Propagation of Atriplex halimus (Mediterranean Saltbush) in Multi-Contaminated Mine Tailings by Unrooted Cuttings
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area
2.2. Substrate Preparation
- CP: mine tailings from Campo Pisano.
- CP + CI: mixture of Campo Pisano mine tailing (6.8 L), compost (2.2 L), sand (9 L), and gravel (9 L) in a 25:8:33:33% volume ratio (CP + CI).
- B (Blank control): commercial horticultural substrate mixed with sand and gravel in a 33:33:33% volume ratio.
2.3. Plants and Experimental Design
2.4. Analytical Methods
2.4.1. Substrate Characterization
2.4.2. Plant Sampling and Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Substrate Properties
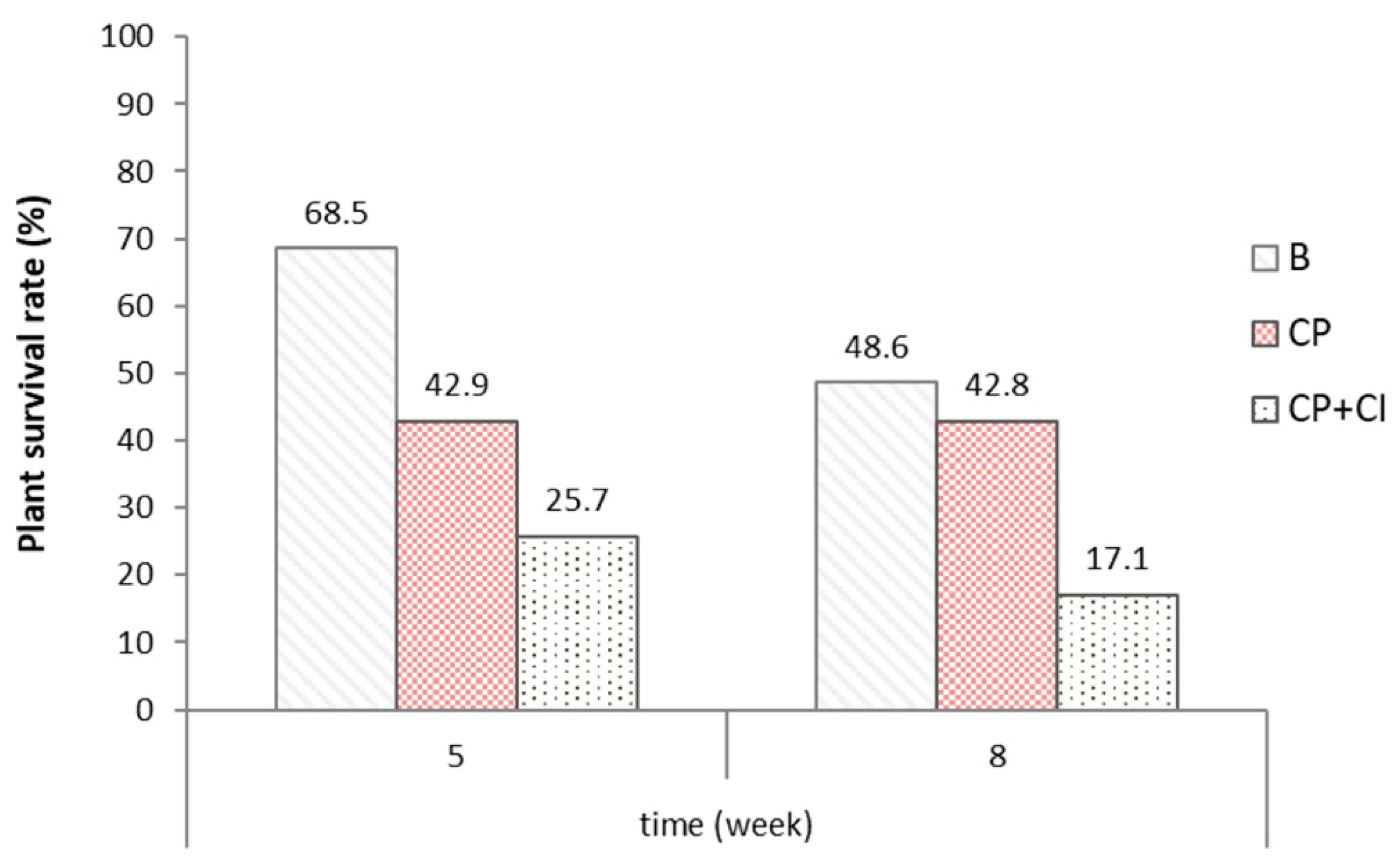
3.2. Plant Survival
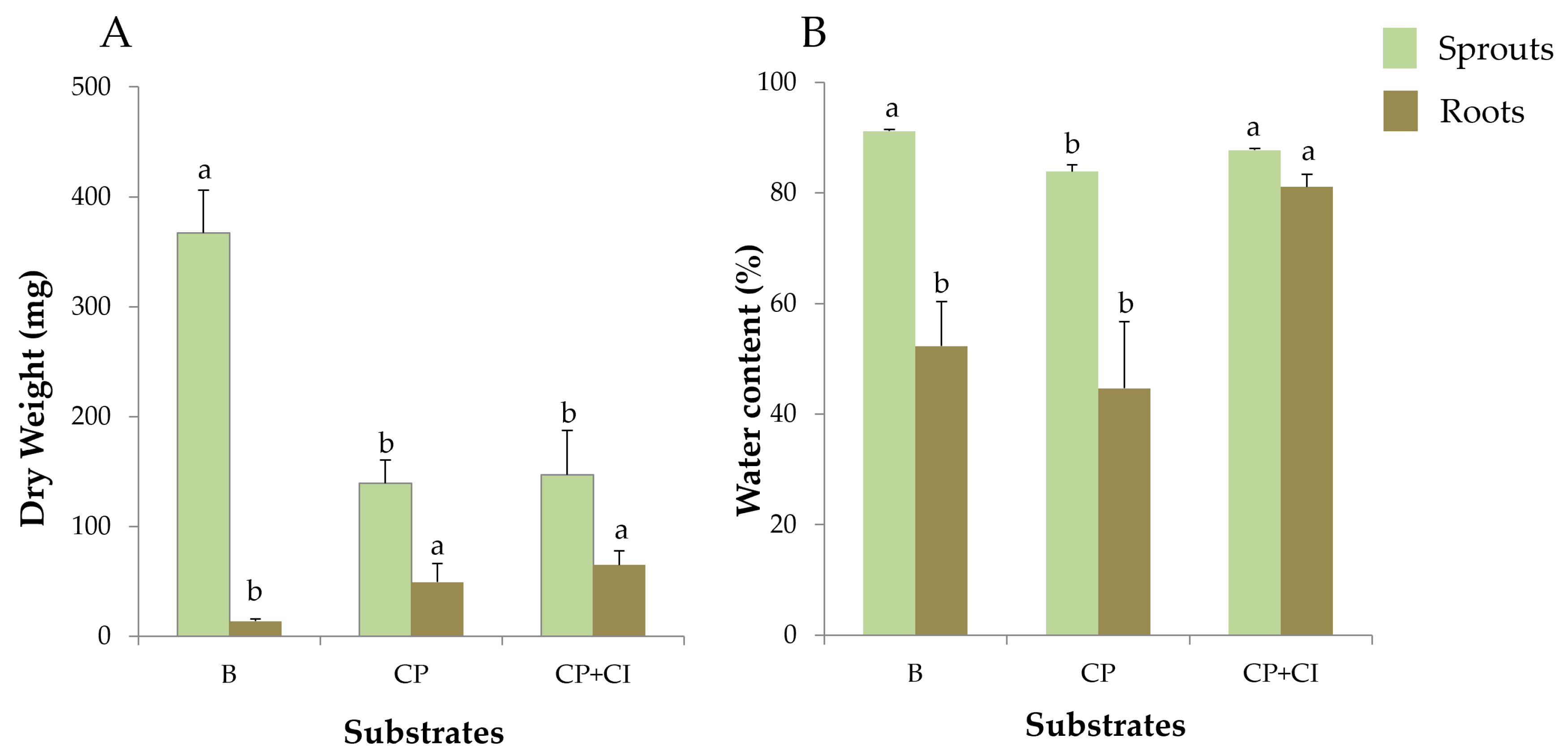
3.3. Biomass Growth
3.4. Chlorophyll and Carotenoids
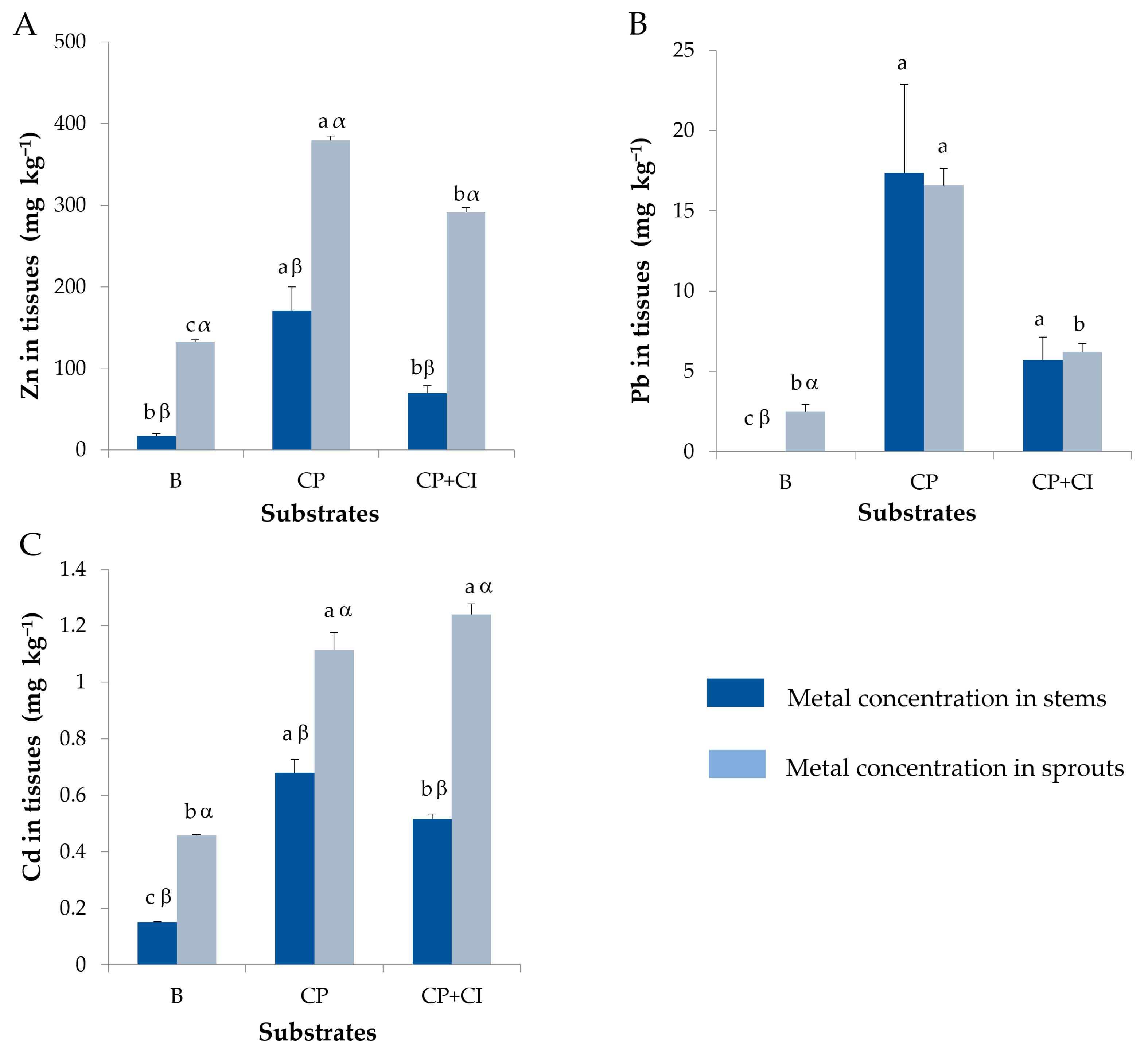
3.5. Plants Metals Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| Cc | Carotenes |
| CEC | Cation exchange capacity |
| Chla | Chlorophyll a |
| Chlb | Chlorophyll b |
| CRM | Critical raw materials |
| Cx | xanthophylls |
| DW | Dry weight |
| EDTA | Ethylene-diamine-tetra-acetic acid |
| FW | Fresh weight |
| PAST | Paleontological statistics |
| SE | Standard error |
| SIN | National Interest Site |
| SRM | Secondary raw materials |
References
- WHO. Human Health Effects of Benzene, Arsenic, Cadmium, Nickel, Lead and Mercury: Report of an Expert Consultation; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview Assessment of Risk Evaluation and Treatment Technologies for Heavy Metal Pollution of Water and Soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- Zaghdoudi, R.; Sghayar, S.; Necib, M.; Dekaezmaeker, P.; Dailly, H.; Zorrig, W.; Abdelly, C.; Debez, A.; Lutts, S. Screening for Heavy Metal-Resistant Clones in the Xero-Halophyte Atriplex halimus L.: A Prerequisite for Phytoremediation of Polymetallic Mining Pollution in Arid Areas. Int. J. Environ. Res. 2025, 19, 72. [Google Scholar] [CrossRef]
- Dang, P.; Li, C. A Mini-Review of Phytomining. Int. J. Environ. Sci. Technol. 2022, 19, 12825–12838. [Google Scholar] [CrossRef]
