Abstract
Background: Recurrent aphthous stomatitis (RAS) has been recognized as a pathology characterized by ulcerations of the oral mucosa with a wide cross-prevalence in the general population. The aim of the present investigation was to investigate a new gel formulation for the treatment of minor oral ulcers after 2 weeks of treatment. Materials and Methods: A randomized triple-blinded controlled trial was designed for the present purpose. A total of two different study groups was conceptualized: group I (placebo) and group II (active treatment). After the enrolment process, the subjects were included in a domiciliary treatment and evaluated by a blinded operator to clinical scoring and thermography assessment at the baseline, after 1 week, and 2 weeks. Results: A total of 60 patients were observed and treated in the present study. The mean age of the patients was 34.91 ± 7.27 years old for a total of 38 females and 22 males. At 1 and 2 weeks, a significant difference in clinical scoring was observed comparing group I and group II (p < 0.05). A significant reduction in pain score was detected in group II patients (p < 0.05) after the topical gel administration. Conclusions: According to the findings of this randomized trial, the gel treatment showed a significant decrease in the signs and symptoms of oral ulcerations and could be considered useful to ameliorate the clinical course of the disease.
1. Introduction
Recurrent aphthous stomatitis (RAS) is an inflammatory disease with a multifactorial and immune-mediated etiology whose clinical manifestation is associated with recurrent ulcerations of the oral mucosa [1,2]. This pathology is documented as one of the most frequent diseases of the oral cavity, affecting around 10–20% of the population, especially between the second and third decade [3,4]. RAS has recurrent evidence that shows periods of quiescence following phases of exacerbation with a moderate tendency to decrease in frequency and severity in the following decades [5,6]. An individual predisposition can sometimes generate the presentation of recurrent lesions even in adulthood, especially if associated with systemic disorders or states of immunodepression [2,5,7]. Several factors have been detected for RAS including local trauma, microbiological activity, hypersensitivity, immunological and hormonal disorders, and vitamin deficiencies such as zinc, iron, vitamin B1, B2, B6, B12, folic acid, and beta-blockers, bisphosphonates, NSAIDs, protease inhibitors, and sulfonamides [4,5,6,8,9,10,11]. Moreover, syndromic conditions, such as Behcet’s syndrome, Reiter’s disease, Sweet’s syndrome, PFAPA syndrome, MAGIC syndrome, and HIV/AIDS infection, have been correlated to a higher frequency and severity of RAS disease [4,5,6,8,9,10,11].
A recent study by Altay et al. reported that a higher salivary expression of irisin, interleukin-2 (IL-2), and interferon-ɣ (IF-ɣ) was detected in the RAS group compared with the smoker and non-smoker cohort [12]. The authors supposed that a higher level of irisin could be involved with the mucosal healing process in the digestive system [12], and that this biomarker could be used for this purpose due to its high sensitivity for this disorder group.
From the point of view of clinical treatment, several protocols have been proposed in the literature that are mainly symptomatic therapies that do not produce an elimination of the disease or do not influence its possible recrudescence in both adulthood and pediatric age [5,6,13,14,15,16]. Although no curative treatments have been detected, several therapeutic approaches and active ingredients have been described including chlorhexidine 0.2% in mouthwash, topical applications of benzidamine and zinc chloride, and polycresulene [6,8,9,10,11] devices. Possible therapeutic efficacy has been associated with tetracycline and lidocaine 1%, where many ulcerative lesions may sometimes recur and be refractory to such administrations [16,17,18].
Regarding the efficacy of topical treatment, a clinical difficulty could be represented by the keeping the molecule in situ for a sufficient time to express its therapeutic action, in association with the dilution and wash-out effect of saliva [13,18]. In addition, the bio-adhesiveness of soft tissues affected by aphthous lesions is a decisive factor for the effectiveness of treatment [19].
The aim of the present research was to evaluate the efficacy of a gel formulation for a total treatment of 14 days for the treatment of oral ulcers of traumatic or aphthosic nature. The null hypothesis (H0) means that no difference at 14 days in the reduction in the mean pain sensation and ulcer area score, thermography [20,21], and ulcer severity score (USS) [22] was detected between the two groups.
