Acute Effects of Static Stretching on Submaximal Force Control of the Ankle
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
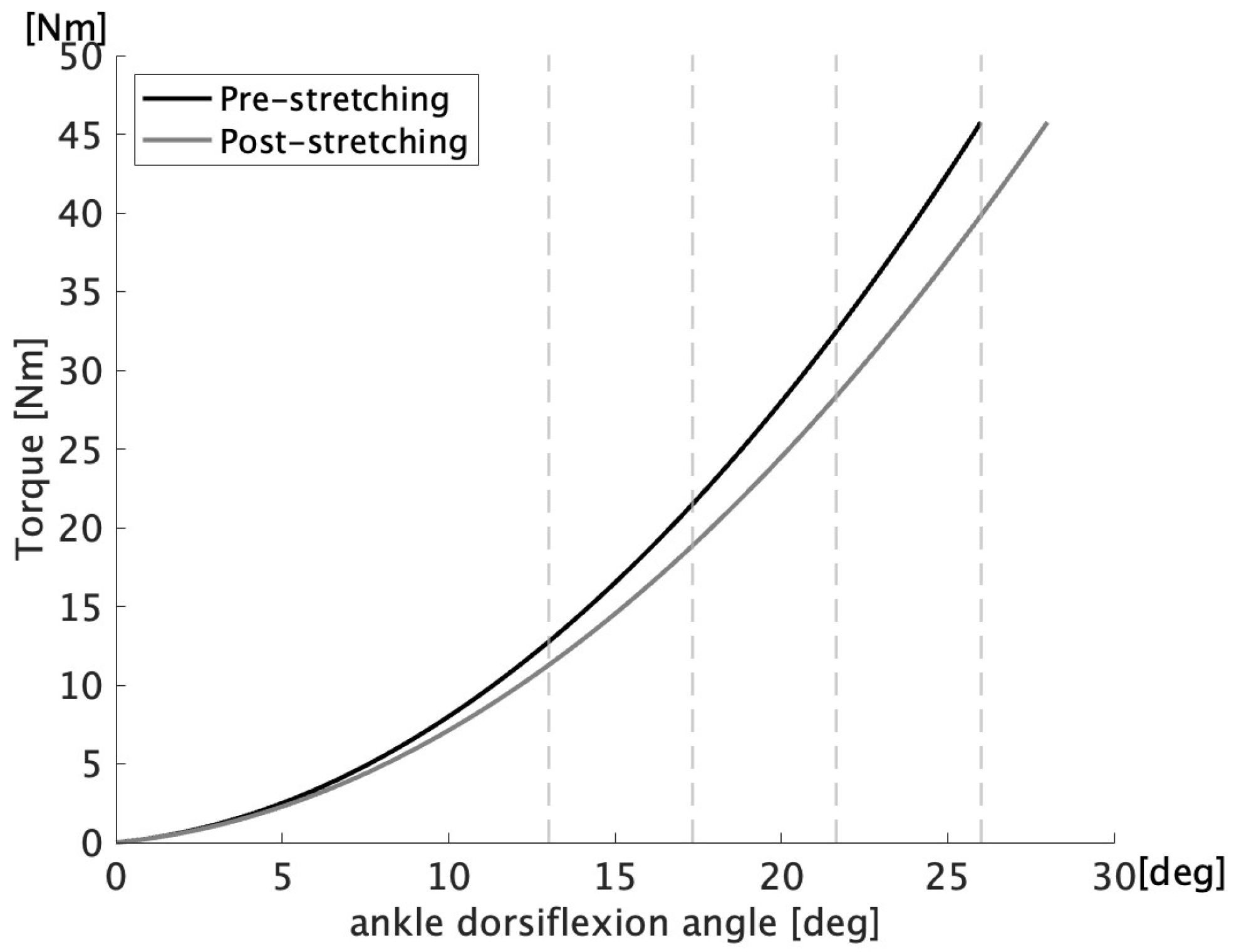
2.3. Measurement of Maximum Angle of Ankle Dorsiflexion and Passive Resistance Torque
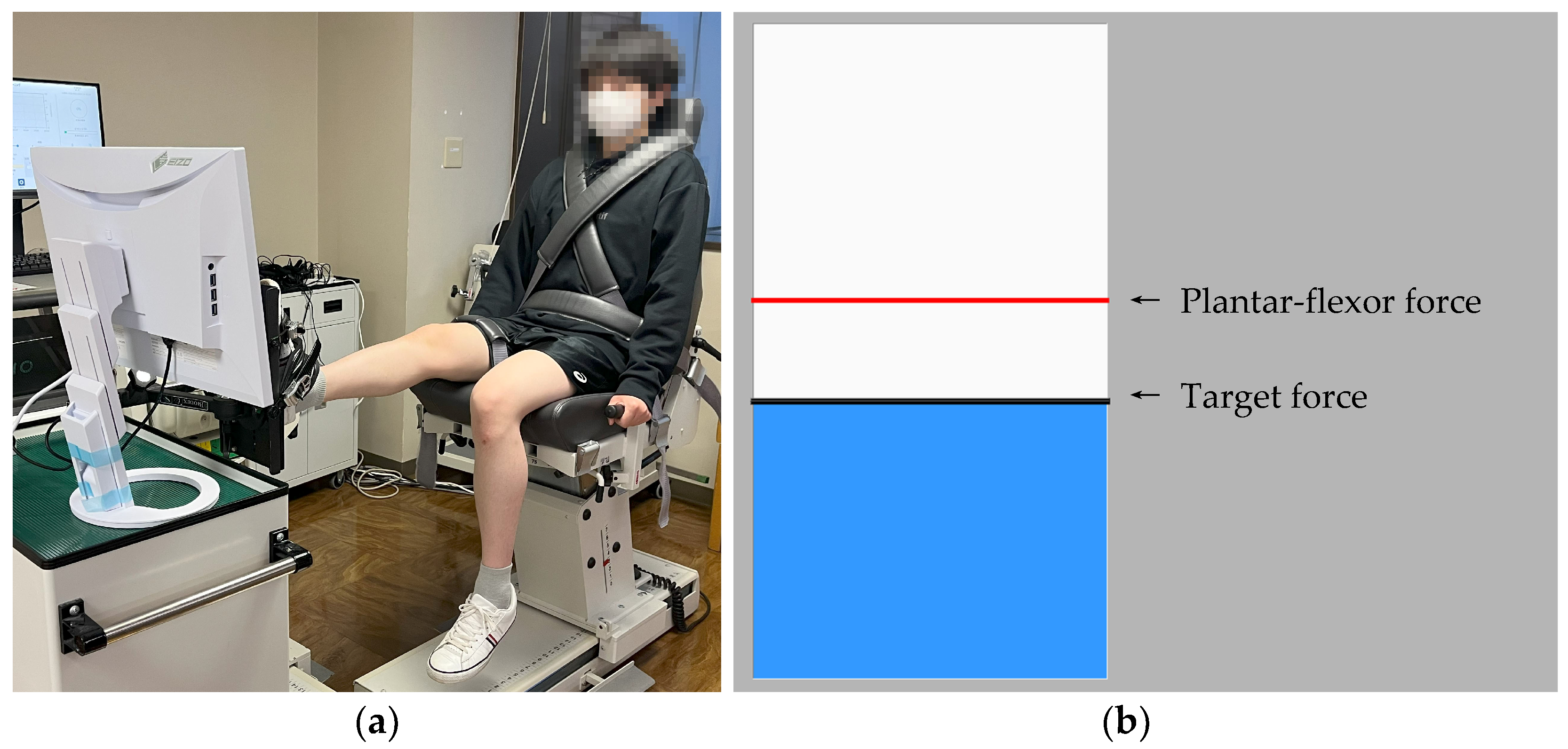
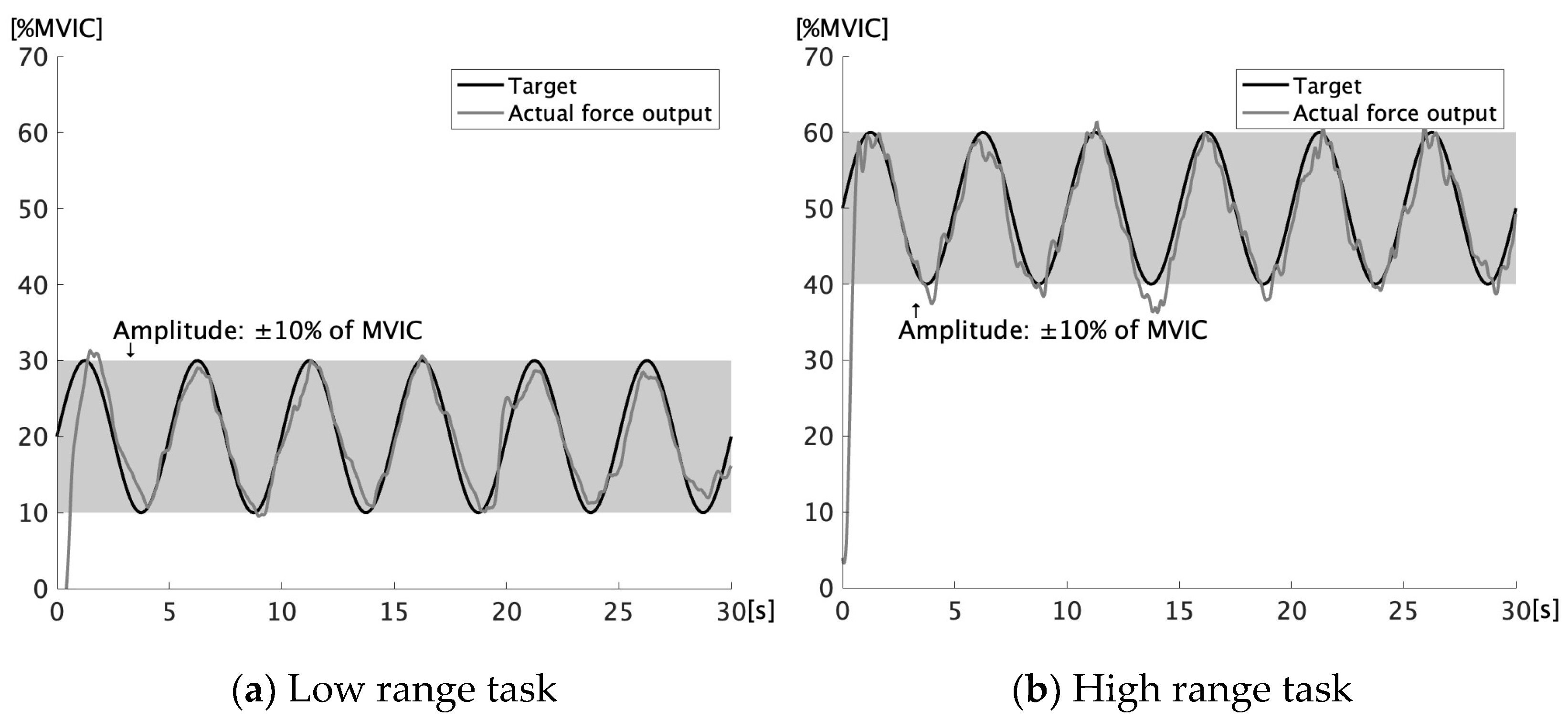
2.4. Force-Tracking Task
2.5. Static Stretching Protocol
2.6. Data Processing and Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konrad, A.; Budini, F.; Tilp, M. Acute Effects of Constant Torque and Constant Angle Stretching on the Muscle and Tendon Tissue Properties. Eur. J. Appl. Physiol. 2017, 117, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kiyono, R.; Takahashi, N.; Yoshida, T.; Takeuchi, K.; Nakamura, M. The Acute and Prolonged Effects of 20-s Static Stretching on Muscle Strength and Shear Elastic Modulus. PLoS ONE 2020, 15, e0228583. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yoshida, R.; Sato, S.; Yahata, K.; Murakami, Y.; Kasahara, K.; Fukaya, T.; Takeuchi, K.; Nunes, J.P.; Konrad, A. Comparison Between High- and Low-Intensity Static Stretching Training Program on Active and Passive Properties of Plantar Flexors. Front. Physiol. 2021, 12, 796497. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, H.; Behm, D.G.; Negra, Y.; Granacher, U. Acute Effects of Static Stretching on Muscle Strength and Power: An Attempt to Clarify Previous Caveats. Front. Physiol. 2019, 10, 1468. [Google Scholar] [CrossRef]
- Oba, K.; Samukawa, M.; Nakamura, K.; Mikami, K.; Suzumori, Y.; Ishida, Y.; Keeler, N.; Saitoh, H.; Yamanaka, M.; Tohyama, H. Influence of Constant Torque Stretching at Different Stretching Intensities on Flexibility and Mechanical Properties of Plantar Flexors. J. Strength Cond. Res. 2021, 35, 709–714. [Google Scholar] [CrossRef]
- Budini, F.; Rafolt, D.; Christova, M.; Gallasch, E.; Tilp, M. The Recovery of Muscle Spindle Sensitivity Following Stretching Is Promoted by Isometric but Not by Dynamic Muscle Contractions. Front. Physiol. 2020, 11, 905. [Google Scholar] [CrossRef]
