Abstract
As people age, more careful health monitoring becomes increasingly important. The article presents the development and implementation of an integrated system for monitoring the health of elderly individuals using Internet of Things (IoT) technology and a wearable bracelet to continuously collect vital data. The device integrates MAX30100 sensors for heart rate monitoring and MPU-6050 for step counting and sleep quality analysis (deep and superficial sleep). The collected data for average heart rate (AR), minimum (mR), maximum (MR), number of steps (S), deep sleep time (DST), and superficial sleep time (SST) is processed in real-time through a health anomaly detection algorithm (HADA), based on the dimensionality reduction method using PCA. The system is connected to the Azure cloud infrastructure, ensuring secure data transmission, preprocessing, and the automatic generation of alerts for prompt medical interventions. Studies conducted over two years demonstrated a sensitivity of 100% and an accuracy of 98.5%, with a tendency to generate additional alerts to avoid overlooking critical events. The results outline the importance of personalizing the analysis, adapting algorithms to individual characteristics, and the system’s potential to prevent medical complications and improve the quality of life for elderly individuals.
1. Introduction
Modern society faces an increase in mortality, and many nations are dealing with an aging population. This aspect puts pressure on the healthcare system due to the challenges posed by the need for medical assistance provided to the elderly. Emerging technologies in the field of smart healthcare contribute to improving patient monitoring and reducing the need for medical interventions [1,2].
1.1. Health Monitoring Using Sensor-Based Wearable Devices
The paper by Areia et al. [3] analyzes smart health monitoring devices. In the studies analyzed in this research, 4433 patients were evaluated, and some of the findings outline a series of benefits of the devices, including reduced mortality. However, the study analyzes a few samples, making it impossible to draw a definitive conclusion. There is a variety of devices that promise to monitor users’ health status. For example, Van Velthoven et al. [4] propose a device that measures blood pressure and heart rate but is not precise enough to measure respiration, temperature, and blood oxygen levels. The study by Leenen et al. [5] uses a sample of 21 patients monitored over 5 days in their homes after being discharged from the hospital. According to the results, 92% of the patients reported comfort in using the device. The data was collected to identify possible post-operative complications in real time but remotely (with the patient at home). An unstable internet connection resulted in temporary data loss in 5% of cases. The study notes that remote monitoring reduces unexpected hospital visits by approximately 15%, provided that well-established protocols are used. Similarly to this study, the research by Breteler et al. [6] includes 20 patients with a 7-day post-operative monitoring period. The paper combines patient monitoring with telephone consultations, and the results indicated a data loss rate of 25%. This value is high for a patient who has undergone surgery.
In recovery, 5153 patients were monitored using 31 wearable devices. The monitoring duration ranged between 15 h and 3 months. The studies mainly focused on measuring physical activity (71.8%). The research highlighted the early detection of postoperative complications compared to standard care [7]. In the study conducted by van Rossum et al. [8], 39 patients were monitored for a total of 3999 h, averaging 103 h per patient. The system proposed in this paper generated approximately 0.49 daily alarms per patient. Of these, only 42% were true positive alarms, making the number of false alarms an indicator of the need for algorithm improvement. Brekke et al. [9] synthesize 35 studies from the specialized literature, summarizing results related to wearable devices. These studies used retrospective data on vital signs collected with the help of these devices and demonstrated their importance in the evolutionary analysis of diagnoses. Multiple studies have analyzed the improvements brought about by wireless wearable devices. However, Leenen et al. [10] note the low quality of the materials. The paper concludes that wireless wearable devices for continuous monitoring are still in the clinical validation phase.
A special category of wearable devices is fitness. The study by Polat et al. [11] integrates a flexible photo-detector (PD) into bracelets. The proposed device’s operating principle employs a Light-Emitting Diode (LED) that illuminates the skin, and a photodetector measures light variations caused by blood vessels. In transmission mode, ambient light passes through the tissue and is detected by the PD. Another area of applicability includes safe driving [12], identifying SARS-CoV-2 infection [13], monitoring vital signs in children [14,15], or in low-resource contexts such as those in Burkina Faso and Kenya [16].
1.2. Health Monitoring Using AI-Based Wearable Devices
Modern wearable devices also analyze historical data. This analysis is carried out using artificial intelligence (AI) models [17]. Rathore and Rathore [18] and Khan [19] mention the following advantages of integrating AI models: early diagnosis, extraction of information from electronic health records, analysis of medical images, virtual assistant chatbots, predictive analyses for patient conditions, and personalized medicine [20]. Zaid Salah et al. [21] document the use of the Long short-term memory (LSTM) model for monitoring vital signs. The model achieved an accuracy of 96.78%. Lekkala [22] mentions a series of disadvantages of integrating AI models in the medical context. Among these disadvantages is the black-box approach of AI algorithms, which does not provide explicit data interpretations. Additionally, the author also notes advantages such as early arrhythmia diagnosis.
Devi et al. [23] focus on analyzing patients’ medical history and genetic profiles using AI methods, with the compromise of data confidentiality [24]. The study by Nam et al. [25] presents the level of patient satisfaction with using a conversational system for pain assessment in spinal conditions. According to the authors, the satisfaction score obtained was 5.5 ± 1.4 out of 7 points, indicating high satisfaction.
Addobea et al. [26] outline four major challenges of wearable devices in the context of big data analysis: computational cost, communication cost, storage cost, and energy consumption. For this purpose, smart bracelets include accelerometers, heart rate sensors, and a Global Positioning System (GPS) for data collection [27,28]. The Support Vector Machine (SVM) algorithm from the paper [27] achieved an accuracy of 94.47% with a training time of 45.7 s and a testing time of 0.0242 s. The research by Wang et al. [29] analyzed 45 elderly adults from three nursing care centers in Hong Kong who wore a telemedicine bracelet for three months. The results of this study are encouraging regarding the monitoring of elderly individuals. The cardiopulmonary monitoring system introduced by Zhao et al. [30] offers data analysis through Edge Computing. This approach reduces the burden of multiple cloud service accesses, leading to faster data transfer speeds [31].
Tang et al. [32] propose using data classification methods for monitoring fetal heart rate. In tests, the Multi-Kernel Network (MKNet) algorithm achieved the highest accuracy, 94.70%, with a training time of 19 s per epoch over a set of 70 epochs. The accuracy of the values provided by these sensors is analyzed by Bent et al. [33], who note a high degree of reliability at rest but a decrease when activity changes occur. Althobiani et al. [34] present the results of a survey in which 118 clinicians were questioned about their trust in wearable devices, with 80% rating them as useless. Conversely, 55.8% of patients, or 11,121 respondents, use such devices for cardiac conditions [35]. Dias and Paulo Silva Cunha [36] classify health monitoring devices into smartwatches, ear sensors (which measure oxygen saturation and heart rate), smart lenses, patches, QuardioCore, and smart shirts. These smart devices are generally connected to smartphones for detailed monitoring [37].
1.3. Health Monitoring Using IoT-Based Wearable Devices
In the specialized literature, several Internet of Things (IoT) configuration proposals for monitoring patients’ health status are available. Alsayaydeh et al. [38] propose a hardware configuration that includes temperature and heart rate sensors connected to the Arduino Uno microcontroller and ESP8266. Tests resulted in an accuracy of 96% in monitoring temperatures above 37.5 °C and a heart rate above 120 beats per minute (BPM). The work by Choudhary et al. [39] also employs DS18B sensors, the MAX30102 module, and an Arduino Nano to monitor oxygen saturation and electrocardiogram (ECG). The same configuration, which includes Arduino Nano for monitoring using DHT11 sensors for temperature, MAX30100 for heart rate and blood oxygen level, and a buzzer that sounds when the results are ready, is also described by Sheikh et al. [40]. Taştan [41] monitors the same parameters using a pulse sensor for photoplethysmography (PPG) measurement, a Negative Temperature Coefficient (NTC) sensor for body temperature, an HC-06 Bluetooth module for communication, and an Arduino Pro Mini platform. The collected data is transmitted to the Blynk application in real-time, allowing for graphical display on Android and iOS devices. Similar configurations based on sensors for monitoring vital functions are presented in works [42,43,44]. Naresh et al. [45] include radio frequency identification (RFID) technology to monitor patients’ medical conditions. Data collected from sensors attached to the patient’s body are updated in the database via a Wireless Personal Network (WPN) or mobile connection, depending on the patient’s location. The system updates patients’ medical records using a unique radio frequency identification (RFID) tag.
Seo et al. [46] propose an innovative sensor for monitoring vital signs through blood pressure, heart rate, and respiratory signals. This sensor uses a low-frequency reactive field to detect changes in arterial blood flow. The sensor was validated by comparing measurements with those of a commercial PPG sensor and a traditional sphygmomanometer in 30 subjects. The sensor proposed by the authors yields values that are correlated with those obtained using conventional methods. Fan et al. [47] compare the performance of wearable medical devices against traditional devices. The research shows no major differences between the values reported by these devices for the vital functions of the 60 patients. Park et al. [48] demonstrate that the Impulse-Radio Ultra-Wideband (IR-UWB) radar simultaneously monitors respiratory activity and carotid pulses in a non-contact manner, relying on electromagnetic waves to detect fine body movements. A biometric driver seat is presented by [49] using Six-Port receiver technology.
Yang et al. [50] introduce an automated health risk assessment system for the elderly integrated through wearable sensors and deep learning technology, such as LSTM. The model presented in this research uses five vital signs (blood pressure, heart rate, blood oxygen saturation, respiratory rate, and temperature), and the predicted values are used to calculate a risk score, thereby implementing an early warning system. Experiments demonstrated that the optimal result was achieved using a dataset of 1000 patients, a 12 h time window, and a configuration of 50 epochs and 5 LSTM neurons. Del-Valle-Soto et al. [51] point out that wearable systems offer a level of safety for the elderly, contributing to their improved health. The authors analyze 50 people aged between 50 and 70 years. Using the monitoring system resulted in a 4% improvement in inner peace for women, while for men, this impact was only 2%. Most participants showed a reduction in heart rate when the system was active. These results contribute to the positive implications of monitoring systems in the lives of elderly and lonely individuals.
1.4. Anomaly Detection Employing IoT-Based Wearable Devices
Several works in the specialized literature have previously proposed different IoT configurations to analyze complex scenarios related to anomaly detection in vital parameters. Their identification was carried out by running the query TS = (“anomaly detection” AND (“physiological data” OR “health monitoring”) AND (“wearable devices” OR “IoT”)) AND PY = (2020–2025) AND DT = Article in the Web of Science (WOS) Core Collection. Out of the 18 results provided, the works that directly address the common elements of the present research are analyzed in the following.
The paper by Shumba et al. [52] analyzes architectures for continuously monitoring physiological parameters through wearable devices. They reduce latency by integrating flexible piezoelectric sensors, Autoencoder-type algorithms, and Edge Computing. The paper highlights scenarios such as chronic disease monitoring, real-time anomaly detection, and community assistance for the elderly, which align with the common purpose of the present research. This research examines the issue of algorithm execution at the wearable device level (on-device AI) versus its implementation in a centralized system to which the wearable device reports data (on-cloud). The paper employs the encoders algorithm and reports an accuracy of 99.9% for the two scenarios, using a dataset of five measurements/sample and a time of 3 ms in the best scenario. Said et al. [53] designed an anomaly detection system for critical health events of patients in hospitals. The system aims to minimize false alerts by simulating scenarios associated with detecting a decrease in heart rate. The authors use the One-Class SVM algorithm and Edge Computing implementation in this paper. The reported accuracy for heart attacks is 82%. A dataset of 1000 records was used for training, and 200 records were used for testing. The latency is mentioned as being low, but without an exact numerical value.
