Phase Transition Behavior and Mechanical Properties of 9 Mol% CaO-PSZ with MnO2 Doping Under Thermal Stress
Abstract
1. Introduction
2. Materials and Methods
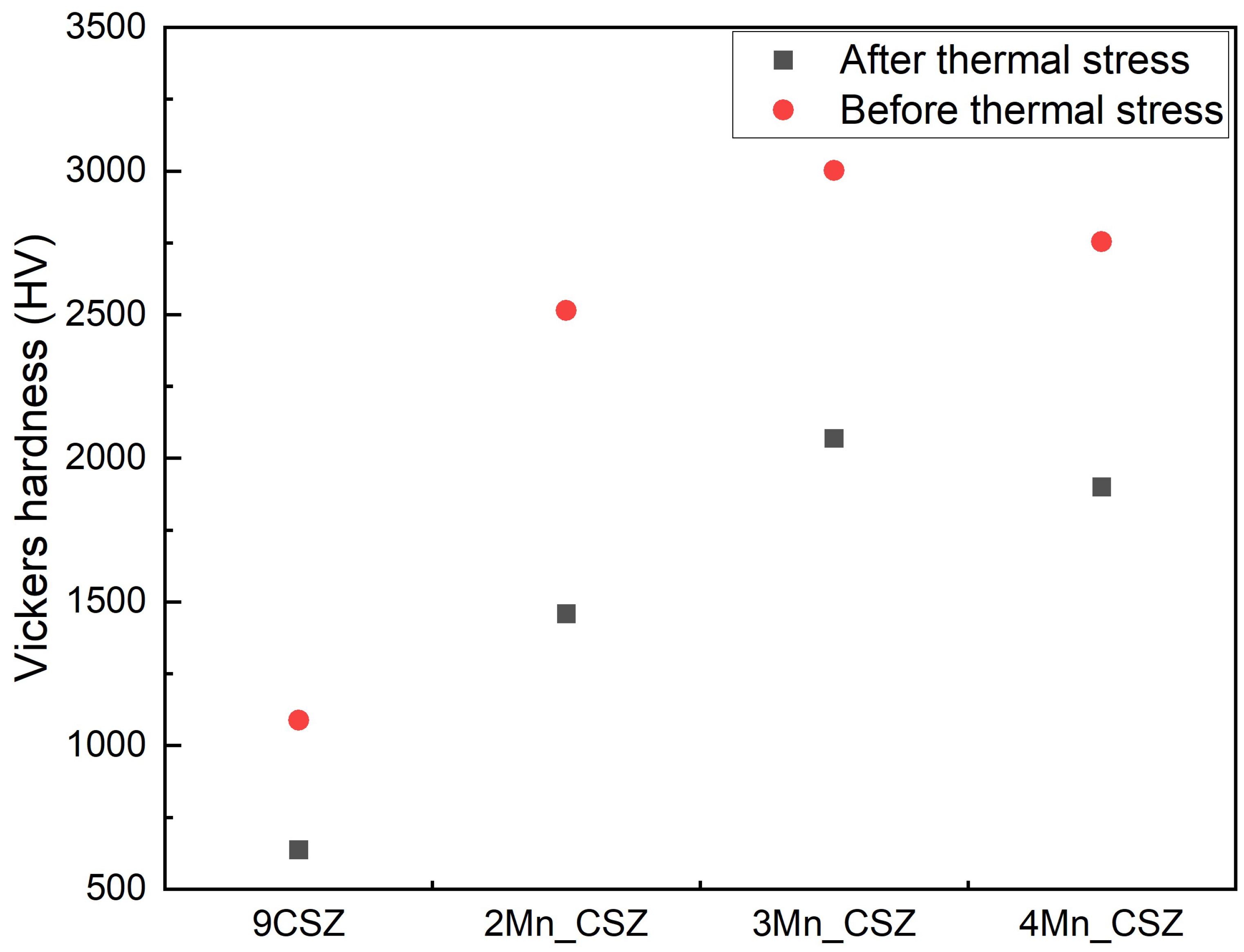
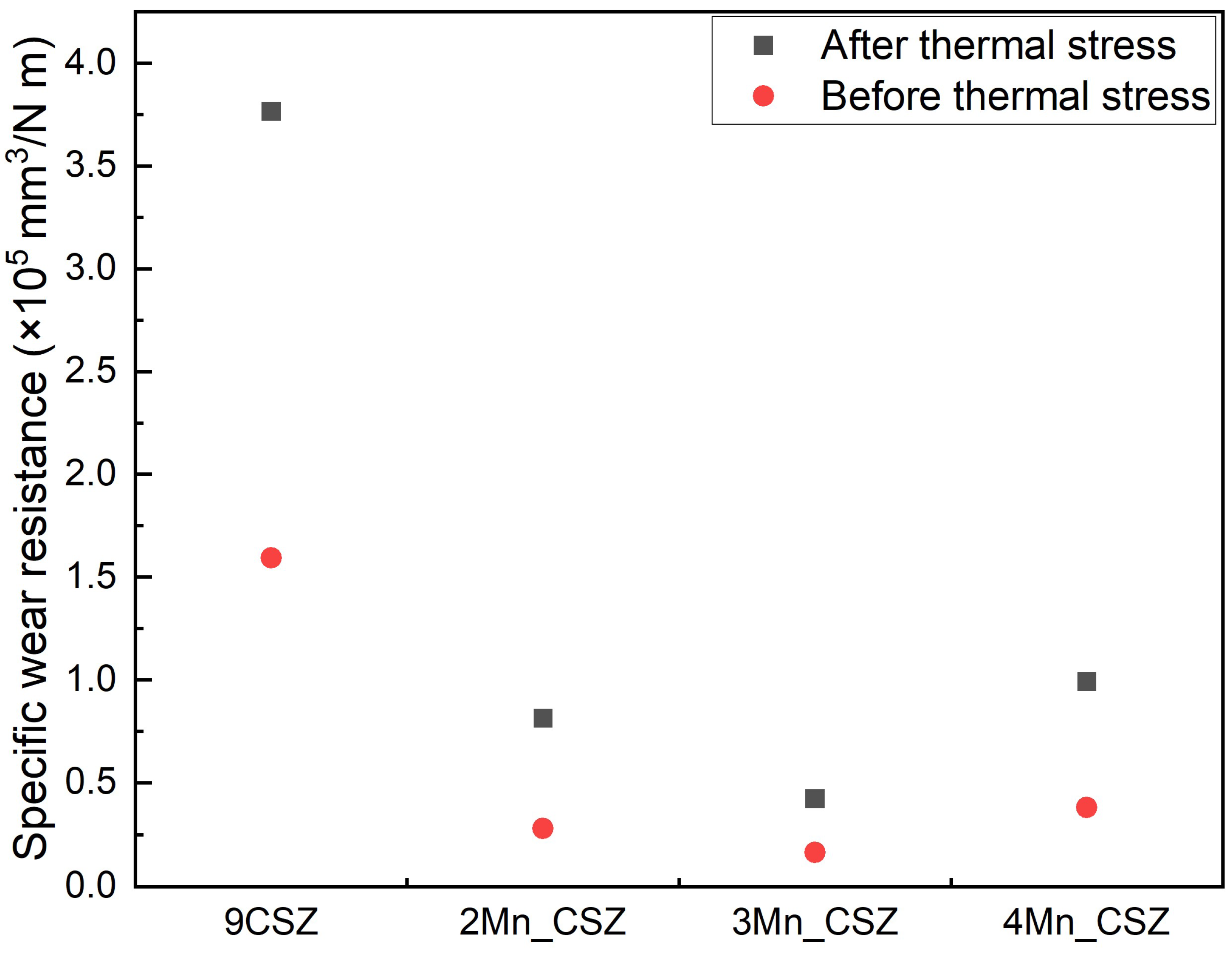
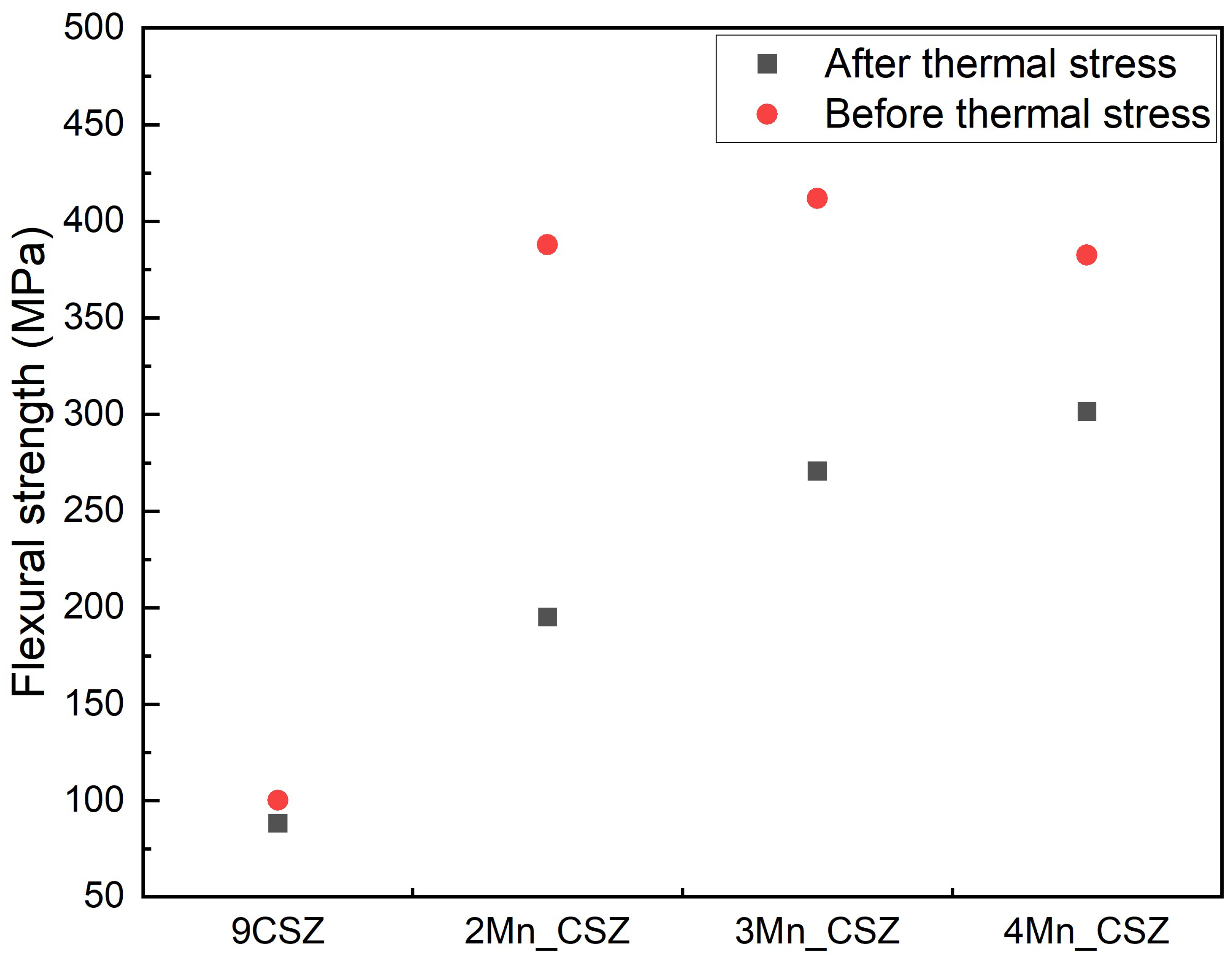
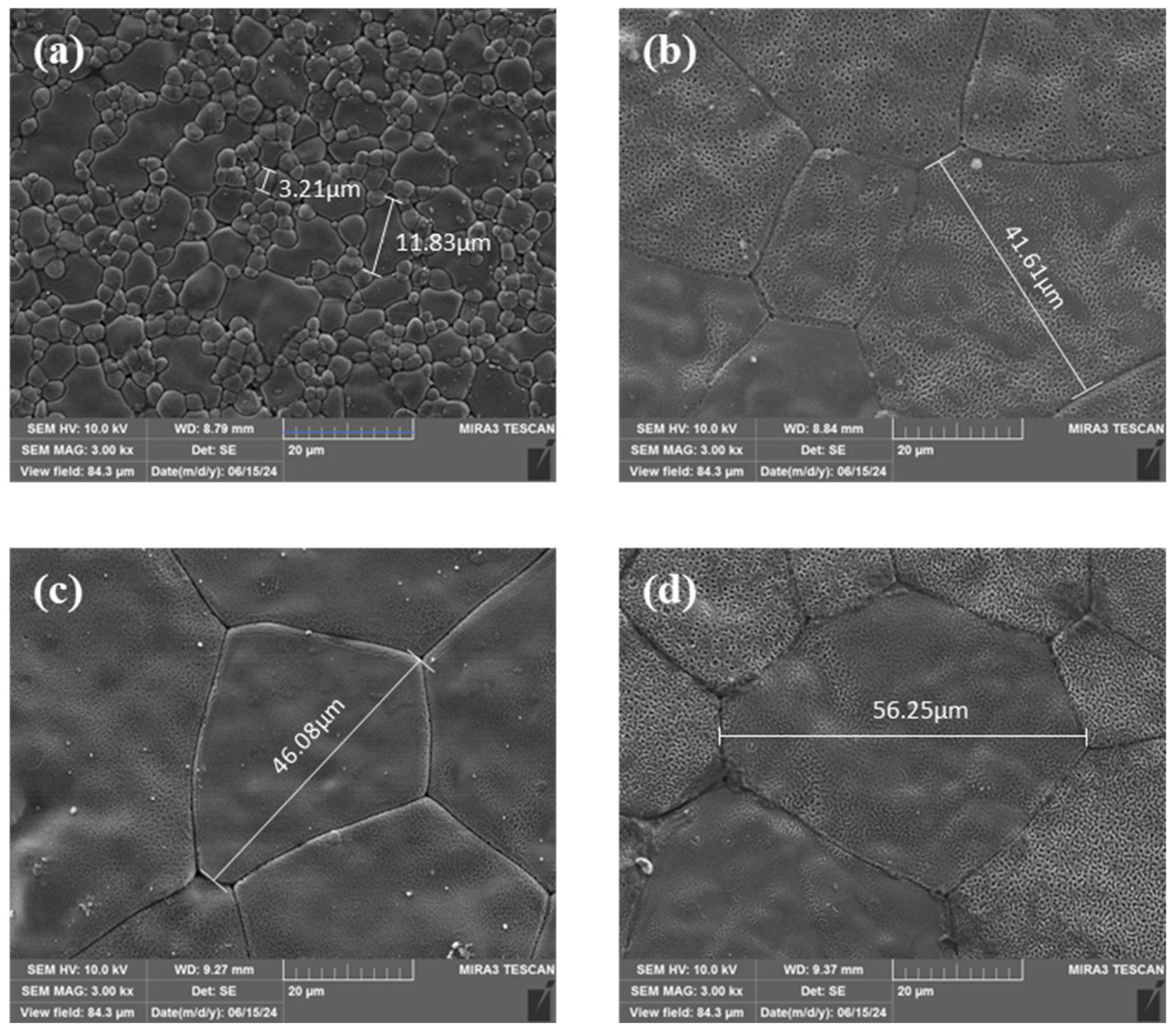
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Gandara, C.; Ramos, F.M.; Cirera, A. YSZ-based oxygen sensors and the use of nanomaterials: A review from classical models to current trends. J. Sens. 2009, 2009, 258489. [Google Scholar] [CrossRef]
- Olah, J.; Aburumman, N.; Popp, J.; Khan, M.A.; Haddad, H. Impact of industry 4.0 on environmental sustainability. Sustainability 2020, 12, 4674. [Google Scholar] [CrossRef]
- Hou, X.; Zhao, X.; Zhang, Y.; Zhang, Z.; Liu, Y.; Qin, Y.; Tan, P.; Chen, C.; Yu, S.; Ding, M.; et al. High-performance harsh-environment-resistant GaOX Solar-Blind photodetectors via defect and doping engineering. Adv. Mater. 2022, 34, 2106923. [Google Scholar] [CrossRef]
- Manicone, P.F.; Iommetti, P.R.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Loganathan, A.; Gandhi, A. Effect of phase transformations on the fracture toughness of t0 yttria stabilized zirconia. Mater. Sci. Eng. A 2012, 556, 927–935. [Google Scholar] [CrossRef]
