Removal of Metal Ions in Spin-on Hardmask Using Functionalized Porous Silica Adsorbents
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
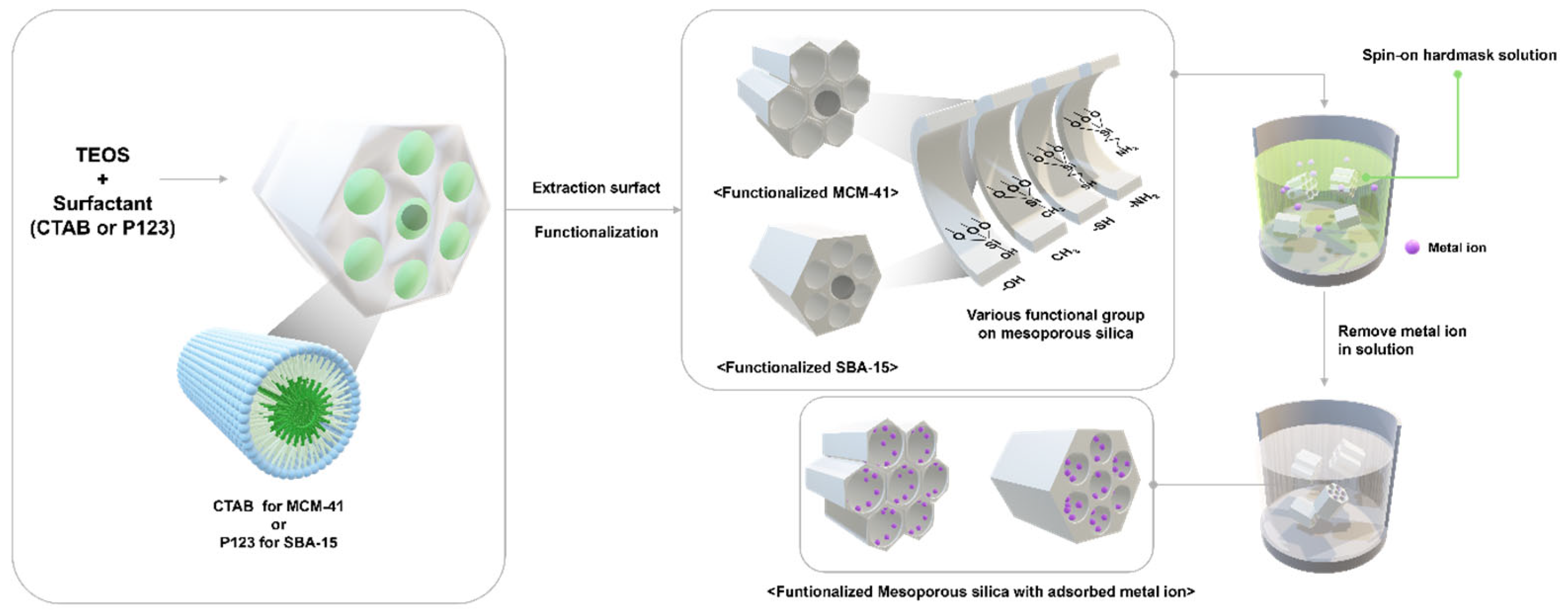
2.2. Preparation of the Adsorbents
2.3. Material Characterization
2.4. Preparation of Metal Ion Solutions
2.5. Adsorption Experiment
2.6. Adsorption Isotherm Modeling
3. Result and Discussion
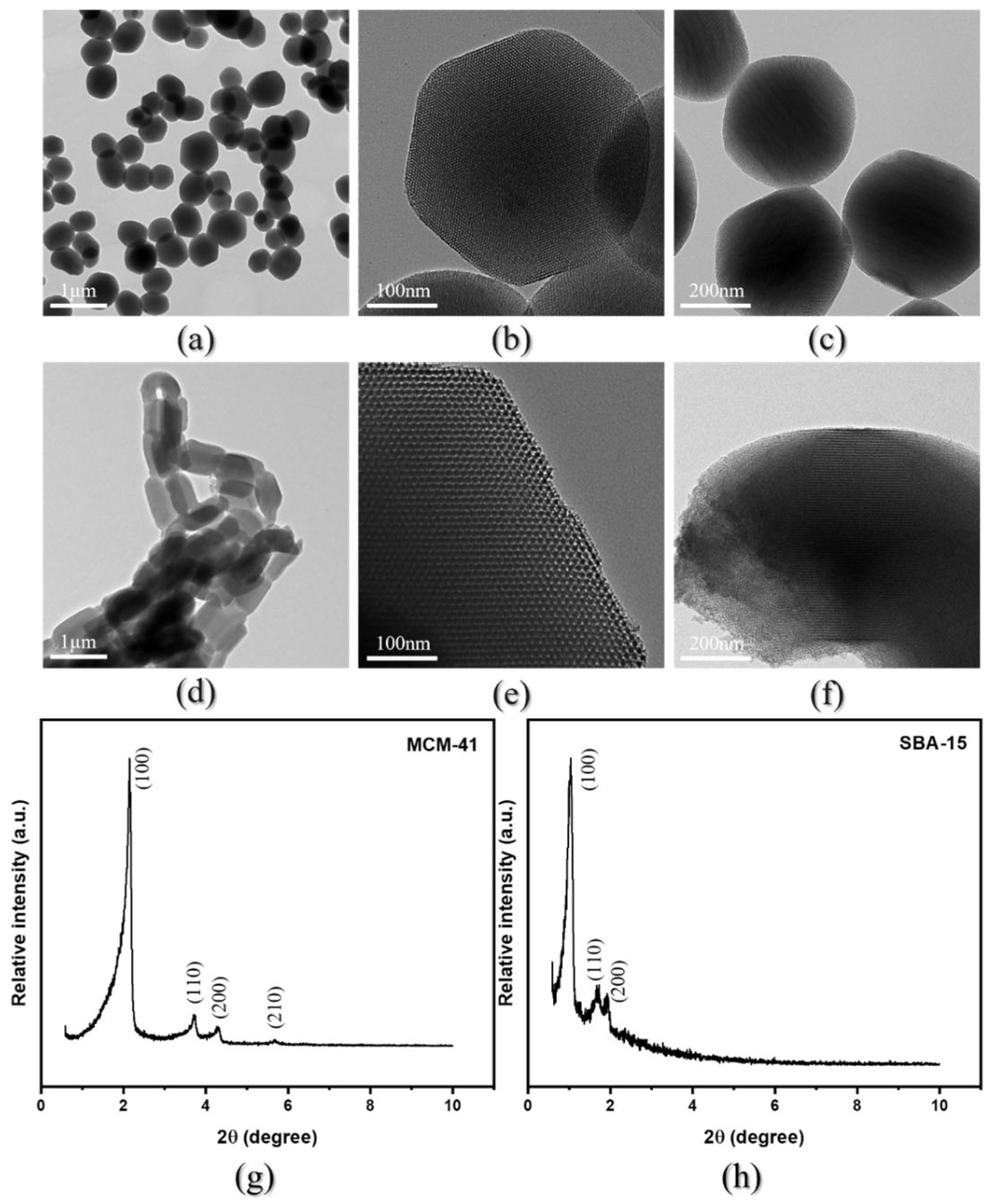
3.1. Synthesis and Characterization of Mesoporous Adsorbents
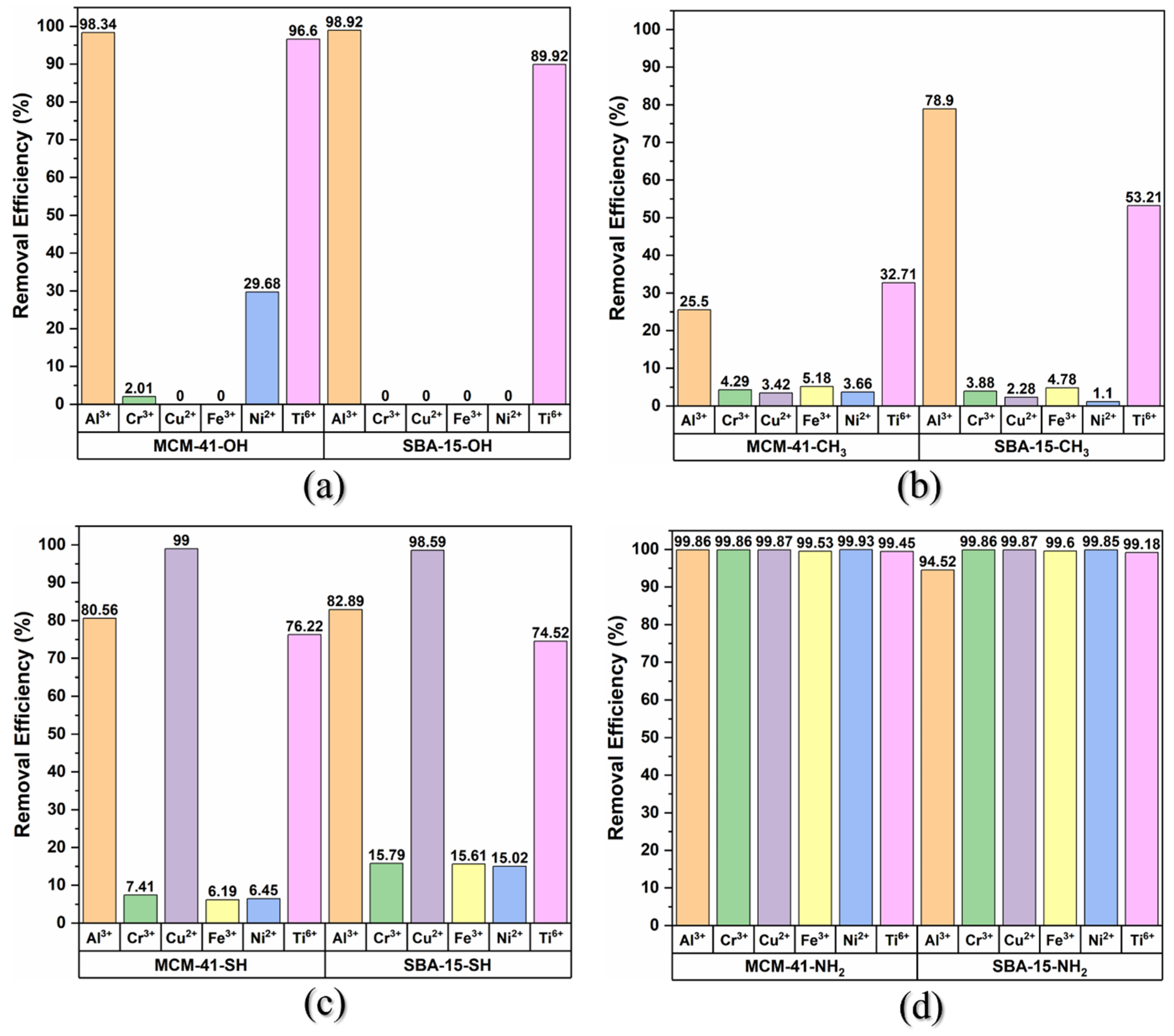
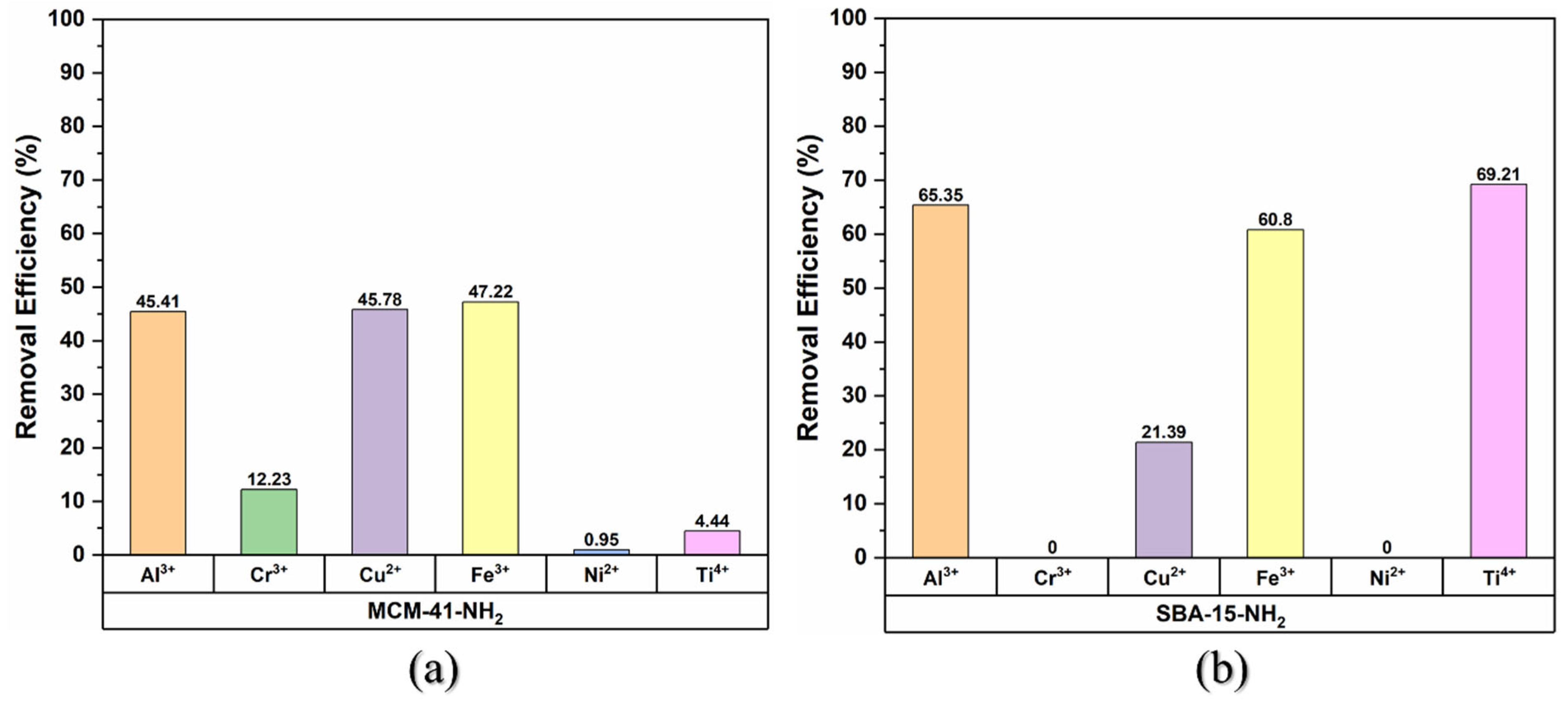
3.2. Metal Ion Adsorption
3.3. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capitanio, D.; Mizuno, Y.; Lee, J. Metal ion removal from photoresist solvents. In Proceedings of the Advances in Resist Technology and Processing XVI, Santa Clara, CA, USA, 14–19 March 1999; SPIE: Bellingham, WA, USA, 1999; pp. 684–688. [Google Scholar]
- Shin, H.; Kim, H.; Lee, H.; Yoo, H.; Kim, J.; Kim, H.; Lee, M. Photoresist-Free Lithographic Patterning of Solution-Processed Nanostructured Metal Thin Films. Adv. Mater. 2008, 20, 3457–3461. [Google Scholar] [CrossRef]
- Chen, A.C.; Wu, W.-H.; Lin, B.; Chang, E.Y.; Cheon, H.-S.; Yen, A.; Kim, S.K.; Cho, H.M.; Yoon, K.-H.; Kim, J.S. Use of spin-on-hard mask materials for nano scale patterning technology. Proc. SPIE 2008, 7140, 658–669. [Google Scholar]
