Articular Cartilage: Structure, Biomechanics, and the Potential of Conventional and Advanced Diagnostics
Abstract
1. Introduction
2. Anatomy of Articular Cartilage
2.1. Zonation and Regional Organisation
2.2. Chondrocytes
2.3. Collagens
2.4. Proteoglycans
2.5. Water
2.6. Metabolism
3. Biomechanics of Articular Cartilage
3.1. Time-Dependent Behaviour
3.2. Time-Independent Behaviour
3.3. Anisotropic, Heterogeneous and Non-Linear Behaviours
3.4. Mechanical Properties of Articular Cartilage
3.5. Lubrication Mechanisms in AC
3.6. AC Behaviour Under Cyclic Loading
4. Articular Synovial Parameters
4.1. Composition and Properties of the Synovial Fluid
4.2. Synovial Membrane
4.3. Synovial Fluid—Articular Cartilage Interactions
5. Degradation of Articular Cartilage
6. Alternative Methods of Articular Cartilage Diagnosis
6.1. MRI with Advanced Imaging Techniques
6.2. Vibroarthrography (VAG)
6.3. Ultrasound Elastography
6.4. Optical Coherence Tomography (OCT)
6.5. Modal Analysis
6.6. Raman Spectroscopy
6.7. Numerical Methods and Artificial Intelligence
7. Summary
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Nachtsheim, J.; Dursun, G.; Markert, B.; Stoffel, M. Chondrocyte Colonisation of a Tissue-Engineered Cartilage Substitute under a Mechanical Stimulus. Med. Eng. Phys. 2019, 74, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Sajewicz, E. Wprowadzenie Do Biotribologii; Oficyna Wydawnicza Politechniki Białostockiej: Białystok, Poland, 2011; ISBN 978-83-62582-13-6. [Google Scholar]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular Cartilage and Osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar]
- Weber, J.F.; Perez, R.; Waldman, S.D. Mechanobioreactors for Cartilage Tissue Engineering. In Cartilage Tissue Engineering; Doran, P.M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1340, pp. 203–219. ISBN 978-1-4939-2937-5. [Google Scholar]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef]
- Lu, X.L.; Mow, V.C. Biomechanics of Articular Cartilage and Determination of Material Properties. Med. Sci. Sports Exerc. 2008, 40, 193–199. [Google Scholar] [CrossRef]
- Chiang, H.; Jiang, C.-C. Repair of Articular Cartilage Defects: Review and Perspectives. J. Formos. Med. Assoc. 2009, 108, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ohta, Y.; Larmour, C.; Enomoto-Iwamoto, M. Toward Regeneration of Articular Cartilage. Birth Defects Res. Part C 2013, 99, 192–202. [Google Scholar] [CrossRef]
- Borrelli, J.; Olson, S.A.; Godbout, C.; Schemitsch, E.H.; Stannard, J.P.; Giannoudis, P.V. Understanding Articular Cartilage Injury and Potential Treatments. J. Orthop. Trauma 2019, 33, S6–S12. [Google Scholar] [CrossRef]
- Petitjean, N.; Canadas, P.; Royer, P.; Noël, D.; Le Floc’h, S. Cartilage Biomechanics: From the Basic Facts to the Challenges of Tissue Engineering. J. Biomed. Mater. Res. 2023, 111, 1067–1089. [Google Scholar] [CrossRef]
- Meftah, M.; Ranawat, A.S.; Ranawat, C.S. The Natural History of Anterior Knee Pain in 2 Posterior-Stabilized, Modular Total Knee Arthroplasty Designs. J. Arthroplast. 2011, 26, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Gu, W.; Chen, F.H. Structure and Function of Articular Cartilage and Meniscus. In Basic Orthopaedic Biomechanics & Mechano-Biology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Rubin, D.A.; Maas, M. Imaging of the Knee. In Musculoskeletal Diseases 2013–2016: Diagnostic Imaging and Interventional Techniques; Springer: Milan, Italy, 2013; pp. 59–66. [Google Scholar]
- Mosher, T.J.; Dardzinski, B.J. Cartilage MRI T2 Relaxation Time Mapping: Overview and Applications. Semin. Musculoskelet. Radiol. 2004, 8, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Saarakkala, S.; Laasanen, M.S.; Jurvelin, J.S.; Töyräs, J. Quantitative Ultrasound Imaging Detects Degenerative Changes in Articular Cartilage Surface and Subchondral Bone. Phys. Med. Biol. 2006, 51, 5333. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Eckstein, F. Exercise and Osteoarthritis. J. Anat. 2009, 214, 197–207. [Google Scholar] [CrossRef]
- Guermazi, A.; Hayashi, D.; Eckstein, F.; Hunter, D.J.; Duryea, J.; Roemer, F.W. Imaging of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 67–105. [Google Scholar] [CrossRef]
- Nieminen, M.T.; Rieppo, J.; Töyräs, J.; Hakumäki, J.M.; Silvennoinen, J.; Hyttinen, M.M.; Helminen, H.J.; Jurvelin, J.S. T2 Relaxation Reveals Spatial Collagen Architecture in Articular Cartilage: A Comparative Quantitative MRI and Polarized Light Microscopic Study. Magn. Reson. Med. 2001, 46, 487–493. [Google Scholar] [CrossRef]
- Omoumi, P.; Mourad, C.; Ledoux, J.-B.; Hilbert, T. Morphological Assessment of Cartilage and Osteoarthritis in Clinical Practice and Research: Intermediate-Weighted Fat-Suppressed Sequences and Beyond. Skelet. Radiol. 2023, 52, 2185–2198. [Google Scholar] [CrossRef]
- Omoumi, P.; Berg, B.C.V.; Lecouvet, F.E. Value of CT Arthrography in the Assessment of Cartilage Pathology. In Cartilage Imaging; Link, T.M., Ed.; Springer: New York, NY, USA, 2011; pp. 37–48. ISBN 978-1-4419-8437-1. [Google Scholar]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Bonnin, M.; Chambat, P. (Eds.) Osteoartritis of the Knee; Approche pratique en orthopédie-traumatologie; Springer: Paris, France; Berlin, Germany, 2008; ISBN 978-2-287-74174-6. [Google Scholar]
- Befrui, N.; Elsner, J.; Flesser, A.; Huvanandana, J.; Jarrousse, O.; Le, T.N.; Müller, M.; Schulze, W.H.W.; Taing, S.; Weidert, S. Vibroarthrography for Early Detection of Knee Osteoarthritis Using Normalized Frequency Features. Med. Biol. Eng. Comput. 2018, 56, 1499–1514. [Google Scholar] [CrossRef]
- Hayashi, D.; Roemer, F.W.; Guermazi, A. Imaging of Osteoarthritis by Conventional Radiography, MR Imaging, PET–Computed Tomography, and PET–MR Imaging. PET Clin. 2019, 14, 17–29. [Google Scholar] [CrossRef]
- Richette, P.; Latourte, A. Osteoarthritis: Value of imaging and biomarkers. Rev. Prat. 2019, 69, 507–509. [Google Scholar] [PubMed]
- Iagnocco, A. Imaging the Joint in Osteoarthritis: A Place for Ultrasound? Best. Pract. Res. Clin. Rheumatol. 2010, 24, 27–38. [Google Scholar] [CrossRef]
- Grassi, W.; Salaffi, F.; Filippucci, E. Ultrasound in Rheumatology. Best. Pract. Res. Clin. Rheumatol. 2005, 19, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Muthupillai, R.; Lomas, D.J.; Rossman, P.J.; Greenleaf, J.F.; Manduca, A.; Ehman, R.L. Magnetic Resonance Elastography by Direct Visualization of Propagating Acoustic Strain Waves. Science 1995, 269, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Benjamin Ma, C.; Link, T.M.; Castillo, D.-D.; Blumenkrantz, G.; Lozano, J.; Carballido-Gamio, J.; Ries, M.; Majumdar, S. In Vivo T1ρ and T2 Mapping of Articular Cartilage in Osteoarthritis of the Knee Using 3T MRI. Osteoarthr. Cartil. 2007, 15, 789–797. [Google Scholar] [CrossRef]
- Bashir, A.; Gray, M.L.; Burstein, D. Gd-DTPA2− as a Measure of Cartilage Degradation. Magn. Reson. Med. 1996, 36, 665–673. [Google Scholar] [CrossRef]
- Doyley, M.M. Model-Based Elastography: A Survey of Approaches to the Inverse Elasticity Problem. Phys. Med. Biol. 2012, 57, R35-73. [Google Scholar] [CrossRef]
- Karpiński, R.; Prus, A.; Jonak, K.; Krakowski, P. Vibroarthrography as a Noninvasive Screening Method for Early Diagnosis of Knee Osteoarthritis: A Review of Current Research. Appl. Sci. 2024, 15, 279. [Google Scholar] [CrossRef]
