1. Introduction
Psychological responses to cold exposure may influence athletic performance and can be critical in extreme environmental conditions. According to psychologist Jan Blecharz, understanding the psychological mechanisms of the cold response is the first step toward adaptation [
1]. The sudden mental stress of entering icy water releases a number of phenomena in the body [
2].
Noradrenaline, along with adrenaline, thyroid hormones, and adrenal cortical steroids, plays a significant role in cold adaptation. These agents increase energy production. Tolerance to cold results in higher sensitivity to noradrenaline and in raised capacity to generate adrenaline and noradrenaline. Nevertheless, cold-tolerant individuals usually exhibit suppressed rates of resting sympathetic nervous system arousal, unlike those who are not cold-tolerant or trained [
3].
Winter swimming has been shown to exert a positive effect on the human psyche [
4,
5,
6,
7] and to bring about antidepressant effects [
8]. Regular winter swimming leads to an improvement in the overall well-being of those suffering from rheumatism, fibromyalgia, or asthma [
4]. There is a well-known case report of a 24-year-old woman with symptoms of severe depression and anxiety [
8]. Individuals who perceive themselves as tolerating hot and cold experience stable emotions, as well as greater activity and vigor [
3]. Habituation or acclimatization is considered to be the mechanism of adaptation [
9].
This is, however, related to quick changes in cardiac autonomic balance, characterized by significant vagal tone withdrawal. Caution is thus recommended, especially when a high cardiac risk exists. Moreover, the cold shock response should be reduced with the use of suitable strategies [
10]. From a psychological perspective, examples of such strategies might include adopting an appropriate attitude toward the stimuli that one is about to experience, such as preparing for the appearance of cold, cramps, etc. (becoming comfortable with what lies ahead), or breathing exercises. These techniques help reduce the psychological shock that can occur during a sudden change in temperature. As for the physiological risk, a head bath with cold water is applied in turn.
Immersion in cold water is always accompanied by a significant risk. Cold shock can lead to death caused by cardiac arrhythmia or water inhalation [
11]. In 2021, six deaths were reported in English rivers or lakes during a summer weekend [
12]. Those who wish to practise cold-water immersion should take some simple protective means: (1) enter the water slowly and in a controlled way in order to inhibit the effect of cold shock; (2) splash water onto the whole body before the immersion; (3) move slowly into the water; (4) avoid progressing further into the water until breathing is under control; (5) start practising immersions in the summer, when the water temperature is higher; (6) seek a physician’s advice on underlying health conditions that might increase the risk, e.g., high blood pressure or heart disease; (7) follow information on cold water immersion for the unexperienced, e.g., on the Outdoor Swimming Society website [
7].
The present study aimed to determine if regular winter swimmers differed from those who did not practise winter swimming in terms of (1) daily perceived emotional states, (2) subjectively perceived satisfaction with life, and (3) hormone concentrations. An additional objective was to investigate how a single immersion in cold water in regular winter swimmers affected their subjectively assessed emotional states and levels of stress hormones.
2. Material and Methods
2.1. Participants
The study involved 30 males aged 30–50 years. The experimental group consisted of 15 males (
n = 15) who regularly practised winter swimming within the Krakow Society of Winter Swimmers ‘Kaloryfer’. The control group (
n = 15) had no contact with cold water. The inclusion criteria were as follows: male sex, age of 30–50 years, and written informed consent to participate in the study. The decision to enrol males only had two reasons: (1) the access to female winter swimmers was much lower than to male winter swimmers (a group involving 3 individuals would not be representative); (2) it is significantly more difficult to control hormonal changes in women because of the menstrual cycle (fluctuations in oestrogen and progesterone levels affect brain function, cognition, emotional processing, stress reactivity, mood, and memory, influencing outcomes in psychological studies [
13]). Thus, the male study group seems to have been the most representative group of winter swimmers.
The selection of people aged 30–50 for the study group in this research was based on several theoretical and practical considerations. This age range includes adults in so-called middle adulthood, who, in the opinion of Erikson [
14] and Levinson [
15], are characterized by relative professional and family related stability and a well-established sense of identity. At this age, individuals are usually professionally and socially active while also demonstrating emotional and cognitive maturity, which contributes to reliable participation in psychological research [
16]. What is more, these people often function under the pressure of numerous responsibilities, both at work and at home, and this makes them particularly vulnerable to chronic stress, burnout, and emotional overload [
17,
18]. Therefore, selecting this age group allows us to capture the dynamics of mental functioning in the context of everyday challenges of adulthood and to limit the impact of confounding variables related to development (in younger groups) or aging processes (in older groups) [
19].
The exclusion criteria involved cardiac arrhythmias, uncontrolled hypertension, cancer, oncological treatment, diabetes, and rheumatoid conditions.
Table 1 presents the basic characteristics of the study participants. Analysis with the Mann–Whitney
U-test indicated no significant differences (
p > 0.05) among the subjects in terms of age, physical activity level, body height, or body weight.