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Xu, Z.; Sun, Y. Adsorption-Pyrolysis Technology for Recovering Heavy Metals in Solution Using Contaminated Biomass Phytoremediation. Resour. Conserv. Recycl. 2018, 129, 20–26. [Google Scholar] [CrossRef]
- Liu, Z.; Tran, K.-Q. A Review on Disposal and Utilization of Phytoremediation Plants Containing Heavy Metals. Ecotoxicol. Environ. Saf. 2021, 226, 112821. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.A.; Mench, M.; Garbisu, C.; Kidd, P.; Castro, P.M.L. Phytomanagement of Metal(Loid)-Contaminated Soils: Options, Efficiency and Value. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Vázquez-Núñez, E.; Fernández-Luqueño, F.; Peña-Castro, J.M.; Vera-Reyes, I. Coupling Plant Biomass Derived from Phytoremediation of Potential Toxic-Metal-Polluted Soils to Bioenergy Production and High-Value by-Products—A Review. Appl. Sci. 2021, 11, 2982. [Google Scholar] [CrossRef]
- Ding, Z.; Alharbi, S.; Almaroai, Y.A.; Eissa, M.A. Improving Quality of Metal-Contaminated Soils by Some Halophyte and Non-Halophyte Forage Plants. Sci. Total Environ. 2021, 764, 142885. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Phytoextraction of Pb and Cd by the Mediterranean Saltbush (Atriplex halimus L.): Metal Uptake in Relation to Salinity. Environ. Sci. Pollut. Res. 2009, 16, 844–854. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Maggio, A. Functional Biology of Halophytes in the Phytoremediation of Heavy Metal Contaminated Soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Waris, M.; Baig, J.A.; Talpur, F.N.; Afridi, H.I.; Kazi, T.G.; Yousaf, H. Evaluation of Selected Halophytes for Phytoextraction of Co, Cu, Zn and Capability of Desalination of Saline Soil. Int. J. Environ. Sci. Technol. 2022, 19, 2737–2746. [Google Scholar] [CrossRef]
- Kachout, S.S.; Mansoura, A.B.; Mechergui, R.; Leclerc, J.C.; Rejeb, M.N.; Ouerghi, Z. Accumulation of Cu, Pb, Ni and Zn in the Halophyte Plant Atriplex Grown on Polluted Soil. J. Sci. Food Agric. 2012, 92, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.; Rutter, A.; Zeeb, B. Evaluating the Efficacy of Atriplex Spp. in the Phytoextraction of Road Salt (NaCl) from Contaminated Soil. Environ. Pollut. 2020, 265, 114963. [Google Scholar] [CrossRef]
