Evolution of Microbial Community Structure and Denitrifying Functional Microorganisms in the Biological Sponge Iron System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Physicochemical Properties of the Experimental Wastewater
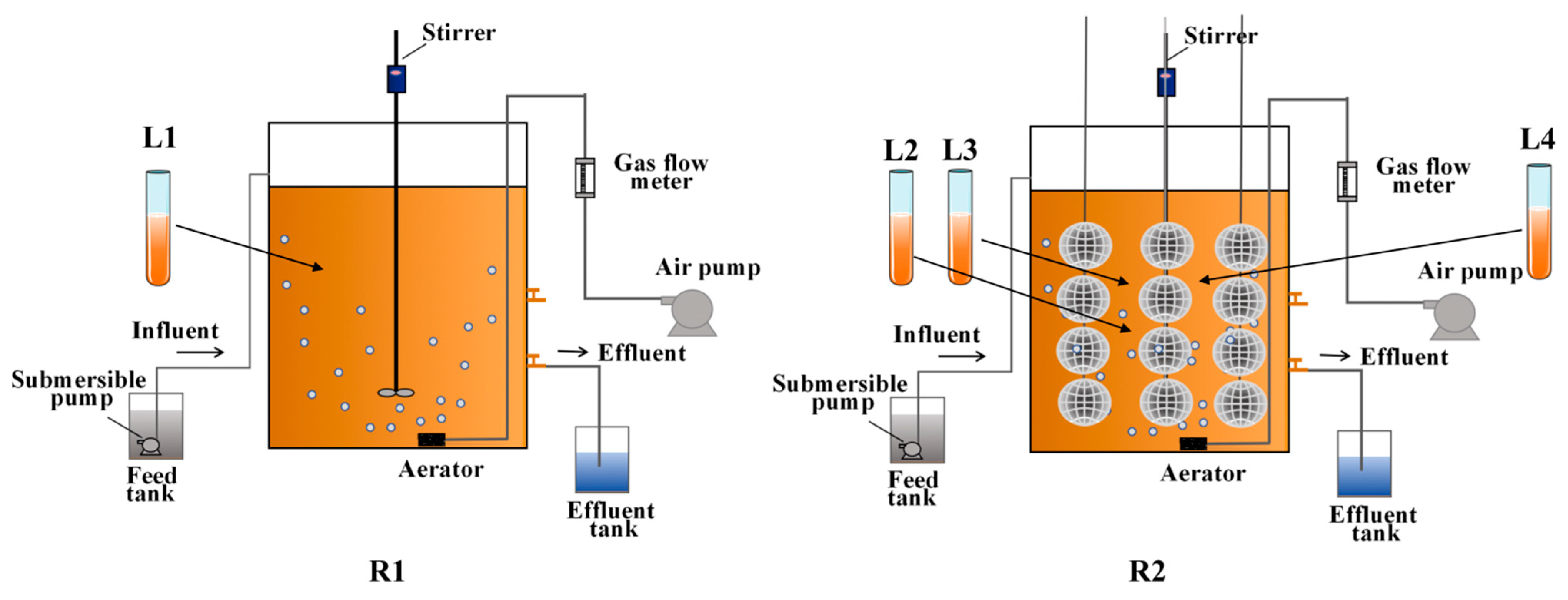
2.3. Experimental Setup
2.4. Test Method
2.5. Analysis of Microbial Community Composition
3. Results and Analysis
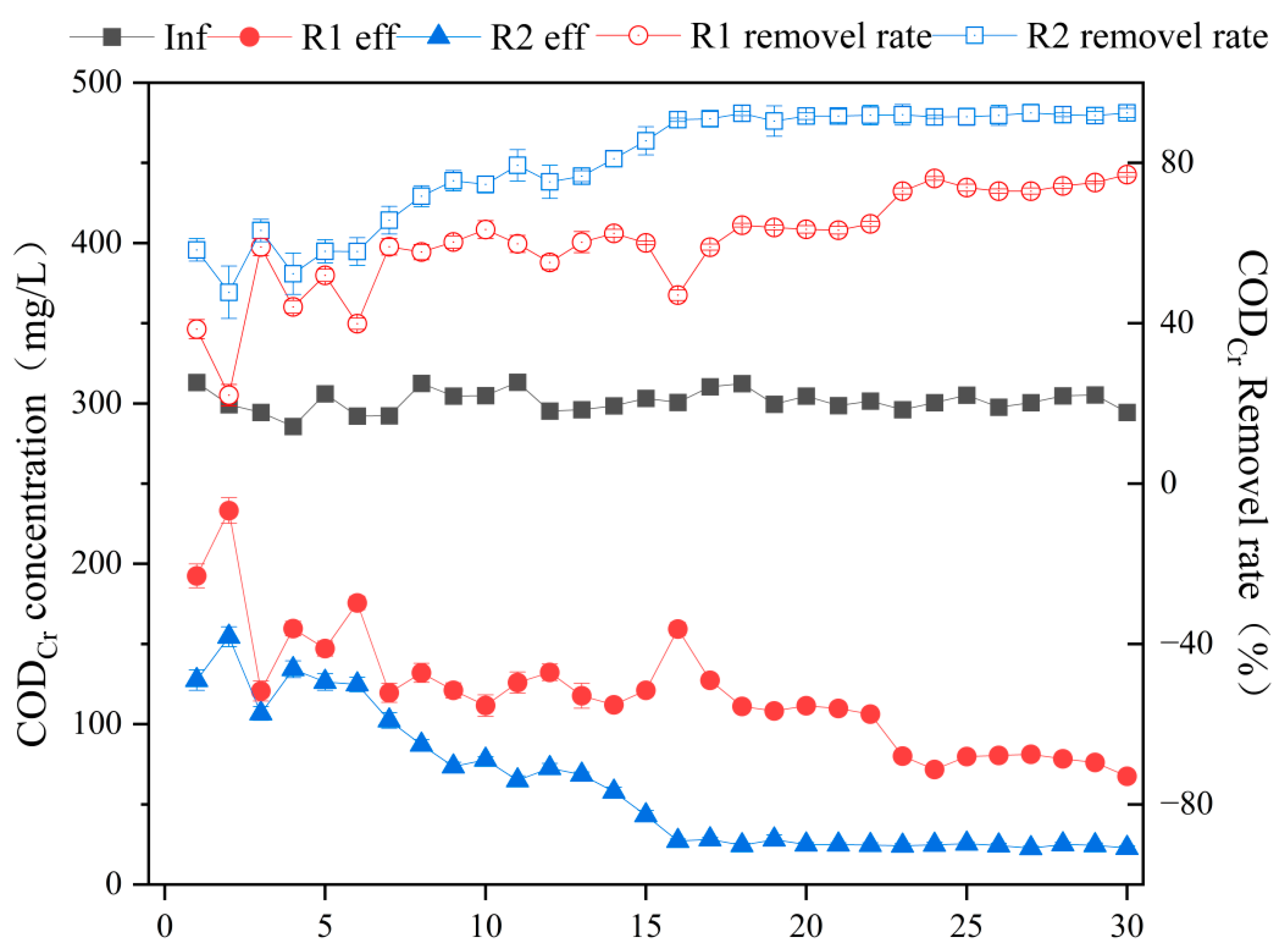
3.1. Study on the CODCr Removal Performance of a Biological Sponge Iron Reactor
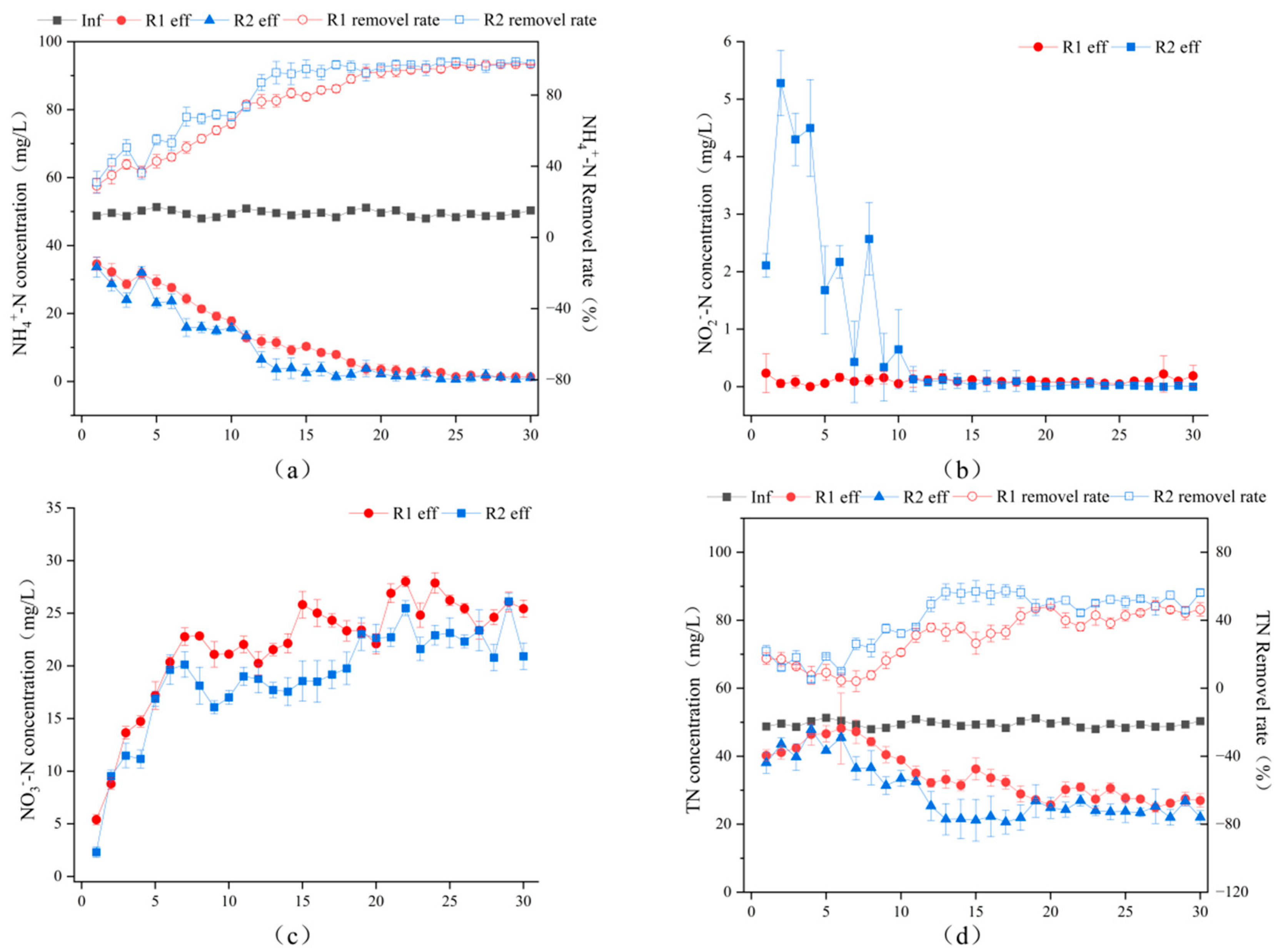
3.2. Study on the Nitrogen Removal Performance of a Biological Sponge Iron Reactor
3.3. The Impact of Sponge Iron on the Microbial Community Structure
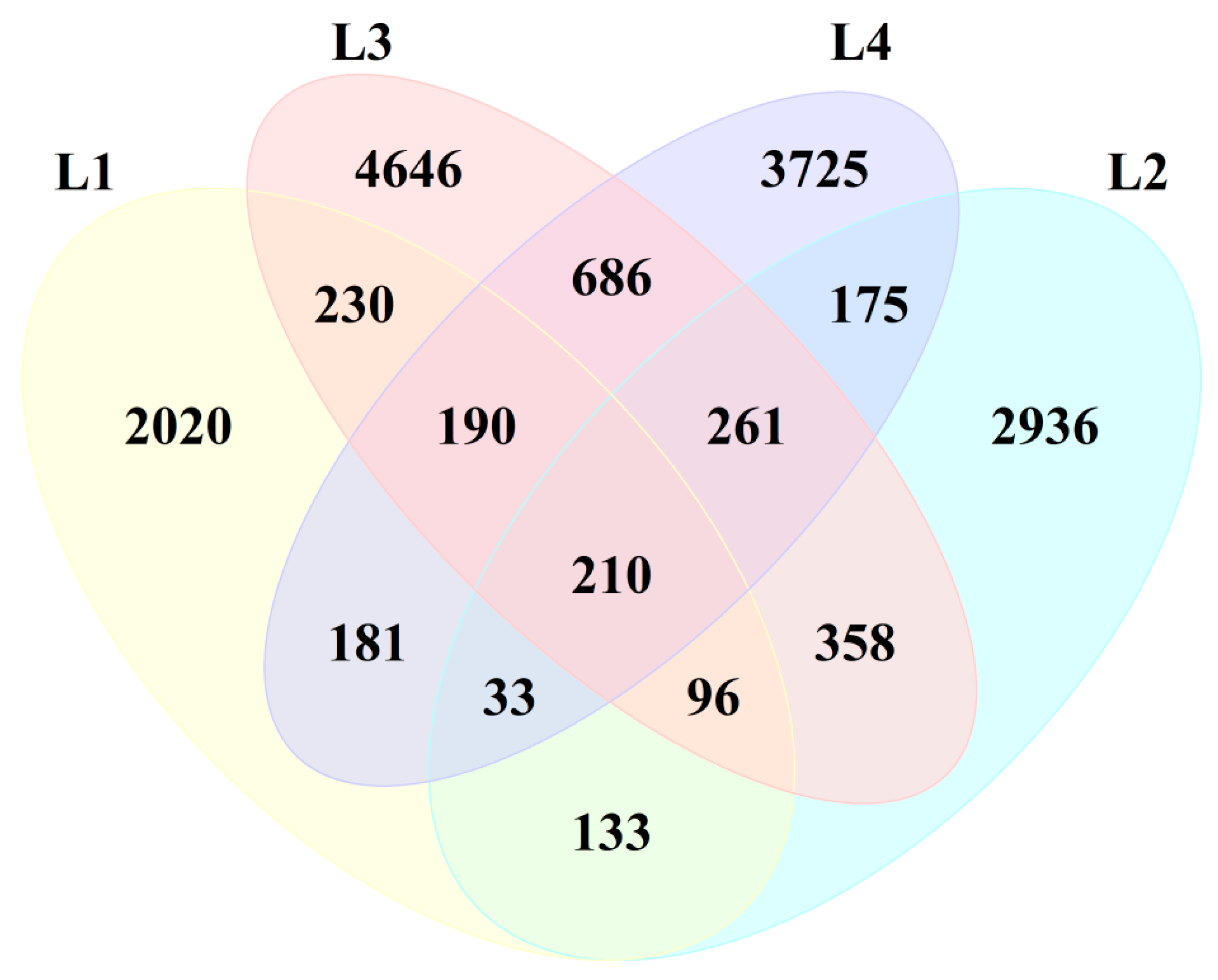
3.3.1. Analysis of Microbial Community Richness and Diversity
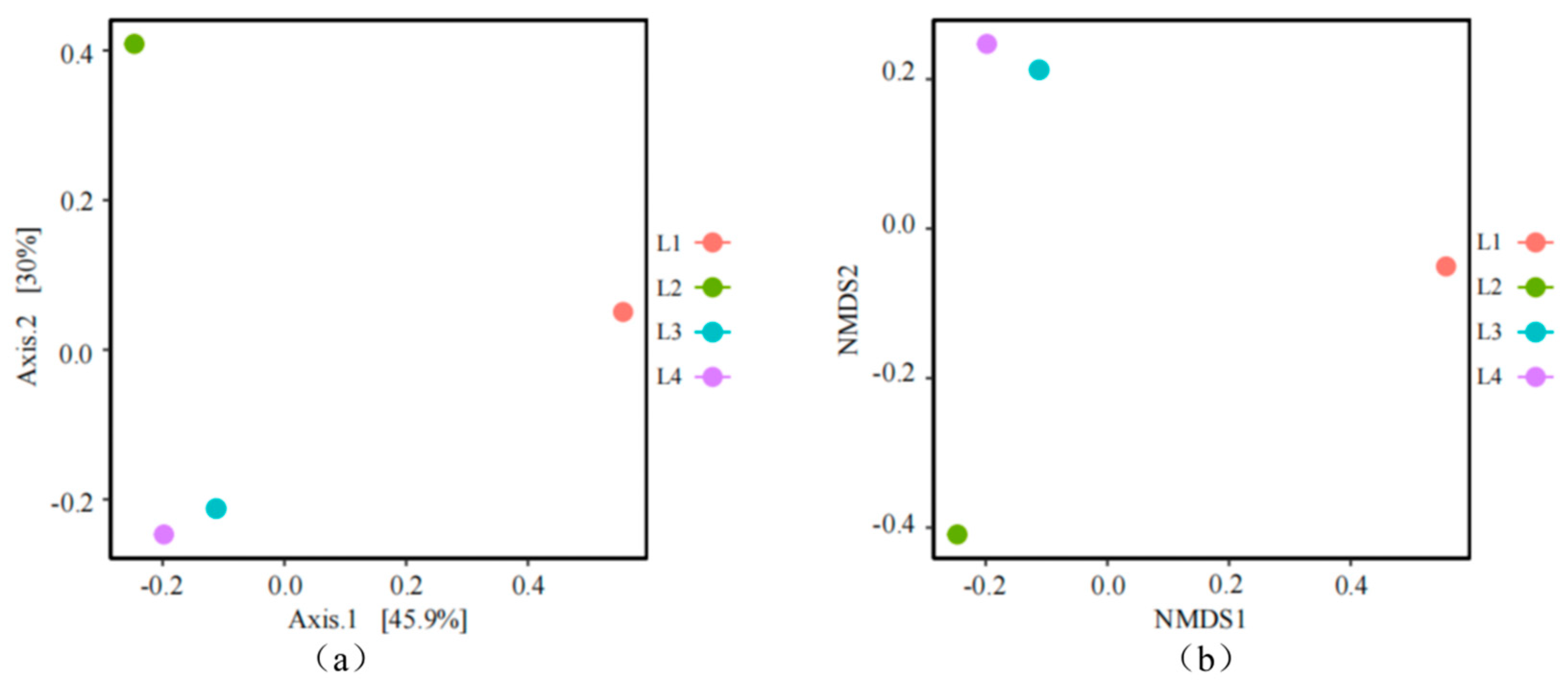
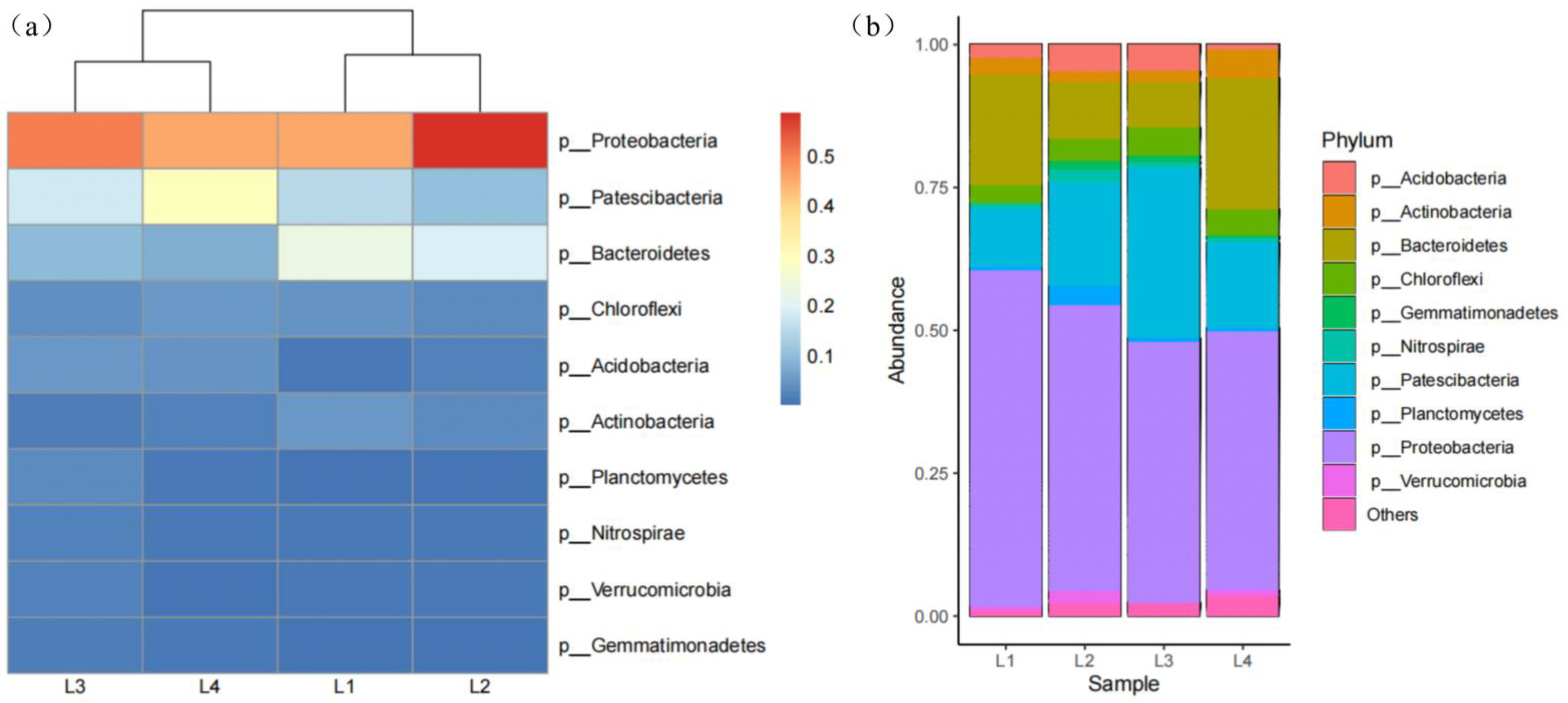
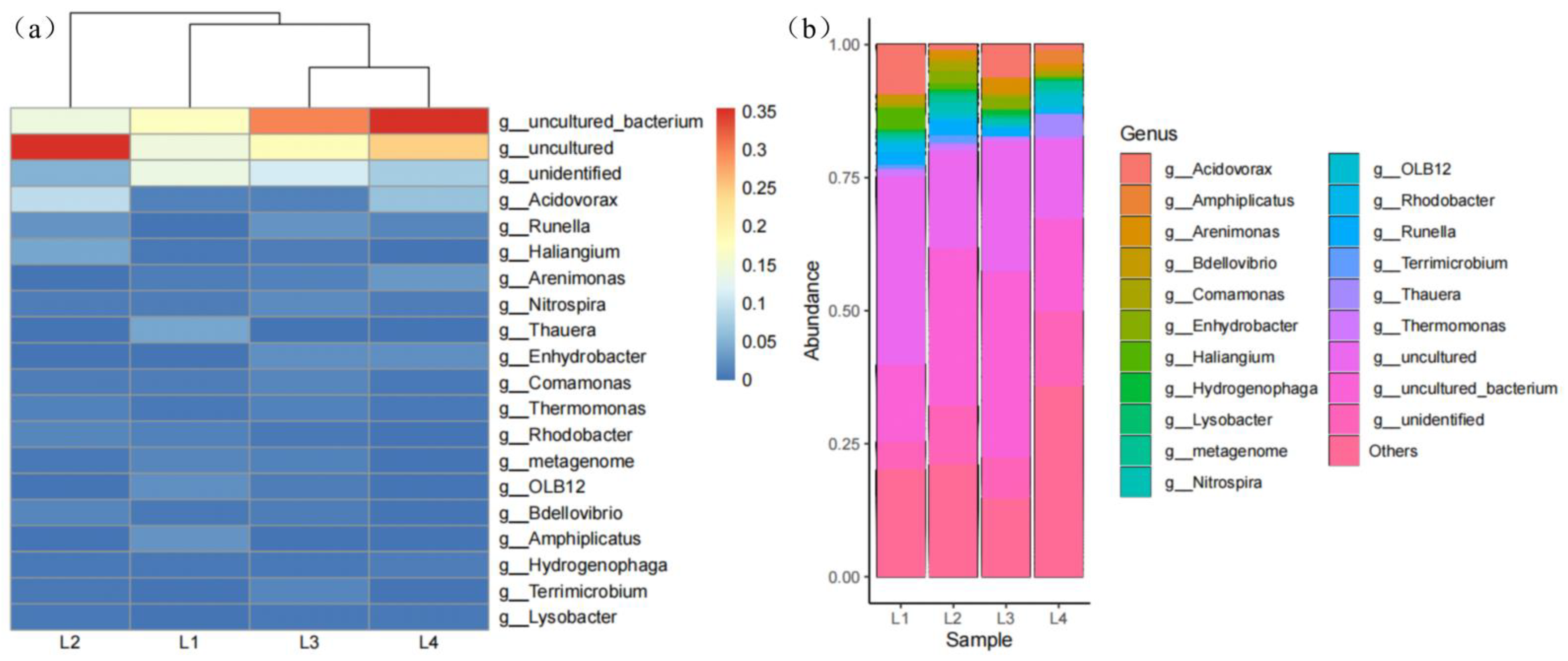
3.3.2. Analysis of the Microbial Community Structure
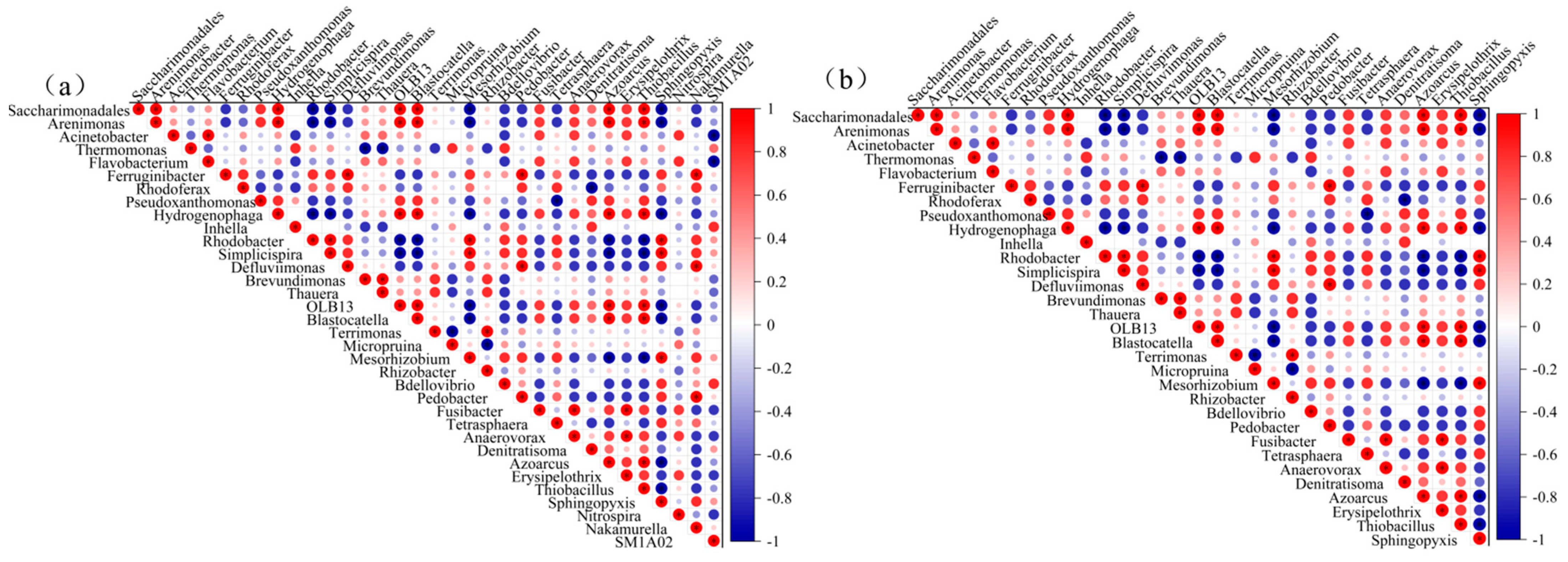
3.3.3. Analysis of Key Functional Bacteria and Their Correlations
4. Conclusions
5. Future Research Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SFe | sponge iron |
| BSIS | biological sponge iron system |
| NH4+-N | ammonium nitrogen |
| NO3−-N | nitrate nitrogen |
| NO2−-N | nitrite nitrogen |
| TN | total nitrogen |
| ZVI | zero-valent iron |
| A2/O | anaerobic–anoxic–oxic |
| SBR | sequencing batch reactor |
| DO | dissolved oxygen |
| PCoA | principal coordinates analysis |
| NMDS | non-metric multidimensional scaling |
References
- Rasul, G. Food, water, and energy security in South Asia: A nexus perspective from the Hindu Kush Himalayan region. Environ. Sci. Policy 2014, 39, 35–48. [Google Scholar] [CrossRef]
- Tan, A.H.P.; Yap, E.H.; Abakr, Y.A.; Lechner, A.M.; Mustafa, M.A.; Massawe, F. Chapter 13—The Water–Energy–Food nexus as a rallying point for sustainable development: Emerging lessons from South and Southeast Asia. In Water-Energy-Food Nexus Narratives and Resource Securities; Mabhaudhi, T., Senzanje, A., Modi, A., Jewitt, G., Massawe, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 235–256. [Google Scholar]
