Enhancing Hydrophobicity of Nanocellulose-Based Films by Coating with Natural Wax from Halimium viscosum
Abstract
1. Introduction
2. Materials and Methods
2.1. Wax Extraction
2.2. Hydrolysis of Waxes
2.3. Extraction of Fatty Acids
2.4. Characterization of Waxes
2.5. Characterization of Nanocellulose Gels
2.6. Production and Characterization of Nanocellulose Films
3. Results and Discussion
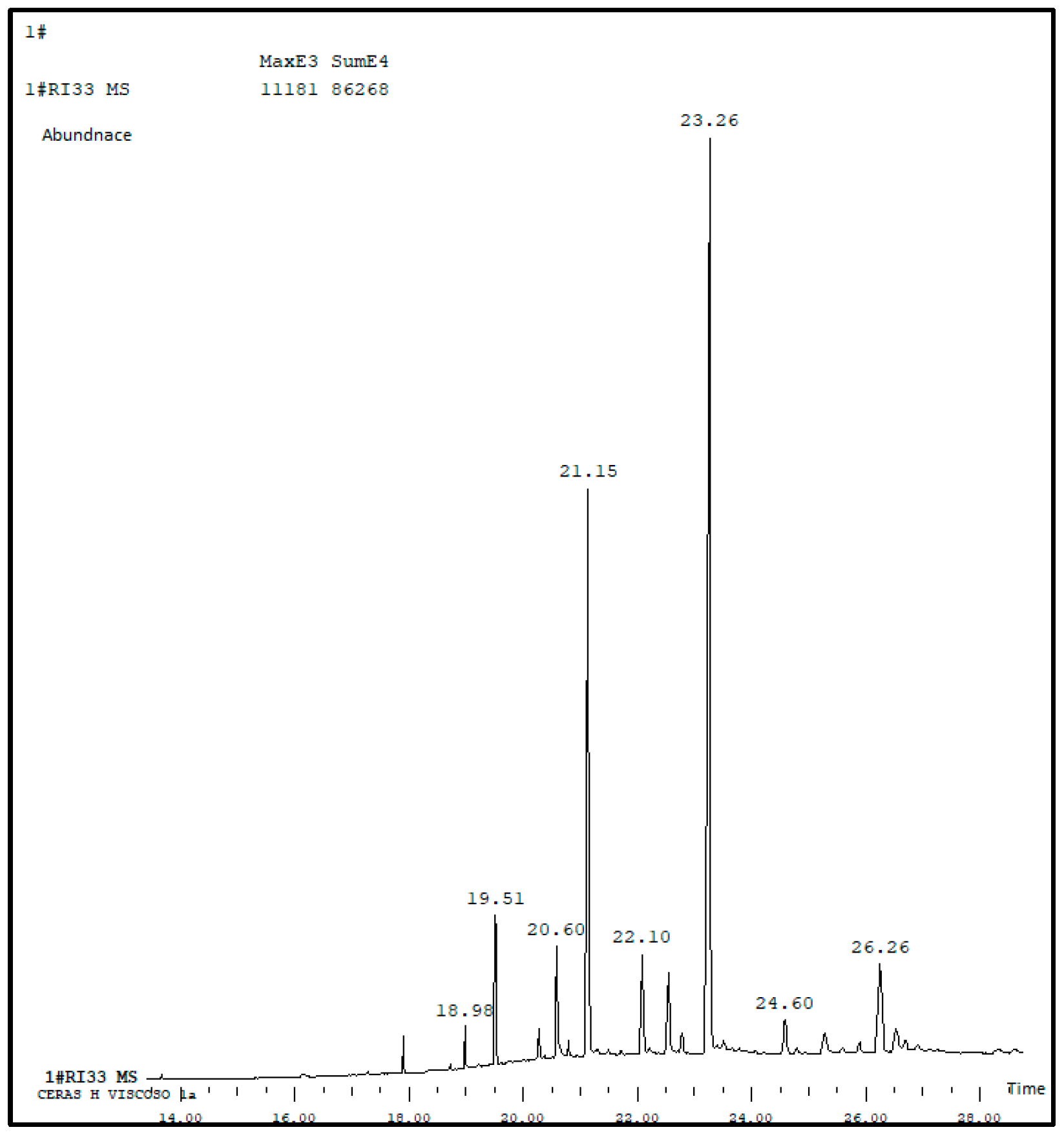
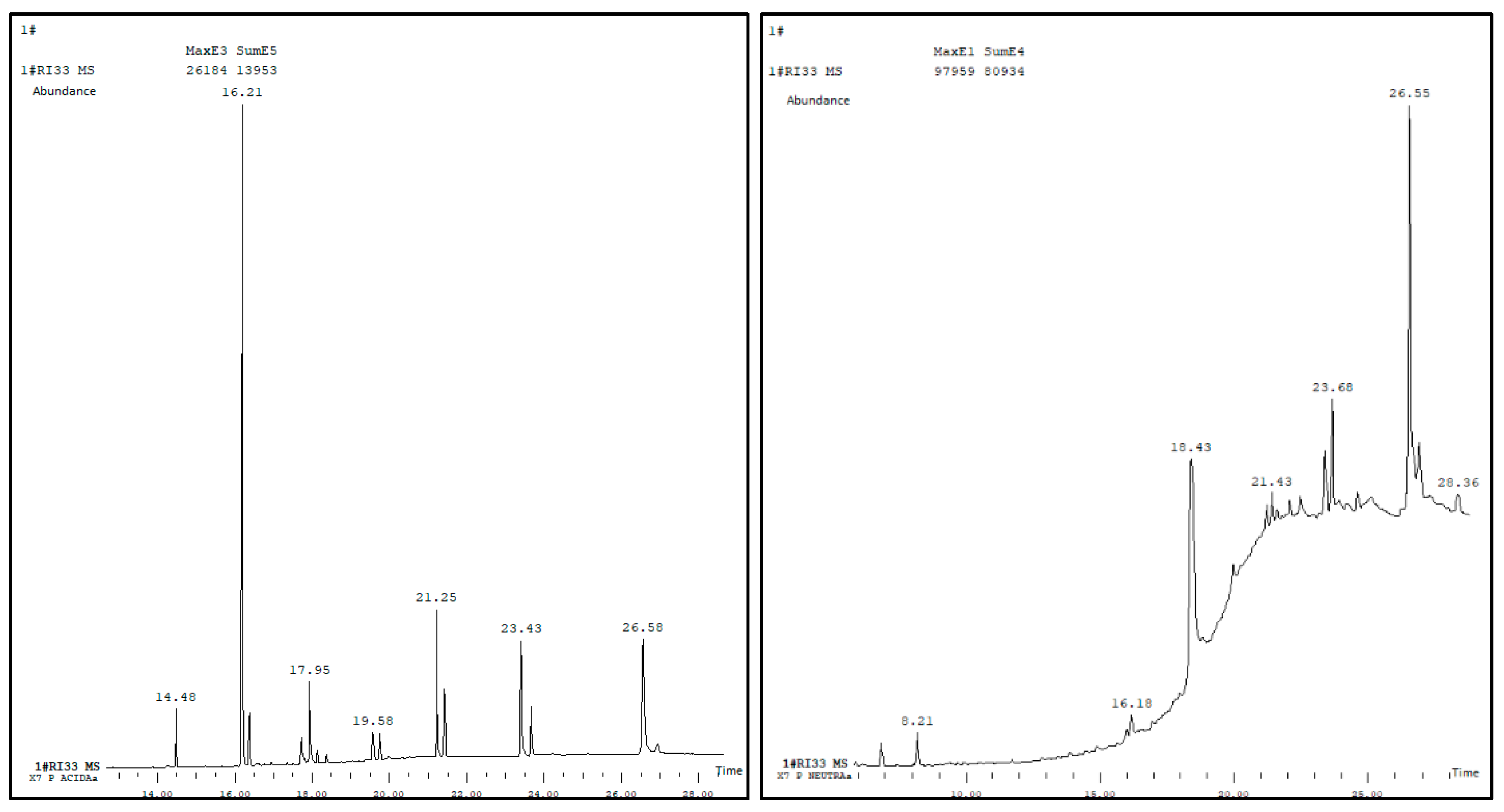
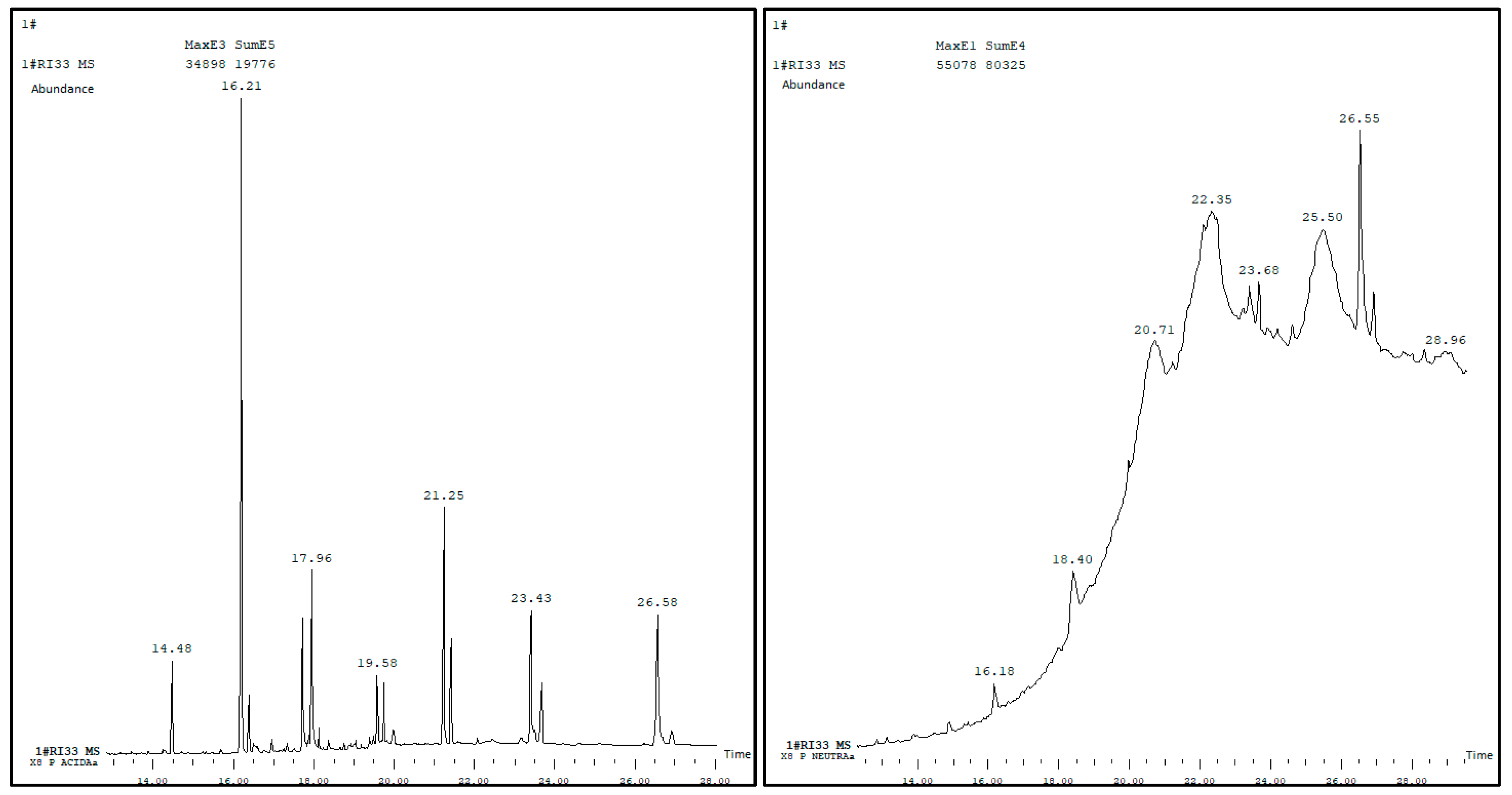
3.1. Chemical Analysis of the Wax
3.2. Characterization of Nanocellulose Gels and Films
3.2.1. Solids Content of CNF Gels
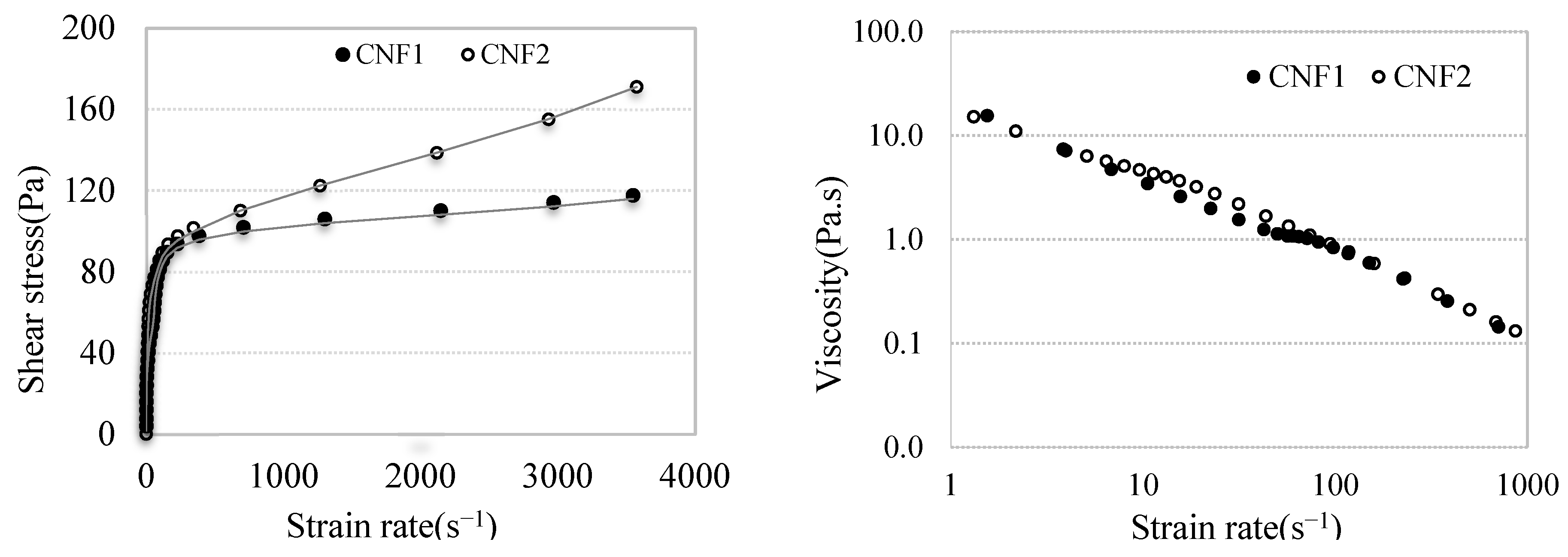
3.2.2. Rheology of CNF Gel
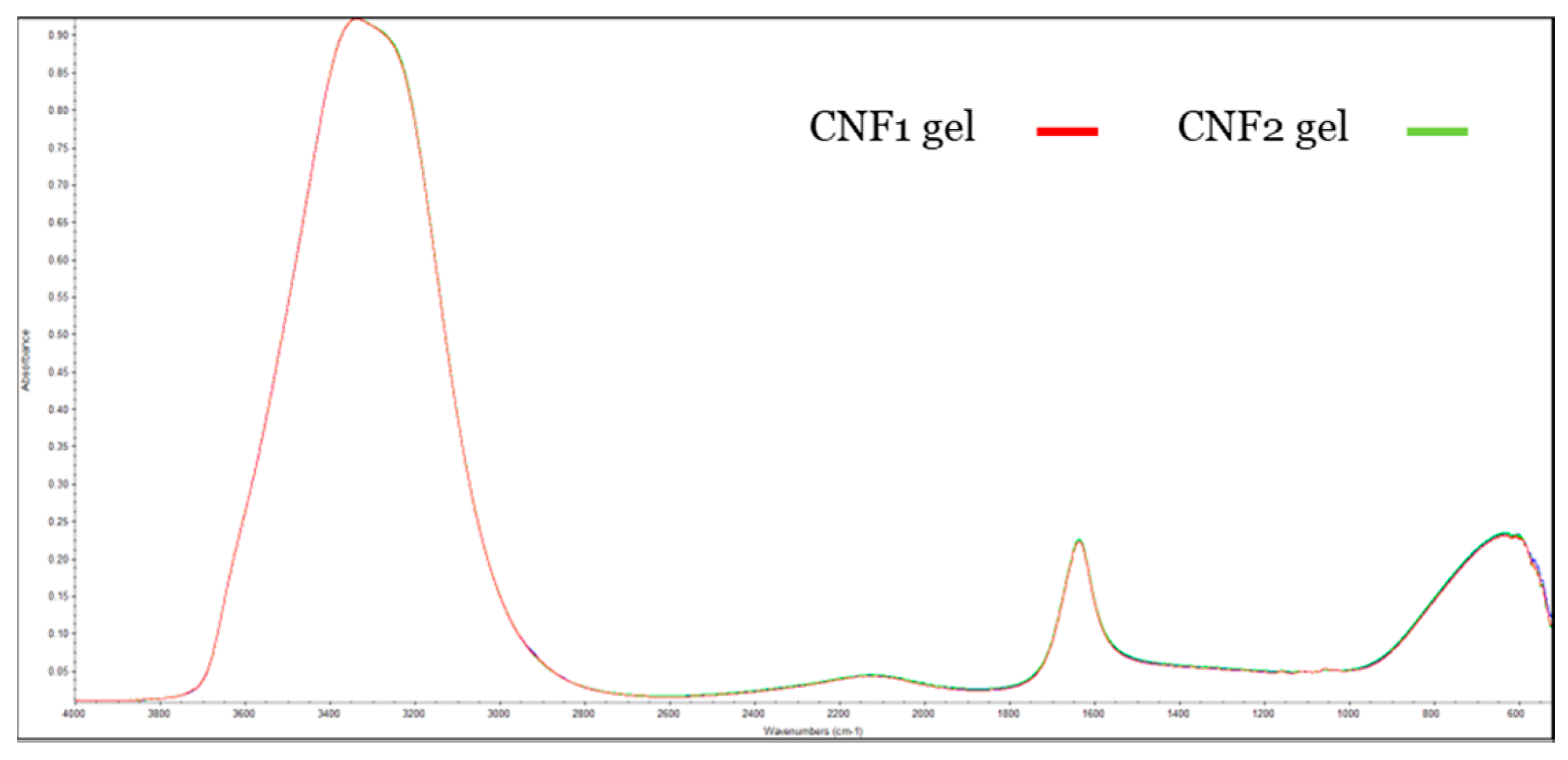
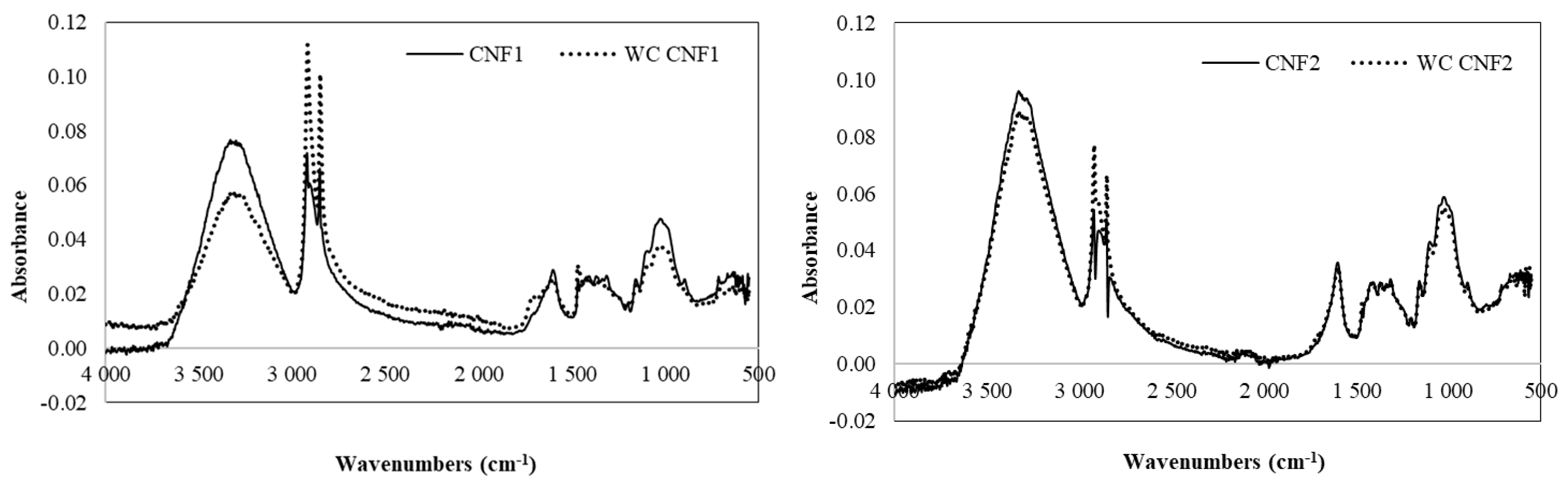
3.2.3. FTIR of CNF Gels and CNF Films
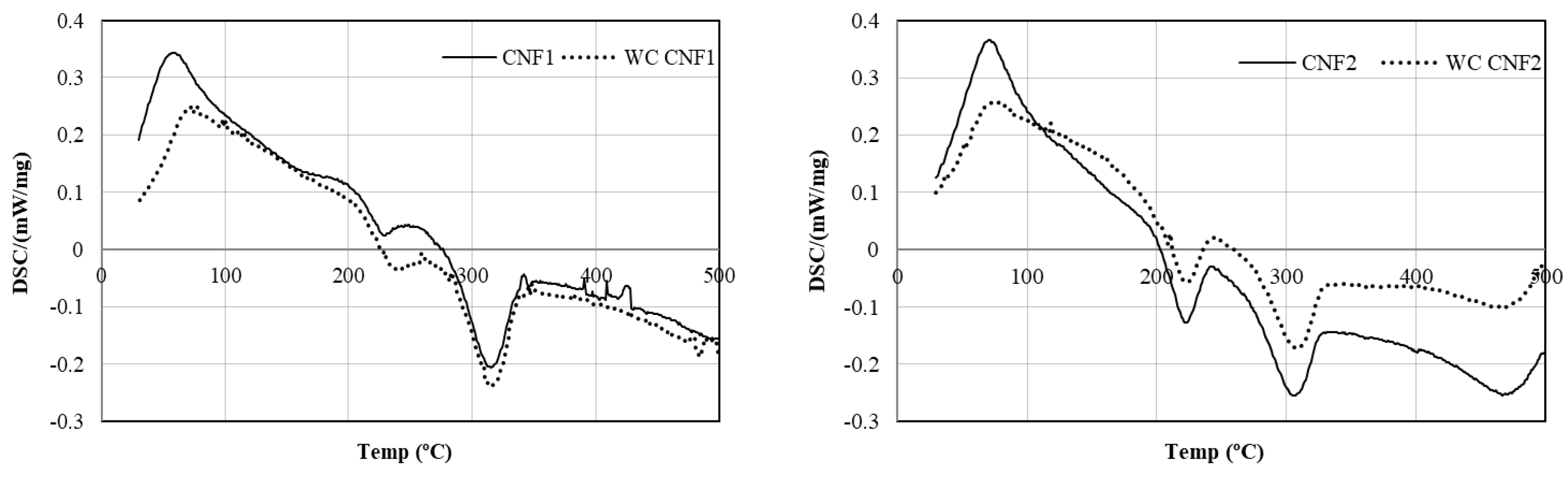
3.2.4. DSC of CNF Films
3.2.5. Grammage, Thickness, Density and Transparency of CNF Films
3.2.6. Water Vapor Permeability of Films
3.2.7. Contact Angle and Surface Energy of Films
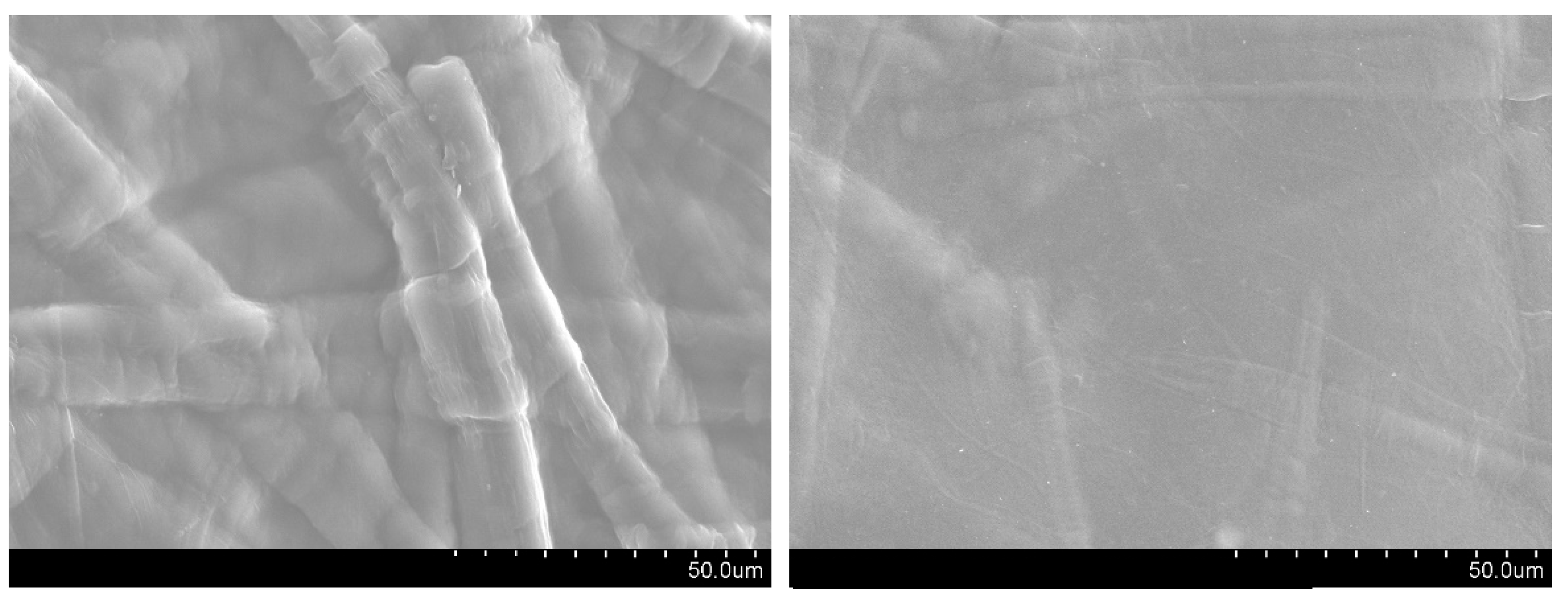