The participants were not blinded during the study. Nevertheless, when counting the results, the researchers did not know their identities or group affiliations.
2.2. Study Procedure
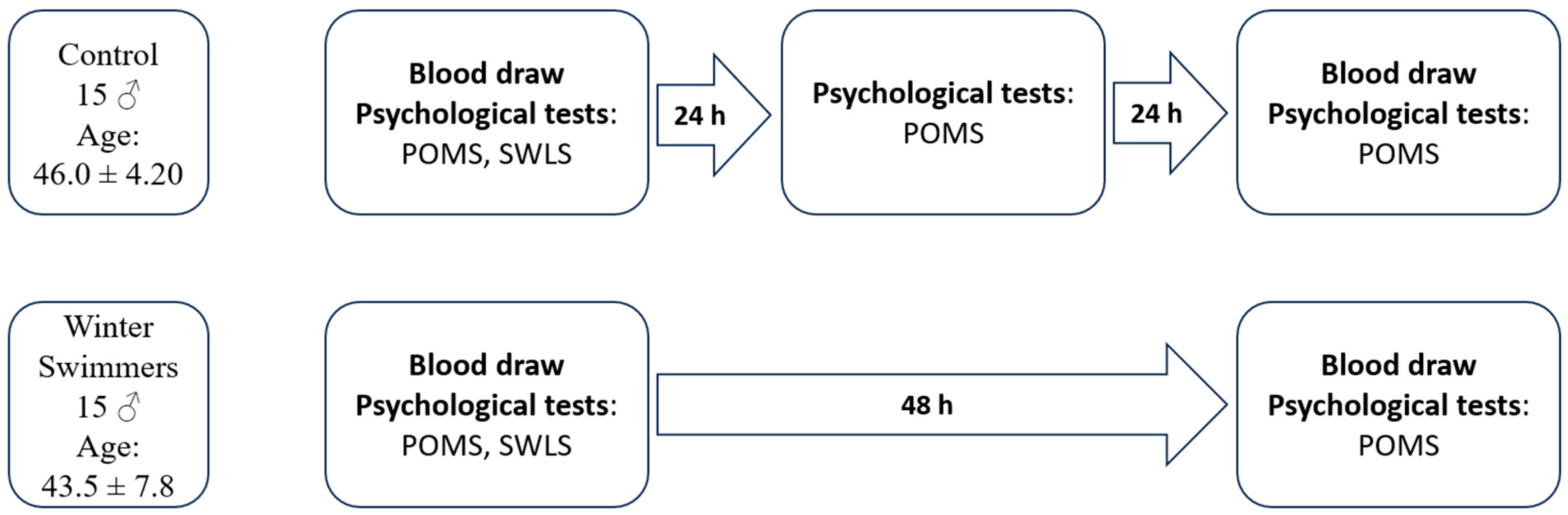
Three measurements were taken in the study: measurement 1, carried out 24 h before cold-water immersion (winter swimming) (blood collection on 18 February 2023, psychological questionnaires); measurement 2, carried out only in the group of winter swimmers, immediately after coming out of the 4 °C water, with an air temperature of 5 °C (pressure 1011 hPa, wind speed 25 km/h, humidity 90%, light rain showers on that day; psychological questionnaires); measurement 3, carried out 24 h after cold-water immersion (winter swimming) (blood collection on 20 February 2023, psychological questionnaires). In order to eliminate uncontrolled variables (weather conditions, air pressure, etc.), measurements 1 and 3 were performed over the same period in both the control and experimental groups.
Fasting blood was collected twice from all subjects, from an ulnar vein into vacuum tubes, in the middle of the 2022/2023 winter swimming season, 24 h before cold-water immersion (winter swimming) and 24 h after the immersion. The blood was collected in 10-mL Vacuette tubes by a qualified nurse, under a physician’s supervision, in accordance with the applicable standards. At the same time, the control group participants were subjected to the same testing procedure (without contact with cold water). Serum blood indicators were determined in the Diagnostyka S.A. laboratory in Krakow, Poland. Adrenaline (pmol/L), noradrenaline (pmol/L), and serotonin (ng/mL) were assessed with the use of the ELISA method (test by LDN, Nordhorn, Germany). Dopamine (pmol/L) was identified with the HPLC method (Agilent, Santa Clara, CA, USA). Cortisol (µg/dL) was determined with chemiluminescence (Abbott, Abbott Park, IL, USA). The subjects were also asked to complete a battery of psychological tests consisting of the following questionnaires: the Profile of Mood States (POMS) questionnaire [
20] in the Polish adaptation by Dudek and Koniarek [
21], and the Satisfaction with Life Scale (SWLS) by Diener et al. (1985) [
22] in the Polish adaptation by Juczyński [
23].
The POMS questionnaire is a self-description tool consisting of 65 items to which the respondent is asked to refer on a 5-point Likert scale (0–4). This questionnaire measures the intensity of 6 emotional states: tension–anxiety, depression–dejection, anger–hostility, fatigue, confusion, and vigor. The measurement can refer to any specified period. In the case of this research, the respondents were asked to indicate their state ‘here and now’.
