Agro-Food and Lignocellulosic Urban Wastes as Sugar-Rich Substrates for Multi-Product Oil-Based Biorefineries
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Media Preparation
2.2. Pulse-Feeding Cultivation Strategy
2.3. Analytical Methods
3. Results and Discussion
3.1. Potential of Biowastes as Culture Media
3.2. Optimization of the Pulse-Feeding Strategy for the Cultivation of R. toruloides Using Biowastes with Different Nature
3.3. Effects of Media Composition on the Intracellular Accumulation of Lipids and Carotenoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C/N | Carbon/nitrogen ratio |
| DCW | Dry cell weight |
| FAs | Fatty acids |
| FAMES | Fatty acid methyl esters |
| SCO | Single-cell oil |
References
- Farzad, S.; Mandegari, M.A.; Guo, M.; Haigh, K.F.; Shah, N.; Görgens, J.F. Multi-product biorefineries from lignocelluloses: A pathway to revitalisation of the sugar industry? Biotechnol. Biofuels 2017, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Gallego-García, M.; Susmozas, A.; Negro, M.J.; Moreno, A.D. Challenges and prospects of yeast-based microbial oil production within a biorefinery concept. Microb. Cell Fact. 2023, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Talluri, G. Market Uptake Support for Intermediate Bioenergy Carriers. Scarperia e San Piero. 2022. Available online: https://www.music-h2020.eu/ (accessed on 31 December 2024).
- Larroude, M.; Celinska, E.; Back, A.; Thomas, S.; Nicaud, J.-M.; Ledesma-Amaro, R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol. Bioeng. 2018, 115, 464–472. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A potential red yeast chassis for lipids and beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, B.; Li, J.; Zhang, J. Rhodotorula toruloides: An ideal microbial cell factory to produce oleochemicals, carotenoids, and other products. World J. Microbiol. Biotechnol. 2021, 38, 13. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Mota, M.N.; Fernandes, P.A.R.; Coelho, E.; Coimbra, M.A.; Sá-Correia, I. Cell wall alterations occurring in an evolved multi-stress tolerant strain of the oleaginous yeast Rhodotorula toruloides. Sci. Rep. 2024, 14, 23366. [Google Scholar] [CrossRef]
- Singh, R.V.; Sambyal, K. An overview of β-carotene production: Current status and future prospects. Food Biosci. 2022, 47, 101717. [Google Scholar] [CrossRef]
- Lopes da Silva, T.; Fontes, A.; Reis, A.; Siva, C.; Gírio, F. Oleaginous yeast biorefinery: Feedstocks, processes, techniques, bioproducts. Fermentation 2023, 9, 1013. [Google Scholar] [CrossRef]
- PMR&C. Natural Beta Carotene Market Share, S., Trends, Industry Analysis Report: By Source (Algae, Fruit & Vegetable, Fungi, and Palm Oil), Form, Application, and Region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa)—Market Forecast, 2025–2034. Dover, DE, USA. 2024. Available online: https://www.polarismarketresearch.com/industry-analysis/natural-beta-carotene-market/analysis-type (accessed on 31 December 2024).