- Drexler, W.; Fujimoto, J.G. Optical Coherence Tomography: Technology and Applications; Springer Science & Business Media: Berlin, Germany, 2008; ISBN 3-540-77550-1. [Google Scholar]
- Shehata, E.; Nippolainen, E.; Shaikh, R.; Ronkainen, A.-P.; Töyräs, J.; Sarin, J.K.; Afara, I.O. Raman Spectroscopy and Machine Learning Enables Estimation of Articular Cartilage Structural, Compositional, and Functional Properties. Ann. Biomed. Eng. 2023, 51, 2301–2312. [Google Scholar] [CrossRef]
- Ewins, D.J. Modal Testing: Theory, Practice and Application; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 0-86380-218-4. [Google Scholar]
- Shepherd, D.E.T.; Seedhom, B.B. Thickness of Human Articular Cartilage in Joints of the Lower Limb. Ann. Rheum. Dis. 1999, 58, 27–34. [Google Scholar] [CrossRef]
- Wayne, J.S.; Brodrick, C.W.; Mukherjee, N. Measurement of Articular Cartilage Thickness in the Articulated Knee. Ann. Biomed. Eng. 1998, 26, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kuettner, K.E.; Cole, A.A. Cartilage Degeneration in Different Human Joints. Osteoarthr. Cartil. 2005, 13, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage: Tissue Design and Chondrocyte-Matrix Interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar]
- Doran, P.M. (Ed.) Cartilage Tissue Engineering: Methods and Protocols; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1340, ISBN 978-1-4939-2937-5. [Google Scholar]
- Grässel, S.; Aszódi, A. (Eds.) Cartilage; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-29566-4. [Google Scholar]
- Xu, W.; Zhu, J.; Hu, J.; Xiao, L. Engineering the Biomechanical Microenvironment of Chondrocytes towards Articular Cartilage Tissue Engineering. Life Sci. 2022, 309, 121043. [Google Scholar] [CrossRef]
- Mansour, J.M. Biomechanics of Cartilage. Kinesiol. Mech. Pathomechanics Hum. Mov. 2003, 2, 66–79. [Google Scholar]
- Redler, I.; Mow, V.C.; Zimny, M.L.; Mansell, J. The Ultrastructure and Biomechanical Significance of the Tidemark of Articular Cartilage. Clin. Orthop. Relat. Res. 1975, 112, 357–362. [Google Scholar] [CrossRef]
- Eggli, P.S.; Herrmann, W.; Hunziker, E.B.; Schenk, R.K. Matrix Compartments in the Growth Plate of the Proximal Tibia of Rats. Anat. Rec. 1985, 211, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Mow, V.C. The Mechanical Environment of the Chondrocyte: A Biphasic Finite Element Model of Cell-Matrix Interactions in Articular Cartilage. J. Biomech. 2000, 33, 1663–1673. [Google Scholar] [CrossRef]
- Muir, H. The Chondrocyte, Architect of Cartilage. Biomechanics, Structure, Function and Molecular Biology of Cartilage Matrix Macromolecules. BioEssays 1995, 17, 1039–1048. [Google Scholar] [CrossRef]
- Szirmai, J. Aging of Connective and Skeletal Tissue. Struct. Cartil. 1969, 163–184. [Google Scholar]
- Mow, V.C.; Guo, X.E. Mechano-Electrochemical Properties Of Articular Cartilage: Their Inhomogeneities and Anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Alford, J.W.; Cole, B.J. Cartilage Restoration, Part 1: Basic Science, Historical Perspective, Patient Evaluation, and Treatment Options. Am. J. Sports Med. 2005, 33, 295–306. [Google Scholar] [CrossRef]
- Chen, H.; Tan, X.-N.; Hu, S.; Liu, R.-Q.; Peng, L.-H.; Li, Y.-M.; Wu, P. Molecular Mechanisms of Chondrocyte Proliferation and Differentiation. Front. Cell Dev. Biol. 2021, 9, 664168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tuan, R.S. Origin and Function of Cartilage Stem/Progenitor Cells in Osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 206–212. [Google Scholar] [CrossRef]
- Chijimatsu, R.; Saito, T. Mechanisms of Synovial Joint and Articular Cartilage Development. Cell. Mol. Life Sci. 2019, 76, 3939–3952. [Google Scholar] [CrossRef]
- Lane Smith, R.; Trindade, M.; Ikenoue, T.; Mohtai, M.; Das, P.; Carter, D.; Goodman, S.; Schurman, D. Effects of Shear Stress on Articular Chondrocyte Metabolism. Biorheology 2000, 37, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Bačenková, D.; Trebuňová, M.; Demeterová, J.; Živčák, J. Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 17096. [Google Scholar] [CrossRef]
- Lin, Z.; Willers, C.; Xu, J.; Zheng, M.-H. The Chondrocyte: Biology and Clinical Application. Tissue Eng. 2006, 12, 1971–1984. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A Pathway to Bone: Signaling Molecules and Transcription Factors Involved in Chondrocyte Development and Maturation. Development 2015, 142, 817–831. [Google Scholar] [CrossRef]
- Liu, C.-F.; Samsa, W.E.; Zhou, G.; Lefebvre, V. Transcriptional Control of Chondrocyte Specification and Differentiation. Semin. Cell Dev. Biol. 2017, 62, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Knoch, N.; Sims, T.; Rosshirt, N.; Richter, W. Time-dependent Contribution of BMP, FGF, IGF, and HH Signaling to the Proliferation of Mesenchymal Stroma Cells during Chondrogenesis. J. Cell. Physiol. 2018, 233, 8962–8970. [Google Scholar] [CrossRef]
- Akiyama, H.; Chaboissier, M.-C.; Martin, J.F.; Schedl, A.; De Crombrugghe, B. The Transcription Factor Sox9 Has Essential Roles in Successive Steps of the Chondrocyte Differentiation Pathway and Is Required for Expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef]
- Soetjahjo, B.; Hidayat, M.; Sujuti, H.; Fibrianto, Y. Immunohistochemistry Evaluation of TGF-Β1, SOX-9, Type II Collagen and Aggrecan in Cartilage Lesions Treated with Conditioned Medium of Umbilical Cord Mesencyhmal Stem Cells in Wistar Mice (Rattus Novergicus). J. Trop. Life Sci. 2018, 8, 21–27. [Google Scholar] [CrossRef]
- Stegen, S.; Rinaldi, G.; Loopmans, S.; Stockmans, I.; Moermans, K.; Thienpont, B.; Fendt, S.-M.; Carmeliet, P.; Carmeliet, G. Glutamine Metabolism Controls Chondrocyte Identity and Function. Dev. Cell 2020, 53, 530–544.e8. [Google Scholar] [CrossRef]
- Zhao, Q.; Eberspaecher, H.; Lefebvre, V.; De Crombrugghe, B. Parallel Expression ofSox9 andCol2a1 in Cells Undergoing Chondrogenesis. Dev. Dyn. 1997, 209, 377–386. [Google Scholar] [CrossRef]
- Akiyama, H.; Lyons, J.P.; Mori-Akiyama, Y.; Yang, X.; Zhang, R.; Zhang, Z.; Deng, J.M.; Taketo, M.M.; Nakamura, T.; Behringer, R.R.; et al. Interactions between Sox9 and β-Catenin Control Chondrocyte Differentiation. Genes Dev. 2004, 18, 1072–1087. [Google Scholar] [CrossRef]
- Chen, H.; Ghori-Javed, F.Y.; Rashid, H.; Adhami, M.D.; Serra, R.; Gutierrez, S.E.; Javed, A. Runx2 Regulates Endochondral Ossification Through Control of Chondrocyte Proliferation and Differentiation. J. Bone Miner. Res. 2014, 29, 2653–2665. [Google Scholar] [CrossRef]
- Komori, T. Roles of Runx2 in Skeletal Development. In RUNX Proteins in Development and Cancer; Groner, Y., Ito, Y., Liu, P., Neil, J.C., Speck, N.A., Van Wijnen, A., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; Volume 962, pp. 83–93. ISBN 978-981-10-3231-8. [Google Scholar]
- Ding, M.; Lu, Y.; Abbassi, S.; Li, F.; Li, X.; Song, Y.; Geoffroy, V.; Im, H.; Zheng, Q. Targeting Runx2 Expression in Hypertrophic Chondrocytes Impairs Endochondral Ossification during Early Skeletal Development. J. Cell. Physiol. 2012, 227, 3446–3456. [Google Scholar] [CrossRef]
- Jiang, Q.; Qin, X.; Yoshida, C.A.; Komori, H.; Yamana, K.; Ohba, S.; Hojo, H.; Croix, B.S.; Kawata-Matsuura, V.K.S.; Komori, T. Antxr1, Which Is a Target of Runx2, Regulates Chondrocyte Proliferation and Apoptosis. Int. J. Mol. Sci. 2020, 21, 2425. [Google Scholar] [CrossRef]