The SWLS scale is a 5-item self-report inventory (on a 7-point scale) that measures satisfaction with life [
23].
The procedure for the conducted tests is shown in
Figure 1.
2.3. Statistics
The statistical analyses were performed with the Jamovi Project ver. 2.3 computer software (2022). To test for between-group differences, a statistical test was selected based on the distribution of the results of the variable in question: when the results of the Shapiro–Wilk test did not indicate that the distribution of a variable was close to normal, the Mann–Whitney
U-test was applied (for the following variables: adrenaline, dopamine, and serotonin); otherwise, the Student’s
t-test was used with Bonferroni correction. General linear model (multivariate GLM) analyses for dependent variables were employed to examine changes in the studied parameters over time. In the case of variables whose result distribution was not close to normal, the multivariate GLM was used: many studies indicate that type I error and the power of the F-test are not modified when the assumptions of normal distribution and sphericity are not met [
24]. Mauchly’s test served to test the assumption of sphericity of variance. When this assumption was not met, the Greenhouse–Geisser correction was applied in GLM analyses [
25]. A limitation of our study is the relatively small number of participants (30 individuals). This increases the probability of type I and type II errors. However, it is acceptable to perform this type of analysis with a considerable degree of caution [
26].
The model assumed two forms: (1) for 2 measurements: the first measurement (24 h before) and the last measurement (24 h after); time [measurement 1 vs. measurement 3] × group [winter swimmers vs. control group]; (2) in addition, for the results of the POMS questionnaire in the winter swimming group, an analysis was carried out for 3 measurements: time [measurement 1 vs. measurement 2 vs. measurement 3]. To determine the effect size, the partial eta square (η
p2) was calculated, with values > 0.01, 0.06, and 0.14 corresponding to small, medium, and large effect sizes [
27]. When significant interactions were found, probabilities were calculated for post hoc tests using the Tukey test [
28].
In view of the need for considerable caution when interpreting the results of parametric analyses, a decision was taken to perform analogous analyses with non-parametric tests (the Wilcoxon test for comparing two measurements and the Friedman test for comparing three measurements with the post hoc Durbin–Conover test). A limitation of these tools is the inability to examine the time × group interaction, which is crucial for drawing conclusions indicating whether the observed changes over time differ between the studied groups. Hence, the decision to apply both types of analysis was made.
The significance level was set at 5% (α = 0.05); additionally, a situation where the p-values fell within the range of 0.05–0.10 was considered as a trend.
2.4. Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the Regional Medical Chamber in Krakow (No. 226/KBL/OIL/2023). Written informed consent was obtained from all subjects involved in the study.
The men were informed, as required by the Declaration of Helsinki, about the purpose of the study, the methodology used, the potential side effects, and the possibility of withdrawing from the study at any time without giving a reason. The entire experiment was conducted under the supervision of medical personnel.
3. Results
The first step was to examine whether the individuals in the study groups differed significantly from one another at the moment of the first measurement (before winter swimming).
As for the results of the POMS questionnaire, which examines emotional moods, the first measurement (
Table 2) revealed significant differences in the scales of depression–dejection and vigor. In all the cases, the differences were in favor of the winter swimming participants.
As for the results of the SWLS questionnaire, which examines the level of satisfaction with life (winter swimmers: SWLS of 21.4 ± 5.69; control group: SWLS of 20.94 ± 8.39), no statistically significant differences were found between the study groups (p > 0.05).
The results of the analysis using
t-tests indicated significant differences between the study groups for adrenaline. The winter swimmers were characterized by higher concentrations of this hormone (
Table 3). No statistically significant differences were indicated in the concentrations of the other hormones (noradrenaline, dopamine, serotonin, and cortisol) (
p > 0.05).
With reference to the GLM analysis concerning the results of the POMS tests (
Table 2), the following differences were reported (
p < 0.05). As for tension-anxiety, a decrease in this scale (by 0.3 points) was noted in all subjects at measurement 3 (24 h after). Additionally, a trend for the time × group interaction was reported. The results indicate that the reduction in the perceived tension–anxiety in the winter swimming group (by 0.49 points; post hoc Tukey:
p = 0.009; Wilcoxon:
p = 0.001; r
rb = 0.997) is significantly greater than that in the control group (by 0.13 points;
p > 0.05). As for depression–dejection, no statistically significant changes over time were indicated, either when considering the whole sample or for the time × group interaction (
p > 0.05). However, there was a trend (0.10 >
p > 0.05) indicating a very slight decrease in the results of this scale (by 0.15 points) in the whole sample of respondents. As for anger–hostility, a significant decrease in the intensity of this emotional state (by 0.42 points) was observed in all subjects. No significant time × group interaction was reported (
p > 0.05). As for fatigue, a statistically significant decrease in the perceived fatigue was revealed for both whole sample analysis (by 0.57 points) and time × group interaction. In the case of the observed interaction, it was found that in the winter swimming group, the reduction in the perceived fatigue (by 0.86 points; post hoc Tukey:
p < 0.001; Wilcoxon:
p = 0.001; r
rb = 0.950) was significantly larger than that in the control group (by 0.28 points;
p > 0.05). As for confusion, in the third measurement, a decrease of 0.29 points in the results was reported by all participants. No significant time × group interaction was noted (
p > 0.05). As for vigor, no significant changes were reported concerning vigor intensity for all studied subjects and with the consideration of time × group interactions (
p > 0.05) (
Figure 2).
