Multimodal Comparison of Cold Atmospheric Plasma Sources for Disinfection
Abstract
1. Introduction
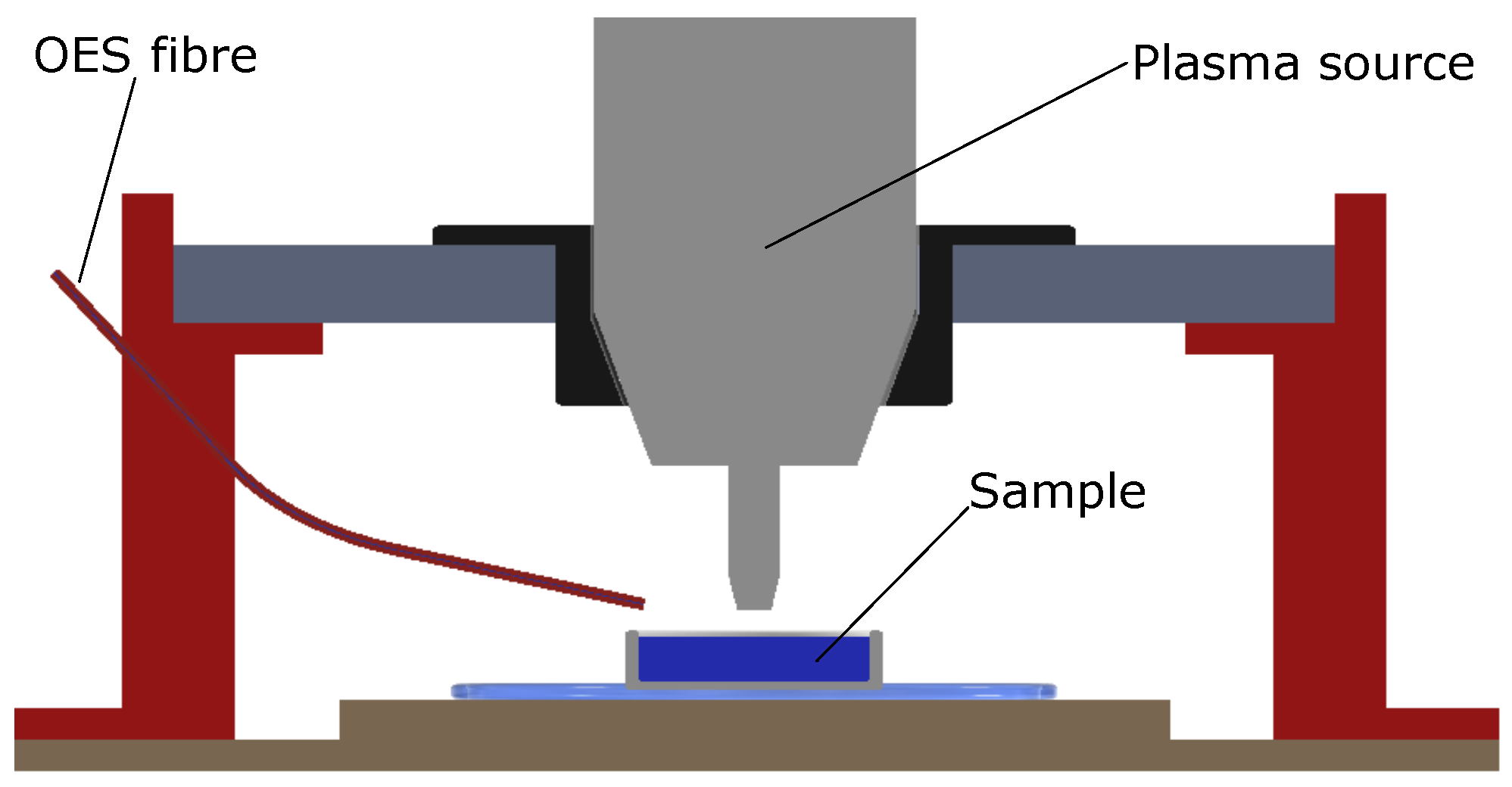
2. Setup
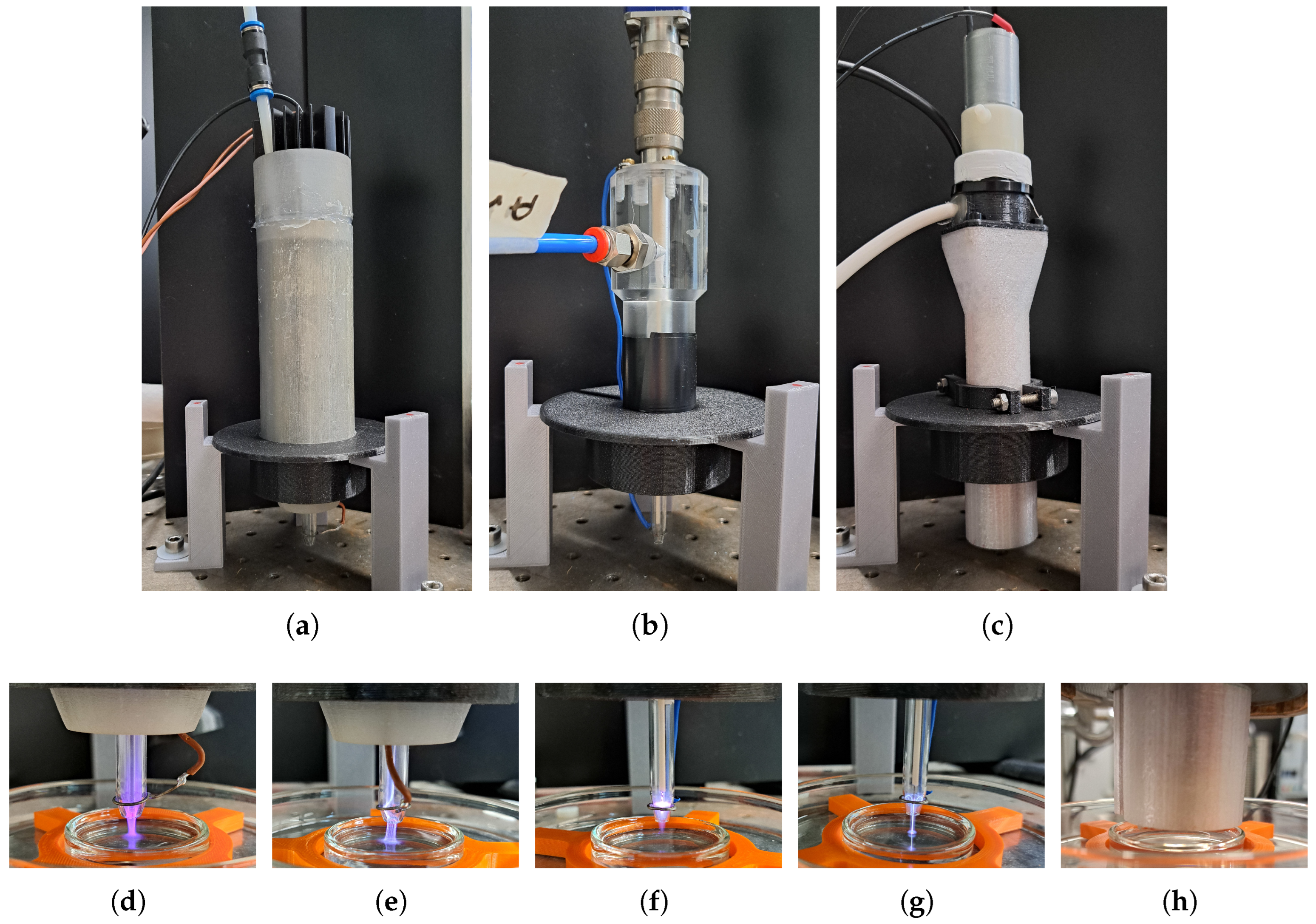
3. Plasma Sources
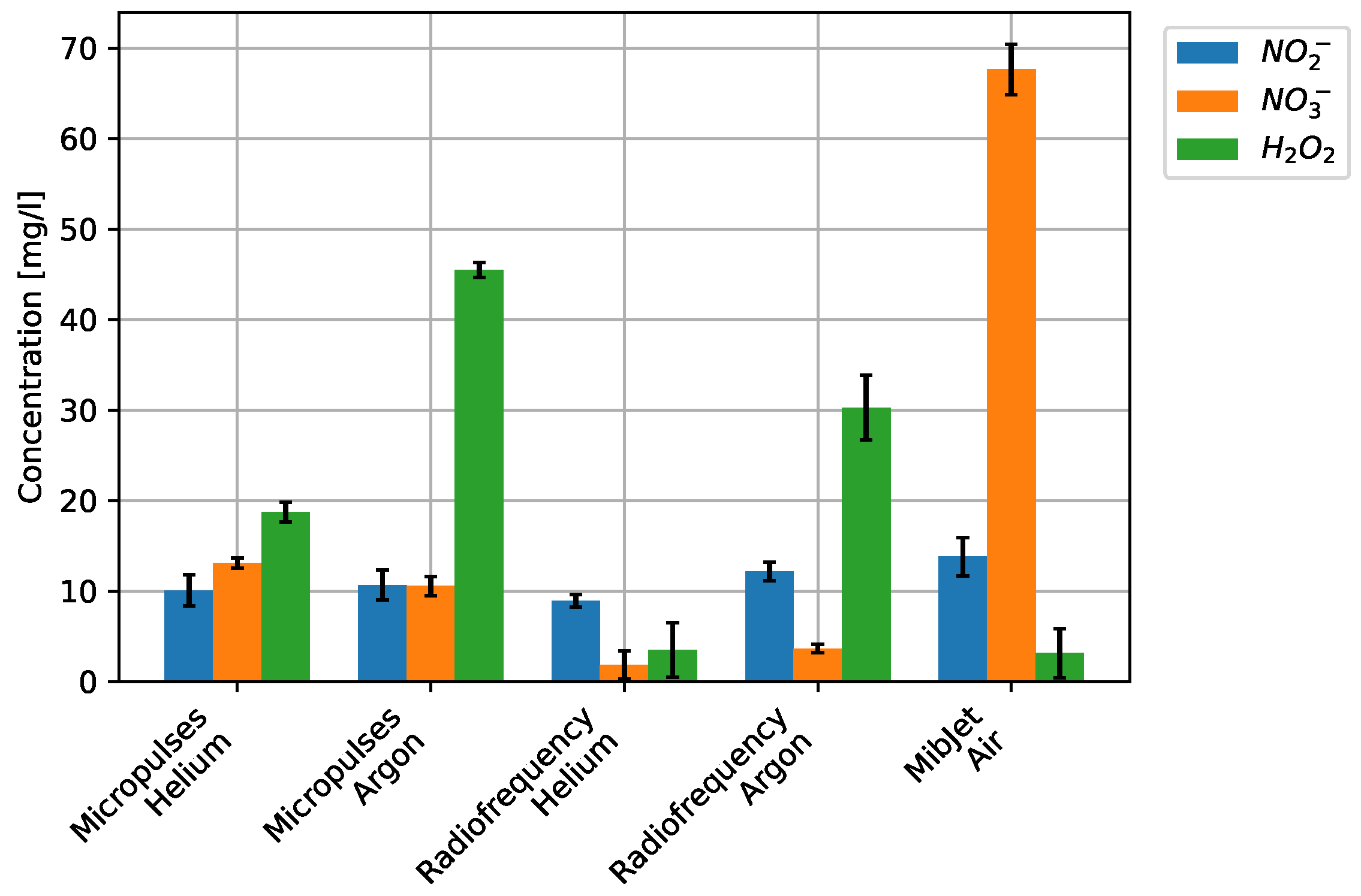
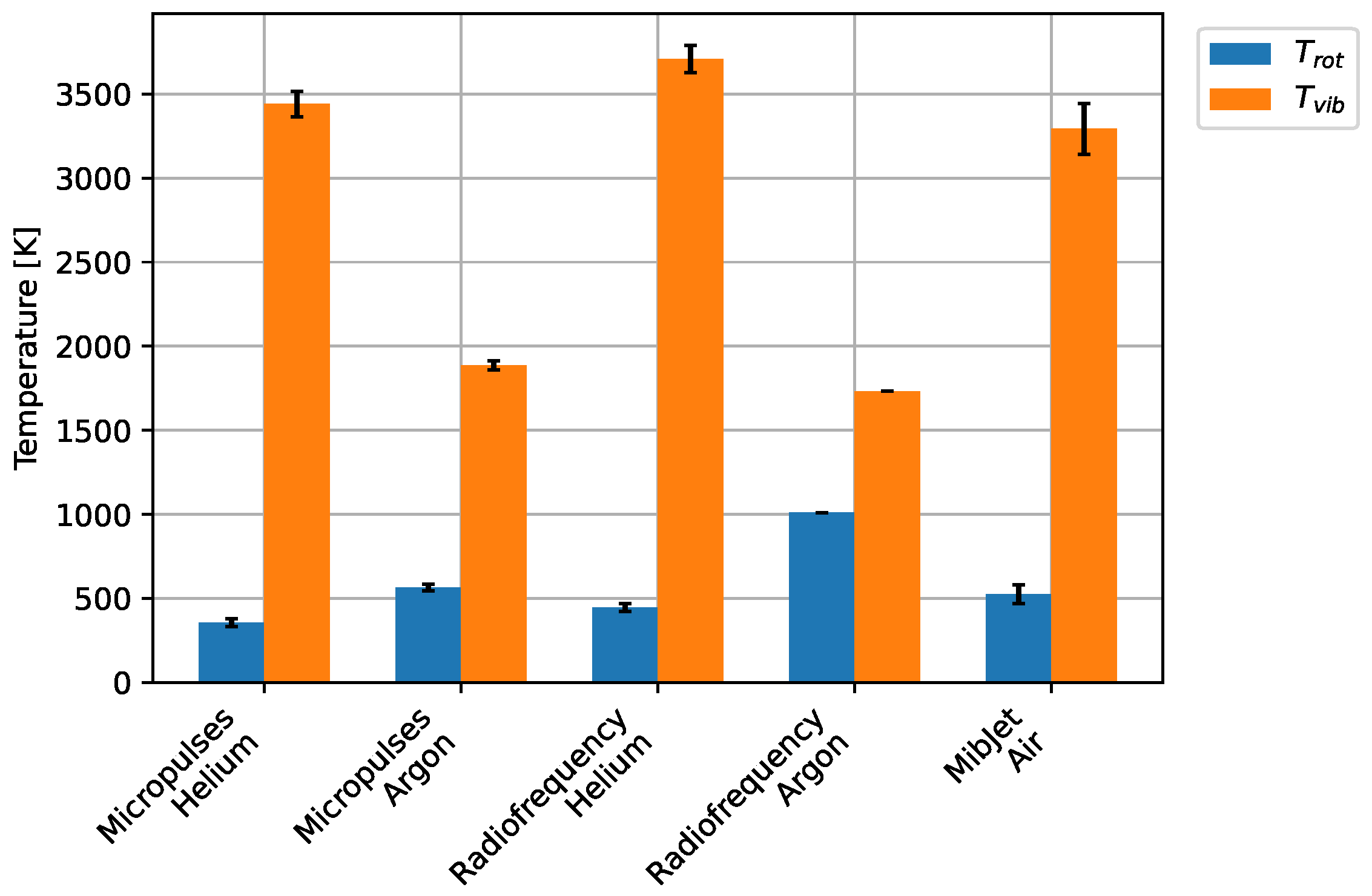
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nastuta, A.V.; Gerling, T. Cold Atmospheric Pressure Plasma Jet Operated in Ar and He: From Basic Plasma Properties to Vacuum Ultraviolet, Electric Field and Safety Thresholds Measurements in Plasma Medicine. Appl. Sci. 2022, 12, 644. [Google Scholar] [CrossRef]
- Jin, S.; Nie, L.; Zhou, R.; Luo, J.; Lu, X. An Ionization-Driven Air Plasma Jet. Front. Phys. 2022, 10, 928402. [Google Scholar] [CrossRef]
- Viegas, P.; Slikboer, E.; Bonaventura, Z.; Guaitella, O.; Sobota, A.; Bourdon, A. Physics of Plasma Jets and Interaction with Surfaces: Review on Modelling and Experiments. Plasma Sources Sci. Technol. 2022, 31, 053001. [Google Scholar] [CrossRef]
- Li, J.; Lei, B.; Wang, J.; Xu, B.; Ran, S.; Wang, Y.; Zhang, T.; Tang, J.; Zhao, W.; Duan, Y. Atmospheric Diffuse Plasma Jet Formation from Positive-Pseudo-Streamer and Negative Pulseless Glow Discharges. Commun. Phys. 2021, 4, 64. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Iza, F.; Brandenburg, R. Foundations of Atmospheric Pressure Non-Equilibrium Plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef]
- Weiss, M.; Arnholdt, M.; Hißnauer, A.; Fischer, I.; Schönfisch, B.; Andress, J.; Gerstner, S.; Dannehl, D.; Bösmüller, H.; Staebler, A.; et al. Tissue-Preserving Treatment with Non-Invasive Physical Plasma of Cervical Intraepithelial Neoplasia—A Prospective Controlled Clinical Trial. Front. Med. 2023, 10, 1242732. [Google Scholar] [CrossRef]