- Kamekura, S.; Kawasaki, Y.; Hoshi, K.; Shimoaka, T.; Chikuda, H.; Maruyama, Z.; Komori, T.; Sato, S.; Takeda, S.; Karsenty, G.; et al. Contribution of Runt-related Transcription Factor 2 to the Pathogenesis of Osteoarthritis in Mice after Induction of Knee Joint Instability. Arthritis Rheum. 2006, 54, 2462–2470. [Google Scholar] [CrossRef] [PubMed]
- Heinegård, D. Fell-Muir Lecture: Proteoglycans and More–from Molecules to Biology. Int. J. Exp. Path 2009, 90, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Chen, Y.-J.; Chang, W.-A.; Tsai, W.-C.; Ou, T.-T.; Wu, C.-C.; Sung, W.-Y.; Yen, J.-H.; Kuo, P.-L. Dual Role of Chondrocytes in Rheumatoid Arthritis: The Chicken and the Egg. Int. J. Mol. Sci. 2020, 21, 1071. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Cancedda, F.D.; Castagnola, P. Chondrocyte Differentiation. Int. Rev. Cytol. 1995, 159, 265–358. [Google Scholar]
- Ecke, A.; Lutter, A.-H.; Scholka, J.; Hansch, A.; Becker, R.; Anderer, U. Tissue Specific Differentiation of Human Chondrocytes Depends on Cell Microenvironment and Serum Selection. Cells 2019, 8, 934. [Google Scholar] [CrossRef]
- Camper, L.; Hellman, U.; Lundgren-Åkerlund, E. Isolation, Cloning, and Sequence Analysis of the Integrin Subunit A10, a Β1-Associated Collagen Binding Integrin Expressed on Chondrocytes. J. Biol. Chem. 1998, 273, 20383–20389. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Integrins and Chondrocyte–Matrix Interactions in Articular Cartilage. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef]
- Lundgren-Åkerlund, E.; Aszòdi, A. Integrin A10β1: A Collagen Receptor Critical in Skeletal Development. Adv. Exp. Med. Biol. 2014, 819, 61–71. [Google Scholar] [PubMed]
- Knudson, W.; Casey, B.; Nishida, Y.; Eger, W.; Kuettner, K.E.; Knudson, C.B. Hyaluronan Oligosaccharides Perturb Cartilage Matrix Homeostasis and Induce Chondrocytic Chondrolysis. Arthritis Rheum. 2000, 43, 1165. [Google Scholar] [CrossRef]
- Aguiar, D.J.; Knudson, W.; Knudson, C.B. Internalization of the Hyaluronan Receptor CD44 by Chondrocytes. Exp. Cell Res. 1999, 252, 292–302. [Google Scholar] [CrossRef]
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M.; Eto, S. Chondrocytes Are Regulated by Cellular Adhesion Through CD44 and Hyaluronic Acid Pathway. J. Bone Miner. Res. 1997, 12, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Burt, D.W.; Law, A.S. Evolution of the Transforming Growth Factor-Beta Superfamily. Prog. Growth Factor. Res. 1994, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- De Caestecker, M. The Transforming Growth Factor-β Superfamily of Receptors. Cytokine Growth Factor. Rev. 2004, 15, 1–11. [Google Scholar] [CrossRef]
- Mantel, P.-Y.; Schmidt-Weber, C.B. Transforming Growth Factor-Beta: Recent Advances on Its Role in Immune Tolerance. In Suppression and Regulation of Immune Responses; Cuturi, M.C., Anegon, I., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 677, pp. 303–338. ISBN 978-1-60761-868-3. [Google Scholar]
- Yang, D.; Dai, F.; Yuan, M.; Zheng, Y.; Liu, S.; Deng, Z.; Tan, W.; Chen, L.; Zhang, Q.; Zhao, X.; et al. Role of Transforming Growth Factor-Β1 in Regulating Fetal-Maternal Immune Tolerance in Normal and Pathological Pregnancy. Front. Immunol. 2021, 12, 689181. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, G.J.V.M.; Van Der Veen, S.W.; Buma, P.; Verwoerd-Verhoef, H.L. Effect of Transforming Growth Factor-β on Proteoglycan Synthesis by Chondrocytes in Relation to Differentiation Stage and the Presence of Pericellular Matrix. Matrix Biol. 1998, 17, 413–424. [Google Scholar] [CrossRef]
- Puolakkainen, P.A.; Twardzik, D.R.; Ranchalis, J.E.; Pankey, S.C.; Reed, M.J.; Gombotz, W.R. The Enhancement in Wound Healing by Transforming Growth Factor-Β1 (TGF-Β1) Depends on the Topical Delivery System. J. Surg. Res. 1995, 58, 321–329. [Google Scholar] [CrossRef]
- Critchlow, M.A.; Bland, Y.S.; Ashhurst, D.E. The Effect of Exogenous Transforming Growth Factor-Β2 on Healing Fractures in the Rabbit. Bone 1995, 16, 521–527. [Google Scholar] [CrossRef]
- Woo, S.L.; Buckwalter, J.A. Injury and Repair of the Musculoskeletal Soft Tissues. Savannah, Georgia, June 18–20, 1987. J. Orthop. Res. 1988, 6, 907–931. [Google Scholar] [CrossRef]
- Eyre, D. Articular Cartilage and Changes in Arthritis: Collagen of Articular Cartilage. Arthritis Res. Ther. 2001, 4, 30–35. [Google Scholar] [CrossRef][Green Version]
- Mankin, H.J. Articular Cartilage, Cartilage Injury, and Osteoarthritis. Patellofemoral Jt. 1993, 1993, 13–45. [Google Scholar]
- Kelly, D.J.; Crawford, A.; Dickinson, S.C.; Sims, T.J.; Mundy, J.; Hollander, A.P.; Prendergast, P.J.; Hatton, P.V. Biochemical Markers of the Mechanical Quality of Engineered Hyaline Cartilage. J. Mater. Sci. Mater. Med. 2007, 18, 273–281. [Google Scholar] [CrossRef]
- Eyre, D.R.; Weis, M.A.; Wu, J.-J. Articular Cartilage Collagen: An Irreplaceable Framework. Eur. Cell Mater. 2006, 12, 57–63. [Google Scholar] [CrossRef]
- Wu, J.-J.; Woods, P.E.; Eyre, D.R. Identification of Cross-Linking Sites in Bovine Cartilage Type IX Collagen Reveals an Antiparallel Type II-Type IX Molecular Relationship and Type IX to Type IX Bonding. J. Biol. Chem. 1992, 267, 23007–23014. [Google Scholar] [CrossRef]
- Mwale, F.; Tchetina, E.; Wu, C.W.; Poole, A.R. The Assembly and Remodeling of the Extracellular Matrix in the Growth Plate in Relationship to Mineral Deposition and Cellular Hypertrophy: An in Situ Study of Collagens II and IX and Proteoglycan. J. Bone Miner. Res. 2002, 17, 275–283. [Google Scholar] [CrossRef]
- Muragaki, Y.; Mariman, E.C.; van Beersum, S.E.; Perälä, M.; van Mourik, J.B.; Warman, M.L.; Olsen, B.R.; Hamel, B.C. A Mutation in the Gene Encoding the A2 Chain of the Fibril-Associated Collagen IX, COL9A2, Causes Multiple Epiphyseal Dysplasia (EDM2). Nat. Genet. 1996, 12, 103–105. [Google Scholar] [CrossRef]
- Opolka, A.; Ratzinger, S.; Schubert, T.; Spiegel, H.-U.; Grifka, J.; Bruckner, P.; Probst, A.; Grässel, S. Collagen IX Is Indispensable for Timely Maturation of Cartilage during Fracture Repair in Mice. Matrix Biol. 2007, 26, 85–95. [Google Scholar] [CrossRef]
- Gregory, K.E.; Oxford, J.T.; Chen, Y.; Gambee, J.E.; Gygi, S.P.; Aebersold, R.; Neame, P.J.; Mechling, D.E.; Bächinger, H.P.; Morris, N.P. Structural Organization of Distinct Domains within the Non-Collagenous N-Terminal Region of Collagen Type XI. J. Biol. Chem. 2000, 275, 11498–11506. [Google Scholar] [CrossRef]
- Poole, C.A.; Ayad, S.; Schofield, J.R. Chondrons from Articular Cartilage: I. Immunolocalization of Type VI Collagen in the Pericellular Capsule of Isolated Canine Tibial Chondrons. J. Cell Sci. 1988, 90, 635–643. [Google Scholar] [CrossRef]
- Responte, D.J.; Natoli, R.M.; Athanasiou, K.A. Collagens of Articular Cartilage: Structure, Function, and Importance in Tissue Engineering. Crit. Rev. Biomed. Eng. 2007, 35, 363–411. [Google Scholar] [CrossRef]
- Guilak, F.; Alexopoulos, L.G.; Upton, M.L.; Youn, I.; Choi, J.B.; Cao, L.; Setton, L.A.; Haider, M.A. The Pericellular Matrix as a Transducer of Biomechanical and Biochemical Signals in Articular Cartilage. Ann. N. Y. Acad. Sci. 2006, 1068, 498–512. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Rapid Phenotypic Changes in Passaged Articular Chondrocyte Subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef]
- Kirsch, T.; von der Mark, K. Isolation of Human Type X Collagen and Immunolocalization in Fetal Human Cartilage. Eur. J. Biochem. 1991, 196, 575–580. [Google Scholar] [CrossRef]
