Abstract
Osteoporosis is a prevalent disease among the elderly, with fractures being one of the most serious consequences. Early diagnosis and accurate assessment of fracture risk could help prevent fractures. Radiomics employs advanced image analysis techniques for the development of diagnostic tools, thereby improving the accuracy of disease diagnosis and treatment strategies. Specifically, in the application of bone diseases, radiomics has proven effective in the diagnosis and prognostic evaluation of osteoporosis, osteoarthritis, and bone tumors. Radiomics allowed for quantitative characterization of bone geometry, material distribution, and microstructure, making it applicable to osteoporosis as well. In this review, an overview was provided regarding the current progress of radiomics based on clinical bone imaging in osteoporosis, including bone strength assessment, osteoporosis diagnosis, and fracture risk prediction. Additionally, the potential and challenges for their clinical application were summarized.
1. Introduction
Osteoporosis is a widespread global disease that substantially increases the risk of fractures, resulting in fractures that place considerable burdens on both patients and healthcare systems [1]. Data from 19 countries and regions covering hospitalized hip fracture patients between 2005 and 2018 revealed one-year all-cause mortality rates for hip fractures ranging from 14.4% in Singapore to 28.3% in the United Kingdom, yielding a median mortality rate of 22.4%. Mortality in men was particularly high, ranging from 19.2% to 35.8% [2]. It was essential to diagnose osteoporosis early and assess fracture risk accurately to prevent osteoporotic fractures and reduce related mortality. The T-score, derived from bone mineral density (BMD) measurement obtained by dual-energy X-ray absorptiometry (DXA), was considered the gold standard for diagnosing osteoporosis, with a score below −2.5. However, DXA could be sensitive to artifacts from surrounding tissue, leading to an overestimation of BMD and inadequate diagnosis of osteoporosis [3,4]. Similarly, the fracture risk assessment tool (FRAX), widely employed in clinical practice to evaluate fracture risk, has demonstrated limitations by either overestimating or underestimating fracture risk across different populations [5]. These discrepancies primarily arose from variations in calibration methods, inaccuracies in the assessment of clinical risk factors, and the lack of BMD [5]. The limitations in these techniques have spurred the investigation of additional image features and the development of novel imaging methods. For example, the trabecular bone score (TBS) extracted gray-scale texture features from 2D DXA images of the lumbar spine to represent 3D bone microarchitecture, thereby providing an independent perspective for fracture risk assessment distinct from traditional DXA and FRAX methods [6]. As an adjunct to DXA and/or FRAX methods, TBS has been incorporated into clinical guidelines to enhance fracture risk assessment [7]. Furthermore, the Radiofrequency Ultrasound Multispectral Analysis (REMS) represented an emerging non-ionizing imaging modality that provided a portable and radiation-free approach for BMD. The performance of REMS was comparable to that of DXA [8]. Additionally, biomechanical computed tomography analysis utilized opportunistic clinical computed tomography (CT) images to calculate bone strength and equivalent BMD for osteoporosis screening and fracture risk assessment. It has been shown to enhance the accuracy of osteoporosis diagnosis and fracture risk assessment in various populations [9,10]. However, it could only be implemented in specialized laboratories by trained technicians, limiting its widespread clinical application [11]. Therefore, there was an urgent need for convenient and accurate methods to diagnose osteoporosis and assess fracture risk.
Radiomics, an advanced image analysis technique, has demonstrated potential in quantitative, comprehensive, and automated high-throughput extraction of features from clinical images [12,13]. It excelled in mining image information and capturing disease characteristics that may not be readily detectable by the human eye [14]. Radiomics, as a quantitative analytical tool, has facilitated improved diagnostic and prognostic modeling in musculoskeletal disorders, from metabolic bone diseases like osteoporosis to degenerative and neoplastic conditions such as osteoarthritis and bone tumors [15,16,17]. Radiomics features extracted from bone images provided detailed insights into bone-quality-related information such as bone geometry, material distribution, and microstructure. Specifically, in the application of osteoporosis, these radiomics features have been employed to build classification and prediction models, facilitating bone strength assessment, osteoporosis diagnosis, and fracture risk prediction [17,18,19]. Currently, radiomics has demonstrated considerable advantages in osteoporosis. On one hand, bone-quality-related information from widely used clinical imaging modalities was efficiently extracted by radiomics, including radiographs, CT images, and magnetic resonance imaging (MRI) images, thus supporting opportunistic osteoporosis screening [20]. On the other hand, the radiomics-derived features, which differed from traditional bone quality parameters, hold potential as novel biomarkers for bone quality [21]. For instance, the radiomics feature wavelet-LLL_firstorder_10percentile employed wavelet transform to decompose complex signals, enabling effective quantification of the intricate structure of vertebrae [22,23]. This feature demonstrated high performance in detecting osteoporotic vertebral fractures, with an area under the curve (AUC) of 0.76 [23]. Additionally, entropy within the texture features was considered a potential sign for bone damage [24]. Therefore, radiomics offers a promising method for enhancing opportunistic osteoporosis screening, improving fracture risk prediction, and providing therapeutic efficacy assessment. The general workflow for applying radiomics to osteoporosis is depicted in Figure 1. It consists of six steps: image acquisition, image segmentation, image processing, radiomics feature extraction, radiomics feature selection, and modeling and analysis.
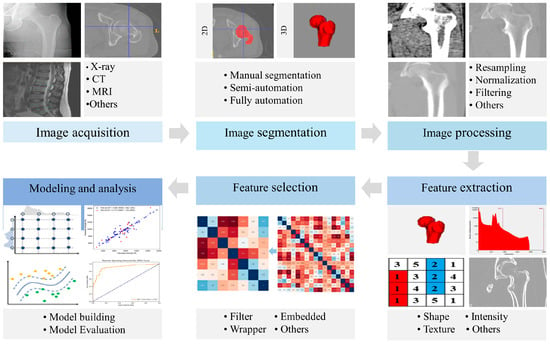
Figure 1.
General workflow of radiomics in osteoporosis.
In this review, current applications and the development of radiomics in osteoporosis were summarized. It was critical to conduct an accurate assessment of bone strength for predicting fracture risk. Thus, the utilization of radiomics for assessing bone strength was reviewed. Additionally, the application of radiomics derived from CT images, radiographs, and MRI images for diagnosing osteoporosis and predicting fracture risk was provided. Finally, the advantages, limitations, and potential clinical applications of radiomics in bone strength assessment, osteoporosis diagnosis, and fracture risk prediction were analyzed and summarized. The outline of this review is shown in Figure 2.
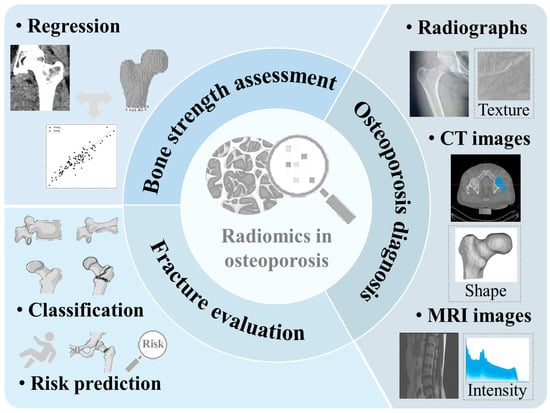
Figure 2.
Research tasks related to the applications of radiomics in osteoporosis are investigated in this review.