- Oh, C.-I.; Uh, D.-S.; Kim, D.-H.; Lee, J.-K.; Yun, H.-C.; Nam, I.; Kim, M.-S.; Yoon, K.-H.; Hyung, K.-H.; Tokareva, N.; et al. Spin-on Organic Hardmask Materials in 70 nm Devices; SPIE: Bellingham, WA, USA, 2007; p. 6519. [Google Scholar]
- Oh, C.-I.; Lee, J.-K.; Kim, M.-S.; Yoon, K.-H.; Cheon, H.-S.; Tokareva, N.; Song, J.-Y.; Kim, J.-S.; Chang, T.-W. Novel Spin-On Organic Hardmask with High Plasma Etch Resistance; SPIE: Bellingham, WA, USA, 2008; p. 6923. [Google Scholar]
- Chakraborty, R.; Verma, R.; Asthana, A.; Vidya, S.S.; Singh, A.K. Adsorption of hazardous chromium (VI) ions from aqueous solutions using modified sawdust: Kinetics, isotherm and thermodynamic modelling. Int. J. Environ. Anal. Chem. 2019, 101, 911–928. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Addy, M.; Losey, B.; Mohseni, R.; Zlotnikov, E.; Vasiliev, A. Adsorption of heavy metal ions on mesoporous silica-modified montmorillonite containing a grafted chelate ligand. Appl. Clay Sci. 2012, 59, 115–120. [Google Scholar] [CrossRef]
- Nyirenda, J.; Kalaba, G.; Munyati, O. Synthesis and characterization of an activated carbon-supported silver-silica nanocomposite for adsorption of heavy metal ions from water. Results Eng. 2022, 15, 100553. [Google Scholar] [CrossRef]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: A review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128. [Google Scholar] [CrossRef]
