Seedling Selection of the Large Yellow Croaker (Larimichthys crocea) for Sustainable Aquaculture: A Review
Abstract
1. Introduction
2. Selective Breeding Strategies for Large Yellow Croaker Seedlings
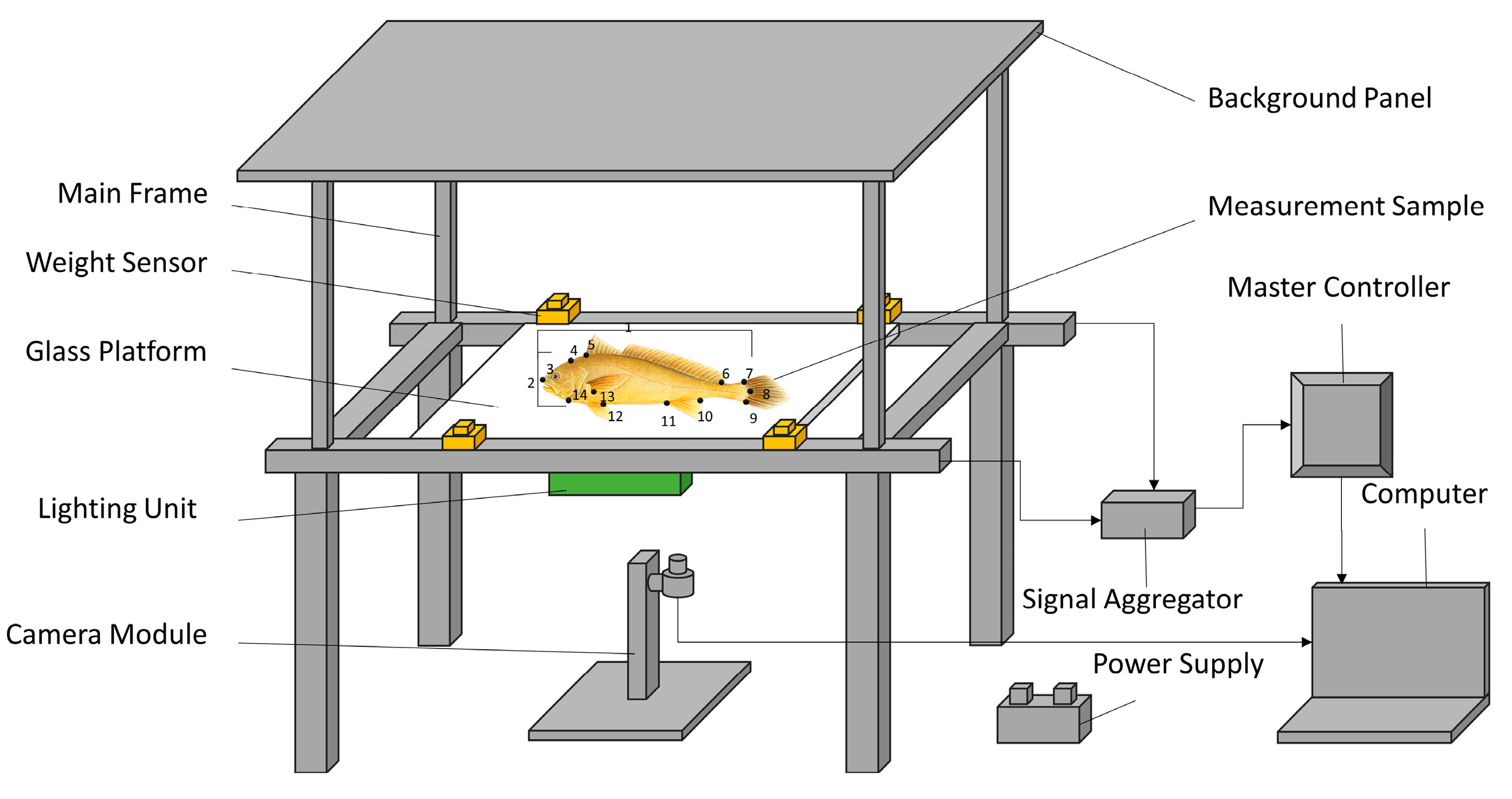
2.1. Morphological Trait Selection
2.2. Growth Performance Selection
2.3. Genetic Diversity Selection
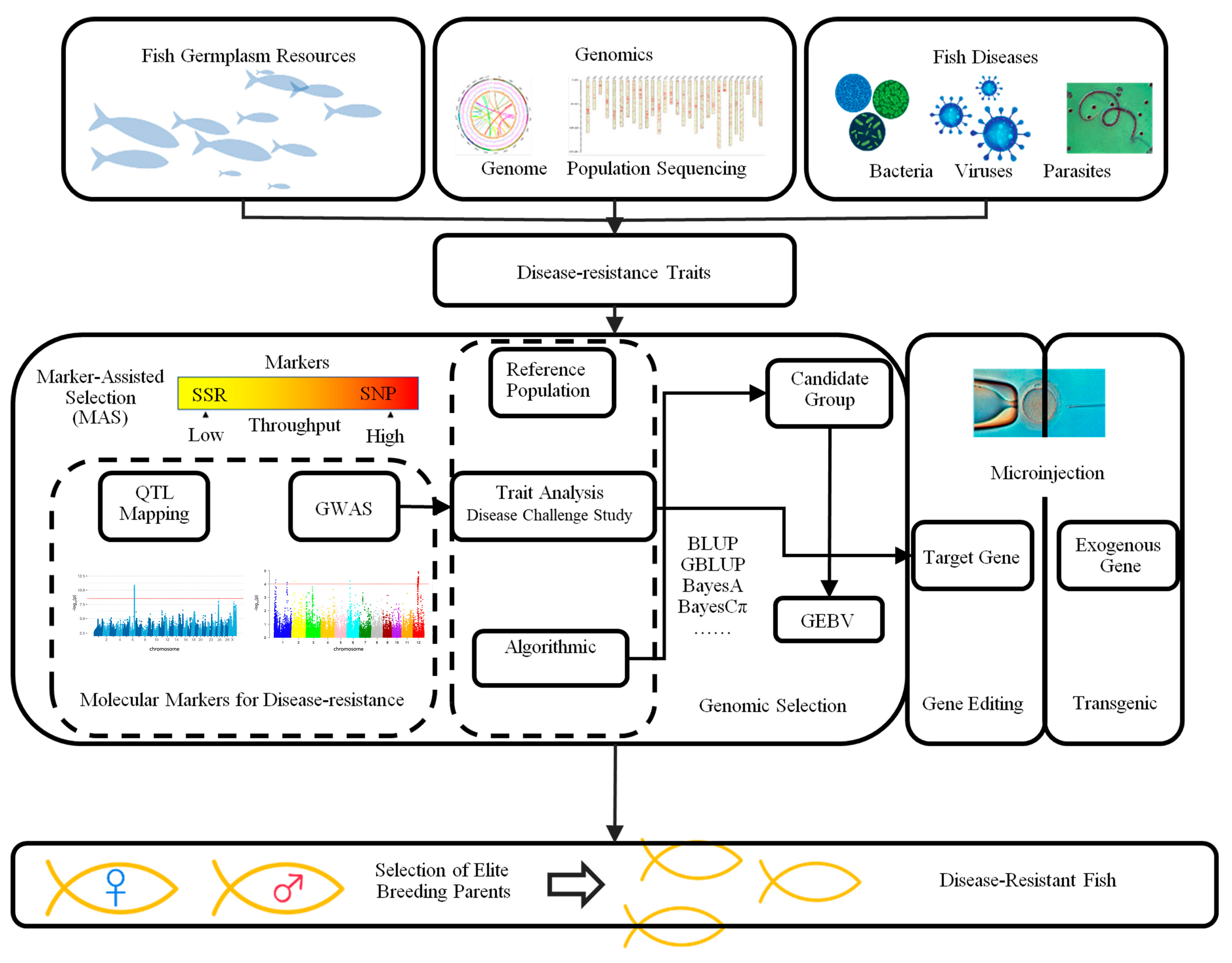
2.4. Disease Resistance Selection
2.5. Environmental Adaptability Selection
| Trait | Species | Marker Type | Marker Number | Marker/Gene | |
|---|---|---|---|---|---|
| temperature tolerance | low temperature tolerance | Larimichthys crocea [45,46,47] | SSR | 1 | LYC002112bp |
| SSR | 1 | LYC0015 | |||
| SSR | 1 | KPC43 | |||
| high temperature tolerance | Larimichthys crocea [40,48] | SSR | 3 | LYC0148, LYC0200, LYC0435 | |
| SNP | 38 | ||||
| Oxygen tolerance | hypoxia-tolerance | Larimichthys crocea [49] | SNP | 4 | chr13:2535902, chr15:11774198, chr18:20360178, chr24:9514192 |
3. Application of Emerging Technologies in Seedling Selection for Large Yellow Croaker
3.1. Molecular Breeding Technology
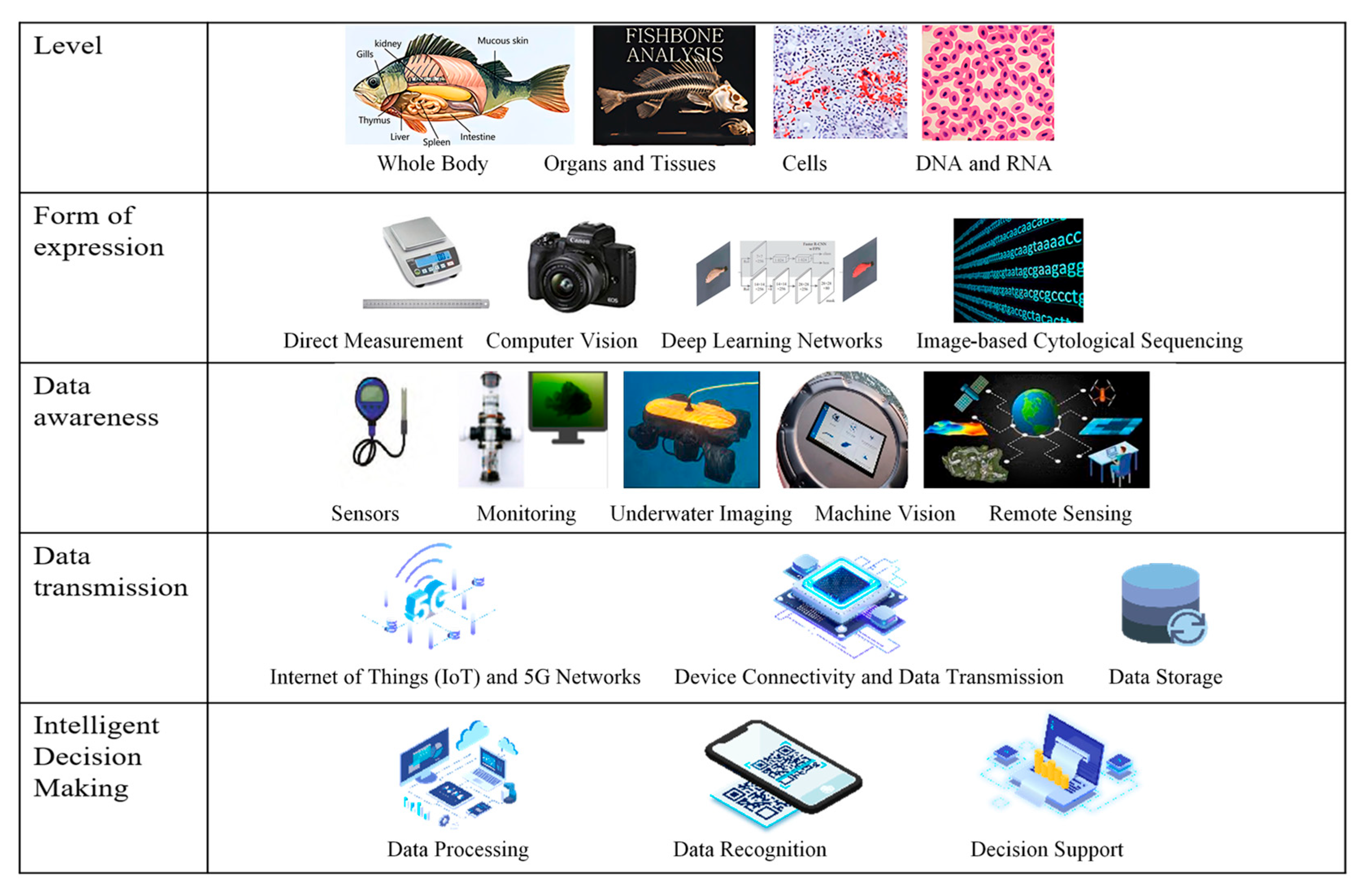
3.2. Intelligent Monitoring and Data Analysis Technology
3.3. Environmental Control and Optimization Technologies
3.4. Intelligent Feeding Technology
3.5. Disease Prevention and Immunization Technologies
4. Key Issues in the Selection of Large Yellow Croaker Fry
4.1. Over-Reliance on Single Trait Selection
4.2. Neglecting Genetic Diversity and Inbreeding Risks
4.3. Ignoring the Impact of Environmental Factors
4.4. Over-Reliance on Traditional Breeding Methods
4.5. Insufficient Attention to Disease Resistance Breeding
5. Problems and Development Trends
5.1. Problems and Challenges
5.1.1. High Technical Costs
5.1.2. Uncontrollable Environmental Factors
5.1.3. Risk of Loss of Genetic Diversity
5.1.4. Limited Effectiveness of Disease Resistance Breeding
5.1.5. Lack of Standardized Systems
5.2. Future Prospects and Trends
5.2.1. Widespread Application of Genomics and Genome Editing Technologies
5.2.2. Popularization of Intelligent Technologies and Precision Aquaculture
5.2.3. Application of Green and Sustainable Technologies
5.2.4. Genetic Diversity Protection and Germplasm Resource Management
5.2.5. Cross-Disciplinary Technological Integration Driving Innovative Development
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.; Wang, J.; Jing, Y. Larimichthys crocea (large yellow croaker): A bibliometric study. Heliyon 2024, 10, e37393. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ke, Q.-Z.; Su, Y.-Q.; Liu, J.-Q.; Zheng, W.-Q. Protection and utilization status and prospect of large yellow croaker (Larimichthys crocea) germplasm resources. Aquac. Fish. 2022, 46, 674–682. [Google Scholar]
- Gong, D.; Cui, X.; Song, M.; Xing, B.; Xu, P.; Tang, Y.; Yin, L. Behavior of large yellow croaker (Larimichthys crocea) in pen aquaculture as measured by meter-scale telemetry. Front. Mar. Sci. 2023, 10, 1177037. [Google Scholar] [CrossRef]
- Yuan, J.; Lin, H.; Wu, L.; Zhuang, X.; Ma, J.; Kang, B.; Ding, S. Resource status and effect of long-term stock enhancement of large yellow croaker in China. Front. Mar. Sci. 2021, 8, 743836. [Google Scholar] [CrossRef]
- Janssen, K. The Economic Optimization of Breeding Programs in Aquaculture. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Yao, J.-X.; Lin, H.-D.; Wu, L.-S.; Wu, L.-N.; Yuan, J.-G.; Ding, S.-X. Stability of population genetic structure in large yellow croaker (Larimichthys crocea): Insights from temporal, geographical factors, and artificial restocking processes. Ecol. Evol. 2024, 14, e70207. [Google Scholar] [CrossRef]
- Azra, M.N.; Okomoda, V.T.; Ikhwanuddin, M. Breeding technology as a tool for sustainable aquaculture production and ecosystem services. Front. Mar. Sci. 2022, 9, 679529. [Google Scholar] [CrossRef]
- Liu, H.; Fan, S.-J.; Xu, Q.-L.; Wang, X.; Zhang, Y.-L.; Chen, W.; Hu, Y.; Deng, X.-Y.; Liu, H.-Y.; Yang, C.-Z.; et al. Germplasm innovation of large yellow croaker and its research progress. Reprod. Breed. 2025, 5, 44–53. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Guo, H.-Y.; Liu, B.-S.; Zhang, N.; Zhu, K.-C.; Zhang, D.-C. Analysis of morphological differences in five large yellow croaker (Larimichthys crocea) populations. Isr. J. Aquac.-Bamidgeh 2024, 76, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Wang, J.-Y.; Xin, R.; Ke, Q.-Z.; Jiang, P.-X.; Zhou, T.; Xu, P. Application of computer vision in morphological and body weight measurements of large yellow croaker (Larimichthys crocea). J. Fish. China 2023, 47, 207–216. (In Chinese) [Google Scholar]
- Yu, X.-J.; Wu, X.-F.; Shen, W.-L. Pattern recognition method for the identification of Daiqu large yellow croaker based on computer vision. J. Zhejiang Univ. 2018, 44, 490–498. (In Chinese) [Google Scholar]
- Yu, X. “Minyou No. 1” Large Yellow Croaker Passed the Provincial Evaluation for Original and Fine Aquatic Seed. Mod. Fish. Inf. 2010, 25, 28. (In Chinese) [Google Scholar]
- Li, M. Large Yellow Croaker “Donghai No. 1”. Rural. Inf. 2016, 36. (In Chinese) [Google Scholar]
- Yu, X.-J.; Wu, X.-F.; Wang, J.-P.; Chen, L.; Wang, L. Rapid Detecting Method for Pseudosciaena crocea Morphological Parameters Based on the Machine Vision. J. Integr. Technol. 2014, 3, 45–51. (In Chinese) [Google Scholar]