When analyzing the results of the Wilcoxon test, more differences were noted than in the GLM analysis. In the winter swimming group, significant decreases were observed in the outcomes of depression–dejection (p = 0.025; rrb = 0.714), anger–hostility (p = 0.004; rrb = 0.850), and confusion (p = 0.033; rrb = 0.657). In turn, no changes were reported in the control group (p > 0.05). Analyses involving non-parametric tests do not permit examining the interaction of time × group. It is, therefore, difficult to assess the effect of winter swimming on these emotional states.
Additionally, in the group of winter swimmers, an analysis was performed for three measurements (24 h before the single cold-water immersion, immediately after the single cold-water immersion, and 24 h after the single cold-water immersion) with the use of the POMS questionnaire.
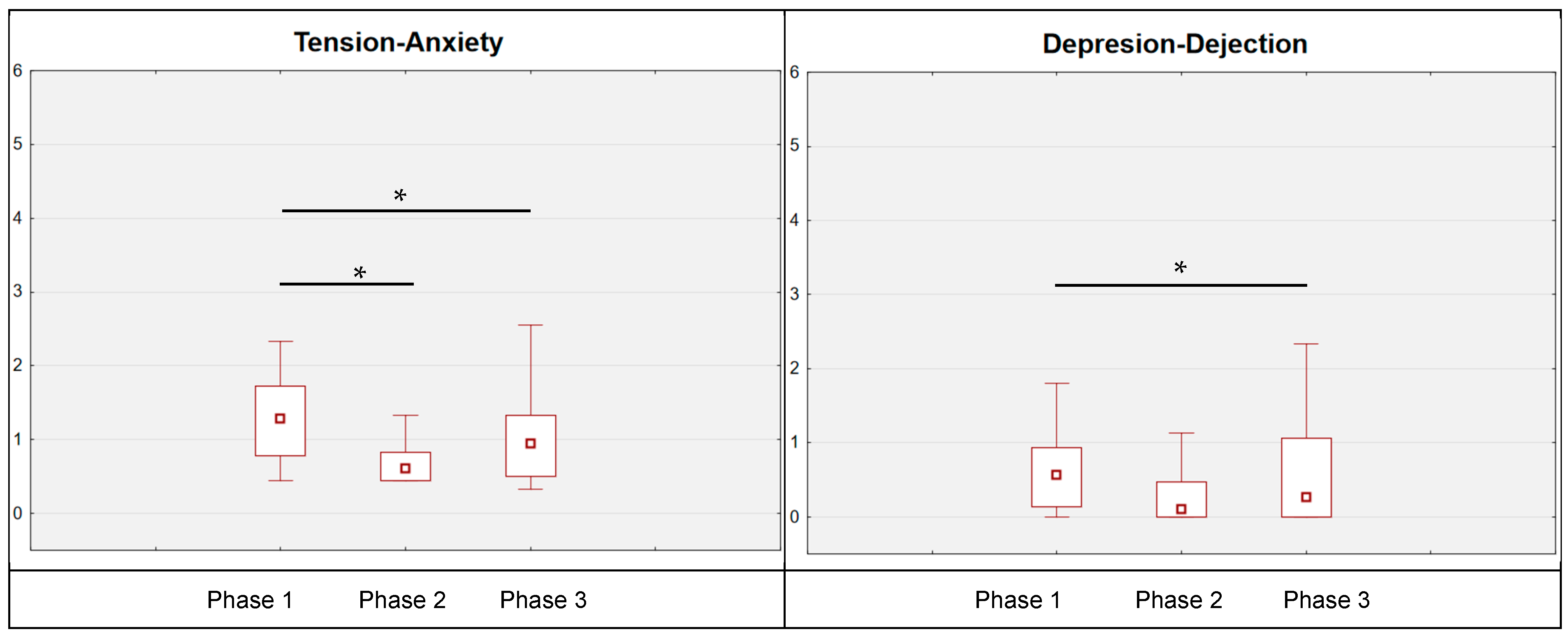
With reference to POMS results (
Figure 3,
Table 2), several significant changes were reported. As for tension–anxiety, statistically significant changes were noted; immediately after leaving the water, the subjects’ tension–anxiety level was reduced (by 0.47 points; post hoc Tukey:
p = 0.002; post hoc Durbin–Conover:
p < 0.001). This result was maintained up to the moment of the measurement carried out 24 h after the single cold-water immersion (phase 1 vs. phase 3: post hoc Tukey:
p < 0.001; post hoc Durbin–Conover:
p < 0.001). For the results of the depression–dejection scale, a statistically significant change was also reported. In this case, a reduction in measurement 2 was observed (by 0.20 points; nonsignificant
p > 0.05), which was further reduced in measurement 3 (significant post hoc Tukey:
p = 0.042; post hoc Durbin–Conover:
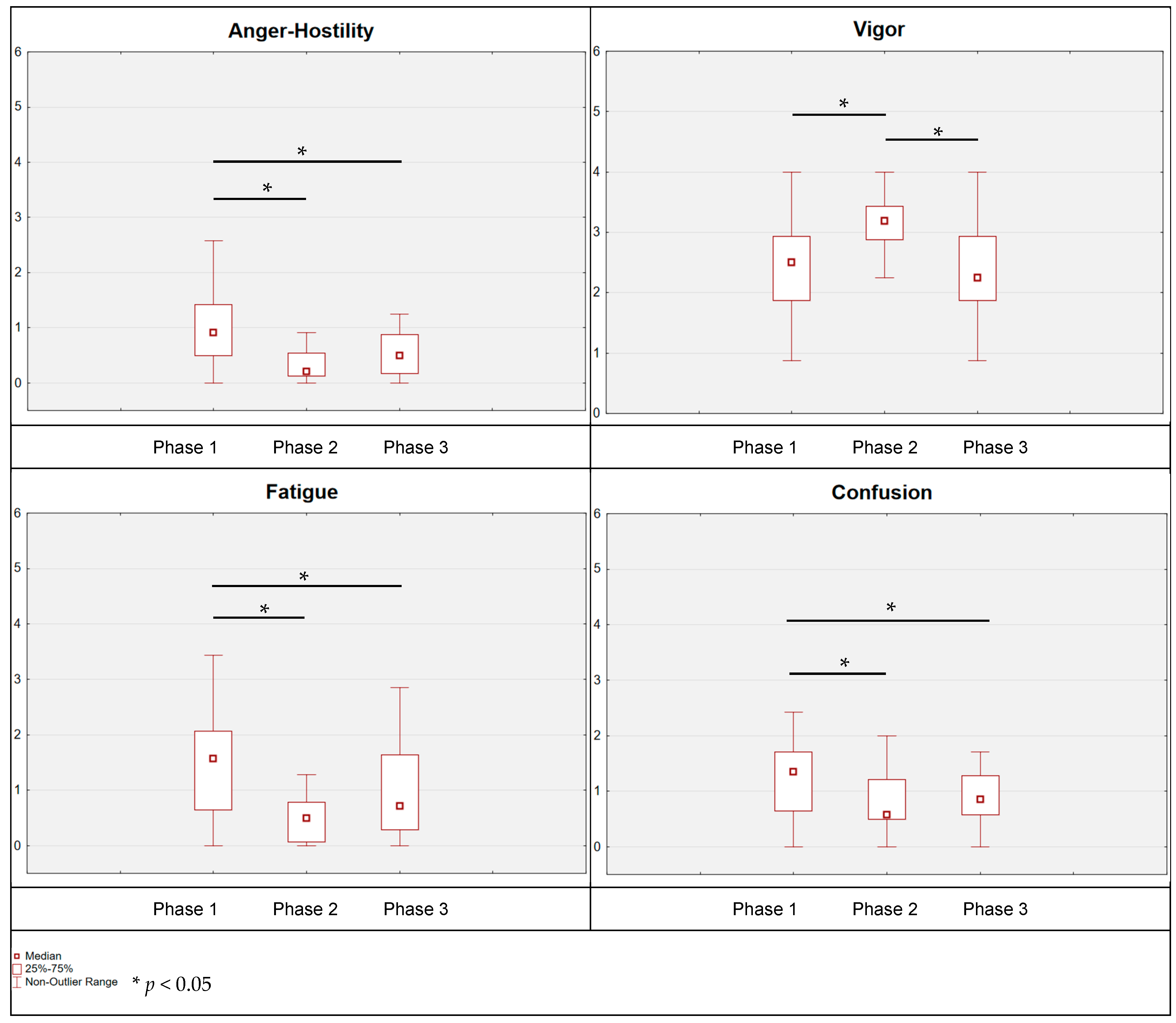
p = 0.003). Moreover, a corresponding reduction in anger–hostility was noted immediately after emerging from the water, the anger–hostility status was reduced by 0.59 points (post hoc Tukey:
p = 0.018; post hoc Durbin–Conover:
p = 0.007) and remained at this low level until the third measurement (phase 1 vs. phase 3: post hoc Tukey:
p = 0.009; post hoc Durbin–Conover:
p = 0.015). The state of vigor also changed immediately after emerging from the water, and it was improved by 0.27 points (post hoc Tukey:
p = 0.095; post hoc Durbin–Conover:
p = 0.031); then, after 24 h, a return of this state to the level of the first measurement was recorded (phase 2 vs. phase 3: post hoc Tukey:
p = 0.066; post hoc Durbin–Conover:
p = 0.039). When analyzing the results relating to fatigue, a statistically significant reduction was also indicated. Immediately after leaving the water, the level of fatigue was reduced by 0.70 points (post hoc Tukey:
p = 0.021; post hoc Durbin–Conover:
p = 0.008); also, for measurement 3, a reduction was identified by 0.15 points in relation to measurement 2 (nonsignificant) and by 0.86 points in relation to measurement 1 (phase 1 vs. phase 3: post hoc Tukey:
p < 0.001; post hoc Durbin–Conover:
p < 0.001).
