Molten Salt Mixtures as an Energy Carrier for Thermochemical Processes of Renewable Gas Production: Review and Perspectives
Abstract
1. Introduction
2. Molten Salt Mixtures
2.1. Molten Salt Mixtures as Heat Transfer Fluid and Storage Medium
2.2. Molten Salt Mixtures’ Thermophysical Properties
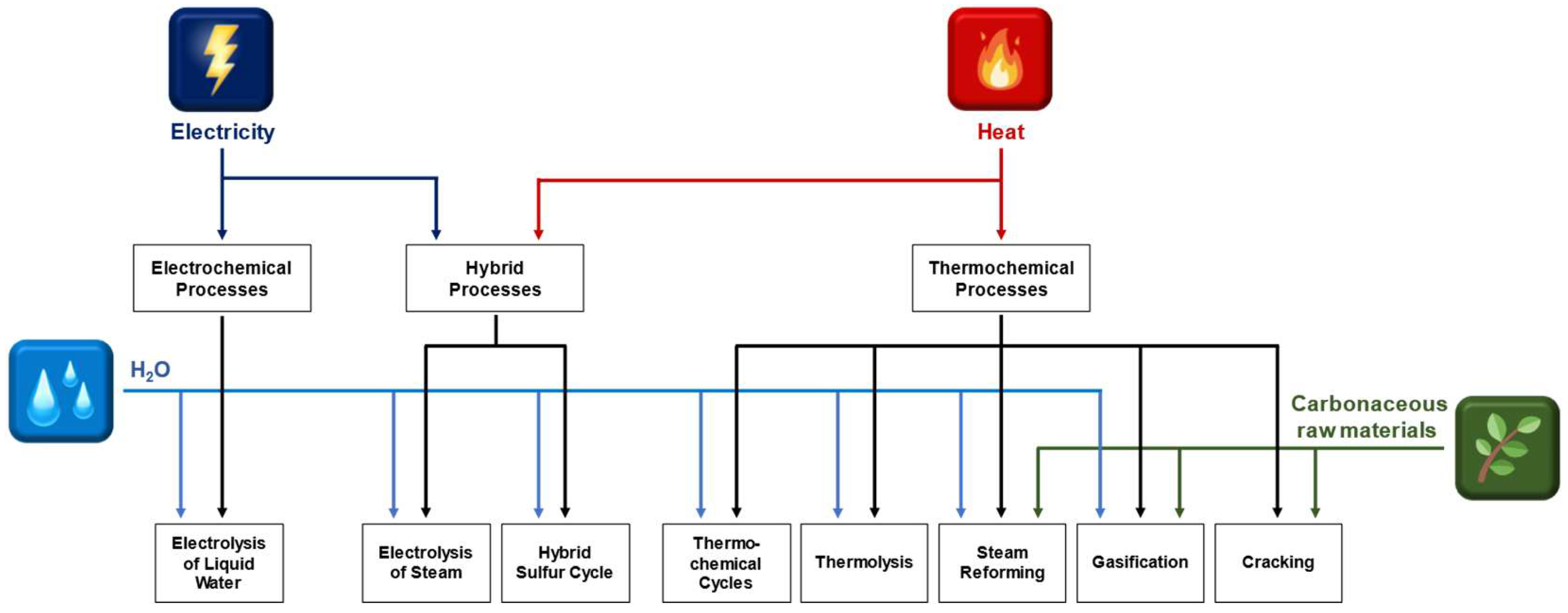
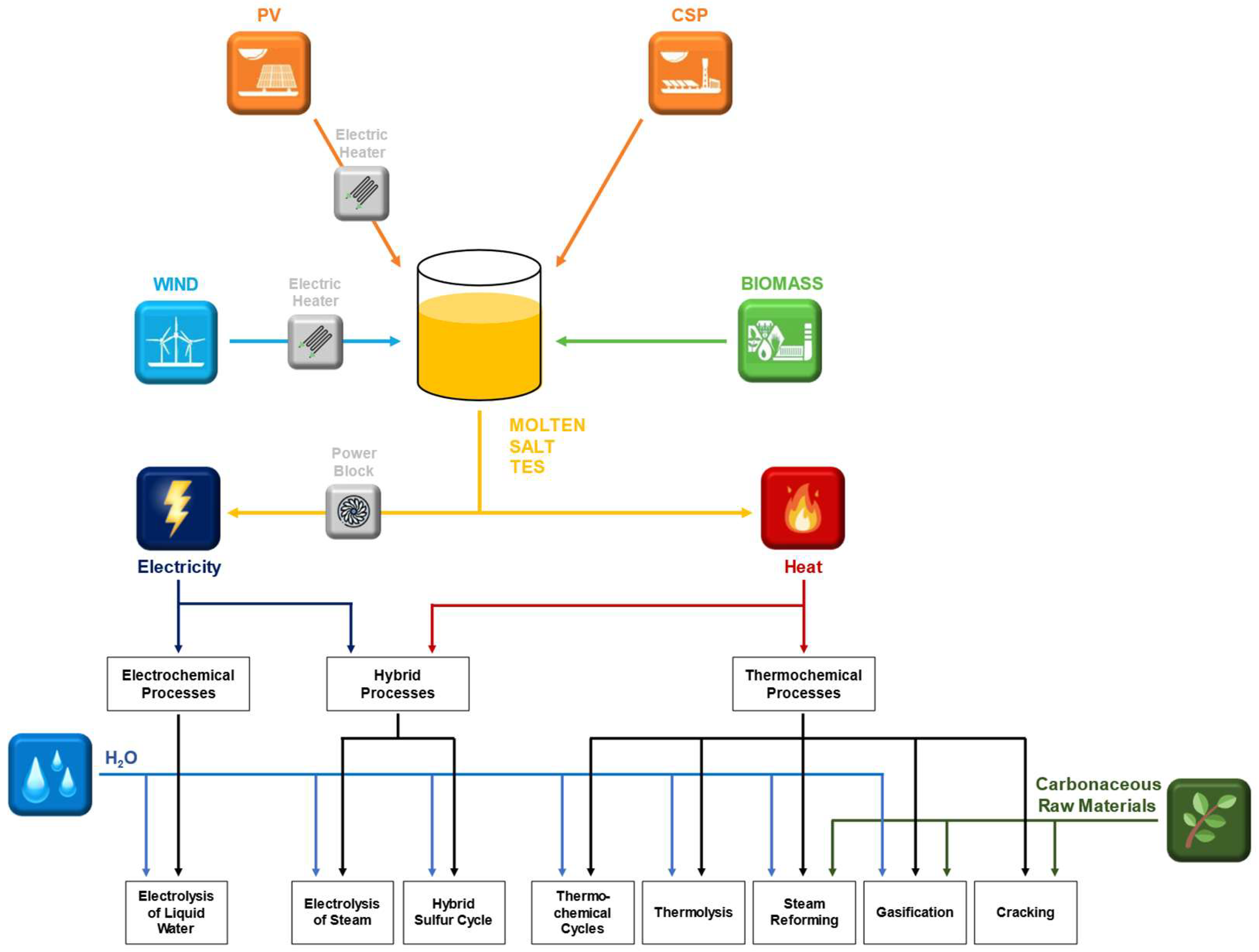