- Chen, J.-W. QTL Analysis of Some Morphological Traits in the Large Yellow Croaker (Larimichthys crocea). Master’s Thesis, Jimei University, Xiamen, China, 2016. (In Chinese). [Google Scholar]
- Jiang, S.; Wang, J.-Y.; Yang, W.-L.; Kong, D.-W.; Yang, Q.-B.; Huang, J.-H.; Yang, Y.-D.; Zhou, F.-L. Evaluation of genetic parameters of growth traits in G2 selected generation of Penaeus monodon. Isr. J. Aquac.-Bamidgeh 2024, 76. [Google Scholar] [CrossRef]
- Huang, W.-Q.; Han, K.-H.; Chen, S.-X.; Zhang, Y.; Zhou, S.-F.; Zhou, R.-F.; Luo, F. Realized Heritability and Growth of Offsprings of Wild Large Yellow Croaker Pseudosciaena crocea. Fish. Sci. 2016, 35, 204–209. (In Chinese) [Google Scholar]
- Dong, L.; Fang, M.; Wang, Z. Prediction of genomic breeding values using new computing strategies for the implementation of MixP. Sci. Rep. 2017, 7, 17200. [Google Scholar] [CrossRef]
- Yan, M.-Z.; Li, B.-J.; Wang, J.-Y.; Bai, Y.-L.; Ke, Q.-Z.; Zhou, T.; Xu, P. Disruption of mstn gene by CRISPR/Cas9 in large yellow croaker (Larimichthys crocea). Mar. Biotechnol. 2022, 24, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, Q.-Y.; Zheng, Y.; Sun, W.-B.; Yang, L.; Song, X.-Y. Analysis of the Muscle Structure and Protein Composition of Different Varieties of Cultured Large Yellow Croaker (Larimichthys crocea). Sci. Technol. Food Ind. 2025, 46, 316–323. (In Chinese) [Google Scholar]
- Zhang, Y.; Han, M.-X.; Cao, M.-Y.; Zhao, Y.-Y.; Li, J.; Xue, L.-Y. SNP Detection of FST Gene and Its Association With Growth Traits in Larimichthys crocea. J. Nucl. Agric. Sci. 2018, 32, 883–891. (In Chinese) [Google Scholar]
- Sui, B.-L. Genetic Parameters Estimation and Correlation Analysis Between Growth-Related Traits and Microsatellite Markers in Large Yellow Croaker Larimichthys crocea. Master’s Thesis, Jimei University, Xiamen, China, 2012. (In Chinese). [Google Scholar]
- Wei, B.; Zheng, S.; Gao, Y.; Zheng, Y.; Yang, X.; Jiang, Z.; Guo, Q. A comparative study on the quality of large yellow croaker (Larimichthys crocea) of different sizes cultured in different cage systems. Aquac. Res. 2023, 2023, 6628371. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Liu, L.X.; Wang, W.Z.; Cai, M.-Y.; Yao, C.-L. Genetic structure and genetic diversity analysis of four consecutive breeding generations of large yellow croaker (Pseudosciaena crocea) using microsatellite markers. J. Fish. China 2010, 34, 500–507. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, W.; Ma, L. Genetic diversity and population structure of small yellow croaker (Larimichthys polyactis) in the Yellow and East China seas based on microsatellites. Aquat. Living Resour. 2019, 32, 16. [Google Scholar] [CrossRef]
- Guo, D.-D.; Liu, F.; Niu, B.-L.; Zhan, W.; Xie, Q.-P.; Zhang, Y.; Lou, B. Establishment of diploid hybrid strains derived from female Larimichthys crocea× male Larimichthys polyactis and transmission of parental mtDNA in hybrid progenies. Aquaculture 2022, 561, 738693. [Google Scholar] [CrossRef]
- Hou, H.-H.; Miao, L.; Li, M.-Y.; Mu, F.-S.; Xu, Y.-M. The genetic diversity of F4 generations of “Donghai No. 1” large yellow croaker (Larimichthys crocea) analyzed by AFLP. J. Ningbo Univ. 2018, 31, 31–35. (In Chinese) [Google Scholar]
- Zheng, J.; Yan, Y.-R.; Li, Z.-L.; Song, N. Genetic structure of the small yellow croaker (Larimichthys polyactis) across the Yellow Sea and the East China Sea by microsatellite DNA variation: Implications for the division of management units. PeerJ 2022, 10, e13789. [Google Scholar] [CrossRef]
- Xu, S.-W.; Ge, M.-F.; Feng, J.; Wei, X.-X.; Tan, H.-L.; Liang, Z.; Tong, G.-X. Epidemiological investigation on diseases of Larimichthys crocea in Ningbo culture area. Front. Cell. Infect. Microbiol. 2024, 14, 1420995. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-L.; Chen, X.-T.; Qu, A.; Liu, Y.; Zhao, J.; Ke, Q.-Z.; Pu, F.; Wu, L.-N.; Chi, H.-S.; Gong, H.; et al. Identification and Expression Analysis of LncRNAs Reveal the Immune Mechanism of Visceral White-Nodules Disease Resistance in Large Yellow Croaker. Mar. Biotechnol. 2023, 25, 57–69. [Google Scholar] [CrossRef]
- Li, F.-X.; Yin, X.-L.; Lu, D.-Z.; Lu, D.-Z.; Liu, C.; Zhang, J.-S.; Shen, B. Single nucleotide polymorphisms and its association with disease resistant trait of ISG15 genes in Larimichthys crocea. J. Oceanol. Limnol. 2023, 54, 173–182. (In Chinese) [Google Scholar]
- Wei, Z.-Y.; Lu, L.-X.; Ren, Q.-L.; He, T.-L.; Chen, X.-H. Molecular characteristics and antiviral effects of ATG10 in large yellow croaker (Larimichthys crocea). Acta Hydrobiol. Sin. 2022, 46, 521–528. (In Chinese) [Google Scholar]
- Fu, C.-Y.; Wang, J.-P.; Sun, C.; Chen, L.; Qian, D. Isolation, Identification, Inhibition Spectrum and Safety Test of Antagonistic Bacteria to Major Pathogenic Bacteria in Larimichtys crocea. aBIOTECH 2019, 35, 67–75. (In Chinese) [Google Scholar]
- Liu, L.-D. “Core” technology breaks through the genetic code of large yellow croaker and largemouth bass. Sci. Fish Farming 2024, 8–11. (In Chinese) [Google Scholar]
- Huang, Y.; Li, Z.-Y.; Li, M.-C.; Zhang, X.-H.; Shi, Q.; Xu, Z. Fish Genomics and Its Application in Disease-Resistance Breeding. Rev. Aquac. 2025, 17, e12973. [Google Scholar] [CrossRef]
- Yanez, J.M.; Barria, A.; Lopez, M.E.; Moen, T.; Garcia, B.F.; Yoshida, G.M.; Xu, P. Genome-wide association and genomic selection in aquaculture. Rev. Aquac. 2023, 15, 645–675. [Google Scholar] [CrossRef]