As for confusion, no statistically significant changes were found (p > 0.05); there was only a trend towards differences (0.10 > p > 0.05), pointing at a potential reduction in confusion intensity (phase 1 vs. phase 2: post hoc Tukey: p = 0.339; post hoc Durbin–Conover: p = 0.050; phase 1 vs. phase 3: post hoc Tukey: p = 0.061; post hoc Durbin–Conover: p = 0.032).
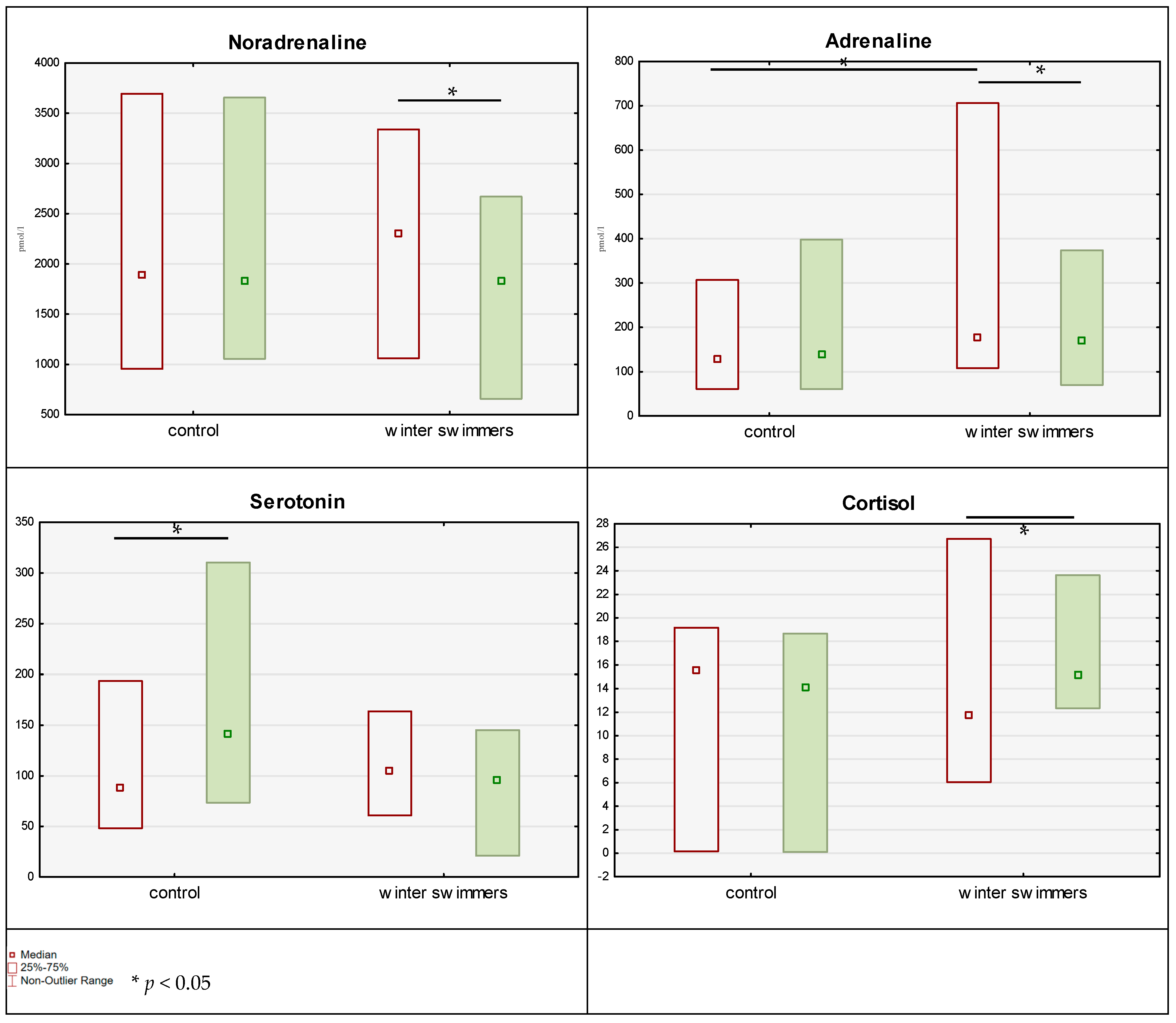
With reference to the results concerning the concentration of the hormones studied (
Table 3,
Figure 4), the GLM analysis indicated a trend indicative of a potential time × group interaction in the case of noradrenaline. The control group showed a slight (statistically nonsignificant) increase in the results (by 42 pmol/L), while the winter swimming group demonstrated a statistically significant (post hoc Tukey:
p = 0.049; Wilcoxon:
p = 0.044; r
rb = 0.574) decrease in the blood concentrations of the hormone (by 369 pmol/L). Moreover, time × group interactions were reported for adrenaline. Similarly, as with noradrenaline, a negligible, nonsignificant change (
p > 0.05) was noted in the control group (by −6.25 pmol/L), with a significant reduction (post hoc Tukey:
p = 0.045; Wilcoxon:
p = 0.047; r
rb = 0.592) in adrenaline levels in the winter swimming group (by 55.1 pmol/L). However, one should observe that in the winter swimming group, despite this reduction, the adrenaline concentration was higher than in the control group. As for the results regarding serotonin, time × group interactions were reported. Serotonin concentration significantly increased (post hoc Tukey:
p < 0.001; Wilcoxon:
p < 0.001; r
rb = −0.971) in the control group (by 55.1 ng/mL) and decreased (but not significantly;
p > 0.05) in the winter swimming group (by 10 ng/mL). In the case of cortisol, a statistically significant time × group interaction was also found. Cortisol concentration significantly increased (post hoc Tukey:
p = 0.019; Wilcoxon:
p = 0.050; r
rb = −0.583) in the winter swimming group (by three units) and slightly (insignificantly;
p > 0.05) decreased in the control group (by 0.9 unit). Dopamine levels were the same in both groups at each measurement (
p > 0.05).
4. Discussion and Conclusions
Contact with cold or icy water provides numerous health-related benefits by triggering a physiological response in the body aimed at homeostasis. It is known what happens in the human blood after a single immersion in icy water [
29] and after regular winter swimming [
30]. Another relevant issue concerns the psychological responses of winter swimmers.
