Valorization of Spent Osmotic Solutions by Production of Powders by Spray Drying
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Plan
2.2. Spray Drying
2.3. Analytical Methods
2.3.1. Particle Morphology—SEM Analysis
2.3.2. Particle Size Measurement
2.3.3. Moisture Content and Water Activity
2.3.4. Hygroscopicity
2.3.5. Solubility
2.3.6. Bulk Density and Flowability
2.4. Statistical Methods
2.4.1. Analysis of Variance (ANOVA)
2.4.2. Chemometric Analysis
3. Results
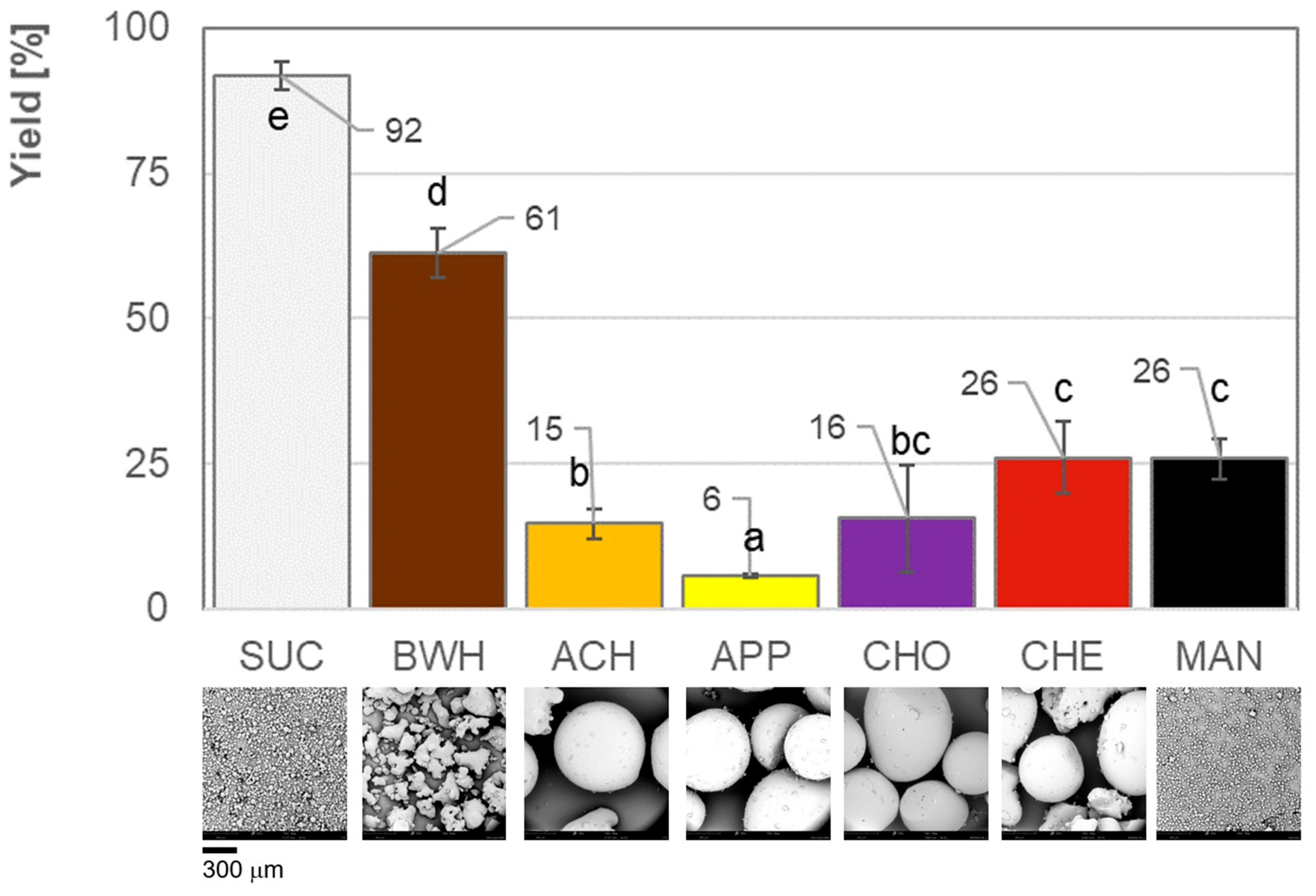
3.1. Yield
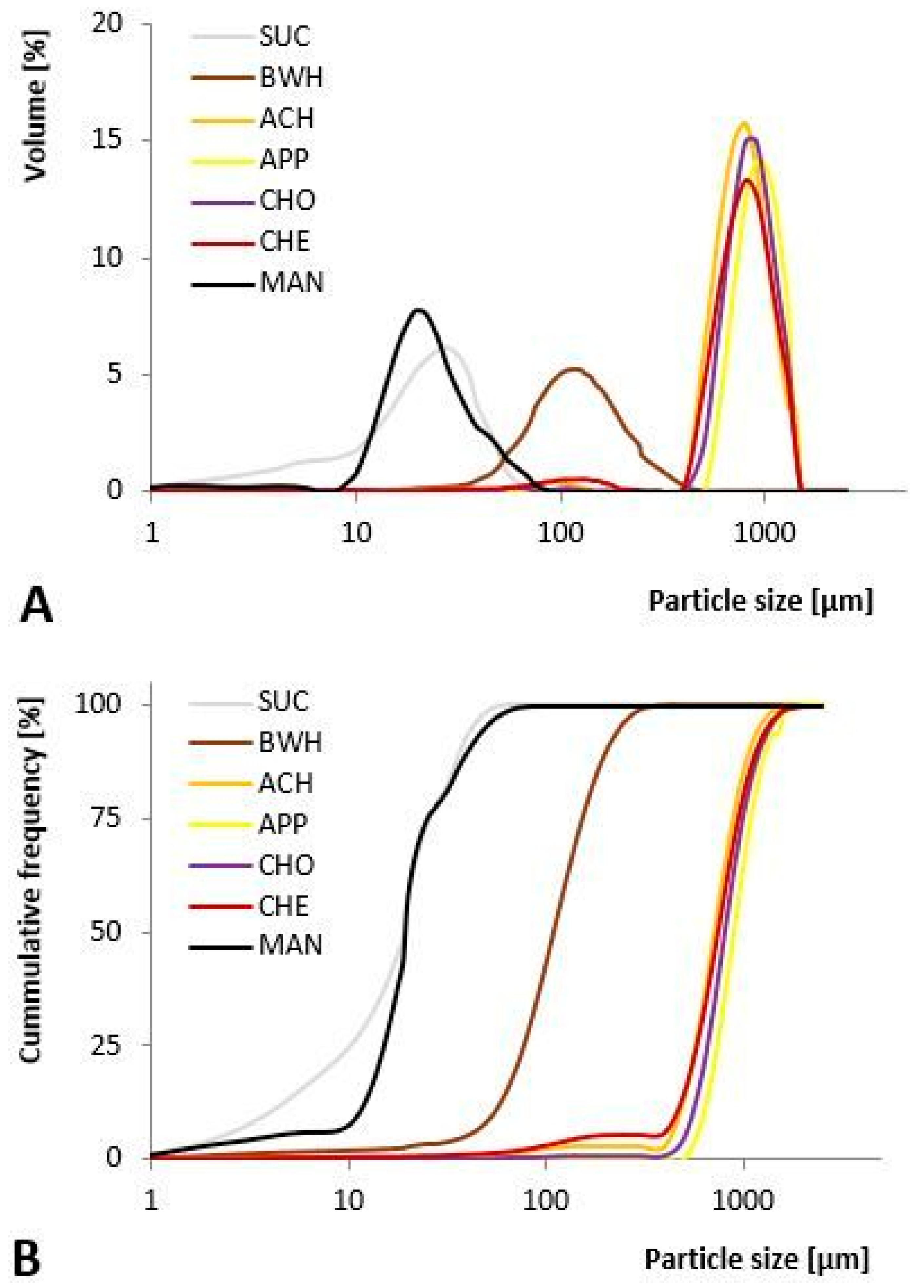
3.2. Particle Morphology and Particle Size
3.3. Moisture Content and Water Activity
3.4. Hygroscopicity
3.5. Solubility
3.6. Bulk Density and Flowability
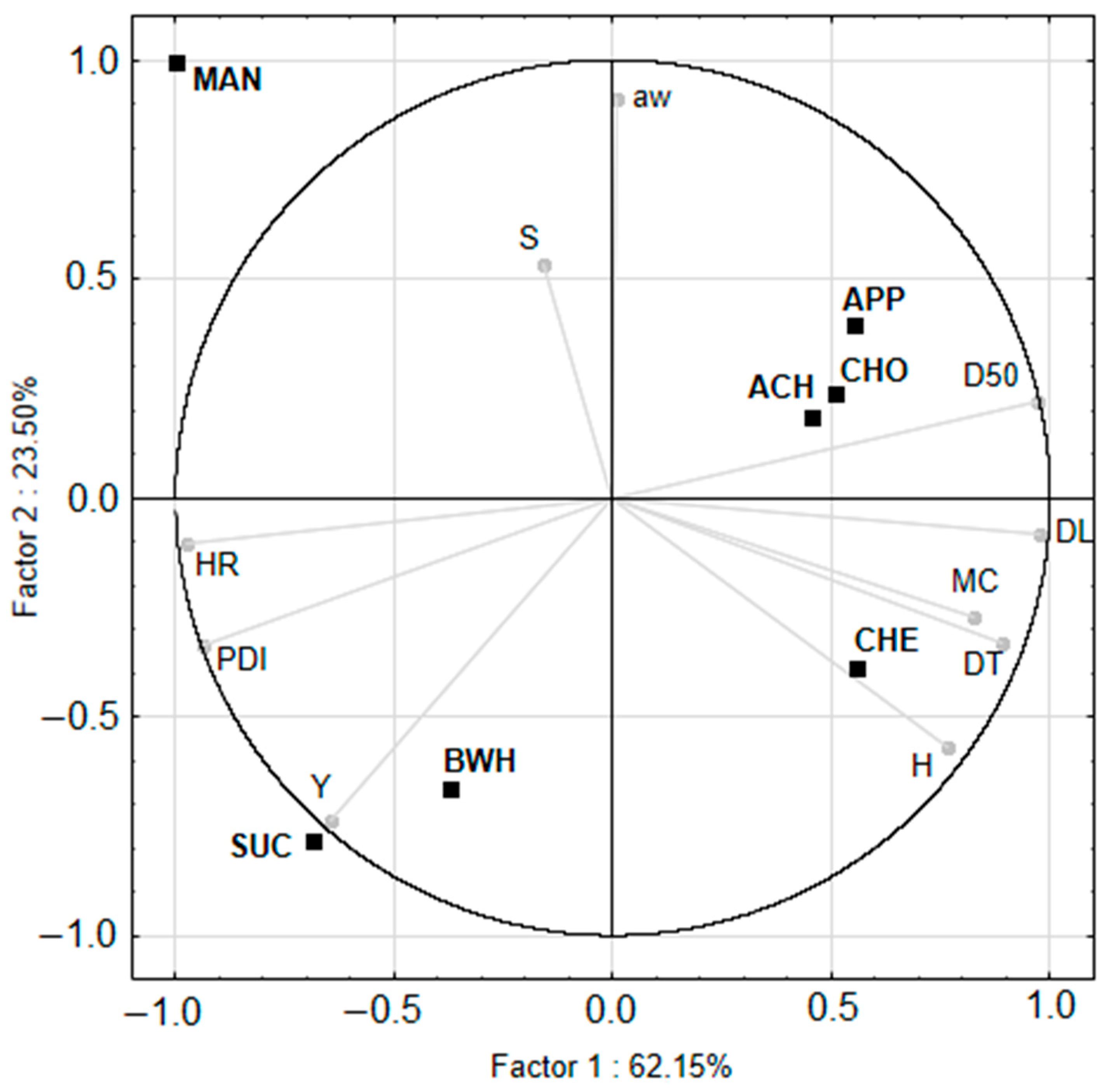
3.7. Hierarchical Cluster Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACH | Acacia honey |
| ANOVA | One-way analysis of variance |
| APP | Apple |
| BWH | Buckwheat honey |
| CHE | Cherry |
| CHO | Chokeberry |
| D10 | 10th percentile of the particle size distribution |
| D50 | Median diameter |
| D90 | 90th percentile of the particle size distribution |
| DL | Loose bulk density |
| DT | Tapped bulk density |
| H | Hygroscopicity |
| HCA | Hierarchical Cluster Analysis |
| HR | Hausner Ratio |
| IAT | Inlet air temperature |
| MAN | Mannitol |
| MC | Moisture content |
| MD | Maltodextrin |
| OAT | Outlet air temperature |
| OD | Osmotic dehydration |
| OS | Osmotic solution |
| PCA | Principal Component Analysis |
| PDI | Polydispersity index |
| SD | Spray drying |
| SUC | Sucrose |
| Tg | Glass transition temperature |
| WSI | Water solubility index |
| Y | Yield |
References
- Phisut, N. Factors affecting mass transfer during osmotic dehydration of fruits. Int. Food Res. J. 2012, 19, 7–18. [Google Scholar]
- Salehi, F. Recent advances in the ultrasound-assisted osmotic dehydration of agricultural products: A review. Food Biosci. 2023, 51, 102307. [Google Scholar] [CrossRef]
- Manzoor, A.; Jan, B.; Rizvi, Q.U.E.H.; Junaid, P.M.; Pandith, J.A.; Dar, I.H.; Bhat, S.A.; Ahmad, S. Osmotic dehydration technology for preservation of fruits and vegetables. In Quality Control in Fruit and Vegetable Processing; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 167–184. [Google Scholar]
- Wiktor, A.; Chadzynska, M.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries. Molecules 2022, 27, 1376. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Singh, S.V. Osmotic dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2014, 51, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, F.R.; Correa, J.L.G. Osmotic dehydration: More than water loss and solid gain. Crit. Rev. Food Sci. Nutr. 2023, 63, 2970–2989. [Google Scholar] [CrossRef]