2. Materials and Methods
2.1. Ethic Statement
The principles outlined in the Helsinki Declaration on clinical research involving human subjects have been respected. Written informed consent was obtained for each patient. The study was reported according to the CONSORT statement to improve the quality of reports of randomized parallel-group trials (http://www.consort-statement.org/, accessed on 17 February 2025). The treatments envisaged by this clinical protocol were carried out according to the principles of Good Clinical Practice (:Update ICH GCP E6 R2). The study protocol was reviewed and approved by the “Comitato Etico Territoriale Regione Abruzzo C.Et.R.A.” (Prot. no. RA/0362845, 9 May 2023).
The screening procedure included a phase of enrolment of the subjects that was carried out at the Department of Innovative Technologies in Medicine and Dentistry of the University “G. D’Annunzio” of Chieti-Pescara, which, as observed by the clinical plan, provided for voluntary adherence to the study protocol and the collection of written informed consent. On 28 November 2023, the recruitment phase was launched and included in phase V1 of the clinical investigation.
2.2. Gel Device
The formulation ensures an effective antiseptic action on the film-forming component. The presence of DNA fractions increases the viscosity of the solutions and increases the hydration of the mucous membranes with a consequent improvement in trophism for oral ulcers. Indications: To delay and prevent oral ulcers and the formation of bacterial plaque; prevent irritation of the gums and oral cavity, even in wearers of prostheses and orthodontic implants accompanied by problems with dental support structures; after tooth extraction; and before and after any dental surgery.
2.3. Study Design
The present investigation was structured as a 2-week double-arm randomized controlled trial. The study developed the following randomization and masking criteria.
2.3.1. Randomization Protocol
We used a computer-generated, centralized random number with random codes (A, B) placed in sequentially numbered sealed opaque envelopes provided by the study consultant. A randomization procedure of the two different study groups was used for this purpose.
2.3.2. Masking of Assignment
Physicians involved in the patient’s treatment were unaware of the treatment. Masking of the bottles and made indistinguishable with mouthwash was conducted by a person designated by the sponsor. The gel was poured into identical vials labeled with a letter (A, B), and only the person designated by the sponsor was aware of the match of the product or placebo. Random assignment was made by opening the sequentially numbered sealed envelope just before treatment.
2.3.3. Blinded Assessment
A single experienced, independent, blinded assessor performed all of the clinical measurements. Patients were not aware of the product being administered. In addition, the masking of the evaluators, operators, and statistician remained blinded until the statistical analysis was performed. The codices (A, B) were broken at the time of writing the manuscript. The patient’s instructions and gel posology were performed following the protocol of the present gel device. As a topical product, calibration based on salivary flow was not foreseen.
2.3.4. Inclusion Criteria
The enrollment of sixty (60) subjects was conducted to evaluate the following inclusion criteria for inclusion in the study:
- Patients with at least one well-defined ulcer;
- Any patient who was at least 18 years old and able to sign informed consent.
According to the Scully et al. consensus report, the diagnosis of RAS and the patients’ admission in the protocol were made on the basis of history and clinical criteria, since there are no specific laboratory tests available. The diagnosis was performed by a single specialist operator (AS) [23].
2.3.5. Exclusion Criteria
The presence of one of the following saw a priori exclusion from the clinical study:
- Intolerance or allergy to the product;
- Patients with hematologic deficiency such as anemia, iron, vitamin B12 and/or folic acid deficiency; systemic diseases such as ulcerative colitis, Crohn’s disease, Behcet’s syndrome in which RAS is part of their clinical presentation; alcohol consumption and smoking; history of allergy; treatment of ulcers with systemic steroids, vitamins, antibiotics, antihistamines, oral retinoids, or immunomodulatory agents within three months prior to study entry; the use of nonsteroidal anti-inflammatory drugs or mouthwash for the treatment of ulcer prior to 72 h of study entry; and patients who were pregnant or breastfeeding.
2.4. Device Characteristics and Composition
The hydrogel compound used for the present investigation was composed of purified water, propylene glycol, VP/VA copolymer, carbomer, cellulose gum, PVM/MA copolymer, hydrolyzed RNA/DNA, leuconostoc/radish root ferment filtrate, sodium hyaluronate, allantoin, glycyrrhetinic acid, beta-glucan, glycerin, ruscogenin, bisabolol, leptospermum scoparium branch/leaf oil, melaleuca alternifolia leaf oil, o-cymen-5-ol, phenoxyethanol, sodium benzoate, ammonium glycyrrhizate, sodium saccharin, peg40 hydrogenated castor oil, caprylyl glycol, 1,2-hexanediol, and aroma (Afterapid Curasept, Saronno CO, Italy). The placebo was deprived of all active components.