- Tetsuo, K.; Liyi, P.; Ryota, I.; Chunyan, C.; Ping, W.; Ikuyo, T.; Yingying, Y.; Xiaojun, Y.; Baoying, G.; Weiye, L. Whole-genome resequencing of large yellow croaker (Larimichthys crocea) reveals the population structure and signatures of environmental adaptation. Sci. Rep. 2021, 11, 11235. [Google Scholar]
- Zhang, J.-J.; Wang, Y.-B.; Qiao, G.-D.; Wang, Q.; Han, D.-C.; Peng, S.-M. Comparison of antioxidant capacity in tissues, musele ultrastructure and related gene expression of Larimichthys crocea with different anti-flowing abilities. Mar. Fish. 2025, 47, 20–28. (In Chinese) [Google Scholar]
- Chen, X.-M.; Li, J.-K.; Wang, Z.-Y.; Cai, M.-Y.; Han, F.; Liu, X.-D. Genome-wide association study of thermal tolerance in large yellow croaker (Larimichthys crocea) based on SLAF-seq technology. Acta Hydrobiol. Sin. 2017, 41, 735–740. (In Chinese) [Google Scholar]
- Ji, Q.; Xie, Z.-l.; Li, L.-Z.; Han, X.-L.; Song, W. A Characterization of the RNA Modification Response to Starvation under Low Temperatures in Large Yellow Croaker (Larimichthys crocea). Fishes 2024, 9, 41. [Google Scholar] [CrossRef]
- Zhang, H.; Ceng, L.; Xiong, Y.-F.; Song, W. Mechanism of salinity acclimation in Larimichthys crocea improving tolerance to salinity stress. J. Fish. Sci. China 2023, 30, 334–343. (In Chinese) [Google Scholar]
- Ding, J.; Zhang, Y.-B.; Wang, J.-Y.; Liu, C.; Gao, X.-M.; Wu, Y.-J.; Wang, J.-Q.; Wu, X.-F.; Zhu, J.-Q.; Shen, W.-L. Genome-wide association study identified candidate SNPs and genes associated with hypoxia tolerance in large yellow croaker (Larimichthys crocea). Aquaculture 2022, 560, 738472. [Google Scholar] [CrossRef]
- Han, D.-C.; Zhang, J.-J.; Wang, Y.-B.; Qiao, G.-D.; Wang, Q.; Peng, S.-M. Comparative analysis of energy metabolism differences among populations of Larimichthys crocea with different flow resistances. Mar. Fish. 2024, 46, 608–615. (In Chinese) [Google Scholar]
- Guo, H.-H.; Hu, Z.; Zhang, J.-G.; Zou, G.-W.; Liang, H.-W. Advances in environmental tolerance and resistance breeding in fish. J. Fish. China 2023, 47, 70–90. (In Chinese) [Google Scholar]
- Gao, G.-Q.; Chang, Y.-M.; Han, Q.-X.; Chi, B.-J.; Li, M.-Y.; Xue, L.-Y.; Liang, L.-Q. Screening of microsatellite markers associated with cold tolerance oflarge yellow croaker (Pseudosciaena crocea R.). Hereditas 2010, 32, 248–253. (In Chinese) [Google Scholar]
- Wang, H.-R.; Liu, M.-H.; You, J.-J.; Luo, H.-Z.; Xu, Y.-A.; Fu, R.-B. Genetic Diversity Analysis and Screening of Microsatellite Markers Associated with Cold Tolerance of Large Yellow Croaker Pseudosciaena crocea Richardson. J. Zhejiang Ocean Univ. 2014, 33, 6–13. (In Chinese) [Google Scholar]
- Mu, F.-S.; Miao, L.; Ming-Yunli et, a.l. Screening of microsatellite markers associated with cold tolerance of large yellow croaker (Pseudosciaena crocea). J. Biol. 2017, 34, 34–38. (In Chinese) [Google Scholar]
- Li, J.-K. Effects of High Temperature on Physiology Biochemistry and Screening Related Microsatellite Markers in Large Yellow Croaker Larimichthys crocea. Master’s Thesis, Jimei University, Xiamen, China, 2015. (In Chinese). [Google Scholar]
- Wu, Y.-D.; Zhou, Z.-X.; Pan, Y.; Zhao, J.; Bai, H.-Q.; Chen, B.-H.; Zhang, X.-Y.; Pu, F.; Chen, J.; Xu, P. GWAS identified candidate variants and genes associated with acute heat tolerance of large yellow croaker. Aquaculture 2021, 540, 736696. [Google Scholar] [CrossRef]
- Jiang, D.; Li, W.-B.; Wang, Z.-Y.; Fang, M. Genome-wide identification of cis-acting expression QTLs in large yellow croaker. Mar. Biotechnol. 2021, 23, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, Y.; Liu, X.-D.; Wang, X.-Q.; Wang, Z.-Y. Genetic mapping and QTL analysis of growth traits in the large yellow croaker Larimichthys crocea. Mar. Biotechnol. 2014, 16, 729–738. [Google Scholar] [CrossRef]
- Zeng, J.-J.; Long, F.; Wang, J.-Y.; Zhao, J.; Ke, Q.-Z.; Gong, J.; Bai, Y.-L.; Deng, Y.-C.; Jiang, P.-X.; Qu, A.; et al. GWAS reveals heritable individual variations in the inherent swimming performance of juvenile large yellow croaker. Aquaculture 2022, 559, 738419. [Google Scholar] [CrossRef]
- Ke, Q.-Z.; Wang, J.-Y.; Bai, Y.-L.; Zhao, J.; Gong, J.; Deng, Y.-C.; Qu, A.; Suo, N.; Chen, J.; Zhou, T.; et al. GWAS and genomic prediction revealed potential for genetic improvement of large yellow croaker adapting to high plant protein diet. Aquaculture 2022, 553, 738090. [Google Scholar] [CrossRef]
- Yu, M.; Xie, Q.-P.; Wei, F.-L.; Wu, X.-F.; Xu, W.-T.; Zhan, W.; Liu, F.; Guo, D.-D.; Niu, B.-L.; Lou, B. Development and identification of a sex-specific molecular marker in Dai-qu stock large yellow croaker (Larimichthys crocea). Aquaculture 2022, 555, 738172. [Google Scholar] [CrossRef]
- Ke, Q.-Z.; Liu, J.-X.; Zhao, J.; Wang, J.-Y.; Jiang, P.-X.; Deng, Y.-C.; Zhou, X.-Y.; Zeng, J.-J.; Zhou, T.; Xu, P. Genomic Selection of Large Yellow Croaker (Larimichthys crocea) with a High Plant Protein Diet Enhances the Growth Performance of Offspring. Mar. Biotechnol. 2024, 26, 732–740. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Wang, J.-Y.; Zhao, J.; Ke, Q.-Z.; Qu, A.; Deng, Y.-C.; Zeng, J.-J.; Gong, J.; Chen, J.; Pan, Y.; et al. Genomic selection for visceral white-nodules diseases resistance in large yellow croaker. Aquaculture 2022, 559, 738421. [Google Scholar] [CrossRef]