3. Thermo(-Electro-)Chemical Processes for the Production of Hydrogen and Syngas
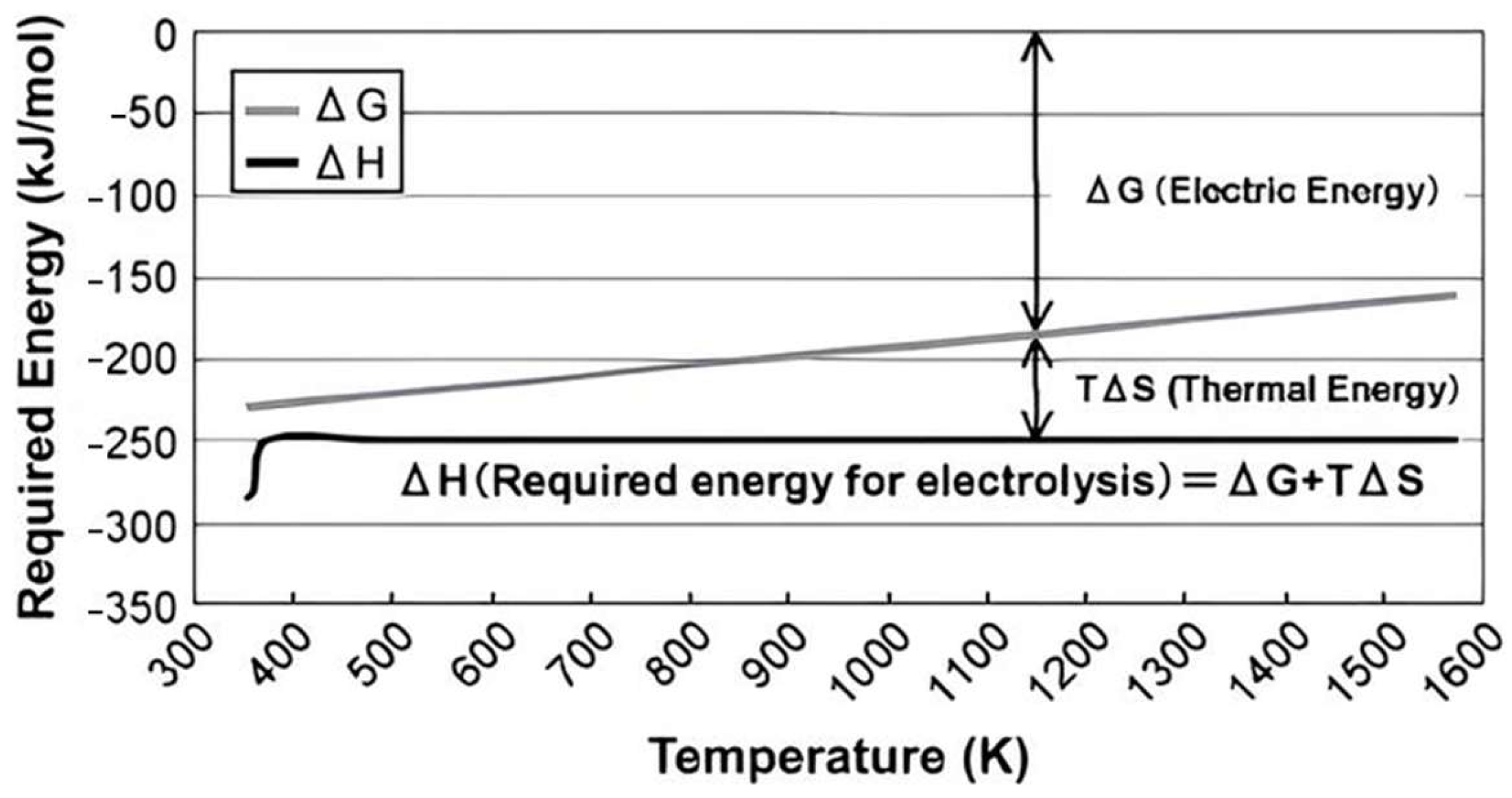
4. Steam Electrolysis and Thermo(-Electro-)Chemical Cycles
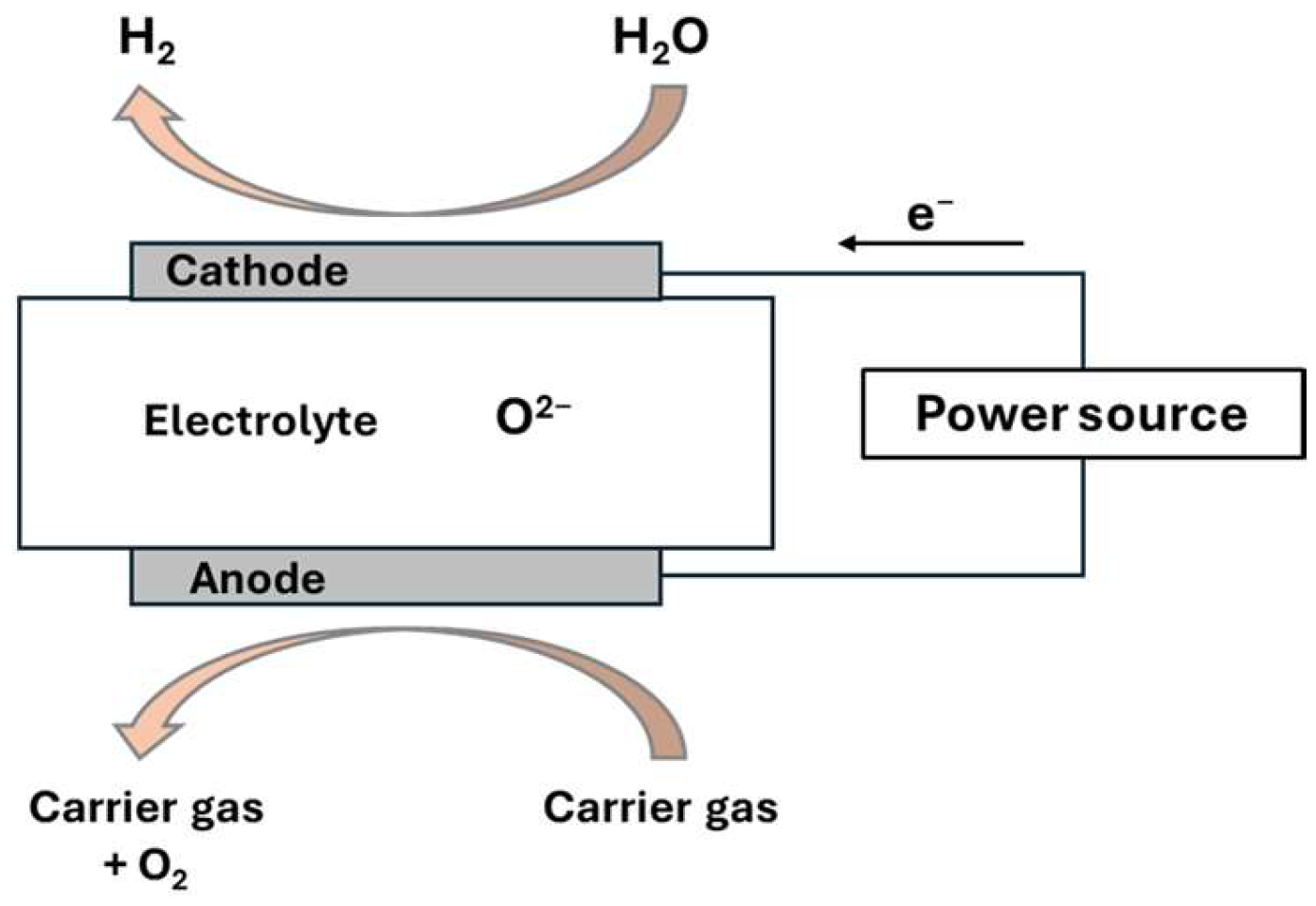
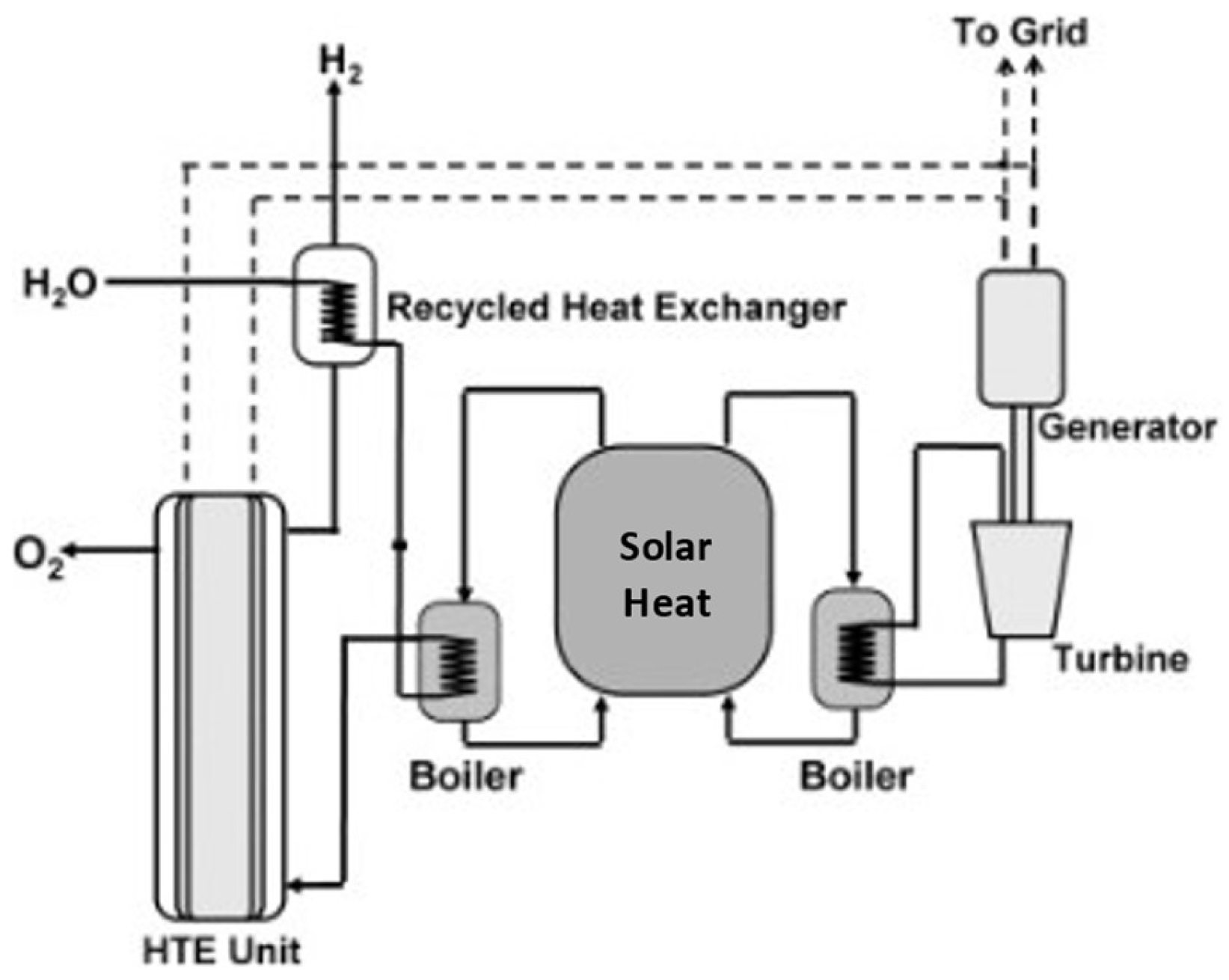
4.1. High-Temperature Electrolysis
4.2. Water Splitting Thermochemical Cycles
4.3. Hybrid Thermochemical Cycles
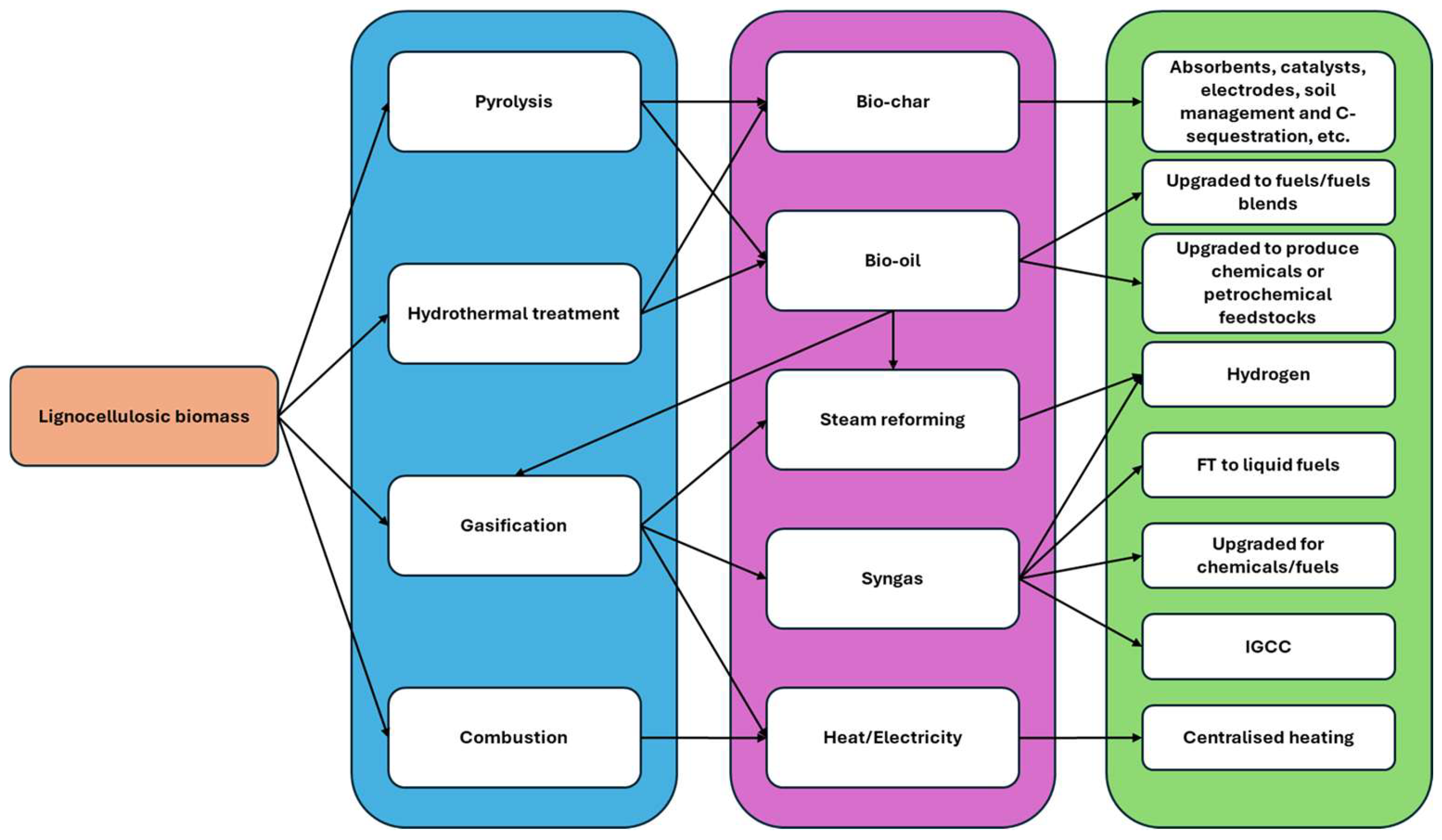
