The Impact of A1- and A2 β-Casein on Health Outcomes: A Comprehensive Review of Evidence from Human Studies
Abstract
Featured Application
Abstract
1. Introduction
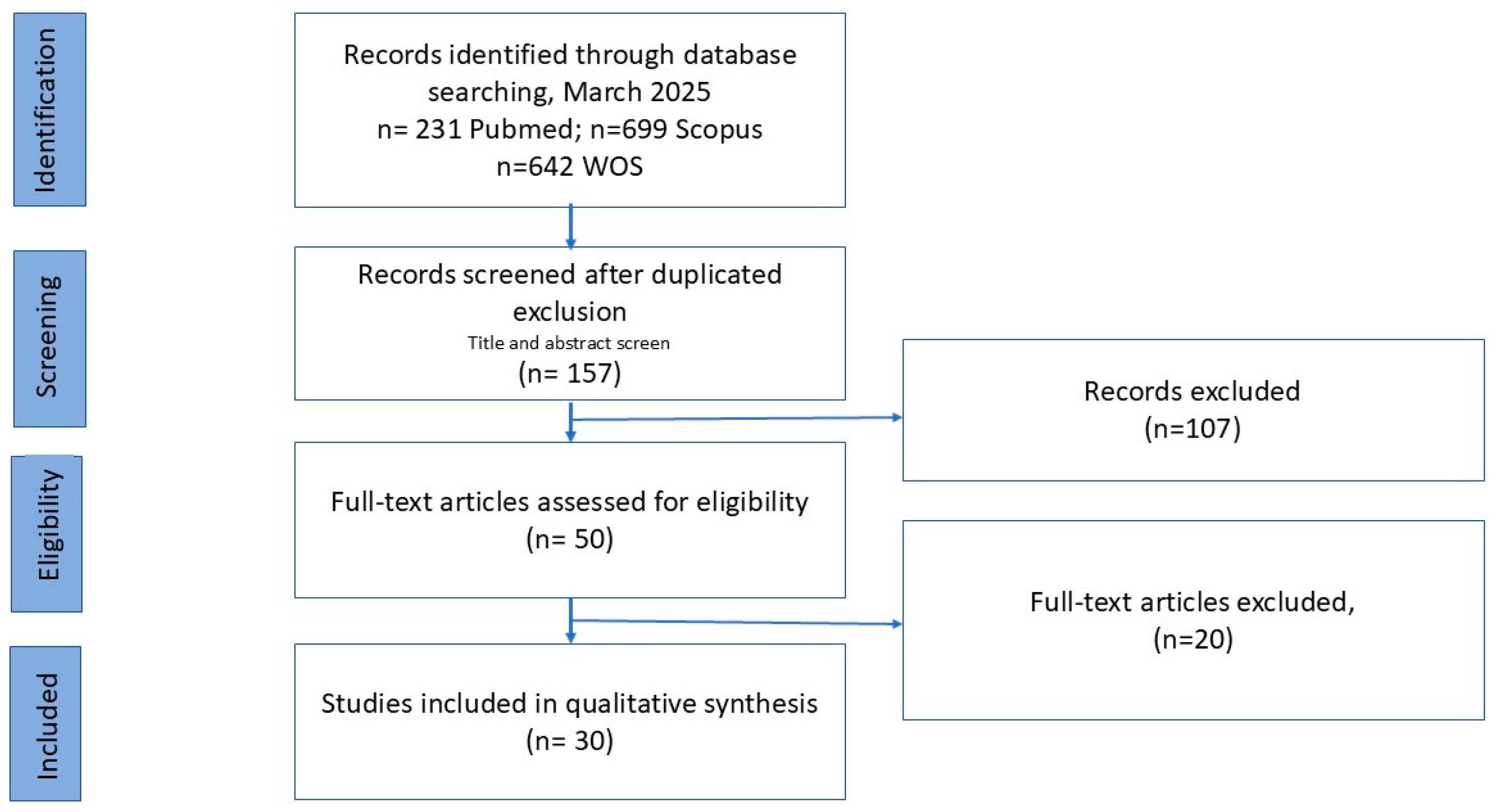
2. Methodology
Search Strategy and Inclusion Criteria
- The core question that guided this review was
- What are the health implications for humans of consuming milk with the A1 β-casein protein variant compared to consuming milk that contains only the A2 β-casein variant?
- Secondary questions:
- How do βCM-7 and the microbiota interact in gut health and disease regulation?
- How does individual susceptibility to βCM-7 exposure influence the observed effects of A1 β-casein consumption?
- How does the consumption of A1 β-casein versus A2 influence digestive health in different populations?