- Pai, K.; Timmons, C.; Roehm, K.D.; Ngo, A.; Narayanan, S.S.; Ramachandran, A.; Jacob, J.D.; Ma, L.M.; Madihally, S.V. Investigation of the Roles of Plasma Species Generated by Surface Dielectric Barrier Discharge. Sci. Rep. 2018, 8, 16674. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial Effects of Low-Temperature Plasma Generated by Atmospheric-Pressure Plasma Jet Are Mediated by Reactive Oxygen Species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Wong, K.S.; Chew, N.S.L.; Low, M.; Tan, M.K. Plasma-Activated Water: Physicochemical Properties, Generation Techniques, and Applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Kc, S.K.; Ghimire, B.; Hong, S.H.; Oh, J.S.; Szili, E.J. How to Control the Plasma Jet Production of Reactive Species for Medical Therapy? A Topical Review. J. Phys. D Appl. Phys. 2025, 58, 143006. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and Antibacterial Effects of Plasma Activated Water: Correlation with Gaseous and Aqueous Reactive Oxygen and Nitrogen Species, Plasma Sources and Air Flow Conditions. J. Phys. D Appl. Phys. 2019, 52, 034002. [Google Scholar] [CrossRef]
- Verlackt, C.C.W.; Van Boxem, W.; Bogaerts, A. Transport and Accumulation of Plasma Generated Species in Aqueous Solution. Phys. Chem. Chem. Phys. 2018, 20, 6845–6859. [Google Scholar] [CrossRef]
- Chautrand, T.; Souak, D.; Chevalier, S.; Duclairoir-Poc, C. Gram-Negative Bacterial Envelope Homeostasis under Oxidative and Nitrosative Stress. Microorganisms 2022, 10, 924. [Google Scholar] [CrossRef]
