Effect of Tai Chi Practice on the Adaptation to Sensory and Motor Perturbations While Standing in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Participants
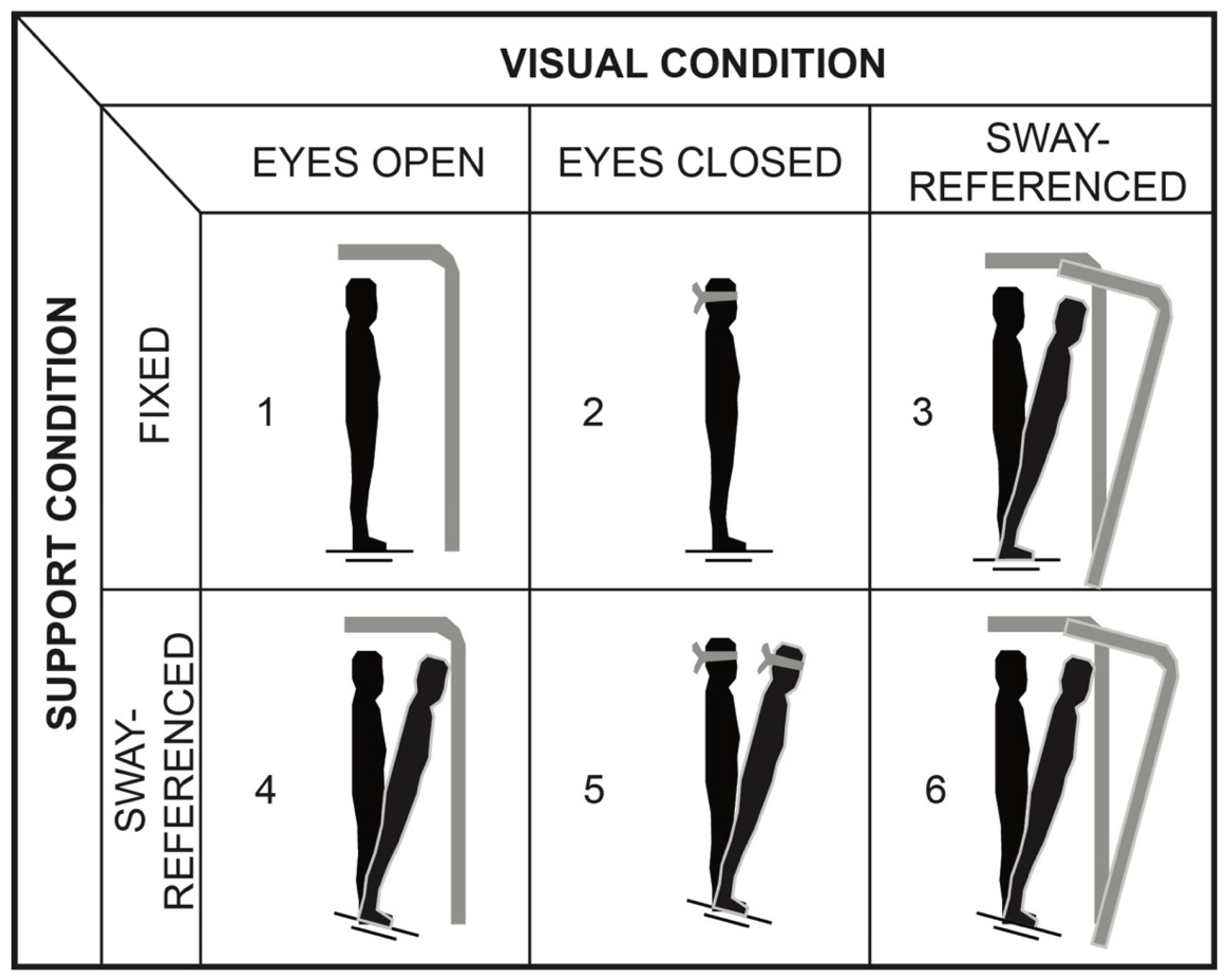
2.3. Postural Control Tasks
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
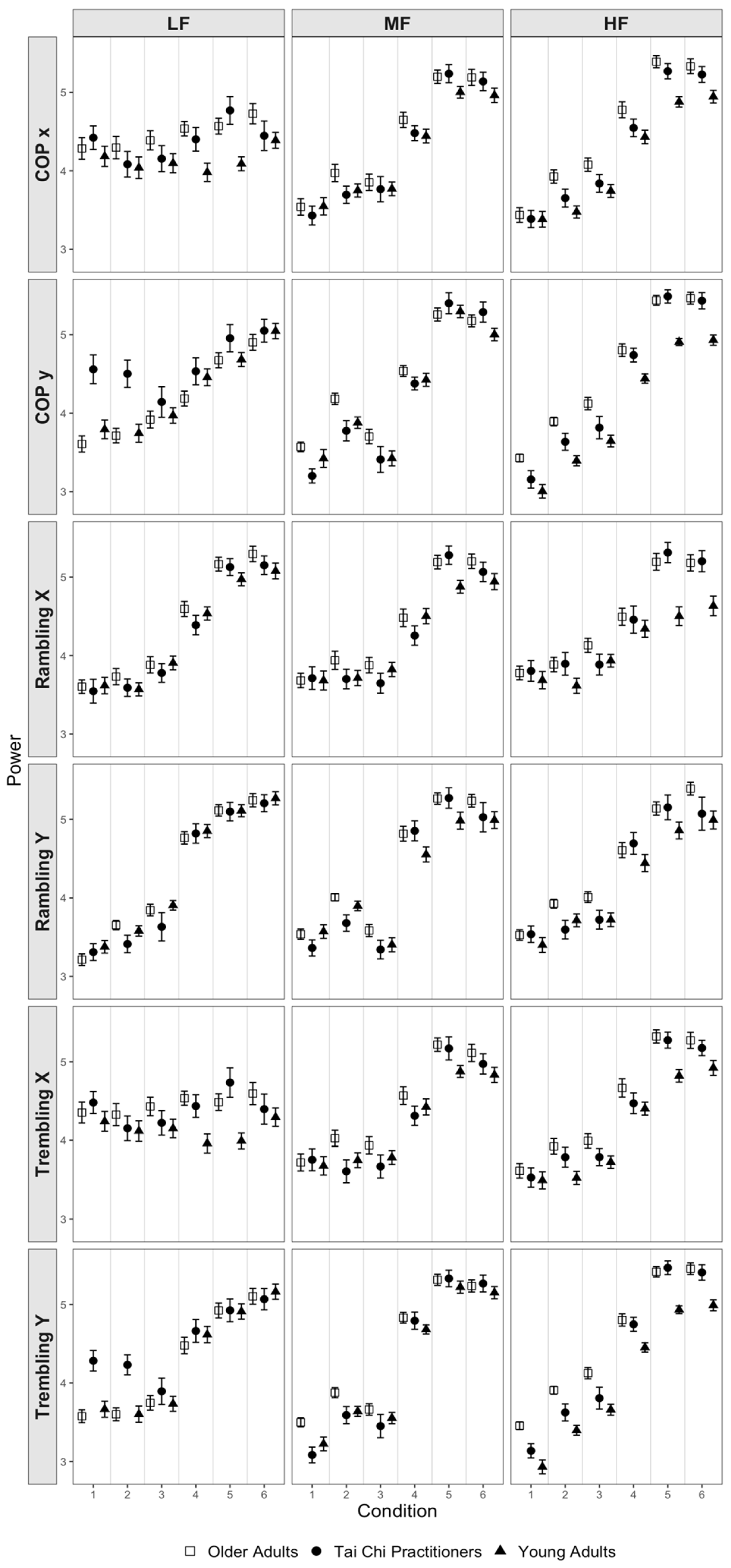
3.1. Overall Results
3.2. Center of Pressure Trajectories
3.3. Rambling Trajectories
3.4. Trembling Trajectories
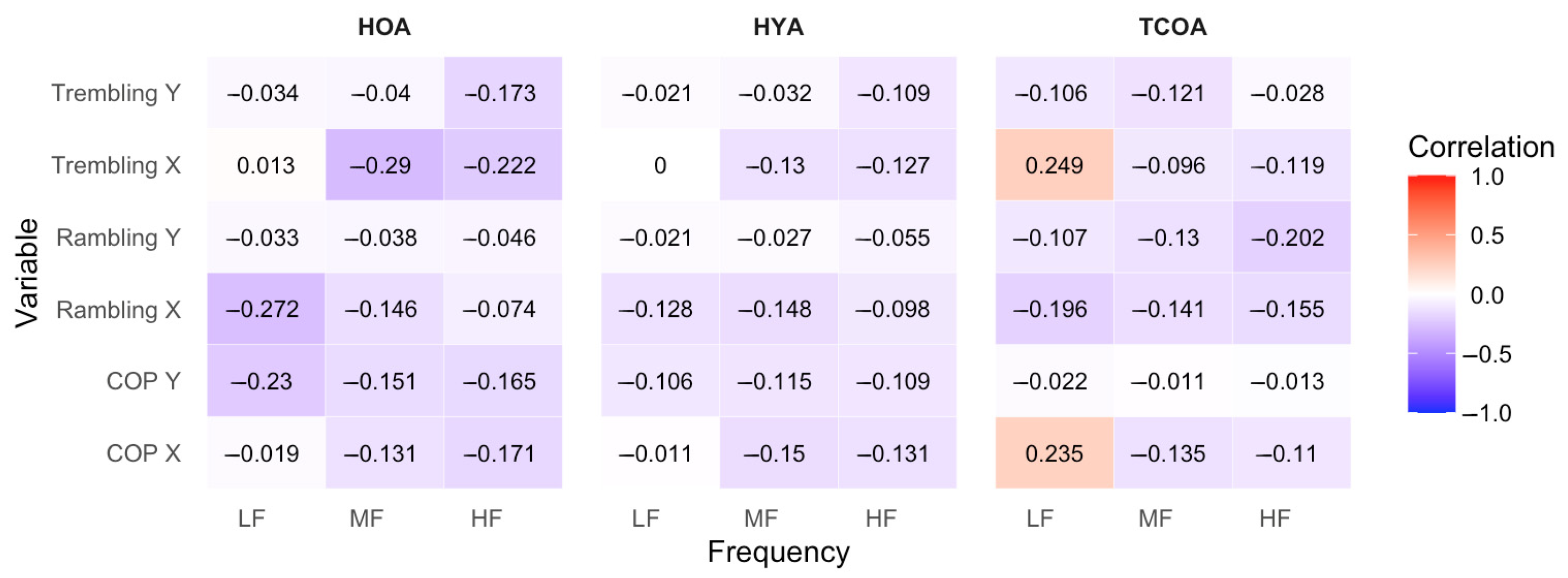
3.5. Correlation Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COP | Center of pressure |
| HYA | Healthy young adults |
| HOA | Healthy older adults |
| TCOA | Tai Chi practicing older adults |
References
- Day, B.L.; Lord, S.R. Balance, Gait, and Falls; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159. [Google Scholar]
- Bergen, G. Falls and Fall Injuries among Adults Aged ≥65 Years—United States, 2014. Morb. Mortal. Wkly. Rep. 2016, 65, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Parkkari, J.; Koskinen, S.; Niemi, S.; Palvanen, M.; Järvinen, M.; Vuori, I. Fall-Induced Injuries and Deaths among Older Adults. JAMA 1999, 281, 1895–1899. [Google Scholar] [CrossRef] [PubMed]
- Hartholt, K.A.; van Beeck, E.F.; Polinder, S.; van der Velde, N.; van Lieshout, E.M.M.; Panneman, M.J.M.; van der Cammen, T.J.M.; Patka, P. Societal Consequences of Falls in the Older Population: Injuries, Healthcare Costs, and Long-Term Reduced Quality of Life. J. Trauma. Acute Care Surg. 2011, 71, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Rose, J.; Rohlfing, T.; Pfefferbaum, A. Postural Sway Reduction in Aging Men and Women: Relation to Brain Structure, Cognitive Status, and Stabilizing Factors. Neurobiol. Aging 2009, 30, 793–807. [Google Scholar] [CrossRef]
- Quijoux, F.; Vienne-Jumeau, A.; Bertin-Hugault, F.; Zawieja, P.; Lefevre, M.; Vidal, P.-P.; Ricard, D. Center of Pressure Displacement Characteristics Differentiate Fall Risk in Older People: A Systematic Review with Meta-Analysis. Ageing Res. Rev. 2020, 62, 101117. [Google Scholar] [CrossRef]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What Is Balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef]