5. Thermochemical Conversion of Carbonaceous Feedstocks
5.1. Steam Reforming
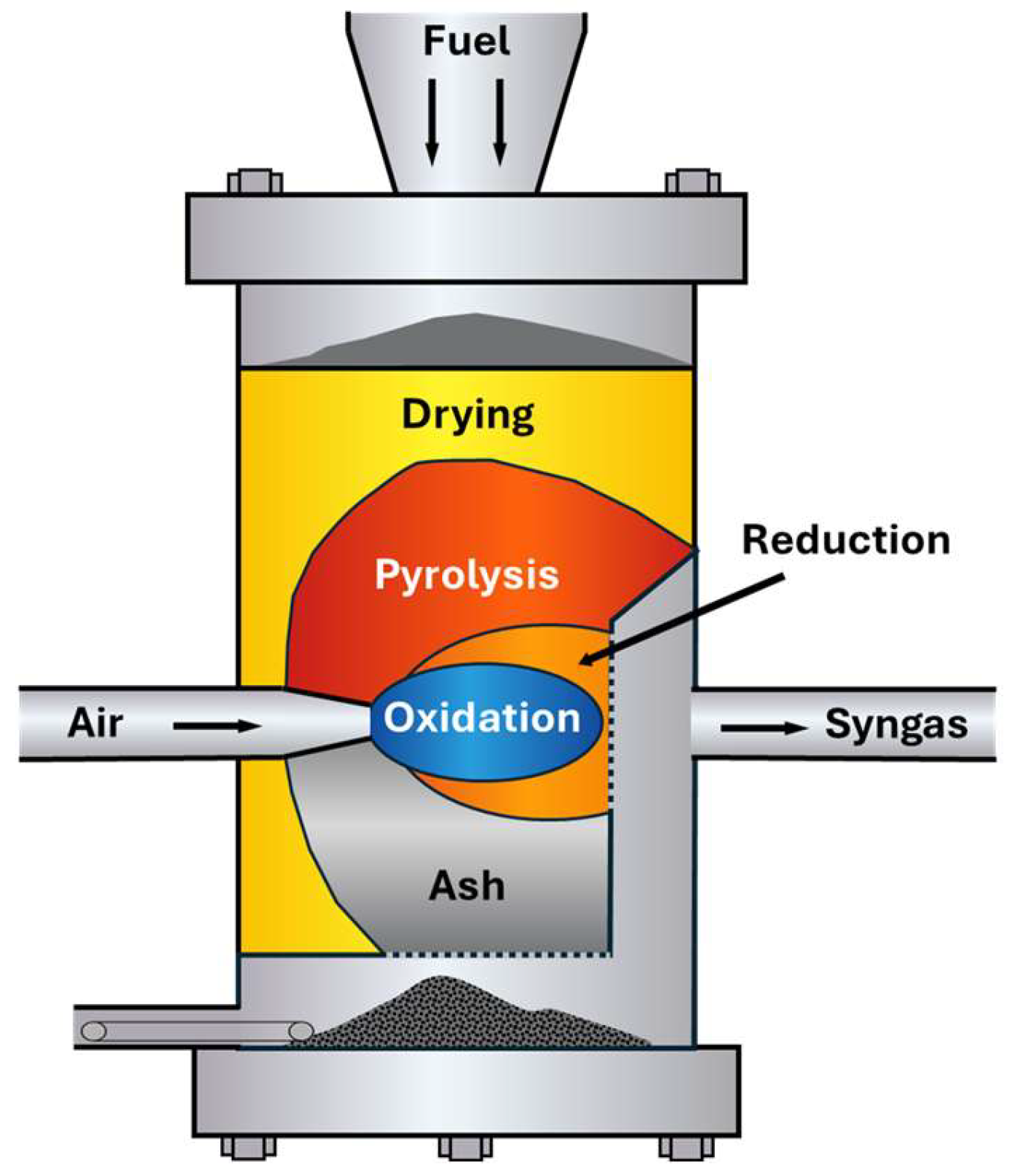
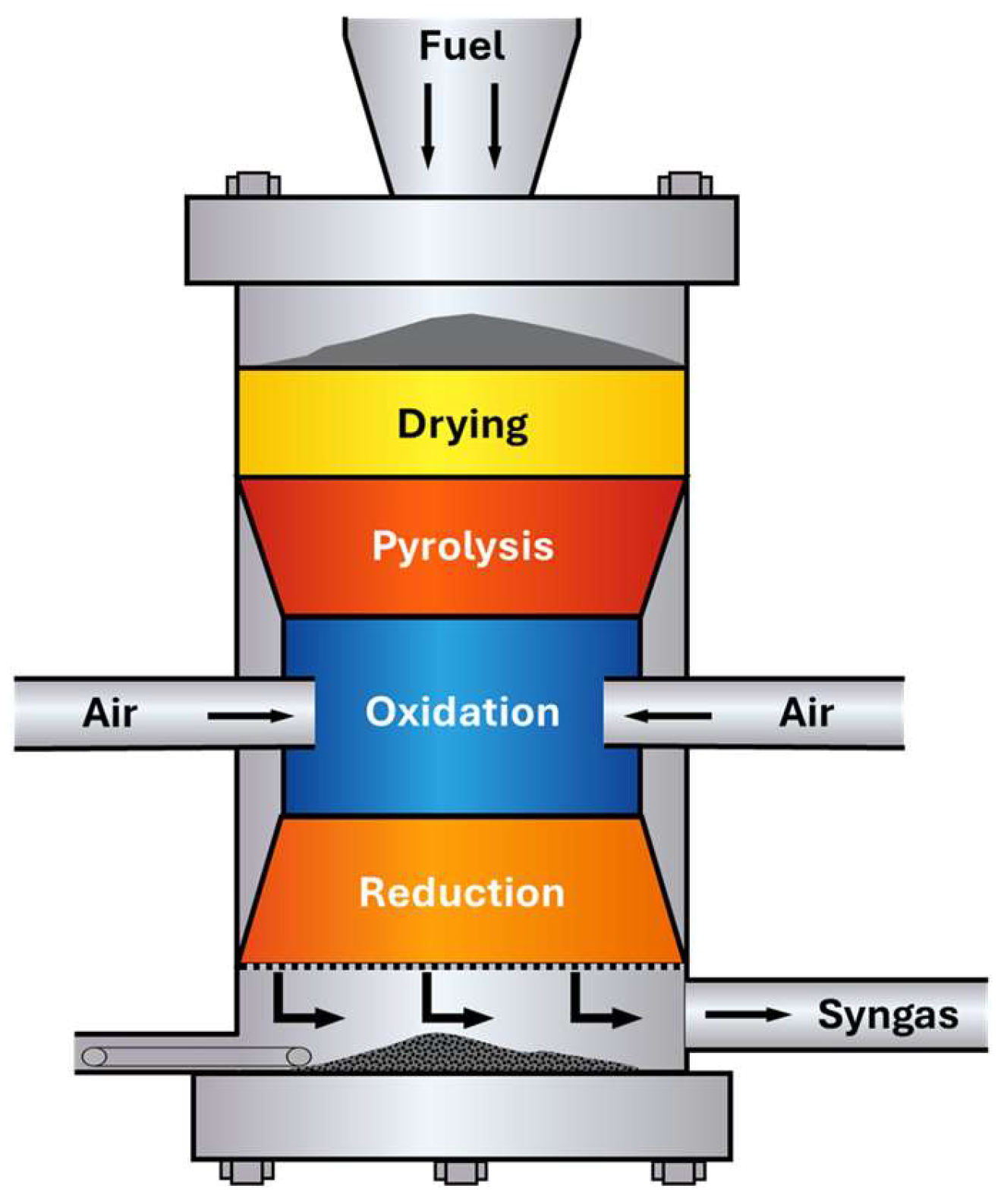
5.2. Gasification
- Oxidation (exothermic);
- Drying (endothermic);
- Pyrolysis (endothermic);
- Reduction (endothermic).
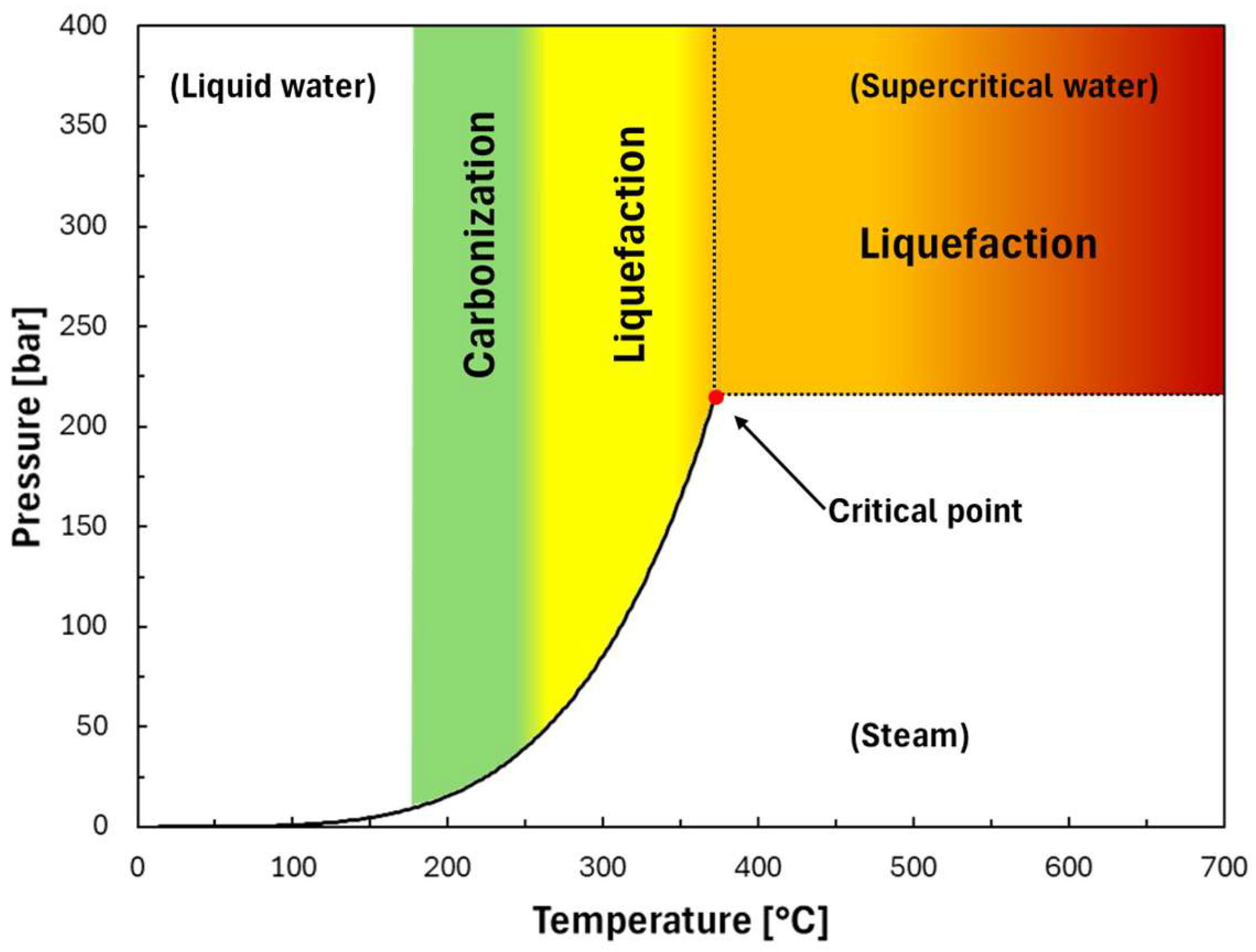
5.3. Hydrothermal Gasification
5.4. Pyrolysis
- Biochar (10–30%);
- Bio-oil (20–50%);
- Syngas (20–30%).