- Morrison, E.; Ferguson, M.; Bayliss, M.; Archer, C. The Development of Articular Cartilage: I. The Spatial and Temporal Patterns of Collagen Types. J. Anat. 1996, 189, 9. [Google Scholar]
- Walker, G.D.; Fischer, M.; Gannon, J.; Thompson, R.C., Jr.; Oegema, T.R., Jr. Expression of type-X Collagen in Osteoarthritis. J. Orthop. Res. 1995, 13, 4–12. [Google Scholar] [CrossRef]
- Girkontaite, I.; Frischholz, S.; Lammi, P.; Wagner, K.; Swoboda, B.; Aigner, T.; von der Mark, K. Immunolocalization of Type X Collagen in Normal Fetal and Adult Osteoarthritic Cartilage with Monoclonal Antibodies. Matrix Biol. 1996, 15, 231–238. [Google Scholar] [CrossRef]
- Espanha, M.M. Articular cartilage: Structure and histochemical composition. Acta Reum. Port. 2010, 35, 424–433. [Google Scholar]
- Benninghoff, A. Form Und Bau Der Gelenkknorpel in Ihren Beziehungen Zur Funktion: Zweiter Teil: Der Aufbau Des Gelenkknorpels in Seinen Beziehungen Zur Funktion. Z. Für Zellforsch. Und Mikrosk. Anat. 1925, 2, 783–862. [Google Scholar] [CrossRef]
- Wong, M.; Carter, D. Articular Cartilage Functional Histomorphology and Mechanobiology: A Research Perspective. Bone 2003, 33, 1–13. [Google Scholar] [CrossRef]
- Moger, C.; Barrett, R.; Bleuet, P.; Bradley, D.; Ellis, R.; Green, E.; Knapp, K.; Muthuvelu, P.; Winlove, C. Regional Variations of Collagen Orientation in Normal and Diseased Articular Cartilage and Subchondral Bone Determined Using Small Angle X-Ray Scattering (SAXS). Osteoarthr. Cartil. 2007, 15, 682–687. [Google Scholar] [CrossRef]
- Långsjö, T.K.; Hyttinen, M.; Pelttari, A.; Kiraly, K.; Arokoski, J.; Helminen, H.J. Electron Microscopic Stereological Study of Collagen Fibrils in Bovine Articular Cartilage: Volume and Surface Densities Are Best Obtained Indirectly (from Length Densities and Diameters) Using Isotropic Uniform Random Sampling. J. Anat. 1999, 195, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Jones, W.R.; Ting-Beall, H.P.; Lee, G.M. The Deformation Behavior and Mechanical Properties of Chondrocytes in Articular Cartilage. Osteoarthr. Cartil. 1999, 7, 59–70. [Google Scholar] [CrossRef]
- Alexopoulos, L.G.; Haider, M.A.; Vail, T.P.; Guilak, F. Alterations in the Mechanical Properties of the Human Chondrocyte Pericellular Matrix with Osteoarthritis. J. Biomech. Eng. 2003, 125, 323–333. [Google Scholar] [CrossRef]
- Alexopoulos, L.G.; Setton, L.A.; Guilak, F. The Biomechanical Role of the Chondrocyte Pericellular Matrix in Articular Cartilage. Acta Biomater. 2005, 1, 317–325. [Google Scholar] [CrossRef]
- Muir, H. Proteoglycans as Organizers of the Intercellular Matrix. Biochem. Soc. Trans. 1983, 11, 613–622. [Google Scholar] [CrossRef]
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and Dynamics of Articular Cartilage: Structure, Function, and Maintaining Healthy State. J. Orthop. Sports Phys. Ther. 1998, 28, 203–215. [Google Scholar] [CrossRef]
- Buckwalter, J. Articular Cartilage: Composition, Structure, Response to Injury, and Methods of Facilitating Repair. Articul. Cartil. Knee Jt. Funct. Basic. Sci. Arthrosc. 1990, 19–56. [Google Scholar]
- Pottenger, L.A.; Lyon, N.B.; Hecht, J.D.; Neustadt, P.M.; Robinson, R.A. Influence of Cartilage Particle Size and Proteoglycan Aggregation on Immobilization of Proteoglycans. J. Biol. Chem. 1982, 257, 11479–11485. [Google Scholar] [CrossRef]
- Maroudas, A. Physicochemical Properties of Articlar Cartilage. In Adult Articular Cartilage; Pitman Medical: Tunbridge Wells, UK, 1979. [Google Scholar]
- Maroudas, A. Balance between Swelling Pressure and Collagen Tension in Normal and Degenerate Cartilage. Nature 1976, 260, 808–809. [Google Scholar] [CrossRef]
- Lai, W.M.; Hou, J.S.; Mow, V.C. A Triphasic Theory for the Swelling and Deformation Behaviors of Articular Cartilage. J. Biomech. Eng. 1991, 113, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Ratcliffe, A.; Robin Poole, A. Cartilage and Diarthrodial Joints as Paradigms for Hierarchical Materials and Structures. Biomaterials 1992, 13, 67–97. [Google Scholar] [CrossRef] [PubMed]
- Setton, L.A.; Zhu, W.; Mow, V.C. The Biphasic Poroviscoelastic Behavior of Articular Cartilage: Role of the Surface Zone in Governing the Compressive Behavior. J. Biomech. 1993, 26, 581–592. [Google Scholar] [CrossRef]
- Setton, L.A.; Mow, V.C.; Müller, F.J.; Pita, J.C.; Howell, D.S. Mechanical Properties of Canine Articular Cartilage Are Significantly Altered Following Transection of the Anterior Cruciate Ligament. J. Orthop. Res. 1994, 12, 451–463. [Google Scholar] [CrossRef]
- Torzilli, P.A. Influence of Cartilage Conformation on Its Equilibrium Water Partition. J. Orthop. Res. 1985, 3, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Maroudas, A.; Wachtel, E.; Grushko, G.; Katz, E.P.; Weinberg, P. The Effect of Osmotic and Mechanical Pressures on Water Partitioning in Articular Cartilage. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1991, 1073, 285–294. [Google Scholar] [CrossRef]
- Mow, V.C.; Kuei, S.C.; Lai, W.M.; Armstrong, C.G. Biphasic Creep and Stress Relaxation of Articular Cartilage in Compression: Theory and Experiments. J. Biomech. Eng. 1980, 102, 73–84. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Telomere Erosion and Senescence in Human Articular Cartilage Chondrocytes. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, B172–B179. [Google Scholar] [CrossRef]
- Buckwalter, J.; Mankin, H. Articular Cartilage: Part I. J. Bone Jt. Surg. 1997, 79, 600. [Google Scholar] [CrossRef]
- Masuda, K.; Sah, R.L.; Hejna, M.J.; Thonar, E.J. A Novel Two-step Method for the Formation of Tissue-engineered Cartilage by Mature Bovine Chondrocytes: The Alginate-recovered-chondrocyte (ARC) Method. J. Orthop. Res. 2003, 21, 139–148. [Google Scholar] [CrossRef]
- Poole, C.A.; Flint, M.H.; Beaumont, B.W. Chondrons in Cartilage: Ultrastructural Analysis of the Pericellular Microenvironment in Adult Human Articular Cartilages. J. Orthop. Res. 1987, 5, 509–522. [Google Scholar] [CrossRef]
- Recht, M.; Bobic, V.; Burstein, D.; Disler, D.; Gold, G.; Gray, M.; Kramer, J.; Lang, P.; McCauley, T.; Winalski, C. Magnetic Resonance Imaging of Articular Cartilage. Clin. Orthop. Relat. Res.® 2001, 391, S379–S396. [Google Scholar] [CrossRef]
- Torzilli, P.A.; Grigiene, R.; Borrelli, J., Jr.; Helfet, D. Effect of Impact Load on Articular Cartilage: Cell Metabolism and Viability, and Matrix Water Content. J. Biomech. Eng. 1999, 121, 433–441. [Google Scholar] [CrossRef]
- Howell, D.S. Pathogenesis of Osteoarthritis. Am. J. Med. 1986, 80, 24–28. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Articular Cartilage: Injuries and Potential for Healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef]
- Ateshian, G.A.; Warden, W.H.; Kim, J.J.; Grelsamer, R.P.; Mow, V.C. Finite Deformation Biphasic Material Properties of Bovine Articular Cartilage from Confined Compression Experiments. J. Biomech. 1997, 30, 1157–1164. [Google Scholar] [CrossRef]
- Mow, V.C.; Holmes, M.H.; Michael Lai, W. Fluid Transport and Mechanical Properties of Articular Cartilage: A Review. J. Biomech. 1984, 17, 377–394. [Google Scholar] [CrossRef]
- Mow, V.C. , Hayes, W.C., Eds. Basic Orthopaedic Biomechanics, 2nd ed; Lippincott-Raven: Philadelphia, PA, USA, 1997; ISBN 978-0-397-51684-1. [Google Scholar]
- Mankin, H. Form and Function of Articular Cartilage. Orthop. Basic Sci. 1994, 1–44. [Google Scholar]