- Perucca, L.; Robecchi Majnardi, A.; Frau, S.; Scarano, S. Normative Data for the NeuroCom® Sensory Organization Test in Subjects Aged 80–89 Years. Front. Hum. Neurosci. 2021, 15, 761262. [Google Scholar] [CrossRef]
- Sung, P.; Rowland, P. Impact of Sensory Reweighting Strategies on Postural Control Using the Sensory Organization Test in Older Adults with and without Fall Risks. Physiother. Res. Int. 2024, 29, e2075. [Google Scholar] [CrossRef]
- Alcock, L.; O’Brien, T.D.; Vanicek, N. Association between Somatosensory, Visual and Vestibular Contributions to Postural Control, Reactive Balance Capacity and Healthy Ageing in Older Women. Health Care Women Int. 2018, 39, 1366–1380. [Google Scholar] [CrossRef]
- Stevens, J.A.; Burns, E. A CDC Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2015.
- Li, F.; Harmer, P.; Mack, K.A.; Sleet, D.; Fisher, K.J.; Kohn, M.A.; Millet, L.M.; Xu, J.; Yang, T.; Sutton, B.; et al. Tai Chi: Moving for Better Balance—Development of a Community-Based Falls Prevention Program. J. Phys. Act. Health 2008, 5, 445–455. [Google Scholar] [CrossRef]
- Li, F.; Harmer, P.; Eckstrom, E.; Fitzgerald, K.; Chou, L.-S.; Liu, Y. Effectiveness of Tai Ji Quan vs. Multimodal and Stretching Exercise Interventions for Reducing Injurious Falls in Older Adults at High Risk of Falling: Follow-up Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e188280. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.W.; Bartlett, D.J. Effectiveness of Tai Chi as a Therapeutic Exercise in Improving Balance and Postural Control. Phys. Occup. Ther. Geriatr. 2000, 17, 1–22. [Google Scholar] [CrossRef]