While answering the research questions posed, the authors indicated that individuals who regularly practised winter swimming seemed to present lower levels of the depression–dejection scale while, at the same time, having higher levels of vigor (question 1). However, life satisfaction levels did not differ between winter swimmers and controls (question 2). It was also implied that a single immersion in cold water by regular winter swimmers brought about considerable improvements in mood: immediately after emerging from the water, all negative emotional states were reduced while vigor improved. This better mood (in addition to the vigor score) persisted for at least 24 h after immersion, as evidenced in measurement 3. When comparing the changes in the results (between the measurements performed 24 h before cold-water immersion and 24 h after the immersion), the authors indicated that in the winter swimming group, the results were significantly reduced between the two measurements for tension–anxiety and fatigue as compared with the control group (question 4). Similar results were obtained by Huttunen et al. [
4]; they reported that after 4 months of immersing in cold water, winter swimmers exhibited more vigor and less fatigue than the control group individuals.
The presented study confirms the assumption that adaptation to cold water contributes to a weaker response to stressors arising in daily life (lower depression–dejection score). In 2018, a case study was published demonstrating for the first time the effect of cold-water swimming in open areas on reducing symptoms of depression [
8]. Summarizing the results obtained from the POMS questionnaire, one could conclude that regular winter swimming positively influences emotional states. Among the explanations for the observed changes is the activation of the parasympathetic nervous system. The results of this study indicate that systematic winter swimmers exhibit significantly higher blood adrenaline concentrations as compared with the individuals who did not practise winter swimming. Adrenaline is the hormone responsible for the immediate stress response of the body in an emergency situation: in this case, repeated contact with cold water. The effect of adrenaline is short-lasting, but among winter swimmers, the frequent contact with cold water influenced the activation of the sympathetic nervous system and increased blood adrenaline concentrations as compared with the control group; this confirms that the stimulus was sufficient to induce significant adaptive changes in the activity of the sympathetic nervous system and the production of adrenaline. According to Shevchuk [
31], owing to the increase in catecholamines, cold-water swimming can be curative for depression as it activates the sympathetic nervous system, as well as increases the concentration of noradrenaline and endorphins. A recent study involving 1114 female cold-water swimmers demonstrated that this hobby alleviated the mood fluctuations associated with menstruation and menopause [
32].