- Orrego, F.; Ortíz-Calderón, C.; Lutts, S.; Ginocchio, R. Effect of Single and Combined Cu, NaCl and Water Stresses on Three Atriplex Species with Phytostabilization Potential. South Afr. J. Bot. 2020, 131, 161–168. [Google Scholar] [CrossRef]
- Tapia, Y.; Diaz, O.; Pizarro, C.; Segura, R.; Vines, M.; Zúñiga, G.; Moreno-Jiménez, E. Atriplex Atacamensis and Atriplex halimus Resist as Contamination in Pre-Andean Soils (Northern Chile). Sci. Total Environ. 2013, 450–451, 188–196. [Google Scholar] [CrossRef]
- Ellili, A.; Rabier, J.; Prudent, P.; Salducci, M.-D.; Heckenroth, A.; Lachaâl, M.; Laffont-Schwob, I. Decision-Making Criteria for Plant-Species Selection for Phytostabilization: Issues of Biodiversity and Functionality. J. Environ. Manag. 2017, 201, 215–226. [Google Scholar] [CrossRef]
- Walker, D.J.; Lutts, S.; Sánchez-García, M.; Correal, E. Atriplex halimus L.: Its Biology and Uses. J. Arid Environ. 2014, 100–101, 111–121. [Google Scholar] [CrossRef]
- Babi, K.; Guittonny, M.; Bussière, B.; Larocque, G.R. Effect of Soil Quality and Planting Material on the Root Architecture and the Root Anchorage of Young Hybrid Poplar Plantations on Waste Rock Slopes. Int. J. Min. Reclam. Environ. 2023, 37, 1–20. [Google Scholar] [CrossRef]
- Labrecque, M.; Hu, Y.; Vincent, G.; Shang, K. The Use of Willow Microcuttings for Phytoremediation in a Copper, Zinc and Lead Contaminated Field Trial in Shanghai, China. Int. J. Phytoremediat. 2020, 22, 1331–1337. [Google Scholar] [CrossRef]
- Ruttens, A.; Boulet, J.; Weyens, N.; Smeets, K.; Adriaensen, K.; Meers, E.; Van Slycken, S.; Tack, F.; Meiresonne, L.; Thewys, T.; et al. Short Rotation Coppice Culture of Willows and Poplars as Energy Crops on Metal Contaminated Agricultural Soils. Int. J. Phytoremediat. 2011, 13, 194–207. [Google Scholar] [CrossRef]