- Liu, X.X.; Wang, G.; Zhang, R.; Ren, Z.; Wang, D.; Liu, J.; Wang, J.; Gao, Y. Sensory Reweighting and Self-Motion Perception for Postural Control under Single-Sensory and Multisensory Perturbations in Older Tai Chi Practitioners. Front. Hum. Neurosci. 2024, 18, 1482752. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.M.; Krebs, D.E.; Wolf, S.L.; Gill-Body, K.M.; Scarborough, D.M.; McGibbon, C.A.; Kaptchuk, T.J.; Parker, S.W. Can Tai Chi Improve Vestibulopathic Postural Control? Arch. Phys. Med. Rehabil. 2004, 85, 142–152. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F. Cortical Control of Postural Responses. J. Neural Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. Cognitive Demands and Cortical Control of Human Balance-Recovery Reactions. J. Neural Transm. 2007, 114, 1279–1296. [Google Scholar] [CrossRef]
- Papegaaij, S.; Taube, W.; Baudry, S.; Otten, E.; Hortobagyi, T. Aging Causes a Reorganization of Cortical and Spinal Control of Posture. Front. Aging Neurosci. 2014, 6, 28. [Google Scholar] [CrossRef]
- Zatsiorsky, V.M.; Duarte, M. Instant Equilibrium Point and Its Migration in Standing Tasks: Rambling and Trembling Components of the Stabilogram. Mot. Control 1999, 3, 28–38. [Google Scholar] [CrossRef]
- Zatsiorsky, V.M.; Duarte, M. Rambling and Trembling in Quiet Standing. Mot. Control 2000, 4, 185–200. [Google Scholar] [CrossRef]
- Ferronato, P.A.M.; Barela, J.A. Age-Related Changes in Postural Control: Rambling and Trembling Trajectories. Mot. Control 2011, 15, 481–493. [Google Scholar] [CrossRef]
- Tahayori, B.; Riley, Z.A.; Mahmoudian, A.; Koceja, D.M.; Hong, S.L. Rambling and Trembling in Response to Body Loading. Mot. Control 2012, 16, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.E.; Marsiske, M.; McCoy, K.J.M. The Use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the Detection of Amnestic Mild Cognitive Impairment. J. Geriatr. Psychiatry Neurol. 2009, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Black, F.O. Clinical Status of Computerized Dynamic Posturography in Neurotology. Curr. Opin. Otolaryngol. Head Neck Surg. 2001, 9, 314–318. [Google Scholar] [CrossRef]