- Mow, V.; Ateshian, G.; Ratcliffe, A. Anatomic Form and Biomechanical Properties of Articular Cartilage of the Knee Joint. Biol. Biomech. Traumatized Synovial Jt. Knee A Model. 1992, 55–81. [Google Scholar]
- Maroudas, A.; Bullough, P. Permeability of Articular Cartilage. Nature 1968, 219, 1260–1261. [Google Scholar] [CrossRef]
- Frank, E.H.; Grodzinsky, A.J. Cartilage Electromechanics—I. Electrokinetic Transduction and the Effects of Electrolyte pH and Ionic Strength. J. Biomech. 1987, 20, 615–627. [Google Scholar] [CrossRef]
- Mow, V.; Rosenwasser, M. Articular Cartilage: Biomechanics. Inj. Repair. Musculoskelet. Soft Tissues 1988, 1, 427–463. [Google Scholar]
- Woo, S.L.-Y.; Lee, T.Q.; Gomez, M.A.; Sato, S.; Field, F.P. Temperature Dependent Behavior of the Canine Medial Collateral Ligament. J. Biomech. Eng. 1987, 109, 68–71. [Google Scholar] [CrossRef]
- Klika, V.; Gaffney, E.A.; Chen, Y.-C.; Brown, C.P. An Overview of Multiphase Cartilage Mechanical Modelling and Its Role in Understanding Function and Pathology. J. Mech. Behav. Biomed. Mater. 2016, 62, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Coats, R.; Woo, S. Relaxation and Creep Quasilinear Viscoelastic Models for Normal Articular Cartilage. J. Biomech. Eng. 1984, 106, 159–164. [Google Scholar] [CrossRef]
- Hurschler, C.; Abedian, R. Möglichkeiten Der Biomechanischen Charakterisierung von Knorpelgewebe. Der Orthopäde 2013, 42, 232–241. [Google Scholar] [CrossRef]
- Hayes, W.; Bodine, A. Flow-Independent Viscoelastic Properties of Articular Cartilage Matrix. J. Biomech. 1978, 11, 407–419. [Google Scholar] [CrossRef]
- LY, W.S. Biomechanical Properties of Articular Cartilage. Handb. Bioeng. 1988, 4, 1–44. [Google Scholar]
- Buckwalter, J.A.; Mow, V.C.; Ratcliffe, A. Restoration of Injured or Degenerated Articular Cartilage. JAAOS-J. Am. Acad. Orthop. Surg. 1994, 2, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, S.; Mow, V.C.; Müller, F.; Pita, J.C.; Howell, D.S.; Manicourt, D.H. Tensile Properties of Human Knee Joint Cartilage: I. Influence of Ionic Conditions, Weight Bearing, and Fibrillation on the Tensile Modulus. J. Orthop. Res. 1986, 4, 379–392. [Google Scholar] [CrossRef]
- Kempson, G. Mechanical Properties of Articular Cartilage. Adult Articul. Cartil. 1979, 333–414. [Google Scholar]
- Roth, V.; Mow, V.C. The Intrinsic Tensile Behavior of the Matrix of Bovine Articular Cartilage and Its Variation with Age. J. Bone Jt. Surg. 1980, 62, 1102–1117. [Google Scholar] [CrossRef]
- Hayes, W.; Mockros, L. Viscoelastic Properties of Human Articular Cartilage. J. Appl. Physiol. 1971, 31, 562–568. [Google Scholar] [CrossRef]
- Setton, L.; Mow, V.C.; Howell, D. Mechanical Behavior of Articular Cartilage in Shear Is Altered by Transection of the Anterior Cruciate Ligament. J. Orthop. Res. 1995, 13, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.F. The Apparent Viscoelastic Behavior of Articular Cartilage—The Contributions From the Intrinsic Matrix Viscoelasticity and Interstitial Fluid Flows. J. Biomech. Eng. 1986, 108, 123–130. [Google Scholar] [CrossRef]
- Maier, F.; Drissi, H.; Pierce, D.M. Shear Deformations of Human Articular Cartilage: Certain Mechanical Anisotropies Apparent at Large but Not Small Shear Strains. J. Mech. Behav. Biomed. Mater. 2017, 65, 53–65. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, M.R.; Zhu, Q.; Suh, J.-K.F. Biphasic Poroviscoelastic Simulation of the Unconfined Compression of Articular Cartilage: II—Effect of Variable Strain Rates. J. Biomech. Eng. 2001, 123, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Nia, H.T.; Han, L.; Li, Y.; Ortiz, C.; Grodzinsky, A. Poroelasticity of Cartilage at the Nanoscale. Biophys. J. 2011, 101, 2304–2313. [Google Scholar] [CrossRef]
- Park, S.; Hung, C.T.; Ateshian, G.A. Mechanical Response of Bovine Articular Cartilage under Dynamic Unconfined Compression Loading at Physiological Stress Levels. Osteoarthr. Cartil. 2004, 12, 65–73. [Google Scholar] [CrossRef]
- Fung, Y. Biomechanics: Mechanical Properties of Living Tissues; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 1-4757-2257-5. [Google Scholar]
- Huang, C.-Y.; Soltz, M.A.; Kopacz, M.; Mow, V.C.; Ateshian, G.A. Experimental Verification of the Roles of Intrinsic Matrix Viscoelasticity and Tension-Compression Nonlinearity in the Biphasic Response of Cartilage. J. Biomech. Eng. 2003, 125, 84–93. [Google Scholar] [CrossRef]
- Wilson, W.; Van Donkelaar, C.C.; Van Rietbergen, R.; Huiskes, R. The Role of Computational Models in the Search for the Mechanical Behavior and Damage Mechanisms of Articular Cartilage. Med. Eng. Phys. 2005, 27, 810–826. [Google Scholar] [CrossRef]
- Chen, A.C.; Bae, W.C.; Schinagl, R.M.; Sah, R.L. Depth- and Strain-Dependent Mechanical and Electromechanical Properties of Full-Thickness Bovine Articular Cartilage in Confined Compression. J. Biomech. 2001, 34, 1–12. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Luo, Z.-P.; Fertala, A.; An, K.-N. Stretching Type II Collagen with Optical Tweezers. J. Biomech. 2004, 37, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, F.; Peretti, G.M. Tensile and Compressive Properties of Healthy and Osteoarthritic Human Articular Cartilage. Biorheology 2008, 45, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Jurvelin, J.S.; Buschmann, M.D.; Hunziker, E.B. Mechanical Anisotropy of the Human Knee Articular Cartilage in Compression. Proc. Inst. Mech. Eng. H 2003, 217, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.-B.; Hung, C.T.; Mow, V.C. An Analysis of the Effects of Depth-Dependent Aggregate Modulus on Articular Cartilage Stress-Relaxation Behavior in Compression. J. Biomech. 2001, 34, 75–84. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Mow, V.C.; Ateshian, G.A. The Role of Flow-Independent Viscoelasticity in the Biphasic Tensile and Compressive Responses of Articular Cartilage. J. Biomech. Eng. 2001, 123, 410–417. [Google Scholar] [CrossRef]
- Akizuki, S.; Mow, V.C.; Muller, F.; Pita, J.C.; Howell, D.S. Tensile Properties of Human Knee Joint Cartilage. II. Correlations between Weight Bearing and Tissue Pathology and the Kinetics of Swelling. J. Orthop. Res. 1987, 5, 173–186. [Google Scholar] [CrossRef]
- Jurvelin, J.S.; Buschmann, M.D.; Hunziker, E.B. Optical and Mechanical Determination of Poisson’s Ratio of Adult Bovine Humeral Articular Cartilage. J. Biomech. 1997, 30, 235–241. [Google Scholar] [CrossRef]
- Mow, V.C.; Gibbs, M.C.; Lai, W.M.; Zhu, W.B.; Athanasiou, K.A. Biphasic Indentation of Articular Cartilage—II. A Numerical Algorithm and an Experimental Study. J. Biomech. 1989, 22, 853–861. [Google Scholar] [CrossRef]
- Williamson, A.K.; Chen, A.C.; Masuda, K.; Thonar, E.J.-M.A.; Sah, R.L. Tensile Mechanical Properties of Bovine Articular Cartilage: Variations with Growth and Relationships to Collagen Network Components. J. Orthop. Res. 2003, 21, 872–880. [Google Scholar] [CrossRef]
- Zhu, W.; Mow, V.C.; Koob, T.J.; Eyre, D.R. Viscoelastic Shear Properties of Articular Cartilage and the Effects of Glycosidase Treatments. J. Orthop. Res. 1993, 11, 771–781. [Google Scholar] [CrossRef]
