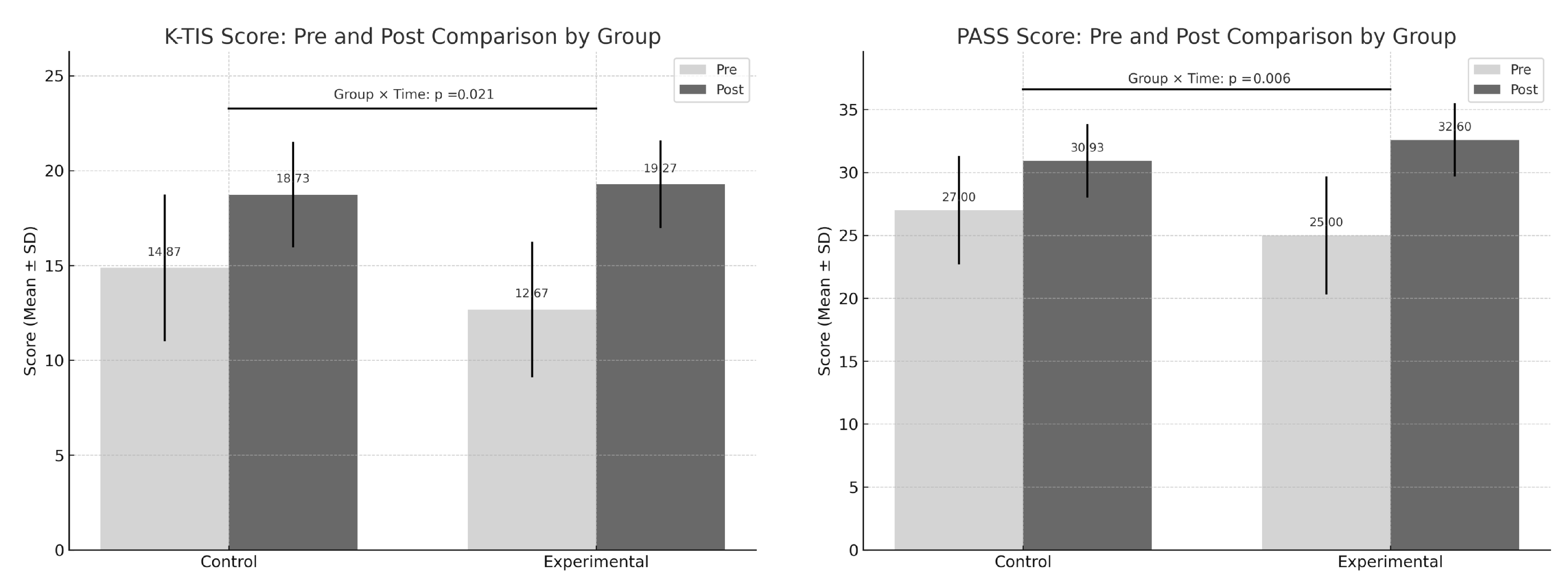
| Variable | Experimental Group (n = 15) | Control Group (n = 15) | Group X Time F(df), p |
|---|---|---|---|
| K-TIS (score) | |||
| Pre | 12.67 ± 3.57 | 14.87 ± 3.87 | |
| Post | 19.27 ± 2.31 | 18.73 ± 2.78 | |
| Change | 6.60 ± 3.56 | 3.86 ± 2.47 | F(1,28) = 5.958, p = 0.021 |
| PASS (score) | |||
| Pre | 25.00 ± 4.69 | 27.00 ± 4.29 | |
| Post | 32.60 ± 2.92 | 30.93 ± 2.93 | |
| Change | 7.60 ± 3.92 | 3.93 ± 2.63 | F(1,28) = 9.034, p = 0.006 |
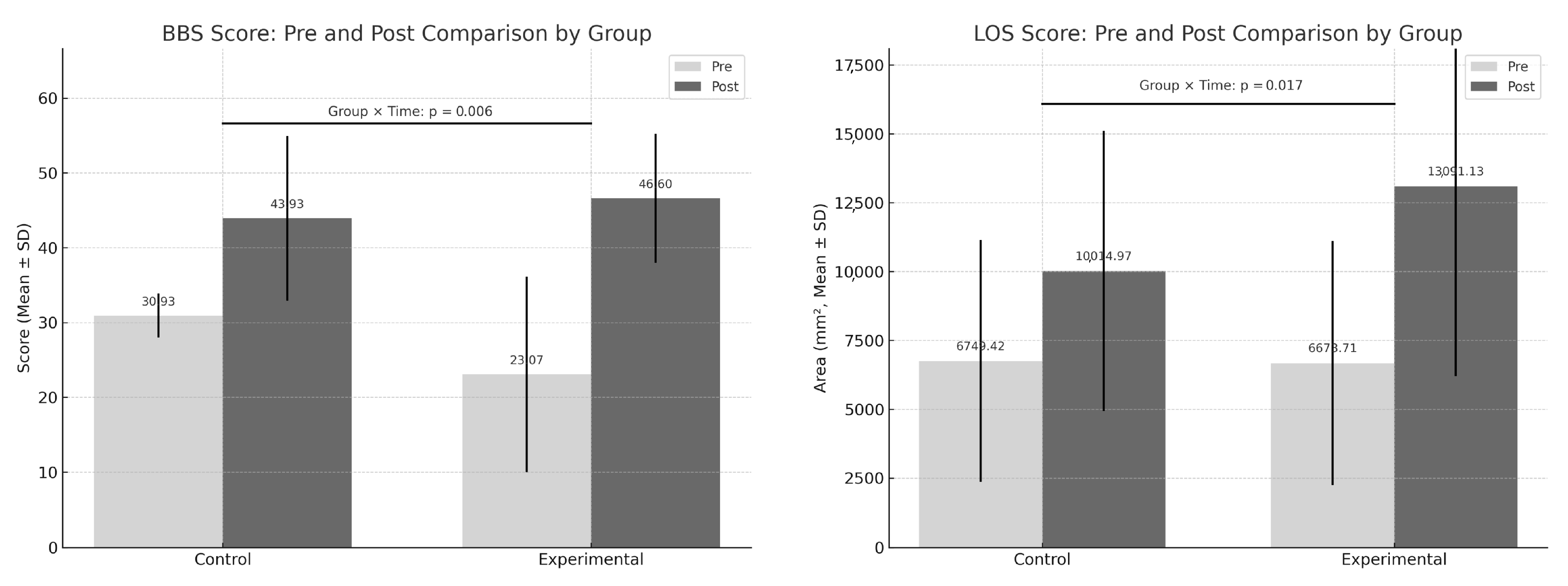
| Variable | Experimental Group (n = 15) | Control Group (n = 15) | Group X Time F(df), p |
|---|---|---|---|
| BBS (score) | |||
| Pre | 23.07 ± 13.06 | 30.93 ± 2.93 | |
| Post | 46.60 ± 8.63 | 43.93 ± 11.01 | |
| Change | 23.53 ± 9.94 | 13.00 ± 9.53 | F(1,28) = 8.767, p = 0.006 |
| LOS (mm2) | |||
| Pre | 6673.71 ± 4430.90 | 6749.42 ± 4393.63 | |