- Honaker, J.A.; Criter, R.E.; Patterson, J.N. Life in Balance: By Assessing Older Patients’ Risk of Falling and Offering Advice to Reduce the Likelihood of Falls inside and Outside the Home, Audiologists Can Help Keep Patients Active and Upright. ASHA Lead. 2013, 18, 40–46. [Google Scholar] [CrossRef]
- Huntley, J.; Ostfeld, A.M.; Taylor, J.O.; Wallace, R.B.; Blazer, D.; Berkman, L.F.; Evans, D.A.; Kohout, J.; Lemke, J.H.; Scherr, P.A.; et al. Established Populations for Epidemiologic Studies of the Elderly: Study Design and Methodology. Aging Clin. Exp. Res. 1993, 5, 27–37. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Murphy, S.L.; Alexander, N.B. Characteristics of Older Adults with Self-Reported Stooping, Crouching, or Kneeling Difficulty. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2008, 63, 759–763. [Google Scholar] [CrossRef]
- Delbaere, K.; Close, J.C.T.; Mikolaizak, A.S.; Sachdev, P.S.; Brodaty, H.; Lord, S.R. The Falls Efficacy Scale International (FES-I). A Comprehensive Longitudinal Validation Study. Age Ageing 2010, 39, 210–216. [Google Scholar] [CrossRef]
- Kanekar, N.; Lee, Y.-J.; Aruin, A.S. Frequency Analysis Approach to Study Balance Control in Individuals with Multiple Sclerosis. J. Neurosci. Methods 2014, 222, 91–96. [Google Scholar] [CrossRef]
- do Nascimento, C.F.; de Moraes Batista, A.F.; Duarte, Y.A.O.; Chiavegatto Filho, A.D.P. Early Identification of Older Individuals at Risk of Mobility Decline with Machine Learning. Arch. Gerontol. Geriatr. 2022, 100, 104625. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Goldberg, A.; Alexander, N.B. Decreased Muscle Strength Relates to Self-Reported Stooping, Crouching, or Kneeling Difficulty in Older Adults. Phys. Ther. 2010, 90, 67–74. [Google Scholar] [CrossRef]
- Tsang, W.W.; Wong, V.S.; Fu, S.N.; Hui-Chan, C.W. Tai Chi Improves Standing Balance Control under Reduced or Conflicting Sensory Conditions. Arch. Phys. Med. Rehabil. 2004, 85, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hao, Z.; Tian, H.; Yang, Y.; Wang, J.; Lin, X. The Effects of Tai Chi on Standing Balance Control in Older Adults May Be Attributed to the Improvement of Sensory Reweighting and Complexity Rather than Reduced Sway Velocity or Amplitude. Front. Aging Neurosci. 2024, 16, 1330063. [Google Scholar] [CrossRef] [PubMed]