3.2.8. Surface Morphology of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Kim, J.H.; Shim, B.S.; Kim, H.S.; Lee, Y.J.; Min, S.K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Green. Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2020, 27, 1149–1194. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically Transparent Nanofiber Paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Stark, N.M. Opportunities for cellulose nanomaterials in packaging films: A review and future trends. J. Renew. Mater. 2016, 4, 313–326. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in thin films, coatings, and plies for packaging applications: A review. BioResources 2017, 12, 2143–2233. [Google Scholar] [CrossRef]
- Song, J.; Rojas, O.J. Approaching super-hydrophobicity from cellulosic materials: A review. Nord. Pulp Pap. Res. J. 2013, 28, 216–238. [Google Scholar] [CrossRef]
- Costa, V.L.D.; Simões, R.M.S. Hydrophobicity improvement of cellulose nanofibrils films by stearic acid and modified precipitated calcium carbonate coating. J. Mater. Sci. 2022, 57, 11443–11459. [Google Scholar] [CrossRef]
- Forsman, N.; Lozhechnikova, A.; Khakalo, A.; Johansson, L.S.; Vartiainen, J.; Österberg, M. Layer-by-layer assembled hydrophobic coatings for cellulose nanofibril films and textiles, made of polylysine and natural wax particles. Carbohydr. Polym. 2017, 173, 392–402. [Google Scholar] [CrossRef]