2.5. Study Visits and Treatments
Screening and Baseline Visit (V1)
Potentially eligible patients were screened to determine their eligibility for the study. The patient eligibility CRF was completed, and the number and reason for patients not included were recorded.
Prior to enrollment, all patients were asked to sign an informed consent form to document that they understood the purpose of the study (including procedures, follow-up assessments, and any potential risks involved), had been given the opportunity to ask questions related to this study, and had been informed of treatment alternatives.
At the recruitment visit, the clinician was to assess the presence of an oral ulcer, rule out lesions of neoplastic, infectious or allergic origin, and remove, if present, any causative agents (such as sharp edges of teeth, fillings, crowns, dentures, or braces).
Primary Indices
- Pain intensity. This was measured using the VAS consisting of a 10 cm line [13], where 0 indicates no pain and 10 indicates severe pain.
- Size of each ulcer. A sheet of clear plastic was applied directly to the ulcer, and using a permanent waterproof marker, the circumference of the ulcer was traced and then placed on graph paper, and the number of mm2 units included within the drawn area was counted.
Secondary Indices
- Thermography;
- Ulcer severity score (USS).
Treatment was started on the same day with strict infection control measures.
The assessor proceeded with the allocation phase as required by the assignment protocol, indicating in the CRF the progressive number of the patient contained in the envelope and to which group (A or B) the patient was assigned.
Patients received an anonymous tube vial, coded with A, B, containing the gel and the appropriate applicator. Patients therefore applied the gel. This procedure was performed under supervision, providing further explanations to patients if necessary.
Participants were asked to apply the gel twice a day directly to the injured part, inside the mouth, with the help of the applicator provided. Patients were prevented from eating, drinking, or rinsing their mouths for at least one h after each application.
2.6. Thermography
The infrared thermal measurements were performed by adopting a controlled environment (temperature: 20–24 °C, relative humidity percentage: 52%, with no direct ventilation). The humidity was constantly monitored using an integrated sensor (Atmo-Tube, San Francisco, CA, USA). This device sensor measures the relative humidity (RH) at regular intervals. The intraoral thermography measurements were assessed using a 14-bit digital infrared camera (FLIR SC660 QWIP, Flir Systems, Danderyd, Sweden) [24]. The device specifications were 320 × 240 pixels focal plane array; 8–9 m spectral range; 0.02 K noise equivalent.
2.6.1. 1-Week (V2) and 2-Week (V3) Visits
In the event of any complication observed during a scheduled visit or during an emergency visit, a protocol deviation and drop out notation in CRF were provided.
Unscheduled visits could be made during the study at the discretion of the investigator.
The relevant information was therefore collected in the CRF as required by the investigation plan. The study plan and the scheduled items are summarized in the following flowchart (Table 1).

Table 1.
Summary of the study design and clinical visits.
2.6.2. Summary of Procedures and Follow-Up
- Day 0: Patient recruitment, informed consent, pain intensity measurement (VAS), injury measurement, thermography, USS and gel application.
- Day 7: Pain intensity measurement (VAS), lesion measurement, thermography, USS, and recording of complications and adverse effects.
- Day 14: Pain intensity measurement (VAS), lesion measurement, thermography, USS, and recording of complications and adverse effects.
2.6.3. Drop-Out
All dropouts needed to be reported by recording the study group’s reason for drop out. No included patients were excluded from the final evaluation, for any reason, by the clinical investigators.
2.6.4. Evaluation of the Primary Objectives
- Pain intensity. This was measured using the VAS consisting of a 10 cm line [13], where 0 indicates no pain and 10 indicates severe pain.
- Size of each ulcer. A sheet of transparent plastic was applied directly to the ulcer, and using a permanent waterproof marker, the circumference of the ulcer was traced and then placed on graph paper, counting the units of mm2 included within the drawn area. Treatment began on the same day with strict infection control measures.