Gayathri et al. [54] combined LSTM and Convolutional neural network (CNN) models for remote patient monitoring using wearable IoT devices. The objective is to detect anomalies and trends in physiological data, such as heart rate, blood pressure, or oxygen levels, with goals similar to those of the present research. The Ensemble LSTM-CNN model achieved an accuracy of 97.23%, with an anomaly detection rate of 95% and a response time of 2.5 s. The results demonstrate a 20% reduction in hospital readmissions and early identification of health issues in 92% of cases. Another research proposes a ResNet-LSTM model for blood pressure monitoring in the context of personalized health [55]. The model achieves a mean absolute error (MAE) of 6.2 mmHg and a root mean square error (RMSE) of 8.9 mmHg. Anomalies are detected through data analysis in an IoT and cloud infrastructure for storage and analysis. The paper by Kishor Kumar Reddy et al. [55] does not mention specific times. However, the dataset volume is not explicitly stated, but it emphasizes the importance of large datasets for anomaly detection.
Another cloud-based patient monitoring system for real-time anomaly detection is presented in the paper by Ebadinezhad and Mobolade [56]. This paper aims to reduce risks for elderly individuals through a concept-proof IoT device similar to the one presented in the current research. The system employs a Random Forest (RF) and SVM. The authors declare an accuracy of 100%. The average decision time is 16.3 s for 46 features. The infrastructure used is also cloud-based. This research [56] does not mention the volume of the dataset. A real-time monitoring system for the elderly that uses IoT sensors and commercial devices is analyzed by Shahid et al. [57]. The system’s objective is to learn the users’ daily routines. The paper aims to detect behavioral anomalies and notify these deviations via SMS in near real-time, similar to the prototype proposed in the current research. The system analyzed 1000 activities (meals, bathroom visits) and sent notifications to 9 locations. Users rated the system with a score of 4 out of 5. The data was collected over 80–355 days, and 93% of the observed activities were standard.
Based on the observations obtained from the literature analysis, the potential of the setup of IoT—Edge Computing—AI algorithms in health monitoring systems is noted. Although these works had a common objective of monitoring patients by analyzing various parameters, they do not address a typical setup issue. The present research aims to integrate the setup mentioned into a unified system, encompassing all the aspects discussed in the papers presented in this section. The synthesis of the results presented in this subsection is compared with those obtained from the algorithm proposed in this research in the Discussion Section. In this way, the positive and negative elements of the proposed setup are highlighted in the existing literature.
1.5. Paper Contributions
The authors of this paper possess expertise in the field of the integration of IoT technologies [58], AI-specific methods [59], and addressing the compatibility issues between them [60], enabling the development of a new infrastructure to integrate anomaly detection technologies through user data analysis. The analyzed data focuses on the vital functions of users, with applicability in generating alerts for elderly or at-risk individuals.
The research questions (RQs) of this paper are as follows:
- RQ1. How can an Azure cloud-based IoT infrastructure be used for a critical situation alert system associated with the elderly?
- RQ2. Can a principal component analysis (PCA)-based anomaly detection algorithm be used to monitor vital parameters for the elderly?
- RQ3. To what extent can a wearable device equipped with integrated sensors prevent medical complications by providing real-time alerts?
Based on these questions, the paper analyzes, in the following sections, the use of IoT technologies for monitoring the health of the elderly and anomaly detection in a cloud context, providing concrete solutions for each.
To evaluate the uniqueness of our proposed solution, we conducted a comprehensive search in WOS using the query TS = (“wearable bracelet” OR “wearable device”) AND “anomaly” AND “elderly.” This search yielded only three results [61,62,63], highlighting the limited research in this specific domain. Notably, none of these studies propose a cloud-based solution akin to the one presented in this manuscript.
Among the identified studies, only one explores an anomaly detection approach based on life patterns [61]. However, the methodology and implementation described in that study differ from those proposed in our solution. Specifically, the existing work relies on localized data processing and does not leverage cloud computing for real-time monitoring or advanced analytics. In contrast, our work introduces a multidimensional anomaly detection algorithm integrated with a cloud-based architecture, enabling real-time tracking of elderly individuals using wearable bracelets.
The authors disseminate through this article the following innovative elements from their research in the field of elderly health monitoring:
- A customized IoT system is proposed for the continuous health monitoring of an elderly person;
- A wearable bracelet prototype with specialized sensors (MAX30100 and MPU-6050) is proposed. Collecting vital parameters—heart rate (average, minimum, and maximum), step count, and sleep quality (deep and superficial sleep)—provides a holistic view of the patient’s health status. Although such a bracelet already exists, this prototype is compatible with the IoT Azure infrastructure proposed in this work;
- A multidimensional anomaly detection algorithm is proposed using a dimensionality reduction method based on PCA. The health anomaly detection algorithm (HADA) identifies anomalies in the monitored data by analyzing complex correlations between the proposed parameters. This approach allows for personalized analysis tailored to the specifics of each patient;
- Integration of the Microsoft Azure cloud infrastructure into the system ensures secure data transmission. Preprocessing of data and real-time automatic alert generation is facilitated by the Azure IoT infrastructure, enabling prompt medical interventions in critical situations;
- The tests conducted in the Results section disseminate energy analysis for wearable devices. Implementing energy-saving strategies, including deep sleep mode, extends the bracelet’s operational life, making the system practical for continuous use at home;
- The study presents results from continuous patient monitoring over two years, demonstrating a high sensitivity of 100% and an overall accuracy of 98.5%.
These contributions represent a milestone in research in the field, combining embedded technologies, IoT, and Cloud. The paper proposes the development of an intelligent, personalized, and specialized monitoring system for the Azure infrastructure to prevent medical complications in the elderly. Unlike existing solutions, this work integrates a multidimensional anomaly detection algorithm with cloud infrastructure, ensuring efficient data processing and medical alerting. The system contributes to proactive healthcare management by identifying physiological deviations before they escalate into critical conditions.
The paper structure is as follows: The methodology is presented in Section 2, where the novel elements are introduced, along with the description of the dataset, the proposed system, the analyzed algorithms, and the architecture and communication of the proposed IoT infrastructure. Section 3 focuses on HADA’s results in detecting anomalies, monitoring elderly individuals’ data, and performing IoT infrastructure speed tests. In addition, HADA was generalized to outline its use in real-world scenarios. The discussion of the results is depicted in Section 4, and the conclusions are detailed in Section 5.
2. Materials and Methods
In the 21st century, European countries are experiencing population aging, which is exacerbated by the crisis in their health systems. Most countries face a lack of personnel and a decrease in birth rates. The long-term effects of these two problems have led to a shortage of personnel to provide palliative care for the elderly. This paper proposes a comprehensive solution that integrates embedded technology with modern systems for analyzing and providing real-time alerts on the health status of elderly individuals who do not have serious health issues. The bracelet device uses a heart rate sensor mounted on an M5Stack (produced by M5Stack Technology Co., Ltd., Shenzhen, China) to capture the average heart rate. The system also integrates a preventive component that monitors other parameters whose values could indicate precursor events of an imminent incident. For this identification, the prototype bracelet monitors average heart rate (AR), minimum heart rate (mR), maximum heart rate (MR), number of steps taken (S), deep sleep time (DST), and superficial sleep time (SST). The integrated system uses Azure cloud infrastructure for automatic alert generation and critical event management. In this way, medical staff can intervene to save the lives of those in need.
This section provides an overview of the proposed system’s technical specifications before detailing individual components to ensure a clear and structured approach.
The proposed system comprises a wearable bracelet equipped with a MAX30100 heart rate sensor (manufactured by Analog Devices Holdings Co., Ltd., Shanghai, China) and an MPU-6050 accelerometer (produced by TDK InvenSense, Shenzhen, China) for tracking steps and sleep. Data is continuously transmitted to an Azure-based cloud platform via secure Wi-Fi communication. The cloud infrastructure preprocesses the data, applies anomaly detection algorithms, and triggers alerts when necessary.
HADA is designed to analyze six key physiological parameters: AR, mR, MR, S, DST, and SST. The algorithm utilizes PCA to identify deviations from expected trends, enabling personalized health assessments based on individual baseline data. The system periodically retrains HADA to account for changes in patient conditions, ensuring adaptive and accurate monitoring.
Once processed, data is securely stored in an Azure SQL Database. Alerts are generated and transmitted via Azure Communication Services, including SMS notifications to caregivers if an anomaly is detected. This ensures rapid medical intervention in critical situations.
2.1. Monitored Parameters and Analyzed Dataset
The identification of health status and prevention of incidents in elderly individuals is achieved through real-time processing of the following parameters:
- AR represents the average pulse value calculated at predefined time intervals. Through this parameter, a general overview of cardiovascular activity is identified. In this way, trends that may indicate a suboptimal health condition are detected.
- mR is the minimum recorded pulse value and is monitored to detect any episodes of bradycardia. Such an incident requires prompt medical intervention and is a zero-level alert.
- MR records the maximum pulse values, abnormal values being associated with tachycardia situations. These fluctuations can signal cardiovascular stress or other acute cardiac issues, also constituting zero-level alerts.
- S monitors the number of daily steps taken. This parameter serves as an indicator of physical activity level. This parameter contributes to assessing mobility and overall health status, not being one that generates a major alert.
- DST indicates the time spent in the deep sleep phase. This parameter identifies the body’s recovery capacity and physical resource restoration.
- SST monitors the time spent in the superficial sleep phase. This parameter helps evaluate overall sleep quality, providing clues about disturbances or imbalances in rest cycles.
The first three parameters are quantified in BPM, S is measured in the number of steps, and the last two in minutes, as shown in Figure 1, which presents the feature diagram for the dataset built for a single patient. If an anomaly is identified, the output of the employee’s anomaly analyzer algorithm is 1. In this case, an alert is released. Otherwise, the output is 0.
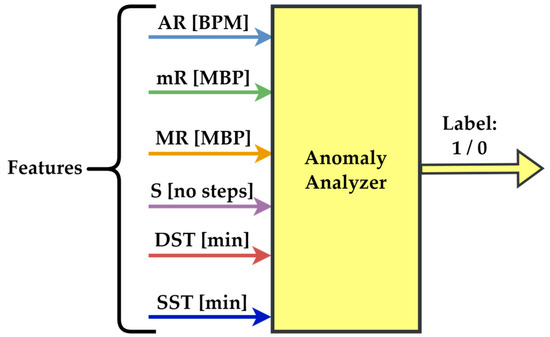
Figure 1.
Features for anomaly detection were examined through a custom-made analyzer.
The dataset is built using the six parameters presented in Figure 1. These enable the monitoring of the health status of elderly patients. The analysis must be personalized because each patient has unique traits, depending on the medication, their specific body configuration, lifestyle, and activity levels. Thus, thresholds for parameters can vary significantly from one patient to another.
It is important to note that these parameters were selected based on their correlation with early warning signs. They encompass cardiovascular health, physical activity, and sleep patterns, collectively providing a holistic health assessment. Moreover, the variability of threshold values among individuals is addressed through personalized baseline adjustments. The threshold values for each patient can be customized by the treating doctor, who is familiar with the patient’s medical history. By analyzing the collected data, the doctor can establish personalized thresholds that accurately reflect the patient’s health status and specific medical needs.
Based on these considerations, a unique analysis is required for each monitored person. This unique model enables precise alerts tailored to personalized statistics for each individual. Therefore, the dataset is built for a single patient because individualized monitoring enables obtaining a detailed and accurate profile of vital parameters without the interference caused by inter-individual variability. The analyzed dataset spans a two-year period, from 1 January 2023 to 31 December 2024. The frequency of activity data for life-threatening parameters (AR, mR, and MR) is 725,338 records, while the general state parameter S contains 9455 records, and the rest (DST and SST) contain 680. The output of the anomaly analyzer is referred to as a label.