2. Literature Search Strategies
A hybrid search was conducted in the Web of Science, PubMed, and Scopus databases for studies published between 2018 and January 2025. The following search query was employed in Web of Science: ‘TS = ((Osteoporo* OR (Fracture* NEAR/3 (humer* OR spin* OR vertebra* OR “hip” OR “femur” OR “femoral” OR “lumbar” OR “forearm” OR “fragility”)) OR (“bone” NEAR/3 densit*) OR (“bone” NEAR/3 (strength OR load))) AND (radiomic* OR textur*)) AND PY = (2018–2025).’ An equivalent search query was applied for PubMed and Scopus. Additionally, citation searches for key studies were conducted, and references from systematic reviews and meta-analyses were examined to identify further relevant studies. The titles and abstracts of the retrieved studies were screened and evaluated based on the following inclusion criteria: (1) studies that utilized radiomics in osteoporosis; and (2) studies published in English. Exclusion criteria included review articles, conference abstracts, letters, and other non-empirical research. Following full-text assessment, the retained studies were included in this review.
3. Radiomics in Bone Strength Assessment
The commonly applied clinical method for evaluating bone strength relies on BMD measurement by DXA, which may not accurately reflect actual bone strength [25]. Finite element analysis based on quantitative computed tomography (QCT) images offered a more precise evaluation of bone strength; however, the complexity, high costs, and elevated radiation exposure associated with this method presented challenges for its widespread clinical application [26]. The lumbar strength prediction model, which combined gray-scale and geometric features manually extracted from specific regions of the lumbar spine using QCT images and machine learning algorithms, provided an alternative to the QCT-based finite element analysis method for calculating lumbar spine strength [27]. Radiomics, which automatically extracted a wide range of gray-scale, geometric, and textural features, offered a less time-consuming and more user-friendly method for assessing bone strength. It is noteworthy that the application of radiomics in bone strength prediction has primarily focused on femoral strength. The femoral failure load was effectively estimated by the prediction model, which integrated the internal femoral shaft thickness with texture features extracted from standard radiographs at 200-micron resolution, such as entropy, homogeneity, and contrast from the gray-level co-occurrence matrix (GLCM). The accuracy of the texture features may be enhanced by utilizing femur images with higher resolution than the standard radiographs [22,28]. Furthermore, the performance of the femoral failure load prediction model was improved by incorporating texture features such as fractal parameters, GLCM, and gray-scale run matrix with BMD, compared to using BMD alone. Specifically, this integration resulted in a significant increase in the coefficient of determination value from 0.74 to 0.82 (p < 0.05) [29]. Similarly, the combination of the BMD and bone volume fraction from the principal tensile region with the texture feature (correlation feature computed in the GLCM) from the principal compressive region yielded superior predictive performance for failure load under stance configuration compared to individual features [30]. In a recent study, radiomics features extracted from QCT images of the proximal femur reflecting geometric, gray, and texture characteristics were utilized with a machine learning algorithm to develop a femoral strength prediction model, achieving a coefficient of determination of 0.820 and the normalized root mean square error of 10.645% [31].
The validity of the radiomics texture features in the bone strength assessment model was supported by the correlation between those features and bone structure parameters. The homogeneity of texture features derived from hip radiographs exhibited a significant correlation (p < 0.05) with BMD and various bone structure parameters, such as bone volume fraction and structural model index calculated from CT images [32]. Additionally, GLCM features extracted from low-resolution MRI images were correlated with bone volume fraction, trabecular separation, and trabecular number as measured by high-resolution MRI (correlation coefficient R > 0.57) [33]. These findings suggested that radiomics features can effectively quantify bone structural characteristics, offering critical bone strength information distinct from BMD. Consequently, the bone strength assessment model incorporating radiomics features represented a non-invasive and effective method for evaluating bone strength, highlighting its potential for clinical application.
When assessing femoral strength in different subjects using radiomics, it was crucial to emphasize the selection of a repeatable region of interest (ROI) that strongly correlated with femoral strength. To ensure the repeatability of the ROI, consistency was required among different operators. Furthermore, the same operator must demonstrate consistency when selecting the ROI on the same image at different times [16]. A repeatable ROI was necessary for accurate quantitative analysis of radiomics features [29,34]. Five ROIs, including the femoral head, upper neck, lower neck, greater trochanter, and femoral shaft, were selected from the proximal femur of the subjects to extract texture features. It was demonstrated that the correlations between texture features from different regions and femoral strengths varied [35]. Comparing the texture features extracted from the upper femoral neck (the principal tensile region) of the proximal femur and the lower femoral neck (the principal compressive region) between the fracture and non-fracture groups revealed that the mean entropy of the principal tensile region in the fracture group was significantly lower than that in the non-fracture group (p = 0.029). In contrast, no significant differences (p > 0.190) were observed in any texture features from the principal compressive region between the two groups [30]. Hence, it was essential to choose an ROI that was both closely related to bone strength and exhibited high repeatability when assessing femur strength through radiomics.
4. Radiomics in Osteoporosis Diagnosis
CT images, radiographs, and MRI images were the most commonly utilized orthopedic clinical images. The application of radiomics based on CT images, radiographs, and MRI images in osteoporosis diagnosis was reviewed in this section (Table 1).

Table 1.
Studies of osteoporosis diagnosis using radiomics, their general characteristics, and performances.
4.1. Radiomics Based on CT Images in Osteoporosis Diagnosis
CT images of the chest, abdomen, and hip were frequently utilized in routine physical examination or disease follow-up. It was cost-effective to leverage these clinical opportunistic CT images for osteoporosis screening, as it avoided additional radiation exposure and enhanced screening rates for osteoporosis [38,51]. Currently, machine learning algorithms that utilize Hounsfield Unit values or radiomics features, along with fully automated deep learning algorithms, have become widely adopted as artificial intelligence (AI)-driven tools for osteoporosis screening based on CT images [40,52].
Hounsfield Unit values derived from CT images exhibited a moderate correlation with BMD measured by DXA, providing a useful diagnostic marker for osteoporosis [53]. The advantage of this method lies in its ability to identify patients with low BMD before orthopedic surgery, offering a fast and effective screening tool. However, radiomics features, which encompass three-dimensional geometric and textural features, have proven superior to Hounsfield Unit values in diagnosing osteoporosis, with AUC values of 0.92 and 0.84, respectively [36]. These radiomics features demonstrated strong diagnostic performance across various anatomical regions. For instance, radiomics features extracted from lumbar or hip CT images, when combined with machine learning algorithms such as random forest, support vector machines, and extreme gradient boosting, have demonstrated AUC values of no less than 0.886 in osteoporosis diagnosis [40,41].
Notably, the performance of both Hounsfield Unit values and radiomics features in osteoporosis diagnosis was influenced by the selection of ROI and CT scan parameters, such as tube voltage and reconstructed slice thickness [39,54]. Variations in tube voltage can negatively impact the repeatability of radiomics features, reducing the model’s diagnostic accuracy. Therefore, it was critical to implement the standardized processes of radiomics to maintain consistency in ROI selection and scan parameter settings for improving diagnostic reliability [43,55]. Fully automated deep learning algorithms offered advantages in ROI selection, given their high automation and repeatability. Deep learning algorithms typically require large, labeled CT image datasets for training. However, it is challenging to obtain such high-quality clinical data [56]. Moreover, deep learning algorithms might struggle with learning texture features effectively due to their inherent higher dimensionality [57]. In contrast, radiomics excels in extracting a wide array of high-dimensional texture features, offering a unique advantage in osteoporosis diagnosis. To enhance the efficiency and accuracy of osteoporosis diagnosis, researchers have increasingly explored the integration of radiomics with deep learning algorithms [44].