- Doumas, M.; Krampe, R.T. Adaptation and Reintegration of Proprioceptive Information in Young and Older Adults’ Postural Control. J. Neurophysiol. 2010, 104, 1969–1977. [Google Scholar] [CrossRef]
- Henry, M.; Baudry, S. Age-Related Changes in Leg Proprioception: Implications for Postural Control. J. Neurophysiol. 2019, 122, 525–538. [Google Scholar] [CrossRef]
- Gerber, E.D.; Huang, C.K.; Moon, S.; Devos, H.; Luchies, C.W. Sensory Reweighting of Postural Control Requires Distinct Rambling and Trembling Sway Adaptations. Gait Posture 2024, 112, 16–21. [Google Scholar] [CrossRef]
- Hu, Y.; Petruzzello, S.J.; Hernandez, M.E. Beta Cortical Oscillatory Activities and Their Relationship to Postural Control in a Standing Balance Demanding Test: Influence of Aging. Front. Aging Neurosci. 2023, 15, 1126002. [Google Scholar] [CrossRef]
- Sasagawa, S.; Arakawa, A.; Furuyama, A.; Matsumoto, Y. Age-Related Changes in Static Balance in Older Women Aged in Their Early Sixties to Their Late Eighties: Different Aging Patterns in the Anterior–Posterior and Mediolateral Directions. Front. Aging Neurosci. 2024, 16, 1361244. [Google Scholar] [CrossRef]
- Allen, D.; Ribeiro, L.; Arshad, Q.; Seemungal, B.M. Age-Related Vestibular Loss: Current Understanding and Future Research Directions. Front. Neurol. 2016, 7, 231. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chan, P.Y.S.; Baillet, S.; Lin, Y.Y. Age-related Reduced Somatosensory Gating Is Associated with Altered Alpha Frequency Desynchronization. Neural Plast. 2015, 2015, 302878. [Google Scholar] [CrossRef]
- Benjuya, N.; Melzer, I.; Kaplanski, J. Aging-Induced Shifts from a Reliance on Sensory Input to Muscle Cocontraction during Balanced Standing. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 166. [Google Scholar] [CrossRef]