5.5. Hydrothermal Liquefaction
6. Identification of MS-Driven Processes of Renewable Gas Production
| Process Class | Process Family | Process | Feedstock | Main Products | Process Temperature | Conventional Process Interface | Ref. |
|---|---|---|---|---|---|---|---|
| Steam electrolysis | Solid oxide steam electrolysis | Solid oxide steam electrolysis | H2O (+CO2) | H2, O2, and (CO) | 600–800 °C | Steam generator (~100 °C) | [148] |
| Steam electrolysis | Molten carbonate steam electrolysis | Molten carbonate steam electrolysis | H2O (+CO2) | H2, O2, and (+CO2) | 650 °C | Steam generator (~100 °C) and amine regeneration column reboilers, if CO2 separation is included | [149,150] |
| Thermo(-electro-) chemical cycles | Sulfur- family cycles | Sulfur- family cycles | H2O | H2 and O2 | Up to >800 °C | Not applicable | [151] |
| Thermo(-electro-) chemical cycles | Sulfur- family cycles | Hybrid sulfur (Westinghouse) cycle | H2O | H2 and O2 | 800–900 °C | Not applicable | [102,104,152] |
| Thermo(-electro-) chemical cycles | Sulfur- family cycles | Sulfur– iodine cycle | H2O | H2 and O2 | 800–900 °C | Not applicable | [153] |
| Thermo(-electro-) chemical cycles | Sulfur- family cycles | S-A cycle | H2O | H2 and O2 | 800–900 °C | Not applicable | [154] |
| Thermo(-electro-) chemical cycles | Sulfur- family cycles | Modified S-A cycle | H2O | H2 and O2 | 800–900 °C | Not applicable | [105] |
| Thermo(-electro-) chemical cycles | Sulfur- family cycles | Modified sulfur–iodine with solid intermediates | H2O | H2 and O2 | 900 °C | Not applicable | [100,155] |
| Thermo(-electro-) chemical cycles | Non-volatile metal oxide cycles | Non-volatile metal oxide cycles | H2O | H2 and O2 | >1000 °C | Not applicable | [156] |
| Thermo(-electro-) chemical cycles | Non-volatile metal oxide cycles | Mixed ferrites | H2O | H2 and O2 | 800 °C | Nost applicable | [157] |
| Thermo(-electro-) chemical cycles | Metal halide-based hybrid cycles | UT-3 | H2O | H2 and O2 | 760 °C | Not applicable | [158,159] |
| Thermo(-electro-) chemical cycles | Metal halide-based hybrid cycles | Cu-Cl cycle | H2O | H2 and O2 | Up to 500 °C | Not applicable | [160] |
| Thermochemical conversion of carbonaceous feedstocks | Steam reforming | Low-temperature steam methane (or biogas) reforming | CH4, (CO2), and H2O | H2 + syngas | 500–550 °C | Not applicable (the conventional high-temperature process is operated at T > 800 °C by using gas-fired furnaces) | [147,161,162] |
| Thermochemical conversion of carbonaceous feedstocks | Gasification | Gasification | Biomass waste 1 | H2 + syngas | 800–2000 °C | Fluidized-bed and fixed-bed reactors | [109,111] |
| Thermochemical conversion of carbonaceous feedstocks | Hydrothermal gasification | Supercritical water gasification | Biomass waste 1 | H2 + CO2 | 374–500 °C (Water pressure >25 MPa) | Heat exchangers | [121,122] |
| Thermochemical conversion of carbonaceous feedstocks | Hydrothermal liquefaction | Hydrothermal liquefaction | Wet biomass waste | Bio-oil | 75–250 °C (Water pressure 1.5–10 MPa) | Heat exchangers | [141,142] |
| Thermochemical conversion of carbonaceous feedstocks | Biomass pyrolysis | Slow pyrolysis | Biomass waste | Biochar (50–70%), bio-oil (20–30%), and syngas (10–20%) | 200–400 °C | One-stage pyrolysis process characterized by slow pyrolysis process (5 to 30 min) | [130] |
| Thermochemical conversion of carbonaceous feedstocks | Biomass pyrolysis | Intermediate pyrolysis | Biomass waste | Biochar (20–30%), bio-oil (50–70%), and syngas (10–20%) | 400–600 °C | One-stage pyrolysis process characterized by intermediate pyrolysis process (1 to 5 min) | [130] |
| Thermochemical conversion of carbonaceous feedstocks | Biomass pyrolysis | Fast or flash pyrolysis | Biomass waste | Biochar (15–40%), bio-oil (15–20%), and syngas (50–70%) | 600–1000 °C | One-stage pyrolysis process characterized by fast pyrolysis (<2 s) or flash pyrolysis (<1 s) | [163,164] |
| Process Class | Process Family | Process | MS Mixture (Tfreeze-Tmax) | MS Interface |
|---|---|---|---|---|
| Steam electrolysis | Solid oxide steam electrolysis | Solid oxide steam electrolysis | Quaternary mixtures (coupling with CSP plants using solar salt requires intermediate HTF) | Steam generator, with the possibility of using salts from cold tank |
| Steam electrolysis | Molten carbonate steam electrolysis | Molten carbonate steam electrolysis | Quaternary mixtures (coupling with CSP plants using solar salt requires intermediate HTF) | Steam generator (and amine regeneration column reboilers, if included), with the possibility of using salts from cold tank |
| Thermo(-electro-) chemical cycles | Sulfur-family cycles | Sulfur-family cycles | Solar salt (240–565 °C). In the future, chlorides or other very-high-temperature mixtures could be used. | Sulfuric acid concentration reboilers (~200 °C), sulfuric acid vaporization, and decomposition exchanger–reactor (300–500 °C) |
| Thermo(-electro-) chemical cycles | Sulfur-family cycles | Hybrid sulfur (Westinghouse) cycle | Solar salt (240–565 °C). In the future, chlorides or other very-high-temperature mixtures could be used. | Sulfuric acid concentration reboilers (~200 °C), sulfuric acid vaporization, and decomposition exchanger–reactor (300–500 °C) |
| Thermo(-electro-) chemical cycles | Sulfur-family cycles | Sulphur–iodine cycle | Solar salt (240–565 °C). In the future, chlorides or other very-high-temperature mixtures could be used. | Sulfuric acid concentration reboilers (~200 °C), sulfuric acid vaporization, decomposition exchanger–reactor (300–500 °C) and separation of HI from I2, followed by HI cracking (endothermic, at 300–450 °C) |
| Thermo(-electro-) chemical cycles | Sulfur-family cycles | S-A cycle | Solar Salt (240–565 °C). In the future, chlorides or other very-high-temperature mixtures could be used. | Sulfuric acid concentration reboilers (~200 °C), sulfuric acid vaporization, decomposition exchanger–reactor (300–500 °C), and ammonium sulfate decomposition (400–500 °C) |
| Thermo(-electro-) chemical cycles | Sulfur-family cycles | Modified S-A cycle | Solar Salt (240–565 °C). In the future, chlorides or other very-high-temperature mixtures could be used. | Dehydration of metal sulfate (350–450 °C), and ammonium sulfate decomposition (400–500 °C) |
| Thermo(-electro-) chemical cycles | Sulfur-family cycles | Modified sulfur–iodine with solid intermediates | Solar salt (240–565 °C). In the future, chlorides or other very-high-temperature mixtures could be used. | Metal sulfate Pre-heating and dehydration (up to 500 °C) and Metal iodide pre-heating and dehydration (up to 500 °C) |
| Thermo(-electro-) chemical cycles | Non-volatile metal oxide cycles | Non-volatile metal oxide cycles | Solar salt (240–565 °C) | MSs could be considered for heat recovery/thermal buffering/reactant pre-heating in some parts of the plant |
| Thermo(-electro-) chemical cycles | Non-volatile metal oxide cycles | Mixed ferrites | In the future, chlorides or other very-high-temperature mixtures could be used | MSs could be considered for H2/CO2 separation from excess water |
| Thermo(-electro-) chemical cycles | Metal halide-based hybrid cycles | UT-3 | In the future, chlorides or other very-high-temperature mixtures could be used | MSs could be considered for pre-heating regarding water splitting with HBr formation and hydrogen formation from FeBr2 |
| Thermo(-electro-) chemical cycles | Metal halide-based hybrid cycles | Cu-Cl cycle | Solar salt (240–565 °C) | Reactor for oxygen production at 530 °C (configuration to be defined, e.g., jacketed reactor, integrated heat exchanger/coil, etc.) |
| Thermochemical conversion of carbonaceous feedstocks | Steam reforming | Low-temperature steam methane (or biogas) reforming | Solar salt (240–565 °C) | Heat exchangers, steam generators, and integrated membrane reactors/heat exchangers |
| Thermochemical conversion of carbonaceous feedstocks | Gasification | Gasification | Solar salt (240–565 °C). In the future, chlorides or other very-high-temperature mixtures could be used. | MSs could be considered for feeding the reactions in the pyrolysis step (temperature of up to 500 °C) in down-draft reactors |
| Thermochemical conversion of carbonaceous feedstocks | Hydrothermal gasification | Supercritical water gasification | Solar salt (240–565 °C) | Heat exchangers and steam generators |
| Thermochemical conversion of carbonaceous feedstocks | Hydrothermal liquefaction | Hydrothermal liquefaction | Ternary mixtures | Heat exchangers |
| Thermochemical conversion of carbonaceous feedstocks | Biomass pyrolysis | Slow pyrolysis | Solar salt (240–565 °C) | Heat exchangers, steam generators, and integrated membrane reactors/heat exchangers |
| Thermochemical conversion of carbonaceous feedstocks | Biomass pyrolysis | Intermediate pyrolysis | Solar salt (240–565 °C) | Heat exchangers, steam generators, and integrated membrane reactors/heat exchangers |
| Thermochemical conversion of carbonaceous feedstocks | Biomass pyrolysis | Fast or flash pyrolysis | In the future, chlorides or other very-high-temperature mixtures could be used | Heat exchangers and integrated membrane reactors/heat exchangers |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission the European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 11 June 2024).
- European Commission Fit for 55: Delivering on the Proposals. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal/delivering-european-green-deal/fit-55-delivering-proposals_en (accessed on 11 June 2024).
- European Commission. Next Generation EU. Available online: https://next-generation-eu.europa.eu/index_en (accessed on 11 June 2024).
- European Commission. REPowerEU; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- European Commission. Directive-EU-2023/2413-EN-Renewable Energy Directive-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32023L2413 (accessed on 11 June 2024).
- Bhandari, R.; Adhikari, N. A Comprehensive Review on the Role of Hydrogen in Renewable Energy Systems. Int. J. Hydrogen Energy 2024, 82, 923–951. [Google Scholar] [CrossRef]
- Ajanovic, A.; Sayer, M.; Haas, R. On the Future Relevance of Green Hydrogen in Europe. Appl. Energy 2024, 358, 122586. [Google Scholar] [CrossRef]
- Islam, A.; Islam, T.; Mahmud, H.; Raihan, O.; Islam, M.S.; Marwani, H.M.; Rahman, M.M.; Asiri, A.M.; Hasan, M.M.; Hasan, M.N.; et al. Accelerating the Green Hydrogen Revolution: A Comprehensive Analysis of Technological Advancements and Policy Interventions. Int. J. Hydrogen Energy 2024, 67, 458–486. [Google Scholar] [CrossRef]
- Majewski, S.; Zhao, X.; Vivanco-Martín, B.; Iranzo, A. Analysis of the European Strategy for Hydrogen: A Comprehensive Review. Energies 2023, 16, 3866. [Google Scholar] [CrossRef]
- Nunes, V.M.B.; Queirós, C.S.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Molten Salts as Engineering Fluids—A Review: Part I. Molten Alkali Nitrates. Appl. Energy 2016, 183, 603–611. [Google Scholar] [CrossRef]
- Bernagozzi, M.; Panesar, A.S.; Morgan, R. Molten Salt Selection Methodology for Medium Temperature Liquid Air Energy Storage Application. Appl. Energy 2019, 248, 500–511. [Google Scholar] [CrossRef]
- Caraballo, A.; Galán-Casado, S.; Caballero, Á.; Serena, S. Molten Salts for Sensible Thermal Energy Storage: A Review and an Energy Performance Analysis. Energies 2021, 14, 1197. [Google Scholar] [CrossRef]
- Myers, P.D.; Goswami, D.Y. Thermal Energy Storage Using Chloride Salts and Their Eutectics. Appl. Therm. Eng. 2016, 109, 889–900. [Google Scholar] [CrossRef]
- Sau, S.; Corsaro, N.; Crescenzi, T.; D’Ottavi, C.; Liberatore, R.; Licoccia, S.; Russo, V.; Tarquini, P.; Tizzoni, A.C.; D’Ottavi, C.; et al. Techno-Economic Comparison between CSP Plants Presenting Two Different Heat Transfer Fluids. Appl. Energy 2016, 168, 96–109. [Google Scholar] [CrossRef]
- Roper, R.; Harkema, M.; Sabharwall, P.; Riddle, C.; Chisholm, B.; Day, B.; Marotta, P. Molten Salt for Advanced Energy Applications: A Review. Ann. Nucl. Energy 2022, 169, 108924. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Siddiqui, S.; Sreedhar, I.; Parameshwaran, R. Molten Salts: Potential Candidates for Thermal Energy Storage Applications. Int. J. Energy Res. 2022, 46, 17755–17785. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat Transfer Fluids for Concentrating Solar Power Systems—A Review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Collares-Pereira, M.; Canavarro, D.; Guerreiro, L.L. Linear Fresnel Reflector (LFR) Plants Using Superheated Steam, Molten Salts, and Other Heat Transfer Fluids. In Advances in Concentrating Solar Thermal Research and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 339–352. ISBN 9780081005170. [Google Scholar]
- Laing, D.; Bauer, T.; Breidenbach, N.; Hachmann, B.; Johnson, M. Development of High Temperature Phase-Change-Material Storages. Appl. Energy 2013, 109, 497–504. [Google Scholar] [CrossRef]
- Fiorucci, L.C.; Goldstein, S.L.; Fiorucci, L.C.; Goldstein, S.L. Manufacture, Distribution, and Handling of Nitrate Salts for Solar-Thermal Applications. STIN 1982, 83, 21625. [Google Scholar]
- Bradshaw, R.W.; Siegel, N.P. Molten Nitrate Salt Development for Thermal Energy Storage in Parabolic Trough Solar Power Systems. In Proceedings of the ASME 2008 2nd International Conference on Energy Sustainability Collocated with the Heat Transfer, Fluids Engineering, and 3rd Energy Nanotechnology Conferences, Jacksonville, FL, USA, 10–14 August 2008; Volume 2, pp. 631–637. [Google Scholar]