- Saulino, L.; Allevato, E.; Rossi, S.; Minotta, G.; Fiorentino, N.; Saracino, A. Lengthening Single-Stem Rotation Improves Biomass Yield and Water Use Efficiency in Black Poplar Genotype Multi-Stem Rotation Coppice Plantations. Biomass Bioenergy 2021, 154, 106256. [Google Scholar] [CrossRef]
- Andrades-Moreno, L.; Cambrollé, J.; Figueroa, M.E.; Mateos-Naranjo, E. Growth and Survival of Halimione Portulacoides Stem Cuttings in Heavy Metal Contaminated Soils. Mar. Pollut. Bull. 2013, 75, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Pérez-Clemente, R.; Caçador, I.; Sleimi, N. Accumulation Potential of Atriplex halimus to Zinc and Lead Combined with NaCl: Effects on Physiological Parameters and Antioxidant Enzymes Activities. South Afr. J. Bot. 2019, 123, 51–61. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Pardo, T.; Martínez-Fernández, D.; Bernal, M.P. The Use of a Halophytic Plant Species and Organic Amendments for the Remediation of a Trace Elements-Contaminated Soil under Semi-Arid Conditions. J. Hazard. Mater. 2012, 223–224, 63–71. [Google Scholar] [CrossRef]
- Frutos, I.; García-Delgado, C.; Cala, V.; Gárate, A.; Eymar, E. The Use of Spent Mushroom Compost to Enhance the Ability of Atriplex halimus to Phytoremediate Contaminated Mine Soils. Environ. Technol. 2017, 38, 1075–1084. [Google Scholar] [CrossRef]
- Pérez-Esteban, J.; Escolástico, C.; Ruiz-Fernández, J.; Masaguer, A.; Moliner, A. Bioavailability and Extraction of Heavy Metals from Contaminated Soil by Atriplex halimus. Environ. Exp. Bot. 2013, 88, 53–59. [Google Scholar] [CrossRef]
- Kidd, P.; Mench, M.; Álvarez-López, V.; Bert, V.; Dimitriou, I.; Friesl-Hanl, W.; Herzig, R.; Olga Janssen, J.; Kolbas, A.; Müller, I.; et al. Agronomic Practices for Improving Gentle Remediation of Trace Element-Contaminated Soils. Int. J. Phytoremediation 2015, 17, 1005–1037. [Google Scholar] [CrossRef]
- Acosta, J.A.; Abbaspour, A.; Martínez, G.R.; Martínez-Martínez, S.; Zornoza, R.; Gabarrón, M.; Faz, A. Phytoremediation of Mine Tailings with Atriplex halimus and Organic/Inorganic Amendments: A Five-Year Field Case Study. Chemosphere 2018, 204, 71–78. [Google Scholar] [CrossRef]