- Agus, R.; Avino, F.; Ibba, L.; Myers, B.; Zampieri, L.; Martines, E.; Howling, A.; Furno, I. Implementing Water Recirculation in a Novel Portable Plasma-Activated Water Reactor Enhances Antimicrobial Effect against Escherichia coli. Chem. Eng. J. 2024, 486, 149915. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.P.; Canal, C. Important Parameters in Plasma Jets for the Production of RONS in Liquids for Plasma Medicine: A Brief Review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Bosi, F.J.; Tampieri, F.; Marotta, E.; Bertani, R.; Pavarin, D.; Paradisi, C. Characterization and Comparative Evaluation of Two Atmospheric Plasma Sources for Water Treatment. Plasma Process. Polym. 2018, 15, e1700130. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, T. Comparative Study of Discharge Schemes for Production Rates and Ratios of Reactive Oxygen and Nitrogen Species in Plasma Activated Water. J. Phys. D Appl. Phys. 2019, 52, 385202. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. A Comparative Study of Dielectric Barrier Discharge Plasma Device and Plasma Jet to Generate Plasma Activated Water and Post-Discharge Trapping of Reactive Species. Phys. Plasmas 2022, 29, 033510. [Google Scholar] [CrossRef]
- Park, J.H.; Kumar, N.; Hoon Park, D.; Yusupov, M.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A.; Ho Kang, M.; Sup Uhm, H.; Ha Choi, E.; et al. A Comparative Study for the Inactivation of Multidrug Resistance Bacteria Using Dielectric Barrier Discharge and Nano-Second Pulsed Plasma. Sci. Rep. 2015, 5, 13849. [Google Scholar] [CrossRef]
- Mann, M.; Schnabel, U.; Weihe, T.; Weltmann, K.D.; Von Woedtke, T. A Reference Technique to Compare the Antimicrobial Properties of Atmospheric Pressure Plasma Sources. Plasma Med. 2015, 5, 27–47. [Google Scholar] [CrossRef]