- Du, Q.; Zhangsun, X.; Cao, J.; Zhang, F.; Huang, T. Water-lifting aerator coupled with sponge iron-enhanced biological aerobic denitrification to remove nitrogen in low C/N water source reservoirs: Effect and mechanism. Environ. Res. 2025, 277, 121557. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Su, J.; Ali, A.; Wang, Z.; Chen, C.; Xu, L. Role of porous polymer carriers and iron-carbon bioreactor combined micro-electrolysis and biological denitrification in efficient removal of nitrate from wastewater under low carbon to nitrogen ratio. Bioresour. Technol. 2021, 321, 124447. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci. Total Environ. 2019, 671, 388–403. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Liu, X.; Ma, B.; Huang, T. Iron-Activated Carbon Systems to Enhance Aboriginal Aerobic Denitrifying Bacterial Consortium for Improved Treatment of Micro-Polluted Reservoir Water: Performances, Mechanisms, and Implications. Environ. Sci. Technol. 2022, 56, 3407–3418. [Google Scholar] [CrossRef]
- Tang, C.; Yue, Q.; Liu, H.; Dang, H.; Lv, W.; Li, X.; Chen, Y. Optimizing operation strategy to improve storage of intracellular carbon sources in anaerobic/oxic/anoxic system: Enhanced nitrogen removal by endogenous denitrification. Chemosphere 2024, 365, 143306. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Qin, Q.; Chen, L.; Cai, M.; Chen, X.; Liu, Y.; Su, Z.; Fan, X.; Cheng, L. Nitrogen Removal Performance and Feeding Strategy Optimisation in a Nitritation Denitrification Step-feed Sequencing Batch Reactor System. Biochem. Eng. J. 2025, 109761. [Google Scholar] [CrossRef]
- Alaya, S.B.; Haouech, L.; Cherif, H.; Shayeb, H. Aeration management in an oxidation ditch. Desalination 2010, 252, 172–178. [Google Scholar] [CrossRef]
- Jiang, M.; Zheng, X.; Chen, Y. Enhancement of denitrification performance with reduction of nitrite accumulation and N2O emission by Shewanella oneidensis MR-1 in microbial denitrifying process. Water Res. 2020, 169, 115242. [Google Scholar] [CrossRef]
- He, Z.; Geng, S.; Pan, Y.; Cai, C.; Wang, J.; Wang, L.; Liu, S.; Zheng, P.; Xu, X.; Hu, B. Improvement of the trace metal composition of medium for nitrite-dependent anaerobic methane oxidation bacteria: Iron (II) and copper (II) make a difference. Water Res. 2015, 85, 235–243. [Google Scholar] [CrossRef]