- Is there an association between A1 β-casein consumption and an increased risk of inflammation or cardiovascular disease?
- How does A1 β-casein affect muscle performance and recovery in athletes?
- How does A1 β-casein influence immune function?
- What is the impact of A1 β-casein on neurological and cognitive health?
- Types of β-casein (A1 β-casein, A2 β-casein, β-casein variants, milk proteins);
- βCM-7 (βCM-7, BCM-7, β-casomorphin-7, β-casomorphin, opioid peptides);
- Human studies (clinical trials, intervention studies, observational studies, cohort studies, case-control studies);
- Microbiota perturbations and βCM-7 (gut–brain axis, intestinal barrier, gut microbiome, gut microbiota);
- Human health outcomes (chronic diseases, gastrointestinal health, cardiovascular health, diabetes, mental health, autism, autoimmune diseases, muscle performance and recovery);
- Population (children, middle-aged adults, athletes).
3. Results
3.1. Synthesis
| Reference | Study Design | Population | Methodology | Outcome | Results | Main Finding |
|---|---|---|---|---|---|---|
| (McLachlan, 2001) [48] | Epidemiological | WHO MONICA PROJECT. Ecological data (1985–1990), 17 countries | Correlation between β-casein A1 and IHD mortality rates | IHD mortality rates | Strong correlation (r2 = 0.86) | A1 β-casein may be a dietary risk factor in IHD |
| (Boland MJ et al., 2002) [27] | Correlational epidemiological | Twenty “healthcare-affluent” countries | Estimated A1/A2 intake and mental disorder mortality | Mental disorder | Positive correlation with A1 intake | A1 β-casein may be linked to mental disorder mortality |
| (Crawford et al., 2002) [27] | Epidemiological | 67 autistic children | Milk with histidine or proline β-casein; urine BCM levels | βCM-7 in urine | Higher βCM-7 (urine from autistic children) in histidine group | Histidine variant may contribute to neurological disorders in susceptible individuals |
| (Laugesen & Elliott, 2003) [49] | Epidemiological | Data from: 20 countries IHD mortality; 51 countries DM-1 | Correlation between per capita A1 β-casein and incidence IHD and DM-1 | IHD and DM-1 rates | Strong correlations | Further studies needed to confirm A1′s role in CHD and DM-1 |
| (Chin-Dusting et al., 2006) [26] | RCT | 15 high-CVD-risk adults | 12-week A1/A2 intake; vascular function markers | CVD risk biomarkers | No significant differences | No cardiovascular disadvantage with A1 |
| (Venn et al., 2006) [31] | Crossover trial | 62 adults | 4.5-week A1/A2 vs. A2 dairy consumption | Plasma lipid profile | No significant differences | A1/A2 do not impact cholesterol |
| (Kost et al., 2009) [46] | Longitudinal observational | 90 infants | Plasma βCM-7, psychomotor evaluation | βCM-7 levels and development | Elevated βCM-7 in formula-fed with delays | A1 milk may affect development; breastfeeding beneficial |
| (Crowley et al., 2013) [25] | RCT crossover | 52 children | Soy vs. cow’s milk, then A1 vs. A2 | Constipation resolution | Soy milk resolved CFC; no diff. A1/A2 | Cow’s milk (not casein type) linked to CFC |
| (Ho et al., 2014) [28] | RCT crossover | 41 adults | 2 weeks A1 then A2 milk | Bowel habits, pain | Higher stool consistency and pain with A1 | A1 linked to GI symptoms |
| (Sokolov et al., 2014) [47] | Case-control | 20 children aged 4–8 years: 10 healthy controls, 10 ASD | βCM-7 in urine, autism severity | βCM-7 vs. CARS | Higher βCM-7 in autistic children | βCM-7 may play role in ASD |