- Shin, S.; Milosevic, M.; Chung, C.M.; Lee, Y. Contractile Properties of Superficial Skeletal Muscle Affect Postural Control in Healthy Young Adults: A Test of the Rambling and Trembling Hypothesis. PLoS ONE 2019, 14, e0223850. [Google Scholar] [CrossRef] [PubMed]
| Young Adult (N = 23) | Older Adult (N = 21) | Tai Chi Practitioner (N = 15) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value | |
| Age (y) | 21.4 | 2.0 | 70.8 | 5.5 | 76.7 | 5.6 | <0.001 |
| Height (cm) | 170.9 | 11.0 | 171.0 | 10.4 | 168.1 | 9.0 | 0.649 |
| Weight (kg) | 71.5 | 11.5 | 72.0 | 11.9 | 64.2 | 7.0 | 0.073 |
| Foot Size (cm) | 25.9 | 1.7 | 25.9 | 1.6 | 25.5 | 1.4 | 0.654 |
| SOT Score [0–100] | 75.8 | 4.7 | 75.4 | 6.7 | 73.0 | 6.8 | 0.369 |
| SCK Difficulty [0–4] | 0.2 | 0.4 | 1.1 | 0.9 | 0.9 | 0.9 | <0.001 |
| Brisk Activity/Week | 4.0 | 2.3 | 4.8 | 3.4 | 3.0 | 2.2 | 0.265 |
| FES-I Score [16–64] | 18.0 | 2.1 | 19.9 | 3.2 | 22.7 | 3.9 | <0.001 |
| Number | Percent | Number | Percent | Number | Percent | p-Value | |
| Females | 12 | 52.2 | 11 | 52.4 | 9 | 60 | 0.874 |
| Low Frequency | Medium Frequency | High Frequency | ||||
|---|---|---|---|---|---|---|
| Source of Variance | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value |
| Center of Pressure in x | ||||||
| Condition | 6.47 | <0.001 | 247.45 | <0.001 | 373.98 | <0.001 |
| Trial | 0.04 | 0.962 | 25.71 | <0.001 | 49.83 | <0.001 |
| Group | 0.39 | 0.680 | 2.22 | 0.118 | 1.66 | 0.200 |
| Age | 1.22 | 0.274 | 4.60 | 0.036 | 1.92 | 0.172 |
| Condition × Trial | 0.43 | 0.935 | 1.36 | 0.196 | 2.40 | 0.008 |
| Condition × Group | 2.11 | 0.021 | 1.02 | 0.423 | 2.13 | 0.020 |
| Trial × Group | 0.64 | 0.631 | 1.41 | 0.229 | 0.23 | 0.924 |
| Condition × Trial × Group | 0.16 | 1.000 | 1.36 | 0.131 | 1.33 | 0.148 |
| Center of Pressure in y | ||||||
| Condition | 69.96 | <0.001 | 283.50 | <0.001 | 541.60 | <0.001 |
| Trial | 6.51 | 0.002 | 17.78 | <0.001 | 75.75 | <0.001 |
| Group | 2.61 | 0.083 | 1.95 | 0.152 | 2.47 | 0.093 |
| Age | 0.03 | 0.869 | 2.62 | 0.111 | 0.14 | 0.709 |
| Condition × Trial | 2.75 | 0.002 | 1.13 | 0.339 | 3.14 | 0.001 |