Moreover, in the own study, 24 h after cold-water immersion, the winter swimming group was characterized by a significant reduction in noradrenaline and adrenaline concentrations and by a slight increase in cortisol concentration, as compared with the control group. Of interest, a slight increase in serotonin levels in the control group was observed, unlike in the winter swimming group. Dopamine levels remained unchanged. The effects of adrenaline and noradrenaline are similar: both constrict peripheral blood vessels, accelerate the heart rate, and raise blood pressure. It is most likely that frequent contact with water induced the winter swimmers’ adaptation to the prevailing conditions, i.e., cold water. Additionally, Leppäluoto et al. [
33] reported that 3 months of regular winter swimming resulted in a decrease in the catecholamine concentration, as measured immediately after immersion in water at a temperature of 0–2 °C. In turn, Huttunen et al. [
34] observed that adaptation through habitual exposure to cold during winter swimming attenuated the physiological response and inhibited the increase in catecholamine concentrations. Cold habituation activates the parasympathetic nervous system and reduces the blood concentration of catecholamines in acute hypoxia [
35]. In contrast, according to Janský et al. [
36], a single immersion in cold water (with the head over the water, at 14 °C, for 1 h) increased sympathetic nervous system activity, as evidenced by a four-fold increase in plasma noradrenaline concentrations with no change in plasma adrenaline and dopamine concentrations; however, there was no apparent change in sympathetic nervous system reactivity as a result of repeated cold-water immersions (three times a week for 6 weeks). Šrámek et al. [
37] observed that a cold-water immersion (14 °C) induced an increase in plasma noradrenaline and dopamine concentrations by 530% and 250%, respectively, and a rise in diuresis by 163% (i.e., more than at a temperature of 32 °C). Cortisol concentrations tended to decrease. Plasma adrenaline concentrations remained unchanged, which, according to the authors, is explained by the fact that cold-induced responses are mainly due to increased sympathetic nervous system activity. In turn, swimmers who participated in winter swimming three times a week at water temperatures of 0–3 °C for 12 weeks presented increased ACTH and cortisol, as well as norepinephrine levels [
33]. Water immersion (20 s per week for 3 winter months in water at a temperature of 0–2 °C) increased noradrenaline concentration, which can reduce pain perception, e.g., in the case of whole-body cold therapy or ice swimming [
33].
The lack of differences in life satisfaction levels raises concerns. By ‘satisfaction with life,’ the authors of the SWLS questionnaire understood one’s own quality of life evaluated in a conscious cognitive process on the basis of individually adopted criteria [
38]. By completing the questionnaire, the respondents were therefore able to refer to a number of undefined factors, such as family relations, work, socioeconomic status, health, sense of self-efficacy, etc. In addition, this term describes a condition that is relatively stable over time (in contrast to the emotional states, which were investigated with the POMS questionnaire). It can, therefore, be assumed that participation in winter swimming does not substantially translate into enhanced satisfaction with life in general. A scoping review performed by Ono et al. [
39], referring to the relationship between cold-water immersion and well-being (which involves positive affect, negative affect, and cognitive life assessment, including satisfaction with life) [
40,
41], indicated the positive impact of such practices on well-being. However, the authors point out that further research related to this topic is needed to better understand the phenomenon. The observed potential differences between winter swimmers and the control group in terms of emotional states suggest that winter swimming contributes to an improvement in well-being in two of its three spheres: positive affect and negative affect.
The study indicated that regular winter swimmers exhibited significantly higher blood concentrations of adrenaline than the control group participants. Moreover, 24 h after cold-water immersion, the winter swimming group was characterized by a significant reduction in noradrenaline and adrenaline concentrations and by a slight increase in cortisol concentration as compared with the control group. Of interest, a slight increase in serotonin levels in the control group was observed, unlike in the winter swimming group. Dopamine levels remained unchanged.
5. Limitations and Further Research
Limitations of the present study include the small group size mentioned earlier. The obtained results should, therefore, be treated with caution (as a pilot study). During the study preparation and result analysis, efforts were made to reduce the risk of type I errors (showing significant differences that do not actually exist). However, it is likely that not all the changes that occur during winter swimming have been captured (type II error). According to G*Power software analyses (ver 3.1.9.7), obtaining high-power parametric test results for repeated measurements (effect size: f = 0.25; α = 0.05; power (1 − β) = 0.80; number of groups: 2; number of measurements: 2) would require a sample size of 98 individuals, which is very difficult to achieve in the case of winter swimmers.
Winter swimmers are a group that is not easily accessible: there is only one club for winter swimmers in the city where the study was conducted. Virtually all winter swimmers meeting the eligibility criteria were included in the presented study. Given the difficulties in recruiting winter swimmers for scientific research, if it is not possible to involve a larger group in further studies, the number of measurements should be increased. This should allow to reduce the minimum sample size while maintaining high test power [
42].
The fact that women were not investigated is another limitation of the presented study.
Not implementing the second measurement in the control group (monitoring of emotional states) can be considered a further limitation. The research team was not able to investigate this owing to human resource constraints: it was impossible to carry out two measurements at two different locations at the same time. In further studies, two options can be suggested: (1) to examine mood states in the control group at the same time as among the winter swimmers; (2) to invite the control group individuals to a swimming pool with warm (air-temperature) water, adjust the swimming time, etc., to winter swimmers, and take measurements after the participants leave the water.
In future studies, it would be advisable to test the long-term effect of winter swimming, e.g., over an entire season. The research presented here mainly focused on short-term results arising from a single cold-water immersion. Although differences between winter swimmers and the control group were examined, this was only a cross-sectional evaluation, which did not allow us to identify a cause–effect relationship.