- Kaur, D.; Singh, M.; Zalpouri, R.; Singh, I. Osmotic dehydration of fruits using unconventional natural sweeteners and non-thermal-assisted technologies: A review. J. Food Process. Preserv. 2022, 46, e16890. [Google Scholar] [CrossRef]
- Bchir, B.; Besbes, S.; Karoui, R.; Paquot, M.; Attia, H.; Blecker, C. Osmotic Dehydration Kinetics of Pomegranate Seeds Using Date Juice as an Immersion Solution Base. Food Bioprocess Technol. 2010, 5, 999–1009. [Google Scholar] [CrossRef]
- Kowalska, H.; Trusinska, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Shaping the Properties of Osmo-Dehydrated Strawberries in Fruit Juice Concentrates. Appl. Sci. 2023, 13, 2728. [Google Scholar] [CrossRef]
- Durrani, A.M.; Srivastava, P.K.; Verma, S. Development and quality evaluation of honey based carrot candy. J. Food Sci. Technol. 2011, 48, 502–505. [Google Scholar] [CrossRef]
- Dhungana, A.; Kharel, G.P.; Ojha, P. Effect of osmotic agents on dehydration of yacon (Smallanthus sonchifolia) slices. GoldenGate J. Sci. Technol. 2017, 3, 46–51. [Google Scholar]
- Aguirre-García, M.; Cortés-Zavaleta, O.; Ruiz-Espinosa, H.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. The role of coupled water and solute diffusion and product shrinkage during osmotic dehydration. J. Food Eng. 2022, 331, 111121. [Google Scholar] [CrossRef]
- Li, H.; Ramaswamy, H.S. Osmotic dehydration. Stewart Postharvest Rev. 2005, 1, 1–9. [Google Scholar]
- Dalla Rosa, M.; Giroux, F. Osmotic treatments (OT) and problems related to the solution management. J. Food Eng. 2001, 49, 223–236. [Google Scholar] [CrossRef]
- Garcıa-Martınez, E.; Martínez-Monzó, J.; Camacho, M.M.; Martınez-Navarrete, N. Characterisation of reused osmotic solution as ingredient in new product formulation. Food Res. Int. 2002, 35, 307–313. [Google Scholar] [CrossRef]
- Zimmer, A.; Masztalerz, K.; Serowik, M.; Nejman, M.; Lech, K. Utilization of Post-Process Osmotic Solution Based on Tomato Juice Through Spray Drying. Agriculture 2024, 14, 1883. [Google Scholar] [CrossRef]
- Samborska, K.; Barańska, A.; Boostani, S.; Riazi, M.; Jafari, S.M. Introduction to the spray drying process. In Spray Drying for the Food Industry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–28. [Google Scholar]
- Samborska, K.; Sarabandi, K.; Tonon, R.; Topuz, A.; Eroğlu, E.; Kaymak-Ertekin, F.; Malekjani, N.; Jafari, S.M. Recent progress in the stickiness reduction of sugar-rich foods during spray drying. Dry. Technol. 2023, 41, 2566–2585. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Majerska, J.; Brzezowska, J.; Wojdyło, A.; Figiel, A. The Influence of Maltodextrin and Inulin on the Physico-Chemical Properties of Cranberry Juice Powders. ChemEngineering 2020, 4, 12. [Google Scholar] [CrossRef]