- Fowles, J.R.; Sale, D.G.; MacDougall, J.D. Reduced Strength after Passive Stretch of the Human Plantarflexors. J. Appl. Physiol. 2000, 89, 1179–1188. [Google Scholar] [CrossRef]
- Ghaffarinejad, F.; Taghizadeh, S.; Mohammadi, F. Effect of Static Stretching of Muscles Surrounding the Knee on Knee Joint Position Sense. Br. J. Sports Med. 2007, 41, 684–687. [Google Scholar] [CrossRef]
- Trajano, G.S.; Nosaka, K.; B. Seitz, L.; Blazevich, A.J. Intermittent Stretch Reduces Force and Central Drive More than Continuous Stretch. Med. Sci. Sports Exerc. 2014, 46, 902–910. [Google Scholar] [CrossRef]
- Masugi, Y.; Obata, H.; Inoue, D.; Kawashima, N.; Nakazawa, K. Neural Effects of Muscle Stretching on the Spinal Reflexes in Multiple Lower-Limb Muscles. PLoS ONE 2017, 12, e0180275. [Google Scholar] [CrossRef]
- Latash, M.L. Motor Control: On the Way to Physics of Living Systems. Adv. Exp. Med. Biol. 2014, 826, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wuebbenhorst, K.; Zschorlich, V. The Effect of Increasing External Degrees of Freedom on Force Production and Neuromuscular Stabilisation. J. Sports Sci. 2012, 30, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- An, K.-N. Muscle Force and Its Role in Joint Dynamic Stability. Clin. Orthop. Relat. Res. 2002, 403, S37–S42. [Google Scholar] [CrossRef][Green Version]
- Enoka, R.M.; Duchateau, J. Muscle Fatigue: What, Why and How It Influences Muscle Function: Muscle Fatigue. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hirono, T.; Ikezoe, T.; Taniguchi, M.; Yamagata, M.; Miyakoshi, K.; Umehara, J.; Ichihashi, N. Relationship between Ankle Plantar Flexor Force Steadiness and Postural Stability on Stable and Unstable Platforms. Eur. J. Appl. Physiol. 2020, 120, 1075–1082. [Google Scholar] [CrossRef]
- Pethick, J.; Tallent, J. The Neuromuscular Fatigue-Induced Loss of Muscle Force Control. Sports 2022, 10, 184. [Google Scholar] [CrossRef]