- Walcarius, A.; Mercier, L. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.-H.; Choi, K.; Kim, H.-G.; Park, J.-A.; Cho, S.-H.; Hong, S.W.; Lee, J.-H.; Lee, J.H.; Lee, S.; et al. Investigation of the mechanism of chromium removal in (3-aminopropyl)trimethoxysilane functionalized mesoporous silica. Sci. Rep. 2018, 8, 12078. [Google Scholar] [CrossRef]
- Wang, T.-K.; Wang, M.-Y.; Ko, F.-H.; Tseng, C.-L. Characterization and modeling of the metal diffusion from deep ultraviolet photoresist and silicon-based substrate. Appl. Radiat. Isot. 2001, 54, 811–820. [Google Scholar] [CrossRef]
- Mathew, A.; Parambadath, S.; Kim, S.Y.; Ha, H.M.; Ha, C.-S. Diffusion mediated selective adsorption of Zn2+ from artificial seawater by MCM-41. Microporous Mesoporous Mater. 2016, 229, 124–133. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous Organosilica with Amidoxime Groups for CO2 Sorption. ACS Appl. Mater. Interfaces 2014, 6, 13069–13078. [Google Scholar] [CrossRef]
- Sepehrian, H.; Waqif-Husain, S.; Ghannadi-Maragheh, M. Development of Thiol-Functionalized Mesoporous Silicate MCM-41 as a Modified Sorbent and Its Use in Chromatographic Separation of Metal Ions from Aqueous Nuclear Waste. Chromatographia 2009, 70, 277–280. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, Y.; Zhou, L.; Huang, Y.; Zeng, Q. Study on the adsorption behavior of cadmium, copper, and lead ions on the crosslinked polyethylenimine dithiocarbamate material. Environ. Sci. Pollut. Res. 2018, 27, 2444–2454. [Google Scholar] [CrossRef]
- Mureseanu, M.; Reiss, A.; Stefanescu, I.; David, E.; Parvulescu, V.; Renard, G.; Hulea, V. Modified SBA-15 mesoporous silica for heavy metal ions remediation. Chemosphere 2008, 73, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Albayati, T.M.; Salih, I.K.; Alazzawi, H.F. Synthesis and characterization of a modified surface of SBA-15 mesoporous silica for a chloramphenicol drug delivery system. Heliyon 2019, 5, e02539. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore Structure and Fractal Characteristics of Different Shale Lithofacies in the Dalong Formation in the Western Area of the Lower Yangtze Platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Zhao, H.; Han, H. Synthesis and characterization of functionalized SBA-15 silica through template removal. J. Solid. State Chem. 2020, 282, 121074. [Google Scholar] [CrossRef]