| (Jianqin et al., 2015) [33] | RCT crossover | 45 adults | A1/A2 vs. A2 milk | GI symptoms, inflammation, cognition | A1 increased symptoms and inflammation | A2 milk improved GI and cognitive outcomes |
| (Deth et al., 2015) [39] | RCT crossover | 45 adults | A1/A2 vs. A2 milk | Glutathione, βCM-7 | A2 milk ↑GSH, ↓βCM-7 | A2 may boost antioxidant capacity |
| (He et al., 2017) [32] | Multicenter RCT | 600 adults | A1/A2 vs. A2 milk | GI symptoms | A2 milk reduced GI symptoms | A2 milk better for lactose intolerance |
| (Kirk et al., 2017) [44] | RCT placebo-controlled | 21 athletes | Post-exercise milk intake | Muscle recovery | A2 and regular milk > placebo | A2 helps in recovery for A1-intolerant |
| (Clarke AJ & Yelland GY, 2017) [53] | RCT crossover | 40 adults + 30 preschool children | A1/A2 vs. A2 milk | Cognitive tests (DSST) | A2 improved processing speed | A2 enhances cognition and comfort |
| (Jarmołowska et al., 2019) [22] | Case-control | 137 children | βCM-7, DPPIV assays | Serum/urine levels | ↑βCM-7 and DPPIV in ASD | Dairy peptides may relate to ASD |
| (Sheng et al., 2019) [34] | RCT crossover | 75 preschoolers | 5 days A1/A2 vs. A2 milk | GI symptoms, cognition | ↓Symptoms, inflammation with A2 | A2 improves comfort and cognition |
| (Milan et al., 2020) [29] | RCT | 40 women | Milk challenge by type | Digestive symptoms | A2 ↓symptoms in lactose-intolerant | A2 may benefit LI but not NLDI |
| (Ramakrishnan et al., 2020) [43] | RCT crossover | 33 adults | Single meal A1/A2 vs. A2 | GI symptoms, H2 breath | Less pain with A2 | A2 reduces symptoms in LI/maldigestion |
| (Ramakrishnan et al., 2020) [40] | RCT | 21 adults | A1A2/Jersey/A2/Lactose free milk | GI symptoms, H2 breath | Less H2 with A2 and Jersey | A2 reduces symptoms in LI/maldigestion |
| (Lijun C et al., 2021) [52] | Cohort | 60 adults | A1/A2 vs. A2 milk | GI symptoms, microbiota | A2 ↑Bifidobacterium | A2 improves gut health |
| (Prodhan et al., 2022) [30] | RCT crossover | 40 women | 3 milk types, plasma AAs | Protein digestion | No differences overall | Digestion not affected by intolerance |
| (Ramakrishnan et al., 2023) [41,50] | RCT crossover | 10 LI adults | MRI gastric emptying | Gastric emptying time | A1 milk emptied faster | A1 leads to more symptoms |
| (Meng et al., 2023) [37] | RCT | 387 toddlers | GUM A2 vs. conventional | GI symptoms, constipation | Less constipation in A2 | A2 well tolerated in toddlers |
| (Choi et al., 2024) [38] | RCT crossover | Adults (n/s) | A2 vs. A1/A2 milk | GI symptoms, calprotectin | Less discomfort with A2 | A2 milk better tolerated |
| (Ramakrishnan et al., 2024) [50] | RCT crossover | 16 LI adults | 2 weeks A2 vs. A1/A2 | Symptoms, biomarkers | ↓Bloating with A2 | A2 improves symptoms over time |
| (Saiprasad et al., 2024) [51] | RCT crossover | 16 LI adults | 16 LI adults | GI symptoms, H2 breath, inflammatory markers and GSH levels | ↓acute fecal urgencyH2 breath | A1/A2 increases digestive discomfort in LI |
| (Sheng et al., 2024) [36] | RCT | 180 infants | A2 formula vs. standard | Growth, stool, bone | Better outcomes with A2 | A2 formula improves infant health |
| (Novika et al., 2025) [45] | Quasi-experimental | 30 children | 3-month A2 milk | Growth, inflammation | ↑Growth, ↓TNF-α and cortisol | A2 supports growth in stunted kids |
| (Yu et al., 2025) [35] | Open-label RCT | 200 toddlers | A1PF vs. conventional formula | ARI, diarrhea, tolerance | ↓ARI duration, better GI tolerance | A1-free formula may reduce illness duration |