- Xu, A.; Gao, D.; Wu, W.-M.; Gong, X.; Liang, H. Enhanced denitrification using iron modified biochar under low carbon source condition: Modulating community assembly, allocating carbon metabolism and facilitating electron transfer. J. Environ. Manag. 2025, 381, 125354. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lu, L.; Zhang, Y.; Fang, K.; An, D.; Li, H. Removal of bisphenol A by waste zero-valent iron regulating microbial community in sequencing batch biofilm reactor. Sci. Total Environ. 2021, 753, 142073. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, H.; Liu, Y.; Yu, L.; Wang, K.; Xue, G. Iron scraps packing rapidly enhances nitrogen removal in an aerobic sludge system and the mechanism. Sci. Total Environ. 2023, 856, 159081. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Zhang, M.; Li, Z.; Chang, Z.; Kang, S. Application of sponge iron-carbon to enrich anaerobic ammonia-oxidizing bacteria from sludge mixture and coupled denitrification for degradation of industrial wastewater. J. Contam. Hydrol. 2025, 272, 104571. [Google Scholar] [CrossRef]
- Liu, X.; Wei, J.; Wu, Y.; Zhang, J.; Xing, L.; Zhang, Y.; Pan, G.; Li, J.; Xu, M.; Li, J. Performances and mechanisms of microbial nitrate removal coupling sediment-based biochar and nanoscale zero-valent iron. Bioresour. Technol. 2022, 345, 126523. [Google Scholar] [CrossRef]
- Deng, S.; Li, D.; Yang, X.; Cai, Q.; Peng, S.; Peng, X.; Yao, H.; Xie, B. Novel characteristics on micro-electrolysis mediated Fe(0)-oxidizing autotrophic denitrification with aeration: Efficiency, iron-compounds transformation, N2O and NO2− accumulation, and microbial characteristics. Chem. Eng. J. 2020, 387, 123409. [Google Scholar] [CrossRef]
- Zhang, Y.; Douglas, G.B.; Kaksonen, A.H.; Cui, L.; Ye, Z. Microbial reduction of nitrate in the presence of zero-valent iron. Sci. Total Environ. 2019, 646, 1195–1203. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Yang, Z.; Liu, Z.; Chen, Y.; Wang, Y.-E.; Xie, H. Denitrification performance and mechanism of sequencing batch reactor with a novel iron-polyurethane foam composite carrier. Biochem. Eng. J. 2021, 176, 108209. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Cheng, Y.; Jiang, M.; Li, X.; Xue, G. Iron Robustly Stimulates Simultaneous Nitrification and Denitrification Under Aerobic Conditions. Environ. Sci. Technol. 2018, 52, 1404–1412. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Yang, T.; Zhu, H.; He, Z.; Zhao, R.; Chen, Y. Sponge iron enriches autotrophic/aerobic denitrifying bacteria to enhance denitrification in sequencing batch reactor. Bioresour. Technol. 2024, 407, 131097. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Al-Gabr, H.M.; Jin, H.; Zhang, K. Performances and enhanced mechanisms of nitrogen removal in a submerged membrane bioreactor coupled sponge iron system. J. Environ. Manag. 2022, 318, 115505. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-N.; Li, J.; Wang, Y.-E.; Zhao, W.; Zhang, L.-H.; Li, J. Influencing factors for the Fenton-like of biological sponge iron system and its degradation mechanism of aniline. Process Biochem. 2021, 101, 230–236. [Google Scholar] [CrossRef]
- Guo, K.-H.; Li, W.-X.; Wang, Y.-E.; Zheng, Y.; Ji, B.; Mao, F.-J.; Xie, H.-N.; Li, J. Substantially enhanced degradation of nitrobenzene by biosponge iron system: Process and mechanisms. Process Biochem. 2021, 108, 146–152. [Google Scholar] [CrossRef]
- Kong, Q.; Ngo, H.H.; Shu, L.; Fu, R.-S.; Jiang, C.-H.; Miao, M.-S. Enhancement of aerobic granulation by zero-valent iron in sequencing batch airlift reactor. J. Hazard. Mater. 2014, 279, 511–517. [Google Scholar] [CrossRef]
- Zhu, H.; Li, W.; Chen, X.; Mu, H.; Hu, K.; Ren, S.; Peng, Y.; Zhao, R.; Wang, Y. Effects of sponge iron dosage on nitrogen removal performance and microbial community structure in sequencing batch reactors. Bioresour. Technol. 2023, 368, 128307. [Google Scholar] [CrossRef]
- Ahmad, H.A.; Ni, S.-Q.; Ahmad, S.; Zhang, J.; Ali, M.; Ngo, H.H.; Guo, W.; Tan, Z.; Wang, Q. Gel immobilization: A strategy to improve the performance of anaerobic ammonium oxidation (anammox) bacteria for nitrogen-rich wastewater treatment. Bioresour. Technol. 2020, 313, 123642. [Google Scholar] [CrossRef]
- Chien, C.C.; Kao, C.M.; Chen, C.W.; Dong, C.D.; Chien, H.Y. Evaluation of biological stability and corrosion potential in drinking water distribution systems: A case study. Environ. Monit. Assess. 2009, 153, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, D.; Zhang, Z.; Fan, D.; Du, B.; Zeng, H.; Li, D.; Zhang, J. Enhancing 2,6-dichlorophenol degradation and nitrate removal in the nano-zero-valent iron (nZVI) solid-phase denitrification system. Chemosphere 2022, 287, 132249. [Google Scholar] [CrossRef]