- LeRoux, M.A.; Guilak, F.; Setton, L.A. Compressive and Shear Properties of Alginate Gel: Effects of Sodium Ions and Alginate Concentration. J. Biomed. Mater. Res. 1999, 47, 46–53. [Google Scholar] [CrossRef]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical Properties of Natural Cartilage and Tissue-Engineered Constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.F.; Lai, W.M.; Mow, V.C. Biphasic Indentation of Articular Cartilage—I. Theoretical Analysis. J. Biomech. 1987, 20, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; de Wijn, J.R.; Blitterswijk, C.A. van 3D Fiber-Deposited Scaffolds for Tissue Engineering: Influence of Pores Geometry and Architecture on Dynamic Mechanical Properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef]
- Jurvelin, J.S.; Arokoski, J.P.A.; Hunziker, E.B.; Helminen, H.J. Topographical Variation of the Elastic Properties of Articular Cartilage in the Canine Knee. J. Biomech. 2000, 33, 669–675. [Google Scholar] [CrossRef]
- Williamson, A.K.; Masuda, K.; Thonar, E.J.-M.A.; Sah, R.L. Growth of Immature Articular Cartilage in Vitro: Correlated Variation in Tensile Biomechanical and Collagen Network Properties. Tissue Eng. 2003, 9, 625–634. [Google Scholar] [CrossRef]
- Korhonen, R.K.; Jurvelin, J.S. Compressive and Tensile Properties of Articular Cartilage in Axial Loading Are Modulated Differently by Osmotic Environment. Med. Eng. Phys. 2010, 32, 155–160. [Google Scholar] [CrossRef]
- Spirt, A.A.; Mak, A.F.; Wassell, R.P. Nonlinear Viscoelastic Properties of Articular Cartilage in Shear. J. Orthop. Res. 1989, 7, 43–49. [Google Scholar] [CrossRef]
- Patel, J.M.; Wise, B.C.; Bonnevie, E.D.; Mauck, R.L. A Systematic Review and Guide to Mechanical Testing for Articular Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2019, 25, 593–608. [Google Scholar] [CrossRef]
- Forster, H.; Fisher, J. The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1996, 210, 109–119. [Google Scholar] [CrossRef]
- Krishnan, R.; Kopacz, M.; Ateshian, G.A. Experimental Verification of the Role of Interstitial Fluid Pressurization in Cartilage Lubrication. J. Orthop. Res. 2004, 22, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Molecular Mechanisms of Synovial Joint Lubrication. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 691–710. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, 2005513. [Google Scholar] [CrossRef] [PubMed]
- Wright, V.; Dowson, D. Lubrication and Cartilage. J. Anat. 1976, 121, 107. [Google Scholar]
- Hou, J.; Holmes, M.; Lai, W.; Mow, V. Boundary Conditions at the Cartilage-Synovial Fluid Interface for Joint Lubrication and Theoretical Verifications. J. Biomech. Eng. 1989, 111, 78–87. [Google Scholar] [CrossRef]
- Katta, J.; Jin, Z.; Ingham, E.; Fisher, J. Biotribology of Articular Cartilage—A Review of the Recent Advances. Med. Eng. Phys. 2008, 30, 1349–1363. [Google Scholar] [CrossRef]
- Daniel, M. Boundary Cartilage Lubrication: Review of Current Concepts. Wien. Med. Wochenschr. 2014, 164, 88–94. [Google Scholar] [CrossRef]
- Burris, D.L.; Moore, A.C. Cartilage and Joint Lubrication: New Insights into the Role of Hydrodynamics. Biotribology 2017, 12, 8–14. [Google Scholar] [CrossRef]
- MacConaill, M. The Function of Intra-Articular Fibrocartilages, with Special Reference to the Knee and Inferior Radio-Ulnar Joints. J. Anat. 1932, 66, 210. [Google Scholar]
- Dowson, D. Bio-Tribology. Faraday Discuss. 2012, 156, 9–30. [Google Scholar] [CrossRef]
- Jin, Z.; Dowson, D. Bio-Friction. Friction 2013, 1, 100–113. [Google Scholar] [CrossRef]
- McCutchen, C. Mechanism of Animal Joints: Sponge-Hydrostatic and Weeping Bearings. Nature 1959, 184, 1284–1285. [Google Scholar] [CrossRef]
- McCutchen, C.W. The Frictional Properties of Animal Joints. Wear 1962, 5, 1–17. [Google Scholar] [CrossRef]
- Maroudas, A. Biophysical Chemistry of Cartilaginous Tissues with Special Reference to Solute and Fluid Transport. Biorheology 1975, 12, 233–248. [Google Scholar]
- Dowson, D.; Jin, Z.-M. Micro-Elastohydrodynamic Lubrication of Synovial Joints. Eng. Med. 1986, 15, 63–65. [Google Scholar] [CrossRef]
- Ateshian, G.; Wang, H.; Lai, W. The Role of Interstitial Fluid Pressurization and Surface Porosities on the Boundary Friction of Articular Cartilage. J. Tribol. 1998, 120, 241–248. [Google Scholar] [CrossRef]
- Ateshian, G.A. The Role of Interstitial Fluid Pressurization in Articular Cartilage Lubrication. J. Biomech. 2009, 42, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Nakashima, K.; Sawae, Y.; Sakai, N.; Hosoda, N. Roles of Adsorbed Film and Gel Layer in Hydration Lubrication for Articular Cartilage. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2009, 223, 287–295. [Google Scholar] [CrossRef]
- Morrell, K.C.; Hodge, W.A.; Krebs, D.E.; Mann, R.W. Corroboration of in Vivo Cartilage Pressures with Implications for Synovial Joint Tribology and Osteoarthritis Causation. Proc. Natl. Acad. Sci. USA 2005, 102, 14819–14824. [Google Scholar] [CrossRef]
- Radin, E.L.; Swann, D.A.; Weisser, P.A. Separation of a Hyaluronate-Free Lubricating Fraction from Synovial Fluid. Nature 1970, 228, 377–378. [Google Scholar] [CrossRef]
- Radin, E.L.; Paul, I.L.; Pollock, D. Animal Joint Behaviour under Excessive Loading. Nature 1970, 226, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Lai, W.M. Recent Developments in Synovial Joint Biomechanics. SIAM Rev. 1980, 22, 275–317. [Google Scholar] [CrossRef]
- Ateshian, G.A.; Chahine, N.O.; Basalo, I.M.; Hung, C.T. The Correspondence between Equilibrium Biphasic and Triphasic Material Properties in Mixture Models of Articular Cartilage. J. Biomech. 2004, 37, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Caligaris, M.; Ateshian, G.A. Effects of Sustained Interstitial Fluid Pressurization under Migrating Contact Area, and Boundary Lubrication by Synovial Fluid, on Cartilage Friction. Osteoarthr. Cartil. 2008, 16, 1220–1227. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Baro, V.J.; Wang, L.; Burris, D.L. Fluid Load Support during Localized Indentation of Cartilage with a Spherical Probe. J. Biomech. 2012, 45, 1036–1041. [Google Scholar] [CrossRef]
- Krishnan, R.; Mariner, E.N.; Ateshian, G.A. Effect of Dynamic Loading on the Frictional Response of Bovine Articular Cartilage. J. Biomech. 2005, 38, 1665–1673. [Google Scholar] [CrossRef]
- Soltz, M.A.; Ateshian, G.A. Interstitial Fluid Pressurization During Confined Compression Cyclical Loading of Articular Cartilage. Ann. Biomed. Eng. 2000, 28, 150–159. [Google Scholar] [CrossRef]
- Schmidt, T.A.; Gastelum, N.S.; Nguyen, Q.T.; Schumacher, B.L.; Sah, R.L. Boundary Lubrication of Articular Cartilage: Role of Synovial Fluid Constituents. Arthritis Rheum. 2007, 56, 882–891. [Google Scholar] [CrossRef]
- Gleghorn, J.P.; Jones, A.R.C.; Flannery, C.R.; Bonassar, L.J. Boundary Mode Lubrication of Articular Cartilage by Recombinant Human Lubricin. J. Orthop. Res. 2009, 27, 771–777. [Google Scholar] [CrossRef]
- Moore, A.C.; Burris, D.L. Tribological Rehydration of Cartilage and Its Potential Role in Preserving Joint Health. Osteoarthr. Cartil. 2017, 25, 99–107. [Google Scholar] [CrossRef]
- Milner, P.E.; Parkes, M.; Puetzer, J.L.; Chapman, R.; Stevens, M.M.; Cann, P.; Jeffers, J.R.T. A Low Friction, Biphasic and Boundary Lubricating Hydrogel for Cartilage Replacement. Acta Biomater. 2018, 65, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Feeney, E.; Guan, Y.; Cook, S.G.; Gourdon, D.; Bonassar, L.J.; Putnam, D. Boundary Mode Lubrication of Articular Cartilage with a Biomimetic Diblock Copolymer. Proc. Natl. Acad. Sci. USA 2019, 116, 12437–12441. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Gardiner, B.S.; Zhang, L.; Grodzinsky, A.J. Articular Cartilage Dynamics; Springer: Singapore, 2019; ISBN 9789811314735. [Google Scholar]