- Tian, S.; Gao, W.; Liu, Y.; Kang, W.; Yang, H. Effects of surface modification Nano-SiO2 and its combination with surfactant on interfacial tension and emulsion stability. Colloids Surf. Physicochem. Eng. Aspects 2020, 595, 124682. [Google Scholar] [CrossRef]
- Salsabiila, N.; Morsin, M.; Nafisah, S.; Razali, N.L.; Mahmud, F.; Alip, A.A. Investigation of the effect of amine and thiol as functional groups on gold Nanobipyramids properties. Materialia 2024, 34, 102072. [Google Scholar] [CrossRef]
- Zhang, C.; Su, J.; Zhu, H.; Xiong, J.; Liu, X.; Li, D.; Chen, Y.; Li, Y. The removal of heavy metal ions from aqueous solutions by amine functionalized cellulose pretreated with microwave-H2O2. RSC Adv. 2017, 7, 34182–34191. [Google Scholar] [CrossRef]
- Melnyk, I.V.; Pogorilyi, R.P.; Zub, Y.L.; Vaclavikova, M.; Gdula, K.; Dąbrowski, A.; Seisenbaeva, G.A.; Kessler, V.G. Protection of Thiol Groups on the Surface of Magnetic Adsorbents and Their Application for Wastewater Treatment. Sci. Rep. 2018, 8, 8592. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yu, W.; Yang, S.; Wan, Q. Understanding the relationship between pore size, surface charge density, and Cu2+ adsorption in mesoporous silica. Sci. Rep. 2024, 14, 13521. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Batonneau-Gener, I.; Ghssein, G.; Pouilloux, Y. Recent Advances in Heavy Metal Adsorption via Organically Modified Mesoporous Silica: A Review. Water 2025, 17, 669. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E. Effect of micropore size distribution on phenol adsorption on steam activated carbons. Adsorption 2015, 22, 599–607. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ito, R.; Sugimoto, H.; Morimoto, Y.; Itoh, S. Oxidation mechanism of phenols by copper(ii)–halide complexes. Chem. Commun. 2024, 60, 7586–7589. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Selim, A.Q.; Mohamed, E.A.; Mobarak, M.; Zayed, A.M.; Seliem, M.K.; Komarneni, S. Cr(VI) uptake by a composite of processed diatomite with MCM-41: Isotherm, kinetic and thermodynamic studies. Microporous Mesoporous Mater. 2018, 260, 84–92. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, İ. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
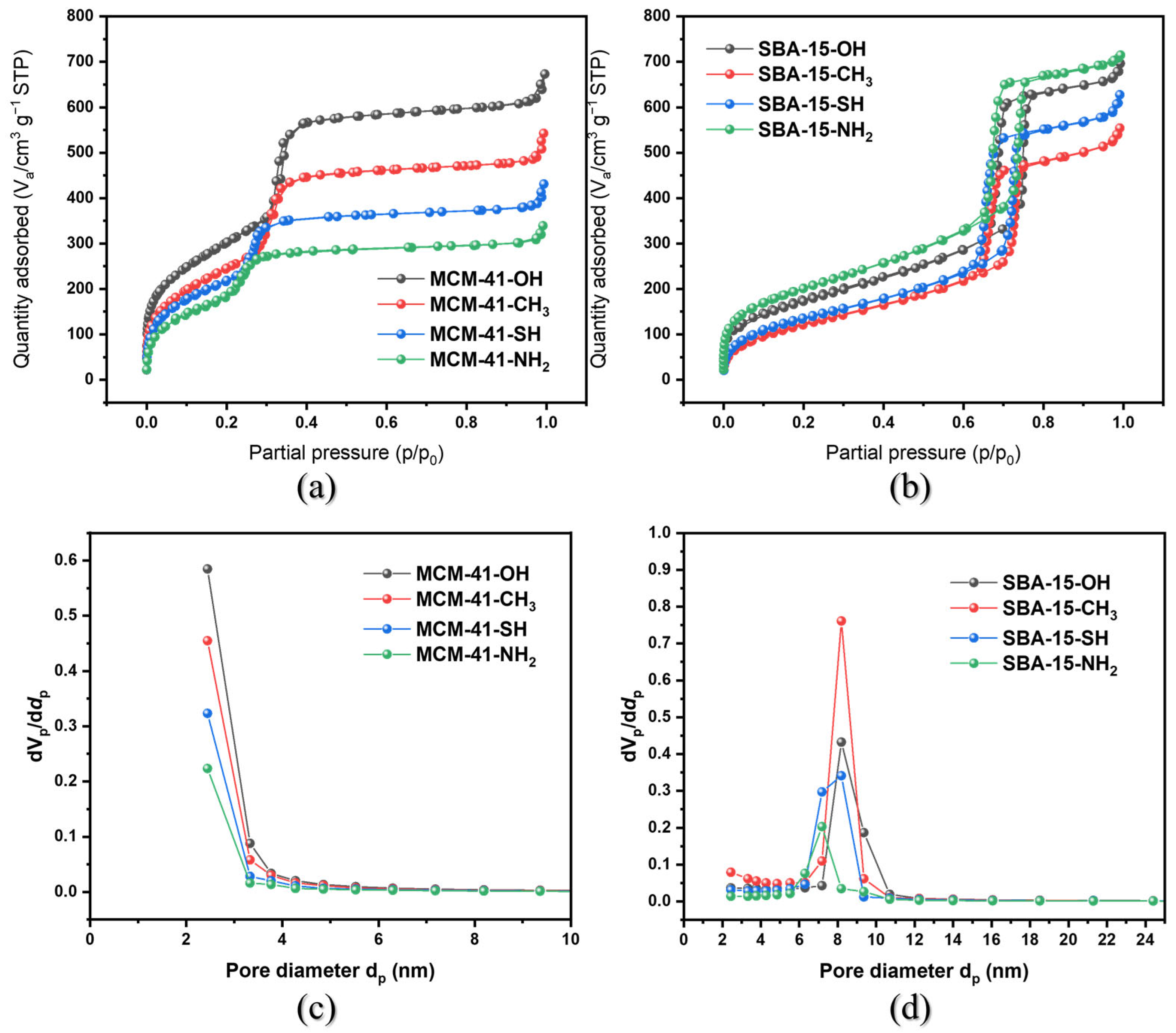



| Plot Data | Specific Surface Area (m2 g−1) | Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| MCM-41-OH | 1120.4 | 3.5605 | 0.9973 |
| MCM-41-CH3 | 927.21 | 3.4795 | 0.8066 |
| MCM-41-NH2 | 656.81 | 3.1381 | 0.5153 |
| MCM-41-SH | 785.3 | 3.2415 | 0.6364 |
| SBA-15-OH | 593.75 | 7.737 | 1.1455 |