- Simpson, E. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Chao, A.; Yang, M.C.K. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika 1993, 80, 193–201. [Google Scholar] [CrossRef]
- Zhang, L.; Long, B.; Wu, J.; Cheng, Y.; Zhang, B.; Zeng, Y.; Huang, S.; Zeng, M. Evolution of microbial community during dry storage and recovery of aerobic granular sludge. Heliyon 2019, 5, e03023. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z.; Zhang, G.; Qin, L.; Fang, J. Application progress of microbial immobilization technology based on biomass materials. BioResources 2021, 16, 8509. [Google Scholar] [CrossRef]
- Song, Z.; Su, X.; Li, P.; Sun, F.; Dong, W.; Zhao, Z.; Wen, Z.; Liao, R. Facial fabricated biocompatible homogeneous biocarriers involving biochar to enhance denitrification performance in an anoxic moving bed biofilm reactor. Bioresour. Technol. 2021, 341, 125866. [Google Scholar] [CrossRef]
- Tikhonov, M.; Leach, R.W.; Wingreen, N.S. Interpreting 16S metagenomic data without clustering to achieve sub-OTU resolution. ISME J. 2015, 9, 68–80. [Google Scholar] [CrossRef]
- Tang, S.; Qian, J.; Wang, P.; Lu, B.; He, Y.; Yi, Z.; Zhang, Y. Exposure to nanoplastic induces cell damage and nitrogen inhibition of activated sludge: Evidence from bacterial individuals and groups. Environ. Pollut. 2022, 306, 119471. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Dong, L.; Han, C.; Li, M.; Wu, H. Effects of biochar dosage on treatment performance, enzyme activity and microbial community in aerated constructed wetlands for treating low C/N domestic sewage. Environ. Technol. Innov. 2021, 24, 101919. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, K.; Li, J.; Sun, S.; Jia, T.; Huang, Y.; Peng, Y.; Zhang, L. Pilot-scale evaluation of partial denitrification/anammox on nitrogen removal from low COD/N real sewage based on a modified process. Bioresour. Technol. 2021, 338, 125580. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Hou, R.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Effect of intermittent operation and shunt wastewater on pollutant removal and microbial community changes in subsurface wastewater infiltration system. Process Saf. Environ. Prot. 2022, 165, 255–265. [Google Scholar] [CrossRef]