- Talibi, M.; Kaur, K.; Parmar, H. Do you know your ceramics? Part 5: Zirconia. Br. Dent. J. 2022, 232, 311–316. [Google Scholar] [CrossRef]
- Juri, A.Z.; Zhang, Y.; Kotousov, A.; Yin, L. Zirconia responses to edge chipping damage induced in conventional and ultrasonic vibration-assisted diamond machining. J. Mater. Res. Technol. 2021, 13, 573–589. [Google Scholar] [CrossRef]
- Chen, G.; Ling, Y.; Li, Q.; Zheng, H.; Li, K.; Jiang, Q.; Gao, L.; Omran, M.; Peng, J.; Chen, J. Stability properties and structural characteristics of CaO-partially stabilized zirconia ceramics synthesized from fused ZrO2 by microwave sintering. Ceram. Int. 2020, 46, 16842–16848. [Google Scholar] [CrossRef]
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration curve for quantitative analysis of the monoclinic-tetragonal ZrO2 System by X-Ray Diffraction. J. Am. Ceram. Soc. 1984, 67, 119–121. [Google Scholar] [CrossRef]
- Kulyk, V.; Duriagina, Z.; Kostryzhev, A.; Vasyliv, B.; Vavrukh, V.; Marenych, O. The effect of yttria content on microstructure, strength, and fracture behavior of yttria-stabilized zirconia. Materials 2022, 15, 5212. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, Y.; Yin, G.; Tong, S.; Chen, J. Synthesis and characterization of a MgO-MgAl2O4-ZrO2 composite with a continuous network microstructure. Ceram. Int. 2017, 43, 5914–5919. [Google Scholar] [CrossRef]
- Gao, L.; Guan, R.; Zhang, S.; Zhi, H.; Jin, C.; Jin, L.; Wei, Y.; Wang, J. As-sintered manganese-stabilized zirconia ceramics with excellent electrical conductivity. Crystals 2022, 12, 620. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, L.; Kang, J.; Pu, J.; Peng, J.; Omran, M.; Chen, G. Stability optimisation of CaO-doped partially stabilized zirconia by microwave sintering. Ceram. Int. 2019, 45, 23278–23282. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Peng, J.; Koppala, S.; Omran, M.; Chen, G. One-step preparation of CaO-doped partially stabilized zirconia from fused zirconia. Ceram. Int. 2020, 46, 6484–6490. [Google Scholar] [CrossRef]
- Changsu, H. Degradation of ZrO2-C material for submerged entry nozzle in thin slab casting. J. Korean Ceram. Soc. 1998, 35, 251–258. [Google Scholar]
- Yoo, H.; Kim, J.; Lee, H.; Jo, I.; Lee, H. Phase formation and stabilization behavior of Ca-PSZ by post-heat treatment II: CaOx-ZrO2(1 - x) (x = 5–10mol%). Metals 2023, 13, 1659. [Google Scholar] [CrossRef]