2.2. Proposed Embedded System
The hardware prototype features the M5Stack module as its central element. This represents a modular embedded solution that integrates processing, wireless communication, and energy management functionalities. This energy management method, which utilizes deep sleep and wake-up mechanisms triggered by events, is superior to all other solutions proposed in the literature to date. This embedded solution enables the connection of multiple sensors, whose values are read at predetermined time intervals or when an event occurs, with minimal energy consumption. Furthermore, the real-time handling of data transmitted to the cloud infrastructure for detailed analysis and alert generation makes the M5Stack a top choice for elderly monitoring solutions. Additionally, when the battery level drops below the 5% threshold, the person responsible for monitoring the elderly individual is notified to intervene and resolve the issue. This situation must be addressed when the elderly person neglects to recharge the bracelet.
A heart rate bit sensor, MAX30100, is used for heart rate monitoring. This sensor emits light pulses, while the photodetector measures the variations in the intensity of light reflected by blood flow.
For step counting (S), the prototype integrates an MPU-6050 module, which combines an accelerometer and a gyroscope. The accelerometer detects the characteristic movements of walking, and data processing algorithms convert this information into the number of steps. By correlating this data with heart rate data, the algorithms estimate energy consumption.
Sleep quality is assessed based on the DST and SS. The values of these two parameters are determined through actigraphic analysis of the accelerometer data. Detecting periods of minimal movement enables differentiation between deep sleep phases and superficial sleep phases.
Figure 2 presents the simplified logical schema of the embedded device. Each device has a unique identification code (ID). This code establishes the correlation between the monitored patient, the device, and the evolution of the six monitored parameters over time for that specific patient. The embedded device’s algorithm initializes serial communication and Wi-Fi connection to transmit data to the central server. Subsequently, the two sensors are initialized and read the battery level. Heart rate measurements are collected over a defined interval in a continuous loop to determine the maximum, minimum, and average values. Afterward, the six monitored parameters are calculated. The M5Stack receives and preprocesses data from all sensors. It forms a JSON payload with all necessary values, including the battery level information. Using the integrated Wi-Fi module, the M5Stack sends the data via an HTTP POST to the ASP.NET API server, which collects the information for anomaly analysis. The system disables data reading until the condition is met again. During the entire period of system deactivation, it remains in power-saving mode. Exiting this mode occurs when a new reading is required or the battery is fully depleted. Figure 2 presents a simplified schema, as each parameter has different time conditions in reality. For example, heart rate monitoring is performed more frequently than sleep monitoring.
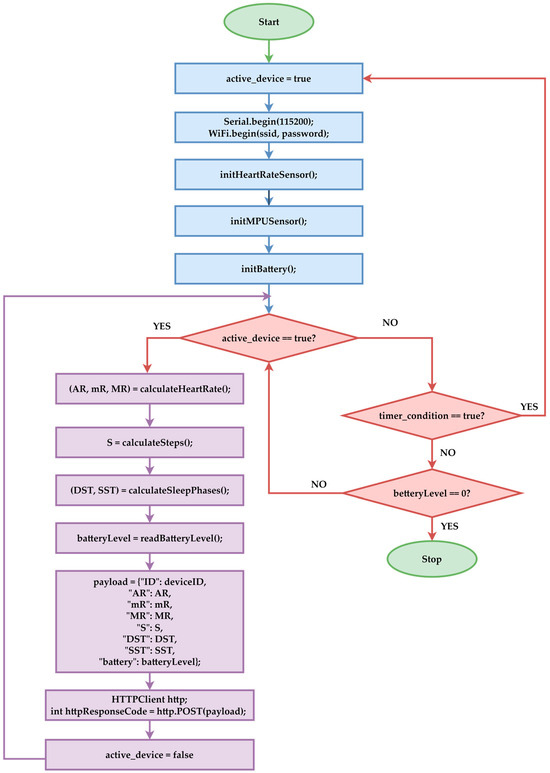
Figure 2.
Device operation flowchart.
The prototype proposed in this paper uses a Wi-Fi connection in this configuration to transmit data to the Azure infrastructure. For situations where the system is in a scenario where the Wi-Fi network is unavailable, the M5Stack module has the option to use the cellular communication module with a Subscriber Identity Module (SIM) card. This technology is compatible with Long-Term Evolution (LTE) for Machines (LTE-M) or Narrowband IoT (NB-IoT). In this way, the collected data is transmitted to the cloud platform via the mobile network, eliminating the need for a local network. Future versions will consider integrating the automatic communication mechanism between Wi-Fi and the mobile connection, depending on network availability. In this way, the continuity of real-time monitoring and the resilience of the system in diverse environments will be ensured.
The pseudocode for calculating AR, mR, and MR involves initializing a list of successive heart rate readings from the heart rate bit sensor, followed by the standard initialization of the total number of readings and the minimum and maximum values that need to be calculated. Subsequently, the list of readings is built, and the average, maximum, and minimum values are updated accordingly (Heart_Rate.txt file from the Supplementary Material).
The prototype used in this study employs the M5Stack module, which integrates the ESP32 microcontroller, enabling operation in five modes that optimize energy consumption. In the standard version, it operates in active mode, with all peripherals functioning. The Modem Sleep variant keeps the processor active while deactivating the communication modules. For light sleep, the Central Processing Unit (CPU) is turned off, whereas the random-access memory (RAM) and peripherals are retained. It can be reactivated via General Purpose Input/Output (GPIO) or a timer. Regarding the deep sleep mode, it minimizes energy consumption by keeping only the Real-Time Clock (RTC) and wake-up sensors active, which is the operating mode chosen for the proposed prototype. The Hibernation option has even lower power consumption than deep sleep, but all data associated with RAM is lost. For these reasons, the deep sleep operating mode was considered the most suitable for the proposed variant in this work, and this operating mode is exclusively available in the architecture that includes the M5Stack hardware ecosystem with the ESP32 board.
The pseudocode in the Steps.txt file from the Supplementary Material calculates the parameter S, which reads the values of the coordinates ax, ay, and az from the MPU 6070 accelerometer. Subsequently, it calculates the magnitude of acceleration for each reading. A step is considered if the difference between the current reading and the previous one exceeds a predefined threshold (STEP_THRESHOLD).
The pseudocode in the Sleep_Phases.txt file from the Supplementary Material calculates the DST and SST parameters. For the determination of DST and SST parameters, the authors propose that the system use data obtained from the MPU-6050 accelerometer. In the proposed algorithm, variations in the magnitude of acceleration on the three axes lead to a value that must be below a preset threshold (DEEP_SLEEP_THRESHOLD). The respective period is classified as deep sleep. If the calculated value falls between two predefined thresholds, it is considered shallow sleep (DEEP_SLEEP_THRESHOLD < acceleration < SHALLOW_SLEEP_THRESHOLD). The intervals are accumulated over a monitoring period, and the total time of each sleep phase is calculated in minutes. Threshold values can be studied in new research to be customized according to each user’s sleep quality.
The analog pin reads the battery voltage to calculate the battery level. This value is converted into an actual voltage relative to the analog-to-digital converter (ADC) reference. Subsequently, the measured voltage is mapped between the values of MIN_BATTERY_VOLTAGE (e.g., 3.0 V for a discharged battery) and MAX_BATTERY_VOLTAGE (e.g., 4.2 V for a fully charged battery). If the percentage exceeds 100%, it is set to 100%. If the value is below 0% (error or heavily discharged battery), the value is set to 0% (Battery_Level.txt file from the Supplementary Material).
The analysis of threshold values is presented in the Results section, where the final values established in the algorithm and the method of their determination will be detailed.
2.3. Proposed Health Anomaly Detector Algorithm
Focusing on a single dataset facilitates the identification of trends specific to each individual. This approach reduces the complexity of analysis, allowing HADA to focus on human organism specificity for prompt medical interventions. This analysis must be personalized because each patient has different physiological characteristics and lifestyles, requiring the adjustment of alert thresholds based on individual reference values. Thus, a correct interpretation of the data is ensured, optimizing treatments and preventing complications through interventions tailored to the specific needs of each individual.
The authors chose this option due to its compatibility with Microsoft technologies. This choice was based on the native compatibility with the development environment used for the back-end, namely Visual Studio C#. A second reason stems from the ability to quickly implement anomaly detection models by accessing data from the Microsoft Azure infrastructure.
The steps of processing and training of HADA are schematically illustrated in Figure 3 and described as follows:

Figure 3.
HADA data processing and training workflow.
- The data is acquired from M5Stack and transmitted to Microsoft Azure via an API request. Subsequently, through an Azure Function, the data is preprocessed, centralized, and organized into a suitable format for model training.
- The feature variables (AR, mR, MR, S, DST, and SST) are identified.
- Unlike the data used in classification and clustering models, which is scaled and normalized to eliminate any potential disproportionalities that could negatively impact model training, the data is not altered in this case. This is a particularly important remark! It must be emphasized that the authors did not remove or modify the acquired data to avoid altering potential alerts.
- The authors started the investigation by simultaneously examining all input parameters. Later, they also analyzed models with one input at a time. Additionally, various anomaly detection hyperparameters were evaluated for the Randomized PCA method. For each algorithm, hyperparameters were adjusted to identify the scenario with the best performance. Performance was determined based on standard AI model evaluation metrics. The algorithm with the best performance was selected for integration into HADA’s software pipeline.
- After establishing the final HADA configuration, the model is integrated into a .NET application that runs on a local server in collaboration with Azure functionalities.
- Implement the alert emission component to ensure the system responds with the lowest possible latency.
During the development of HADA, mechanisms for monitoring the model’s performance were implemented. It is noted that the model is periodically retrained for each individual separately, as physiological changes can occur in every person’s life due to aging, changes in medication, or other factors that cause alterations in the organism’s usual behavior. A concrete example in this regard is a scenario where an elderly person experiences a hip fracture. Their physical activity will not be the same for an extended period. Therefore, it is periodically adjusted to maintain an accurate record of the model’s performance in various usage scenarios.
2.4. Data Flow Architecture and Communication System
The data flow is designed such that, as soon as the M5Stack collects and preprocesses the data, it is transmitted via a secure Wi-Fi connection to Azure SQL Database through an ASP.NET API, as depicted in Figure 4. Once received, the data is routed to a dedicated Azure Function, which retrieves the values, further preprocesses them, and passes them to the HADA model for evaluation. If the model identifies an anomaly, Azure Functions immediately triggers a set of automated actions:
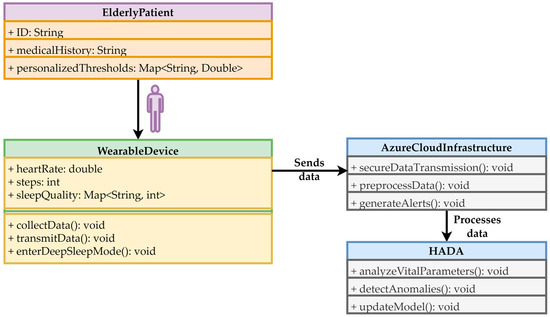
Figure 4.
The integrated system flow for monitoring the health of elderly people.
- Sending a notification to the alert system configured in Azure Notification Hubs;
- Generating a Short Message Service (SMS) alert through Azure Communication Services;
- Saving the incident in the database;
- After the incident is evaluated, the person responsible for monitoring the patient deactivates the alert, intervenes, and generates an intervention report so it is precisely known whether it was an actual incident or a false alarm (as the patient may have disconnected the bracelet), but also to identify the employee who intervened in case of a medical error.