- Wu, S.; Zhao, X.; Shen, H.; Wang, Q.; Zhao, Z.K. Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Bioresour. Technol. 2011, 102, 1803–1807. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Zhu, Z.; Shen, H.; Lin, X.; Jin, X.; Jiao, X.; Zhao, Z.K. Systems analysis of phosphate-limitation-induced lipid accumulation by the oleaginous yeast Rhodosporidium toruloides. Biotechnol. Biofuels 2018, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Bansfield, D.; Spilling, K.; Mikola, A.; Piiparinen, J. Growth of fungi and yeasts in food production waste streams: A feasibility study. BMC Microbiol. 2023, 23, 328. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Z.; Chen, S.; Jin, M. Microbial lipid production from dilute acid and dilute alkali pretreated corn stover via Trichosporon dermatis. Bioresour. Technol. 2020, 295, 122253. [Google Scholar] [CrossRef]
- Poontawee, R.; Lorliam, W.; Polburee, P.; Limtong, S. Oleaginous yeasts: Biodiversity and cultivation. Fungal Biol. Rev. 2023, 44, 100295. [Google Scholar] [CrossRef]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol. Biofuels 2015, 8, 104. [Google Scholar] [CrossRef]
- Lu, T.; Liu, F.; Jiang, C.; Cao, J.; Ma, X.; Su, E. Strategies for cultivation, enhancing lipid production, and recovery in oleaginous yeasts. Bioresour. Technol. 2025, 416, 131770. [Google Scholar] [CrossRef]
- Negro, M.J.; Álvarez, C.; Doménech, P.; Iglesias, R.; Ballesteros, I. Sugars production from municipal forestry and greening wastes pretreated by an integrated steam explosion-based process. Energies 2020, 13, 4432. [Google Scholar] [CrossRef]
- Gallego-García, M.; Moreno, A.D.; González, A.; Negro, M.J. Efficient use of discarded vegetal residues as cost-effective feedstocks for microbial oil production. Biotechnol. Biofuels Biopod 2023, 16, 21. [Google Scholar] [CrossRef]
- Ballesteros, I.; Duque, A.; Negro, M.J.; Coll, C.; Latorre-Sánchez, M.; Hereza, J.; Iglesias, R. Valorisation of cellulosic rejections from wastewater treatment plants through sugar production. J. Environ. Manag. 2022, 312, 114931. [Google Scholar] [CrossRef]
- Moliné, M.; Libkind, D.; van Broock, M. Production of torularhodin, torulene, and β-carotene by Rhodotorula yeasts. Methods Mol. Biol. 2012, 898, 275–283. [Google Scholar] [CrossRef]