- ISO 20808:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Friction and Wear Characteristics of Monolithic Ceramics by Ball-on-Disc Method. ISO: Geneva, Switzerland, 2016.
- Murugan, B.; Ramaswamy, A.V.; Srinivas, D.; Gopinath, C.S.; Ramaswamy, V. Nature of manganese species in Ce1-x MnxO2-δ solid solutions synthesized by the solution combustion route. Chem. Mater. 2005, 17, 3983–3993. [Google Scholar] [CrossRef]
- Di Castro, V.; Polzonetti, G. XPS study of MnO oxidation. J. Electron Spectrosc. Relat. Phenom. 1989, 48, 117–123. [Google Scholar] [CrossRef]
- Richter, J.; Holtappels, P.; Graule, T.; Nakamura, T.; Gauckler, L.J. Materials design for perovskite SOFC cathodes. Monatsh. Chem. 2009, 140, 985–999. [Google Scholar] [CrossRef]
- Occhiuzzi, M.; Cordischi, D.; Dragone, R. Manganese ions in the monoclinic, tetragonal and cubic phases of zirconia: An XRD and EPR study. Phys. Chem. Chem. Phys. 2003, 5, 4938–4945. [Google Scholar] [CrossRef]
- Colbea, C.; Avram, D.; Cojocaru, B.; Negrea, R.; Ghica, C.; Kessler, V.G.; Seisenbaeva, G.A.; Parvulescu, V.; Tiseanu, C. Full tetragonal phase stabilization in ZrO2 nanoparticles using wet impregnation: Interplay of host structure, dopant concentration and sensitivity of characterization technique. Nanomaterials 2018, 8, 988. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S. Investigation of crystal structure confinement and optical attributes of monoclinic–tetragonal Zirconia nanocrystals via chemical co-precipitation technique. Bull. Mater. Sci. 2022, 45, 182. [Google Scholar] [CrossRef]
- Song, X.; Ding, Y.; Zhang, J.; Jiang, C.; Liu, Z.; Lin, C.; Zheng, W.; Zeng, Y. Thermophysical and mechanical properties of cubic, tetragonal and monoclinic ZrO2. J. Mater. Res. Technol. 2023, 23, 648–655. [Google Scholar] [CrossRef]

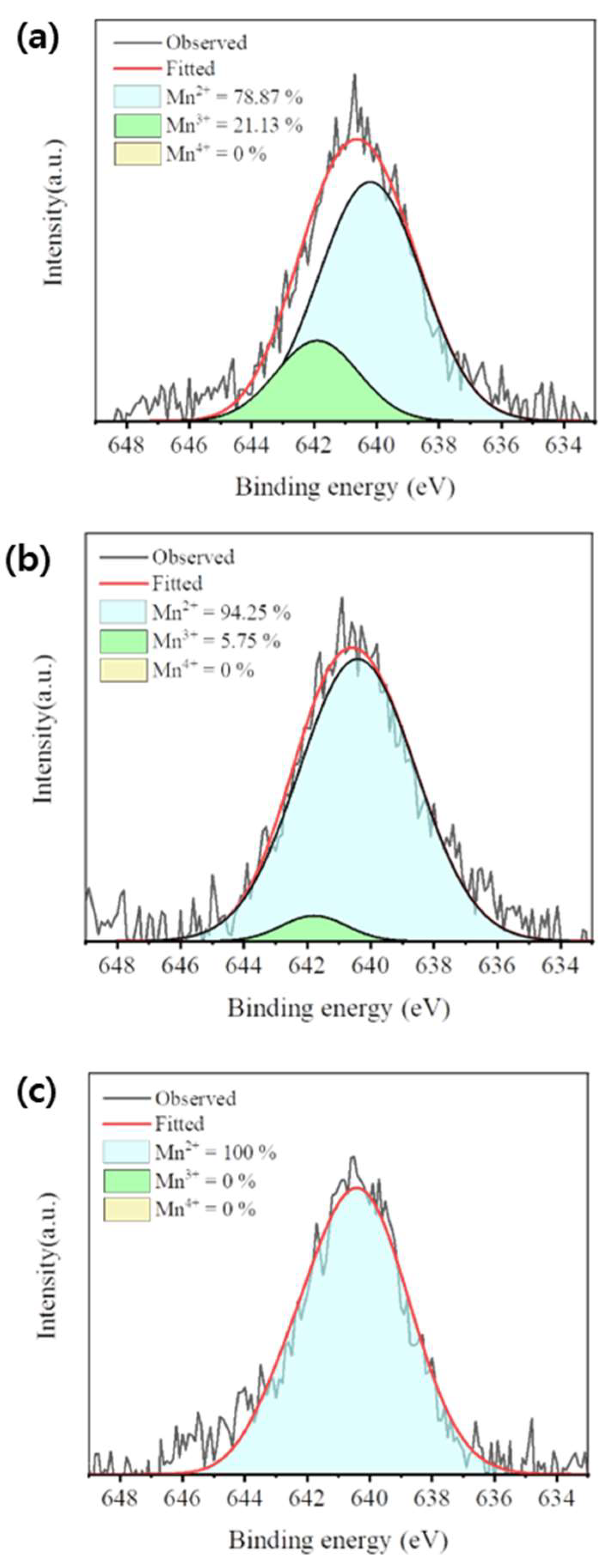
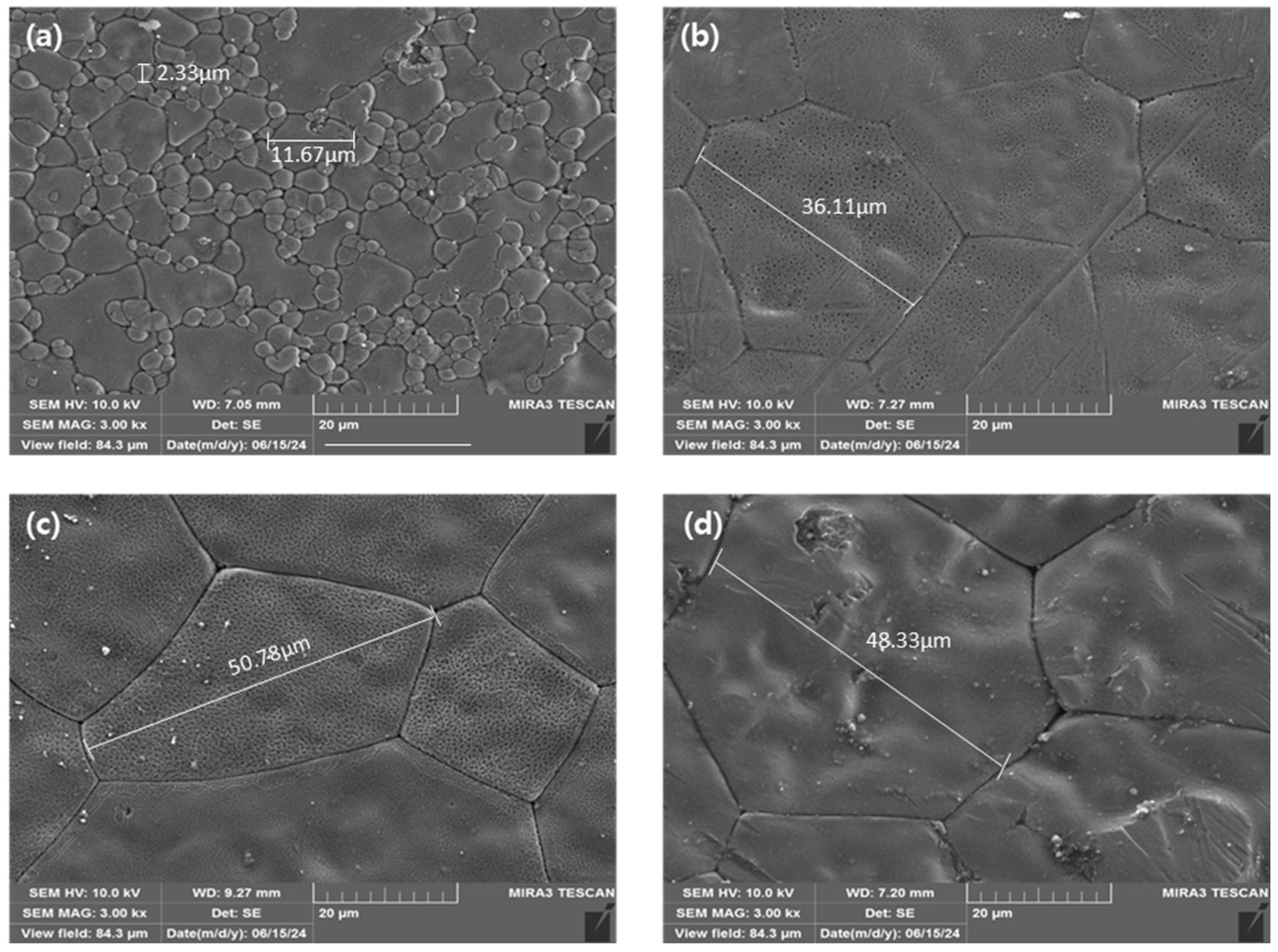
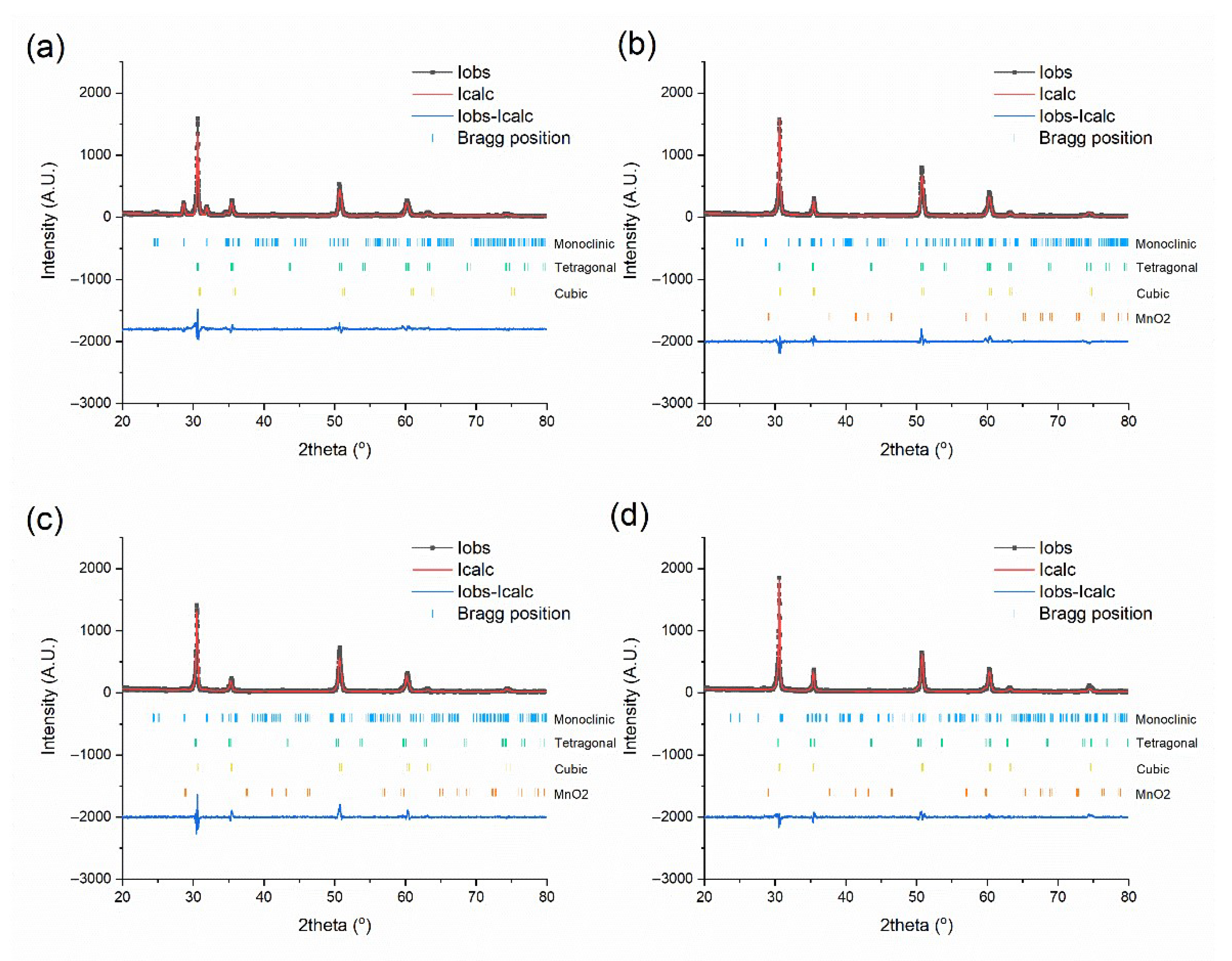
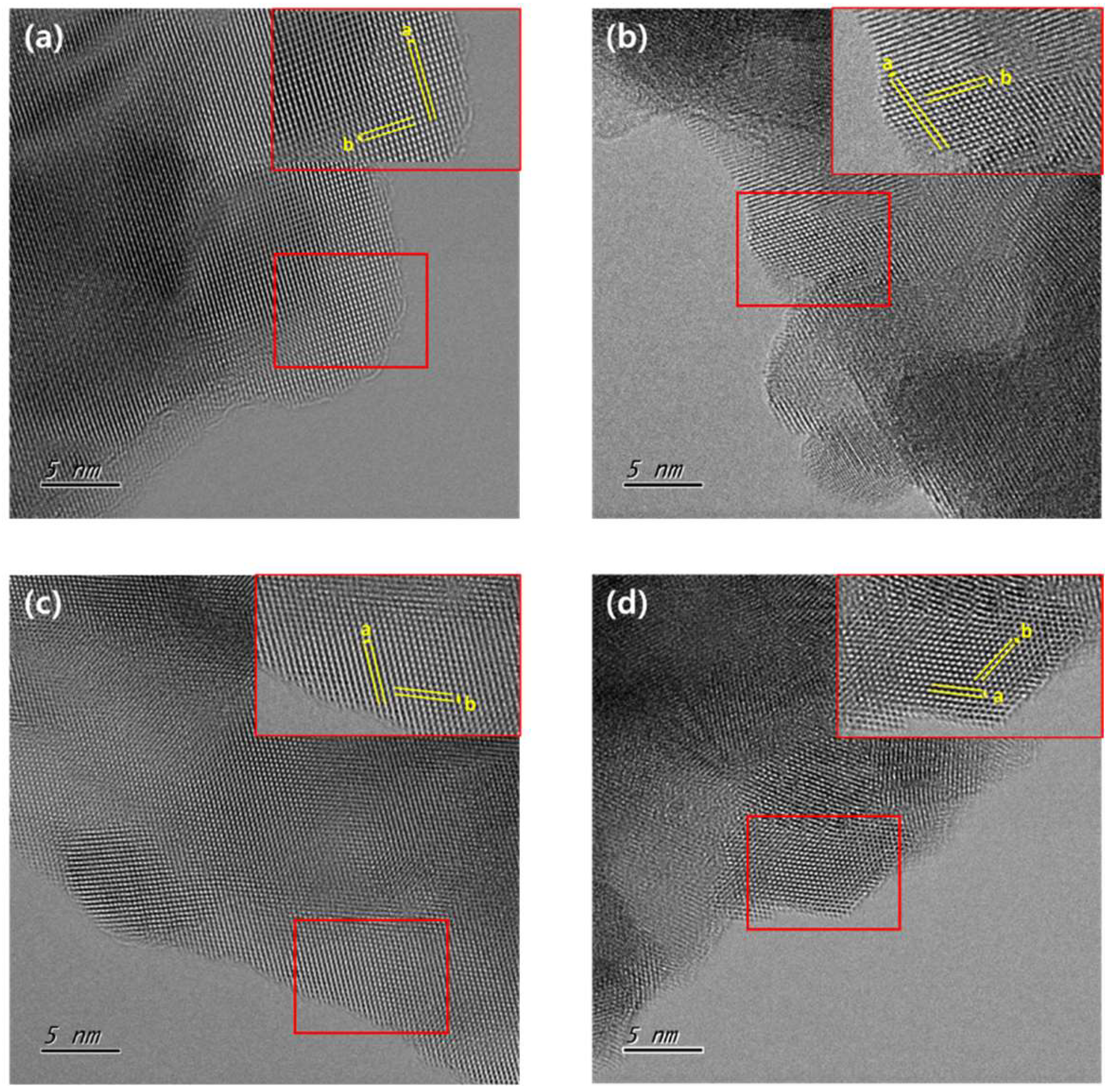
| Name | Composition | Fabrication Conditions | ||
|---|---|---|---|---|
| Raw Material | MnO2 Addition (mol%) | Calcination Temp (°C) | Holding Time (h) | |
| 9-CSZ | 9 mol% CSZ | - | 1200 | 2 |
| 9-CSZ_2 MnO2 | 2 | |||
| 9-CSZ_3 MnO2 | 3 | |||
| 9-CSZ_4 MnO2 | 4 | |||
| 9-CSZ | 2MnCSZ | 3MnCSZ | 4MnCSZ | ||
|---|---|---|---|---|---|
| m-ZrO2 | volume % | 32.6 | 3.8 | 6.5 | 2.5 |
| a (Å) | 5.149 | 5.137 | 5.471 | 5.223 | |
| b (Å) | 5.203 | 5.234 | 5.662 | 5.154 | |
| c (Å) | 5.319 | 5.269 | 5.143 | 5.219 | |
| α = γ | 90° | 90° | 90° | 90° | |