2.6.5. Evaluation of Secondary Objectives
- Thermography [20,21,25];
- Ulcer severity Score (USS) [22].
Complications and adverse events: Any post-operative complications and adverse events (mucosal irritation, allergic reactions, etc.) were recorded and reported by study group by the blinded evaluators in complications, protocol deviation, and drop out. All randomized subjects who complete the clinical procedure and use at least one day of the gel and with at least one available assessment of variables after baseline will be considered in this population (Intention-to-Treat population).
2.6.6. Primary Objectives
- Pain intensity. This was measured using the VAS consisting of a 10 cm line [13], where 0 indicates no pain and 10 indicates severe pain.
- Size of each ulcer. A sheet of transparent plastic was applied directly to the ulcer, and using a permanent waterproof marker, the circumference of the ulcer was traced and then placed on graph paper, counting the units of mm2 included within the drawn area. Treatment began on the same day with strict infection control measures.
2.6.7. Secondary Objectives
Lesion temperature measurement using infrared thermography [20,21,25] and ulcer severity score (USS) [22].
2.7. Statistical Analysis
A sample size of 29 subjects for each group (power 80%) was used to detect a significant difference in means of 0.820, assuming that the common standard deviation was 1.80 using a two-group Student’s t-test with a significance level of p < 0.05. Considering a 3% drop out, a sample of 30 subjects per study group was enrolled.
The descriptive statistics were included in summary tables by group according to the type of outcome measure summarized including the following indicators: n (number of observed values), mean with its 95% CI, and standard deviation. For categorical variables, the number and percentage of subjects with a specific level of the variable were presented.
This primary efficacy analysis was performed on the intention-to-treat population, using the null hypothesis of no between-group difference in the overall VAS change from the baseline between both groups:
where μ is the mean change from baseline on Day 7 within the given product.
H0: μAfteRapidDNA = μ Placebo
H1: μAfteRapidDNA μ Placebo
This hypothesis was tested by the nonparametric Kruskal–Wallis test with baseline values of VAS as a covariate. The groups were compared at the bilateral significance level of p < 0.05.
The assumption of normality was verified by means of QQ graphs and by plotting the studentized residuals with respect to the expected values of the variable. For strong indications of non-normality of the data, the Wilcoxon rank-sum test was used to test the hypothesis of no median difference between the test and placebo at Day 7 in a nonparametric manner. Summaries for each treatment group included the sample size, mean, median, standard deviation, minimum, maximum, Wilcoxon rank-sum test p-value, Hodges–Lehman point estimates of median treatment difference, and its 95% confidence interval. From the Kruskal–Wallis model of the Day 7 endpoint, a least squares (LSMEANS) estimate of the group effect and group difference (both with 95% confidence intervals) was displayed along with the p-value of the difference between the groups. Analysis was also performed for the PP analysis set to test the robustness of the results. For secondary quantitative efficacy variables, cross-treatment group comparison of the changes from baseline was carried out by the same Kruskal–Wallis model used in the primary efficacy analysis.
3. Results
The patients were examined at the Odontostomatology Clinic Unit of the Department of Innovative Technologies in Medicine and Dentistry, where a total of 60 patients were enrolled (Table 2). The CRFs, including the anamnestic and analytical section of the primary and secondary indicators, were compiled. Assignment to the different treatment groups was carried out by means of a randomization function using a special Excel spreadsheet (Vers. 16.0; Microsoft, Redmond, WA, USA). At the end of the procedure, the subjects were therefore included in group I or group II, with the first application of the device in the session phase twice a day for 2 weeks. The subjects were therefore instructed in the correct home oral hygiene maneuvers and the correct procedure for using the device, and re-evaluated at 7 and 14 days. The average age of the patients enrolled was 34.91 ± 7.27 years, consisting of 68.33% women (38) and 36.67% men (22) (Table 3).

Table 2.
Summary of the study design and clinical visits.

Table 3.
Summary of the descriptive statistics of the outcome parameters of group I evaluated at the V1 timepoint [* last reported presentation].
3.1. Group I
3.1.1. V1—Baseline
The baseline assessment of the USS score showed an average of 26.07 ± 5.73 (95% CI: 23.93–28.21). The VAS score of recorded pain was 5.9 ± 2.44 (95% CI: 4.99–6.81). The number of lesions and mean size measured in millimeters were 2.47 ± 2.28 (95% CI: 1.99–2.94) and 5.23 ± 2.5 (95% CI: 3.15–3.65), respectively (Table 3).