- Liang, D.; Guo, W.; Li, D.; Ding, F.; Li, P.; Zheng, Z.; Li, J. Enhanced aerobic granulation for treating low-strength wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor by selecting slow-growing organisms and adding carriers. Environ. Res. 2022, 205, 112547. [Google Scholar] [CrossRef]
- Deng, S.; Peng, Y.; Zhang, L.; Wu, L. Advanced nitrogen removal from municipal wastewater via two-stage partial nitrification-simultaneous anammox and denitrification (PN-SAD) process. Bioresour. Technol. 2020, 304, 122955. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Hu, M.; Lu, D.; Zhang, Y.; Zhang, T.; Ya, T.; Wang, X. Important role of Chloroflexi: Improved the cooperation of anammox community under PFOS stress. Chem. Eng. J. 2024, 499, 155950. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, S.; Wang, Y.; Lan, S.; Dou, Q.; Peng, Y. Rapid start-up strategy of partial denitrification and microbially driven mechanism of nitrite accumulation mediated by dissolved organic matter. Bioresour. Technol. 2021, 340, 125663. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Yang, S.; He, Z.; Li, Y.; Wang, Y.; Li, J. Iron-dependent autotrophic denitrification as a novel microbial driven and iron-mediated denitrification process: A critical review. Environ. Res. 2025, 273, 120808. [Google Scholar] [CrossRef]
- Xiang, X.; Yi, X.; Zheng, W.; Li, Y.; Zhang, C.; Wang, X.; Chen, Z.; Huang, M.; Ying, G.-G. Enhanced biodegradation of thiamethoxam with a novel polyvinyl alcohol (PVA)/sodium alginate (SA)/biochar immobilized Chryseobacterium sp H5. J. Hazard. Mater. 2023, 443, 130247. [Google Scholar] [CrossRef]
- Peng, S.; Deng, S.; Li, D.; Xie, B.; Yang, X.; Lai, C.; Sun, S.; Yao, H. Iron-carbon galvanic cells strengthened anaerobic/anoxic/oxic process (Fe/C-A2O) for high-nitrogen/phosphorus and low-carbon sewage treatment. Sci. Total Environ. 2020, 722, 137657. [Google Scholar] [CrossRef]
- Chen, H.; Li, A.; Cui, D.; Cui, C.; Ma, F. Evolution of microbial community and key genera in the formation and stability of aerobic granular sludge under a high organic loading rate. Bioresour. Technol. Rep. 2019, 7, 100280. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, P.; He, Y.; Zeng, F.; Xu, J.; He, L. Enantioselective effects of cyflumetofen on microbial community and related nitrogen cycle gene function in acid-soil. Sci. Total Environ. 2021, 771, 144831. [Google Scholar] [CrossRef]