3.2. How Do βCM-7 and the Microbiota Interact in Gut Health and Disease Regulation?
3.3. How Does Individual Susceptibility to βCM-7 Exposure Influence the Observed Effects of A1 β-Casein Consumption?
3.4. How Does the Consumption of A1 β-Casein Versus A2 Influence Digestive Health in Different Populations?
3.5. Is There an Association Between A1 β-Casein Consumption and an Increased Risk of Cardiovascular Disease?
3.6. How Does A1 β-Casein Affect Muscle Performance and Recovery in Athletes and Individuals with High Protein Intakes?
3.7. How Does A1 β-Casein Influence Immune Function?
3.8. What Is the Impact of A1 β-Casein on Neurological and Cognitive Health?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.; Niero, G.; Visentin, G.; Penasa, M.; Cassandro, M.; De Marchi, M. Variation of Detailed Protein Composition of Cow Milk Predicted from a Large Database of Mid-Infrared Spectra. Animals 2019, 9, 176. [Google Scholar] [CrossRef]
- Brooke-Taylor, S.; Dwyer, K.; Woodford, K.; Kost, N. Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 β-Casein. Adv. Nutr. 2017, 8, 739–748. [Google Scholar] [CrossRef]
- Kamiński, S.; Cieślińska, A.; Kostyra, E. Polymorphism of Bovine B-Casein and Its Potential Effect on Human Health. J. Appl. Genet. 2007, 48, 189–198. [Google Scholar] [CrossRef]
- Li, X.; Spencer, G.W.K.; Ong, L.; Gras, S.L. β Casein Proteins—A Comparison between Caprine and Bovine Milk. Trends Food Sci. Technol. 2022, 121, 30–43. [Google Scholar] [CrossRef]
- Cattaneo, S.; Stuknytė, M.; Masotti, F.; De Noni, I. Protein Breakdown and Release of β-Casomorphins during In Vitro Gastro-Intestinal Digestion of Sterilised Model Systems of Liquid Infant Formula. Food Chem. 2017, 217, 476–482. [Google Scholar] [CrossRef]
- Muehlenkamp, M.R.; Warthesen, J.J. β-Casomorphins: Analysis in Cheese and Susceptibility to Proteolytic Enzymes from Lactococcus lactis ssp. Cremoris. J. Dairy Sci. 1996, 79, 20–26. [Google Scholar] [CrossRef]
- Zinßius, L.; Keuter, L.; Krischek, C.; Jessberger, N.; Cramer, B.; Plötz, M. Influence of the β-Casein Genotype of Cow’s Milk (A1, A2) on the Quality and β-Casomorphin-7 (BCM-7) Content of a Semi-Hard Cheese During Production. Foods 2025, 14, 463. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Busetti, F.; Smolenski, G.; Johnson, S.K.; Solah, V.A. Release of Beta-Casomorphins during In-Vitro Gastrointestinal Digestion of Reconstituted Milk after Heat Treatment. LWT 2021, 136, 110312. [Google Scholar] [CrossRef]
- De Noni, I.; Fitzgerald, R.J.; Korhonen, H.J.T.; Le Roux, Y.; Chris, T.; Thorsdottir, I.; Tomé, D.; Witkamp, R. Review of the Potential Health Impact of β-Casomorphins. EFSA Sci. Rep. 2009, 231, 1–107. [Google Scholar]
- Teschemacher, H.; Koch, G.; Brantl, V. Milk Protein-derived Opioid Receptor Ligands. Pept. Sci. 1997, 43, 99–117. [Google Scholar] [CrossRef]
- Henschen, A.; Lottspeich, F.; Brantl, V.; Teschemacher, H. Novel Opioid Peptides Derived from Casein (β-Casomorphins). II. Structure of Active Components from Bovine Casein Peptone. Hoppe-Seyler’s Z. Physiol. Chem. 1979, 360, 1217–1224. [Google Scholar]
- Kadam, B.R.; Ambadkar, R.K.; Rathod, K.S.; Pandiyan, C. A1/A2 Milk and Human Health: A Brief Review. J. Environ. Bio-Sci. 2017, 31, 357–362. [Google Scholar]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Sodhi, M.; Upadhyaya, K.; Semwal, D.K. Effects of A1 and A2 Variants of β-Casein on Human Health—Is β-Casomorphin-7 Really a Harmful Peptide in Cow Milk? Nutrire 2022, 47, 8. [Google Scholar] [CrossRef]
- Kuellenberg de Gaudry, D.; Lohner, S.; Bischoff, K.; Schmucker, C.; Hoerrlein, S.; Roeger, C.; Schwingshackl, L.; Meerpohl, J.J. A1- and A2 β-Casein on Health-Related Outcomes: A Scoping Review of Animal Studies. Eur. J. Nutr. 2022, 61, 1–21. [Google Scholar] [CrossRef]