- Cambrollé, J.; Mancilla-Leytón, J.M.; Muñoz-Vallés, S.; Cambrón-Sena, A.; Figueroa, M.E. Advances in the Use of Halimione portulacoides Stem Cuttings for Phytoremediation of Zn-Polluted Soils. Estuar. Coast. Shelf Sci. 2016, 175, 10–14. [Google Scholar] [CrossRef]
- Mancilla-Leytón, J.M.; Navarro-Ramos, M.J.; Muñoz-Vallés, S.; Figueroa, M.E.; Cambrollé, J. Evaluation of the Potential of Atriplex halimus Stem Cuttings for Phytoremediation of Metal-Polluted Soils. Ecol. Eng. 2016, 97, 553–557. [Google Scholar] [CrossRef]
- Ministerial Decree Perimetrazione Del Sito Di Interesse Nazionale Del Sulcis-Iglesiente-Guspinese. Decreto 12 Marzo 2003. GU Serie Generale n.121 Del 27-05-2003-Suppl. Ordinario n. 83. 2003. Available online: https://www.gazzettaufficiale.it/eli/gu/2003/05/27/121/so/83/sg/pdf (accessed on 10 June 2025).
- Canu, S.; Rosati, L.; Fiori, M.; Motroni, A.; Filigheddu, R.; Farris, E. Bioclimate Map of Sardinia (Italy). J. Maps 2015, 11, 711–718. [Google Scholar] [CrossRef]
- Ministerial Decree Approvazione Dei “Metodi Ufficiali Di Analisi Chimica Del Suolo” (GU Serie Generale n.248 Del 21-10-1999—Suppl. Ordinario n. 185). 1999. Available online: https://www.gazzettaufficiale.it/eli/gu/1999/10/21/248/so/185/sg/pdf (accessed on 10 June 2025).
- Barbafieri, M.; Lubrano, L.; Petruzzelli, G. Characterization of pollution in sites contaminates by heavy metals: A proposal. Ann. Chim. 1996, 86, 585–594. [Google Scholar]
- Caplan, D.; Stemeroff, J.; Dixon, M.; Zheng, Y. Vegetative Propagation of Cannabis by Stem Cuttings: Effects of Leaf Number, Cutting Position, Rooting Hormone, and Leaf Tip Removal. Can. J. Plant Sci. 2018, 98, 1126–1132. [Google Scholar] [CrossRef]
- Gilliam, C.H.; Eakes, D.J.; Olive, J.W. Herbicide Use During Propagation Affects Root Initiation and Development. J. Environ. Hortic. 1993, 11, 157–159. [Google Scholar] [CrossRef]
- Hou, P.-C.; Lin, K.-H.; Huang, Y.-J.; Wu, C.-W.; Chang, Y.-S. Applications of Vegetation Indices and Biostimulators to the Rooting of Camellia Cuttings. J. Appl. Hortic. 2019, 21, 111–115. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 10 June 2025).