- Wade, L.; Lichtwark, G.A.; Farris, D.J. Joint and Muscle-Tendon Coordination Strategies during Submaximal Jumping. J. Appl. Physiol. 2020, 128, 596–603. [Google Scholar] [CrossRef]
- Chow, J.W.; Stokic, D.S. Force Control of Quadriceps Muscle Is Bilaterally Impaired in Subacute Stroke. J. Appl. Physiol. 2011, 111, 1290–1295. [Google Scholar] [CrossRef]
- Nakamura, M.; Suzuki, Y.; Yoshida, R.; Kasahara, K.; Murakami, Y.; Hirono, T.; Nishishita, S.; Takeuchi, K.; Konrad, A. The Time-Course Changes in Knee Flexion Range of Motion, Muscle Strength, and Rate of Force Development after Static Stretching. Front. Physiol. 2022, 13, 917661. [Google Scholar] [CrossRef]
- Kato, E.; Vieillevoye, S.; Balestra, C.; Guissard, N.; Duchateau, J. Acute Effect of Muscle Stretching on the Steadiness of Sustained Submaximal Contractions of the Plantar Flexor Muscles. J. Appl. Physiol. 2011, 110, 407–415. [Google Scholar] [CrossRef]
- Ebisu, S.; Kasahara, S.; Saito, H.; Ishida, T. Decrease in Force Control among Older Adults under Unpredictable Conditions. Exp. Gerontol. 2022, 158, 111649. [Google Scholar] [CrossRef] [PubMed]
- Perraton, L.; Clark, R.; Crossley, K.; Pua, Y.-H.; Whitehead, T.; Morris, H.; Telianidis, S.; Bryant, A. Impaired Voluntary Quadriceps Force Control Following Anterior Cruciate Ligament Reconstruction: Relationship with Knee Function. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Panidi, I.; Kotsala, E.; Bogdanis, G.C.; Gaspari, V.; Spiliopoulou, P.; Terzis, G.; Donti, O. Muscle Architecture of Gastrocnemius Medialis and Rate of Force Development during Different Stretching Protocols. Proceedings 2019, 25, 22. [Google Scholar] [CrossRef]
- Morais de Oliveira, A.L.; Greco, C.C.; Molina, R.; Denadai, B.S. The Rate of Force Development Obtained at Early Contraction Phase Is Not Influenced by Active Static Stretching. J. Strength Cond. Res. 2012, 26, 2174–2179. [Google Scholar] [CrossRef]