The UML diagram presented in Figure 4 includes the patient level, corresponding to the system’s end user. He receives medical help in the event of anomalies and is subject to medical interventions when necessary. The wearable device collects vital data and transmits this data to the cloud infrastructure in real-time. As mentioned, this device features energy-saving capabilities, including a deep sleep mode. The cloud infrastructure ensures data transmission security through its mechanisms, preprocesses the data, transfers it to HADA, and generates alerts for patients or caregivers. HADA analyzes vital parameters using PCA, detects anomalies, and periodically updates the model to adapt to changes in patient conditions.
These conceptual steps are schematically presented in Figure 5. For building the software pipeline, the Azure platform was chosen due to its multitude of features, which are necessary for managing a distributed monitoring system. Azure allows everything from data storage and processing to the automatic generation of alerts and workflow management. To achieve HADA integration, the following components were used:
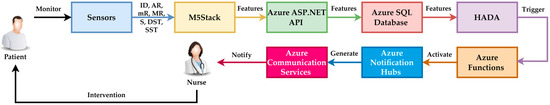
Figure 5.
Conceptual steps involved in the entire pipeline infrastructure.
- Azure ASP.NET API for collecting data from M5Stack devices;
- Azure Functions for serverless code execution, enabling rapid processing of received data and real-time generation of alerts;
- Azure Notification Hubs and Azure Communication Services for distributing notifications to operators, supervisors, and the monitored person’s relatives based on the results of anomaly detection;
- Azure SQL Database for storing the patient’s data history for subsequent system performance analysis.
To ensure the integrity and confidentiality of the data, communication between M5Stack and Azure is carried out through encrypted protocols (TLS/SSL). Additionally, access to Azure resources is protected by multi-factor authentication and strict access rights management. The system is designed to comply with the General Data Protection Regulation (GDPR), ensuring the anonymization of personal data and implementing rigorous backup and recovery policies in the event of incidents.
3. Results
This section disseminates the results obtained following the implementation and testing of the HADA system for monitoring elderly individuals. The data was collected over two years, and the analysis focused on anomaly detection and alert generation based on the monitored parameters: AR, mR, MR, S, DST, and SST.
3.1. HADA Proposed Prototype
M5Stack is a module that uses the ESP32 microcontroller (dual-core, 240 MHz). It features a two-inch LCD with a resolution of 320 × 240 pixels for real-time data visualization, and connectivity is achieved via Wi-Fi and Bluetooth for data transmission. The internal battery is a 1400 mAh Li-ion. Within this project, M5Stack is configured to collect data from an accelerometer and a pulse sensor, ensuring detailed monitoring of the elderly person.
Figure 6 presents the prototype in an extended form for demonstration purposes. The display was also used for presentation purposes. Since the display is a significant battery consumer, it is usually disabled.

Figure 6.
Proposed HADA setup.
- The MPU-6050 Accelerometer and Gyroscope record acceleration and rotation on the three axes, Ox, Oy, and Oz;
- The MAX30100 Pulse Sensor is an optical sensor that measures heart rate and blood oxygen levels. It is useful for measuring the user’s physiological parameters.
The architecture diagram presented in Figure 7 illustrates the data flow of the IoT and cloud-based elderly health monitoring system. The IoT bracelet collects sensor data and transmits it to Azure IoT Hub. Next, the data is processed by Azure Functions and analyzed by the HADA. The results are stored in Azure SQL Database. Azure Notification Hubs and Azure Communication Services notify the caregivers in the event of an alert. This architecture ensures continuous monitoring. Using this architecture, the monitoring system alerts the caregivers.
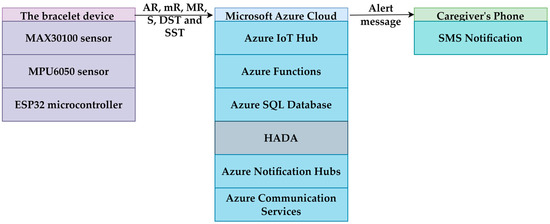
Figure 7.
IoT and Azure cloud architecture with HADA for elderly health monitoring.
Connecting the sensors directly to the M5Stack’s dedicated ports achieves their physical integration. The M5Stack device was programmed using the Arduino IDE development environment, with C/C++ as the primary language. The source code includes the implementation of the functions described earlier.
Several tests were conducted to evaluate battery life based on the measurement frequency and display activity. The obtained results are as follows:
- When the measurement frequency is 1 s and the display is active, the battery life is approximately 1 h.
- When the display was turned off, but the measurement frequency remained at 1 s, the battery life increased to approximately 4 h.
- When the measurement frequency was reduced to 1 h, and display output was eliminated, the battery life increased to approximately 12 h;
- The implementation of the energy-saving mode, using the esp_sleep_enable_timer_wakeup function to allow the ESP32 microcontroller to enter deep sleep mode between measurements, significantly impacted battery life. This increased battery life to 22 h, as the microcontroller consumed much less energy during inactive periods.
These results suggest that optimizing the measurement frequency and employing energy-saving techniques, such as deep sleep, can significantly extend battery life in a portable system.
3.2. HADA Development Dataset
The HADA was integrated using the C# programming language with the ML.NET tool. It was built for a single elderly person who wore a continuous monitoring bracelet for two years (between 1 January 2023 and 31 December 2024). The bracelet was worn almost constantly during this period, except when the user had to charge it or when it was not in use, such as during baths. Thus, monitoring was performed continuously for two years, resulting in 676 recordings.
The elderly individual was born on 24 October 1954 and was 68 years old at the start of the monitoring. From a medical history perspective, the individual underwent surgery in 2000 for a left frontoparietal meningioma, and the postoperative recovery was complete without sequelae. This information is important to understand the user’s health context throughout the monitoring period.
The SQL Server database contains three tables: the table for monitoring the parameters AR, mR, and MR, the table for monitoring the parameters DST and SST, and the table for monitoring the parameter S. Figure 8 presents the frequency measurements: heart rate, DST, SST; and parameter S per day of the monitored patient.
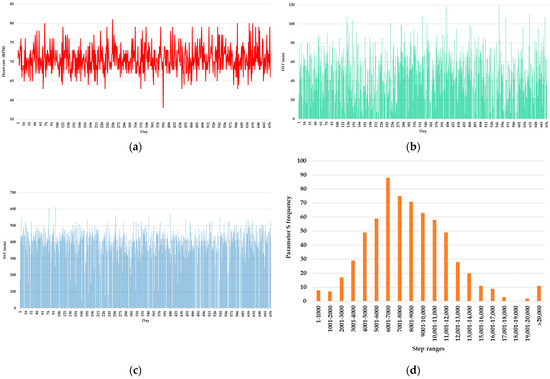
Figure 8.
Frequency measurements per day of the monitored patient: (a) heart rate; (b) DST; (c) SST; (d) parameter S.
For calculating the statistics used in building the model, the values for each day were summarized into a single value by calculating the minimum, maximum, and average for heartbeats, summing the number of steps, and directly taking the unprocessed values of DST and SST.
3.3. HADA Integrated into the Developed Model
Using HADA for the inputs AR, mR, MR, S, DST, and SST, the analysis examines the relationships among these variables, generating a model that is inaccessible to mathematicians or statisticians. The model analyzes values with different representation domains simultaneously. This is almost impossible for a human, who typically performs analyses only on individual values and, at most, can combine them afterward. For example, a specific behavior may be expected in one context (for one parameter) but be abnormal in another context (for another parameter). For instance, MR may be normal in sports but abnormal when accompanied by a low DST. Therefore, analysis in a multidimensional space (with multiple features) helps establish a dependency between parameters, also considering the specificities of the context for which the training is performed.
If each parameter were analyzed individually, then anomalies generated by correlations might be missed. For example, if SST is too low, AR is too high, and S is very elevated simultaneously, this behavior may indicate an anomaly that is not visible in the case of individual analysis. To address these situations, HADA uses a multidimensional anomaly model.
The algorithm for identifying anomalies is based on PCA. It is integrated into ML.NET under the name RandomizedPCA. The algorithm specializes in identifying the main directions of variation in a dataset. These directions are used to reduce the dimensionality of the data. Subsequently, those that determine the residual values are extracted. This determines a deviation from the principal component of the data. This is useful when you want to find observations that do not follow the general patterns of the data trend.
The HADA model was developed to identify anomalies in data collected from an elderly person using six features: AR, mR, MR, DST, SST, and S. In the case of PCA, there is no direct correlation with the number of neurons and layers, as PCA is a dimensionality reduction method rather than a neural network-based model.
The purpose of HADA is to detect anomalies in the monitoring data of elderly individuals, triggering alerts for caregivers to enable rapid intervention. Usually, the number of detected anomalies should be as low as possible. However, detecting many anomalies may indicate that the model is too sensitive, generating excessive false alerts, i.e., alerts that are not caused by real health issues. Conversely, this situation is still preferable. In the opposite case, detecting a few anomalies could mean that the model is not sensitive enough to identify real health problems or hazardous behaviors, putting the elderly person’s life at risk. For this reason, in the study of hyperparameter configuration, it is desirable to identify a larger number of anomalies, even if they are false.
For ensureZeroMean = TRUE, Figure 9 shows that many of the combinations with rank = 1 identified a large number of anomalies (18). This could be useful in scenarios where we want to identify any sign of anomalies, but it might lead to too many alerts and false positives.

Figure 9.
Number of anomalies versus rank for different q values (ensureZeroMean = TRUE).
For ensureZeroMean = FALSE, Figure 10 shows combinations with rank = 1 and rank = 2, detecting only 0 or 1 anomalies. This may indicate less sensitive performance and could reduce false alerts. However, it might also lead to missing important signs of health problems.
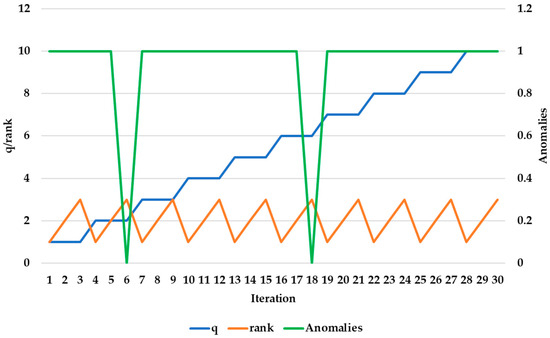
Figure 10.
Number of anomalies versus rank for different q values (ensureZeroMean = FALSE).
The seed q can influence decisions about when to trigger an alert. It helps balance the model’s sensitivity with alert precision. For example, a q that produces many false alerts could cause the model to generate constant warnings, which might be disruptive. On the other hand, a q that produces too few anomalies might result in real health problems of the elderly being overlooked.
A moderate number of anomalies is ideal in elderly monitoring. Most values for q (1–10) with rank = 1 and ensureZeroMean = TRUE led to detecting 18 anomalies, suggesting high sensitivity in anomaly detection. This behavior remains stable and consistent in the context of alert generation, and this hyperparameter configuration was subsequently used.
Regarding the final model hyperparameters, we chose a dimensionality reduction with a rank set to 6, meaning that the model retained all parameters in the analysis without discrimination. The model reports each parameter as a principal component for describing the data. This choice simplifies the model and helps prevent overfitting, but it could reduce the ability to capture the complex variability of the data. The parameter en-sureZeroMean was set to true, ensuring each feature has a mean of 0 before applying PCA. This way, we avoided distortions caused by scale differences between features. Additionally, we used a seed value of 1, ensuring the reproducibility of results to obtain the same results each time the model runs. This validates and enables comparison of the model’s performance on similar datasets. The parameter exampleWeightColumnName was set to null, considering all observations were treated equally without applying additional weights. The combination of these hyperparameters makes HADA a model capable of identifying anomalies specific to variations in AR, mR, MR, DST, SST, and S.