- Arndt, S.; Schmidt, A.; Karrer, S.; Von Woedtke, T. Comparing Two Different Plasma Devices kINPen and Adtec SteriPlas Regarding Their Molecular and Cellular Effects on Wound Healing. Clin. Plasma Med. 2018, 9, 24–33. [Google Scholar] [CrossRef]
- Bakhtiyari-Ramezani, M.; Nohekhan, M.; Akbari, M.E.; Abbasvandi, F.; Bayat, M.; Akbari, A.; Nasiri, M. Comparative Assessment of Direct and Indirect Cold Atmospheric Plasma Effects, Based on Helium and Argon, on Human Glioblastoma: An in Vitro and in Vivo Study. Sci. Rep. 2024, 14, 3578. [Google Scholar] [CrossRef] [PubMed]
- Medvecká, V.; Omasta, S.; Klas, M.; Mošovská, S.; Kyzek, S.; Zahoranová, A. Plasma Activated Water Prepared by Different Plasma Sources: Physicochemical Properties and Decontamination Effect on Lentils Sprouts. Plasma Sci. Technol. 2022, 24, 015503. [Google Scholar] [CrossRef]
- Ďurčányová, S.; Slováková, Ľ.; Klas, M.; Tomeková, J.; Ďurina, P.; Stupavská, M.; Kováčik, D.; Zahoranová, A. Efficacy Comparison of Three Atmospheric Pressure Plasma Sources for Soybean Seed Treatment: Plasma Characteristics, Seed Properties, Germination. Plasma Chem. Plasma Process. 2023, 43, 1863–1885. [Google Scholar] [CrossRef]
- Neal, C.; Thomas, A.G. Field and Laboratory Measurement of pH in Low-Conductivity Natural Waters. J. Hydrol. 1985, 79, 319–322. [Google Scholar] [CrossRef]
- Ventura, L.R.; Fellows, C. The N2 Second Positive (C3Π→ B3Π) System Reviewed: Improved Data and Analysis. J. Quant. Spectrosc. Radiat. Transf. 2019, 239, 106645. [Google Scholar] [CrossRef]
- Naz, M.Y.; Shukrullah, S.; Rehman, S.U.; Khan, Y.; Al-Arainy, A.A.; Meer, R. Optical Characterization of Non-Thermal Plasma Jet Energy Carriers for Effective Catalytic Processing of Industrial Wastewaters. Sci. Rep. 2021, 11, 2896. [Google Scholar] [CrossRef]