- Jay, G.D.; Waller, K.A. The Biology of Lubricin: Near Frictionless Joint Motion. Matrix Biol. 2014, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.P.; Guilak, F.; Jay, G.D.; Zauscher, S. Interaction of Lubricin with Type II Collagen Surfaces: Adsorption, Friction, and Normal Forces. J. Biomech. 2014, 47, 659–666. [Google Scholar] [CrossRef]
- Bell, C.J.; Ingham, E.; Fisher, J. Influence of Hyaluronic Acid on the Time-Dependent Friction Response of Articular Cartilage under Different Conditions. Proc. Inst. Mech. Eng. H 2006, 220, 23–31. [Google Scholar] [CrossRef]
- Forsey, R.; Fisher, J.; Thompson, J.; Stone, M.; Bell, C.; Ingham, E. The Effect of Hyaluronic Acid and Phospholipid Based Lubricants on Friction within a Human Cartilage Damage Model. Biomaterials 2006, 27, 4581–4590. [Google Scholar] [CrossRef]
- Seror, J.; Merkher, Y.; Kampf, N.; Collinson, L.; Day, A.J.; Maroudas, A.; Klein, J. Articular Cartilage Proteoglycans as Boundary Lubricants: Structure and Frictional Interaction of Surface-Attached Hyaluronan and Hyaluronan–Aggrecan Complexes. Biomacromolecules 2011, 12, 3432–3443. [Google Scholar] [CrossRef]
- Lee, D.W.; Banquy, X.; Israelachvili, J.N. Stick-Slip Friction and Wear of Articular Joints. Proc. Natl. Acad. Sci. USA 2013, 110. [Google Scholar] [CrossRef]
- Gaisinskaya, A.; Ma, L.; Silbert, G.; Sorkin, R.; Tairy, O.; Goldberg, R.; Kampf, N.; Klein, J. Hydration Lubrication: Exploring a New Paradigm. Faraday Discuss. 2012, 156, 217. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yonekubo, S.; Kurogouchi, Y. Cryoscanning Electron Microscopy of Loaded Articular Cartilage with Special Reference to the Surface Amorphous Layer. J. Anat. 1996, 188 Pt 2, 311–322. [Google Scholar]
- Gleghorn, J.P.; Bonassar, L.J. Lubrication Mode Analysis of Articular Cartilage Using Stribeck Surfaces. J. Biomech. 2008, 41, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.F.; Dowson, D.; Wright, V. The Pressure-Viscosity Characteristics of Synovial Fluid. Biorheology 1978, 15, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The Knee Meniscus: Structure–Function, Pathophysiology, Current Repair Techniques, and Prospects for Regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Wanivenhaus, F.; Burge, A.J.; Warren, R.F.; Rodeo, S.A. The Human Meniscus: A Review of Anatomy, Function, Injury, and Advances in Treatment. Clin. Anat. 2015, 28, 269–287. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, M.R.; Zhu, Q.; Wong, M.; Jurvelin, J.S.; Suh, J.-K.F. Biphasic Poroviscoelastic Simulation of the Unconfined Compression of Articular Cartilage: I—Simultaneous Prediction of Reaction Force and Lateral Displacement. J. Biomech. Eng. 2001, 123, 191–197. [Google Scholar] [CrossRef]
- McCutchen, C.W. Joint Lubrication. Bull. Hosp. Jt. Dis. Orthop. Inst. 1983, 43, 118–129. [Google Scholar]
- Vazquez, K.J.; Andreae, J.T.; Henak, C.R. Cartilage-on-Cartilage Cyclic Loading Induces Mechanical and Structural Damage. J. Mech. Behav. Biomed. Mater. 2019, 98, 262–267. [Google Scholar] [CrossRef]
- Drewniak, E.I.; Jay, G.D.; Fleming, B.C.; Zhang, L.; Warman, M.L.; Crisco, J.J. Cyclic Loading Increases Friction and Changes Cartilage Surface Integrity in Lubricin-mutant Mouse Knees. Arthritis Rheum. 2012, 64, 465–473. [Google Scholar] [CrossRef]
- Zhang, L.; Miramini, S.; Smith, D.W.; Gardiner, B.S.; Grodzinsky, A.J. Time Evolution of Deformation in a Human Cartilage Under Cyclic Loading. Ann. Biomed. Eng. 2015, 43, 1166–1177. [Google Scholar] [CrossRef]
- Ghosh, S.; Choudhury, D.; Das, N.S.; Pingguan-Murphy, B. Tribological Role of Synovial Fluid Compositions on Artificial Joints—A Systematic Review of the Last 10 Years: Tribological role of synovial fluid compositions on artificial joints. Lubr. Sci. 2014, 26, 387–410. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Goldring, S.R. The Role of Synovitis in Osteoarthritis Pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef]
- Kraus, V.B.; Burnett, B.; Coindreau, J.; Cottrell, S.; Eyre, D.; Gendreau, M.; Gardiner, J.; Garnero, P.; Hardin, J.; Henrotin, Y.; et al. Application of Biomarkers in the Development of Drugs Intended for the Treatment of Osteoarthritis. Osteoarthr. Cartil. 2011, 19, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Henrotin, Y. Biomarkers of (Osteo)Arthritis. Biomarkers 2015, 20, 513–518. [Google Scholar] [CrossRef]
- Carlson, A.K.; Rawle, R.A.; Wallace, C.W.; Brooks, E.G.; Adams, E.; Greenwood, M.C.; Olmer, M.; Lotz, M.K.; Bothner, B.; June, R.K. Characterization of Synovial Fluid Metabolomic Phenotypes of Cartilage Morphological Changes Associated with Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1174–1184. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Reis, R.L. (Eds.) Regenerative Strategies for the Treatment of Knee Joint Disabilities; Studies in Mechanobiology, Tissue Engineering and Biomaterials; Springer International Publishing: Cham, Switzerland, 2017; Volume 21, ISBN 978-3-319-44783-4. [Google Scholar]
- Prekasan, D.; Saju, K.K. Review of the Tribological Characteristics of Synovial Fluid. Procedia Technol. 2016, 25, 1170–1174. [Google Scholar] [CrossRef]
- Fournier, R.L. Basic Transport Phenomena in Biomedical Engineering; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1-315-12047-X. [Google Scholar]
- Tamer, T.M. Hyaluronan and Synovial Joint: Function, Distribution and Healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Milošev, I.; Levašič, V.; Vidmar, J.; Kovač, S.; Trebše, R. pH and Metal Concentration of Synovial Fluid of Osteoarthritic Joints and Joints with Metal Replacements. J. Biomed. Mater. Res. 2017, 105, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, H.A.; Sardinha, H.; Bhatia, S.R. Rheology and Gelation Kinetics in Laponite Dispersions Containing Poly(Ethylene Oxide). J. Polym. Sci. B Polym. Phys. 2005, 43, 233–240. [Google Scholar] [CrossRef]
- Helfet, A.J. (Ed.) Disorders of the Knee, 2nd ed.; Lippincott: Philadelphia, PA, USA, 1982; ISBN 978-0-397-50484-8. [Google Scholar]
- Benz, M.; Chen, N.; Israelachvili, J. Lubrication and Wear Properties of Grafted Polyelectrolytes, Hyaluronan and Hylan, Measured in the Surface Forces Apparatus. J. Biomed. Mater. Res. 2004, 71A, 6–15. [Google Scholar] [CrossRef]
- Gispert, M.P.; Serro, A.P.; Colaço, R.; Saramago, B. Friction and Wear Mechanisms in Hip Prosthesis: Comparison of Joint Materials Behaviour in Several Lubricants. Wear 2006, 260, 149–158. [Google Scholar] [CrossRef]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and Functional Roles of Synoviocytes in the Joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.P.; Abu-Lail, N.I.; Coles, J.M.; Guilak, F.; Jay, G.D.; Zauscher, S. Friction Force Microscopy of Lubricin and Hyaluronic Acid between Hydrophobic and Hydrophilic Surfaces. Soft Matter 2009, 5, 3438–3445. [Google Scholar] [CrossRef]
- Bernardeau, C.; Bucki, B.; Lioté, F. Acute Arthritis after Intra-Articular Hyaluronate Injection: Onset of Effusions without Crystal. Ann. Rheum. Dis. 2001, 60, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.G.; Davies, J.R.; Tudor, D.; Flannery, C.R.; Hughes, C.E.; Dent, C.M.; Caterson, B. Immunolocalisation and Expression of Proteoglycan 4 (Cartilage Superficial Zone Proteoglycan) in Tendon. Matrix Biol. 2002, 21, 593–602. [Google Scholar] [CrossRef]