3.1.2. V2—1st Week
The baseline assessment of the USS score showed an average of 28.17 ± 4.5 (95% CI: 26.5–29.8). The VAS score of recorded pain was 4.83 ± 1.34 (95% CI: 4.33–5.33). The number of lesions and mean size measured in millimeters were 2.46 ± 1.28 (95% CI: 1.99–2.94) and 4.33 ± 1.81 (95% CI: 3.66–5.01), respectively (Table 4).

Table 4.
Summary of the descriptive statistics of the outcome parameters or group I evaluated at the V2 timepoint [** from the protocol inclusion].
3.1.3. V3—2nd Week
The baseline assessment of the USS score showed an average of 19.3 ± 5.82 (95% CI: 17.13–21.47). The VAS score of pain recorded was 2.0 ± 1.76 (95% CI: 1.34–2.66). The number of lesions and mean size measured in millimeters were 0.83 ± 0.87 (95% CI: 0.51–1.16) and 1.3 ± 1.37 (95% CI: 0.79–1.81), respectively (Table 5).

Table 5.
Summary of the descriptive statistics of the outcome parameters or group I evaluated at the V3 timepoint. [** from the protocol inclusion].
3.2. Group II
3.2.1. V1—Baseline
The baseline assessment of the USS score showed an average of 29.80 ± 5.57 (95% CI: 27.72). The VAS score of recorded pain was 6.03 ± 1.73 (95% CI: 5.39–6.68). The number of lesions and mean size measured in millimeters were 2.87 ± 1.31 (95% CI: 2.48–3.35) and 5.7 ± 2.3 (95% CI: 4.87–6.60), respectively (Table 6).

Table 6.
Summary of the descriptive statistics of the outcome parameters or group II evaluated at the V1 timepoint [* last reported presentation].
3.2.2. V2—1st Week
The baseline assessment of the USS score showed an average of 20.42 ± 5.89 (95% CI: 18.22–22.62). The VAS score of recorded pain was 2.73 ± 1.68 (95% CI: 2.11–3.4). The number of lesions and mean size measured in millimeters were 1.2 ± 1.03 (95% CI: 0.81–1.58) and 2.43 ± 1.92 (95% CI: 1.71–3.15), respectively (Table 7).

Table 7.
Summary of the descriptive statistics of the outcome parameters or group II evaluated at the V2 timepoint [** from the protocol inclusion].
3.2.3. V3—2nd Week
The baseline assessment of the USS score showed an average of 14.72 ± 4.068 (95% CI: 13.20–16.24). The VAS score of recorded pain was 0.77 ± 1.33 (95% CI: 0.27–1.26). The number of lesions and the mean size measured in millimeters were 0.26 ± 0.45 (95% CI: 0.1–0.43) and 0.4 ± 0.81 (95% CI: 0.1–0.7), respectively (Table 8).

Table 8.
Summary of the descriptive statistics of the outcome parameters or group II evaluated at the V3 timepoint [** from the protocol inclusion].
3.3. Evaluation of Effectiveness
By means of the Kruskal–Wallis model, the significance of the study variables was tested. At the baseline, the number of lesions was not significant compared with groups I and II (p > 0.05), but significant at V2 and V3 (p = 0.013). There were no significant differences between groups I and II in the size of the lesions measured at the baseline (p > 0.05), but statistically significant differences at V2 and V3 (p = 0.0407). There were no significant differences between groups I and II in the measurement of pain measured with the VAS scale (p > 0.05), whereas a statistically significant difference was detected at experimental times V2 and V3 (p = 0.0094). There were no significant differences between groups A and B in temperature measurement (p > 0.05) and at the experimental times of V2 and V3 (p > 0.05) (Figure 1). No deviations, side effects, or adverse reactions to treatment were recorded in groups I and II. No drop out was recorded in the study population.