- Shah, Q. A Comparative Study on Four Oleaginous Yeasts on Their Lipid Accumulating Capacity; Swedish University of Agricultural Sciences Uppsala: Uppsala, Sweden, 2013. [Google Scholar]
- Gallego-García, M.; Susmozas, A.; Moreno, A.D.; Negro, M.J. Evaluation and Identification of key economic bottlenecks for cost-effective microbial oil production from fruit and vegetable residues. Fermentation 2022, 8, 334. [Google Scholar] [CrossRef]
- Van Wychen, S.; Ramirez, K.; Laurens, L.M. Determination of total lipids as fatty acid methyl esters (FAME) by in situ transesterification: Laboratory analytical procedure (LAP). Natl. Renew. Energy Lab. 2015. [Google Scholar] [CrossRef]
- Sankaran, S.; Khanal, S.K.; Jasti, N.; Jin, B.; Pometto, I.I.I.A.L.; Van Leeuwen, J.H. Use of filamentous fungi for wastewater treatment and production of high value fungal byproducts: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 400–449. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Q.; Fang, Z.; Cong, W.-j.; Zhang, H. Microbial lipid production from rice straw hydrolysates and recycled pretreated glycerol. Bioresour. Technol. 2020, 312, 123580. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.C.A.; Coradini, A.L.V.; Deckmann, A.C.; Franco, T.T. Lipid production from hemicellulose hydrolysate and acetic acid by Lipomyces starkeyi and the ability of yeast to metabolize inhibitors. Biochem. Eng. J. 2017, 118, 11–19. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 2018, 217, 420–426. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Z.; Gao, M.; Ma, Y.; Ma, H.; Zhang, M.; Liu, Y.; Wang, Q. Microbial lipid production from food waste saccharified liquid and the effects of compositions. Energy Convers. Manag. 2018, 172, 306–315. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Aranda, B. Microbial growth under limiting conditions-Future perspectives. Microorganisms 2023, 11, 1641. [Google Scholar] [CrossRef]
- Thumkasem, N.; On-mee, T.; Kongsinkaew, C.; Chittapun, S.; Pornpukdeewattana, S.; Ketudat-Cairns, M.; Thongprajukaew, K.; Antimanon, S.; Charoenrat, T. Enhanced high β-carotene yeast cell production by Rhodotorula paludigena CM33 and in vitro digestibility in aquatic animals. Sci. Rep. 2024, 14, 9188. [Google Scholar] [CrossRef]
