Evaporation and Ignition of Isolated Fuel Drops in an Oxidizing Environment: Analytical Study Based on Varshavskii’s ‘Diffusion Theory’
Abstract
Featured Application
Abstract
1. Introduction
- Many mathematical calculations were performed using pure hydrocarbons: n-heptane, n-pentane, and n-hexane, motivating the fact that those fuel types were best investigated [6,9,10,11,14,17]. Thus, the flammability features of certain investigated fuels of fossil origin are often established by approximation, taking numerical values of the required parameter of a hydrocarbon compound closest to its chemical composition. Such an attitude introduces calculation errors and aggravates the establishment of flammability features of blended fuels.
- Some of the so-called advanced models presented by the authors who investigated droplet burning theory are very complicated [16,36,37,38,39]. However, the authors admit that most of these models are still rather complicated, which limits their wide application [16]. To apply them to establish flammability characteristics of combustible mixtures, experiments determining calorific and physical features that require a lot of time and resources are needed; moreover, they would level the advantage of mathematical modelling against experimental trials.
2. Materials and Methods
2.1. Description of the Experimental Test
- Diesel fuel (DF) not containing a required 5% biodiesel additive, produced by SC ‘Orlen Lietuva’ refinery (EN 590:2014 [42], KN 27101943, see Supplementary Materials).
- Rapeseed oil methyl ester (RME) produced by the JSC ‘Rapsoila’, Lithuania.
- One binary blend: DF80/RME20 (Kaunas University of Technology (Lithuania), Kaunas, Lithuania).
2.2. Mathematical Model and Governing Equations
- The model of the gas phase in the droplet vaporization and combustion is quasi-steady.
- The droplet hanging on a thermocouple is spherically symmetrical in shape (see Figure 2). A spherical drop of radius r is surrounded by a flame zone of radius rc. Concentric to the drop, beyond the flame zone, there exists another boundary space. It consists of the ambient atmosphere.
- During evaporation of the fuel drop (surface), the flame front does not act as a complete separation interface between fuel vapor and air, so that air may penetrate the flame zone. Thus, a hidden energy, namely latent heat, is supplied to change the state of a substance without changing its temperature (this condition does not apply in cases where combustion under critical conditions is considered).
- Thermal diffusion effects are neglected. The effect of radiation heat transfer from the gas phase or neighboring particles is not considered.
- The reaction between the fuel and the oxidizer at the flame front is considered stoichiometric.
- The Lewis number is equal to unity, i.e., the temperature and concentration fields are similar, the thermal physical properties are independent of temperature, and the temperature of the drop over the entire radius is constant at any time.
- The Nusselt number is equal to 2, i.e., thermal and concentration relaxation times around the drop are very short. Soret and Dufour effects are neglected.