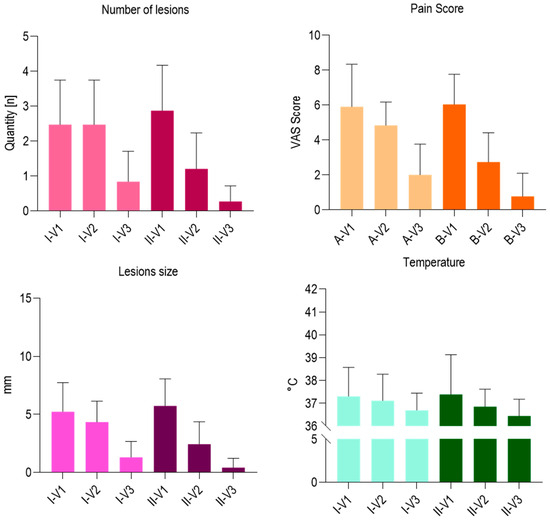
Figure 1.
Summary chart of the outcome parameters or group II evaluated different timepoints.
4. Discussion
A definitive approach for RAS disease is not currently clarified in the literature and could differ considering the severity and grading of the path. The approach purposed by a consensus report [23] reported different support protocols including a soft diet, chlorhexidine rinses with no alcohol base, and topical corticosteroids [26,27]. An alternative approach for type C RAS provides a topical associated with systemic corticosteroids, azathioprine, and immunosuppressants [23]. This systemic approach could enhance the local response to the major lesions, but some side effects should be considered in cases of prolonged drug administration [23]. The minor effects could include gastrointestinal symptoms, diarrhea, male infertility, and nausea. Thalidomide has been reported to an increased teratogenicity, polyneuropathy, and mood change [23]. To reduce the clinical course and local pain, the local infiltration of corticosteroid drugs could produce an effective outcome. In this way, the support therapy of this disorder could produce a more sustainable course of RAS disorders, reducing the related symptoms, especially in the case of multiple and more durable lesions.
The study population enrolled in the present clinical investigation appeared homogeneous considering independent study variables such as age and gender, and no participants were lost to follow-up. The observation of the primary survey indices did not show statistically significant differences between groups I and II with regard to the USS score, pain measured on the VAS scale, and the temperature indices at the baseline. In phase V2 after 1 week of treatment, a statistically significant difference emerged with a decrease in pain parameters on the VAS scale in group II patients as well as a significant reduction in the number and size of lesions. In phase V3, the pain indices measured on the VAS scale in terms of the number and size of residual lesions aligned in both groups. Given the variability of the clinical course, this aspect is widely variable and subject to high recurrence, as evidenced by the literature in the sector. In severe cases, symptoms can be a disabling factor both under a functional point of view and in oral hygiene maneuvers [5,28,29]. In high-risk subjects, it is crucial to control any predisposing factors in order to reduce their frequency and exclude secondary and hygienic problems [22]. The course of the disease was positively influenced by the group II patients undergoing treatment from a symptomatologic point of view in the reduction in the number of lesions and their size. The study findings seem to suggest that hydrolyzed RNA/DNA could produce effective action with the clinical course of the lesions, reducing the symptoms and the healing period. As reported by previous in vitro study, this effect could be supported by effective action against the oxidative stress accompanied by n-cytotoxic activity [30,31]. Given the limited duration of the present study design, it was not possible to evaluate the effects of the treatment on relapse rates. According to the evidence that emerged from the present research, the treatment in question showed a statistically significant reduction in clinical symptoms with an improvement in the clinical course of the disease examined in the absence of adverse or undesirable effects.