- Meyer, D.; Blaauwhoed, J.P. Inulin. In Handbook of Hydrocolloids; Woodhead Publishing: Cambridge, UK, 2009; pp. 829–848. [Google Scholar]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The physiological functions and pharmaceutical applications of inulin: A review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef]
- Vickovic, D.; Czaja, T.P.; Gaiani, C.; Pedersen, S.J.; Ahrné, L.; Hougaard, A.B. The effect of feed formulation on surface composition of powders and wall deposition during spray drying of acidified dairy products. Powder Technol. 2023, 418, 118297. [Google Scholar] [CrossRef]
- Kurek, M.A.; Moczkowska, M.; Pieczykolan, E.; Sobieralska, M. Barley β-d-glucan–modified starch complex as potential encapsulation agent for fish oil. Int. J. Biol. Macromol. 2018, 120, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhang, M.; Mujumdar, A.S.; Sun, J. Spray Drying and Agglomeration of Instant Bayberry Powder. Dry. Technol. 2007, 26, 116–121. [Google Scholar] [CrossRef]
- Pombo, J.C.P.; de Medeiros, H.; Pena, R.D.S. Optimization of the spray drying process for developing cupuassu powder. J. Food Sci. Technol. 2020, 57, 4501–4513. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Ghalegi Ghalenoei, M.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Saw, H.Y.; Davies, C.E.; Paterson, A.H.J.; Jones, J.R. Correlation between Powder Flow Properties Measured by Shear Testing and Hausner Ratio. Procedia Eng. 2015, 102, 218–225. [Google Scholar] [CrossRef]
- Combrzynski, M.; Oniszczuk, T.; Kupryaniuk, K.; Wojtowicz, A.; Mitrus, M.; Milanowski, M.; Soja, J.; Budziak-Wieczorek, I.; Karcz, D.; Kaminski, D.; et al. Physical Properties, Spectroscopic, Microscopic, X-ray, and Chemometric Analysis of Starch Films Enriched with Selected Functional Additives. Materials 2021, 14, 2673. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Oniszczuk, T.; Matwijczuk, A.; Chruściel, E.; Kocira, A.; Niemczynowicz, A.; Wójtowicz, A.; Combrzyński, M.; Wiącek, D. Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil. Sustainability 2019, 11, 6414. [Google Scholar] [CrossRef]
- Bhandari, B.; Datta, N.; Howes, T. Problems associated with spray drying of sugar-rich foods. Dry. Technol. 1997, 15, 671–684. [Google Scholar] [CrossRef]
- Mirlohi, M.; Manickavasagan, A.; Ali, A. The effect of protein drying aids on the quantity and quality of spray dried sugar-rich powders: A systematic review. Dry. Technol. 2022, 40, 1068–1082. [Google Scholar] [CrossRef]
- Adhikari, B.; Howes, T.; Bhandari, B.R.; Truong, V. Characterization of the Surface Stickiness of Fructose–Maltodextrin Solutions During Drying. Dry. Technol. 2003, 21, 17–34. [Google Scholar] [CrossRef]
- Kudra, T. Sticky Region in Drying—Definition and Identification. Dry. Technol. 2003, 21, 1457–1469. [Google Scholar] [CrossRef]