- Chen, X.; Hao, Q.; Xiong, Y.; Hu, J.; Jiang, C. Effects of Iron Ore and Biochar Addition on Wastewater Treatment Efficiency, Greenhouse Gas Emissions, and Microbial Community in Subsurface Flow Constructed Wetlands. Environ. Sci. 2022, 43, 1492–1499. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Q.; Tan, B.; Li, M.; Peng, H.; He, J.; Zhang, Y.; Su, J. Microbial community and metabolic characteristics evaluation in start-up stage of electro-enhanced SBR for aniline wastewater treatment. J. Water Process Eng. 2022, 45, 102489. [Google Scholar] [CrossRef]
- Chi, Y.; Ma, X.; Zhang, X.; Wang, R.; Zhang, D.; Chu, S.; Zhao, T.; Zhou, P.; Zhang, D. Plant growth promoting endophyte modulates soil ecological characteristics during the enhancement process of cadmium phytoremediation. J. Environ. Manag. 2024, 369, 122206. [Google Scholar] [CrossRef]
- Fang, Y.-K.; Wang, H.-C.; Han, J.-L.; Li, Z.-L.; Wang, A.-J. Enhanced nitrogen removal of constructed wetlands by coupling with the bioelectrochemical system under low temperature: Performance and mechanism. J. Clean. Prod. 2022, 350, 131365. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Huang, J.; Huang, M.; Wang, T.; Bao, S.; Tang, W.; Fang, T. The contributions and mechanisms of iron-microbes-biochar in constructed wetlands for nitrate removal from low carbon/nitrogen ratio wastewater. RSC Adv. 2020, 10, 23212–23220. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Le, V.Q.; Hansen, A.A.; Nielsen, J.L.; Nielsen, P.H. High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems. FEMS Microbiol. Ecol. 2011, 76, 256–267. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, X.; Li, H.; Zhu, B.; Bai, J.; Huang, X. Iron-carbon micro-electrolysis coupled to heterotrophic nitrification aerobic denitrification treating low carbon/nitrogen mariculture wastewater. Environ. Res. 2025, 269, 120796. [Google Scholar] [CrossRef]
- Buliauskaitė, R.; Wilfert, P.; Suresh Kumar, P.; de Vet, W.; Witkamp, G.J.; Korving, L.; Loosdrecht, M.C.M. Biogenic iron oxides for phosphate removal. Environ. Technol. 2018, 41, 260–266. [Google Scholar] [CrossRef]