- Li, Q.-H.; Shao, G.-M.; Ding, Y.-Y.; Xu, L.-B.; Shao, J.-C.; Ao, J.-Q.; Chen, X.-H. Effective CRISPR/Cas9-based genome editing in large yellow croaker (Larimichthys crocea). Aquac. Fish. 2023, 8, 26–32. [Google Scholar] [CrossRef]
- Cao, B.-R.; Huang, T.-Q.; Gu, W.; Liu, E.-H.; Wang, G.-C.; Pan, Y.-C.; Wang, B.-Q.; Xu, G.-F. Advances of Gene Editing Technology in Genetic Breeding of Fish. Chin. J. Fish. 2024, 37, 100–108. (In Chinese) [Google Scholar]
- Xiao, S.-J.; Li, J.-T.; Ma, F.-S.; Fang, L.-J.; Xu, S.-B.; Chen, W.; Wang, Z.-Y. Rapid construction of genome map for large yellow croaker (Larimichthys crocea) by the whole-genome mapping in BioNano Genomics Irys system. BMC Genom. 2015, 16, 670. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, J.-J.; Liu, S.-F.; Liu, H.-W.; Zhang, T.-L.; Ye, T.; Lou, B.; Liu, F. QTL Mapping-Based Identification of Visceral White-Nodules Disease Resistance Genes in Larimichthys polyactis. Int. J. Mol. Sci. 2024, 25, 10872. [Google Scholar] [CrossRef]
- Ao, J.-Q.; Li, J.; You, X.-X.; Mu, Y.-M.; Ding, Y.; Mao, K.-Q.; Bian, C.; Mu, P.-F.; Shi, Q.; Chen, X.-H. Construction of the high-density genetic linkage map and chromosome map of large yellow croaker (Larimichthys crocea). Int. J. Mol. Sci. 2015, 16, 26237–26248. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, H.; Chen, J.; Ma, J.; Liu, R.; Ding, S. Genetic variation and population genetic structure of the large yellow croaker (Larimichthys crocea) based on genome-wide single nucleotide polymorphisms in farmed and wild populations. Fish. Res. 2020, 232, 105718. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Li, W.-B.; Liu, G.-J.; Gu, L.-L.; Ye, K.; Zhang, Y.-J.; Li, W.; Jiang, D.; Wang, Z.-Y.; Fang, M. Evaluation for the effect of low-coverage sequencing on genomic selection in large yellow croaker. Aquaculture 2021, 534, 736323. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, B.-H.; Ke, Q.-Z.; Zhao, J.; Pu, F.; Wu, Y.-D.; Chen, L.; Zhou, Z.-X.; Bai, Y.-L.; Pan, Y.; et al. Development and evaluation of a high-throughput single-nucleotide polymorphism array for large yellow croaker (Larimichthys crocea). Front. Genet. 2020, 11, 571751. [Google Scholar] [CrossRef]
- Qiu, C.-L.; Dong, L.-S.; Xiao, S.-J.; Xu, S.-B.; Fang, M.; Wang, Z.-Y. Genetic parameter estimation of nine quantitative traits by a marker-based method in Large Yellow Croaker, Larimichthys crocea (Richardson). Aquac. Res. 2017, 48, 5892–5900. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Han, K.-H.; Wu, Y.-D.; Bai, H.-Q.; Ke, Q.-Z.; Pu, F.; Wang, Y.-L.; Xu, P. Genome-wide association study of growth and body-shape-related traits in large yellow croaker (Larimichthys crocea) using ddRAD sequencing. Mar. Biotechnol. 2019, 21, 655–670. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Tay, Y.X.; Yue, G.H. Genome editing and its applications in genetic improvement in aquaculture. Rev. Aquac. 2022, 14, 178–191. [Google Scholar] [CrossRef]
- Chen, S.-L.; Wang, D.-S.; Kuang, Y.-Y.; Cui, Z.-K.; Li, M.-H. Fish genome editing breeding in china: Status, problems and prospects. J. Fish. China 2023, 47, 13–26. (In Chinese) [Google Scholar]
- Zhao, J.; Feng, M.-S.; Ke, Q.-Z.; Wang, J.-S.; Jiang, T.-S.; Wu, X.-F.; Peng, S.-M.; Bai, Y.-L.; Shen, W.-L.; Zhou, T.; et al. Accurate identification of Larimichthys crocea genetic resources based on “NingXin III” chip and machine learning method. J. Fish. China 2024, 48, 30–41. (In Chinese) [Google Scholar]
- Zhou, L.-Y.; Xie, X.; Jiang, L.-H.; Kurt, B.; Yin, F. Diagnosis of cryptocaryoniasis in large yellow croaker (Larimichthys crocea) by real-time object detection based on YOLOv3. Aquaculture 2024, 581, 740418. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Zhang, X.; Su, Y.-X.; Li, W.-Y.; Yin, X.-L.; Li, Z.-H.; Ying, Y.-F.; Wang, J.-C.; Wu, J.-P.; Miao, F.-J.; et al. Abnormal Behavior Monitoring Method of Larimichthys crocea in Recirculating Aquaculture System Based on Computer Vision. Sensors 2023, 23, 2835. [Google Scholar] [CrossRef]
- Wei, X.-M. Intelligent Monitoring System of Fisher Aquaculture Based on Internet of Things. Master’s Thesis, Tianjin University of Technology, Tianjin, China, 2019. (In Chinese). [Google Scholar]
- Chang, C.-C.; Ubina, N.A.; Cheng, S.-C.; Lan, H.-Y.; Chen, K.-C.; Huang, C.-C. A two-mode underwater smart sensor object for precision aquaculture based on AIoT technology. Sensors 2022, 22, 7603. [Google Scholar] [CrossRef]
- Zhao, L.-H.; Dong, Y.-H.; Zhang, Z.-J. Design and Development of an Intelligent Monitoring System for Marine Aquaculture Environment Based on AIoT. Comput. Knowl. Technol. 2024, 20, 137–139. (In Chinese) [Google Scholar]
- Zhou, R.-J. Studies on the Forecasting of the Main Disease in Cage-Cultured Pseudosciaena crocea. Master’s Thesis, Ningbo University, Ningbo, China, 2011. (In Chinese). [Google Scholar]
- Yang, Z.; Lu, N.; Zhai, L. Study on production strategies for marine aquaculture in China at different scales: A case study of large yellow croaker (Larimichthys crocea). Aquac. Int. 2025, 33, 1–16. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, H.; Zhou, Z.-G.; Chen, N.; Bao, Y.-D. Progress and prospects of the application of digital technology in aquaculture production. China Agric. Inform. 2024, 36, 56–67. (In Chinese) [Google Scholar]