Author Contributions
Conceptualization, A.S.; Methodology, A.S. and F.L.; Software, A.S. and F.L.; Validation, A.S., F.L. and N.G.; Formal analysis, A.S., F.L. and N.G.; Investigation, A.S., F.L. and N.G.; Data curation, A.S. and F.L.; Writing—original draft preparation, A.S., F.L. and N.G.; Writing—review and editing, A.S., F.L. and N.G.; Visualization, A.S., F.L. and N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was reviewed and approved by the “Comitato Etico Territoriale Regione Abruzzo C.Et.R.A.” (Prot. no. RA/0362845, 9 May 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All experimental data to support the findings of this study are available by contacting the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baeza-Trinidad, R.; Mosquera-Lozano, J.D. Symptom alternatives in recurrent oral aphthosis. Med. Clin. 2016, 147, 133. [Google Scholar] [CrossRef] [PubMed]
- Orive Ochoa, J. Aphthae, aphthoid and aphthosis. Rev. Esp. Estomatol. 1975, 23, 447–456. [Google Scholar] [PubMed]
- Izquierdo, C.; Isanta, C.; Guillén, A.; Vecino, R.; Vallés, C. Recurrent oral aphthosis. Its treatment with colchicine. Aten. Primaria 1989, 6, 358–359. [Google Scholar]
- Riera Matute, G.; Riera Alonso, E. Recurrent aphthous stomatitis in Rheumatology. Reumatol. Clin. 2011, 7, 323–328. [Google Scholar] [CrossRef]
- Arguelles Casals, D. Generalized Touraine aphtosis (Behcet’s syndrome), the first Cuban case. Rev. Sifilogr. Leprol. Dermatol. 1948, 5, 228–234. [Google Scholar]
- Suárez-Díaz, S.; Núñez-Batalla, F.; Fernández-García, M.S.; Fernández-Llana, M.B.; Yllera-Gutiérrez, C.; Caminal-Montero, L. Aphthous Stomatitis and Laryngitis, Another Form of Presentation of an IgG4-Related Disease? Reumatol. Clin. 2020, 16, 416–418. [Google Scholar] [CrossRef]
- De La Cuesta Almonacid, L. Cutaneo-mucosal aphthosis. Actas Dermosifiliogr. 1954, 45, 671–675. [Google Scholar] [PubMed]
- Pizarro, A.; Herranz, P.; García-Tobaruelaa, A.; Casado, M. Pentoxifylline in the treatment of orogenital aphthosis and Behçet’s syndrome. Med. Clin. 2000, 115, 678. [Google Scholar] [CrossRef]
- Vilanova, X.; Pinol Aguade, J. Aphthosis; concept, etiology, nosological situation and trial of a new treatment; clinical contribution. Actas Dermosifiliogr. 1955, 46, 704–714. [Google Scholar] [PubMed]
- Allegue, F.; Sarriá Cepeda, C.; García Rodríguez, M. Aphthosis major treated with thalidomide in a patient with AIDS. Enferm. Infecc. Microbiol. Clin. 1991, 9, 133–134. [Google Scholar]
- Torras, H.; Lecha, M.; Mascaró/, J.M. Thalidomide in the treatment of aphthosis and Behçet’s disease. 4 years’ experience. Med. Cutan. Ibero. Lat. Am. 1982, 10, 103–112. [Google Scholar] [PubMed]
- Altay, D.U.; Korkmaz, M.; Ergun, S.; Korkmaz, H.; Noyan, T. Salivary Irisin: Potential Inflammatory Biomarker in Recurrent Apthous Stomatitis Patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2252–2259. [Google Scholar] [CrossRef]
- Blanch Falp, J.; García Pont, X.; Torne Cachot, J.; Moner Coromina, L.; Baucells Azcona, J.M. Pentoxifylline and oral aphthosis in patients with HIV infection. An. Med. Interna 1997, 14, 102. [Google Scholar] [PubMed]
- Sánchez-Bernal, J.; Conejero, C.; Conejero, R. Recurrent Aphthous Stomatitis. Actas Dermosifiliogr. 2020, 111, 471–480. [Google Scholar] [CrossRef]
- Pizarro, A.; Herranz, P.; Ferrer, M.; Casado, M. Recurrent oral aphthosis: Treatment with pentoxifylline. Med. Clin. 1993, 101, 237. [Google Scholar]
- D’Amario, M.; Foffo, G.; Grilli, F.; Capogreco, M.; Pizzolante, T.; Rastelli, S. Treatments for Recurrent Aphthous Stomatitis: A Literature Review. Dent. J. 2025, 13, 66. [Google Scholar] [CrossRef]
- Camacho, F.; Ortega, M.; Elorza, F. Glycophosphopeptical in the treatment of recurrent oral aphthae (a reply). An. Med. Interna 1995, 12, 204. [Google Scholar] [PubMed]