- Österberg, M.; Vartiainen, J.; Lucenius, J.; Hippi, U.; Seppälä, J.; Serimaa, R.; Laine, J. A fast method to produce strong NFC films as a platform for barrier and functional materials. ACS Appl. Mater. Interfaces 2013, 5, 4640–4647. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.D.; To, C.M.; Vrieze, M.D.; Lynen, F.; Danthine, S.; Brown, A.; Dewetthink, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef] [PubMed]
- ISO 536:1995; Paper and Board—Determination of Grammage. International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO 534:2011; Paper and Board—Determination of Thickness, Density and Specific Volume. International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 22891:2013; Paper—Determination of Transparency by Diffuse Reflectance Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- Çıtak, A.; Yarbaş, T. Using contact angle measurement technique for determination of the surface free energy of B-SBA-15-x materials. Int. J. Adhes. Adhes. 2022, 112, 103024. [Google Scholar] [CrossRef]
- ASTM E96-22; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- Sharma, A.; Mandal, T.; Goswami, S. Dispersibility and Stability Studies of Cellulose Nanofibers: Implications for Nanocomposite Preparation. J Polym Env. 2021, 29, 1516–1525. [Google Scholar] [CrossRef]
- Iglesias, M.C.; Gomez-Maldonado, D.; Via, B.K.; Jiang, Z.; Peresin, M.S. Pulping processes and their effects on cellulose fibers and nanofibrillated cellulose properties: A review. Prod. J. 2020, 70, 10–21. [Google Scholar] [CrossRef]
- Kulichikhin, V.G.; Malkin, A.Y. The Role of Structure in Polymer Rheology: Review. Polymers 2022, 14, 1262. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yakymenko, O.S.; Yashchenko, O.V.; Zakharko, R.M.; Myshak, V. D Preparation of hemp nanocellulose and its use to improve the properties of paper for food packaging. Cellulose 2022, 29, 8305–8317. [Google Scholar] [CrossRef]
- Moberg, T.; Sahlin, K.; Yao, K.; Geng, S.; Westman, G.; Zhou, Q.; Oksman, K.; Rigdahl, M. Rheological properties of nanocellulose suspensions: Effects of fibril/particle dimensions and surface characteristics. Cellulose 2017, 24, 2499–2510. [Google Scholar] [CrossRef]
- Honorato, C.; Kumar, V.; Liu, J.; Koivula, H.; Xu, C.; Toivakka, M. Transparent nanocellulose-pigment composite films. J. Mater. Sci. 2015, 50, 7343–7352. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Kamel, S.; Salama, A.; Tohamy, H.A.S. Preparation and infrared study of cellulose based amphiphilic materials. Cellul. Chem. Technol. 2018, 52, 193–200. [Google Scholar]
- Zhu, M.; Ying, D.; Zhang, H.; Xu, X.; Chang, C. Self-healable hydrophobic films fabricated by incorporating natural wax into cellulose matrix. Chem. Eng. J. 2022, 446, 136791. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M. Thermal properties of nanocrystalline cellulose and cellulose nanowhisker. Int. J. Innov. Technol. Explor. Eng. 2019, 9, 5430–5434. [Google Scholar] [CrossRef]
- Miao, X.; Lin, J.; Tian, F.; Li, X.; Bian, F.; Wang, J. Cellulose nanofibrils extracted from the byproduct of cotton plant. Carbohydr. Polym. 2016, 136, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hsieh, Y.L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hult, E.L.; Iversen, T.; Sugiyama, J. Characterization of the supermolecular structure of cellulose in wood pulp fibres. Cellulose 2003, 10, 103–110. [Google Scholar] [CrossRef]
- Kim, H.J.; Roy, S.; Rhim, J.W. Effects of various types of cellulose nanofibers on the physical properties of the CNF-based films. J. Env. Chem. Eng. 2021, 9, 106043. [Google Scholar] [CrossRef]