After processing the data, 18 anomalies were identified over the 2-year monitoring period. Of these anomalies, nine were false, meaning they were records that, although flagged as anomalies by HADA, did not represent a major health issue. The other nine anomalies were not major alerts but rather minor physiological changes that were easily explainable, such as the process of eliminating kidney stones. These changes did not require immediate medical intervention, but the user underwent surgery in 2025 to remove them. This suggests that HADA acts as a predictive component, with alerts indicating subtle changes in the monitored patient’s condition.
Figure 11, Figure 12 and Figure 13 mark the days HADA generated alerts (highlighted by the red vertical line). Figure 11 presents the evolution of AR, mR, and MR regarding the detected anomalies, providing a clear visualization of the moments when the values of these indicators exceeded normal limits and were flagged as anomalies. Thus, HADA identifies changes by analyzing correlations between these parameters, enabling early detection of potential health issues. This visualization underscores the system’s capacity to monitor complex relationships among heart rate metrics, offering actionable insights for prompt medical interventions.
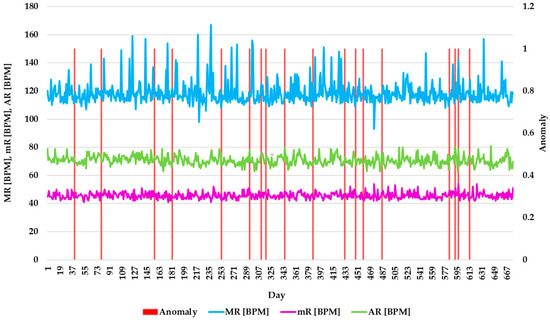
Figure 11.
Evolution of AR, mR, and MR with detected anomalies.
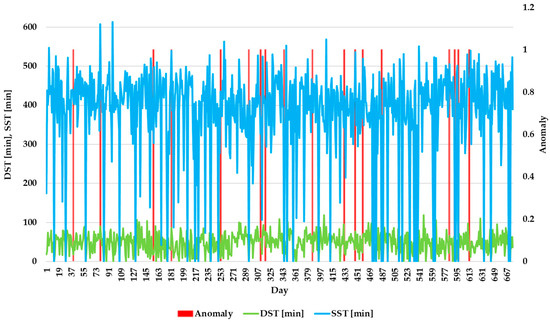
Figure 12.
Distribution of DST and SST associated with anomalies.
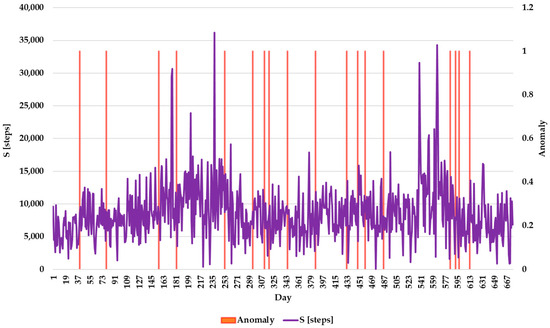
Figure 13.
Correlation between S and anomaly detection.
Figure 12 depicts the distribution of DST and SST associated with anomalies. This graph shows whether the anomalies were associated with changes in sleep behavior. These parameters are crucial for assessing overall sleep quality, as they reflect different stages of sleep. By monitoring these trends over time, the system can detect irregularities that might not be apparent when analyzing individual parameters alone. The results show that DST and SST exhibit fluctuations throughout the monitoring period, with occasional spikes or drops that trigger anomaly alerts. This underscores the importance of continuous monitoring to capture subtle changes in sleep behavior, which could indicate underlying physiological conditions or stressors affecting the elderly’s health.
Figure 13 presents the correlation between S and anomaly detection. This graph demonstrates fluctuations in daily physical activity levels, with some days showing significantly higher or lower step counts compared to others. These variations are likely influenced by factors such as routine activities, health conditions, or environmental changes. Notably, anomalies are detected during periods of unusually high or low step counts, highlighting HADA’s ability to identify deviations that may signal potential health issues or behavioral changes. By analyzing these trends, the system provides insights into the elderly individual’s mobility and overall physical activity, enabling timely interventions if necessary. This underlines the importance of continuous monitoring and anomaly detection in maintaining the health and safety of the elderly.
HADA was evaluated based on the specific metrics of AI models. Out of a total of 676 recordings, 9 anomalies were real (TP = 9), 9 anomalies were false (FP = 9), and 658 were classified as usual (TN = 658). Given the fact that only one person was monitored, who did not require emergency assistance, there were no undiagnosed real anomalies (FN = 0). Precision was identified with a value of 50%, recall was 100%, F1-Score was 33%, and accuracy was 98.5%. Although HADA has low precision, this does not mean that the system cannot be used in practice. It does, however, represent a starting point for exploring future research directions in automating the remote patient monitoring process at home.
In a network with a response time (ping) of 149 ms, a download speed of 138.31 Mbps, and an upload speed of 48.45 Mbps, the alert signaling occurred in 11 s and 46 milliseconds. This time provides a relative picture of the response speed offered by the Azure infrastructure, influenced by factors such as the patient’s internet speed and the speed at which the SMS is sent by the mobile phone operator, among others. This fast response time demonstrates the efficiency of the Microsoft Azure communication infrastructure, which can transmit information in a short interval, ensuring a prompt response for real-time monitoring. The SMS notification includes the patient’s phone number and address. The operator calls the patient so that the individual can confirm their health status. In the meantime, if the patient does not respond, a team is dispatched to the patient.
The tests presented in this results chapter have shown the efficiency of the Microsoft Azure infrastructure. These evaluations highlight how Azure’s capabilities ensure real-time intervention applications for patient monitoring. The low response times and consistent data transmission demonstrate Azure’s infrastructure reliability.
4. Discussion
The main results of the study indicate that the proposed IoT system, which combines a wearable bracelet equipped with an M5Stack module, MAX30100 and MPU-6050 sensors, and an anomaly detection algorithm based on PCA, demonstrated a sensitivity of 100% and an accuracy of 98.5% in the continuous monitoring of vital parameters for a patient over two years. The device utilizes a dual-core ESP32 microcontroller operating at a frequency of 240 MHz, a two-inch LCD screen with a resolution of 320 × 240 pixels, and a 1400 mAh battery. The energy consumption tests have highlighted that the best performance is achieved when the M5Stack module operates in deep sleep mode, resulting in a battery life of 22 h. This system transmits the data acquired from the sensors to the Azure cloud infrastructure, where it is stored in a Microsoft SQL Server database, preprocessed, and evaluated in real-time for the generation of automatic alerts.
The obtained results can be explained by the fact that the multidimensional analysis of the data (including heart rate, step count, and sleep quality) enables the identification of complex correlations between parameters, making it possible to detect even the most subtle physiological changes. Implementing energy-saving techniques, such as deep sleep mode, extended the device’s operational life, enhancing the system’s practical utility.
The research presented in this study represents a conceptual prototype for monitoring the health of elderly individuals through continuous data collection, anomaly detection, and real-time alert generation. While the system’s performance has been evaluated based on a single patient, the results provide valuable insights into the feasibility of such a system in a real-world production context. It is important to note that this study does not provide an exhaustive comparison with other AI methods or existing commercial wearable devices. Instead, it serves as a starting point for future research and development aimed at mass production.
The research demonstrates that the proposed solution is feasible and can offer practical benefits, such as real-time health monitoring and prompt caregiver alerts. While limited in scope, the current prototype has shown promising results in detecting anomalies in the monitored parameters. The precision and recall rates indicate that the system is sensitive to potential health issues, albeit at the cost of generating false positives. This trade-off is important when dealing with elderly individuals, as it prioritizes safety by erring on the side of caution.
These contributions are highly significant in elderly health monitoring, offering an innovative, non-invasive, personalized solution for early anomaly detection. Through the rapid interventions triggered by the alert system, serious medical complications can be prevented, thus contributing to improved quality of life and reduced pressure on healthcare systems.
Although PCA is not compared with other AI techniques like LSTMs, Random Forest, or Isolation Forest, it is worth noting that the choice of PCA was made based on its simplicity, ease of implementation, and suitability for anomaly detection in this particular use case. However, the future development of this system could include testing alternative AI models to further optimize its performance.
Similarly, the absence of comparisons with commercial wearable health devices such as Fitbit, Apple Watch, or Garmin should not detract from the significance of this research. These devices primarily focus on specific health metrics, such as heart rate and step count, but do not offer the same integrated anomaly detection and real-time alerting features tailored for elderly care as the HADA prototype. The proposed system aims to surpass conventional wearables by offering continuous, comprehensive monitoring of multiple physiological parameters and alerting caregivers to potentially critical changes in health conditions.
The authors acknowledge that the proposed prototype requires extensive testing, validation, and refinement for production deployment. The current work presents an initial concept rather than a fully developed production-ready system. The primary objective of this research is to introduce an IoT-based approach and highlight the feasibility of integrating AI, IoT, and cloud technologies for elderly health monitoring.
The proposed solution is a conceptual framework demonstrating how these technologies can be leveraged for continuous health assessment. While the current prototype provides a foundation for further development, future work should focus on extensive clinical trials and ensuring compliance with medical standards.
By presenting this model, the authors emphasize the potential of intelligent health monitoring systems in addressing the challenges of elderly care. The integration of AI enables personalized analysis, IoT devices facilitate real-time data collection, and cloud computing ensures scalability and accessibility.
In this research, the Azure platform was chosen due to its native integration capabilities with the development tools used, namely ASP.NET and ML.NET. Moreover, the platform offers the necessary advantages for the proposed application type, which aims to process data in real-time and generate automatic alerts. It is important to mention that the Azure platform is a proprietary platform, which involves a series of monthly costs, making it inaccessible to user categories with a limited budget. However, the platform demonstrates the proposed prototype’s ability to achieve its objectives. Alternative solutions to this platform include Amazon Web Services (AWS) Free Tier, which is a commercial solution with a free plan but limited in certain features, and Google Cloud Platform (GCP), which is also available for free but with limited features, ThingsBoard, which is free, or developing solutions locally.
In Table 1, the alternative solutions are summarized along with a series of advantages and disadvantages. Most existing solutions are currently available in a commercial version, but there is also an open-source version, such as ThingsBoard, which implies no costs but requires advanced programming skills, resulting in similar overall solution costs.

Table 1.
Comparative analysis of possible cloud platforms and implementation approaches.
Table 2 presents a cost simulation for the scenario of monitoring approximately 23 patients on the Azure platform. Thus, the monthly amount is approximately 18.62 USD per month, which a subscription can cover for the monitored patients.

Table 2.
Estimated monthly cost for Azure-based solution (real-time monitoring of 23 devices).
Integrating cloud technologies with innovative data analysis methods represents an advancement in elderly health monitoring. Cloud-based solutions enable continuous data collection, real-time processing, and remote accessibility, allowing healthcare professionals to monitor patient status in real-time. This facilitates the early detection of health anomalies and reduces the need for emergency interventions.
Recent studies have demonstrated that cloud-integrated health monitoring systems contribute to improved patient outcomes by enabling predictive analytics and personalized healthcare plans. ML algorithms deployed in the cloud analyze vast datasets, recognizing trends and deviations indicative of potential health risks. This proactive approach enhances preventive care and supports timely medical decisions.