Two advantages in osteoporosis diagnosis were offered by integrating radiomics with deep learning algorithms. On one hand, it effectively addressed the challenges of time consumption and low repeatability inherent in the traditional manual or semi-automatic segmentation methods by applying deep learning algorithms to ROI segmentation in radiomics [42,58,59]. On the other hand, deep transfer learning algorithms, utilizing pre-trained networks for feature extraction, offered a solution to the challenge of limited labeled CT images by enabling accurate analysis on smaller datasets [60]. The integration of features derived from deep transfer learning with radiomics features has been shown to enhance diagnostic performance for osteoporosis [44,61]. This integrated method not only leveraged the detailed analysis capabilities of radiomics, but also capitalized on the automation benefits of deep learning algorithms, resulting in an efficient and accurate diagnostic solution.
4.2. Radiomics Based on Radiographs in Osteoporosis Diagnosis
Radiographs have been extensively utilized in clinical practice due to their lower radiation exposure and cost compared to CT images. Standard radiographs offer adequate spatial resolution for analyzing bone structure and geometry, demonstrating potential in osteoporosis diagnosis [62]. The application of radiographs from various anatomical regions, such as the hip, spine, chest, limbs, and mandible, combined with different image analysis techniques, has yielded promising results in osteoporosis detection [63]. However, these techniques differed in complexity, accuracy, and clinical applicability, highlighting the need to choose the appropriate method.
Traditional methods for analyzing bone morphology and structure in radiographs primarily rely on manual measurements or specialized software tools. For instance, the canal–calcar ratio, canal flare index, morphological cortical index, and canal bone ratio of the proximal femur could be manually quantified from anteroposterior radiographs. These parameters offered insights into changes in bone density and structure, which were valuable for diagnosing osteoporosis [64]. The reproducibility results indicated that, despite high intra-operator repeatability reflected by correlation coefficients exceeding 0.800, significant inter-operator differences were observed (p < 0.05), reflecting poor overall reproducibility [64]. Additionally, trabecular bone structure was frequently quantitatively assessed by the trabecular bone score from lumbar spine DXA images, though this process required utilizing specialized software [65]. The above methods have proven useful in diagnosing osteoporosis, but they still have limitations. Manual measurement techniques depended heavily on operator expertise, which could introduce subjective bias and were time consuming, rendering them impractical for large-scale screening. Although specialized software could improve efficiency, it required specific hardware or paid licenses, thus limiting its broader application.
Radiomics and AI-based automation technologies have garnered increasing attention, particularly deep learning algorithms, which show potential in automating the diagnosis of osteoporosis [66]. However, similar to CT-based osteoporosis diagnosis, deep learning algorithms based on clinical radiographs still face challenges due to the limited availability of large, high-quality training datasets, which can result in poor generalization and overfitting to impact model performance and reliability [67].
The application of deep transfer learning algorithms, which leverage pre-trained networks for feature extraction, has addressed the challenge of limited labeled data [60]. The imaging features derived through this approach are commonly referred to as deep learning features. Currently, when radiomics features and/or deep learning features serve as inputs to models constructed using the same machine learning algorithms, the performance of osteoporosis diagnosis varies across different radiograph datasets. In a multi-center study involving 1325 patients, it was found that radiomics features extracted from lumbar radiographs outperformed deep learning-based models in diagnosing osteoporosis [47]. In contrast, another study indicated that, despite employing the same multi-layer perceptron algorithm, the model utilizing solely radiomics features as input exhibited inferior performance compared to the model based exclusively on deep learning features [46]. Nevertheless, an increasing number of studies indicated that combining deep learning features, clinical features, and radiomics features generally yielded superior performance than using these features individually, thereby assisting clinicians in making more reliable and accurate diagnoses. Radiomics features extracted from hip radiographs demonstrated great diagnostic accuracy with an AUC of 0.770, which was further enhanced to 0.890 when combining with deep learning features [46]. Similarly, the osteoporosis classification model developed by integrating texture features and structural information from lumbar radiographs with a deep learning classification network achieved an average accuracy, sensitivity, and specificity that were 9.83% higher than those of direct diagnoses made by clinicians without assistance [68]. These findings suggested that a fusion method combining multidimensional data could leverage the advantages of both radiomics and deep learning to improve the accuracy of osteoporosis diagnosis, which supported clinicians in improving diagnostic capabilities and providing more precise and reliable medical services for patients.
Early and severe osteoporosis is associated with endocrinopathies or metastatic involvement, necessitating accurate assessment of bone status [69,70]. Although traditional methods based on radiographs continued to play a crucial role in osteoporosis diagnosis, their limitations highlighted the need for more efficient and feasible tools. Combining the cost-effectiveness of radiographs with innovative diagnostic technologies, such as radiomics and AI, will offer new opportunities for screening and personalized treatment of osteoporosis.
4.3. Radiomics Based on MRI Images in Osteoporosis Diagnosis
MRI is an imaging modality widely employed in the clinical evaluation of various diseases, including acute trauma, inflammation, metabolic disorders, tumors, and musculoskeletal diseases. Its application has been steadily increasing in recent years [71,72]. By utilizing these clinically available MRI images for early osteoporosis diagnosis and intervention, it is possible to reduce costs and minimize exposure to ionizing radiation. The progression of osteoporosis was associated with the degradation of bone microstructure, alongside reduced bone blood flow and increased bone marrow fat, all leading to bone loss [73]. Unlike radiographs and CT images, MRI images not only show bone microstructure, but also reflect changes in bone marrow composition content [74,75]. However, it was difficult to recognize these heterogeneous features through traditional visual inspection. Radiomics enhanced this process by quantitatively characterizing these features, providing valuable support for the accurate diagnosis of osteoporosis.
Radiomics based on MRI images with different weighted sequences exhibited varying performance in detecting osteoporosis. However, integrating radiomics features from multimodal images might enhance diagnostic accuracy. Sagittal T1-weighted images (T1WI) and T2-weighted images (T2WI), commonly employed in routine spinal MRI protocols, have proven effective in osteoporosis detection [76]. T1WI, with its high signal-to-noise ratio, was particularly sensitive to changes in bone marrow fat, while T2WI provided insights into the changes in bone blood and water composition [48,77]. Radiomics features extracted from multimodal T1WI and T2WI have demonstrated greater ability to distinguish between osteoporosis and non-osteoporosis patients compared to those derived from a single modality [50]. However, another study highlighted that while radiomics based on multimodal images generally outperforms T2WI alone, it may not always surpass the diagnostic performance of T1WI [49]. This indicated that the utility of radiomics features derived from different MRI sequences for osteoporosis diagnosis varied, and their combined application may yield the most effective results, contingent upon the specific images.
Therefore, radiomics extracted from various MRI sequences serves as a reliable diagnostic tool for osteoporosis. By thoroughly extracting and analyzing imaging features from clinically available MRI images, it is possible to more comprehensively capture the changes in the bone microstructure and bone marrow composition associated with osteoporosis.
5. Radiomics in Osteoporotic Fractures Classification and Fracture Risk Prediction
5.1. Osteoporotic Fractures Classification
Comparative analysis of radiomics features between individuals with osteoporosis and a history of fragility fractures (fracture cohort) and healthy controls without osteoporosis or fracture history (non-fracture cohort) held critical value. This approach enabled the identification of imaging biomarkers for osteoporotic fracture detection and may elucidate predictive signatures characterizing fracture risk. Analysis of vertebral MRI images revealed that the trabecular bone texture in the fracture cohort exhibited more heterogeneity compared to the non-fracture cohort. This heterogeneity was quantitatively described by radiomics features, showing 76% higher contrast, 10% higher entropy, and 50% lower angular second moment [78]. Bone texture features correlated with microstructural parameters such as bone volume fraction, trabecular number, trabecular separation, and fractal dimension [79]. Therefore, radiomics offered a more comprehensive method of fracture classification by enabling high-dimensional, quantitative analysis of texture features, thereby enhancing fracture risk evaluation.