- Bacchetta, G.; Cappai, G.; Carucci, A.; Tamburini, E. Use of Native Plants for the Remediation of Abandoned Mine Sites in Mediterranean Semiarid Environments. Bull. Environ. Contam. Toxicol. 2015, 94, 326–333. [Google Scholar] [CrossRef]
- Bacchetta, G.; Boi, M.E.; Cappai, G.; De Giudici, G.; Piredda, M.; Porceddu, M. Metal Tolerance Capability of Helichrysum microphyllum Cambess. Subsp. Tyrrhenicum Bacch., Brullo & Giusso: A Candidate for Phytostabilization in Abandoned Mine Sites. Bull. Environ. Contam. Toxicol. 2018, 101, 758–765. [Google Scholar] [CrossRef]
- Bacchetta, G.; Cao, A.; Cappai, G.; Carucci, A.; Casti, M.; Fercia, M.L.; Lonis, R.; Mola, F. A Field Experiment on the Use of Pistacia lentiscus L. and Scrophularia canina L. Subsp. Bicolor (Sibth. et Sm.) Greuter for the Phytoremediation of Abandoned Mining Areas. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2012, 146, 1054–1063. [Google Scholar] [CrossRef]
- Kharazian, P.; Bacchetta, G.; Cappai, G.; Piredda, M.; De Giudici, G. An Integrated Geochemical and Mineralogical Investigation on Soil-Plant System of Pinus Halepensis Pioneer Tree Growing on Heavy Metal Polluted Mine Tailing. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2022, 157, 272–285. [Google Scholar] [CrossRef]
- Decree Law Norme in Materia Ambientale. GU n.88 Del 14-04-2006-Suppl. Ordinario n. 96. 2006. Available online: https://www.gazzettaufficiale.it/dettaglio/codici/materiaAmbientale (accessed on 10 June 2025).
- Dessena, L.; Mulas, M. Cultivar Selection from Spontaneous Population of Atriplex halimus L. In Proceedings of the ISHS Acta Horticulturae 937, Lisbon, Portugal, 30 September 2012; Volume 2, pp. 205–210. [Google Scholar]
- Francis, B.; Aravindakumar, C.T.; Brewer, P.B.; Simon, S. Plant Nutrient Stress Adaptation: A Prospect for Fertilizer Limited Agriculture. Environ. Exp. Bot. 2023, 213, 105431. [Google Scholar] [CrossRef]
- Shahzad, Z.; Amtmann, A. Food for Thought: How Nutrients Regulate Root System Architecture. Curr. Opin. Plant Biol. 2017, 39, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Andrades-Moreno, L.; Redondo-Gómez, S. Comparison of Germination, Growth, Photosynthetic Responses and Metal Uptake between Three Populations of Spartina densiflora under Different Soil Pollution Conditions. Ecotoxicol. Environ. Saf. 2011, 74, 2040–2049. [Google Scholar] [CrossRef]
- Dos Santos Utmazian, M.N.; Wieshammer, G.; Vega, R.; Wenzel, W.W. Hydroponic Screening for Metal Resistance and Accumulation of Cadmium and Zinc in Twenty Clones of Willows and Poplars. Environ. Pollut. 2007, 148, 155–165. [Google Scholar] [CrossRef]
- Weih, M.; Nordh, N.-E. Characterising Willows for Biomass and Phytoremediation: Growth, Nitrogen and Water Use of 14 Willow Clones under Different Irrigation and Fertilisation Regimes. Biomass Bioenergy 2002, 23, 397–413. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.; Walker, D.J. The Effects of Soil Amendments on the Growth of Atriplex halimus and Bituminaria bituminosa in Heavy Metal-Contaminated Soils. Water Air Soil Pollut. 2012, 223, 63–72. [Google Scholar] [CrossRef]
- Reddy, N.; Crohn, D.M. Compost Induced Soil Salinity: A New Prediction Method and Its Effect on Plant Growth. Compos. Sci. Util. 2012, 20, 133–140. [Google Scholar] [CrossRef]
- Belkheiri, O.; Mulas, M. The Effects of Salt Stress on Growth, Water Relations and Ion Accumulation in Two Halophyte Atriplex Species. Environ. Exp. Bot. 2013, 86, 17–28. [Google Scholar] [CrossRef]