- Haq, M.R.U.; Kapila, R.; Sharma, R.; Saliganti, V.; Kapila, S. Comparative Evaluation of Cow β-Casein Variants (A1/A2) Consumption on Th2-Mediated Inflammatory Response in Mouse Gut. Eur. J. Nutr. 2014, 53, 1039–1049. [Google Scholar] [CrossRef]
- Haq, M.R.U.; Kapila, R.; Saliganti, V. Consumption of β-Casomorphins-7/5 Induce Inflammatory Immune Response in Mice Gut through Th2 Pathway. J. Funct. Foods 2014, 8, 150–160. [Google Scholar] [CrossRef]
- Tailford, K.A.; Berry, C.L.; Thomas, A.C.; Campbell, J.H. A Casein Variant in Cow’s Milk Is Atherogenic. Atherosclerosis 2003, 170, 13–19. [Google Scholar] [CrossRef]
- Scott, F.W.; Rowsell, P.; Wang, G.S.; Burghardt, K.; Kolb, H.; Flohé, S. Oral Exposure to Diabetes-Promoting Food or Immunomodulators in Neonates Alters Gut Cytokines and Diabetes. Diabetes 2002, 51, 73–78. [Google Scholar] [CrossRef]
- Elliott, R.B.; Harris, D.P.; Hill, J.P.; Bibby, N.J.; Wasmuth, H.E. Type I (Insulin-Dependent) Diabetes Mellitus and Cow Milk: Casein Variant Consumption. Diabetologia 1999, 42, 292–296. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, N.D.S.; Gheware, A.; Kulshreshtha, A.; Sharma, P.; Singh, V.P. Oral Feeding of Cow Milk Containing A1 Variant of β Casein Induces Pulmonary Inflammation in Male Balb/c Mice. Sci. Rep. 2020, 10, 8053. [Google Scholar] [CrossRef] [PubMed]
- Jarmołowska, B.; Bukało, M.; Fiedorowicz, E.; Cieślińska, A.; Kordulewska, N.K.; Moszyńska, M.; Świątecki, A.; Kostyra, E. Role of Milk-Derived Opioid Peptides and Proline Dipeptidyl Peptidase-4 in Autism Spectrum Disorders. Nutrients 2019, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Crowley, E.T.; Williams, L.T.; Roberts, T.K.; Dunstan, R.H.; Jones, P.D. Does Milk Cause Constipation? A Crossover Dietary Trial. Nutrients 2013, 5, 253. [Google Scholar] [CrossRef]
- Chin-Dusting, J.; Shennan, J.; Jones, E.; Williams, C.; Kingwell, B.; Dart, A. Effect of Dietary Supplementation with βcasein A1 or A2 on Markers of Disease Development in Individuals at High Risk of Cardiovascular Disease. Br. J. Nutr. 2006, 95, 136–144. [Google Scholar] [CrossRef]
- Crawford, R.A.; Boland, M.J.; Norris, C.S.; Hill, J.P.; Fenwick, R.M. Milk Containing β-Casein with Proline at Position 67 Does Not Aggravate Neurological Disorders. WIPO Patent WO/2002/019832, 10 September 2002. [Google Scholar]
- Ho, S.; Woodford, K.; Kukuljan, S.; Pal, S. Comparative Effects of A1 versus A2 β-Casein on Gastrointestinal Measures: A Blinded Randomised Cross-over Pilot Study. Eur. J. Clin. Nutr. 2014, 68, 994–1000. [Google Scholar] [CrossRef]
- Milan, A.M.; Shrestha, A.; Karlström, H.J.; Martinsson, J.A.; Nilsson, N.J.; Perry, J.K.; Day, L.; Barnett, M.P.G.; Cameron-Smith, D. Comparison of the Impact of Bovine Milk β-Casein Variants on Digestive Comfort in Females Self-Reporting Dairy Intolerance: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 111, 149–160. [Google Scholar] [CrossRef]
- Prodhan, U.K.; Milan, A.M.; Shrestha, A.; Vickers, M.H.; Cameron-Smith, D.; Barnett, M.P.G. Circulatory Amino Acid Responses to Milk Consumption in Dairy and Lactose Intolerant Individuals. Eur. J. Clin. Nutr. 2022, 76, 1415–1422. [Google Scholar] [CrossRef]
- Venn, B.J.; Skeaff, C.M.; Brown, R.; Mann, J.I.; Green, T.J. A Comparison of the Effects of A1 and A2 β-Casein Protein Variants on Blood Cholesterol Concentrations in New Zealand Adults. Atherosclerosis 2006, 188, 175–178. [Google Scholar] [CrossRef]
- He, M.; Sun, J.; Jiang, Z.Q.; Yang, Y.X. Effects of Cow’s Milk β-Casein Variants on Symptoms of Milk Intolerance in Chinese Adults: A Multicentre, Randomised Controlled Study. Nutr. J. 2017, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of Milk Containing Only A2 β Casein versus Milk Containing Both A1 and A2 β Casein Proteins on Gastrointestinal Physiology, Symptoms of Discomfort, and Cognitive Behavior of People with Self-Reported Intolerance to Traditional Cows’ Milk. Nutr. J. 2015, 15, 35, Erratum in Nutr. J. 2016, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Li, Z.; Ni, J.; Yelland, G. Effects of Conventional Milk Versus Milk Containing Only A2 β-Casein on Digestion in Chinese Children: A Randomized Study. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 375–382. [Google Scholar] [CrossRef]