- Delise, T.; Tizzoni, A.C.; Ferrara, M.; Corsaro, N.; D’Ottavi, C.; Sau, S.; Licoccia, S. Thermophysical, Environmental, and Compatibility Properties of Nitrate and Nitrite Containing Molten Salts for Medium Temperature CSP Applications: A Critical Review. J. Eur. Ceram. Soc. 2019, 39, 92–99. [Google Scholar] [CrossRef]
- Jriri, T.; Rogez, J.; Bergman, C.; Mathieu, J.C. Thermodynamic Study of the Condensed Phases of NaNO3, KNO3 and CsNO3 and Their Transitions. Thermochim. Acta 1995, 266, 147–161. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Breidenbach, N.; Eck, M.; Laing, D.; Kaesche, S. Material Aspects of Solar Salt for Sensible Heat Storage. Appl. Energy 2013, 111, 1114–1119. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Ren, N.; Ma, C. Improving the Thermal Properties of NaNO3-KNO3 for Concentrating Solar Power by Adding Additives. Sol. Energy Mater. Sol. Cells 2017, 160, 263–268. [Google Scholar] [CrossRef]
- Bonk, A.; Sau, S.; Uranga, N.; Hernaiz, M.; Bauer, T. Advanced Heat Transfer Fluids for Direct Molten Salt Line-Focusing CSP Plants. Prog. Energy Combust. Sci. 2018, 67, 69–87. [Google Scholar] [CrossRef]
- Bradshaw, R.W.W.; Meeker, D.E.E. High-Temperature Stability of Ternary Nitrate Molten Salts for Solar Thermal Energy Systems. Sol. Energy Mater. 1990, 21, 51–60. [Google Scholar] [CrossRef]
- Raade, J.W.; Padowitz, D. Development of Molten Salt Heat Transfer Fluid With Low Melting Point and High Thermal Stability. J. Sol. Energy Eng. 2011, 133, 031013. [Google Scholar] [CrossRef]
- Li, X.; Xie, L. Experimental Investigation and Thermodynamic Modeling of the LiNO3-RbNO3-AgNO3 System and Its Subsystems. J. Alloys Compd. 2018, 736, 124–135. [Google Scholar] [CrossRef]
- Vallet, C. Phase Diagrams and Thermodynamic Properties of Some Molten Nitrate Mixtures. J. Chem. Thermodyn. 1972, 4, 105–114. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Laing, D.; Steinmann, W.-D.; Eck, M.; Kaesche, S. High-Temperature Molten Salts for Solar Power Application. In Molten Salts Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 415–438. ISBN 9780123985385. [Google Scholar]
- Ding, W.; Bauer, T. Progress in Research and Development of Molten Chloride Salt Technology for Next Generation Concentrated Solar Power Plants. Engineering 2021, 7, 334–347. [Google Scholar] [CrossRef]
- Gomez, J.C. High-Temperature Phase Change Materials (PCM) Candidates for Thermal Energy Storage (TES) Applications; NREL: Golden, CO, USA, 2011; Volume 303. [Google Scholar]
- Misra, A.K.; Whittenberger, J.D. Fluoride Salts and Container Materials for Thermal Energy Storage Applications in the Temperature Range 973–1400 K. In Proceedings of the 22nd Intersociety Energy Conversion Engineering Conference, Reston, VA, USA, 10 August 1987. [Google Scholar]
- Vidal, J.C.; Klammer, N. Molten Chloride Technology Pathway to Meet the U.S. DOE Sunshot Initiative with Gen3 CSP. In Proceedings of the AIP Conference Proceedings, Bodrum, Turkey, 25 July 2019; Volume 2126, p. 080006. [Google Scholar]
- Tripi, V.; Sau, S.; Tizzoni, A.C.; Mansi, E.; Spadoni, A.; Corsaro, N.; D’Ottavi, C.; Capocelli, M.; Licoccia, S.; Delise, T. A General Thermodynamic Model for Eutectics of Phase Change Molten Salts in Concentrating Solar Power Applications. J. Energy Storage 2021, 33, 102065. [Google Scholar] [CrossRef]
- Hosoya, Y.; Terai, T.; Yoneoka, T.; Tanaka, S. Compatibility of Structural Materials with Molten Chloride Mixture at High Temperature. J. Nucl. Mater. 1997, 248, 348–353. [Google Scholar] [CrossRef]
- Jantzen, C.A. An Investigation of Primary Circuit Materials in Molten Chloride Salts with the Design of High Temperature Corrosion Vessels; The University of Manchester: Manchester, UK, 2019. [Google Scholar]
- Kruizenga, A. Corrosion Mechanisms in Chloride and Carbonate Salts; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 2012. [Google Scholar]
- Villada, C.; Ding, W.; Bonk, A.; Bauer, T. Engineering Molten MgCl2–KCl–NaCl Salt for High-Temperature Thermal Energy Storage: Review on Salt Properties and Corrosion Control Strategies. Sol. Energy Mater. Sol. Cells 2021, 232, 111344. [Google Scholar] [CrossRef]
- Huang, Q.; Lu, G.; Wang, J.; Yu, J. Thermal Decomposition Mechanisms of MgCl2·6H2O and MgCl2·H2O. J. Anal. Appl. Pyrolysis 2011, 91, 159–164. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Z.; Lai, X.; Wang, Y.; Tang, Z. Optimum Design and Key Thermal Property of NaCl–KCl–CaCl2 Eutectic Salt for Ultra-High-Temperature Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2022, 236, 111541. [Google Scholar] [CrossRef]
- Kruizenga, A.M.; Andraka, C.E.; Kolb, W.J. Molten Salt Technology; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 2016. [Google Scholar]
- Mahboob, K.; Khan, A.A.; Khan, M.A.; Sarwar, J.; Khan, T.A. Comparison of Li2CO3-Na2CO3-K2CO3, KCl-MgCl2 and NaNO3-KNO3 as Heat Transfer Fluid for Different SCO2 and Steam Power Cycles in CSP Tower Plant under Different DNI Conditions. Adv. Mech. Eng. 2021, 13, 168781402110119. [Google Scholar] [CrossRef]
- Prieto, C.; Fereres, S.; Ruiz-Cabañas, F.J.; Rodriguez-Sanchez, A.; Montero, C. Carbonate Molten Salt Solar Thermal Pilot Facility: Plant Design, Commissioning and Operation up to 700 °C. Renew. Energy 2020, 151, 528–541. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Du, Z.; Jiang, F.; Ding, Y. A Review of Performance Investigation and Enhancement of Shell and Tube Thermal Energy Storage Device Containing Molten Salt Based Phase Change Materials for Medium and High Temperature Applications. Appl. Energy 2019, 255, 113806. [Google Scholar] [CrossRef]
- Sau, G.S.; Tripi, V.; Tizzoni, A.C.; Liberatore, R.; Mansi, E.; Spadoni, A.; Corsaro, N.; Capocelli, M.; Delise, T.; Della Libera, A. High-Temperature Chloride-Carbonate Phase Change Material: Thermal Performances and Modelling of a Packed Bed Storage System for Concentrating Solar Power Plants. Energies 2021, 14, 5339. [Google Scholar] [CrossRef]
- Dunlop, T.O.; Jarvis, D.J.; Voice, W.E.; Sullivan, J.H. Stabilization of Molten Salt Materials Using Metal Chlorides for Solar Thermal Storage. Sci. Rep. 2018, 8, 8190. [Google Scholar] [CrossRef]
- Zavoico, A.B. Solar Power Tower Design Basis Document, Revision 0; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 2001. [Google Scholar]
- Boerema, N.; Morrison, G.; Taylor, R.; Rosengarten, G. Liquid Sodium versus Hitec as a Heat Transfer Fluid in Solar Thermal Central Receiver Systems. Sol. Energy 2012, 86, 2293–2305. [Google Scholar] [CrossRef]
- Serrano-López, R.; Fradera, J.; Cuesta-López, S. Molten Salts Database for Energy Applications. Chem. Eng. Process. Process Intensif. 2013, 73, 87–102. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wu, Z.G. Thermal Property Characterization of a Low Melting-Temperature Ternary Nitrate Salt Mixture for Thermal Energy Storage Systems. Sol. Energy Mater. Sol. Cells 2011, 95, 3341–3346. [Google Scholar] [CrossRef]
- Siegel, N.P.; Bradshaw, R.W.; Cordaro, J.B.; Kruizenga, A.M. Thermophysical Property Measurement of Nitrate Salt Heat Transfer Fluids. In Proceedings of the ASME 2011 5th International Conference on Energy Sustainability, Parts A, B and C, Washington, DC, USA, 7–10 August 2011; pp. 439–446. [Google Scholar]
- Wang, T.; Mantha, D.; Reddy, R.G. Thermal Stability of the Eutectic Composition in LiNO3–NaNO3–KNO3 Ternary System Used for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2012, 100, 162–168. [Google Scholar] [CrossRef]
- Coscia, K.; Nelle, S.; Elliott, T.; Mohapatra, S.; Oztekin, A.; Neti, S. Thermophysical Properties of LiNO3–NaNO3–KNO3 Mixtures for Use in Concentrated Solar Power. J. Sol. Energy Eng. 2013, 135, 034506. [Google Scholar] [CrossRef]
- Roget, F.; Favotto, C.; Rogez, J. Study of the KNO3–LiNO3 and KNO3–NaNO3–LiNO3 Eutectics as Phase Change Materials for Thermal Storage in a Low-Temperature Solar Power Plant. Sol. Energy 2013, 95, 155–169. [Google Scholar] [CrossRef]
- Peng, Q.; Ding, J.; Wei, X.; Jiang, G. Thermodynamic Investigation of the Eutectic Mixture of the LiNO3–NaNO3–KNO3 Ca(NO3)2 System. Int. J. Thermophys. 2017, 38, 142. [Google Scholar] [CrossRef]
- Jiang, Z.; Leng, G.; Ye, F.; Ge, Z.; Liu, C.; Wang, L.; Huang, Y.; Ding, Y. Form-Stable LiNO3–NaNO3–KNO3–Ca(NO3)2/Calcium Silicate Composite Phase Change Material (PCM) for Mid-Low Temperature Thermal Energy Storage. Energy Convers. Manag. 2015, 106, 165–172. [Google Scholar] [CrossRef]
- Cordaro, J.G.; Rubin, N.C.; Bradshaw, R.W. Multicomponent Molten Salt Mixtures Based on Nitrate/Nitrite Anions. J. Sol. Energy Eng. 2011, 133, 011014. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Novel Low Melting Point Quaternary Eutectic System for Solar Thermal Energy Storage. Appl. Energy 2013, 102, 1422–1429. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, X.; Li, P.; Hao, Q.; Xiao, B. Survey and Evaluation of Equations for Thermophysical Properties of Binary/Ternary Eutectic Salts from NaCl, KCl, MgCl2, CaCl2, ZnCl2 for Heat Transfer and Thermal Storage Fluids in CSP. Sol. Energy 2017, 152, 57–79. [Google Scholar] [CrossRef]
- Li, P.; Molina, E.; Wang, K.; Xu, X.; Dehghani, G.; Kohli, A.; Hao, Q.; Kassaee, M.H.; Jeter, S.M.; Teja, A.S. Thermal and Transport Properties of NaCl–KCl–ZnCl2 Eutectic Salts for New Generation High-Temperature Heat-Transfer Fluids. J. Sol. Energy Eng. 2016, 138, 054501. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Li, P.; Li, Y.; Hao, Q.; Xiao, B.; Elsentriecy, H.; Gervasio, D. Experimental Test of Properties of KCl–MgCl2 Eutectic Molten Salt for Heat Transfer and Thermal Storage Fluid in Concentrated Solar Power Systems. J. Sol. Energy Eng. 2018, 140, 051011. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Wang, W.; Ding, J.; Yang, J. Design and Thermal Properties of a Novel Ternary Chloride Eutectics for High-Temperature Solar Energy Storage. Appl. Energy 2015, 156, 306–310. [Google Scholar] [CrossRef]
- Du, L.; Tian, H.; Wang, W.; Ding, J.; Wei, X.; Song, M. Thermal Stability of the Eutectic Composition in NaCl-CaCl2-MgCl2 Ternary System Used for Thermal Energy Storage Applications. Energy Procedia 2017, 105, 4185–4191. [Google Scholar] [CrossRef]
- Du, L.; Ding, J.; Tian, H.; Wang, W.; Wei, X.; Song, M. Thermal Properties and Thermal Stability of the Ternary Eutectic Salt NaCl-CaCl2-MgCl2 Used in High-Temperature Thermal Energy Storage Process. Appl. Energy 2017, 204, 1225–1230. [Google Scholar] [CrossRef]
- Xu, X.; Dehghani, G.; Ning, J.; Li, P. Basic Properties of Eutectic Chloride Salts NaCl-KCl-ZnCl2 and NaCl-KCl-MgCl2 as HTFs and Thermal Storage Media Measured Using Simultaneous DSC-TGA. Sol. Energy 2018, 162, 431–441. [Google Scholar] [CrossRef]
- Forsberg, C.W.; Peterson, P.F.; Zhao, H. High-Temperature Liquid-Fluoride-Salt Closed-Brayton-Cycle Solar Power Towers. J. Sol. Energy Eng. 2007, 129, 141–146. [Google Scholar] [CrossRef]
- Williams, D.F.; Toth, L.M.; Clarno, K.T. Assessment of Candidate Molten Salt Coolants for the Advanced High-Temperature Reactor (AHTR); Brookhaven National Laboratory: Appleton, NY, USA, 2006. [Google Scholar]
- Jerden, J. Molten Salt Thermophysical Properties Database Development: 2019 Update Chemical and Fuel Cycle Technologies Division; Argonne National Laboratory: Argonne, IL, USA, 2019. [Google Scholar]
- An, X.-H.; Cheng, J.-H.; Su, T.; Zhang, P. Determination of Thermal Physical Properties of Alkali Fluoride/Carbonate Eutectic Molten Salt. In Proceedings of the AIP Conference Proceedings, Abu Dhab, United Arab Emirates, 27 June 2017; Volume 1850, p. 070001. [Google Scholar]
- Giaconia, A.; Tizzoni, A.C.; Sau, S.; Corsaro, N.; Mansi, E.; Spadoni, A.; Delise, T. Assessment and Perspectives of Heat Transfer Fluids for CSP Applications. Energies 2021, 14, 7486. [Google Scholar] [CrossRef]
- Delise, T.; Tizzoni, A.C.C.; Menale, C.; Telling, M.T.F.; Bubbico, R.; Crescenzi, T.; Corsaro, N.; Sau, S.; Licoccia, S. Technical and Economic Analysis of a CSP Plant Presenting a Low Freezing Ternary Mixture as Storage and Transfer Fluid. Appl. Energy 2020, 265, 114676. [Google Scholar] [CrossRef]
- Villada, C.; Bonk, A.; Bauer, T.; Bolívar, F. High-Temperature Stability of Nitrate/Nitrite Molten Salt Mixtures under Different Atmospheres. Appl. Energy 2018, 226, 107–115. [Google Scholar] [CrossRef]
- Kruizenga, A.; Cordaro, J.G. Preliminary Development of Thermal Stability Criterion for Alkali Nitrates; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 2011. [Google Scholar]
- Freeman, E.S. The Kinetics of the Thermal Decomposition of Potassium Nitrate and of the Reaction between Potassium Nitrite and Oxygen. J. Am. Chem. Soc. 1957, 79, 838–842. [Google Scholar] [CrossRef]
- Freeman, E.S. The Kinetics of the Thermal Decomposition of Sodium Nitrate and of the Reaction between Sodium Nitrite and Oxygen. J. Phys. Chem. 1956, 60, 1487–1493. [Google Scholar] [CrossRef]
- Steinbrecher, J.; Braun, M.; Bauer, T.; Kunkel, S.; Bonk, A. Solar Salt above 600 °C: Impact of Experimental Design on Thermodynamic Stability Results. Energies 2023, 16, 5241. [Google Scholar] [CrossRef]
- Steinbrecher, J.; Hanke, A.; Braun, M.; Bauer, T.; Bonk, A. Stabilization of Solar Salt at 650 °C—Thermodynamics and Practical Implications for Thermal Energy Storage Systems. Sol. Energy Mater. Sol. Cells 2023, 258, 112411. [Google Scholar] [CrossRef]
- Kust, R.N.; Burke, J.D. Thermal Decomposition in Alkali Metal Nitrate Melts. Inorg. Nucl. Chem. Lett. 1970, 6, 333–335. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Ni, Y.; Wu, A.; Li, J. Investigation on Static and Dynamic Corrosion Behaviors of Thermal Energy Transfer and Storage System Materials by Molten Salts in Concentrating Solar Power Plants. Mater. Corros. 2019, 70, 102–109. [Google Scholar] [CrossRef]
- International Renewable Energy Agency Renewable Energy Statistics 2018; IRENA: Masdar, United Arab Emirates, 2018.