Radiomics features extracted from various imaging modalities have demonstrated promising efficacy in classifying fractures at multiple anatomical sites. In a study comparing bone volume fraction and texture features obtained from CT images, the low-energy acetabular fracture cohort exhibited lower bone volume fraction compared to the non-fracture cohort. Significant differences (p < 0.05) were also observed in the femoral head texture characteristics between the two cohorts [80]. Similarly, texture features extracted from clinical opportunistic CT and MRI images, when combined with machine learning algorithms, were effective in distinguishing the vertebral fracture cohort from the non-fracture cohort, achieving AUC values surpassing 0.9 [81]. These findings indicated that radiomics features offered supplementary bone quality information beyond areal BMD, enhancing fracture identification. In patients with osteoporotic femoral fractures, MRI-based radiomic features, including lower kurtosis, reduced low gray-level emphasis, and higher dependent non-uniformity, were linked to changes in trabecular bone microstructure. These features demonstrated potential for detecting femoral fractures [82]. Furthermore, in a study utilizing thoracolumbar and lumbar spine radiographs from three hospitals, features were extracted using pre-trained deep transfer learning algorithms. A robust classification model for vertebral compression fractures was developed by integrating these extracted features with radiomics features [83]. When combined with clinical baseline data, the classification performance was further enhanced [84].
The incorporation of imaging features that quantitatively describe the spatial heterogeneity of bone marrow across different populations was important for improving fracture classification. A study comparing vertebral MRI texture features from premenopausal and postmenopausal women revealed significant differences (p < 0.05) in bone marrow spatial heterogeneity, further helping the classification of osteoporotic fractures and non-fractures [85,86]. The mean proton density fat fraction effectively quantified spatial heterogeneity in bone marrow. Therefore, a fracture classification model in combination with proton density fat fraction, volumetric BMD, and two additional texture features derived from chemical shift encoding-based water-fat MRI and CT images demonstrated excellent performance [85]. In summary, the combination of multimodal image features and radiomics helped to improve the accuracy of osteoporotic fracture detection and fracture risk prediction.
5.2. Osteoporotic Fracture Risk Prediction
The commonly used FRAX estimates fracture risk by evaluating seven key clinical risk factors: prior fragility fractures, parental hip fracture history, smoking, glucocorticoid use, alcohol consumption, rheumatoid arthritis, and other causes of secondary osteoporosis (e.g., age, sex, body mass index). However, FRAX did not account for important fracture risk factors such as bone microstructure and muscle mass, leading to overestimation or underestimation of fracture risk in certain populations [5,87]. This has prompted researchers to combine imaging features that reflect bone microstructure and muscle mass features with the above clinical risk factors to improve fracture risk prediction.
As an advanced imaging method and image analysis technique, radiomics enables the extraction of additional bone quality features that may not be discernible to the naked eye or through traditional BMD measurements. These features can serve as valuable factors in assessing fracture risk, thereby enhancing the accuracy of fracture risk predictions [88,89]. Three-dimensional texture features extracted from high-resolution peripheral quantitative computed tomography (HR-pQCT) images of the tibia demonstrated superior performance compared to clinical factors or femoral neck BMD alone in distinguishing volunteers with a history of fractures. These texture features have the potential to serve as fracture risk factors, thereby enhancing fracture risk identification [90]. Similarly, texture features derived from femur DXA images were found to be more effective in predicting trochanteric fractures than the commonly used femoral neck T-score, resulting in an increase in the AUC from 0.784 to 0.839, which suggested that texture features obtained from DXA images were valuable in fracture risk assessment [91]. Combining FRAX scores with radiomics features and additional clinical data improved the accuracy of osteoporotic fracture predictions [92]. Therefore, by comparing radiomics features extracted from vertebral body or hip CT images, radiographs, MRI images, and HR-pQCT images of osteoporotic fractures with those of non-fracture populations, additional fracture risk factors can be identified. This method aided in enhancing the accuracy of fracture risk prediction.
Fracture timing played a pivotal role in accurately assessing fracture risk, underlining the necessity for longitudinal studies [93]. In a prospective cohort study with a follow-up of over four years, a multivariate Cox model was developed using a radiomics score derived from the femoral DXA image. After adjusting for clinical risk factors (age, body mass index, fracture history, and femoral neck T-score), it was found that for each point increase in the radiomics score, the risk of hip fracture increased by 4% [21]. Combining radiomics scores with clinical risk factors further improved fracture risk prediction, indicating that radiomics scores were independent predictors of hip fractures. Moreover, in predicting new fractures after percutaneous vertebral augmentation, the integration of clinical parameters with radiomics features from vertebral MRI achieved strong predictive performance, outperforming models based on clinical parameters or imaging features alone [94]. Additionally, the erector spinal muscle area was independently associated with vertebral compression fractures. A nomogram incorporating the erector spinal muscle area, radiomics score, and vertebral CT values demonstrated robust predictive performance for the risk of vertebral compression fracture over three to four years [23]. Collectively, these findings underscore the value of radiomics scores derived from clinical imaging in complementing traditional tools such as FRAX, thereby improving the precision of fracture risk predictions.
6. Future Perspectives and Conclusions
Radiomics has been successfully applied in bone strength assessment, osteoporosis diagnosis, and fracture risk prediction, contributing to enhanced performance and efficiency while demonstrating considerable clinical potential. The integration of radiomics features from multimodal images, such as CT, radiographs, and MRI, along with the incorporation of clinical features, deep learning features, and other relevant features, facilitates a more comprehensive assessment of bone quality. This multimodal method is expected to further improve the performance of radiomics in assessing bone strength, diagnosing osteoporosis, and predicting fracture risk.
Geometric parameters (such as femoral neck-shaft angle, femoral head diameter, and femoral neck width) and microstructural parameters (such as bone volume fraction and trabecular number) are commonly utilized to quantitatively describe bone quality. While radiomics features derived from bone images also provide quantitative depictions of bone quality, the relationship between these radiomics features and the parameters above requires further investigation. Clarifying these relationships will facilitate better interpretation of radiomics features by clinicians, thereby enhancing the understanding, adoption, and clinical utility of radiomics in osteoporosis diagnosis and treatment.
While radiomics is not intended to supplant clinical judgment, it has demonstrated considerable potential as an adjunctive tool in supporting clinical decision-making. Consequently, radiomics may facilitate the development of diagnostic, predictive, and prognostic models, thereby enhancing personalized management strategies [95,96]. Nonetheless, several limitations have constrained its clinical translation, including the variability in feature repeatability due to differences in scanning parameters, challenges in ROI selection, the absence of multi-center external validation, and the limited clinical interpretability of radiomics features [97]. Many studies have focused on improving the reproducibility of radiomics features and associated outcomes through the development and implementation of standardized frameworks and guidelines, including the image biomarker standardization initiative (IBSI), the radiomics quality score (RQS), and the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) [96]. Furthermore, the facilitation of data sharing to enhance dataset robustness is crucial for advancing the clinical translation of radiomics [98]. Lastly, the integration of radiomics into routine clinical radiology workflows, including incorporation within existing radiology information systems (RIS) and picture archiving and communication systems (PACS), remains a critical challenge [20]. In summary, as challenges related to standardization, multi-center validation, technical integration, and interpretability are progressively addressed, radiomics is anticipated to evolve into a robust and efficient adjunctive tool in the clinic, with the potential to revolutionize the diagnosis, prognostication, and personalized management of diseases such as osteoporosis.