- Boufi, S.; Kaddami, H.; Dufresne, A. Mechanical performance and transparency of nanocellulose reinforced polymer nanocomposites. Macromol. Mater. Eng. 2014, 299, 560–568. [Google Scholar] [CrossRef]
- Tian, L.; Xu, X. Optical Properties and Crystallization of Natural Waxes at Several Annealing Temperatures: A Terahertz Time-Domain Spectroscopy Study. J. Infrared Millim. Terahertz Waves 2018, 39, 302–312. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.; Deng, Y.; Ragauskas, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Process 2014, 2, 23. [Google Scholar] [CrossRef]
- Herrera, M.A.; Mathew, A.P.; Oksman, K. Barrier and mechanical properties of plasticized and cross-linked nanocellulose coatings for paper packaging applications. Cellulose 2017, 24, 3969–3980. [Google Scholar] [CrossRef]
- Surya, I.; Hazwan, C.M.; Abdul Khalil, H.P.S.; Yahya, E.B.; Suriani, A.B.; Danish, M.; Mohamed, A. Hydrophobicity and Biodegradability of Silane-Treated Nanocellulose in Biopolymer for High-Grade Packaging Applications. Polymers 2022, 14, 4147. [Google Scholar] [CrossRef] [PubMed]
- Lavrič, G.; Oberlintner, A.; Filipova, I.; Novak, U.; Likozar, B.; Vrabič-Brodnjak, U. Functional Nanocellulose, Alginate and Chitosan Nanocomposites Designed as Active Film Packaging Materials. Polymers 2021, 13, 2523. [Google Scholar] [CrossRef] [PubMed]
- Cherpinski, A.; Torres-Giner, S.; Vartiainen, J.; Peresin, M.S.; Lahtinen, P.; Lagaron, J.M. Improving the water resistance of nanocellulose-based films with polyhydroxyalkanoates processed by the electrospinning coating technique. Cellulose 2018, 25, 1291–1307. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Lainea, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green. Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
| Properties | CNF1 | WC CNF1 | CNF2 | WC CNF2 |
|---|---|---|---|---|
| Grammage (g.m−2) | 40.18 ± 0.32 | 40.68 ± 0.21 | 44.65 ± 0.37 | 45.12 ± 0.17 |
| Thickness (µm) | 38.25 ± 1.39 | 38.39 ± 0.79 | 37.98 ± 1.82 | 38.19 ± 0.68 |
| Apparent density (g.cm−3) | 1.05 | 1.06 | 1.17 | 1.18 |
| Transparency (%) | 79.6 ± 1.51 | 78.24 ± 0.98 | 94.1 ± 1.10 | 91.6 ± 0.90 |
| Properties | CNF1 | WC CNF1 | CNF2 | WC CNF2 |
|---|---|---|---|---|
| WVTR (g.m−2.day−1) | 264.0 | 49.2 | 219.2 | 77.8 |
| WVP (g.Pa−1.day−1.m−1) | 7.03 × 10−6 | 1.39 × 10−6 | 6.03 × 10−6 | 3.0 × 10−6 |
| Films | CAWater | Surface Energy (mN.m−1) | ||
|---|---|---|---|---|