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Fuel Cells and Hydrogen Joint Undertaking Development of Water Electrolysis in the European Union Final Report; New Energy World: London, UK, 2014; pp. 68–69. [Google Scholar]
- Sattler, C.; Monnerie, N.; Houaijia, A.; Romero, M.; Aguilar, J.G.; Miguel, A.; Reyes, L.; Turchetti, L.; Giaconia, A.; Mazzei, D.; et al. Scientific and Technological Alliance for Guaranteeing the European Excellence in Concentrating Solar Thermal Energy D9.4: Final Report on “Technology Roadmap for Solar Fuels”; EERA: Berlin, Germany, 2014. [Google Scholar]
- Compact Multifuel-Energy to Hydrogen Converter | CoMETHy | Project | News & Multimedia | FP7 | CORDIS | European Commission. Available online: https://cordis.europa.eu/project/id/279075 (accessed on 6 June 2024).
- Fuel Cells and Hydrogen Joint Undertaking (Fch Ju) Multi-Annual Work Plan; New Energy World: London, UK, 2014.
- Fujiwara, S.; Kasai, S.; Yamauchi, H.; Yamada, K.; Makino, S.; Matsunaga, K.; Yoshino, M.; Kameda, T.; Ogawa, T.; Momma, S.; et al. Hydrogen Production by High Temperature Electrolysis with Nuclear Reactor. Prog. Nucl. Energy 2008, 50, 422–426. [Google Scholar] [CrossRef]
- Sunfire Electrolyzers-Sunfire. Available online: https://www.sunfire.de/en/hydrogen (accessed on 10 June 2024).
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging Electrochemical Energy Conversion and Storage Technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Dincer, I. A Review and Comparative Evaluation of Thermochemical Water Splitting Cycles for Hydrogen Production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Liberatore, R.; Caputo, G.; Felici, C.; Spadona, A. Demonstration of Hydrogen Production by the Sulphur-Iodine Cycle: Realization of a 10 NL/h Plant. In Proceedings of the 18th World Hydrogen Energy Conference 2010, WHEC 2010 Proceedings, Essen, Germany, 16–21 May 2010; Volume 2, pp. 295–300. [Google Scholar]
- Liberatore, R.; Lanchi, M.; Giaconia, A.; Tarquini, P. Energy and Economic Assessment of an Industrial Plant for the Hydrogen Production by Water-Splitting through the Sulfur-Iodine Thermochemical Cycle Powered by Concentrated Solar Energy. Int. J. Hydrogen Energy 2012, 37, 9550–9565. [Google Scholar] [CrossRef]
- Prosini, P.P.; Cento, C.; Giaconia, A.; Caputo, G.; Sau, S. A Modified Sulphur-Iodine Cycle for Efficient Solar Hydrogen Production. Int. J. Hydrogen Energy 2009, 34, 1218–1225. [Google Scholar] [CrossRef]
- Lanchi, M.; Varsano, F.; Brunetti, B.; Murmura, M.A.; Annesini, M.C.; Turchetti, L.; Grena, R. Thermal Characterization of a Cavity Receiver for Hydrogen Production by Thermochemical Cycles Operating at Moderate Temperatures. Sol. Energy 2013, 92, 256–268. [Google Scholar] [CrossRef]
- Parisi, M.; Giaconia, A.; Sau, S.; Spadoni, A.; Caputo, G.; Tarquini, P. Bunsen Reaction and Hydriodic Phase Purification in the Sulfur–Iodine Process: An Experimental Investigation. Int. J. Hydrogen Energy 2011, 36, 2007–2013. [Google Scholar] [CrossRef]
- Liberatore, R.; Cerioli, A.; Lanchi, M.; Spadoni, A.; Tarquini, P. Experimental Vapour–Liquid Equilibrium Data of HI–H2O–I2 Mixtures for Hydrogen Production by Sulphur–Iodine Thermochemical Cycle. Int. J. Hydrogen Energy 2008, 33, 4283–4290. [Google Scholar] [CrossRef]
- Barbarossa, V.; Brutti, S.; Diamanti, M.; Sau, S.; Demeria, G. Catalytic Thermal Decomposition of Sulphuric Acid in Sulphur–Iodine Cycle for Hydrogen Production. Int. J. Hydrogen Energy 2006, 31, 883–890. [Google Scholar] [CrossRef]
- Giaconia, A.; Sau, S.; Felici, C.; Tarquini, P.; Karagiannakis, G.; Pagkoura, C.; Agrafiotis, C.; Konstandopoulos, A.G.; Thomey, D.; De Oliveira, L.; et al. Hydrogen Production via Sulfur-Based Thermochemical Cycles: Part 2: Performance Evaluation of Fe2O3-Based Catalysts for the Sulfuric Acid Decomposition Step. Int. J. Hydrogen Energy 2011, 36, 6496–6509. [Google Scholar] [CrossRef]
- Favuzza, P.; Felici, C.; Lanchi, M.; Liberatore, R.; Mazzocchia, C.V.; Spadoni, A.; Tarquini, P.; Tito, A.C. Decomposition of Hydrogen Iodide in the S–I Thermochemical Cycle over Ni Catalyst Systems. Int. J. Hydrogen Energy 2009, 34, 4049–4056. [Google Scholar] [CrossRef]
- Lanchi, M.; Caputo, G.; Liberatore, R.; Marrelli, L.; Sau, S.; Spadoni, A.; Tarquini, P.; Sau, G.S.; SPADONI, A.; Tarquini, P. Use of Metallic Ni for H2 Production in S–I Thermochemical Cycle: Experimental and Theoretical Analysis. Int. J. Hydrogen Energy 2009, 34, 1200–1207. [Google Scholar] [CrossRef]
- Varsano, F.; Padella, F.; Alvani, C.; Bellusci, M.; La Barbera, A. Chemical Aspects of the Water-Splitting Thermochemical Cycle Based on Sodium Manganese Ferrite. Int. J. Hydrogen Energy 2012, 37, 11595–11601. [Google Scholar] [CrossRef]
- Liberatore, R.; Bassi, A.; Turchetti, L.; Venturin, M. Multi-Objective Optimization of a Hydrogen Production through the HyS Process Powered by Solar Energy in Different Scenarios. Int. J. Hydrogen Energy 2018, 43, 8683–8697. [Google Scholar] [CrossRef]
- Liberatore, R.; Lanchi, M.; Turchetti, L. Hydrogen Production by the Solar-Powered Hybrid Sulfur Process: Analysis of the Integration of the CSP and Chemical Plants in Selected Scenarios. In Proceedings of the AIP Conference Proceedings, Bodrum, Turkey, 25 July 2019; Volume 1734, p. 120006. [Google Scholar]
- Turchetti, L.; Liberatore, R.; Sau, S.; Tizzoni, A.C. Carbon-Free Production of Hydrogen via the Solar Powered Hybrid Sulfur Cycle: The SOL2HY2 Project. Chem. Eng. Trans. 2015, 43, 2179–2184. [Google Scholar] [CrossRef]
- Tizzoni, A.C.; Corsaro, N.; D’Ottavi, C.; Licoccia, S.; Sau, S.; Tarquini, P. Oxygen Production by Intermediate Metal Sulphates in Sulphur Based Thermochemical Water Splitting Cycles. Int. J. Hydrogen Energy 2015, 40, 4065–4083. [Google Scholar] [CrossRef]
- Taylor, R.; Davenport, R.; Talbot, J.; Herz, R.; Luc, W.; Genders, D.; Symons, P.; Brown, L. Status of the Solar Sulfur Ammonia Thermochemical Hydrogen Production System for Splitting Water. Energy Procedia 2014, 49, 2047–2058. [Google Scholar] [CrossRef]
- Navarro, R.M.; Peña, M.A.; Fierro, J.L.G. Hydrogen Production Reactions from Carbon Feedstocks: Fossil Fuels and Biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef]
- Giaconia, A.; Turchetti, L.; Monteleone, G.; Morico, B.; Iaquaniello, G.; Shabtai, K.; Sheintuch, M.; Boettge, D.; Adler, J.; Palma, V.; et al. Development of a Solar-Powered, Fuel-Flexible Compact Steam Reformer: The CoMETHy Project. Chem. Eng. Trans. 2013, 35, 433–438. [Google Scholar]
- Krishna, B.B.; Biswas, B.; Bhaskar, T. Gasification of Lignocellulosic Biomass. In Biomass, Biofuels, Biochemicals: Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Academic Press: Cambridge, MA, USA, 2019; pp. 285–300. ISBN 9780128168561. [Google Scholar]
- Yan, M.; Afxentiou, N.; Fokaides, P.A. The State of the Art Overview of the Biomass Gasification Technology. Curr. Sustain. Energy Rep. 2021, 8, 282–295. [Google Scholar] [CrossRef]
- Jha, S.; Okolie, J.A.; Nanda, S.; Dalai, A.K. A Review of Biomass Resources and Thermochemical Conversion Technologies. Chem. Eng. Technol. 2022, 45, 791–799. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Blasiak, W. Energy and Exergy Analysis of High Temperature Agent Gasification of Biomass. Energies 2014, 7, 2107–2122. [Google Scholar] [CrossRef]
- Liu, B.; Ji, S. Comparative Study of Fluidized-Bed and Fixed-Bed Reactor for Syngas Methanation over Ni-W/TiO2-SiO2 Catalyst. J. Energy Chem. 2013, 22, 740–746. [Google Scholar] [CrossRef]
- Li, M.; Luo, N.; Lu, Y. Biomass Energy Technological Paradigm (BETP): Trends in This Sector. Sustainability 2017, 9, 567. [Google Scholar] [CrossRef]
- Vivoli, F.P.; Benanti, E. Energia Dalle Biomasse: Tecnologie e Prospettive; Regione Siciliana Assessorato Industria: Palermo, Italy, 2008; ISBN 8882861716. [Google Scholar]
- Mandl, C.; Obrenberger, I.; Biedermann, F. Updraft-Fixed Bed Gasification of Softwood Bellets: Mathematical Modelling and Comparison with Experimental Data. In Proceedings of the 17th European Biomass Conference and Exhibition, Hamburg, Germany, 29 June–3 July 2009; pp. 1–9. [Google Scholar]
- Kruesi, M.; Jovanovic, Z.R.; Steinfeld, A. A Two-Zone Solar-Driven Gasifier Concept: Reactor Design and Experimental Evaluation with Bagasse Particles. Fuel 2014, 117, 680–687. [Google Scholar] [CrossRef]
- Mendonça, M.; Mantilla, V.; Patela, J.; Silva, V.; Resende, F. Design and Experimental Tests of an Imbert Type Downdraft Gasifier Prototype and Clean-up System for Small-Scale Biomass-Based Power Generation. Renew. Energy Environ. Sustain. 2022, 7, 10. [Google Scholar] [CrossRef]
- Pirolisi e Pirogassificazione Delle Biomasse-FitoGen-Università Di Cagliari. Available online: https://sites.unica.it/fitogen/pirolisi-e-pirogassificazione-delle-biomasse/ (accessed on 10 June 2024).