| SBA-15-CH3 | 462.63 | 7.3737 | 0.8528 |
| SBA-15-NH2 | 376.28 | 7.1297 | 0.6707 |
| SBA-15-SH | 500.89 | 7.6897 | 0.9629 |
| Al3+ | Cu2+ | Fe3+ | Ni2+ | |
|---|---|---|---|---|
| qm (mmol g−1) | 0.6206 | 0.8672 | 0.5179 | 0.3653 |
| b | 12.515 | 1691.2 | 736.92 | 221.56 |
| ΔG (J mol−1) | −6260.67 | −18,416.3 | −16,358.1 | −13,379.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Lee, K.; Kim, H.; Choi, M.; Hong, S.-K.; Lee, J.E. Removal of Metal Ions in Spin-on Hardmask Using Functionalized Porous Silica Adsorbents. Appl. Sci. 2025, 15, 7185. https://doi.org/10.3390/app15137185
Kim W, Lee K, Kim H, Choi M, Hong S-K, Lee JE. Removal of Metal Ions in Spin-on Hardmask Using Functionalized Porous Silica Adsorbents. Applied Sciences. 2025; 15(13):7185. https://doi.org/10.3390/app15137185
Chicago/Turabian StyleKim, Won, Kiseok Lee, Hyosik Kim, Mingi Choi, Suk-Koo Hong, and Ji Eun Lee. 2025. "Removal of Metal Ions in Spin-on Hardmask Using Functionalized Porous Silica Adsorbents" Applied Sciences 15, no. 13: 7185. https://doi.org/10.3390/app15137185
APA StyleKim, W., Lee, K., Kim, H., Choi, M., Hong, S.-K., & Lee, J. E. (2025). Removal of Metal Ions in Spin-on Hardmask Using Functionalized Porous Silica Adsorbents. Applied Sciences, 15(13), 7185. https://doi.org/10.3390/app15137185