- Mishra, S.; Deewan, A.; Zhao, H.; Rao, C.V. Nitrogen starvation causes lipid remodeling in Rhodotorula toruloides. Microb. Cell Fact. 2024, 23, 141. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.J. C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoven, E.J.; Pomraning, K.R.; Baker, S.E.; Nielsen, J. Regulation of amino-acid metabolism controls flux to lipid accumulation in Yarrowia lipolytica. NPJ Syst. Biol. Appl. 2016, 2, 16005. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Z.; Guo, M.; Chen, G. Ultraviolet-diethyl sulfate composite mutagenesis of Rhodosporidium toruloides and culture optimization to improve carotenoid production. LWT 2024, 212, 116979. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Villegas-Méndez, M.Á.; Montañez, J.; Contreras-Esquivel, J.C.; Salmerón, I.; Koutinas, A.A.; Morales-Oyervides, L. Scale-up and fed-batch cultivation strategy for the enhanced co-production of microbial lipids and carotenoids using renewable waste feedstock. J. Environ. Manag. 2023, 339, 117866. [Google Scholar] [CrossRef]
- Yen, H.-W.; Liu, Y.X.; Chang, J.-S. The effects of feeding criteria on the growth of oleaginous yeast—Rhodotorula glutinis in a pilot-scale airlift bioreactor. J. Taiwan Inst. Chem. Eng. 2015, 49, 67–71. [Google Scholar] [CrossRef]
- Bhosale, P.; Gadre, R.V. Beta-Carotene production in sugarcane molasses by a Rhodotorula glutinis mutant. J. Ind. Microbiol. Biotechnol. 2001, 26, 327–332. [Google Scholar] [CrossRef]


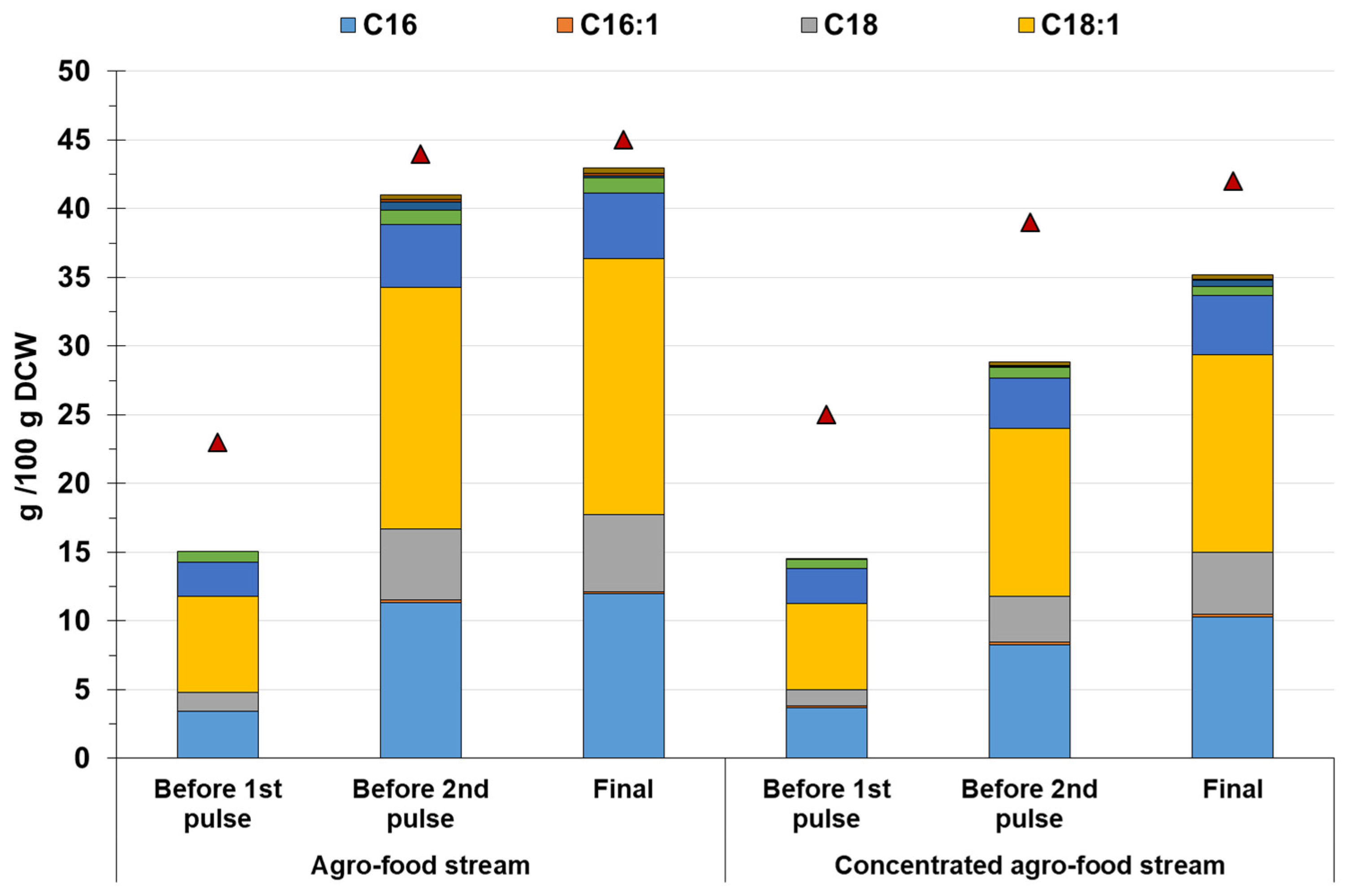
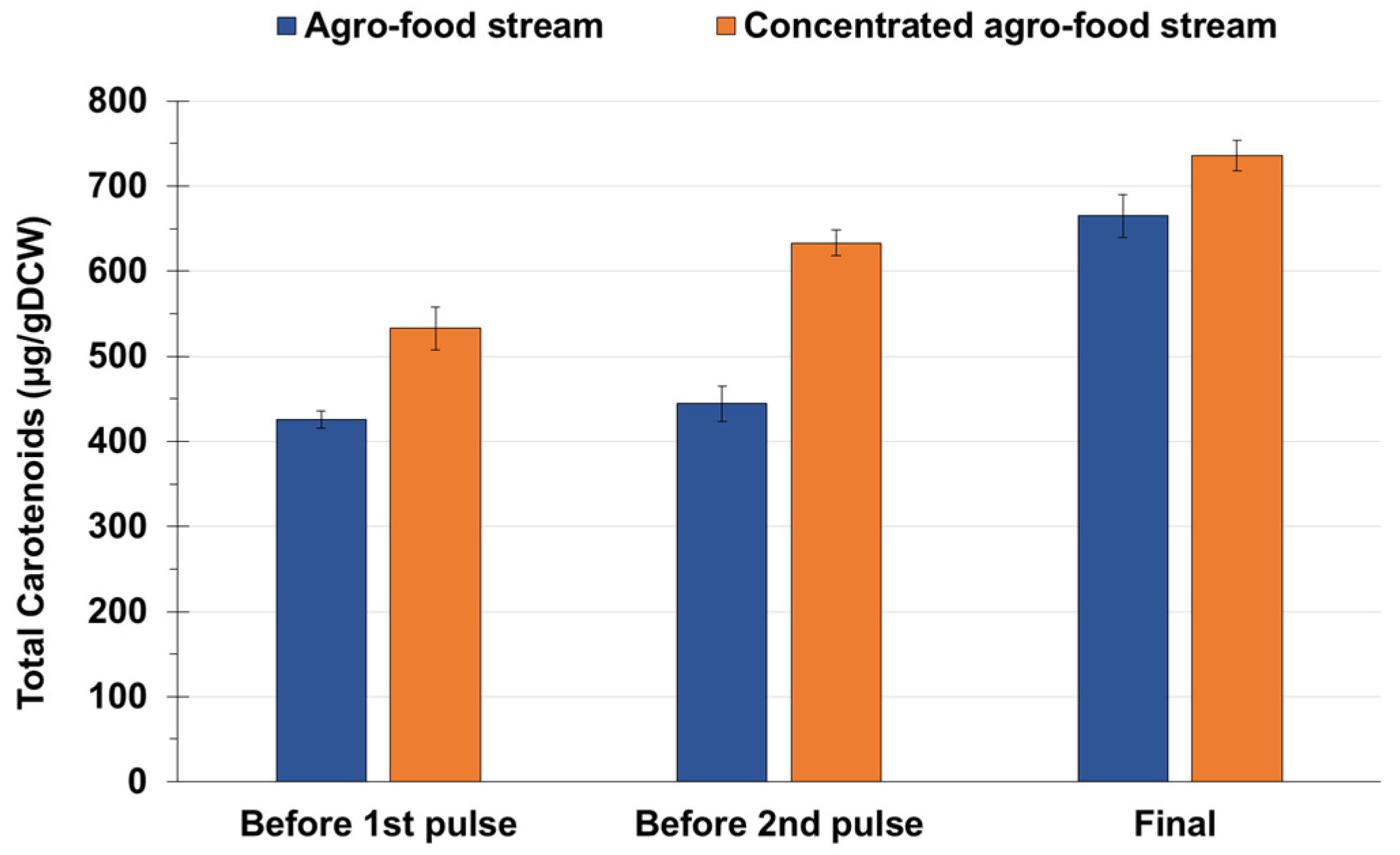
| Carbohydrate | Agro-Food Biowaste | Concentrated Agro-Food Biowaste | Concentrated Enzymatic Hydrolysate from Lignocellulosic Urban Residue |
|---|---|---|---|
| Glucose | 14.40 ± 0.04 | 28.54 ± 0.18 | 244.13 ± 3.21 |
| Xylose | 0.11 ± 0.02 | 0.27 ± 0.02 | 9.17 ± 0.02 |
| Galactose | 0.27± 0.02 | 0.43 ± 0.05 | 0.46 ± 0.02 |
| Arabinose | 0.71 ± 0.03 | 0.39 ± 0.04 | 0.16 ± 0.02 |
| Mannose | 0.16 ± 0.01 | 0.26 ± 0.01 | 0.52 ± 0.02 |
| Fructose | 15.64 ± 0.30 | 31.16 ± 0.47 | n.d. |
| Component | g/100 g of Pretreated Biomass |
|---|---|