- Araujo-Díaz, S.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Wiktor, A.; Jedlińska, A.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Tułodziecki, M.; Błażowski, Ł.; Witrowa-Rajchert, D. Development and characterization of physical properties of honey-rich powder. Food Bioprod. Process. 2019, 115, 78–86. [Google Scholar] [CrossRef]
- Miravet, G.; Alacid, M.; Obón, J.M.; Fernández-López, J.A. Spray-drying of pomegranate juice with prebiotic dietary fibre. Int. J. Food Sci. Technol. 2016, 51, 633–640. [Google Scholar] [CrossRef]
- Barańska, A.; Jedlińska, A.; Samborska, K. Is it Possible to Produce Carrier-Free Fruit and Vegetable Powders by Spray Drying? Pol. J. Food Nutr. Sci. 2023, 73, 214–223. [Google Scholar] [CrossRef]
- Jedlińska, A.; Samborska, K.; Wiktor, A.; Balik, M.; Derewiaka, D.; Matwijczuk, A.; Gondek, E. Spray drying of pure kiwiberry pulp in dehumidified air. Dry. Technol. 2022, 40, 1421–1435. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A new technique for spray drying orange juice concentrate. Innov. Food Sci. Emerg. Technol. 2010, 11, 342–351. [Google Scholar] [CrossRef]
- Tomczyk, M.; Zaguła, G.; Dżugan, M. A simple method of enrichment of honey powder with phytochemicals and its potential application in isotonic drink industry. Lwt 2020, 125, 109204. [Google Scholar] [CrossRef]
- Osés, S.M.; Cantero, L.; Puertas, G.; Fernández-Muiño, M.Á.; Sancho, M.T. Antioxidant, antimicrobial and anti-inflammatory activities of ling-heather honey powder obtained by different methods with several carriers. Lwt 2022, 159, 113235. [Google Scholar] [CrossRef]
- Krauze, A. Sugar spectrum of Polish nectar and honeydew honeys. Acta Aliment. Pol. 1991, 17, 109–117. [Google Scholar]
- Markowski, J.; Baron, A.; Le Quéré, J.-M.; Płocharski, W. Composition of clear and cloudy juices from French and Polish apples in relation to processing technology. LWT Food Sci. Technol. 2015, 62, 813–820. [Google Scholar] [CrossRef]
- Samborska, K.; Barańska, A.; Szulc, K.; Jankowska, E.; Truszkowska, M.; Ostrowska-Ligęza, E.; Wołosiak, R.; Szymańska, E.; Jedlińska, A. Reformulation of spray dried apple concentrate and honey for the enhancement of drying process performance and the physicochemical properties of powders. J. Sci. Food Agric. 2020, 100, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Milek, M.; Bocian, A.; Kleczynska, E.; Sowa, P.; Dzugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Howes, T.; Bhandari, B.R.; Langrish, T.A.G. Effect of addition of proteins on the production of amorphous sucrose powder through spray drying. J. Food Eng. 2009, 94, 144–153. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Lacerda, E.C.Q.; de Araújo Calado, V.M.; Monteiro, M.; Finotelli, P.V.; Torres, A.G.; Perrone, D. Starch, inulin and maltodextrin as encapsulating agents affect the quality and stability of jussara pulp microparticles. Carbohydr. Polym. 2016, 151, 500–510. [Google Scholar] [CrossRef]