- Voráč, J.; Synek, P.; Potočňáková, L.; Hnilica, J.; Kudrle, V. Batch Processing of Overlapping Molecular Spectra as a Tool for Spatio-Temporal Diagnostics of Power Modulated Microwave Plasma Jet. Plasma Sources Sci. Technol. 2017, 26, 025010. [Google Scholar] [CrossRef]
- Voráč, J.; Kusýn, L.; Synek, P. Deducing Rotational Quantum-State Distributions from Overlapping Molecular Spectra. Rev. Sci. Instruments 2019, 90, 123102. [Google Scholar] [CrossRef]
- Voráč, J.; Synek, P.; Procházka, V.; Hoder, T. State-by-State Emission Spectra Fitting for Non-Equilibrium Plasmas: OH Spectra of Surface Barrier Discharge at Argon/Water Interface. J. Phys. D Appl. Phys. 2017, 50, 294002. [Google Scholar] [CrossRef]
- Cordaro, L.; De Masi, G.; Fassina, A.; Mancini, D.; Cavazzana, R.; Desideri, D.; Sonato, P.; Zuin, M.; Zaniol, B.; Martines, E. On the Electrical and Optical Features of the Plasma Coagulation Controller Low Temperature Atmospheric Plasma Jet. Plasma 2019, 2, 156–167. [Google Scholar] [CrossRef]
- De Masi, G.; Gareri, C.; Cordaro, L.; Fassina, A.; Brun, P.; Zaniol, B.; Cavazzana, R.; Martines, E.; Zuin, M.; Marinaro, G.; et al. Plasma Coagulation Controller: A Low-power Atmospheric Plasma Source for Accelerated Blood Coagulation. Plasma Med. 2018, 8, 245–254. [Google Scholar] [CrossRef]
- Peeters, F.; Butterworth, T. Electrical Diagnostics of Dielectric Barrier Discharges. In Atmospheric Pressure Plasma—From Diagnostics to Applications; Nikiforov, A., Chen, Z., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Mentheour, R.; Machala, Z. Coupled Antibacterial Effects of Plasma-Activated Water and Pulsed Electric Field. Front. Phys. 2022, 10, 895813. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, T.; Xie, X. Locally Enhanced Electric Field Treatment (LEEFT) Promotes the Performance of Ozonation for Bacteria Inactivation by Disrupting the Cell Membrane. Environ. Sci. Technol. 2020, 54, 14017–14025. [Google Scholar] [CrossRef]
- Wang, T.; Brown, D.K.; Xie, X. Operando Investigation of Locally Enhanced Electric Field Treatment (LEEFT) Harnessing Lightning-Rod Effect for Rapid Bacteria Inactivation. Nano Lett. 2022, 22, 860–867. [Google Scholar] [CrossRef]
- Velliou, E.; Van Derlinden, E.; Cappuyns, A.; Geeraerd, A.; Devlieghere, F.; Van Impe, J. Heat Inactivation of Escherichia coli K12 MG1655: Effect of Microbial Metabolites and Acids in Spent Medium. J. Therm. Biol. 2012, 37, 72–78. [Google Scholar] [CrossRef]
- Sies, H.; De Groot, H. Role of Reactive Oxygen Species in Cell Toxicity. Toxicol. Lett. 1992, 64–65, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Chandana, L.; Sangeetha, C.; Shashidhar, T.; Subrahmanyam, C. Non-Thermal Atmospheric Pressure Plasma Jet for the Bacterial Inactivation in an Aqueous Medium. Sci. Total Environ. 2018, 640–641, 493–500. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Kim, J.J.; Johnston, P.R.; Makarova, O.; Eravci, M.; Weise, C.; Hengge, R.; Rolff, J. Non-Lethal Exposure to H2O2 Boosts Bacterial Survival and Evolvability against Oxidative Stress. PLoS Genet. 2020, 16, e1008649. [Google Scholar] [CrossRef]
- Jonkers, J.; Sande, M.V.D.; Sola, A.; Gamero, A.; Mullen, J.V.D. On the Differences between Ionizing Helium and Argon Plasmas at Atmospheric Pressure. Plasma Sources Sci. Technol. 2003, 12, 30–38. [Google Scholar] [CrossRef]