- Any internal flows in any dimension within the droplet are neglected.
3. Results
3.1. Experimental Tests
3.2. Mathematical Model
3.2.1. Determination of Thermal Conductivity of the Vapor Phase
3.2.2. Determination of the Critical Temperature and Pressure of Hydrocarbon Fuels
3.2.3. Determination of the Dynamic Viscosity of Hydrocarbon Fuels
3.2.4. Determination of the Specific Heat Capacities of Hydrocarbon Fuel Vapors
3.2.5. Derivation of the Simplified Equation for the Assessment of the Ignition Delay
4. Discussion
5. Conclusions
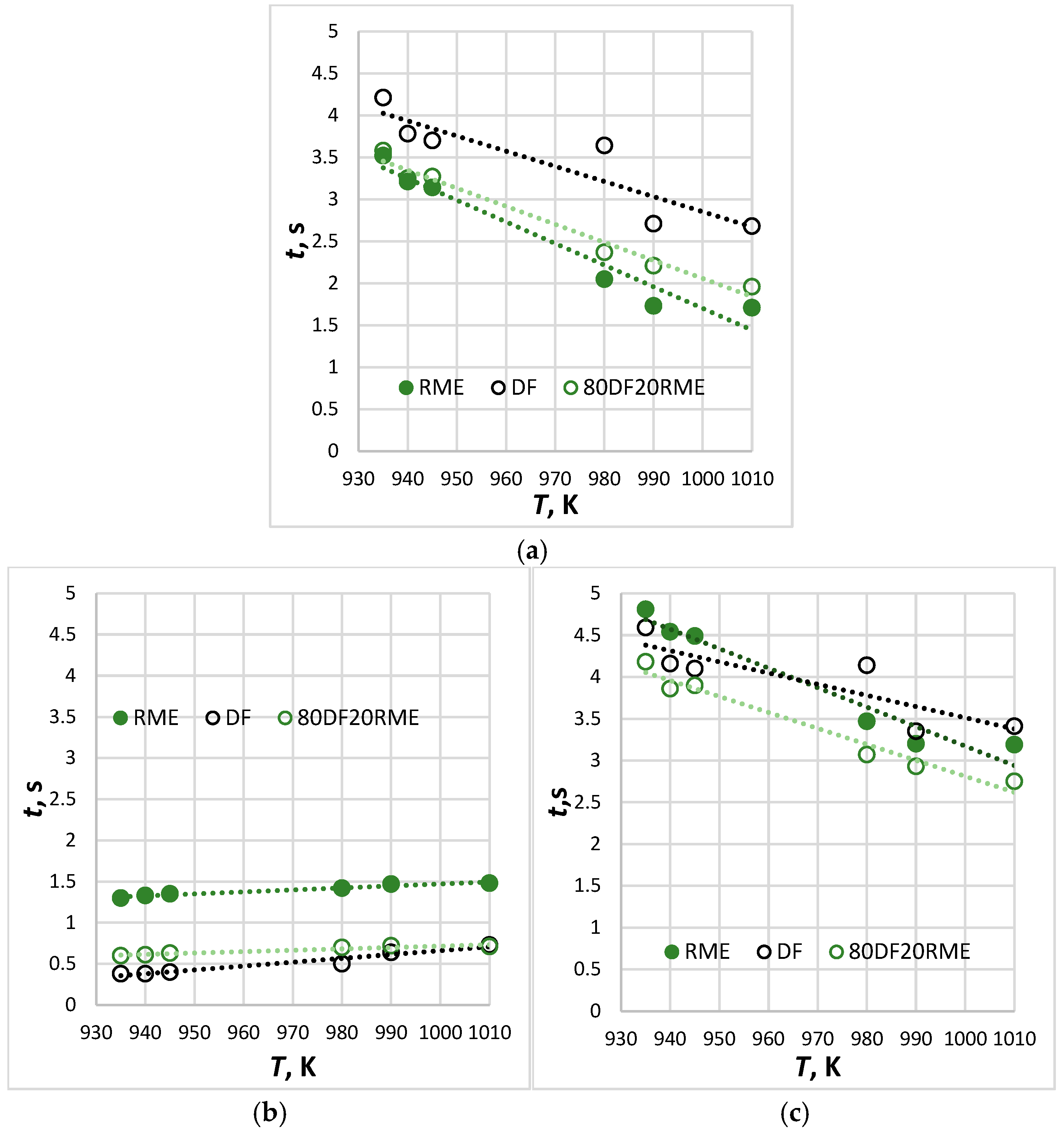
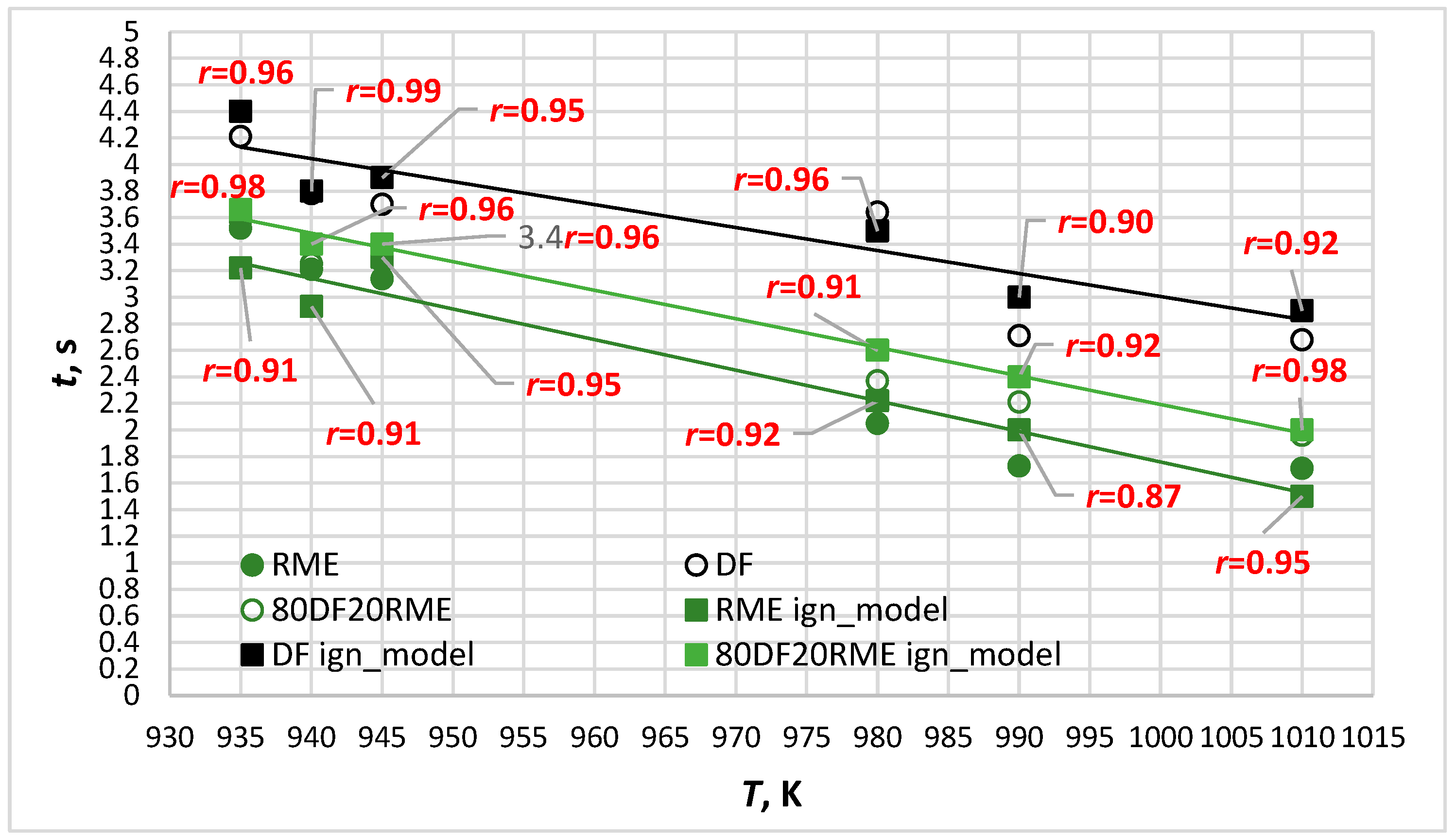
- Single droplets of biodiesel, diesel and their 20% blend were suspended in a relatively static environment with an ambient temperature of 935 K to 1010 K to validate the model proposed in this paper. The semi-theoretical model can well describe the droplet ignition delay and the error between the predicted results, and the experimental data are within 1–13%.
- The upper limit of the temperature range for this experiment exceeds the critical ignition temperature of the RME by 294 K.
- The longest ignition delay was established for diesel fuel and the shortest for RME, while blended fuel exhibited intermediate values. The time of combustion followed the opposite trend: fuels that exhibit longer ignition delays had shorter periods of combustion. This can be explained by the fact that the initial fuel mass per droplet from the thermocouple correlates with the fuel density and viscosity (see Table 2), i.e., biodiesel mass > diesel mass.
- In terms of simulation accuracy assessed for six different temperatures, the average level of correspondence of the ignition delay values with the ones experimentally obtained for different types of fuel arranged in decreasing order: DF80/RME20 (average r = 0.95) > DF (average r = 0.95) > RME (average r = 0.92).
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Latin symbols | |
| A* | dimensionless coefficient |
| a | coefficient |
| amix | coefficient, the size of which depends on the molar fraction of the lighter component in the mixture |
| c1 | fuel–vapour molar concentration |
| c2 | oxidizer’s molar concentration |
| c10 | fuel–vapor molar concentration of the drop surface (at the moment of ignition) |
| specific heat capacity of vapor | |
| specific heat capacity of liquid fuel | |
| cv | constant-volume specific heat |
| d0 | initial drop diameter |
| E | activation energy |
| H | enthalpy |
| K | dynamic modulus (Maxwell) |
| k | coefficient |
| k0 | temperature independent rate constant |
| L | latent heat of vaporization |
| average distance between collisions for a molecule | |
| Le | Lewis number |
| M1, M2 | molecular weights of the components in a gaseous mixture |
| Nu | Nusselt number |
| p1 | partial pressure of the fuel–vapor |
| p2 | partial pressure of the oxidizer |
| Pr | Prandtl criterion |
| r | radius |
| R | universal gas constant |
| r0 | initial drop radius |
| r1 | distance to the zone, where chemical reaction begins |
| rc | radius of a flame zone |