| Condition × Group | 2.14 | 0.020 | 2.06 | 0.025 | 1.99 | 0.032 |
| Trial × Group | 0.31 | 0.873 | 1.95 | 0.101 | 0.31 | 0.870 |
| Condition × Trial × Group | 0.82 | 0.697 | 1.04 | 0.408 | 1.19 | 0.258 |
| Low Frequency | Medium Frequency | High Frequency | ||||
|---|---|---|---|---|---|---|
| Source of Variance | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value |
| Rambling in x-direction | ||||||
| Condition | 202.97 | <0.001 | 148.08 | <0.001 | 94.63 | <0.001 |
| Trial | 22.74 | <0.001 | 9.99 | <0.001 | 14.50 | <0.001 |
| Group | 1.35 | 0.268 | 1.93 | 0.154 | 0.62 | 0.539 |
| Age | 2.06 | 0.157 | 3.28 | 0.075 | 2.37 | 0.129 |
| Condition × Trial | 1.08 | 0.378 | 1.73 | 0.070 | 1.65 | 0.088 |
| Condition × Group | 0.69 | 0.734 | 1.72 | 0.072 | 2.71 | 0.003 |
| Trial × Group | 0.31 | 0.869 | 1.57 | 0.180 | 1.42 | 0.227 |
| Condition × Trial × Group | 1.36 | 0.131 | 1.32 | 0.156 | 1.19 | 0.250 |
| Rambling in y-direction | ||||||
| Condition | 332.66 | <0.001 | 212.60 | <0.001 | 161.44 | <0.001 |
| Trial | 26.71 | <0.001 | 17.62 | <0.001 | 25.19 | <0.001 |
| Group | 0.39 | 0.680 | 4.34 | 0.018 | 3.73 | 0.030 |
| Age | 0.29 | 0.592 | 7.15 | 0.010 | 7.36 | 0.009 |
| Condition × Trial | 1.65 | 0.088 | 1.47 | 0.146 | 1.54 | 0.119 |
| Condition × Group | 0.93 | 0.505 | 1.34 | 0.204 | 1.04 | 0.404 |
| Trial × Group | 2.21 | 0.066 | 2.37 | 0.051 | 3.44 | 0.008 |
| Condition × Trial × Group | 0.68 | 0.847 | 0.56 | 0.942 | 0.84 | 0.663 |
| Low Frequency | Medium Frequency | High Frequency | ||||
|---|---|---|---|---|---|---|
| Source of Variance | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value |
| Trembling in x-direction | ||||||
| Condition | 1.89 | 0.094 | 121.49 | <0.001 | 214.49 | <0.001 |
| Trial | 0.20 | 0.817 | 15.27 | <0.001 | 24.75 | <0.001 |
| Group | 0.33 | 0.722 | 3.85 | 0.027 | 2.08 | 0.134 |
| Age | 1.14 | 0.290 | 6.14 | 0.016 | 3.82 | 0.056 |
| Condition × Trial | 0.37 | 0.961 | 1.24 | 0.261 | 1.65 | 0.087 |