- Rodriguez Correa, C.; Kruse, A. Supercritical Water Gasification of Biomass for Hydrogen Production—Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Biomass for Hydrogen Production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Pandey, A.; Larroche, C.; Ricke, S.; Dussap, C.G.; Gnansounou, E. Biofuels; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A Review of Catalytic Issues and Process Conditions for Renewable Hydrogen and Alkanes by Aqueous-Phase Reforming of Oxygenated Hydrocarbons over Supported Metal Catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Osada, M.; Sato, T.; Watanabe, M.; Shirai, M.; Arai, K. Catalytic Gasification of Wood Biomass in Subcritical and Supercritical Water. Combust. Sci. Technol. 2006, 178, 537–552. [Google Scholar] [CrossRef]
- Elliott, D.C. Catalytic Hydrothermal Gasification of Biomass. Biofuels Bioprod. Biorefin. 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.R.A.; Prins, W.; Van Swaaij, W.P.M.; Van De Beld, B.; Elliott, D.C.; Neuenschwander, G.G.; Kruse, A.; et al. Biomass Gasification in Near- and Super-Critical Water: Status and Prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Chuntanapum, A.; Matsumura, Y. Char Formation Mechanism in Supercritical Water Gasification Process: A Study of Model Compounds. Ind. Eng. Chem. Res. 2010, 49, 4055–4062. [Google Scholar] [CrossRef]
- Wang, A.G.; Austin, D.; Song, H. Catalytic Biomass Valorization. In Biomass Volume Estimation and Valorization for Energy; InTech: Singapore, 2017; ISBN 978-953-51-2938-7. [Google Scholar]
- Kim, J.S.; Choi, G.G. Pyrolysis of Lignocellulosic Biomass for Biochemical Production. In Waste Biorefinery Potential Perspect; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–348. [Google Scholar] [CrossRef]
- Müller, F.L. Solar Reactor Development for Thermochemical Gasification and Calcination Processes. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2018. [Google Scholar] [CrossRef]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-Economic Analysis of Biomass Fast Pyrolysis to Transportation Fuels. Fuel 2010, 89, S2–S10. [Google Scholar] [CrossRef]
- Seo, M.W.; Lee, S.H.; Nam, H.; Lee, D.; Tokmurzin, D.; Wang, S.; Park, Y.K. Recent Advances of Thermochemical Conversion Processes for Biorefinery. Bioresour. Technol. 2022, 343, 126109. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Response to Comment on “Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy”. Ind. Eng. Chem. Res. 2021, 60, 17795–17796. [Google Scholar] [CrossRef]
- Devi, M.; Rawat, S. A Comprehensive Review of the Pyrolysis Process: From Carbon Nanomaterial Synthesis to Waste Treatment. Oxford Open Mater. Sci. 2021, 1, itab014. [Google Scholar] [CrossRef]
- Brown, R.C. The Role of Pyrolysis and Gasification in a Carbon Negative Economy. Processes 2021, 9, 882. [Google Scholar] [CrossRef]
- Korányi, T.I.; Németh, M.; Beck, A.; Horváth, A. Recent Advances in Methane Pyrolysis: Turquoise Hydrogen with Solid Carbon Production. Energies 2022, 15, 6342. [Google Scholar] [CrossRef]
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic Decomposition of Methane to Produce Hydrogen: A Review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- McFarland, E. Molten-Salt Methane Pyrolysis Optimization Through in-Situ Carbon Characterization and Reactor Design. Available online: https://arpa-e.energy.gov/programs-and-initiatives/search-all-projects/molten-salt-methane-pyrolysis-optimization-through-situ-carbon-characterization-and-reactor-design (accessed on 6 June 2024).
- Bhardwaj, R.; Frens, W.; Linders, M.; Goetheer, E. Ember Pyrolysis Technology for Hydrogen and Carbon. Available online: https://www.aiche.nl/images/presentations/EMBER_Pyrolysis-technology-for-production-of-Hydrogen-and-carbon.pdf (accessed on 6 June 2024).
- Giaconia, A.; Caputo, G.; Ienna, A.; Mazzei, D.; Schiavo, B.; Scialdone, O.; Galia, A. Biorefinery Process for Hydrothermal Liquefaction of Microalgae Powered by a Concentrating Solar Plant: A Conceptual Study. Appl. Energy 2017, 208, 1139–1149. [Google Scholar] [CrossRef]
- Raikova, S.; Le, C.D.; Wagner, J.L.; Ting, V.P.; Chuck, C.J. Towards an Aviation Fuel Through the Hydrothermal Liquefaction of Algae. In Biofuels for Aviation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 217–239. ISBN 9780128045688. [Google Scholar]
- NIST. Thermophysical Properties of Fluid Systems. Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 11 April 2025).
- Tzanetis, K.F.; Posada, J.A.; Ramirez, A. Analysis of Biomass Hydrothermal Liquefaction and Biocrude-Oil Upgrading for Renewable Jet Fuel Production: The Impact of Reaction Conditions on Production Costs and GHG Emissions Performance. Renew. Energy 2017, 113, 1388–1398. [Google Scholar] [CrossRef]
- Collares-Pereira, M.; Canavarro, D.; Guerreiro, L.L. Advances in Concentrating Solar Thermal Research and Technology: 15.4 Advanced LFR and Molten Salts: A New Concept Plant, 1st ed.; Blanco, M.J., Santigosa, L.R., Eds.; Woodhead Publishing: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany; London, UK; New York, NY, USA; Oxford, UK; Paris, France; San Diego, CA, USA; San Francisco, CA, USA; Singapore, 2017; ISBN 9780081005163. [Google Scholar]
- Giaconia, A.; De Falco, M.; Caputo, G.; Grena, R.; Tarquini, P.; Marrelli, L. Solar Steam Reforming of Natural Gas for Hydrogen Production Using Molten Salt Heat Carriers. AIChE J. 2008, 54, 1932–1944. [Google Scholar] [CrossRef]
- Giaconia, A.; Iaquaniello, G.; Caputo, G.; Morico, B.; Salladini, A.; Turchetti, L.; Monteleone, G.; Giannini, A.; Palo, E. Experimental Validation of a Pilot Membrane Reactor for Hydrogen Production by Solar Steam Reforming of Methane at Maximum 550 °C Using Molten Salts as Heat Transfer Fluid. Int. J. Hydrogen Energy 2020, 45, 33088–33101. [Google Scholar] [CrossRef]
- Sanz-Bermejo, J.; Muñoz-Antón, J.; Gonzalez-Aguilar, J.; Romero, M. Optimal Integration of a Solid-Oxide Electrolyser Cell into a Direct Steam Generation Solar Tower Plant for Zero-Emission Hydrogen Production. Appl. Energy 2014, 131, 238–247. [Google Scholar] [CrossRef]
- Monforti Ferrario, A.; Santoni, F.; Della Pietra, M.; Rossi, M.; Piacente, N.; Comodi, G.; Simonetti, L. A System Integration Analysis of a Molten Carbonate Electrolysis Cell as an Off-Gas Recovery System in a Steam-Reforming Process of an Oil Refinery. Front. Energy Res. 2021, 9, 655915. [Google Scholar] [CrossRef]
- Frangini, S.; Della Pietra, M.; Della Seta, L.; Paoletti, C.; Pedro Pérez-Trujillo, J. Degradation of MCFC Materials in a 81 Cm2 Single Cell Operated Under Alternated Fuel Cell/Electrolysis Mode. Front. Energy Res. 2021, 9, 653531. [Google Scholar] [CrossRef]
- Roeb, M.; Thomey, D.; de Oliveira, L.; Sattler, C.; Fleury, G.; Pra, F.; Tochon, P.; Brevet, A.; Roux, G.; Gruet, N.; et al. Sulphur Based Thermochemical Cycles: Development and Assessment of Key Components of the Process. Int. J. Hydrogen Energy 2013, 38, 6197–6204. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Corgnale, C.; Summers, W.A. Development of the Hybrid Sulfur Cycle for Use with Concentrated Solar Heat. I. Conceptual design. Int. J. Hydrogen Energy 2017, 42, 20939–20954. [Google Scholar] [CrossRef]
- Norman, J.; Mysels, K.; Sharp, R.; Williamson, D. Studies of the Sulfur-Iodine Thermochemical Water-Splitting Cycle. Int. J. Hydrogen Energy 1982, 7, 545–556. [Google Scholar] [CrossRef]
- Kalyva, A.E.; Vagia, E.C.; Konstandopoulos, A.G.; Srinivasa, A.R.; T-Raissi, A.; Muradov, N.; Kakosimos, K.E. Hybrid Photo-Thermal Sulfur-Ammonia Water Splitting Cycle: Thermodynamic Analysis of the Thermochemical Steps. Int. J. Hydrogen Energy 2017, 42, 9533–9544. [Google Scholar] [CrossRef]
- Tizzoni, A.C.; Mansi, E.; Sau, S.; Spadoni, A.; Corsaro, N.; Lanchi, M.; Giorgi, G.; Turchetti, L.; Delise, T. Thermochemical Cycle Based on Solid Intermediates for Hydrogen Storage and On-Demand Production. E3S Web Conf. 2022, 334, 01006. [Google Scholar] [CrossRef]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef]
- Padella, F.; Alvani, C.; La Barbera, A.; Ennas, G.; Liberatore, R.; Varsano, F. Mechanosynthesis and Process Characterization of Nanostructured Manganese Ferrite. Mater. Chem. Phys. 2005, 90, 172–177. [Google Scholar] [CrossRef]
- Sakurai, M.; Bilgen, E.; Tsutsumi, A.; Yoshida, K. Solar UT-3 Thermochemical Cycle for Hydrogen Production. Sol. Energy 1996, 57, 51–58. [Google Scholar] [CrossRef]
- Doctor, R.D.; Marshall, C.L.; Wade, D.C. Hydrogen Cycle Employing Calcium-Bromine and Electrolysis; Argonne National Laboratory: Argonne, IL, USA, 2002. [Google Scholar]
- Naterer, G.F.; Suppiah, S.; Stolberg, L.; Lewis, M.; Wang, Z.; Rosen, M.A.; Dincer, I.; Gabriel, K.; Odukoya, A.; Secnik, E.; et al. Progress in Thermochemical Hydrogen Production with the Copper–Chlorine Cycle. Int. J. Hydrogen Energy 2015, 40, 6283–6295. [Google Scholar] [CrossRef]
- Angeli, S.D.; Turchetti, L.; Monteleone, G.; Lemonidou, A.A. Catalyst Development for Steam Reforming of Methane and Model Biogas at Low Temperature. Appl. Catal. B Environ. 2016, 181, 34–46. [Google Scholar] [CrossRef]
- Turchetti, L.; Murmura, M.A.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A.; Angeli, S.D.; Palma, V.; Ruocco, C.; Annesini, M.C. Kinetic Assessment of Ni-Based Catalysts in Low-Temperature Methane/Biogas Steam Reforming. Int. J. Hydrogen Energy 2016, 41, 16865–16877. [Google Scholar] [CrossRef]
- Integrating National Research Agendas on Solar Heat for Industrial Processes | INSHIP | Project | Fact Sheet | H2020 | CORDIS | European Commission. Available online: https://cordis.europa.eu/project/id/731287 (accessed on 6 June 2024).