| t | time |
| T | temperature |
| T10 | temperature at which 10% of the oil fraction evaporates |
| T50 | temperature at which 50% of the oil fraction evaporates |
| T70 | temperature at which 70% of the oil fraction evaporates |
| Tcr | critical temperature |
| T: | temperature (K) |
| T0 | droplet temperature at initial time moment |
| T1 | temperature of the drop surface at the moment of ignition |
| T∞ | ambient temperature |
| Tliq | temperature of a liquid |
| Tb | boiling temperature |
| W | rate of a chemical reaction |
| average molecular speed | |
| x | number of carbon atoms in fuel molecule |
| xM (1, 2, 3… i) | molar fractions of the components of a gaseous mixture |
| xMpol | sum of the mole fractions of the polar components in a mixture |
| y | number of hydrogen atoms in fuel molecule |
| Y | dimensionless constant |
| Greek symbols | |
| θ0 | temperature tensions between droplet surface and environment summative coefficient |
| Λ | dimensionless coefficient |
| Λmix | thermal conductivity of gaseous mixture |
| λgas | thermal conductivity of fuel–vapor |
| μ | dynamic viscosity |
| ρ | density |
| relative density of a liquid (at T = 15 K) | |
| τign | ignition delay time |
| φ | stoichiometric coefficient |
| Φ | sum of the parts of atoms, groups, and bonds |
| DF | diesel fuel |
| RME | rapeseed methyl ester |
References
- Kohler, R.E., Jr. The origin of Lavoisier’s first experiments on combustion. Isis 1972, 63, 349–355. [Google Scholar] [CrossRef]
- Kambas, L. Antoine-Laurent Lavoisier’s ‘Sur la nature de l’eau’: An annotated English translation. Ann. Sci. 2025, 82, 102–132. [Google Scholar] [CrossRef]
- Semionov, N.N. Zur Theorie des Verbrennungsprozesses. Z. Phys. 1928, 48, 571–582. [Google Scholar]
- Zeldovich, Y.B.; Semenov, N.N. Kinetics of chemical reactions in a flame. J. Exp. Theor. Phys. 1940, 10, 1116–1136. [Google Scholar]
- Zeldovich, Y.B.; Franz-Kamenetsky, D.A. Theory of thermal flame propagation. J. Phys. Chem. 1938, 12, 100–105. [Google Scholar]
- Varshavskii, G.A. Diffusion Theory of Droplet Combustion; Trudy BNT NKAP: Moscow, Russia, 1945; pp. 87–106. (In Russian) [Google Scholar]
- Godsave, G.A.E. Combustion of droplets in a fuel spray. Nature 1949, 164, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Godsave, G.A.E. The Burning of Single Drops of Fuel, Parts 1 and 2. National Gas Turbine Establishment. Reports No. R66 (March 1950) and R87 (April 1951). Available online: https://apps.dtic.mil/sti/tr/pdf/AD0041743.pdf (accessed on 12 April 2025).
- Godsave, G.A.E. Studies of the combustion of drops in a fuel spray-the burning of single drops of fuel. Symp. Combust. Proc. 1953, 4, 818–830. [Google Scholar] [CrossRef]
- Kumagai, S.; Isoda, H. Combustion of fuel droplets. Nature 1950, 166, 1111. [Google Scholar] [CrossRef]
- Spalding, D.B. The combustion of liquid fuels. Symp. Combust. Proc. 1953, 4, 847–864. [Google Scholar] [CrossRef]
- Ulzama, S.; Specht, E. An analytical study of droplet combustion under microgravity: Quasi-steady transient approach. Proc. Combust. Inst. 2007, 31, 2301–2308. [Google Scholar] [CrossRef]