Author Contributions
Conceptualization, S.L.; methodology, S.L.; investigation, S.L., P.S., C.L., Q.Z., and J.Z.; resources, S.L.; data curation, S.L., P.S., C.L., Q.Z., and J.Z.; writing—original draft preparation, S.L.; writing—review and editing, S.L. and H.G.; project administration, H.G.; funding acquisition, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (12272029), and a research grant from Hangzhou International Innovation Institute, Beihang University (2024KQ093).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Figure 2 was partly generated using Servier Medical Art (https://creativecommons.org/licenses/by/4.0/ last accessed on 3 July 2025), provided by Servier, and licensed under CC BY 4.0.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial intelligence |
| AUC | Area under the curve |
| BMD | Bone mineral density |
| CT | Computed tomography |
| DSC | Dice similarity coefficient |
| DXA | Dual-energy X-ray absorptiometry |
| FRAX | The fracture risk assessment tool |
| GLCM | Gray-level co-occurrence matrix |
| HR-pQCT | High-resolution peripheral quantitative computed tomography |
| IBSI | Image biomarker standardization initiative |
| ICC | Intraclass correlation coefficient |
| MRI | Magnetic resonance imaging |
| PACS | Picture archiving and communication systems |
| QCT | Quantitative computed tomography |
| REMS | Radiofrequency ultrasound multispectral analysis |
| RIS | Radiology information systems |
| RQS | Radiomics quality score |
| ROI | Region of interest |
| T1WI | T1-weighted images |
| T2WI | T2-weighted images |
| TBS | Trabecular bone score |
| TRIPOD | Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis |
References
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteopors. 2021, 16, 82. [Google Scholar] [CrossRef]
- Sing, C.W.; Lin, T.C.; Bartholomew, S.; Bell, J.S.; Bennett, C.; Beyene, K.; Bosco-Levy, P.; Bradbury, B.D.; Chan, A.H.Y.; Chandran, M.; et al. Global epidemiology of hip fractures: Secular trends in incidence rate, post-fracture treatment, and all-cause mortality. J. Bone Miner. Res. 2023, 38, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Slart, R.H.J.A.; Ali, D.S.; Bock, O.; Carey, J.J.; Camacho, P.; Engelke, K.; Erba, P.A.; Harvey, N.C.; Lems, W.F.; et al. Osteoporotic fractures: Diagnosis, evaluation, and significance from the international working group on DXA best practices. Mayo Clin. Proc. 2024, 99, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Martineau, P.; Bazarjani, S.; Zuckier, L.S. Artifacts and incidental findings encountered on dual-energy X-ray absorptiometry: Atlas and analysis. Semin. Nucl. Med. 2015, 45, 458–469. [Google Scholar] [CrossRef]
- Leslie, W.D.; ASBMR Task Force on Clinical Algorithms for Fracture Risk. Effect of race/ethnicity on united states FRAX calculations and treatment qualification: A registry-based study. J. Bone Miner. Res. 2023, 38, 1742–1748. [Google Scholar] [CrossRef]
- Hans, D.; Goertzen, A.L.; Krieg, M.A.; Leslie, W.D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J. Bone Miner. Res. 2011, 26, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 Update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef]
- Adami, G.; Arioli, G.; Bianchi, G.; Brandi, M.L.; Caffarelli, C.; Cianferotti, L.; Gatti, D.; Girasole, G.; Gonnelli, S.; Manfredini, M.; et al. Radiofrequency echographic multi spectrometry for the prediction of incident fragility fractures: A 5-year follow-up study. Bone 2020, 134, 115297. [Google Scholar] [CrossRef]
- Lin, J.K.; Hearn, C.M.; Getzen, E.; Long, Q.; Lee, D.C.; Keaveny, T.M.; Jayadevappa, R.; Robinson, K.W.; Wong, Y.N.; Maxwell, K.N.; et al. Validation of biomechanical computed tomography for fracture risk classification in metastatic hormone-sensitive prostate cancer. Eur. Urol. Oncol. 2024, 7, 794–803. [Google Scholar] [CrossRef]
- Keaveny, T.M.; Adams, A.L.; Fischer, H.; Brara, H.S.; Burch, S.; Guppy, K.H.; Kopperdahl, D.L. Increased risks of vertebral fracture and reoperation in primary spinal fusion patients who test positive for osteoporosis by Biomechanical Computed Tomography analysis. Spine J. 2023, 23, 412–424. [Google Scholar] [CrossRef]
- Keaveny, T.M.; Clarke, B.L.; Cosman, F.; Orwoll, E.S.; Siris, E.S.; Khosla, S.; Bouxsein, M.L. Biomechanical computed tomography analysis (BCT) for clinical assessment of osteoporosis. Osteoporos. Int. 2020, 31, 1025–1048. [Google Scholar] [CrossRef] [PubMed]
- Mian, A.; Kamnitsas, K. Gordon-weeks a: Radiomics for treatment planning in liver cancers. JAMA Surg. 2025, 160, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.J.; Su, G.H.; You, C.; Zhang, X.; Xiao, Y.; Jiang, Y.Z.; Shao, Z.M. Radiomics in breast cancer: Current advances and future directions. Cell Rep. Med. 2024, 5, 101719. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Jiang, T.; Lau, S.H.; Zhang, J.; Chan, L.C.; Wang, W.; Chan, P.K.; Cai, J.; Wen, C. Radiomics signature of osteoarthritis: Current status and perspective. J. Orthop. Transl. 2024, 45, 100–106. [Google Scholar] [CrossRef]
- Gitto, S.; Cuocolo, R.; Huisman, M.; Messina, C.; Albano, D.; Omoumi, P.; Kotter, E.; Maas, M.; Van Ooijen, P.; Sconfienza, L.M. CT and MRI radiomics of bone and soft-tissue sarcomas: An updated systematic review of reproducibility and validation strategies. Insights Imaging 2024, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Shuai, P.; Liu, Y.; Yong, T.; Liu, Y.; Li, H.; Zheng, X. Diagnostic performance of radiomics for predicting osteoporosis in adults: A systematic review and meta-analysis. Osteoporos. Int. 2024, 35, 1693–1707. [Google Scholar] [CrossRef]
- Wang, S.; Tong, X.; Fan, Y.; Hu, M.; Cui, J.; Li, J.; Liu, Y.; Xiao, Q.; Fang, X. Combining deep learning and radiomics for automated, objective, comprehensive bone mineral density assessment from low-dose chest computed tomography. Acad. Radiol. 2024, 31, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mao, S.S.; Budoff, M.J. Trabecular bone mineral density as measured by thoracic vertebrae predicts incident hip and vertebral fractures: The multi-ethnic study of atherosclerosis. Osteoporos. Int. 2024, 35, 1061–1068. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging- “how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Hong, N.; Park, H.; Kim, C.O.; Kim, H.C.; Choi, J.Y.; Kim, H.; Rhee, Y. Bone radiomics score derived from DXA hip images enhances hip fracture prediction in older women. J. Bone Miner. Res. 2021, 36, 1708–1716. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, X.; Cui, W.; Wang, X.; Hu, N.; Tang, H.; Zhang, C.; Shen, J.; Xie, C.; Chen, X. A computed tomography-based radiomics nomogram for predicting osteoporotic vertebral fractures: A longitudinal study. J. Clin. Endocr. Metab. 2023, 108, e283–e294. [Google Scholar] [CrossRef]