- Eiling, E.; Bryant, A.L.; Petersen, W.; Murphy, A.; Hohmann, E. Effects of Menstrual-Cycle Hormone Fluctuations on Musculotendinous Stiffness and Knee Joint Laxity. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 126–132. [Google Scholar] [CrossRef]
- Yim, J.; Petrofsky, J.; Lee, H. Correlation between Mechanical Properties of the Ankle Muscles and Postural Sway during the Menstrual Cycle. Tohoku J. Exp. Med. 2018, 244, 201–207. [Google Scholar] [CrossRef]
- Manoel, M.E.; Harris-Love, M.O.; Danoff, J.V.; Miller, T.A. Acute Effects of Static, Dynamic, and Proprioceptive Neuromuscular Facilitation Stretching on Muscle Power in Women. J. Strength Cond. Res. 2008, 22, 1528–1534. [Google Scholar] [CrossRef]
- Kasahara, S.; Saito, H. Effect of Loading Parameters on Motor Performance during a Dynamic Weight-Shift Task. Gait Posture 2015, 41, 100–105. [Google Scholar] [CrossRef]
- Mizuno, T. Changes in Joint Range of Motion and Muscle-Tendon Unit Stiffness after Varying Amounts of Dynamic Stretching. J. Sports Sci. 2017, 35, 2157–2163. [Google Scholar] [CrossRef]
- Mizuno, T.; Matsumoto, M.; Umemura, Y. Decrements in Stiffness Are Restored within 10 Min. Int. J. Sports Med. 2013, 34, 484–490. [Google Scholar] [CrossRef]
- Pedersen, A.V.; Lorås, H. When Is a Test Score Fair for the Individual Who Is Being Tested? Effects of Different Scoring Procedures across Multiple Attempts When Testing a Motor Skill Task. Front. Psychol. 2017, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Knol, H.; Huys, R.; Temprado, J.-J.; Sleimen-Malkoun, R. Performance, Complexity and Dynamics of Force Maintenance and Modulation in Young and Older Adults. PLoS ONE 2019, 14, e0225925. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Damasceno, M.V.; Duarte, M.; Pasqua, L.A.; Lima-Silva, A.E.; MacIntosh, B.R.; Bertuzzi, R. Static Stretching Alters Neuromuscular Function and Pacing Strategy, but Not Performance during a 3-Km Running Time-Trial. PLoS ONE 2014, 9, e99238. [Google Scholar] [CrossRef] [PubMed]