- Bendaly, A.; Messedi, D.; Smaoui, A.; Ksouri, R.; Bouchereau, A.; Abdelly, C. Physiological and Leaf Metabolome Changes in the Xerohalophyte Species Atriplex halimus Induced by Salinity. Plant Physiol. Biochem. 2016, 103, 208–218. [Google Scholar] [CrossRef]
- Nedjimi, B. Seasonal Variation in Productivity, Water Relations and Ion Contents of Atriplex halimus Spp. Schweinfurthii Grown in Chott Zehrez Wetland, Algeria. J. Saudi Soc. Agric. Sci. 2012, 11, 43–49. [Google Scholar] [CrossRef]
- Miazek, K.; Ledakowicz, S. Chlorophyll Extraction from Leaves, Needles and Microalgae: A Kinetic Approach. Int. J. Agric. Biol. Eng. 2013, 6, 107–115. [Google Scholar]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An Evaluation of Noninvasive Methods to Estimate Foliar Chlorophyll Content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and Near-Infrared Reflectance Techniques for Diagnosing Plant Physiological Status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Küpper, H.; Küpper, F.; Spiller, M. Environmental Relevance of Heavy Metal-Substituted Chlorophylls Using the Example of Water Plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar] [CrossRef]
- Sghaier, D.B.; Duarte, B.; Bankaji, I.; Caçador, I.; Sleimi, N. Growth, Chlorophyll Fluorescence and Mineral Nutrition in the Halophyte Tamarix Gallica Cultivated in Combined Stress Conditions: Arsenic and NaCl. J. Photochem. Photobiol. B Biol. 2015, 149, 204–214. [Google Scholar] [CrossRef]
- Badache, H.; Sbartai, H.; Djebar, M.; Manousaki, E.; Bourguignon, J. Phytoextraction of Cd by Atriplex nummularia L., a Xero-Halophyte Species. Int. J. Biosci. (IJB) 2015, 6, 130–139. [Google Scholar]
- Nedjimi, B.; Daoud, Y. Cadmium Accumulation in Atriplex halimus Subsp. Schweinfurthii and Its Influence on Growth, Proline, Root Hydraulic Conductivity and Nutrient Uptake. Flora-Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 316–324. [Google Scholar] [CrossRef]
- Lotmani, B.; Mesnoua, M. Effects of Copper Stress on Antioxidative Enzymes, Chlorophyll and Protein Content in Atriplex halimus. Afr. J. Biotechnol. 2011, 10, 10143–10148. [Google Scholar] [CrossRef]
- Acuña, E.; Castillo, B.; Queupuan, M.; Casanova, M.; Tapia, Y. Assisted Phytoremediation of Lead Contaminated Soil Using Atriplex halimus and Its Effect on Some Soil Physical Properties. Int. J. Environ. Sci. Technol. 2021, 18, 1925–1938. [Google Scholar] [CrossRef]
- Dessena, L.; Mulas, M. Mineral Composition of Atriplex halimus Plant as Influenced by Genotype and Thermal Regime. Int. J. Phytoremediat. 2021, 23, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Shahrokh, V.; Perez, V.; Zornoza, R.; Acosta, J.A.; Faz, A.; Martinez-Martinez, S. Soil Sodium, Magnesium and Potassium Contents Contribute to Metals Uptake and Accumulation in Leaves of Atriplex halimus in Tailings Ponds. J. Environ. Chem. Eng. 2022, 10, 107948. [Google Scholar] [CrossRef]
| Tissue | Scores | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Roots | No visible roots, no callus. | Presence of root callus | Roots visible but mainly less elongated. Poor branching of rooting system. | Roots in elevated number and good health status. Medium branching of root system. | Roots in high number, long and healthy. Well-developed, branched root system. |