- Saavedra–Leos, M.Z.; Leyva-Porras, C.; Alvarez-Salas, C.; Longoria-Rodríguez, F.; López-Pablos, A.L.; González-García, R.; Pérez-Urizar, J.T. Obtaining orange juice–maltodextrin powders without structure collapse based on the glass transition temperature and degree of polymerization. CyTA-J. Food 2018, 16, 61–69. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, X.; Cheng, Y.; Yu, M.; Meng, Q.; Ke, Q. Mannitol Is a Good Anticaking Agent for Spray-Dried Hydroxypropyl-Beta-Cyclodextrin Microcapsules. Molecules 2023, 28, 1119. [Google Scholar] [CrossRef]
- Littringer, E.M.; Mescher, A.; Eckhard, S.; Schröttner, H.; Langes, C.; Fries, M.; Griesser, U.; Walzel, P.; Urbanetz, N.A. Spray Drying of Mannitol as a Drug Carrier—The Impact of Process Parameters on Product Properties. Dry. Technol. 2012, 30, 114–124. [Google Scholar] [CrossRef]
- Littringer, E.M.; Paus, R.; Mescher, A.; Schroettner, H.; Walzel, P.; Urbanetz, N.A. The morphology of spray dried mannitol particles—The vital importance of droplet size. Powder Technol. 2013, 239, 162–174. [Google Scholar] [CrossRef]
- Littringer, E.M.; Noisternig, M.F.; Mescher, A.; Schroettner, H.; Walzel, P.; Griesser, U.J.; Urbanetz, N.A. The morphology and various densities of spray dried mannitol. Powder Technol. 2013, 246, 193–200. [Google Scholar] [CrossRef]
- Hulse, W.L.; Forbes, R.T.; Bonner, M.C.; Getrost, M. The characterization and comparison of spray-dried mannitol samples. Drug Dev. Ind. Pharm. 2009, 35, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Akhbarifar, S.; Shirvani, M. Improving cyclone efficiency for small particles. Chem. Eng. Res. Des. 2019, 147, 483–492. [Google Scholar] [CrossRef]
- Asensio, C.M.; Paredes, A.J.; Martin, M.P.; Allemandi, D.A.; Nepote, V.; Grosso, N.R. Antioxidant Stability Study of Oregano Essential Oil Microcapsules Prepared by Spray-Drying. J. Food Sci. 2017, 82, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Jedlinska, A.; Samborska, K.; Wieczorek, A.; Wiktor, A.; Ostrowska-Ligeza, E.; Jamroz, W.; Skwarczynska-Maj, K.; Kielczewski, D.; Blaowski, L.; Tulodziecki, M.; et al. The application of dehumidified air in rapeseed and honeydew honey spray drying—Process performance and powders properties considerations. J. Food Eng. 2019, 245, 80–87. [Google Scholar] [CrossRef]
- Barańska, A.; Świeca, M.; Samborska, K. Sour cherry juice concentrate powdered by high and low temperature spray drying with pea protein as a carrier—Physical properties, antioxidant activity and in vitro bioaccessibility. Dry. Technol. 2023, 41, 444–459. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Nishad, J.; Mir, S.A.; Walia, K. Optimization of Spray Drying Technology for Sugarcane Juice Using Natural and Synthetic Encapsulating Agents. Sugar Tech 2019, 21, 749–755. [Google Scholar] [CrossRef]