- Zhang, Y.; Liang, Y.; Li, S.; Yuan, Y.; Zhang, D.; Wu, Y.; Xie, H.; Brindhadevi, K.; Pugazhendhi, A.; Xia, C. A Review of Biomass Pyrolysis Gas: Forming Mechanisms, Influencing Parameters, and Product Application Upgrades. Fuel 2023, 347, 128461. [Google Scholar] [CrossRef]
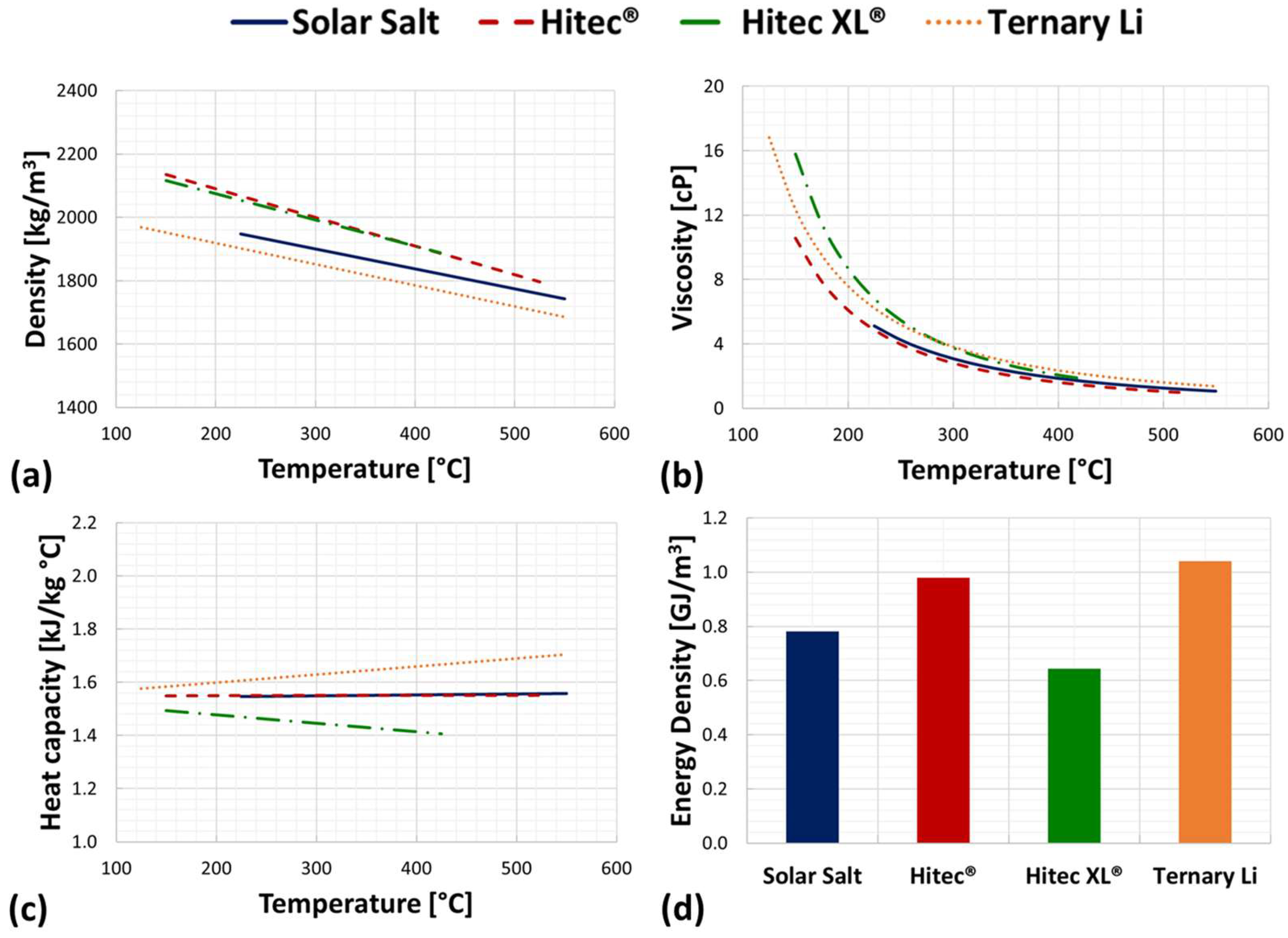


| Mixture [-] | Type of Mixture [-] | Composition [%wt.] | Tmelt [°C] | Tdeco [°C] | [kg m−3] | [J kg−1 °C−1] | Refs. |
|---|---|---|---|---|---|---|---|
| Nitrate-based | |||||||
| Solar salt | Binary | 60 NaNO3 40 KNO3 | 240 | 565 | 1834 | 1512 | [24,49] |
| Hitec® | Ternary | 7 NaNO3 53 KNO3 40 NaNO2 | 142 | 450 | 1721 | 1560 | [28,50,51] |
| Hitec XL® | Ternary | 15 NaNO3 43 KNO3 42 Ca (NO3)2 | 130 | 450 | 2000 | 1449 | [27,52,53] |
| LiNaK//NO3 | Ternary | 30 LiNO3 18 NaNO3 52 KNO3 | 118 | 550 | 1884 | 1580 | [54,55,56] |
| LiNaKCa/NO3 | Quaternary | 15.5 LiNO3 8.2 NaNO3 54.3 KNO3 22 Ca (NO3)2 | 93 | 450 | 1803 | 1518 | [57,58,59] |
| LiNaKNO3NO2 | Quaternary | 9 LiNO3 42.3 NaNO3 33.6 KNO3 15.1 KNO2 | 97 | 450 | 1877 | 1155 | [60] |
| Chloride-based | |||||||
| KMgCl | Binary | 62.5 KCl 37.5 MgCl2 | 430 | >700 | 1857 | 999 | [61,62,63] |
| NaKMgCl | Ternary | 20.5 NaCl 30.9 KCl 48.6 MgCl2 | 383 | >700 | 1669 | 1024 | [35,61] |
| NaMgCaCl | Ternary | 39.6 NaCl 39 MgCl2 21.4 CaCl2 | 407 | 650 | 2557 | 1104 | [64,65,66] |
| NaKZnCl | Ternary | 7.5 NaCl 23.9 KCl 68.6 ZnCl2 | 204 | >700 | 2207 | 901 | [62,66,67] |
| KMgZnCl | Ternary | 49.4 KCl 15.5 MgCl2 35.1 ZnCl2 | 356 | >700 | 1857 | 866 | [61,62,66] |
| Fluoride-based | |||||||
| LiNaKF | Ternary | 29.2 LiF 11.7 NaF 59.1 KF | 454 | >700 | 2109 | 1590 | [68,69] |
| NaBF | Binary | 3 NaF 97 NaBF4 | 385 | >700 | 1866 | 1506 | [51] |
| KBF | Binary | 13 KF 87 KBF4 | 460 | >700 | 1792 | 1305 | [70] |
| KZrF | Binary | 32.5 KF 67.5 ZrF4 | 420 | >700 | 2680 | 1000 | [51] |
| Carbonate-based | |||||||
| LiNaKCO3 | Ternary | 32.1 Li2CO3 33.4 Na2CO3 34.5 K2CO3 | 397 | 670 | 2038 | 1610 | [71] |
| Property | Value | Unit |
|---|---|---|
| Solar Salt | ||
| Chemical composition | NaNO3/KNO3 (60/40) | %wt. |
| Density | kg m−3 | |
| Dynamic viscosity | Pa s | |
| Thermal conductivity (max. operation temperature) | W m−1 K−1 | |
| Heat capacity | kJ K−1 kg−1 | |
| Thermal stability | 600 | °C |
| Liquidus temperature | 238 | °C |
| Hitec® (Na/K nitrate/nitrite) | ||
| Chemical composition | NaNO3/KNO3/NaNO2 (7/53/40) | %wt. |
| Density | kg m−3 | |
| Dynamic viscosity | Pa s | |
| Thermal conductivity | W m−1 K−1 | |
| Heat capacity | kJ K−1 kg−1 | |
| Thermal stability (max. operation temperature) | 450 under air; 530 under inert gas | °C |
| Liquidus temperature (initial solidification point) | 141 | °C |
| Hitec XL® (Na/K/Ca nitrate) | ||
| Chemical composition | NaNO3/KNO3/Ca (NO3)2 (15/43/42) | %wt. |
| Density | kg m−3 | |
| Dynamic viscosity | Pa s | |
| Thermal conductivity | (Constant in the operating range) | W m−1 K−1 |
| Heat capacity | kJ K−1 kg−1 | |
| Thermal stability (max. operation temperature) | ≤425 | °C |
| Liquidus temperature (initial solidification point) | ~125 | °C |
| Na/K/Linitrate | ||
| Chemical composition | NaNO3/KNO3/LiNO3 (18/45/37) | %wt. |
| Density | kg m−3 | |
| Dynamic viscosity | Pa s | |
| Thermal conductivity | W m−1 K−1 | |
| Heat capacity | kJ K−1 kg−1 | |
| Thermal stability (max. operation temperature) | 600 | °C |
| Liquidus temperature (initial solidification point) | 120 | °C |
| Process | No. of Steps | Reactions | Pros (+) & Cons (−) | Ref. |
|---|---|---|---|---|
| Sulfur–iodine | 3 | 2H2O + I2 + SO2 → H2SO4 + 2HI (Bunsen reaction, 20–120 °C) 2HI → I2 + H2 (300–500 °C) H2SO4 → H2O + SO2 + 1/2 O2 (800–1000 °C) |
| [91,92] |
| Modified sulfur–iodine (NIS) | 5 | 2H2O + I2 + SO2 → H2SO4 + 2HI (Bunsen reaction, 20–120 °C) Ni + H2SO4 → NiSO4 + H2 (20–100 °C) NiSO4 → NiO + SO2 + ½ O2 (≈900 °C) NiO + 2HI → NiI2 + H2O (≈100 °C) NiI2 → Ni + I2 (≈600 °C) |
| [93] |
| Mixed ferrites | 2 | 2MnFe2O4 (s) + 3Na2CO3 (s) +H2O → 6Na (Mn1/3Fe2/3) O2 (s) +3CO2 (g) + H2 (g) 6Na(Mn1/3Fe2/3)O2 (s) +3CO2 (g) → 2MnFe2O4 (s) + 3Na2CO3 (s) +0.5O2 |
| [94] |
| Process | No. of Steps 1 | Reactions 2 | Pros (+) & Cons (−) | Ref. |
|---|---|---|---|---|
| Westinghose process | 2 | SO2 + 2H2O → H2SO4 + H2 (elettrochemical, T < 100 °C) H2SO4 → H2O + SO2 + ½ O2 (T ≈ 850 °C) |
| [102,103] |
| Sulphur–ammonia cycle | 3 + 1 (SO2, NH3 absorption in water) | (NH4)2SO3 + H2O → (NH4)2SO4 + H2 (photocatalytic, T < 100 °C) (NH4)2SO4 → 2 NH3 + H2O + SO3 (T ≈ 400–500 °C) SO3 → SO2 + ½ O2 (T ≈ 850 °C) SO2 + H2O + 2NH3 → (NH4)2SO3 (T < 100 °C) |
| [105,106] |
| Operating Condition | Operating Temperature | Residence Time | Main Output | Compatibility with Use of MS |
|---|---|---|---|---|
| Slow pyrolysis | 200–400 °C | 5–30 min | Biochar | Yes, if above 290 °C |
| Intermediate pyrolysis | 400–600 °C | 1–5 min | Bio-oil | Yes, if up to 565 °C |
| Fast/flash pyrolysis | 600–1000 °C | 1–2 s | Syngas | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Auria, M.; Tizzoni, A.C.; Rovense, F.; Sau, S.; Turchetti, L.; Canavarro, D.; Marchã, J.; Horta, P.; Lanchi, M. Molten Salt Mixtures as an Energy Carrier for Thermochemical Processes of Renewable Gas Production: Review and Perspectives. Appl. Sci. 2025, 15, 6916. https://doi.org/10.3390/app15126916
D’Auria M, Tizzoni AC, Rovense F, Sau S, Turchetti L, Canavarro D, Marchã J, Horta P, Lanchi M. Molten Salt Mixtures as an Energy Carrier for Thermochemical Processes of Renewable Gas Production: Review and Perspectives. Applied Sciences. 2025; 15(12):6916. https://doi.org/10.3390/app15126916
Chicago/Turabian StyleD’Auria, Marco, Anna Chiara Tizzoni, Francesco Rovense, Salvatore Sau, Luca Turchetti, Diogo Canavarro, João Marchã, Pedro Horta, and Michela Lanchi. 2025. "Molten Salt Mixtures as an Energy Carrier for Thermochemical Processes of Renewable Gas Production: Review and Perspectives" Applied Sciences 15, no. 12: 6916. https://doi.org/10.3390/app15126916
APA StyleD’Auria, M., Tizzoni, A. C., Rovense, F., Sau, S., Turchetti, L., Canavarro, D., Marchã, J., Horta, P., & Lanchi, M. (2025). Molten Salt Mixtures as an Energy Carrier for Thermochemical Processes of Renewable Gas Production: Review and Perspectives. Applied Sciences, 15(12), 6916. https://doi.org/10.3390/app15126916