- Yu, W.; Wang, W.; Sheng, X. Effect of A1 Protein-free Formula versus Conventional Formula on Acute Respiratory Infections and Diarrhea in Toddlers: An RCT. J. Pediatr. Gastroenterol. Nutr. 2025. [Google Scholar] [CrossRef]
- Sheng, X.-Y.; Mi, W.; Yuan, Q.B.; Liu, B.Y.; Carnielli, V.; Ning, Y.B.; Einerhand, A.W.C. An A2 β-Casein Infant Formula with High Sn-2 Palmitate and Casein Phosphopeptides Supports Adequate Growth, Improved Stool Consistency, and Bone Strength in Healthy, Term Chinese Infants: A Randomized, Double-Blind, Controlled Clinical Trial. Front. Nutr. 2024, 11, 1442584. [Google Scholar] [CrossRef]
- Meng, Y.; Zhou, Y.; Li, H.; Chen, Y.; Dominik, G.; Dong, J.; Tang, Y.; Saavedra, J.M.; Liu, J. Effectiveness of Growing-Up Milk Containing Only A2 β-Casein on Digestive Comfort in Toddlers: A Randomized Controlled Trial in China. Nutrients 2023, 15, 1313. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, N.; Song, C.-H.; Kim, S.; Lee, D.H. The Effect of A2 Milk on Gastrointestinal Symptoms in Comparison to A1/A2 Milk: A Single-Center, Randomized, Double-Blind, Cross-over Study. J. Cancer Prev. 2024, 29, 45. [Google Scholar] [CrossRef]
- Deth, R.; Clarke, A.; Ni, J.; Trivedi, M. Clinical Evaluation of Glutathione Concentrations after Consumption of Milk Containing Different Subtypes of β-Casein: Results from a Randomized, Cross-over Clinical Trial. Nutr. J. 2015, 15, 82. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Eaton, T.; Sermet, O.; Savaiano, D. A Single Meal of Milk Containing A2 β-Casein Causes Fewer Symptoms and Lower Gas Production than Milk Containing Both A1 and A2 β-Casein Among Lactose Intolerant Individuals. Curr. Dev. Nutr. 2020, 4, nzaa052_041. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Zhou, X.; Dydak, U.; Savaiano, D.A. Gastric Emptying of New-World Milk Containing A1 and A2 β-Casein Is More Rapid as Compared to Milk Containing Only A2 β-Casein in Lactose Maldigesters: A Randomized, Cross-Over Trial Using Magnetic Resonance Imaging. Nutrients 2023, 15, 801. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Dydak, U.; Eaton, T.; Savaiano, D.; Zhou, X. A1 β-Casein Milk Transits the Stomach More Quickly Than A2 β-Casein Milk in Lactose Maldigesters Using Magnetic Resonance Imaging. Curr. Dev. Nutr. 2022, 6, 329. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Eaton, T.K.; Sermet, O.M.; Savaiano, D.A. Milk Containing A2 β-Casein Only, as a Single Meal, Causes Fewer Symptoms of Lactose Intolerance than Milk Containing A1 and A2 β-Caseins in Subjects with Lactose Maldigestion and Intolerance: A Randomized, Double-Blind, Crossover Trial. Nutrients 2020, 12, 3855. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Mitchell, J.; Jackson, M.; Amirabdollahian, F.; Alizadehkhaiyat, O.; Clifford, T. A2 Milk Enhances Dynamic Muscle Function Following Repeated Sprint Exercise, a Possible Ergogenic Aid for A1-Protein Intolerant Athletes? Nutrients 2017, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Novika, R.G.H.; Sari, A.N.; Nurhidayati, S.; Maulina, R.; Maulida, L.F.; Wahidah, N.J.; Ilyas, M.F.; Sumarno, L.; Prastowo, S.; Jevitt, C.M. Effect of β-Casein A2 Cow Milk Supplementation on Physical Growth, Inflammation, Growth, and Metabolism Hormonal Profiles in Stunted Children. J. Korean Soc. Pediatr. Endocrinol. 2025. [Google Scholar]
- Kost, N.V.; Sokolov, O.Y.; Kurasova, O.B.; Dmitriev, A.D.; Tarakanova, J.N.; Gabaeva, M.V.; Zolotarev, Y.A.; Dadayan, A.K.; Grachev, S.A.; Korneeva, E. V β-Casomorphins-7 in Infants on Different Type of Feeding and Different Levels of Psychomotor Development. Peptides 2009, 30, 1854–1860. [Google Scholar] [CrossRef]