- Pekünlü, E.; Sağıroğlu, İ.; Kurt, C.; Özsu, İ. Acute Residual Effects of Short and Long Duration Static Stretching on Counter Movement Jump Performances in Well-Trained Female Combat Athletes. Eur. J. Phys. Educ. Sport Sci. 2016, 2. [Google Scholar] [CrossRef]
- Behm, D.G.; Blazevich, A.J.; Kay, A.D.; McHugh, M. Acute Effects of Muscle Stretching on Physical Performance, Range of Motion, and Injury Incidence in Healthy Active Individuals: A Systematic Review. Appl. Physiol. Nutr. Metab. 2016, 41, 1–11. [Google Scholar] [CrossRef]
- Avela, J.; Kyröläinen, H.; Komi, P.V. Altered Reflex Sensitivity after Repeated and Prolonged Passive Muscle Stretching. J. Appl. Physiol. 1999, 86, 1283–1291. [Google Scholar] [CrossRef]
- Marek, S.M.; Cramer, J.; Fincher, A.; Massey, L.; Dangelmaier, S.; Purkayastha, S.; Fitz, K.A.; Culbertson, J. Acute Effects of Static and Proprioceptive Neuromuscular Facilitation Stretching on Muscle Strength and Power Output. J. Athl. Train. 2005, 40, 94–103. [Google Scholar]
- Clark, L.; O’Leary, C.B.; Hong, J.; Lockard, M. The Acute Effects of Stretching on Presynaptic Inhibition and Peak Power. J. Sports Med. Phys. Fit. 2014, 54, 605–610. [Google Scholar]
- Ruan, M.; Zhang, Q.; Wu, X. Acute Effects of Static Stretching of Hamstring on Performance and Anterior Cruciate Ligament Injury Risk during Stop-Jump and Cutting Tasks in Female Athletes. J. Strength Cond. Res. 2017, 31, 1241–1250. [Google Scholar] [CrossRef]
- Patten, C.; Kamen, G. Adaptations in Motor Unit Discharge Activity with Force Control Training in Young and Older Human Adults. Eur. J. Appl. Physiol. 2000, 83, 128–143. [Google Scholar] [CrossRef]
| Outcomes | Condition | Pre | Post | p-Value | Cohen’s d |
|---|---|---|---|---|---|
| Maximal angle of ankle dorsiflexion (°) | SS | 26.4 ± 9.7 | 28.3 ± 10.4 | <0.001 | 1.57 |
| Control | 27.0 ± 9.8 | 27.4 ± 9.8 | >0.999 | 0.50 | |
| Musculotendinous stiffness (Nm/°) | SS | 1.30 ± 0.51 | 1.20 ± 0.47 | 0.034 | 0.78 |