Moreover, cloud-based architectures provide scalability and flexibility, allowing integration with IoT-enabled sensors and wearable devices [64]. This interconnected system ensures that elderly individuals receive tailored healthcare interventions based on real-time physiological data. Additionally, cloud platforms enhance data security and interoperability, addressing concerns related to patient privacy and compliance with medical standards.
Our proposed approach aligns with modern digital health initiatives by leveraging cloud technologies. This advancement underscores the potential of cloud-based health monitoring to revolutionize elderly care.
Integrating cloud computing with health monitoring opens new research directions in digital health. This study provides a framework for future research in personalized healthcare. The proposed methodology optimizes algorithms for real-time health assessment and anomaly detection. It also emphasizes the need for interdisciplinary collaboration between computer science, medicine, and data analytics to enhance predictive healthcare models.
From a practical perspective, our findings offer valuable insights for healthcare providers, policymakers, and technology developers. The proposed system enables remote and continuous patient monitoring, reducing the burden on healthcare facilities while ensuring timely medical interventions. This is particularly relevant for elderly care, where early detection of health deterioration can improve patient outcomes. The scalability of cloud-based solutions allows easy integration with wearable technologies and IoT devices, ensuring accessibility in various healthcare settings.
The study presents some methodological constraints associated with the evaluation conducted on a single subject, which limits the generalization of the results to larger populations. Therefore, the authors acknowledge that the model’s generalization has not been assessed. Although representative over the two years, the number of recordings may not cover all possible clinical variations. During the tests, the total number of aggregated daily records was 676. The number of raw records for the parameters AR, mR, and MR was 725,338, the number of records for the parameter S was 9455, and the number of records for the parameters DST and SST was 680. The patient’s age at the beginning of the monitoring was 69 years, as they were born in 1954.
The performance of the anomaly detection algorithm identified 18 anomalies. Of these, nine were real anomalies, and nine were false anomalies. Based on these values, the standard metrics were identified with the following scores: precision: 50%, recall: 100%, F1-Score: 33%, and accuracy: 98.5%.
The final model hyperparameters include Randomized PCA. Tests showed that detecting non-urgent anomalies is preferred to avoiding missing critical ones. The final hyperparameter configuration after tests is rank = 6, ensureZeroMean = true, seed = 1. Tests confirmed that detecting non-urgent anomalies is preferable to missing urgent events, ensuring robust patient monitoring.
The study’s focus on a single patient was intentional to keep the scope manageable and ensure a more controlled, detailed analysis. While it is true that results based on a single individual may not fully account for inter-individual variability, the findings serve as a proof of concept. Future iterations of the system can include larger datasets that encompass more diverse patients with varying age ranges, pre-existing conditions, and medications.
The detection of anomalies in physiological data is a widely studied topic. Traditional methods such as Isolation Forest and One-Class SVM have been used to identify deviations in vital signs. However, these methods are often sensitive to noise in real-time collected data. In this paper, PCA was investigated to analyze variations in data. The HADA uses Randomized PCA to demonstrate detection efficiency by integrating into an IoT-cloud infrastructure. Table 3 presents the comparative analysis between the state-of-the-art and the HADA as a proof of concept. In Table 3, NS indicates that the value is not specified.

Table 3.
Comparative analysis of algorithms from the state-of-the-art and HADA.
In this research, the PCA method was integrated into the HADA to study the necessity of dimensionality reduction correlated with the identification of monitored physiological parameters, which contribute to the main objective of the study. By analyzing each parameter in isolation, it was demonstrated that subtle anomalies resulting from unitary monitoring cannot be detected. Tests conducted without using PCA resulted in an accuracy of less than 90%. In comparison, the use of PCA within the HADA model resulted in an accuracy of 98.5%. This value demonstrates that the simultaneous and contextual analysis of all variables improves anomaly detection.
HADA balances accuracy and response time compared to existing methods. Although the model presented by Ebadinezhad and Mobolade [56] achieves 100% accuracy, it requires 16.3 s of processing time and an exclusively cloud-based infrastructure, which limits its use in IoT systems. In contrast, HADA offers 98.5% accuracy with a processing time of 3.5 s. This makes the PCA model more suitable for applications that require a quick response.
Compared to RNN-based methods (Autoencoder and LSTM-CNN), HADA offers competitive performance without requiring high computational resources. Although Autoencoder is extremely fast (3 ms), it requires Edge Computing infrastructure, which is challenging to implement in standard IoT solutions. In contrast, the algorithm integrated into HADA can run on an IoT and cloud architecture, reducing response time without affecting anomaly detection.
Lastly, the concern regarding the system’s precision and the possibility of false alarms is valid. However, it is important to understand that the system’s high recall rate and sensitivity ensure that no health issues are left undetected. As we continue to refine the system, future work could focus on improving the precision, potentially through more advanced anomaly detection techniques and by incorporating real-world testing with larger populations.
The authors chose to generalize the HADA using modern techniques represented by synthetic data. This is justified by the need to continuously monitor a large number of elderly people over a long period, at least one year. This would also require their declaration regarding consent for data use, as was performed with the real dataset presented earlier. Moreover, the present research does not represent a clinical study but rather a hardware–software prototype that can be developed for production with specific implications for medical protocols. Any medicated device must undergo a series of standard stages before it can be used and is not the subject of the present research.
As mentioned, synthetic data was added to the tests to generalize the model. Thus, six datasets were introduced for six elderly individuals. The synthetic data were generated according to the values in the specialized literature. For elderly individuals without specific heart conditions, the normal heart rate range is between 60 and 100 beats per minute (bpm). This value is influenced by factors such as medication, hypertension, heart disease, or other stress factors [65]. Additionally, elderly individuals experience changes in sleep architecture, according to the study conducted by Hwang et al. [66]. Thus, elderly people spend 10–15% of the total 7–8 h in deep sleep. Superficial sleep corresponds to a more extended period and is associated with the first two stages of sleep. Therefore, out of the total 7–8 h of sleep, an elderly person spends, on average, 1–2 h in deep sleep and 4–5 h in shallow sleep [67,68].
Regarding regular physical activities, the general recommendation for the elderly is at least 150 min of moderate to intense aerobic activity per week. This corresponds to 7000–8000 daily steps for a healthy older adult [69].
Considering these observations, the six experimental datasets were generated according to the standard values. Each dataset contains 1000 synthetic data points. Of the 1000 data points, 30% contain intentionally generated anomalies to simulate an emergency. According to the literature, the anomalies correspond to exceedances of the values considered normal. These observations generated six experimental datasets based on typical values. Each dataset contains 1000 synthetic data points. Of the 1000 data points, 1% contain intentionally generated anomalies to simulate an emergency. According to the literature, the anomaly corresponds to exceedances of the values considered normal. The methodology used for generating synthetic data is as follows:
For each of the six datasets, 99% of records were generated to comply with the standard limits for all parameters:
- AR has values between 60 and 100 bpm;
- mR has a lower value than AR but above 60 bpm;
- MR has a value greater than AR but less than 100 bpm;
- S—between 6000 and 9000;
- DST—between 20 and 120 min;
- SST—between 240 and 300 min.
To simulate atypical situations, 1% of records were generated with values that exceeded the normal limits in the literature. In each case, only one column was modified. In this way, a single anomaly is reflected by generating values outside the normal range. The standard and abnormal records were randomly mixed to prevent artificial grouping of the data. This ensures that the model learns a realistic distribution of the data. This approach also prevents the model from learning the positions of anomalies. Finally, the six datasets, each file containing 1000 rows, were saved in text format and used to validate the HADA.
Figure 14 presents the accuracy results obtained for executing the HADA using the six synthetic datasets. These results confirm that the model achieves an accuracy of over 98%. The values obtained through the generalization of tests confirm HADA’s capacity for early anomaly detection. This behavior helps prevent medical complications.

Figure 14.
HADA accuracy (>98%) in early anomaly detection using synthetic datasets.
The results presented in Figure 14 confirm that the HADA can be used in real-world scenarios. The authors state that the model cannot be validated for production using such a small number of cases. However, this research does not represent a clinical study but rather a demonstration of integrating an anomaly detection algorithm into a modern cloud infrastructure, represented by the Azure tool suite.
From the Results section, the answers to the RQs stated in the introduction are as follows:
RQ1. The study demonstrates that the Microsoft Azure infrastructure is compatible with implementing a continuous monitoring system for the vital parameters of elderly individuals. This solution uses an integrated data stream that connects IoT sensors to Azure cloud services via ASP.NET APIs. The data collected from the MAX30100 sensors (for heart rate monitoring) and MPU-6050 (for step count and sleep quality) is transmitted in real-time to Azure IoT Hub, where it is processed using Azure Functions. The HADA, implemented in C# using the ML.NET library, analyzes the data via the PCA method to identify anomalies. The results are stored in an Azure SQL Database, and alerts are automatically generated through Azure Communication Services, which sends SMS notifications to the medical staff. The tests conducted showed that the average alert time is approximately 11 s. These results demonstrate HADA’s ability to provide support to the elderly through the proposed hardware–software configuration.
RQ2. The implementation of the HADA demonstrates that PCA is an appropriate method for anomaly detection in vital parameter monitoring. The algorithm simultaneously analyzes six key parameters: average heart rate (AR), minimum heart rate (mR), maximum heart rate (MR), number of steps (S), time spent in deep sleep (DST), and time spent in light sleep (SST). HADA uses Randomized PCA from the ML.NET library to reduce the dimensionality of the data. This process allows for the detection of complex correlations between parameters, providing a multidimensional analysis that would not be possible through the individual examination of each parameter. The test results show that HADA achieves an accuracy of 98.5% in anomaly detection. For model validation, synthetic datasets were introduced to simulate generalized scenarios. These sets contain normal values and values that exceed the standard limits for vital parameters. Tests conducted on this synthetic data have confirmed that HADA maintains an accuracy of over 98%. These values demonstrate HADA’s ability to be applied in real-world scenarios.
RQ3. The wearable device highlighted the ability to detect subtle changes in health status. This aspect highlights the feasibility of these proof-of-concept systems for use in early interventions that can prevent medical complications. The proposed wearable device, based on the M5Stack module, integrates the MAX30100 and MPU-6050 sensors to collect vital data continuously. The device utilizes the ESP32 microcontroller, which operates at a frequency of 240 MHz and is programmed using the Arduino IDE environment and the C++ programming language. To optimize energy consumption, the M5Stack module is configured to enter deep sleep mode between measurements, thereby extending the battery life to 22 h. Tests conducted over two years have shown that the device can detect subtle changes in the patient’s health, such as abnormal increases in heart rate or significant decreases in sleep quality. These anomalies are identified using the HADA, which generates real-time alerts. The study reported a sensitivity of 100% and an accuracy of 98.5%. These values highlight the feasibility of this conceptual system for preventing medical complications.
Research of this nature requires ongoing funding, which becomes accessible after the proof of concept is published. Once the idea is publicly demonstrated and validated, attracting the necessary financial support for further development and scaling becomes easier.
The HADA system provides a strong foundation for further exploration into elderly care and health monitoring, demonstrating the viability of real-time, data-driven interventions, with the proposed infrastructure never investigated before in the literature. While this research does not claim to offer a fully mature commercial product, it provides a promising solution that can be further developed with appropriate funding and support. With additional testing, optimization, and comparisons to alternative methods, this system could be invaluable in enhancing the quality of care for elderly individuals. The authors believe that, with the right investment, this solution has the potential to make a significant impact in the field of remote health monitoring.