| β | 99.198 | 99.093 | 99.439 | 99.156 | |
| lattice volume (Å3) | 140.6650712 | 139.8875853 | 157.1567065 | 138.7020022 | |
| chemical composition | ZrO1.987 ± 0.003 | ZrO1.982 ± 0.018 | ZrO1.978 ± 0.006 | ZrO1.976 ± 0.010 | |
| t-ZrO2 | volume % | 58.2 | 88.2 | 90.1 | 90.3 |
| a = b (Å) | 3.626 | 3.613 | 3.616 | 3.616 | |
| c (Å) | 5.122 | 5.129 | 5.126 | 5.111 | |
| α = β = γ | 90° | 90° | 90° | 90° | |
| lattice volume (Å3) | 67.34342087 | 66.9527812 | 67.02478746 | 66.82865562 | |
| chemical composition | ZrO1.973 ± 0.005 | ZrO1.966 ± 0.018 | ZrO1.966 ± 0.003 | ZrO1.964 ± 0.010 | |
| c-ZrO2 | volume % | 9.2 | 8 | 3.4 | 7.2 |
| a = b = c (Å) | 5.134 | 5.115 | 5.113 | 5.094 | |
| α = β = γ | 90° | 90° | 90° | 90° | |
| lattice volume (Å3) | 135.3217461 | 133.8248959 | 133.6679779 | 132.1833706 | |
| chemical composition | ZrO1.995 ± 0.007 | ZrO1.992 ± 0.014 | ZrO1.985 ± 0.009 | ZrO1.980 ± 0.010 | |
| MnO2 | volume % | - | 0 | 0 | 0 |
| Rwp | 22.474 | 18.498 | 17.342 | 16.886 | |
| Rp | 18.458 | 14.549 | 13.594 | 13.564 | |
| χ2 | 4.0256 | 2.604 | 2.191 | 1.958 | |
| 9-CSZ | 2MnCSZ | 3MnCSZ | 4MnCSZ | ||
|---|---|---|---|---|---|
| m-ZrO2 | volume % | 27.1 | 7.8 | 17.2 | 9.3 |
| a (Å) | 5.145 | 5.212 | 5.171 | 6.084 | |