- Stepanova, O.; Pinchuk, M.; Astafiev, A.; Chen, Z. A Streamer Behavior Evolution during an Applied Voltage Cycle in Helium and Argon Atmospheric Pressure Plasma Jets Fed by DBD. Jpn. J. Appl. Phys. 2020, 59, SHHC03. [Google Scholar] [CrossRef]
- Shimizu, T.; Sakiyama, Y.; Graves, D.B.; Zimmermann, J.L.; Morfill, G.E. The Dynamics of Ozone Generation and Mode Transition in Air Surface Micro-Discharge Plasma at Atmospheric Pressure. New J. Phys. 2012, 14, 103028. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, B.J.; Im, H.G.; Cha, M.S. Ozone Production With Dielectric Barrier Discharge: Effects of Power Source and Humidity. IEEE Trans. Plasma Sci. 2016, 44, 2288–2296. [Google Scholar] [CrossRef]
- Małajowicz, J.; Khachatryan, K.; Kozłowska, M. Properties of Water Activated with Low-Temperature Plasma in the Context of Microbial Activity. Beverages 2022, 8, 63. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K.K. Cold Atmospheric Plasma Activated Water as a Prospective Disinfectant: The Crucial Role of Peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Ikawa, S.; Kitano, K.; Hamaguchi, S. Effects of pH on Bacterial Inactivation in Aqueous Solutions Due to Low-Temperature Atmospheric Pressure Plasma Application. Plasma Process. Polym. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, S.; Choe, W.; Yong, H.I.; Jo, C.; Kim, K. Plasma-Functionalized Solution: A Potent Antimicrobial Agent for Biomedical Applications from Antibacterial Therapeutics to Biomaterial Surface Engineering. ACS Appl. Mater. Interfaces 2017, 9, 43470–43477. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-Phase Chemistry and Bactericidal Effects from an Air Discharge Plasma in Contact with Water: Evidence for the Formation of Peroxynitrite through a Pseudo-Second-Order Post-Discharge Reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing: London, UK, 2012. [Google Scholar] [CrossRef]