- Sokolov, O.; Kost, N.; Andreeva, O.; Korneeva, E.; Meshavkin, V.; Tarakanova, Y.; Dadayan, A.; Zolotarev, Y.; Grachev, S.; Mikheeva, I. Autistic Children Display Elevated Urine Levels of Bovine Casomorphin-7 Immunoreactivity. Peptides 2014, 56, 68–71. [Google Scholar] [CrossRef]
- McLachlan, C.N.S. β-Casein A1, Ischaemic Heart Disease Mortality, and Other Illnesses. Med. Hypotheses 2001, 56, 262–272. [Google Scholar] [CrossRef]
- Laugesen, M.; Elliott, R. Ischaemic Heart Disease, Type 1 Diabetes, and Cow Milk A1 β-Casein. N. Z. Med. J. 2003, 116, U295. [Google Scholar]
- Ramakrishnan, M.; Mysore Saiprasad, S.; Savaiano, D.A. Prolonged Consumption of A2 β-Casein Milk Reduces Symptoms Compared to A1 and A2 β-Casein Milk in Lactose Maldigesters: A Two-Week Adaptation Study. Nutrients 2024, 16, 1963. [Google Scholar] [CrossRef]
- Saiprasad, S.M.; Ramakrishnan, M.; Savaiano, D.A. Lactose Maldigesters Do Not Adapt to a Two-Week Daily Consumption of Milk with A1/A2 β-Casein As Determined by Continued Bloating and Flatulence. Curr. Dev. Nutr. 2024, 8, 102363. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Zhao, J.; Jiang, T.; Zhou, W.; Li, J.; Liu, Y.; Qiao, W. Application of β-Casein A2 and Composition Thereof in Promoting Proliferation of Bifidobacterium. Global Patent WO2021003741A1, 8 June 2022. [Google Scholar]
- Clarke, A.J.; Yelland, G.Y. β-Caseins and Cognitive Function. 2017. Available online: https://patents.google.com/patent/WO2017171563A1/en (accessed on 2 February 2025).
- Sun, J.; Robinson, S.R.; Sheng, X. Comparative Effects of A1 β-Casein and A2 β-Casein versus on the Gut-Brain Axis. Chin. J. Clin. Nutr. 2020, 28. [Google Scholar]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Zhang, L.; Tuoliken, H.; Li, J.; Gao, H. Diet, Gut Microbiota, and Health: A Review. Food Sci. Biotechnol. 2024, 34, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- DiCello, J.J.; Carbone, S.E.; Saito, A.; Rajasekhar, P.; Ceredig, R.A.; Pham, V.; Valant, C.; Christopoulos, A.; Veldhuis, N.A.; Canals, M. Mu and Delta Opioid Receptors Are Coexpressed and Functionally Interact in the Enteric Nervous System of the Mouse Colon. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 465–483. [Google Scholar] [CrossRef]
- Liu, Z.; Udenigwe, C.C. Role of Food-derived Opioid Peptides in the Central Nervous and Gastrointestinal Systems. J. Food Biochem. 2019, 43, e12629. [Google Scholar] [CrossRef]
- Summer, A.; Di Frangia, F.; Ajmone Marsan, P.; De Noni, I.; Malacarne, M. Occurrence, Biological Properties and Potential Effects on Human Health of β-Casomorphin 7: Current Knowledge and Concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3705–3723. [Google Scholar] [CrossRef]
- Barnett, M.P.G.; McNabb, W.C.; Roy, N.C.; Woodford, K.B.; Clarke, A.J. Dietary A1 β-Casein Affects Gastrointestinal Transit Time, Dipeptidyl Peptidase-4 Activity, and Inflammatory Status Relative to A2 β-Casein in Wistar Rats. Int. J. Food Sci. Nutr. 2014, 65, 720–727. [Google Scholar] [CrossRef]
- Lambeir, A.-M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-Peptidase IV from Bench to Bedside: An Update on Structural Properties, Functions, and Clinical Aspects of the Enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef]
- Trivedi, M.; Shah, J.; Hodgson, N.; Byun, H.-M.; Deth, R. Morphine Induces Redox-Based Changes in Global DNA Methylation and Retrotransposon Transcription by Inhibition of Excitatory Amino Acid Transporter Type 3-Mediated Cysteine Uptake. Mol. Pharmacol. 2014, 85, 747–757. [Google Scholar] [CrossRef]
- Hockey, M.; Aslam, H.; Berk, M.; Pasco, J.A.; Ruusunen, A.; Mohebbi, M.; Macpherson, H.; Chatterton, M.L.; Marx, W.; O’Neil, A.; et al. The Moo’D Study: Protocol for a Randomised Controlled Trial of A2 β-Casein Only versus Conventional Dairy Products in Women with Low Mood. Trials 2021, 22, 899. [Google Scholar] [CrossRef]