- Fu, G.; Yuna, Y. Phenotyping and phenomics in aquaculture breeding. Aquac. Fish. 2022, 7, 140–146. [Google Scholar] [CrossRef]
- Zou, H. Intelligent Environmental Monitoring and Control Technologies in Digital Aquaculture. Sci. Fish Farming 2023, 78–80. (In Chinese) [Google Scholar]
- Gan, Q.-L. Innovations in Intelligent Technologies and Digital Management in Aquaculture. J. Agric. Catastrophol. 2024, 14, 115–117. (In Chinese) [Google Scholar]
- Liu, C.; Ding, J.; Gao, X.-M.; Du, C.; Hou, C.-C.; Wu, X.-F.; Shen, W.-L.; Zhu, J.-Q. Effects of acute low temperature stress on the hormones and gene expression of glucocorticoid receptor of large yellow croaker Larimichthys crocea. J. Therm. Biol. 2021, 99, 103018. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Wang, Y.-B.; Chen, R.; Yue, Y.-F.; Gao, Q.-X.; Wang, C.-H.; Peng, S.-M. Proteomic and transcriptomic analysis of large yellow croaker (Larimichthys crocea) during early development under hypoxia and acidification stress. Aquaculture 2023, 577, 739982. [Google Scholar] [CrossRef]
- Ruan, S.-J.; Lu, Z.; Huang, W.-Q.; Zhang, Y.; Shan, X.-J.; Song, W.; Ji, C.-L. Renal metabolomic profiling of large yellow croaker Larimichthys crocea acclimated in low salinity waters. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 46, 101083. [Google Scholar] [CrossRef]
- Su, X.; Sutarlie, L.; Loh, X.J. Sensors, biosensors, and analytical technologies for aquaculture water quality. Research 2020, 2020, 8272705. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Zhou, R.-L.; Xie, X.; Yin, F. Characteristics and risk assessment of cryptocaryoniasis in large yellow croaker (Larimichthys crocea) at different densities in industrialized aquaculture. Aquaculture 2024, 582, 740501. [Google Scholar] [CrossRef]
- Guo, J.-L.; Yang, D.-F.; Xue, J.-G.; Song, M.-Y.; Xu, P.-X. Bibliometric Study On Fish Color Vision. J. Anhui Agric. Sci. 2024, 52, 10–14. (In Chinese) [Google Scholar]
- Kou, F.; Ma, Z.-J.; Wang, S. Research on Intelligent Aquaculture Monitoring System Based on Internet of Things. Sci. Technol. Inf. 2024, 22, 79–81+85. (In Chinese) [Google Scholar]
- Fan, L.-Z. Research on Real-Time Monitoring Technology for the Growth Process of Large Yellow Croaker Based on Computer Vision; Scientific Research Report; NingboTech University: Ningbo, China, 2015. (In Chinese) [Google Scholar]
- Song, C.-B.; Liu, L.-L.; Lu, P.-Z.; Yang, H.; Li, X.; Gao, X.-L.; Chen, T.; Xiong, W.; Lei, B.-L.; Ma, H.; et al. Design of artificial LED lighting in aquaculture workshop. J. Dalian Ocean Univ. 2018, 33, 145–150. (In Chinese) [Google Scholar]
- Song, W.; Chen-Han, X.; Zheng, L.-X.; Wang, L.; Liu, Y.-L.; Wang, L.-M. Development Status and the Prospect of Deep SeaLarge-Scale Fence Culture in China. Prog. Fish. Sci. 2022, 43, 111–120. (In Chinese) [Google Scholar]
- Zhang, P.-P.; Li, Z.-R.; Song, H.-Y.; Cai, H.-W.; Xia, F.-F. AHP-based evaluation index system on site selection for offshore cage cul-ture. South China Fish. Sci. 2023, 19, 1–9. (In Chinese) [Google Scholar]
- Ren, Z.-Y.; Cui, Y.-Q.; Chen, Y.-F.; Shi, L.-F.; Hao, G.-X.; Yang, S.; Qiu, X.-J.; Liu, S.-J.; Weng, W.-Y. Effect of pH on the structural properties and emulsification of myofibrillar proteins of large yellow croaker (Larimichthys crocea). J. Fish. China 2024, 48, 164–173. [Google Scholar]
- Ceng, L.; Xiong, Y.-F.; Song, W.; Xie, Z.-L.; Wang, Y.-H. Effect of salinity stress on energy metabolism and mitochondrial autophagy in large yellow croaker. Acta Hydrobiol. Sin. 2024, 48, 725–733. (In Chinese) [Google Scholar]
- Björn, B.; Lisa, H.; Alexander, R.; Carolina, W.L.; MarcChristopher, H.; Marieke, V.; Wilhelm, P.H. Effects of stocking density, size, and external stress on growth and welfare of African catfish (Clarias gariepinus Burchell, 1822) in a commercial RAS. Fishes 2023, 8, 74. [Google Scholar]
- Zhang, G.-B.; Ye, Z.-Q.; Jiang, Z.-J.; Wu, C.-Q.; Ge, L.-F.; Wang, J.-X.; Xu, X.-W.; Wang, T.-M.; Yang, J.-W. Circadian patterns and photoperiodic modulation of clock gene expression and neuroendocrine hormone secretion in the marine teleost Larimichthys crocea. Chronobiol. Int. 2024, 41, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Chávez, C.C.; Rodríguez-Ibarra, L.E.; Rodríguez Montes de Oca, G.; Abdo-de la Parra, M.I. Evaluación de diferentes fotoperiodos en el cultivo larvario del botete diana (Sphoeroides annulatus). Ecosistemas Y Recur. Agropecu. 2022, 9, 1–7. [Google Scholar]
- Feng, M.-S.; Jiang, P.-X.; Ke, Q.-Z.; Liu, S.-Y.; Chen, Y.-W.; Du, Y.-Q.; Luo, W.-J.; Liu, Y.-X.; Cai, Q.-X.; Zeng, Z.-H.; et al. A computer vision and RFID fusion-based method for measuring individual feed intake and its application for detecting individual differences in feed efficiency of large yellow croaker (Larimichthys crocea). Water Biol. Secur. 2024, 4, 100332. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Yao, C.-W.; Cui, K.; Hao, T.-T.; Yin, Z.-Y.; Xu, W.-X.; Huang, W.-X.; Mai, K.-S.; Ai, Q.-H. Nutritional programming of large yellow croaker (Larimichthys crocea) larvae by dietary vegetable oil: Effects on growth performance, lipid metabolism and antioxidant capacity. Br. J. Nutr. 2023, 129, 967–980. [Google Scholar] [CrossRef]