- Perinban, S.; Orsat, V.; Raghavan, V. Nonthermal Plasma–Liquid Interactions in Food Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1985–2008. [Google Scholar] [CrossRef]
| Abatement [logAbat] | ||
|---|---|---|
| Direct | Indirect | |
| Micropulsed/He | >6 | >6 |
| Micropulsed/Ar | ||
| Radiofrequency/He | <0.5 | <0.5 |
| Radiofrequency/Ar | <0.5 | <0.5 |
| MibJet | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampieri, L.; Agus, R.; Myers, B.; Cavazzana, R.; Cordaro, L.; De Masi, G.; Zuin, M.; Riccardi, C.; Furno, I.; Martines, E. Multimodal Comparison of Cold Atmospheric Plasma Sources for Disinfection. Appl. Sci. 2025, 15, 7037. https://doi.org/10.3390/app15137037
Zampieri L, Agus R, Myers B, Cavazzana R, Cordaro L, De Masi G, Zuin M, Riccardi C, Furno I, Martines E. Multimodal Comparison of Cold Atmospheric Plasma Sources for Disinfection. Applied Sciences. 2025; 15(13):7037. https://doi.org/10.3390/app15137037
Chicago/Turabian StyleZampieri, Leonardo, Rita Agus, Brayden Myers, Roberto Cavazzana, Luigi Cordaro, Gianluca De Masi, Matteo Zuin, Claudia Riccardi, Ivo Furno, and Emilio Martines. 2025. "Multimodal Comparison of Cold Atmospheric Plasma Sources for Disinfection" Applied Sciences 15, no. 13: 7037. https://doi.org/10.3390/app15137037
APA StyleZampieri, L., Agus, R., Myers, B., Cavazzana, R., Cordaro, L., De Masi, G., Zuin, M., Riccardi, C., Furno, I., & Martines, E. (2025). Multimodal Comparison of Cold Atmospheric Plasma Sources for Disinfection. Applied Sciences, 15(13), 7037. https://doi.org/10.3390/app15137037