5. Conclusions
This work addresses the issue of implementing an integrated IoT system for monitoring and anomaly detection. The proposed prototype improves medical intervention time by facilitating the early detection of situations that pose a risk to the elderly’s lives. The results show that the proposed prototype—a wearable bracelet equipped with MAX30100 and MPU-6050 sensors, combined with an anomaly detection algorithm based on PCA and integration with the Azure cloud infrastructure—can monitor the vital parameters of the elderly. The proposed system generates alerts within an average interval of approximately 11 s. The system reported a sensitivity of 100% and an accuracy of 98.5%. These results confirm the method’s feasibility for early detection of physiological changes.
The research involved developing and testing a hardware prototype, implementing a custom anomaly detection algorithm, and conducting continuous evaluations over two years for an elderly subject.
Integrating cloud technologies with innovative data analysis methods enables continuous and personalized health monitoring of the elderly, contributing to the fields of medicine and healthcare.
Future studies will include a more diverse sample of patients, covering different age groups and medical conditions, to validate and extend the system’s applicability. Additionally, the authors plan to optimize the detection algorithms to reduce the rate of false alerts, which could be achieved by integrating additional machine learning methods. Extending functionality by adding other types of sensors and improving communication between the device and the cloud infrastructure will also represent important directions for development.
In conclusion, the study demonstrates the superior quality of Microsoft Azure’s technologies in implementing IoT solutions, even when these involve monitoring sensitive data that can impact people’s lives. The research falls within the field of medicine and healthcare, supporting medical staff and elderly individuals who require monitoring but are not in an advanced stage of illness. As long as they are healthy, mobile, and wish to remain home, they can be automatically monitored remotely.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15137272/s1, Heart_Rate.txt; Steps.txt; Sleep_Phases.txt; Battery_Level.txt.
Author Contributions
Conceptualization, C.-M.R.; methodology, C.-M.R. and A.S.; software, C.-M.R.; validation, C.-M.R. and A.S.; formal analysis, C.-M.R. and A.S.; investigation, A.S.; resources, A.S.; data curation, C.-M.R.; writing—original draft preparation, C.-M.R. and A.S.; writing—review and editing, A.S.; visualization, C.-M.R. and A.S.; supervision, C.-M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Petroleum-Gas University of Ploiesti, Romania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADC | Analog-to-digital converter |
| AI | Artificial intelligence |
| AR | Average heart rate |
| AWS | Amazon Web Services |
| BPM | Beats per minute |
| CNN | Convolutional neural network |
| CPU | Central Processing Unit |
| DST | Deep sleep time |
| ECG | Electrocardiogram |
| FN | False negative |
| FP | False positive |
| GCP | Google Cloud Platform |
| GDPR | General Data Protection Regulation |
| GPIO | General Purpose Input/Output |
| GPS | Global Positioning System |
| HADA | Health anomaly detection algorithm |
| ID | Identification code |
| IoT | Internet of Things |
| IR-UWB | Impulse-Radio Ultra-Wideband |
| LED | Light-Emitting Diode |
| LoRa | Long Range |
| LSD | Liquid crystal display |
| LSTM | Long short-term memory |
| LTE | Long-Term Evolution |
| LTE-M | Long-Term Evolution for Machines |
| MAE | Mean absolute error |
| MKNe | Multi-Kernel Network |
| MQTT | Message Queuing Telemetry Transport |
| mR | Minimum heart rate |
| MR | Maximum heart rate |
| NB-IoT | Narrowband IoT |
| NS | Not specified |
| NTC | Negative Temperature Coefficient |
| PCA | Principal component analysis |
| PD | Photo-detector |
| PPG | Photoplethysmogram |
| RAM | Random-access memory |
| RF | Random forest |
| RFID | Radio Frequency Identification |
| RFID | Radio frequency identification |
| RMSE | Root mean squared error |
| RQ | Research question |
| RTC | Real-Time Clock |
| S | Number of steps |
| SIM | Subscriber Identity Module |
| SMS | Short message service |
| SSL | Secure sockets layer |
| SST | Superficial sleep time |
| SVM | Support Vector Machine |
| TLS | Transport layer security |
| TN | True negative |
| TP | True positive |
| Wi-Fi | Wireless fidelity |
| WOS | Web of Science |
| WPN | Wireless personal network |
References
- Anghelache, C.; Anghel, M.-G.; Iacob, Ș.V.; Panait, M.; Rădulescu, I.G.; Brezoi, A.G.; Miron, A. The Effects of Health Crisis on Economic Growth, Health and Movement of Population. Sustainability 2022, 14, 4613. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Bold, R.-A.; Gerea, A.-E. A Comprehensive Patient Triage Algorithm Incorporating ChatGPT API for Symptom-Based Healthcare Decision-Making. In Emerging Trends and Technologies on Intelligent Systems, Proceedings of the 4th International Conference on Emerging Trends and Technologies on Intelligent Systems (ETTIS 2024), Noida, India, 27–28 March 2024; Lecture Notes in Networks and Systems; Springer: Singapore, 2025; pp. 167–178. [Google Scholar] [CrossRef]
- Areia, C.; Biggs, C.; Santos, M.; Thurley, N.; Gerry, S.; Tarassenko, L.; Watkinson, P.; Vollam, S. The impact of wearable continuous vital sign monitoring on deterioration detection and clinical outcomes in hospitalised patients: A systematic review and meta-analysis. Crit. Care 2021, 25, 351. [Google Scholar] [CrossRef]
- Van Velthoven, M.H.; Oke, J.; Kardos, A. ChroniSense National Early Warning Score Study: Comparison Study of a Wearable Wrist Device to Measure Vital Signs in Patients Who Are Hospitalized. J. Med. Internet Res. 2023, 25, e40226. [Google Scholar] [CrossRef] [PubMed]
- Leenen, J.P.L.; Ardesch, V.; Patijn, G. Remote Home Monitoring of Continuous Vital Sign Measurements by Wearables in Patients Discharged After Colorectal Surgery: Observational Feasibility Study. JMIR Perioper. Med. 2023, 6, e45113. [Google Scholar] [CrossRef] [PubMed]
- Breteler, M.J.M.; Numan, L.; Ruurda, J.P.; Van Hillegersberg, R.; Van Der Horst, S.; Dohmen, D.A.J.; Van Rossum, M.C.; Kalkman, C.J. Wireless Remote Home Monitoring of Vital Signs in Patients Discharged Early After Esophagectomy: Observational Feasibility Study. JMIR Perioper. Med. 2020, 3, e21705. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.I.; Xu, W.; Penfold, J.A.; Keane, C.; Gharibans, A.A.; Bissett, I.P.; O’Grady, G. Wearable devices to monitor recovery after abdominal surgery: Scoping review. BJS Open 2022, 6, zrac031. [Google Scholar] [CrossRef]
- van Rossum, M.C.; Vlaskamp, L.B.; Posthuma, L.M.; Visscher, M.J.; Breteler, M.J.M.; Hermens, H.J.; Kalkman, C.J.; Preckel, B. Adaptive threshold-based alarm strategies for continuous vital signs monitoring. J. Clin. Monit. Comput. 2022, 36, 407–417. [Google Scholar] [CrossRef]
- Brekke, I.J.; Puntervoll, L.H.; Pedersen, P.B.; Kellett, J.; Brabrand, M. The value of vital sign trends in predicting and monitoring clinical deterioration: A systematic review. PLoS ONE 2019, 14, e0210875. [Google Scholar] [CrossRef]
- Leenen, J.P.L.; Leerentveld, C.; Van Dijk, J.D.; Van Westreenen, H.L.; Schoonhoven, L.; Patijn, G.A. Current Evidence for Continuous Vital Signs Monitoring by Wearable Wireless Devices in Hospitalized Adults: Systematic Review. J. Med. Internet Res. 2020, 22, e18636. [Google Scholar] [CrossRef]
- Polat, E.O.; Mercier, G.; Nikitskiy, I.; Puma, E.; Galan, T.; Gupta, S.; Montagut, M.; Piqueras, J.J.; Bouwens, M.; Durduran, T.; et al. Flexible graphene photodetectors for wearable fitness monitoring. Sci. Adv. 2019, 5, eaaw7846. [Google Scholar] [CrossRef]
- Chao, C.-H. Development of smart IoT based biomedical heart rate oximeter for driving safety. Acad. J. Eng. Technol. Sci. 2023, 6, 1–6. [Google Scholar] [CrossRef]
- Gielen, W.; Longoria, K.A.; Van Mourik, R.A. Two cases of COVID-19 monitored by a wearable biosensor—A case report. mHealth 2021, 7, 62. [Google Scholar] [CrossRef]
- Mack, I.; Juchler, N.; Rey, S.; Hirsch, S.; Hoelz, B.; Eckstein, J.; Bielicki, J. Wearable Technologies for Pediatric Patients with Surgical Infections—More than Counting Steps? Biosensors 2022, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Rosca, C.M.; Stancu, A.; Ariciu, A.V. Algorithm for child adoption process using artificial intelligence and monitoring system for children. Internet Things 2024, 26, 101170. [Google Scholar] [CrossRef]
- Barteit, S.; Boudo, V.; Ouedraogo, A.; Zabré, P.; Ouremi, L.; Sié, A.; Munga, S.; Obor, D.; Kwaro, D.; Huhn, S.; et al. Feasibility, acceptability and validation of wearable devices for climate change and health research in the low-resource contexts of Burkina Faso and Kenya: Study protocol. PLoS ONE 2021, 16, e0257170. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, A. Artificial Intelligence vs. Efficient Markets: A Critical Reassessment of Predictive Models in the Big Data Era. Electronics 2025, 14, 1721. [Google Scholar] [CrossRef]
- Rathore, F.A.; Rathore, M.A. The Emerging Role of Artificial Intelligence in Healthcare. J. Pak. Med. Assoc. 2023, 73, 1368–1369. [Google Scholar] [CrossRef]
- Khan, A. Transforming Healthcare through AI: Unleashing the Power of Personalized Medicine. Int. J. Multidiscip. Sci. Arts 2023, 2, 67–77. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A. A Review of Neuro-ML Breakthroughs in Addressing Neurological Disorders. Appl. Sci. 2025, 15, 5442. [Google Scholar] [CrossRef]
- Zaid Salah, O.; Selvaperumal, S.K.; Abdulla, R. Accelerometer-based elderly fall detection system using edge artificial intelligence architecture. Int. J. Electr. Comput. Eng. 2022, 12, 4430–4438. [Google Scholar] [CrossRef]
- Lekkala, L.R. How AI Analytical Models Can Use FHIR (Fast Healthcare Interoperability Resources) Data. Voice Publ. 2023, 9, 197–206. [Google Scholar] [CrossRef]
- Devi, K.J.; Alghamdi, W.; N, D.; Alkhayyat, A.; Sayyora, A.; Sathish, T. Artificial Intelligence in Healthcare: Diagnosis, Treatment, and Prediction. In Proceedings of the International Conference on Newer Engineering Concepts and Technology, Tiruchirappalli, India, 27–28 April 2023; p. 04043. [Google Scholar] [CrossRef]