- Muehlematter, U.J.; Mannil, M.; Becker, A.S.; Vokinger, K.N.; Finkenstaedt, T.; Osterhoff, G.; Osterhoff, G.; Fischer, M.A.; Guggenberger, R. Vertebral body insufficiency fractures: Detection of vertebrae at risk on standard CT images using texture analysis and machine learning. Eur. Radiol. 2019, 29, 2207–2217. [Google Scholar] [CrossRef]
- Rezaei, A.; Dragomir-Daescu, D. Femoral strength changes faster with age than BMD in both women and men: A biomechanical study. J. Bone Miner. Res. 2015, 30, 2200–2206. [Google Scholar] [CrossRef]
- Johannesdottir, F.; Thrall, E.; Muller, J.; Keaveny, T.M.; Kopperdahl, D.L.; Bouxsein, M.L. Comparison of non-invasive assessments of strength of the proximal femur. Bone 2017, 105, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gong, H.; Zhang, K.; Zhang, M. Prediction of lumbar vertebral strength of elderly men based on quantitative computed tomography images using machine learning. Osteoporos. Int. 2019, 30, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Chappard, C.; Bousson, V.; Bergot, C.; Mitton, D.; Marchadier, A.; Moser, T.; Benhamou, C.L.; Laredo, J.D. Prediction of femoral fracture load: Cross-sectional study of texture analysis and geometric measurements on plain radiographs versus bone mineral density. Radiology 2010, 255, 536–543. [Google Scholar] [CrossRef]
- Le Corroller, T.; Halgrin, J.; Pithioux, M.; Guenoun, D.; Chabrand, P.; Champsaur, P. Combination of texture analysis and bone mineral density improves the prediction of fracture load in human femurs. Osteoporos. Int. 2012, 23, 163–169. [Google Scholar] [CrossRef]
- Fujii, M.; Aoki, T.; Okada, Y.; Mori, H.; Kinoshita, S.; Hayashida, Y.; Hajime, M.; Tanaka, K.; Tanaka, Y.; Korogi, Y. Prediction of femoral neck strength in patients with diabetes mellitus with trabecular bone analysis and tomosynthesis images. Radiology 2016, 281, 933–939. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Zhang, M.; Gong, H.; Jia, S.; Zhang, J.; Jia, Z. Explainable machine-learning-based prediction of QCT/FEA-calculated femoral strength under stance loading configuration using radiomics features. J. Orthop. Res. 2025, 43, 161–172. [Google Scholar] [CrossRef]
- Thevenot, J.; Hirvasniemi, J.; Finnilä, M.; Pulkkinen, P.; Kuhn, V.; Link, T.; Eckstein, F.; Jämsä, T.; Saarakkala, S. Trabecular homogeneity index derived from plain radiograph to evaluate bone quality. J. Bone Miner. Res. 2013, 28, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Tameem, H.Z.; Selva, L.E.; Sinha, U.S. Texture measure from low resolution MR images to determine trabecular bone integrity in osteoporosis. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. 2007, 2007, 2027–2030. [Google Scholar]
- Harrison, L.C.V.; Nikander, R.; Sikiö, M.; Luukkaala, T.; Helminen, M.T.; Ryymin, P.; Soimakallio, S.; Eskola, H.J.; Dastidar, P.; Sievänen, H. MRI texture analysis of femoral neck: Detection of exercise load-associated differences in trabecular bone. J. Magn. Reson. Imaging 2011, 34, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.B.; Carballido-Gamio, J.; Fritscher, K.; Schubert, R.; Haenni, M.; Hengg, C.; Majumdar, S.; Link, T.M. Development and testing of texture discriminators for the analysis of trabecular bone in proximal femur radiographs. Med. Phys. 2009, 36, 5089–5098. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Xu, X.J.; Wang, R.; Chen, C.M. Radiomics analysis based on lumbar spine CT to detect osteoporosis. Eur. Radiol. 2022, 32, 8019–8026. [Google Scholar] [CrossRef]
- Cheng, L.; Cai, F.; Xu, M.; Liu, P.; Liao, J.; Zong, S. A diagnostic approach integrated multimodal radiomics with machine learning models based on lumbar spine CT and X-ray for osteoporosis. J. Bone Miner. Metab. 2023, 41, 877–889. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, S.; Chen, S.; He, Y.; Gao, H.; Yan, L.; Hu, X.; Li, P.; Shen, H.; Luo, M.; et al. Prediction of osteoporosis using radiomics analysis derived from single source dual energy CT. BMC Musculoskel Dis. 2023, 24, 100. [Google Scholar] [CrossRef]
- Tong, X.; Wang, S.; Zhang, J.; Fan, Y.; Liu, Y.; Wei, W. Automatic osteoporosis screening system using radiomics and deep learning from low-dose chest CT images. Bioengineering 2024, 11, 50. [Google Scholar] [CrossRef]
- Chen, B.; Cui, J.; Li, C.; Xu, P.; Xu, G.; Jiang, J.; Xue, P.; Sun, Y.; Cui, Z. Application of radiomics model based on lumbar computed tomography in diagnosis of elderly osteoporosis. J. Orthop. Res. 2024, 42, 1356–1368. [Google Scholar] [CrossRef]
- Fang, K.; Zheng, X.; Lin, X.; Dai, Z. Unveiling osteoporosis through radiomics analysis of hip CT imaging. Acad. Radiol. 2024, 31, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Ha, H.I.; Lim, H.K.; Han, J.; Pak, S. Femoral osteoporosis prediction model using autosegmentation and machine learning analysis with PyRadiomics on abdomenpelvic computed tomography (CT). Quant. Imag. Med. Surg. 2024, 14, 3959–3969. [Google Scholar] [CrossRef]
- Tong, X.; Wang, S.; Cheng, Q.; Fan, Y.; Fang, X.; Wei, W.; Li, J.; Liu, Y.; Liu, L. Effect of fully automatic classification model from different tube voltage images on bone density screening: A self-controlled study. Eur. J. Radiol. 2024, 177, 111521. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Yan, L.; Chen, S.; Zhang, K. Predicting osteoporosis and osteopenia by fusing deep transfer learning features and classical radiomics features based on single-source dual-energy CT imaging. Acad. Radiol. 2024, 31, 4159–4170. [Google Scholar] [CrossRef]
- Areeckal, A.S.; Jayasheelan, N.; Kamath, J.; Zawadynski, S.; Kocher, M.; David, S.S. Early diagnosis of osteoporosis using radiogrammetry and texture analysis from hand and wrist radiographs in Indian population. Osteoporos. Int. 2018, 29, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, B.R.; Chae, H.D.; Lee, J.; Ye, S.J.; Kim, D.H.; Hong, S.H.; Choi, J.Y.; Yoo, H.J. Deep radiomics-based approach to the diagnosis of osteoporosis using hip radiographs. Radiol.-Artif. Intell. 2022, 4, e210212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, Z.; Yan, R.; Lai, B.; Wu, G.; You, J.; Wu, X.; Duan, J.; Zhang, S. Development and validation of a feature-based broad-learning system for opportunistic osteoporosis screening using lumbar spine radiographs. Acad. Radiol. 2024, 31, 84–92. [Google Scholar] [CrossRef]
- He, L.; Liu, Z.; Liu, C.; Gao, Z.; Ren, Q.; Lei, L.; Ren, J. Radiomics based on lumbar spine magnetic resonance imaging to detect osteoporosis. Acad. Radiol. 2021, 28, e165–e171. [Google Scholar] [CrossRef]