| Glucan (Cellulose) | 43.96 ± 0.44 |
| Hemicellulose | 3.74 |
| Xylan | 2.84 ± 0.14 |
| Galactan | 0.69 ± 0.05 |
| Arabinan | 0.06 ± 0.01 |
| Mannan | 0.16 ± 0.01 |
| Acid-insoluble lignin | 42.17 ± 0.81 |
| Ash | 2.41 ± 0.31 |
| Growth Medium | Lipids (g/L) | Yield Lipids/Sugars (g/g) | SFAs (%) | MFAs (%) | PFAs (%) |
|---|---|---|---|---|---|
| Non-concentrated | 16.42 ± 0.76 | 0.25 ± 0.02 | 44.42 ± 0.27 | 43.21 ± 0.27 | 13.57 ± 0.03 |
| Concentrated | 23.32 ± 1.16 | 0.20 ± 0.01 | 45.86 ± 0.10 | 40.96 ± 0.10 | 13.78 ± 1.04 |
| Growth Medium | Process Time | Torularhodin | Torulene | γ-Carotene | β-Carotene |
|---|---|---|---|---|---|
| Agro-food waste stream | Before 1st pulse | 62 ± 4 | 115 ± 7 | 109 ± 2 | 62 ± 8 |
| Before 2nd pulse | 68 ± 5 | 141 ± 4 | 106 ± 6 | 43 ± 3 | |
| Final | 94 ± 4 | 289 ± 6 | 100 ± 8 | 40 ± 3 | |
| Concentrated agro-food waste stream | Before 1st pulse | 72 ± 8 | 166 ± 6 | 145 ± 4 | 71 ± 3 |
| Before 2nd pulse | 119 ± 5 | 211 ± 4 | 127 ± 5 | 61 ± 4 | |
| Final | 132 ± 16 | 341 ± 11 | 103 ± 21 | 59 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-López, A.; Negro, M.J.; Fernández-Rojo, J.L.; Ballesteros, I.; Moreno, A.D. Agro-Food and Lignocellulosic Urban Wastes as Sugar-Rich Substrates for Multi-Product Oil-Based Biorefineries. Appl. Sci. 2025, 15, 7240. https://doi.org/10.3390/app15137240
Rodríguez-López A, Negro MJ, Fernández-Rojo JL, Ballesteros I, Moreno AD. Agro-Food and Lignocellulosic Urban Wastes as Sugar-Rich Substrates for Multi-Product Oil-Based Biorefineries. Applied Sciences. 2025; 15(13):7240. https://doi.org/10.3390/app15137240
Chicago/Turabian StyleRodríguez-López, Alberto, María José Negro, José Luis Fernández-Rojo, Ignacio Ballesteros, and Antonio D. Moreno. 2025. "Agro-Food and Lignocellulosic Urban Wastes as Sugar-Rich Substrates for Multi-Product Oil-Based Biorefineries" Applied Sciences 15, no. 13: 7240. https://doi.org/10.3390/app15137240
APA StyleRodríguez-López, A., Negro, M. J., Fernández-Rojo, J. L., Ballesteros, I., & Moreno, A. D. (2025). Agro-Food and Lignocellulosic Urban Wastes as Sugar-Rich Substrates for Multi-Product Oil-Based Biorefineries. Applied Sciences, 15(13), 7240. https://doi.org/10.3390/app15137240