- Aragüez-Fortes, Y.; Robaina-Morales, L.M.; Pino, J.A. Optimization of the spray-drying parameters for developing guava powder. J. Food Process Eng. 2019, 42, e13230. [Google Scholar] [CrossRef]
- Almeida, R.F.; Gomes, M.H.G.; Kurozawa, L.E. Rice bran protein increases the retention of anthocyanins by acting as an encapsulating agent in the spray drying of grape juice. Food Res. Int. 2023, 172, 113237. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Mohamed Ahmed, I.A.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Bhusari, S.; Muzaffar, K.; Kumar, P. Effect of carrier agents on physical and microstructural properties of spray dried tamarind pulp powder. Powder Technol. 2014, 266, 354–364. [Google Scholar] [CrossRef]
- Gonnissen, Y.; Remon, J.P.; Vervaet, C. Development of directly compressible powders via co-spray drying. Eur. J. Pharm. Biopharm. 2007, 67, 220–226. [Google Scholar] [CrossRef]
- Moghaddam, A.D.; Pero, M.; Askari, G.R. Optimizing spray drying conditions of sour cherry juice based on physicochemical properties, using response surface methodology (RSM). J. Food. Sci. Technol. 2017, 54, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Nadali, N.; Pahlevanlo, A.; Sarabi-Jamab, M.; Balandari, A. Effect of maltodextrin with different dextrose equivalents on the physicochemical properties of spray-dried barberry juice (Berberis vulgaris L.). J. Food. Sci. Technol. 2022, 59, 2855–2866. [Google Scholar] [CrossRef]
- Ganaie, T.A.; Masoodi, F.A.; Rather, S.A.; Gani, A. Exploiting maltodextrin and whey protein isolate macromolecules as carriers for the development of freeze dried honey powder. Carbohydr. Polym. Technol. Appl. 2021, 2, 100040. [Google Scholar] [CrossRef]
- George, S.; Thomas, A.; Kumar, M.V.P.; Kamdod, A.S.; Rajput, A.; Abdullah, S. Impact of processing parameters on the quality attributes of spray-dried powders: A review. Eur. Food Res. Technol. 2022, 249, 241–257. [Google Scholar] [CrossRef]
- Murugesan, R.; Orsat, V. Spray Drying for the Production of Nutraceutical Ingredients—A Review. Food Bioprocess Technol. 2011, 5, 3–14. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Brzezowska, J.; Majerska, J.; Ciska, E. The influence of inulin on the retention of polyphenolic compounds during the drying of blackcurrant juice. Molecules 2019, 24, 4167. [Google Scholar] [CrossRef]
- Acosta-Vega, L.; Martínez-Suárez, J.F.; Sánchez-Garzón, F.S.; Hernández-Carrión, M.; Nerio, L.S. Optimization of the encapsulation process of Cupuassu (Theobroma grandiflorum) pulp by spray drying as an alternative for the valorization of Amazonian fruits. Lwt 2023, 184, 114994. [Google Scholar] [CrossRef]
- Taengsopha, P.; Junyusen, T.; Moolkaew, P.; Junyusen, P. Comparative effects of prebiotic addition on the physicochemical and microstructural properties of spray-dried yogurt powder. Eng. Appl. Sci. Res. 2023, 50, 657–663. [Google Scholar] [CrossRef]