- Kang, S.R.; Wang, K. Radiomic nomogram based on lumbar spine magnetic resonance images to diagnose osteoporosis. Acad. Radiol. 2024, 65, 02841851241242052. [Google Scholar] [CrossRef]
- Zhen, T.; Fang, J.; Hu, D.; Shen, Q.; Ruan, M. Comparative evaluation of multiparametric lumbar MRI radiomic models for detecting osteoporosis. BMC Musculoskel Dis. 2024, 25, 185. [Google Scholar] [CrossRef]
- Yuan, X.; Liang, Y.; Yang, H.; Feng, L.; Sun, H.; Li, C.; Qin, J. Applying machine learning analysis based on proximal femur of abdominal computed tomography to screen for abnormal bone mass in femur. Acad. Radiol. 2024, 31, 2003–2010. [Google Scholar] [CrossRef]
- Paderno, A.; Ataide Gomes, E.J.; Gilberg, L.; Maerkisch, L.; Teodorescu, B.; Koç, A.M.; Meyer, M. Artificial intelligence-enhanced opportunistic screening of osteoporosis in CT scan: A scoping review. Osteoporos. Int. 2024, 35, 1681–1692. [Google Scholar] [CrossRef]
- Yaprak, G.; Gemici, C.; Seseogullari, O.O.; Karabag, I.S.; Cini, N. CT derived Hounsfield unit: An easy way to determine osteoporosis and radiation related fracture risk in irradiated patients. Front. Oncol. 2020, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Sande, E.P.S.; Martinsen, A.C.T.; Hole, E.O.; Olerud, H.M. Interphantom and interscanner variations for Hounsfield units-establishment of reference values for HU in a commercial QA phantom. Phys. Med. Biol. 2010, 55, 5123–5135. [Google Scholar] [CrossRef] [PubMed]
- Levi, R.; Mollura, M.; Savini, G.; Garoli, F.; Battaglia, M.; Ammirabile, A.; Cappellini, L.A.; Superbi, S.; Grimaldi, M.; Barbieri, R.; et al. CT cadaveric dataset for radiomics features stability assessment in lumbar vertebrae. Sci. Data 2024, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, J.; Zhu, S.; Zhu, J.; Xu, Z. Deep learning in the radiologic diagnosis of osteoporosis: A literature review. J. Int. Med. Res. 2024, 52, 03000605241244754. [Google Scholar] [CrossRef]
- Basu, S.; Mukhopadhyay, S.; Karki, M.; DiBiano, R.; Ganguly, S.; Nemani, R.; Gayaka, S. Deep neural networks for texture classification-a theoretical analysis. Neural Netw. 2018, 97, 173–182. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Shan, X.; Zhang, L.; Cui, S.; Shi, Z.; Liu, Y.; Zhang, Y.; Wang, L. Hybrid transformer convolutional neural network-based radiomics models for osteoporosis screening in routine CT. BMC Med. Imaging 2024, 24, 62. [Google Scholar] [CrossRef]
- Peng, T.; Zeng, X.; Li, Y.; Li, M.; Pu, B.; Zhi, B.; Wang, Y.; Qu, H. A study on whether deep learning models based on CT images for bone density classification and prediction can be used for opportunistic osteoporosis screening. Osteoporos. Int. 2024, 35, 117–128. [Google Scholar] [CrossRef]
- Li, J.; Dong, D.; Fang, M.; Wang, R.; Tian, J.; Li, H.; Gao, J. Dual-energy CT-based deep learning radiomics can improve lymph node metastasis risk prediction for gastric cancer. Eur. Radiol. 2020, 30, 2324–2333. [Google Scholar] [CrossRef]
- Fang, K.; Zheng, X.; Lin, X.; Dai, Z. A comprehensive approach for osteoporosis detection through chest CT analysis and bone turnover markers: Harnessing radiomics and deep learning techniques. Front. Endocrinol. 2024, 15, 1296047. [Google Scholar] [CrossRef]
- Pulkkinen, P.; Saarakkala, S.; Nieminen, M.T.; Jämsä, T. Standard radiography: Untapped potential in the assessment of osteoporotic fracture risk. Eur. Radiol. 2013, 23, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.W.; Ong, W.; Makmur, A.; Kumar, N.; Low, X.Z.; Shuliang, G.; Liang, T.Y.; Ting, D.F.; Tan, J.H.; Hallinan, J.T. Application of artificial intelligence methods on osteoporosis classification with radiographs-a systematic review. Bioengineering 2024, 11, 484. [Google Scholar] [CrossRef]
- Yeung, Y.; Chiu, K.Y.; Yau, W.P.; Tang, W.M.; Cheung, W.Y.; Ng, T.P. Assessment of the proximal femoral morphology using plain radiograph-can it predict the bone quality? J. Arthroplast. 2006, 21, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Shevroja, E.; Reginster, J.Y.; Lamy, O.; Al-Daghri, N.; Chandran, M.; Demoux-Baiada, A.L.; Kohlmeier, L.; Lecart, M.-P.; Messina, D.; Camargos, B.M.; et al. Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: Results of an expert group meeting organized by the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO), and the international osteoporosis foundation (IOF) under the auspices of who collaborating center for epidemiology of musculoskeletal health and aging. Osteoporos. Int. 2023, 34, 1501–1529. [Google Scholar]
- Hong, N.; Cho, S.W.; Shin, S.; Lee, S.; Jang, S.A.; Roh, S.; Lee, Y.H.; Rhee, Y.; Cummings, S.R.; Kim, H.; et al. Deep-learning-based detection of vertebral fracture and osteoporosis using lateral spine X-ray radiography. J. Bone Miner. Res. 2023, 38, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Smets, J.; Shevroja, E.; Hügle, T.; Leslie, W.D.; Hans, D. Machine learning solutions for osteoporosis-a review. J. Bone Miner. Res. 2021, 36, 833–851. [Google Scholar] [CrossRef]
- Xue, L.; Qin, G.; Chang, S.; Luo, C.; Hou, Y.; Xia, Z.; Yuan, J.; Wang, Y.; Liu, S.; Liu, K.; et al. Osteoporosis prediction in lumbar spine X-ray images using the multi-scale weighted fusion contextual transformer network. Artif. Intell. Med. 2023, 143, 102639. [Google Scholar] [CrossRef]
- Modica, R.; Benevento, E.; Altieri, B.; Minotta, R.; Liccardi, A.; Cannavale, G.; Di Iasi, G.; Colao, A. Role of bone metastases in lung neuroendocrine neoplasms: Clinical presentation, treatment and impact on prognosis. Int. J. Mol. Sci. 2024, 25, 8957. [Google Scholar] [CrossRef]
- Modica, R.; Altieri, B.; D’Aniello, F.; Benevento, E.; Cannavale, G.; Minotta, R.; Liccardi, A.; Colao, A.; Faggiano, A. Vitamin D and bone metabolism in adult patients with neurofibromatosis type 1. Metabolites 2023, 13, 255. [Google Scholar] [CrossRef]
- Huang, C.C.; Effendi, F.F.; Kosik, R.O.; Lee, W.J.; Wang, L.J.; Juan, C.J.; Chan, W.P. Utilization of CT and MRI scanning in Taiwan, 2000-2017. Insights Imaging 2023, 14, 23. [Google Scholar] [CrossRef]
- Sneag, D.B.; Abel, F.; Potter, H.G.; Fritz, J.; Koff, M.F.; Chung, C.B.; Pedoia, V.; Tan, E.T. MRI advancements in musculoskeletal clinical and research practice. Radiology 2023, 308, e230531. [Google Scholar] [CrossRef] [PubMed]
- Fathi Kazerooni, A.; Pozo, J.M.; McCloskey, E.V.; Saligheh Rad, H.; Frangi, A.F. Diffusion MRI for assessment of bone quality; a review of findings in healthy aging and osteoporosis. J. Magn. Reson. Imagin. 2020, 51, 975–992. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.C. Advances and shortfalls in MRI evaluation of osteoporosis. Radiology 2023, 307, e223144. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Binkley, N.; Hernando, D.; Anderson, P.A. Opportunistic use of lumbar magnetic resonance imaging for osteoporosis screening. Osteoporos. Int. 2022, 33, 861–869. [Google Scholar] [CrossRef]