- Wang, X.-M.; Liu, H.; Zhang, C.-L.; Zhu, C.; Liu, H.-Y. Mechanisms of the Effect of Starvation Duration on the Regulation of Feeding Rhythm and Metabolic Physiology of Cultured Large Yellow Croaker (Larimichthys crocea). J. Mar. Sci. Eng. 2025, 13, 90. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Zhao, J.-Y.; Yao, M.-Y.; Liu, X.; Huo, Y.-K.; Chen, Y.-Y.; Wang, H.-H. Research advances on fish feeding behavior recognition and intensity quantification methods in aquaculture. arXiv 2025, arXiv:250215311. [Google Scholar]
- He, Y.-L.; Tang, Y.-H.; Xu, N.; Yao, C.-W.; Gong, Y.; Yin, Z.-Y.; Li, Q.-F.; Zhang, Y.-Q.; Lai, W.-C.; Liu, Y.-T. Effects of supplemental phytosterol on growth performance, body composition, serum biochemical indexes and lipid metabolism of juvenile large yellow croaker (Larimichthys crocea) fed with high lipid diet. Aquaculture 2022, 551, 737889. [Google Scholar] [CrossRef]
- Zhang, C. Behavior Tracking of Large Yellow Croaker Based on Machine Vision; Zhejiang Ocean University: Zhoushan, China, 2022. (In Chinese) [Google Scholar]
- Tian, Y.-X.; Feng, D.-J.; Zhang, H.; Gui, F.-K.; Qu, X.-Y. Distribution detection of Larimichthys crocea cultured in largenet-enclosure aquaculture by Small Unmanned Surface Vehicle. J. Fish. China 2022, 46, 2084–2096. (In Chinese) [Google Scholar]
- Ren, X.-M.; Gao, D.-Z.; Yao, Y.-L.; Yang, F.; Liu, J.-F.; Xie, F.-J. Occurrence and characteristic of sound in large yellow croaker (Pseudosciaena crocea). J. Dalian Ocean. Univ. 2007, 22, 123–128. (In Chinese) [Google Scholar]
- Dhinakaran, D.; Gopalakrishnan, S.; Manigandan, M.; Anish, T. IoT-Based Environmental Control System for Fish Farms with Sensor Integration and Machine Learning Decision Support. arXiv 2023, arXiv:231104258. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Yu, H.; Zhang, X.; Zhang, P.; Tu, W.; Gu, L.-S. Fish behavior recognition based on an audio-visual multimodal interactive fusion network. Aquac. Eng. 2024, 107, 102471. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Miryala, K.R.; Swain, B. Advances and Challenges in Aeromonas hydrophila Vaccine Development: Immunological Insights and Future Perspectives. Vaccines 2025, 13, 202. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Zhu, C.-H.; Xiao, F.-N.; Liu, X.-D.; Xie, A.-H.; Chen, F.-M.; Dong, P.-P.; Lin, P.-D.; Zheng, C.-Y.; Zhang, H.; et al. PH-controlled release of antigens using mesoporous silica nanoparticles delivery system for developing a fish oral vaccine. Front. Immunol. 2021, 12, 644396. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Du, Y.; Hu, X.-M.; Miao, L.; Chen, J. Current status and development prospects of aquatic vaccines. Front. Immunol. 2022, 13, 1040336. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-F.; Cui, K.; Wu, M.-J.; Xu, D.; Mai, K.-S.; Ai, Q.-H. Polyunsaturated fatty acids influence LPS-induced inflammation of fish macrophages through differential modulation of pathogen recognition and p38 MAPK/NF-κB signaling. Front. Immunol. 2020, 11, 559332. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Chen, H.; An, H.-M.; Wang, Y.-Y.; Shao, J.-C.; Yan, M.-J.; Ao, J.-Q.; Chen, X.-H.; Zhang, W.-N. Dietary Astragalus polysaccharides enhance potency of inactivated Pseudomonas plecoglossicida vaccine in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2025, 157, 107–110. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Chi, H.; Mweemba, M.H.; Xu, C.; Qin, P.; Chen, X.-H. Early immune response in large yellow croaker (Larimichthys crocea) after immunization with oral vaccine. Mol. Cell. Probes 2021, 56, 101708. [Google Scholar] [CrossRef]
- Li, X.-M.; Tan, Y.-Z.; Zhang, Z.; Huang, Y.-P.; Mu, P.-F.; Cui, Z.-W.; Chen, X.-H. Development of recombinant dihydrolipoamide dehydrogenase subunit vaccine against Vibrio infection in large yellow croaker. Fishes 2022, 7, 17. [Google Scholar] [CrossRef]
- He, L.-Y.; Li, Y.-S.; Kang, J.-L.; Li, J.-X. Development and potential use of Pseudomonas plecoglossicida mutant ΔOmpRΔrpoS as a live attenuated vaccine against visceral white nodules disease in large yellow croaker (Larimichthys crocea). Aquaculture 2023, 574, 739718. [Google Scholar] [CrossRef]
- Bøgwald, J. DALMORA Review on immersion vaccines for fish: An update 2019. Microorganisms 2019, 7, 627. [Google Scholar] [CrossRef]
- Su, H.; Yakovlev, I.A.; André, V.E.; Jianguo, S.; Liu, C.J. Plant-produced vaccines: Future applications in aquaculture. Front. Plant Sci. 2021, 12, 718775. [Google Scholar] [CrossRef]
- Clarke, J.L.; Waheed, M.T.; Lössl, A.G.; Martinussen, I.; Daniell, H. How can plant genetic engineering contribute to cost-effective fish vaccine development for promoting sustainable aquaculture? Plant Mol. Biol. 2013, 83, 33–40. [Google Scholar] [CrossRef]
- Nuryati, S.; Juliadiningtyas, A.D. Potential transmission test of GP25 vaccine in normal flora bacteria of common carp culture media. J. Akuakultur Indones. 2015, 14, 90–97. [Google Scholar] [CrossRef]
- Zhang, K.-F.; Zhou, Y.-D.; Song, W.-H.; Jiang, L.-H.; Yan, X.-J. Genome-wide radseq reveals genetic differentiation of wild and cultured populations of large yellow croaker. Genes 2023, 14, 1508. [Google Scholar] [CrossRef]
- Xu, Z.; Dou, S.-Z.; Ding, S.-X.; Liu, J.-X. Temporal genetic stability despite decades of overexploitation for large yellow croaker in the East China sea. Front. Mar. Sci. 2022, 9, 861840. [Google Scholar] [CrossRef]