- Faik, A.M.E.-D. Quantitative Investigation of the Multicomponent Fuel Droplet Combustion Using High Speed Imaging and Digital Image Processing. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2017; 240p. Available online: https://core.ac.uk/download/96890617.pdf (accessed on 12 April 2025).
- Chiu, H.H. Advances and challenges in droplet and spray combustion. I. Toward a unified theory of droplet aerothermochemistry. Prog. Energy Combust. 2000, 26, 381–416. [Google Scholar] [CrossRef]
- Varshavskii, G.A.; Fedoseev, D.V.; Frank-Kamenetskii, A.D. Autoignition of a fuel droplet. In Problems of Evaporation, Combustion and Gas Dynamics in Disperse Systems, Proceedings of the Sixth Conference on Evaporation, Combustion and Gas Dynamics in Disperse Systems, Odessa, Ukraine, 3–5 October 1966; Fedoseev, V.A., Ed.; Odessa University Publishing House: Odessa, Ukraine, 1968; pp. 91–95. (In Russian) [Google Scholar]
- Sazhin, S.S. Advanced models of fuel droplet heating and evaporation. Prog. Energy Combust. 2006, 32, 162–214. [Google Scholar]
- Williams, A. Combustion of droplets of liquid fuels: A review. Combust. Flame 1973, 21, 1–31. [Google Scholar] [CrossRef]
- Finneran, J.; Garner, C.P.; Nada, F. Deviations from classicaldroplet evaporation theory. Proc. R. Soc. A 2021, 477, 2251. [Google Scholar] [CrossRef]
- Sun, K.; Jia, F.; Zhang, P.; Shu, L.; Wang, T. Marangoni effect in bipropellant droplet mixing during hypergolic ignition. Phys. Rev. Appl. 2021, 15, 034076. [Google Scholar] [CrossRef]
- Glushkov, D.; Klepikov, D.; Nigay, A.; Paushkina, K.; Pleshko, A. Experimental research of the initial temperature and additives effect on the ignition and combustion mechanisms of composite liquid fuel in a high-temperature oxidizer. Appl. Sci. 2023, 13, 3501. [Google Scholar] [CrossRef]
- He, C.; He, Z.X.; Zhang, P. Droplet collision of hypergolic propellants. Droplet 2024, 3, e116. [Google Scholar] [CrossRef]
- Schwind, R.A.; Sinrud, J.B.; Fuller, C.C.; Klassen, M.S.; Walker, R.A.; Goldsmith, C.F. Comparison of flame inception behavior of liquid nitromethane in inert and air environments. Combust. Flame 2022, 241, 112101. [Google Scholar] [CrossRef]
- Ochowiak, M.; Bielecki, Z.; Bielecki, M.; Włodarczak, S.; Krupinska, A.; Matuszak, M.; Choiński, D.; Lewtak, R.; Pavlenko, I. The D2-law of droplet evaporation when calculating the droplet evaporation process of liquid containing solid state catalyst particles. Energies 2022, 15, 7642. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion; MacGraw Hill: Boston, MA, USA, 2000. [Google Scholar]
- Dalla, B.F.; Wang, J.; Picano, F. Revisiting d2-law for the evaporation of dilute droplets. Fluid Dyn. 2021, 33, 051701. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Chiang, C.-H.; Yang, C.-I.; Yang, S.-Y.; Wei, H.-H. Defying the D2-law in fuel droplet combustion under gravity. Phys. Fluids 2024, 36, 097171. [Google Scholar] [CrossRef]
- Pathak, A.; Raessi, M. Steady-state and transient solutions to drop evaporation in a finite domain: Alternative benchmarks to the d2 law. Int. J. Heat Mass Trans. 2018, 127, 1147–1158. [Google Scholar] [CrossRef]