| Sprouts | No visible sprouts, no buds. | Start of bud sprouting. | All buds open and sprouts developed, but less elongated and poorly branched. | Sprouts in elevated number and good health status. Sprouts medium branched. | Sprouts in high number, long and healthy. Sprouts well branched. |
| Substrates | pH | CEC | C | N | P (Bioavailable) |
|---|---|---|---|---|---|
| [meq 100 g Substrate−1] | [%] | [%] | [mg kg−1] | ||
| B | 5.5 ± 0.1 | 23.2 ± 0.5 | 3.9 ± 0.5 | 0.07 ± 0.01 | 29.0 ± 1.08 |
| CP | 7.3 ± 0.1 | nd * | 6.5 ± 0.1 | nd | nd |
| CP + CI | 7.5 ± 0.1 | 11.0 ± 0.7 | 7.1 ± 0.3 | 0.08 ± 0.01 | 13.6 ± 0.8 |
| Substrates | Aqua Regia–Extractable | EDTA–Extractable | KNO3–Extractable | H2O–Extractable | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | |||||||||
| Cd | Pb | Zn | Cd | Pb | Zn | Cd | Pb | Zn | Cd | Pb | Zn | |
| B | nd * | 6.36 ± 2.24 | 36.5 ± 1.3 | nd | nd | 8.4 ± 0.8 | nd | nd | 8.8 ± 3.4 | nd | nd | nd |
| CP | 70.1 ± 2.7 | 3041 ± 44.9 | 12,779 ± 33.4 | 33.2 ± 2.0 | 1066 ± 56.1 | 2407 ± 59.1 | nd | nd | 7.1 ± 0.8 | nd | nd | 6.6 ± 1.4 |
| CP + CI | 31.6 ± 2.5 | 1236 ± 102 | 5268 ± 513 | 13.5 ± 6.1 | 361 ± 88.6 | 1003 ± 497 | nd | nd | 4.6 ± 0 | nd | nd | 5 ± 0.3 |
| Substrates | Cu | Mg | Mn | Cu | Mg | Mn | Cu | Mg | Mn | Cu | Mg | Mn |
| B | 11.8 ± 0.8 | 1460 ± 83.1 | 280 ± 10.4 | 3.33 ± 0.2 | 330 ± 14.9 | 106 ± 18.8 | nd | 515 ± 10.5 | 26.3 ± 1.8 | nd | 31.1 ± 1.04 | 1.47 ± 0.07 |
| CP | 55.4 ± 5.3 | 60,194 ± 729 | 1316 ± 29.6 | 14.5 ± 0.8 | 1970 ± 58.7 | 115 ± 6.8 | nd | 149 ± 6.9 | 0.35 ± 0.02 | nd | 581 ± 31 | 0.5 ± 0.06 |
| CP + CI | 34.6 ± 1.9 | 31,795 ± 2732 | 761 ± 46.1 | 6.43 ± 1.6 | 1799 ± 120 | 133 ± 20.6 | nd | 228 ± 3.8 | 1.15 ± 0.7 | 1.08 ± 0.1 | 713 ± 131 | 2.03 ± 0.4 |
| Roots | Sprouts | |||||
|---|---|---|---|---|---|---|
| B | CP | CP + CI | B | CP | CP + CI | |
| Score | 2.8 ± 0.2 (a) | 1.6 ± 0.2 (b) | 2.8 ± 0.2 (a) | 4.0 ± 0 (a) | 1.8 ± 0.4 (b) | 2.4 ± 0.2 (b) |
| Substrates | Ca | Cb | Cx+c | Ca+Cb | Ca/Cb | (Cx+c)/(Ca+b) |
|---|---|---|---|---|---|---|
| (mg g−1) | (mg g−1) | (mg g−1) | (mg g−1) | |||
| B | 1.11 ± 0.03 b | 0.4 ± 0.02 b | 0.37 ± 0.01 b | 1.52 ± 0.05 b | 2.81 ± 0.1 a | 0.24 ± 0.01 c |
| CP | 1.46 ± 0.05 a | 0.52 ± 0.02 a | 0.5 ± 0.02 a | 1.99 ± 0.07 a | 2.81.0 ± 2 a | 0.25 ± 0 c |
| CP + CI | 0.87 ± 0.04 c | 0.32 ± 0.02 b | 0.38 ± 0.01 b | 1.19 ± 0.06 c | 2.75 ± 0.04 a | 0.32 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canu, M.; Milia, S.; Ubaldini, S.; Tamburini, E.; Carucci, A.; Cappai, G. Propagation of Atriplex halimus (Mediterranean Saltbush) in Multi-Contaminated Mine Tailings by Unrooted Cuttings. Appl. Sci. 2025, 15, 7027. https://doi.org/10.3390/app15137027
Canu M, Milia S, Ubaldini S, Tamburini E, Carucci A, Cappai G. Propagation of Atriplex halimus (Mediterranean Saltbush) in Multi-Contaminated Mine Tailings by Unrooted Cuttings. Applied Sciences. 2025; 15(13):7027. https://doi.org/10.3390/app15137027
Chicago/Turabian StyleCanu, Marta, Stefano Milia, Stefano Ubaldini, Elena Tamburini, Alessandra Carucci, and Giovanna Cappai. 2025. "Propagation of Atriplex halimus (Mediterranean Saltbush) in Multi-Contaminated Mine Tailings by Unrooted Cuttings" Applied Sciences 15, no. 13: 7027. https://doi.org/10.3390/app15137027
APA StyleCanu, M., Milia, S., Ubaldini, S., Tamburini, E., Carucci, A., & Cappai, G. (2025). Propagation of Atriplex halimus (Mediterranean Saltbush) in Multi-Contaminated Mine Tailings by Unrooted Cuttings. Applied Sciences, 15(13), 7027. https://doi.org/10.3390/app15137027