- Xinxiu, Y.; Rajesh, J.; Magnus, H.G.; Zhenming, L.; Matthew, K. Construction of genetic linkage maps from a hybrid family of large yellow croaker (Larimichthys crocea). Front. Genet. 2022, 12, 792666. [Google Scholar]
- Liu, R.-Z.; Wang, S.; Huang, D.-L.; Huang, Y.-L.; He, T.-L.; Chen, X.-H. The probiotic roles of Lactiplantibacillus plantarum E2 as a dietary supplement in growth promotion and disease resistance of juvenile large yellow croaker (Larimichthys crocea). Aquaculture 2024, 578, 740082. [Google Scholar] [CrossRef]
- Jia, C.-F.; Liu, H.-L.; Xu, J.; Zhang, Z.-Y.; Zhang, Z.-W.; Chen, S.-Y.; Zhu, F. A review on the germplasm genetic diversity of large yellow croaker (Larimichthys crocea). Mar. Sci. Bull. 2017, 36, 12–18. (In Chinese) [Google Scholar]
- Zhao, J.; Bai, H.-Q.; Ke, Q.-Z.; Li, B.-J.; Zhou, Z.-X.; Wang, H.; Chen, B.-H.; Pu, F.; Zhou, T.; Xu, P. Genomic selection for parasitic ciliate Cryptocaryon irritans resistance in large yellow croaker. Aquaculture 2021, 531, 735786. [Google Scholar] [CrossRef]
- Nguyen, N.H. Genetics and Genomics of Infectious Diseases in Key Aquaculture Species. Biology 2024, 13, 29. [Google Scholar] [CrossRef]
- Shao, J.-C.; Wang, X.-X.; Liu, Q.-Q.; Lv, H.-Y.; Qi, Q.; Li, C.-H.; Zhang, J.-N.; Chen, X.-J.; Chen, X.-H. Eucommia ulmoides leaf extracts combined with Astragalus polysaccharides: Effects on growth, antioxidant capacity, and intestinal inflammation in juvenile large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2025, 161, 110229. [Google Scholar] [CrossRef]
- Zhu, A.-J.; Li, W.-Y.; Zhang, X.-L.; Yan, X.-J. Dietary ursodeoxycholic acid supplementation enhanced nonspecific immunity, antioxidant capacity, and anti-inflammatory-related gene expression in juvenile large yellow croaker (Larimichthys crocea). Aquac. Rep. 2025, 41, 102698. [Google Scholar] [CrossRef]
- Peng, S.-M.; Wang, Y.-B.; Wang, Q.; Gao, Q.-X.; Zheng, H.-F.; Xu, J.; Wang, L.-M. A review: Molecular breeding related techniques of Larimichthys crocea. Mar. Fish. 2022, 44, 375. (In Chinese) [Google Scholar]
- Zhang, Y. Effects of Different Carbon-to Nitrogen Ratios on Biofloc Formation and Water Quality in Larval Rearing Ponds of Large Yellow Croaker. Sci. Fish Farm. 2023, 12, 77–79. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.-X.; Li, Y.; Tang, J.-C.; Li, K.-Q.; Shen, J.-J.; Liu, C.; Jiang, Y.-H.; Zhang, Z.-P.; Wang, Y.-L.; Zou, P.-F. SARM suppresses TRIF, TRAF3, and IRF3/7 mediated antiviral signaling in large yellow croaker Larimichthys crocea. Front. Immunol. 2023, 13, 1021443. [Google Scholar] [CrossRef]
- Muktar, Y.; Tesfaye, S.; Tesfaye, B. Present Status and Future Prospects of Fish Vaccination: A Review. J. Vet. Sci. Technol. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Wan, L.; Wang, W.-J.; Liu, G.-J.; Dong, L.-S.; Li, W.-B.; Han, Z.-F.; Ye, K.; Wang, Z.-Y. A genome-wide association study of resistance to Pseudomonas plecoglossicida infection in the large yellow croaker (Larimichthys crocea). Aquac. Int. 2019, 27, 1195–1208. [Google Scholar] [CrossRef]
- Shen, W.-L.; Wu, X.-F.; Shen, T.-J.-K.; Lin, S.-Q. The Effects of Different Diets and Culture Environments on the Morphological Variations in the Large Yellow Croaker (Larimichthys crocea). Prog. Fish. Sci. 2017, 38, 70–77. (In Chinese) [Google Scholar]
- Wang, Y. Paradigm shift of fish nutrition and feed: The necessity revealed by the application of formulated feed in Micropterus salmoides and Larimichthys crocea farming. J. Fish. China 2024, 48, 222–240. (In Chinese) [Google Scholar]
- Zhou, X.; Gao, F.-Y.; Lu, M.-X. Progress on breeding of disease-resistant fishes: A review. J. Dalian Ocean Univ. 2021, 36, 510–523. (In Chinese) [Google Scholar]
- Houston, R.D.; Christina, K.; Diego, R. Animal board invited review: Widespread adoption of genetic technologies is key to sustainable expansion of global aquaculture. Animal 2022, 16, 100642. [Google Scholar] [CrossRef]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-J. Research Progress in Precision Feeding Technologies for Aquaculture. Anim. Breed. Feed 2024, 23, 58–60. (In Chinese) [Google Scholar]
- Rather, M.A.; Ahmad, I.; Shah, A.; Hajam, Y.A.; Amin, A.; Khursheed, S.; Ahmad, I.; Rasool, S. Exploring opportunities of Artificial Intelligence in aquaculture to meet increasing food demand. Food Chem. X 2024, 22, 101309. [Google Scholar] [CrossRef]
- Xu, Y.-B.; Zhang, X.-P.; Li, H.-H.; Zheng, J.-N.; Olsen Michael, S.; Varshney Rajeev, K.; Prasanna Boddupalli, M.; Qian, Q. Smart breeding driven by big data, artificial intelligence and integrated genomic-enviromic prediction. Mol. Plant 2022, 15, 1664–1695. [Google Scholar] [CrossRef]
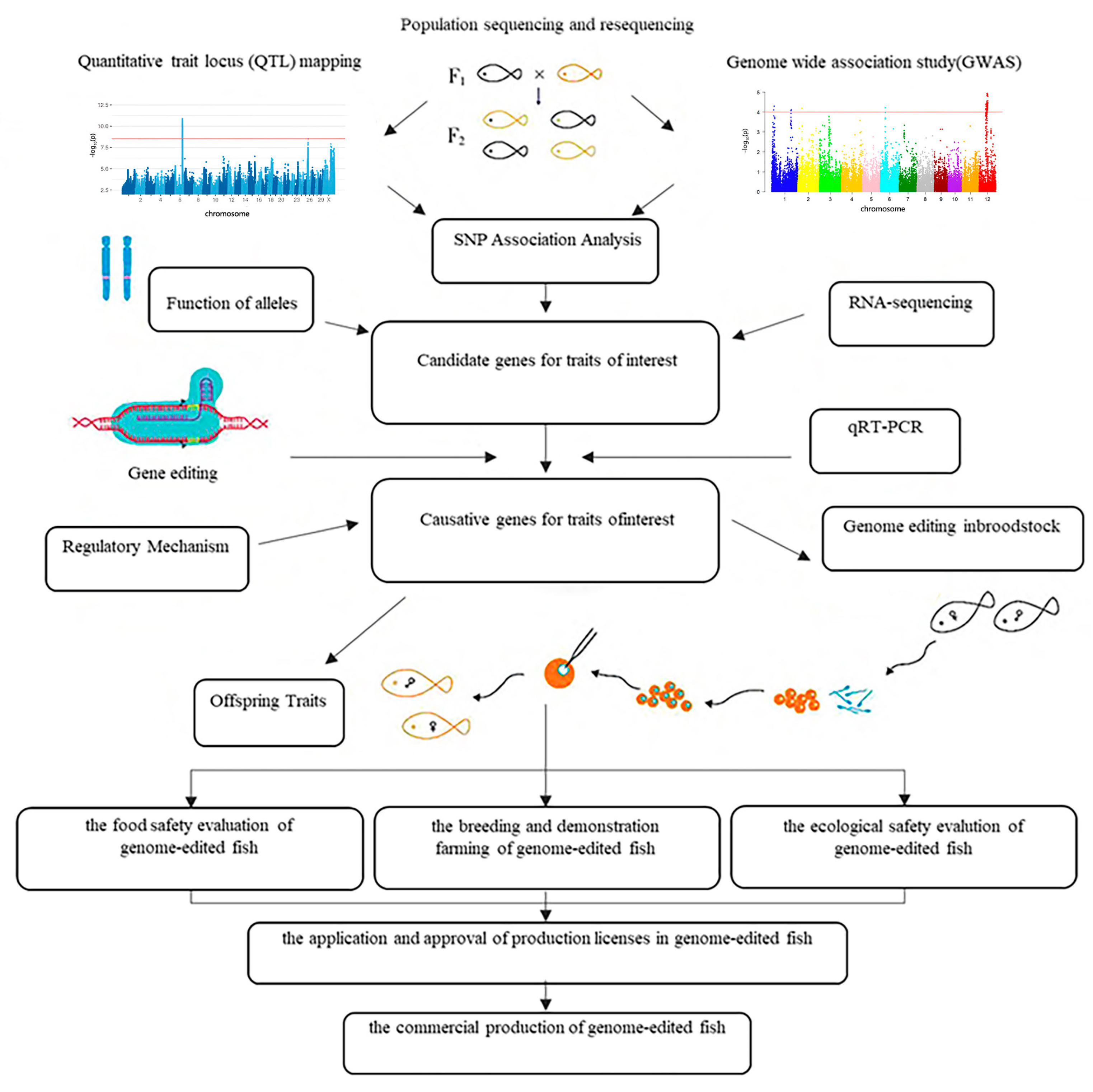
| Technical Approach | Core Principles | Characteristics | Limitations |
|---|---|---|---|
| Quantitative trait gene mapping [50,51] | Linkage between molecular markers and QTLs | Requires segregating families with trait variation. | Low resolution; requires construction of linkage maps. |
| Genome-wide association studies [52,53] | Linkage disequilibrium between markers and traits | Utilizes molecular and phenotypic variation in natural populations. | Requires dense markers and deep sequencing of large populations. |
| Marker-assisted selection [54] | Selection based on genotype–phenotype association | Effective for traits that are hard to measure or have low heritability (e.g., disease resistance). | Limited to traits controlled by major genes or a few QTLs. |