- Raslavičius, L.; Bazaras, Ž. Variations in oxygenated blend composition to meet energy and combustion characteristics very similar to the diesel fuel. Fuel Process. Technol. 2010, 91, 1049–1054. [Google Scholar] [CrossRef]
- Fang, W.; Tieshi, Z.; Min, L.; Rui, L.; Jie, J. Single droplet ignition models via evaporation and ignition competition. Meas. Sens. 2021, 13, 100029. [Google Scholar] [CrossRef]
- Xu, G.; Ikegami, M.; Honma, S.; Ikeda, K.; Ma, X.; Nagaishi, H.; Dietrich, D.L.; Struk, P.M. Inverse Influence of initial diameter on droplet burning rate in cold and hot ambiences: A thermal action of flame in balance with heat loss. Int. J. Heat Mass Transf. 2003, 46, 1155–1169. [Google Scholar] [CrossRef]
- Gallego, A.; Cacua, K.; Gamboa, D.; Rentería, J.; Herrera, B. Ignition delay and burning rate analysis of diesel–carbon nanotube blends stabilized by a surfactant: A droplet-scale study. Energies 2023, 16, 7740. [Google Scholar] [CrossRef]
- Araya, K.; Yoshida, T. Single droplet combustion of sunflower oil (part I). J. Senshu Univ. 1985, 18, 83–89. [Google Scholar]
- Law, C.L. Multicomponent droplet combustion with rapid internal mixing. Combust. Flame 1976, 26, 219–233. [Google Scholar] [CrossRef]
- Botero, M.L.; Huang, Y.; Zhu, D.L.; Molina, A.; Law, C.K. Synergistic combustion of droplets of ethanol, diesel and biodiesel mixtures. Fuel 2012, 94, 342–347. [Google Scholar] [CrossRef]
- Hallett, W.L.H. Simple model for the vaporization of droplets with large numbers of components. Combust. Flame 2000, 121, 334–344. [Google Scholar] [CrossRef]
- Sirignano, W.A. Advances in droplet array combustion theory and modelling. Prog. Energy Combust. 2014, 42, 54–86. [Google Scholar] [CrossRef]
- Brenn, G. Concentration fields in evaporating droplets. Int. J. Heat Mass Transf. 2005, 48, 395–402. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sanyal, D. A spherical cell model for multicomponent droplet combustion in a dilute spray. Int. J. Energy Res. 2001, 25, 1275–1294. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sanyal, D. A parametric study of burning of multicomponent droplets in a dilute spray. Int. J. Energy Res. 2001, 25, 1295–1314. [Google Scholar] [CrossRef]
- Lebedevas, S.; Klyus, O.; Raslavičius, L.; Krause, P.; Rapalis, P. Findings on droplet breakup behaviour of the preheated microalgae oil jet for efficiency improvement in diesel engines. Biomass Convers. Biorefin. 2023, 13, 17075–17086. [Google Scholar] [CrossRef]
- Sacomano Filho, F.L.; Krieger Filho, G.C.; van Oijen, J.A.; Sadik, A. Numerical investigation of droplet evaporation modeling in combustion environment. In Proceedings of the ICLASS 2018, 14th Triennial International Conference on Liquid Atomization and Spray Systems, Chicago, IL, USA, 22–26 July 2018. [Google Scholar]
- EN 590:2014; Automotive Fuels—Diesel—Requirements and Test Methods; German Version EN 590:2013 + AC:2014 (Foreign Standard). Available online: https://webstore.ansi.org/standards/din/dinen5902014?srsltid=AfmBOorKshQ54vfSh9u-WS2LfqxLyr5o7WDu2HcEREtPZAUAUMqacV8m (accessed on 30 June 2025).