- Xu, D.; Lin, L.; Xu, P.; Zhou, Y.; Xiao, E.; He, F.; Wu, Z. Effect of drained-flooded time ratio on ammonia nitrogen removal in a constructed wetland-microbial fuel cell system by tidal flow operation. J. Water Process Eng. 2021, 44, 102450. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, S.; Deng, Z.; Wang, Y.; Deng, S.; Song, Y.; Sun, C.; Bu, N.; Wang, X. Evaluating responses of nitrification and denitrification to the co-selective pressure of divalent zinc and tetracycline based on resistance genes changes. Bioresour. Technol. 2020, 314, 123769. [Google Scholar] [CrossRef]
- Dang, C.; Liu, S.; Chen, Q.; Sun, W.; Zhong, H.; Hu, J.; Liang, E.; Ni, J. Response of microbial nitrogen transformation processes to antibiotic stress in a drinking water reservoir. Sci. Total Environ. 2021, 797, 149119. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, Y.; Zhang, G.; Lian, J.; Liu, Z.; Zhang, L.; Tian, S. Effects of low-intensity ultrasound on nitrite accumulation and microbial characteristics during partial nitrification. Sci. Total Environ. 2020, 705, 135985. [Google Scholar] [CrossRef]
- Mao, Y.; Xia, Y.; Zhang, T. Characterization of Thauera-dominated hydrogen-oxidizing autotrophic denitrifying microbial communities by using high-throughput sequencing. Bioresour. Technol. 2013, 128, 703–710. [Google Scholar] [CrossRef]

| Sample Number | Simpson | Chao1 | ACE | Shannon |
|---|---|---|---|---|
| L1 | 0.99 | 1184 | 1184 | 8.46 |
| L2 | 0.97 | 1286 | 1286 | 7.64 |
| L3 | 0.98 | 2537 | 2742 | 8.25 |
| L4 | 0.96 | 2075 | 2287 | 7.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xie, H.; Zhao, W.; Li, J. Evolution of Microbial Community Structure and Denitrifying Functional Microorganisms in the Biological Sponge Iron System. Appl. Sci. 2025, 15, 7244. https://doi.org/10.3390/app15137244
Li J, Xie H, Zhao W, Li J. Evolution of Microbial Community Structure and Denitrifying Functional Microorganisms in the Biological Sponge Iron System. Applied Sciences. 2025; 15(13):7244. https://doi.org/10.3390/app15137244
Chicago/Turabian StyleLi, Jing, Huina Xie, Wei Zhao, and Jie Li. 2025. "Evolution of Microbial Community Structure and Denitrifying Functional Microorganisms in the Biological Sponge Iron System" Applied Sciences 15, no. 13: 7244. https://doi.org/10.3390/app15137244
APA StyleLi, J., Xie, H., Zhao, W., & Li, J. (2025). Evolution of Microbial Community Structure and Denitrifying Functional Microorganisms in the Biological Sponge Iron System. Applied Sciences, 15(13), 7244. https://doi.org/10.3390/app15137244