| Control | 1.44 ± 0.56 | 1.42 ± 0.58 | >0.999 | 0.14 |
| Task | Force Phase | Condition | Pre | Post | Change (%) | 95%CI of Change (%) | p-Value | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Low range | Generation | SS | 1.40 ± 0.44 | 1.30 ± 0.33 | −3.12 | −16.04 to 9.81 | >0.999 | 0.32 |
| Control | 1.39 ± 0.36 | 1.19 ± 0.20 | −11.00 | −21.55 to −0.45 | 0.101 | 0.65 | ||
| Release | SS | 1.53 ± 0.48 | 1.44 ± 0.34 | −1.55 | −13.43 to 10.33 | >0.999 | 0.26 | |
| Control | 1.51 ± 0.59 | 1.40 ± 0.39 | −3.37 | −13.23 to 6.49 | >0.999 | 0.28 | ||
| High range | Generation | SS | 1.82 ± 0.57 | 1.92 ± 0.71 | +5.65 | −6.85 to 18.16 | >0.999 | 0.21 |
| Control | 1.77 ± 0.39 | 1.69 ± 0.36 | −2.07 | −12.09 to 7.95 | >0.999 | 0.24 | ||
| Release | SS | 1.82 ± 0.72 | 1.47 ± 0.42 | −14.52 | −26.33 to −2.79 | 0.030 | 0.79 | |
| Control | 1.54 ± 0.38 | 1.37 ± 0.45 | −10.38 | −21.80 to 1.05 | 0.446 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sentoku, F.; Koshino, Y.; Sato, M.; Ishida, T.; Kasahara, S.; Tohyama, H.; Samukawa, M. Acute Effects of Static Stretching on Submaximal Force Control of the Ankle. Appl. Sci. 2025, 15, 7294. https://doi.org/10.3390/app15137294
Sentoku F, Koshino Y, Sato M, Ishida T, Kasahara S, Tohyama H, Samukawa M. Acute Effects of Static Stretching on Submaximal Force Control of the Ankle. Applied Sciences. 2025; 15(13):7294. https://doi.org/10.3390/app15137294
Chicago/Turabian StyleSentoku, Fuma, Yuta Koshino, Masahiro Sato, Tomoya Ishida, Satoshi Kasahara, Harukazu Tohyama, and Mina Samukawa. 2025. "Acute Effects of Static Stretching on Submaximal Force Control of the Ankle" Applied Sciences 15, no. 13: 7294. https://doi.org/10.3390/app15137294
APA StyleSentoku, F., Koshino, Y., Sato, M., Ishida, T., Kasahara, S., Tohyama, H., & Samukawa, M. (2025). Acute Effects of Static Stretching on Submaximal Force Control of the Ankle. Applied Sciences, 15(13), 7294. https://doi.org/10.3390/app15137294