| b (Å) | 5.194 | 4.928 | 5.903 | 4.989 | |
| c (Å) | 5.309 | 5.55 | 5.26 | 5.324 | |
| α = γ | 90° | 90° | 90° | 90° | |
| β | 99.174 | 99.693 | 99.137 | 100.791 | |
| lattice volume (Å3) | 140.058358 | 140.5152436 | 158.5211616 | 158.7421548 | |
| chemical composition | ZrO1.995 ± 0.008 | ZrO1.992 ± 0.003 | ZrO1.993 ± 0.011 | ZrO1.988 ± 0.020 | |
| t-ZrO2 | volume % | 65.9 | 53.6 | 21.8 | 17 |
| a = b (Å) | 3.626 | 3.619 | 3.633 | 3.657 | |
| c (Å) | 5.11 | 5.129 | 5.129 | 5.101 | |
| α = β = γ | 90° | 90° | 90° | 90° | |
| lattice volume (Å3) | 67.18564636 | 67.17533877 | 67.69607588 | 68.21898355 | |
| chemical composition | ZrO1.983 ± 0.013 | ZrO1.979 ± 0.012 | ZrO1.977 ± 0.009 | ZrO1.968 ± 0.021 | |
| c-ZrO2 | volume % | 7 | 38.7 | 61 | 73.7 |
| a = b = c (Å) | 5.142 | 5.109 | 5.107 | 5.113 | |
| α = β = γ | 90° | 90° | 90° | 90° | |
| lattice volume (Å3) | 135.9553233 | 133.35451 | 133.19796 | 133.6679779 | |
| chemical composition | ZrO1.998 ± 0.003 | ZrO1.995 ± 0.017 | ZrO1.991 ± 0.008 | ZrO1.983 ± 0.016 | |
| MnO2 | volume % | - | 0 | 0 | 0 |
| Rwp | 16.599 | 17.205 | 19.18 | 17.156 | |
| Rp | 12.902 | 13.28 | 14.79 | 12.973 | |
| χ2 | 1.423 | 1.557 | 1.806 | 1.547 | |
| Length of a (Å) | Length of b (Å) | Angle Between a and b(°) | Estimated Phase | |