| Genomic selection [55,56] | Uses genome-wide dense markers for phenotype prediction | Does not require QTL detection; it provides higher prediction accuracy. | Requires close-related reference populations; model-dependent performance. |
| Genome Editing [57] | Precise and stable modification of genomic sequences | Enables targeted improvement of complex traits; shortens breeding cycles. | Few functional targets for complex traits; subject to regulatory approval. |
| Environmental Factors | Specific Parameters | Mechanisms of Influence | Optimization Recommendations |
|---|---|---|---|
| Water conditions [92,93] | Temperature, dissolved oxygen, ammonia nitrogen concentration, pH, and salinity | High temperatures may trigger stress responses, while low temperatures slow growth. Insufficient dissolved oxygen suppresses metabolism; excessive ammonia causes toxicity. pH affects immune function, and improper salinity disrupts feed conversion efficiency. | Apply smart temperature control systems to maintain optimal ranges; ensure adequate oxygen supply; stabilize water quality, and adjust salinity appropriately. |
| Stocking density [85,94] | Larval stage: 1000–6000 fish/m3; Grow-out stage: 12–30 fish/m3 | Excessive density leads to water quality deterioration, poor growth, and lower survival rates; overly low density wastes resources and reduces farming efficiency. | Adjust stocking density according to growth stage; use aeration systems to maintain water quality; prevent overcrowding to improve survival rates. |
| Light [95,96] | Light intensity, photoperiod | Insufficient light suppresses feeding motivation; overly strong light causes fry to cluster, leading to localized hypoxia. | Adjust light intensity and duration to simulate natural lighting conditions. |
| Inoculation Methods | Advantages | Disadvantages |
|---|---|---|
| Immunization by immersion [117] | Invasive, low stress, suitable for fry mass vaccination; cost-effective. | Short duration of protection and moderate efficacy Limited effectiveness in larger fish |
| Oral immunization [111,118] | Easy for mass dosing, low stress, no labor costs. | Efficacy varies depending on feeding behavior Requires precise feed formulation to maintain vaccine activity |
| Injection immunization [118] | immune response, long-lasting protection, targeted delivery. | Time-consuming, suited for larger fish, high cost Risk of injury or stress if improperly handled |
| Injectable particulate vaccine [119] | Emerging tech: nanoparticles/adjuvants enhance antigen delivery and immune response. | Requires specialized equipment; relatively high technical cost |
| Genetic vaccines [120] | Strong, long-term immunity; broad-spectrum potential. | Technically complex with regulatory challenges Not yet widely adopted in all countries |
| Myths | Description | Results | Solution |
|---|---|---|---|
| Over-reliance on single-trait preferences [6] | Overemphasis on traits like growth, neglecting disease resistance, and adaptability. | Reduced fry quality; compromised long-term health and growth. | Incorporate multiple traits to ensure balanced performance. |
| Neglect of genetic diversity and risk of inbreeding [121] | Inbreeding reduces diversity and increases disease susceptibility. | Lower resilience, disease outbreak risk, and slower breeding progress. | Maintain diverse germplasm; avoid inbreeding. |
| Ignoring the influence of environmental factors [122] | Ignoring environmental context in selection. | Even genetically superior fish may perform poorly under stress. | Apply environmental monitoring and adopt field-relevant selection criteria. |
| Over-reliance on traditional breeding methods [123] | Overdependence on phenotype-based methods without genomic tools. | Low selection efficiency, slow genetic gain, and limited precision. | Integrate genomics and molecular markers to accelerate improvement. |
| Insufficient attention to disease resistance selection [124] | Lack of systematic breeding for disease resistance. | Susceptibility in mass culture, frequent disease outbreaks, and economic loss. | Employ molecular markers and immune-based breeding for resilience. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Zhang, S.; Wang, Y.; Fang, H.; Peng, S.; Yang, S.; Wu, Z. Seedling Selection of the Large Yellow Croaker (Larimichthys crocea) for Sustainable Aquaculture: A Review. Appl. Sci. 2025, 15, 7307. https://doi.org/10.3390/app15137307
Han X, Zhang S, Wang Y, Fang H, Peng S, Yang S, Wu Z. Seedling Selection of the Large Yellow Croaker (Larimichthys crocea) for Sustainable Aquaculture: A Review. Applied Sciences. 2025; 15(13):7307. https://doi.org/10.3390/app15137307
Chicago/Turabian StyleHan, Xinran, Shengmao Zhang, Yabing Wang, Hui Fang, Shiming Peng, Shenglong Yang, and Zuli Wu. 2025. "Seedling Selection of the Large Yellow Croaker (Larimichthys crocea) for Sustainable Aquaculture: A Review" Applied Sciences 15, no. 13: 7307. https://doi.org/10.3390/app15137307
APA StyleHan, X., Zhang, S., Wang, Y., Fang, H., Peng, S., Yang, S., & Wu, Z. (2025). Seedling Selection of the Large Yellow Croaker (Larimichthys crocea) for Sustainable Aquaculture: A Review. Applied Sciences, 15(13), 7307. https://doi.org/10.3390/app15137307