| Post | 13,091.13 ± 6896.00 | 10,014.97 ± 5086.36 | |
| Change | 6417.42 ± 4358.72 | 3265.55 ± 2056.75 | F(1,28) = 6.415, p = 0.017 |
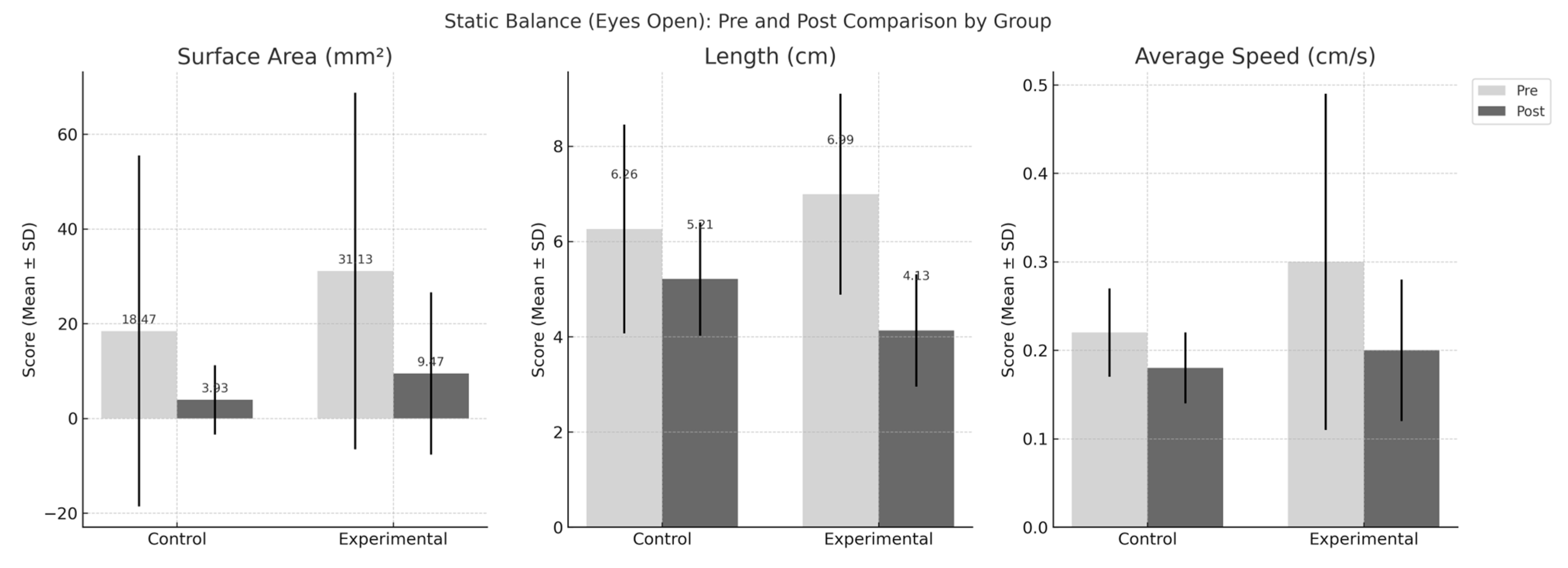
| Variable | Experimental Group (n = 15) | Control Group (n = 15) | Group X Time F(df), p |
|---|---|---|---|
| Eyes-open | |||
| Surface area (mm2) | |||
| Pre | 31.13 ± 37.63 | 18.47 ± 37.05 | |
| Post | 9.47 ± 17.12 | 3.93 ± 7.32 | |
| Change | −21.67 ± 34.84 | −14.53 ± 30.09 | F(1,28) = 0.360, p = 0.553 |
| Length (cm) | |||
| Pre | 6.99 ± 2.11 | 6.26 ± 2.19 | |
| Post | 4.13 ± 1.18 | 5.21 ± 1.19 | |
| Change | −2.86 ± 1.85 | −1.04 ± 1.85 | F(1,28) = 7.177, p = 0.012 |
| Average speed (cm/s) | |||
| Pre | 0.30 ± 0.19 | 0.22 ± 0.05 | |
| Post | 0.20 ± 0.08 | 0.18 ± 0.04 | |
| Change | −0.10 ± 0.16 | −0.04 ± 0.05 | F(1,28) = 1.503, p = 0.230 |
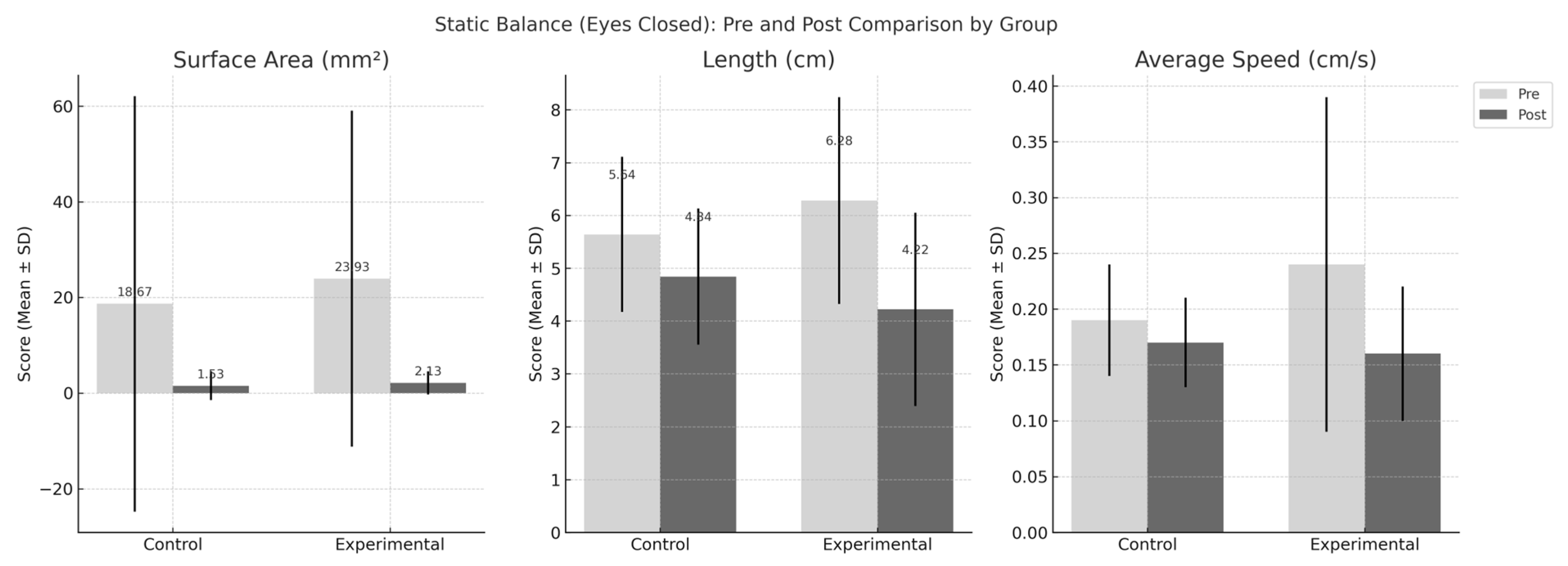