- Rangavittal, P.B. Evolving Role of AI in Enhancing Patient Care within Digital Health Platforms. J. Artif. Intell. Cloud Comput. 2022, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, D.Y.; Kim, D.H.; Lee, J.H.; Lee, J.I.; Kim, M.J.; Park, J.Y.; Hwang, J.H.; Yun, S.S.; Choi, B.K.; et al. Conversational Artificial Intelligence for Spinal Pain Questionnaire: Validation and User Satisfaction. Neurospine 2022, 19, 348–356. [Google Scholar] [CrossRef]
- Addobea, A.A.; Li, Q.; Amankona, I.O.; Hou, J. A Batch Processing Technique for Wearable Health Crowd-Sensing in the Internet of Things. Cryptography 2022, 6, 33. [Google Scholar] [CrossRef]
- Ma, X. Analysis of Human Exercise Health Monitoring Data of Smart Bracelet Based on Machine Learning. Comput. Intell. Neurosci. 2022, 2022, 7971904. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, A.; Cusumano, G.; La Barbera, A.L.; La Parola, V.L.; Lombardi, S. Application of Machine Learning Ensemble Methods to ASTRI Mini-Array Cherenkov Event Reconstruction. Appl. Sci. 2023, 13, 8172. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Yu, L.; Liu, J.; Zwetsloot, I.M.; Cabrera, J.; Tsui, K.-L. A Personalized Health Monitoring System for Community-Dwelling Elderly People in Hong Kong: Design, Implementation, and Evaluation Study. J. Med. Internet Res. 2020, 22, e19223. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Zhou, H.; Wei, Y.; Yu, Y.; Lu, S.; An, X.; Liu, Q. Intelligent Gateway Based Human Cardiopulmonary Health Monitoring System. J. Sens. 2023, 2023, 3534224. [Google Scholar] [CrossRef]
- Anzalone, A.; Pagliaro, A.; Tutone, A. An Introduction to Machine and Deep Learning Methods for Cloud Masking Applications. Appl. Sci. 2024, 14, 2887. [Google Scholar] [CrossRef]
- Tang, H.; Wang, T.; Li, M.; Yang, X. The Design and Implementation of Cardiotocography Signals Classification Algorithm Based on Neural Network. Comput. Math. Methods Med. 2018, 2018, 568617. [Google Scholar] [CrossRef]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Althobiani, M.A.; Khan, B.; Shah, A.J.; Ranjan, Y.; Mendes, R.G.; Folarin, A.; Mandal, S.; Porter, J.C.; Hurst, J.R. Clinicians’ Perspectives of Wearable Technology to Detect and Monitor Exacerbations of Chronic Obstructive Pulmonary Disease: Mixed-Method Survey. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1401–1412. [Google Scholar] [CrossRef]
- Venn, R.A.; Khurshid, S.; Grayson, M.; Ashburner, J.M.; Al-Alusi, M.A.; Chang, Y.; Foulkes, A.; Ellinor, P.T.; McManus, D.D.; Singer, D.E.; et al. Characteristics and Attitudes of Wearable Device Users and Nonusers in a Large Health Care System. J. Am. Heart Assoc. 2024, 13, e032126. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Paulo Silva Cunha, J. Wearable Health Devices—Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef]
- Hamasaki, H. Efficacy of Wearable Devices to Measure and Promote Physical Activity in the Management of Diabetes. EMJ Diabetes 2018, 6, 62–69. [Google Scholar] [CrossRef]
- Alsayaydeh, J.A.J.; Yusof, M.F.B.; Halim, M.Z.B.A.; Zainudin, M.N.S.; Herawan, S.G. Patient Health Monitoring System Development using ESP8266 and Arduino with IoT Platform. Int. J. Adv. Comput. Sci. Appl. 2023, 14, 617–624. [Google Scholar] [CrossRef]
- Choudhary, A.S.; Itoo, A.R.; Singh, U.A.; Shedge, R.; Tundalwar, A.; Patil, R. IOT Based Remote Health Monitoring System for Discharged Patients. Int. J. Res. Appl. Sci. Eng. Technol. 2023, 11, 597–603. [Google Scholar] [CrossRef]
- Sheikh, P.P.; Riyad, T.; Tushar, B.D.; Alam, S.S.; Ruddra, I.M.; Shufian, A. Analysis of Patient Health Using Arduino and Monitoring System. J. Eng. Res. Rep. 2024, 26, 25–33. [Google Scholar] [CrossRef]
- Taştan, M. IoT Based Wearable Smart Health Monitoring System. Celal Bayar Üniversitesi Fen. Bilim. Derg. 2018, 14, 343–350. [Google Scholar] [CrossRef]
- Wan, J.; Al-Awlaqi, M.A.A.H.; Li, M.; O’Grady, M.; Gu, X.; Wang, J.; Cao, N. Wearable IoT enabled real-time health monitoring system. EURASIP J. Wirel. Commun. Netw. 2018, 2018, 298. [Google Scholar] [CrossRef]
- Reddy, Y.M.; Pavani, B. Machine Learning Algorithm for Early Detection of Heart Diseases Using 3-Tier IoT Architecture. Int. J. Eng. Adv. Technol. 2019, 8, 1877–1882. [Google Scholar] [CrossRef]
- Oikonomou, F.P.; Mantas, G.; Cox, P.; Bashashi, F.; Gil-Castineira, F.; Gonzalez, J. A Blockchain-based Architecture for Secure IoT-based Health Monitoring Systems. In Proceedings of the 26th International Workshop on Computer Aided Modeling and Design of Communication Links and Networks, Porto, Portugal, 25–27 October 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Naresh, V.S.; Reddi, S.; Murthy, N.V.E.S. Secure Lightweight IoT Integrated RFID Mobile Healthcare System. Wirel. Commun. Mob. Comput. 2020, 2020, 1468281. [Google Scholar] [CrossRef]
- Seo, S.; Jo, H.; Kim, J.; Lee, B.; Bien, F. An ultralow power wearable vital sign sensor using an electromagnetically reactive near field. Bioeng. Transl. Med. 2023, 8, e10502. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, P.; Jin, H.; Ma, J.; Qin, L. Vital Sign Measurement in Telemedicine Rehabilitation Based on Intelligent Wearable Medical Devices. IEEE Access 2019, 7, 54819–54823. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, Y.; Choi, Y.-W.; Heo, R.; Park, H.-K.; Cho, S.-H.; Cho, S.H.; Lim, Y.-H. Preclinical Evaluation of a Noncontact Simultaneous Monitoring Method for Respiration and Carotid Pulsation Using Impulse-Radio Ultra-Wideband Radar. Sci. Rep. 2019, 9, 11892. [Google Scholar] [CrossRef]
- Vinci, G.; Lenhard, T.; Will, C.; Koelpin, A. Microwave interferometer radar-based vital sign detection for driver monitoring syst. In Proceedings of the MTT-S International Conference on Microwaves for Intelligent Mobility, Heidelberg, Germany, 27–29 April 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Y.; Hou, W.; Wu, X.; Chen, L. An Automatic Remote Health Risk Assessment system based on LSTM for elderly. In Proceedings of the 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Del-Valle-Soto, C.; Valdivia, L.J.; López-Pimentel, J.C.; Visconti, P. Comparison of Collaborative and Cooperative Schemes in Sensor Networks for Non-Invasive Monitoring of People at Home. Int. J. Environ. Res. Public Health 2023, 20, 5268. [Google Scholar] [CrossRef]
- Shumba, A.-T.; Montanaro, T.; Sergi, I.; Fachechi, L.; De Vittorio, M.; Patrono, L. Leveraging IoT-Aware Technologies and AI Techniques for Real-Time Critical Healthcare Applications. Sensors 2022, 22, 7675. [Google Scholar] [CrossRef]
- Said, A.M.; Yahyaoui, A.; Abdellatif, T. Efficient Anomaly Detection for Smart Hospital IoT Systems. Sensors 2021, 21, 1026. [Google Scholar] [CrossRef]
- Gayathri, R.; Maheswari, S.; Mathivanan, S.K.; Shivahare, B.D.; Chandan, R.R.; Shah, M.A. A comprehensive health assessment approach using ensemble deep learning model for remote patient monitoring with IoT. Sci. Rep. 2024, 14, 15661. [Google Scholar] [CrossRef]
- Kishor Kumar Reddy, C.; Kaza, V.S.; Madana Mohana, R.; Alhameed, M.; Jeribi, F.; Alam, S.; Shuaib, M. Detecting anomalies in smart wearables for hypertension: A deep learning mechanism. Front. Public Health 2025, 12, 1426168. [Google Scholar] [CrossRef]
- Ebadinezhad, S.; Mobolade, T.E. A Novel Cloud-Based IoT Framework for Secure Health Monitoring. Sustainability 2024, 16, 1349. [Google Scholar] [CrossRef]
- Shahid, Z.K.; Saguna, S.; Åhlund, C. Detecting Anomalies in Daily Activity Routines of Older Persons in Single Resident Smart Homes: Proof-of-Concept Study. JMIR Aging 2022, 5, e28260. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A. Earthquake Prediction and Alert System Using IoT Infrastructure and Cloud-Based Environmental Data Analysis. Appl. Sci. 2024, 14, 10169. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A.; Neculaiu, C.-F.; Gortoescu, I.-A. Designing and Implementing a Public Urban Transport Scheduling System Based on Artificial Intelligence for Smart Cities. Appl. Sci. 2024, 14, 8861. [Google Scholar] [CrossRef]
- Rosca, C.M.; Popescu, M.; Patrascioiu, C.; Stancu, A. Comparative Analysis of pH Level Between Pasteurized and UTH Milk Using Dedicated Developed Application. Rev. De Chim. 2019, 70, 3917–3920. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Chu, C.-H.; Liu, S.-W.; Hsieh, S.-Y.; Tseng, V.S. Mining Life Patterns from Wearable Sensors Data for Elderly Anomaly Detection. In Proceedings of the Conference on Technologies and Applications of Artificial Intelligence, Taipei, Taiwan, 1–3 December 2017; pp. 66–71. [Google Scholar] [CrossRef]
- Cola, G.; Avvenuti, M.; Vecchio, A.; Yang, G.-Z.; Lo, B. An On-Node Processing Approach for Anomaly Detection in Gait. IEEE Sens. J. 2015, 15, 6640–6649. [Google Scholar] [CrossRef]
- Fáñez, M.; Villar, J.R.; De La Cal, E.; González, V.M.; Sedano, J.; Khojasteh, S.B. Mixing user-centered and generalized models for Fall Detection. Neurocomputing 2021, 452, 473–486. [Google Scholar] [CrossRef]
- Rosca, C.-M.; Stancu, A. Integration of AI in Self-Powered IoT Sensor Systems. Appl. Sci. 2025, 15, 7008. [Google Scholar] [CrossRef]
- Njoba, S.; Ani, C.; Uzoigwe, Z.; Okeke, A.; Ugwuchukwu, N.; Anyaeji, P.; Odoh, C.; Ndubuisi, A.; Nworgu, C.; Ikwuka, D.; et al. Electrocardiogram parameters in patients with hypertensive heart failure in Abakaliki, South-Eastern Nigeria. J. Clin. Med. Res. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, K.M.; Yun, C.-H.; Yang, K.I.; Chu, M.K.; Kim, W.-J. Sleep state of the elderly population in Korea: Nationwide cross-sectional population-based study. Front. Neurol. 2023, 13, 1095404. [Google Scholar] [CrossRef]
- Li, X.; Ono, C.; Warita, N.; Shoji, T.; Nakagawa, T.; Usukura, H.; Yu, Z.; Takahashi, Y.; Ichiji, K.; Sugita, N.; et al. Comprehensive evaluation of machine learning algorithms for predicting sleep–wake conditions and differentiating between the wake conditions before and after sleep during pregnancy based on heart rate variability. Front. Psychiatry 2023, 14, 1104222. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhang, L.; Cui, Y.; Gong, Q.; Ma, J.; Wang, Y.; Sang, H. The association of sleep duration and quality with depressive symptoms in older Chinese women. PLoS ONE 2022, 17, e0262331. [Google Scholar] [CrossRef] [PubMed]
- Purnamasari, A.T.; Ulfiana, E.; Wahyudi, A.S. The Relationship of Physical Activity and Sleep Quality Among Elderly Who Are Still Working. Indones. J. Community Health Nurs. 2021, 6, 45–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).