- Elversson, J.; Millqvist-Fureby, A. Particle size and density in spray drying-effects of carbohydrate properties. J. Pharm. Sci. 2005, 94, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Tze, N.L.; Han, C.P.; Yusof, Y.A.; Ling, C.N.; Talib, R.A.; Taip, F.S.; Aziz, M.G. Physicochemical and nutritional properties of spray-dried pitaya fruit powder as natural colorant. Food Sci. Biotechnol. 2012, 21, 675–682. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mobli, H.; Madadlou, A.; Rafiee, S. Influence of wall material and inlet drying air temperature on the microencapsulation of fish oil by spray drying. Food Bioprocess Technol. 2013, 6, 1561–1569. [Google Scholar] [CrossRef]
- Shah, N.; Sandhu, H.; Choi, D.S.; Chokshi, H.; Malick, A.W. Amorphous solid dispersions. In Theory and Practice; Springer: Berlin, Germany, 2014. [Google Scholar]
- Guiling, X.; Xiaoping, C.; Cai, L.; Pan, X.; Changsui, Z. Experimental investigation on the flowability properties of cohesive carbonaceous powders. Part. Sci. Technol. 2016, 35, 322–329. [Google Scholar] [CrossRef]
- Goh, H.P.; Heng, P.W.S.; Liew, C.V. Comparative evaluation of powder flow parameters with reference to particle size and shape. Int. J. Pharm. 2018, 547, 133–141. [Google Scholar] [CrossRef]
- Jedlińska, A.; Barańska, A.; Witrowa-Rajchert, D.; Ostrowska-Ligęza, E.; Samborska, K. Dehumidified Air-Assisted Spray-Drying of Cloudy Beetroot Juice at Low Temperature. Appl. Sci. 2021, 11, 6578. [Google Scholar] [CrossRef]
- Fan, A.; Pallerla, S.; Carlson, G.; Ladipo, D.; Dukich, J.; Capella, R.; Leung, S. Effect of particle size distribution and flow property of powder blend on tablet weight variation. Am. Pharm. Rev. 2005, 8, 73–78. [Google Scholar]

| Name | Osmotic Material | Concentration Before Osmotic Dehydration (% Solids, w/w) | Concentration After Osmotic Dehydration of Apples (% Solids, w/w) | Concentration of Feed Solution Before Spray Drying (% Solids, w/w) | Osmotic Material: Inulin Ratio in Feed Solutions Before Spray Drying (Solids, w/w) |
|---|---|---|---|---|---|
| SUC | Sucrose | 50 | 46 | 50 | 50:50 |
| BWH | Buckwheat honey (Pasieka Warmińska, Rentyny, Poland) | 50 | 46 | 50 | 50:50 |
| ACH | Acacia honey (Pasieka Warmińska, Rentyny, Poland) | 50 | 46 | 50 | 50:50 |
| APP | Apple juice concentrate (Białuty, Błonie, Poland) | 50 | 45 | 50 | 50:50 |
| CHO | Chokeberry juice concentrate (Gomar, Pinczów, Poland) | 50 | 45 | 50 | 50:50 |
| CHE | Cherry juice concentrate (Białuty, Błonie, Poland) | 50 | 43 | 50 | 50:50 |
| MAN | Mannitol | 25 | 20 | 20 | 100:0 |
| D50 | PDI | MC | aw | H | WSI | DL | DT | HR | |
|---|---|---|---|---|---|---|---|---|---|
| [μm] | [%] | [%] | [%] | [g/mL3] | [g/mL3] | ||||
| SUC | 22 ± 5 a* | 1.8 ± 0.1 b | 1.2 ± 0.2 b | 0.040 ± 0.009 a | 17.5 ± 0.2 b | 97.2 ± 1.2 a | 0.62 ± 0.02 b | 0.79 ± 0.01 b | 1.27 ± 0.05 b |