- Yoo, H.; Yoo, R.E.; Choi, S.H.; Hwang, I.; Lee, J.Y.; Seo, J.Y.; Koh, S.Y.; Choi, K.S.; Kang, K.M.; Yun, T.J. Deep learning-based reconstruction for acceleration of lumbar spine MRI: A prospective comparison with standard MRI. Eur. Radiol. 2023, 33, 8656–8668. [Google Scholar] [CrossRef]
- Nian, S.; Zhao, Y.; Li, C.; Zhu, K.; Li, N.; Li, W.; Chen, J. Development and validation of a radiomics-based model for predicting osteoporosis in patients with lumbar compression fractures. Spine J. 2024, 24, 1625–1634. [Google Scholar] [CrossRef]
- Zaworski, C.; Cheah, J.; Koff, M.F.; Breighner, R.; Lin, B.; Harrison, J.; Donnelly, E.; Stein, E.M. MRI-based texture analysis of trabecular bone for opportunistic screening of skeletal fragility. J. Clin. Endocr. Metab. 2021, 106, 2233–2241. [Google Scholar] [CrossRef]
- Ollivier, M.; Le Corroller, T.; Blanc, G.; Parratte, S.; Champsaur, P.; Chabrand, P.; Argenson, J.N. Radiographic bone texture analysis is correlated with 3D microarchitecture in the femoral head, and improves the estimation of the femoral neck fracture risk when combined with bone mineral density. Eur. J. Radiol. 2013, 82, 1494–1498. [Google Scholar] [CrossRef]
- Gebre, R.K.; Hirvasniemi, J.; Lantto, I.; Saarakkala, S.; Leppilahti, J.; Jämsä, T. Discrimination of low-energy acetabular fractures from controls using computed tomography-based bone characteristics. Ann. Biomed. Eng. 2021, 49, 367–381. [Google Scholar] [CrossRef]
- Poullain, F.; Champsaur, P.; Pauly, V.; Knoepflin, P.; Le Corroller, T.; Creze, M.; Pithioux, M.; Bendahan, D.; Guenoun, D. Vertebral trabecular bone texture analysis in opportunistic MRI and CT scan can distinguish patients with and without osteoporotic vertebral fracture: A preliminary study. Eur. J. Radiol. 2023, 158, 110642. [Google Scholar] [CrossRef]
- Martel, D.; Monga, A.; Chang, G. Radiomic analysis of the proximal femur in osteoporosis women using 3T MRI. Front. Radiol. 2023, 3, 1293865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, L.; Liu, J.; Niu, X.; Tang, J.; Xia, J.; Liu, Y.; Zhang, W.; Liang, Z.; Zhang, X.; et al. Exploring deep learning radiomics for classifying osteoporotic vertebral fractures in X-ray images. Front. Endocrinol. 2024, 15, 1370838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, L.; Tang, J.; Xia, J.; Liu, Y.; Zhang, W.; Liu, J.; Liang, Z.; Zhang, X.; Zhang, L.; et al. Constructing a deep learning radiomics model based on X-ray images and clinical data for predicting and distinguishing acute and chronic osteoporotic vertebral fractures: A multicenter study. Acad. Radiol. 2024, 31, 2011–2026. [Google Scholar] [CrossRef]
- Burian, E.; Subburaj, K.; Mookiah, M.R.K.; Rohrmeier, A.; Hedderich, D.M.; Dieckmeyer, M.; Diefenbach, M.N.; Ruschke, S.; Rummeny, E.J.; Zimmer, C.; et al. Texture analysis of vertebral bone marrow using chemical shift encoding-based water-fat MRI: A feasibility study. Osteoporos. Int. 2019, 30, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Becherucci, E.A.; Boehm, C.; Husseini, M.E.; Ruschke, S.; Burian, E.; Kirschke, J.S.; Link, T.M.; Subburaj, K.; Karampinos, D.C.; et al. Texture analysis using CT and chemical shift encoding-based water-fat MRI can improve differentiation between patients with and without osteoporotic vertebral fractures. Front. Endocrinol. 2022, 12, 778537. [Google Scholar] [CrossRef]
- Schini, M.; Johansson, H.; Harvey, N.C.; Lorentzon, M.; Kanis, J.A.; McCloskey, E.V. An overview of the use of the fracture risk assessment tool (FRAX) in osteoporosis. J. Endocrinol. Invest. 2024, 47, 501–511. [Google Scholar] [CrossRef]
- Whittier, D.E.; Samelson, E.J.; Hannan, M.T.; Burt, L.A.; Hanley, D.A.; Biver, E.; Szulc, P.; Sornay-Rendu, E.; Merle, B.; Chapurlat, R.; et al. Bone microarchitecture phenotypes identified in older adults are associated with different levels of osteoporotic fracture risk. J. Bone Miner. Res. 2022, 37, 428–439. [Google Scholar] [CrossRef]
- Hong, N.; Whittier, D.E.; Glüer, C.C.; Leslie, W.D. The potential role for artificial intelligence in fracture risk prediction. Lancet Diabetes Endocrinol. 2024, 12, 596–600. [Google Scholar] [CrossRef]
- Lu, S.; Fuggle, N.R.; Westbury, L.D.; Ó Breasail, M.; Bevilacqua, G.; Ward, K.A.; Dennison, E.M.; Mahmoodi, S.; Niranjan, M.; Cooper, C. Machine learning applied to HR-pQCT images improves fracture discrimination provided by DXA and clinical risk factors. Bone 2023, 168, 116653. [Google Scholar] [CrossRef]
- Farzi, M.; Pozo, J.M.; McCloskey, E.; Eastell, R.; Harvey, N.C.; Frangi, A.F.; Wilkinson, J.M. Quantitating age-related BMD textural variation from DXA region-free-analysis: A study of hip fracture prediction in three cohorts. J. Bone Miner. Res. 2022, 37, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Ferizi, U.; Besser, H.; Hysi, P.; Jacobs, J.; Rajapakse, C.S.; Chen, C.; Saha, P.K.; Honig, S.; Chang, G. Artificial intelligence applied to osteoporosis: A performance comparison of machine learning algorithms in predicting fragility fractures from MRI data. J. Magn. Reson. Imaging 2019, 49, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, L.; Zhang, X.; Liu, J.; Tang, J.; Xia, J.; Liu, Y.; Zhang, W.; Liang, Z.; Tang, G.; et al. Development and validation of a predictive model for vertebral fracture risk in osteoporosis patients. Eur. Spine J. 2024, 33, 3242–3260. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, J.; Xia, B.; Gu, Z.; Yin, H.; Zhang, H.; Yang, H.; Song, B. Novel radiomics-clinical model for the noninvasive prediction of new fractures after vertebral augmentation. Acad. Radiol. 2023, 30, 1092–1100. [Google Scholar] [CrossRef]
- Ibrahim, A.; Vallières, M.; Woodruff, H.; Primakov, S.; Beheshti, M.; Keek, S.; Refaee, T.; Sanduleanu, S.; Walsh, S.; Morin, O.; et al. Radiomics analysis for clinical decision support in nuclear medicine. Semin. Nucl. Med. 2019, 49, 438–449. [Google Scholar] [CrossRef]
- Lohmann, P.; Franceschi, E.; Vollmuth, P.; Dhermain, F.; Weller, M.; Preusser, M.; Smits, M.; Galldiks, N. Radiomics in neuro-oncological clinical trials. Lancet Digit. Health 2022, 4, e841–e849. [Google Scholar] [CrossRef]
- Santinha, J.; Pinto dos Santos, D.; Laqua, F.; Visser, J.J.; Groot Lipman, K.B.W.; Dietzel, M.; Smits, M.; Galldiks, N. ESR essentials: Radiomics-practice recommendations by the European Society of Medical Imaging Informatics. Eur. Radiol. 2025, 35, 1122–1132. [Google Scholar] [CrossRef]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C.; et al. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).