- Casalá, A.; Delmar, O.; Bianchi, O.J.; Bianchi, C.A. Touraine’s great aphthosis. Apropos of a case with severe digestive hemorrhage. Arch. Argent. Dermatol. 1965, 15, 87–90. [Google Scholar]
- Liu, H.; Tan, L.; Fu, G.; Chen, L.; Tan, H. Efficacy of Topical Intervention for Recurrent Aphthous Stomatitis: A Network Meta-Analysis. Medicina 2022, 58, 771. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Facial Skin Temperature and Discomfort When Wearing Protective Face Masks: Thermal Infrared Imaging Evaluation and Hands Moving the Mask. Int. J. Environ. Res. Public Health 2020, 17, 4624. [Google Scholar] [CrossRef]
- Antonio, S.; Felice, L.; Merla, A.; Camillo, D.; Renato, C.; Santos, O.P. Lateral Sinus Floor Elevation Performed with Trapezoidal and Modified Triangular Flap Designs: A Randomized Pilot Study of Post-Operative Pain Using Thermal Infrared Imaging. Int. J. Environ. Res. Public Health 2018, 15, 1277. [Google Scholar] [CrossRef]
- Tappuni, A.R.; Kovacevic, T.; Shirlaw, P.J.; Challacombe, S.J. Clinical Assessment of Disease Severity in Recurrent Aphthous Stomatitis. J. Oral Pathol. Med. 2013, 42, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Gorsky, M.; Lozada-Nur, F. The Diagnosis and Management of Recurrent Aphthous Stomatitis: A Consensus Approach. J. Am. Dent. Assoc. 2003, 134, 200–207. [Google Scholar] [CrossRef] [PubMed]
- D’Accardi, E.; Ammannato, L.; Giannasi, A.; Pieri, M.; Masciopinto, G.; Ancona, F.; Santonicola, G.; Palumbo, D.; Galietti, U. Infrared Thermography for Non-Destructive Testing of Cooling Hole Integrity and Flow Evaluation in Specimens Made with Innovative Technologies. Eng. Proc. 2025, 85, 15. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Amuso, D.; Scogna, G.; Amore, R.; Lorusso, F. Static Crow’s Feet Treated with Voltaic Arc Dermabrasion (Atmospheric Plasma): Post-Operative Pain Assessment by Thermal Infrared Imaging. J. Clin. Med. 2021, 10, 3074. [Google Scholar] [CrossRef]
- Lorusso, F.; Tartaglia, G.; Inchingolo, F.; Scarano, A. Peri-Implant Mucositis Treatment with a Chlorexidine Gel with A.D.S. 0.5%, PVP-VA and Sodium DNA vs a Placebo Gel: A Randomized Controlled Pilot Clinical Trial. Front. Biosci. Elite Ed. 2022, 14, 30. [Google Scholar] [CrossRef]
- Mahapatra, A.; Panda, S.; Tumedei, M.; Panda, S.; Das, A.C.; Kumar, M.; Del Fabbro, M. Clinical and Microbiological Evaluation of 0.2% Tea Tree Oil Mouthwash in Prevention of Dental Biofilm-Induced Gingivitis. Dent. J. 2025, 13, 149. [Google Scholar] [CrossRef]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A 30-Year (1988–2018) Retrospective Microscopical Evaluation of Dental Implants Retrieved for Different Causes: A Narrative Review. Int. J. Periodontics Restor. Dent. 2020, 40, e211–e227. [Google Scholar] [CrossRef]
- Biazussi, B.R.; Perrotti, V.; D’Arcangelo, C.; Elias, C.N.; Bianchini, M.A.; Tumedei, M.; de Vasconcellos, D.K. Evaluation of the Effect of Air Polishing With Different Abrasive Powders on the Roughness of Implant Abutment Surface: An In Vitro Study. J. Oral Implantol. 2019, 45, 202–206. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Vezzoli, E.; Conte, V.; Sartori, P.; Procacci, P.; Brambilla, E. Activity of Experimental Mouthwashes and Gels Containing DNA-RNA and Bioactive Molecules against the Oxidative Stress of Oral Soft Tissues: The Importance of Formulations. A Bioreactor-Based Reconstituted Human Oral Epithelium Model. Molecules 2021, 26, 2976. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Vezzoli, E.; Conte, V.; Procacci, P.; Garcia-Godoy, F.; Brambilla, E. Effects of Na-DNA Mouthwash Solutions on Oral Soft Tissues. A Bioreactor-Based Reconstituted Human Oral Epithelium Model. Am. J. Dent. 2020, 33, 277–284. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).