| BWH | 111 ± 4 b | 1.5 ± 0.2 b | 1.9 ± 0.2 c | 0.115 ± 0.005 b | 27.3 ± 0.2 d | 94.1 ± 3.2 a | 0.62 ± 0.02 b | 0.78 ± 0.02 b | 1.28 ± 0.05 b |
| ACH | 717 ± 15 c | 0.9 ± 0.1 a | 2.1 ± 0.5 c | 0.141 ± 0.004 c | 25.0 ± 0.1 c | 96.8 ± 3.2 a | 0.88 ± 0.02 c | 0.92 ± 0.01 c | 1.05 ± 0.01 a |
| APP | 896 ± 10 d | 0.8 ± 0.1 a | 2.8 ± 0.2 c | 0.169 ± 0.007 d | 24.7 ± 0.2 c | 96.3 ± 2.1 a | 0.86 ± 0.02 c | 0.86 ± 0.02 c | 1.00 ± 0.04 a |
| CHO | 802 ± 38 d | 0.8 ± 0.1 a | 2.8 ± 0.6 c | 0.127 ± 0.009 c | 25.0 ± 0.2 c | 97.9 ± 0.5 a | 0.87 ± 0.02 c | 0.90 ± 0.01 c | 1.04 ± 0.01 a |
| CHE | 734 ± 44 c | 0.9 ± 0.1 a | 4.8 ± 0.5 d | 0.106 ± 0.007 b | 26.0 ± 0.2 c | 94.5 ± 1.8 a | 0.82 ± 0.02 c | 0.87 ± 0.02 c | 1.05 ± 0.02 a |
| MAN | 20 ± 1 a | 1.5 ± 0.1 b | 0.4 ± 0.1 a | 0.208 ± 0.004 e | 0.5 ± 0.1 a | 97.4 ± 3.0 a | 0.45 ± 0.02 a | 0.60 ± 0.00 a | 1.34 ± 0.06 b |
| MC | aw | H | DL | DT | HR | WSI | D50 | PDI | |
|---|---|---|---|---|---|---|---|---|---|
| Y | −0.38 | −0.75 | −0.09 | −0.53 | −0.29 | 0.68 | −0.14 | −0.77 | 0.86 |
| MC | −0.19 | 0.70 | 0.73 | 0.68 | −0.75 | −0.50 | 0.74 | −0.71 | |
| aw | −0.44 | −0.13 | −0.36 | −0.05 | 0.17 | 0.18 | −0.32 | ||
| H | 0.79 | 0.89 | −0.66 | −0.49 | 0.60 | −0.52 | |||
| DL | 0.96 | −0.97 | −0.04 | 0.93 | −0.87 | ||||
| DT | −0.85 | −0.12 | 0.79 | −0.71 | |||||
| HR | −0.01 | −0.99 | 0.94 | ||||||
| WSI | 0.01 | 0.00 | |||||||
| D50 | −0.98 | ||||||||
| negative significant correlation | |||||||||
| −0.90 | −0.80 | −0.70 | |||||||
| positive significant correlation | |||||||||
| 0.70 | 0.80 | 0.90 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samborska, K.; Barańska-Dołomisiewicz, A.; Jedlińska, A.; Costa, R.; Klimantakis, K.; Mourtzinos, I.; Nowacka, M. Valorization of Spent Osmotic Solutions by Production of Powders by Spray Drying. Appl. Sci. 2025, 15, 6927. https://doi.org/10.3390/app15126927
Samborska K, Barańska-Dołomisiewicz A, Jedlińska A, Costa R, Klimantakis K, Mourtzinos I, Nowacka M. Valorization of Spent Osmotic Solutions by Production of Powders by Spray Drying. Applied Sciences. 2025; 15(12):6927. https://doi.org/10.3390/app15126927
Chicago/Turabian StyleSamborska, Katarzyna, Alicja Barańska-Dołomisiewicz, Aleksandra Jedlińska, Rui Costa, Konstantinos Klimantakis, Ioannis Mourtzinos, and Małgorzata Nowacka. 2025. "Valorization of Spent Osmotic Solutions by Production of Powders by Spray Drying" Applied Sciences 15, no. 12: 6927. https://doi.org/10.3390/app15126927
APA StyleSamborska, K., Barańska-Dołomisiewicz, A., Jedlińska, A., Costa, R., Klimantakis, K., Mourtzinos, I., & Nowacka, M. (2025). Valorization of Spent Osmotic Solutions by Production of Powders by Spray Drying. Applied Sciences, 15(12), 6927. https://doi.org/10.3390/app15126927