| Eyes-closed | |||
| Surface area (mm2) | |||
| Pre | 23.93 ± 35.14 | 18.67 ± 43.43 | |
| Post | 2.13 ± 2.41 | 1.53 ± 3.02 | |
| Change | −21.80 ± 33.57 | −17.13 ± 40.59 | F(1,28) = 0.118, p = 0.734 |
| Length (cm) | |||
| Pre | 6.28 ± 1.96 | 5.64 ± 1.47 | |
| Post | 4.22 ± 1.83 | 4.84 ± 1.29 | |
| Change | −2.06 ± 1.76 | −0.80 ± 1.06 | F(1,28) = 5.613, p = 0.025 |
| Average speed (cm/s) | |||
| Pre | 0.24 ± 0.15 | 0.19 ± 0.05 | |
| Post | 0.16 ± 0.06 | 0.17 ± 0.04 | |
| Change | −0.07 ± 0.13 | −0.02 ± 0.04 | F(1,28) = 2.185, p = 0.150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Yim, J. Effect of Virtual-Reality-Based Training, Including Preceding Trunk Stabilization Education, on Postural Control and Balance in Patients with Stroke: A Randomized Controlled Trial. Appl. Sci. 2025, 15, 7620. https://doi.org/10.3390/app15137620
Lee S, Yim J. Effect of Virtual-Reality-Based Training, Including Preceding Trunk Stabilization Education, on Postural Control and Balance in Patients with Stroke: A Randomized Controlled Trial. Applied Sciences. 2025; 15(13):7620. https://doi.org/10.3390/app15137620
Chicago/Turabian StyleLee, SeongMin, and JongEun Yim. 2025. "Effect of Virtual-Reality-Based Training, Including Preceding Trunk Stabilization Education, on Postural Control and Balance in Patients with Stroke: A Randomized Controlled Trial" Applied Sciences 15, no. 13: 7620. https://doi.org/10.3390/app15137620
APA StyleLee, S., & Yim, J. (2025). Effect of Virtual-Reality-Based Training, Including Preceding Trunk Stabilization Education, on Postural Control and Balance in Patients with Stroke: A Randomized Controlled Trial. Applied Sciences, 15(13), 7620. https://doi.org/10.3390/app15137620