|---|---|---|---|---|
| 9-CSZ | 2.91 | 3.81 | 87.58 | Monoclinic |
| 2MnCSZ | 3.01 | 3.16 | 69.96 | Tetragonal |
| 3MnCSZ | 2.99 | 3.13 | 71.32 | Tetragonal |
| 4MnCSZ | 2.98 | 2.96 | 59.93 | Cubic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, J.-j.; Jo, K.; Lee, H.; Lee, H. Phase Transition Behavior and Mechanical Properties of 9 Mol% CaO-PSZ with MnO2 Doping Under Thermal Stress. Appl. Sci. 2025, 15, 7437. https://doi.org/10.3390/app15137437
Kim J, Kim J-j, Jo K, Lee H, Lee H. Phase Transition Behavior and Mechanical Properties of 9 Mol% CaO-PSZ with MnO2 Doping Under Thermal Stress. Applied Sciences. 2025; 15(13):7437. https://doi.org/10.3390/app15137437
Chicago/Turabian StyleKim, Janghoon, Jong-jin Kim, Kanghee Jo, Hwanseok Lee, and Heesoo Lee. 2025. "Phase Transition Behavior and Mechanical Properties of 9 Mol% CaO-PSZ with MnO2 Doping Under Thermal Stress" Applied Sciences 15, no. 13: 7437. https://doi.org/10.3390/app15137437
APA StyleKim, J., Kim, J.-j., Jo, K., Lee, H., & Lee, H. (2025). Phase Transition Behavior and Mechanical Properties of 9 Mol% CaO-PSZ with MnO2 Doping Under Thermal Stress. Applied Sciences, 15(13), 7437. https://doi.org/10.3390/app15137437