| Total | Dispersive | Polar | ||
| CNF1 | 52.53 ± 2.01 | 46.05 | 28.85 | 17.19 |
| CNF2 | 49.96 ± 2.45 | 49.63 | 29.11 | 20.52 |
| WC CNF1 | 110.93 ± 1.61 | 28.76 | 28.75 | 0.01 |
| WC CNF2 | 108.17 ± 1.03 | 28.93 | 28.91 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, A.; Rodilla, J.M.; Ferreira, R.; Luís, Â. Enhancing Hydrophobicity of Nanocellulose-Based Films by Coating with Natural Wax from Halimium viscosum. Appl. Sci. 2025, 15, 7576. https://doi.org/10.3390/app15137576
Ramos A, Rodilla JM, Ferreira R, Luís Â. Enhancing Hydrophobicity of Nanocellulose-Based Films by Coating with Natural Wax from Halimium viscosum. Applied Sciences. 2025; 15(13):7576. https://doi.org/10.3390/app15137576
Chicago/Turabian StyleRamos, Ana, Jesus M. Rodilla, Rodrigo Ferreira, and Ângelo Luís. 2025. "Enhancing Hydrophobicity of Nanocellulose-Based Films by Coating with Natural Wax from Halimium viscosum" Applied Sciences 15, no. 13: 7576. https://doi.org/10.3390/app15137576
APA StyleRamos, A., Rodilla, J. M., Ferreira, R., & Luís, Â. (2025). Enhancing Hydrophobicity of Nanocellulose-Based Films by Coating with Natural Wax from Halimium viscosum. Applied Sciences, 15(13), 7576. https://doi.org/10.3390/app15137576