- Majta, J.; Odrzywolek, K.; Milanovic, B.; Hubar, V.; Wrobel, S.; Strycharz-Angrecka, E.; Wojciechowski, S.; Milanowska, K. Identification of Differentiating Metabolic Pathways between Infant Gut Microbiome Populations Reveals Depletion of Function-Level Adaptation to Human Milk in the Finnish Population. mSphere 2019, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Katsahian, S.; Fassot, C.; Tropeano, A.I.; Gautier, I.; Laloux, B.; Boutouyrie, P. Aortic Stiffness Is an Independent Predictor of Fatal Stroke in Essential Hypertension. Stroke 2003, 34, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.J.; McRae, J.L.; Enjapoori, A.K.; Lefèvre, C.M.; Kukuljan, S.; Dwyer, K.M. Dietary Cows’ Milk Protein A1 β-Casein Increases the Incidence of T1D in NOD Mice. Nutrients 2018, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Rosenwasser, L. The Allergic Asthma Phenotype. J. Allergy Clin. Immunol. Pract. 2014, 2, 645–648. [Google Scholar] [CrossRef]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Kurek, M.; Ruëff, F.; Czerwionka-Szaflarska, M.; Doroszewska, G.; Przybilla, B. Exorphins Derived from Cow’s Milk Casein Elicit Pseudo-Allergic Wheal-and-Flare Reactions in Healthy Children. Rev. Fr. Allergol. Immunol. Clin. 1996, 36, 191–196. [Google Scholar] [CrossRef]
- Cade, R.; Privette, M.; Fregly, M.; Rowland, N.; Sun, Z.; Zele, V.; Wagemaker, H.; Edelstein, C. Autism and Schizophrenia: Intestinal Disorders. Nutr. Neurosci. 2000, 3, 57–72. [Google Scholar] [CrossRef]
- Reichelt, K.L.; Ekrem, J.; Scott, H. Gluten, Milk Proteins and Autism: Dietary Intervention Effects on Behavior and Peptide Secretion. J. Appl. Nutr. 1990, 42, 1–11. [Google Scholar]
- Lindstrom, L.H.; Nyberg, F.; Terenius, L. CSF and Plasma β-Casomorphin-like Opioid Peptides in Postpartum Psychosis. Am. J. Psychiatry 1984, 141, 1059–1066. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M.; Geffard, M.; Bosmans, E. In Depression, Bacterial Translocation May Drive Inflammatory Responses, Oxidative and Nitrosative Stress (O&NS), and Autoimmune Responses Directed against O&NS-Damaged Neoepitopes. Acta Psychiatr. Scand. 2013, 127, 344–354. [Google Scholar] [CrossRef]
- Aslam, H.; Ruusunen, A.; Berk, M.; Loughman, A.; Rivera, L.; Pasco, J.A.; Jacka, F.N. Unravelled Facets of Milk Derived Opioid Peptides: A Focus on Gut Physiology, Fractures and Obesity. Int. J. Food Sci. Nutr. 2020, 71, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.C. The Gut-Brain Barrier in Major Depression: Intestinal Mucosal Dysfunction with an Increased Translocation of LPS from Gram Negative Enterobacteria (Leaky Gut) Plays a Role in the Inflammatory Pathophysiology of Depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, N.; Vázquez-Liz, N.; Rodríguez-Sampedro, A.; Regal, P.; Fente, C.; Lamas, A. The Impact of A1- and A2 β-Casein on Health Outcomes: A Comprehensive Review of Evidence from Human Studies. Appl. Sci. 2025, 15, 7278. https://doi.org/10.3390/app15137278
González-Rodríguez N, Vázquez-Liz N, Rodríguez-Sampedro A, Regal P, Fente C, Lamas A. The Impact of A1- and A2 β-Casein on Health Outcomes: A Comprehensive Review of Evidence from Human Studies. Applied Sciences. 2025; 15(13):7278. https://doi.org/10.3390/app15137278
Chicago/Turabian StyleGonzález-Rodríguez, Nerea, Natalia Vázquez-Liz, Ana Rodríguez-Sampedro, Patricia Regal, Cristina Fente, and Alexandre Lamas. 2025. "The Impact of A1- and A2 β-Casein on Health Outcomes: A Comprehensive Review of Evidence from Human Studies" Applied Sciences 15, no. 13: 7278. https://doi.org/10.3390/app15137278
APA StyleGonzález-Rodríguez, N., Vázquez-Liz, N., Rodríguez-Sampedro, A., Regal, P., Fente, C., & Lamas, A. (2025). The Impact of A1- and A2 β-Casein on Health Outcomes: A Comprehensive Review of Evidence from Human Studies. Applied Sciences, 15(13), 7278. https://doi.org/10.3390/app15137278