- ISO 12966-4:2015; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography. ISO: Geneva, Switzerland, 2015.
- Misic, D.; Thodos, G. The thermal conductivity of hydrocarbon gases at normal pressures. AIChE J. 1961, 7, 264–271. [Google Scholar] [CrossRef]
- Vargaftik, N.B. Handbook of Thermal Conductivity of Liquids and Gases, 1st ed.; Taylor & Francis Ltd.: Abingdon, UK, 1993; 368p. [Google Scholar]
- Brokaw, R.S.; O’Neal, C., Jr. Rotational relaxation and the relation between thermal conductivity and viscosity for some nonpolar polyatomic gases. Symp. Combust. Proc. 1963, 9, 725–732. [Google Scholar] [CrossRef]
- Cape, J.; Lehman, G. Temperature and Finite Pulse-Time Effects in the Flash Method for Measuring Thermal Diffusivity. J. Appl. Phys. 1963, 34, 1909–1913. [Google Scholar] [CrossRef]
- Thomas, H.L.; Smith, H. Correlation of vapour pressure, temperature, and latent heat of vaporisation. J. Appl. Chem. 1970, 20, 33–36. [Google Scholar] [CrossRef]
- Sherwood, T.K.; Reed, C.E. Applied Mathematics in Chemical Engineering; McGraw-Hill Chemical Engineering Series; McGraw-Hill: New York, NY, USA, 1939; 403p. [Google Scholar]
- Lydersen, A.L. Estimation of Critical Properties of Organic Compounds by the Method of Group Contributions; University of Wisconsin, Engineering Experiment Station: Madison, WI, USA, 1955. [Google Scholar]
- Bretschneider, S. Properties of Gases and Liquids; Khimiya Publishing House: Moscow/Leningrad, Russia, 1966; 536p. (In Russian) [Google Scholar]
- Shelomentsev, A.M. The Calculation of Thermophysical Properties of Petroleum Products, Review; Publishing House of Standards: Moscow, Russia, 1985; 76p. (In Russian) [Google Scholar]