| Condition × Group | 2.22 | 0.015 | 1.15 | 0.323 | 1.25 | 0.258 |
| Trial × Group | 0.57 | 0.686 | 1.03 | 0.393 | 1.08 | 0.365 |
| Condition × Trial × Group | 0.19 | 1.000 | 1.00 | 0.456 | 1.07 | 0.380 |
| Trembling in y-direction | ||||||
| Condition | 116.23 | <0.001 | 389.96 | <0.001 | 546.63 | <0.001 |
| Trial | 6.37 | 0.002 | 24.40 | <0.001 | 76.31 | <0.001 |
| Group | 1.65 | 0.201 | 1.65 | 0.201 | 3.15 | 0.050 |
| Age | 0.03 | 0.873 | 0.14 | 0.711 | 0.18 | 0.670 |
| Condition × Trial | 2.40 | 0.008 | 0.90 | 0.536 | 3.56 | <0.001 |
| Condition × Group | 2.09 | 0.023 | 1.11 | 0.350 | 1.78 | 0.060 |
| Trial × Group | 1.27 | 0.279 | 1.36 | 0.245 | 0.80 | 0.526 |
| Condition × Trial × Group | 0.58 | 0.928 | 0.63 | 0.896 | 1.16 | 0.285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, A.; Chang, H.; Shaaban, L.; Suga, A.; Braden, G.; Bustamante, A.; Park, J.; Zhang, S.; Hu, Y.; Hernandez, M.E. Effect of Tai Chi Practice on the Adaptation to Sensory and Motor Perturbations While Standing in Older Adults. Appl. Sci. 2025, 15, 7458. https://doi.org/10.3390/app15137458
Dey A, Chang H, Shaaban L, Suga A, Braden G, Bustamante A, Park J, Zhang S, Hu Y, Hernandez ME. Effect of Tai Chi Practice on the Adaptation to Sensory and Motor Perturbations While Standing in Older Adults. Applied Sciences. 2025; 15(13):7458. https://doi.org/10.3390/app15137458
Chicago/Turabian StyleDey, Arion, Huiyeong Chang, Laila Shaaban, Armaan Suga, Genavieve Braden, Andres Bustamante, Jisang Park, Shenhua Zhang, Yang Hu, and Manuel E. Hernandez. 2025. "Effect of Tai Chi Practice on the Adaptation to Sensory and Motor Perturbations While Standing in Older Adults" Applied Sciences 15, no. 13: 7458. https://doi.org/10.3390/app15137458
APA StyleDey, A., Chang, H., Shaaban, L., Suga, A., Braden, G., Bustamante, A., Park, J., Zhang, S., Hu, Y., & Hernandez, M. E. (2025). Effect of Tai Chi Practice on the Adaptation to Sensory and Motor Perturbations While Standing in Older Adults. Applied Sciences, 15(13), 7458. https://doi.org/10.3390/app15137458