- Brush, S.G. Kinetic Theory. The Chapman–Enskog Solution of the Transport Equation for Moderately Dense Gases, 1st ed.; ter Haar, D., Ed.; Pergamon: Oxford, UK, 2013. [Google Scholar]
- Falkovskii, V.B. Approximate calculation of the viscosity of vapors of organic compounds. Zh. Fiz. Khimii 1953, 27, 768–770. (In Russian) [Google Scholar]
- Flynn, L.W.; Thodos, G. The viscosity of hydrocarbon gases at normal pressures. J. Chem. Eng. Data 1961, 6, 457–459. [Google Scholar] [CrossRef]
- Presser, C.; Nazarian, A.; Millo, A. Laser-driven calorimetry measurements of petroleum and biodiesel fuels. Fuel 2018, 214, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Rudin, M.G. Oil Refiner’s Pocket Guide; Khimiya Publishing House: Leningrad, Russia, 1989; 464p. (In Russian) [Google Scholar]
- Kay, W.B. Density of hydrocarbon gases and vapors at high temperature and pressure. Ind. Eng. Chem. 1936, 28, 1014–1019. [Google Scholar] [CrossRef]
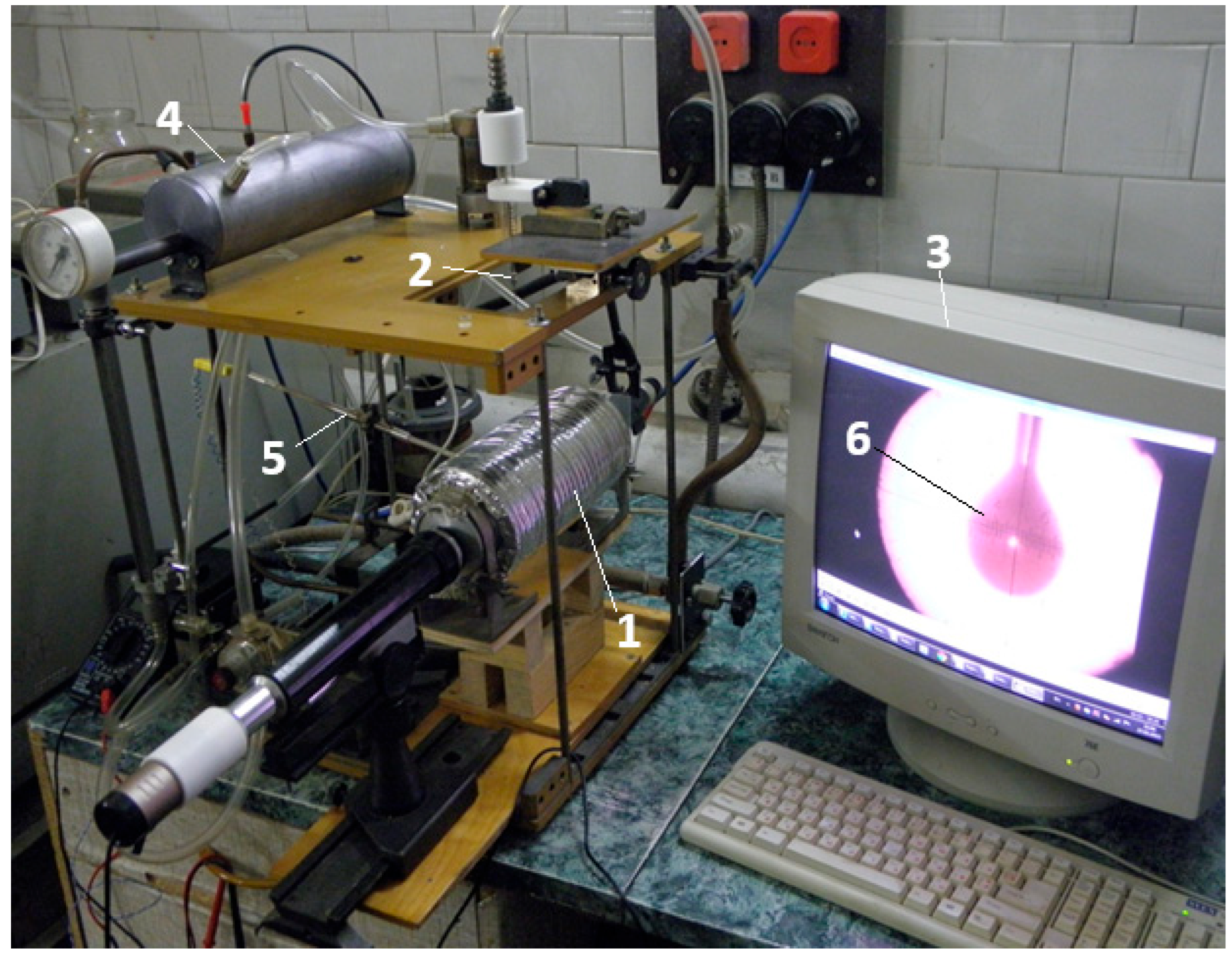
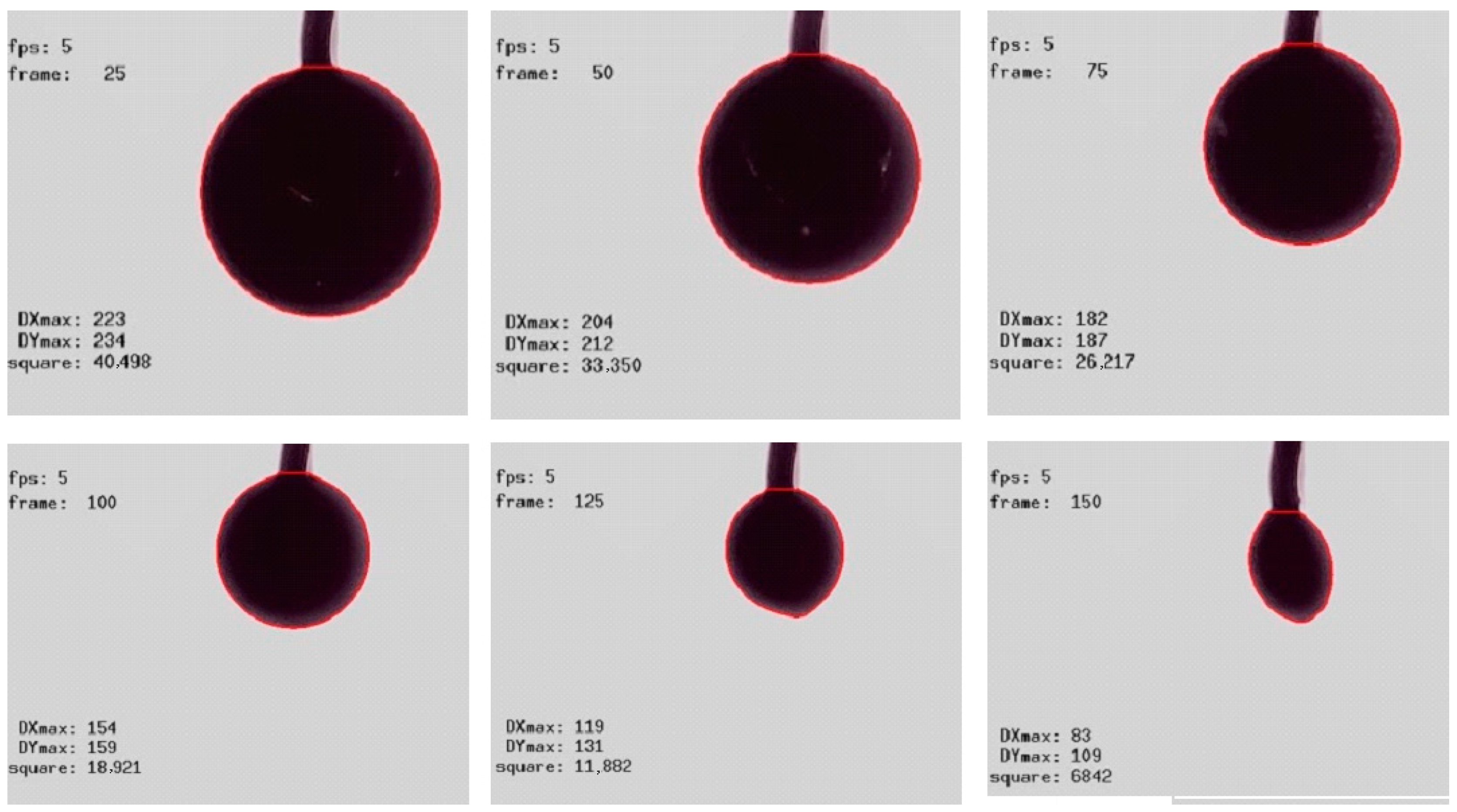


| Parameter | Diesel Fuel | RME |
|---|---|---|
| Density at 15 °C, kg/m3 | 835 | 884.3 |
| Viscosity at 40 °C, mm2/s | 4.2 | 4.7 |
| Cetane number | 51.0 | 55.0 |
| Water content, mg/kg | 200 | 223 |
| Methanol content, % mass | – | 0.05 |
| Total glycerol, % mass | – | 0.51 |
| Sulphur content, mg/kg | 6.9 (negligible) | negligible |
| Atoms, Groups, Bonds | Number of Parts | ||
|---|---|---|---|
| C | −0.462 | 18 | −8.316 |
| H | +0.249 | 36 | +8.964 |
| O | +0.054 | 1 | +0.054 |
| Double bonds | +0.478 | 1 | +0.478 |
| CO (ketones, ethers) | +0.105 | 1 | +0.105 |
| Atoms, Groups, Bonds | Number of Parts | ||
|---|---|---|---|
| –CH3 | 0.020 | 2 | 0.040 |
| –CH2– | 0.020 | 14 | 0.280 |
| –O– | 0.021 | 1 | 0.021 |
| =CO | 0.040 | 1 | 0.040 |
| –CH | 0.012 | 2 | 0.024 |
| Atoms, Groups, Bonds | Number of Parts | ||
|---|---|---|---|
| –CH3 | 0.227 | 2 | 0.454 |
| –CH2– | 0.227 | 14 | 3.178 |
| –O– | 0.16 | 1 | 0.16 |
| =CO | 0.29 | 1 | 0.29 |
| –CH= | 0.198 | 2 | 0.396 |
| Atoms, Groups, Bonds | Diesel Fuel | RME |
|---|---|---|
| Chemical formula | C16H34 | C19H36O2 |
| Molecular weight | 226.43 | 292.00 |
| Density at T = 293 K, kg/m3 | 860 | 900 |
| Thermal conductivity, W/m·K | 95.72 | 66.46 |
| Heat capacity (liquid phase) at T = 293 K, J/(g·K) | 1.85 | 2.12 |
| Dynamic viscosity at T = 288 K, Pa·s | 2.44·10−6 [55] | 4.54·10−6 4.21·10−6 |
| Specific heat capacity at K, | 1.63 | 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raslavičius, L. Evaporation and Ignition of Isolated Fuel Drops in an Oxidizing Environment: Analytical Study Based on Varshavskii’s ‘Diffusion Theory’. Appl. Sci. 2025, 15, 7488. https://doi.org/10.3390/app15137488
Raslavičius L. Evaporation and Ignition of Isolated Fuel Drops in an Oxidizing Environment: Analytical Study Based on Varshavskii’s ‘Diffusion Theory’. Applied Sciences. 2025; 15(13):7488. https://doi.org/10.3390/app15137488
Chicago/Turabian StyleRaslavičius, Laurencas. 2025. "Evaporation and Ignition of Isolated Fuel Drops in an Oxidizing Environment: Analytical Study Based on Varshavskii’s ‘Diffusion Theory’" Applied Sciences 15, no. 13: 7488. https://doi.org/10.3390/app15137488
APA StyleRaslavičius, L. (2025). Evaporation and Ignition of Isolated Fuel Drops in an Oxidizing Environment: Analytical Study Based on Varshavskii’s ‘Diffusion Theory’. Applied Sciences, 15(13), 7488. https://doi.org/10.3390/app15137488