- Jones, A.R.C.; Gleghorn, J.P.; Hughes, C.E.; Fitz, L.J.; Zollner, R.; Wainwright, S.D.; Caterson, B.; Morris, E.A.; Bonassar, L.J.; Flannery, C.R. Binding and Localization of Recombinant Lubricin to Articular Cartilage Surfaces. J. Orthop. Res. 2007, 25, 283–292. [Google Scholar] [CrossRef]
- Trunfio-Sfarghiu, A.-M.; Berthier, Y.; Meurisse, M.-H.; Rieu, J.-P. Multiscale Analysis of the Tribological Role of the Molecular Assemblies of Synovial Fluid. Case of a Healthy Joint and Implants. Tribol. Int. 2007, 40, 1500–1515. [Google Scholar] [CrossRef]
- Elsaid, K.A.; Fleming, B.C.; Oksendahl, H.L.; Machan, J.T.; Fadale, P.D.; Hulstyn, M.J.; Shalvoy, R.; Jay, G.D. Decreased Lubricin Concentrations and Markers of Joint Inflammation in the Synovial Fluid of Patients with Anterior Cruciate Ligament Injury. Arthritis Rheum. 2008, 58, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Nakagawa, T.; Reddi, A.H. Induction of Chondrogenesis and Expression of Superficial Zone Protein (SZP)/Lubricin by Mesenchymal Progenitors in the Infrapatellar Fat Pad of the Knee Joint Treated with TGF-Β1 and BMP-7. Biochem. Biophys. Res. Commun. 2008, 376, 148–153. [Google Scholar] [CrossRef]
- Das, R.H.J.; Jahr, H.; Verhaar, J.A.N.; Van Der Linden, J.C.; Van Osch, G.J.V.M.; Weinans, H. In Vitro Expansion Affects the Response of Chondrocytes to Mechanical Stimulation. Osteoarthr. Cartil. 2008, 16, 385–391. [Google Scholar] [CrossRef]
- Wang, A.; Essner, A.; Schmidig, G. The Effects of Lubricant Composition on in Vitro Wear Testing of Polymeric Acetabular Components. J. Biomed. Mater. Res. 2004, 68B, 45–52. [Google Scholar] [CrossRef]
- Elsaid, K.A.; Jay, G.D.; Warman, M.L.; Rhee, D.K.; Chichester, C.O. Association of Articular Cartilage Degradation and Loss of Boundary-lubricating Ability of Synovial Fluid Following Injury and Inflammatory Arthritis. Arthritis Rheum. 2005, 52, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.Y.; McCarty, W.J.; Masuda, K.; Firestein, G.S.; Sah, R.L. A Systems Biology Approach to Synovial Joint Lubrication in Health, Injury, and Disease. WIREs Mech. Dis. 2012, 4, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Myant, C.W.; Underwood, R.; Cann, P.M.; Hart, A. Inlet Protein Aggregation: A New Mechanism for Lubricating Film Formation with Model Synovial Fluids. Proc. Inst. Mech. Eng. H 2011, 225, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Myant, C.; Underwood, R.; Fan, J.; Cann, P.M. Lubrication of Metal-on-Metal Hip Joints: The Effect of Protein Content and Load on Film Formation and Wear. J. Mech. Behav. Biomed. Mater. 2012, 6, 30–40. [Google Scholar] [CrossRef]
- Hyc, A.; Iwan, A.; Moskalewski, S. Morphology and Function of Normal Synovial Membrane. Reumatologia 2012, 50, 501–506. [Google Scholar] [CrossRef]
- Berumen-Nafarrate, E.; Leal-Berumen, I.; Luevano, E.; Solis, F.J.; Muñoz-Esteves, E. Synovial Tissue and Synovial Fluid. J. Knee Surg. 2002, 15, 46–48. [Google Scholar]
- De Sousa, E.B.; Casado, P.L.; Neto, V.M.; Duarte, M.E.L.; Aguiar, D.P. Synovial Fluid and Synovial Membrane Mesenchymal Stem Cells: Latest Discoveries and Therapeutic Perspectives. Stem Cell Res. Ther. 2014, 5, 112. [Google Scholar] [CrossRef]
- Blom, A.B.; Van Lent, P.L.E.M.; Holthuysen, A.E.M.; Van Der Kraan, P.M.; Roth, J.; Van Rooijen, N.; Van Den Berg, W.B. Synovial Lining Macrophages Mediate Osteophyte Formation during Experimental Osteoarthritis. Osteoarthr. Cartil. 2004, 12, 627–635. [Google Scholar] [CrossRef]
- Van Lent, P.L.; Van Den Berg, W.B. Mesenchymal Stem Cell Therapy in Osteoarthritis: Advanced Tissue Repair or Intervention with Smouldering Synovial Activation? Arthritis Res. Ther. 2013, 15, 112. [Google Scholar] [CrossRef]
- Revell, P.A.; al-Saffar, N.; Fish, S.; Osei, D. Extracellular Matrix of the Synovial Intimal Cell Layer. Ann. Rheum. Dis. 1995, 54, 404–407. [Google Scholar] [CrossRef]
- Jay, G.D.; Tantravahi, U.; Britt, D.E.; Barrach, H.J.; Cha, C. Homology of Lubricin and Superficial Zone Protein (SZP): Products of Megakaryocyte Stimulating Factor (MSF) Gene Expression by Human Synovial Fibroblasts and Articular Chondrocytes Localized to Chromosome 1q25. J. Orthop. Res. 2001, 19, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.P.; Yanase, H.; Kitamura, H.; Iwanaga, T. Unique Localization of Protein Gene Product 9.5 in Type B Synoviocytes in the Joints of the Horse. J. Histochem. Cytochem. 1999, 47, 343–351. [Google Scholar] [CrossRef]
- Sabaratnam, S.; Coleman, P.J.; Mason, R.M.; Levick, J.R. Interstitial Matrix Proteins Determine Hyaluronan Reflection and Fluid Retention in Rabbit Joints: Effect of Protease. J. Physiol. 2007, 578, 291–299. [Google Scholar] [CrossRef]
- Levick, J.R. Flow Through Interstitium and Other Fibrous Matrices. Exp. Physiol. 1987, 72, 409–437. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Levick, J.; Wang, W. The Mechanism of Synovial Fluid Retention in Pressurized Joint Cavities. Microcirculation 2005, 12, 581–595. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; Van Den Berg, W.B.; Bresnihan, B. Synovial Tissue Inflammation in Early and Late Osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- De Lange-Brokaar, B.J.E.; Ioan-Facsinay, A.; Van Osch, G.J.V.M.; Zuurmond, A.-M.; Schoones, J.; Toes, R.E.M.; Huizinga, T.W.J.; Kloppenburg, M. Synovial Inflammation, Immune Cells and Their Cytokines in Osteoarthritis: A Review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Kraus, V. Osteoarthritis Year 2010 in Review: Biochemical Markers. Osteoarthr. Cartil. 2011, 19, 346–353. [Google Scholar] [CrossRef]
- Urban, J.P.G.; Hall, A.C.; Gehl, K.A. Regulation of Matrix Synthesis Rates by the Ionic and Osmotic Environment of Articular Chondrocytes. J. Cell. Physiol. 1993, 154, 262–270. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Gluzband, Y.A.; Grodzinsky, A.J.; Hunziker, E.B. Mechanical Compression Modulates Matrix Biosynthesis in Chondrocyte/Agarose Culture. J. Cell Sci. 1995, 108, 1497–1508. [Google Scholar] [CrossRef]
- Graham, B.T.; Moore, A.C.; Burris, D.L.; Price, C. Sliding Enhances Fluid and Solute Transport into Buried Articular Cartilage Contacts. Osteoarthr. Cartil. 2017, 25, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Burris, D.; Ramsey, L.; Graham, B.; Price, C.; Moore, A. How Sliding and Hydrodynamics Contribute to Articular Cartilage Fluid and Lubrication Recovery. Tribol. Lett. 2019, 67, 46. [Google Scholar] [CrossRef]
- Sise, C.V.; Petersen, C.A.; Ashford, A.K.; Yun, J.; Zimmerman, B.K.; Vukelic, S.; Hung, C.T.; Ateshian, G.A. A Major Functional Role of Synovial Fluid Is to Reduce the Rate of Cartilage Fatigue Failure under Cyclical Compressive Loading. Osteoarthr. Cartil. 2025, 33, 94–100. [Google Scholar] [CrossRef]
- Soltz, M.A.; Ateshian, G.A. A Conewise Linear Elasticity Mixture Model for the Analysis of Tension-Compression Nonlinearity in Articular Cartilage. J. Biomech. Eng. 2000, 122, 576–586. [Google Scholar] [CrossRef] [PubMed]
- O’hara, B.; Urban, J.; Maroudas, A. Influence of Cyclic Loading on the Nutrition of Articular Cartilage. Ann. Rheum. Dis. 1990, 49, 536–539. [Google Scholar] [CrossRef]
- Eisenberg, S.R.; Grodzinsky, A.J. Swelling of Articular Cartilage and Other Connective Tissues: Electromechanochemical Forces. J. Orthop. Res. 1985, 3, 148–159. [Google Scholar] [CrossRef]
- Buckwalter, J.; Mankin, H. Articular Cartilage: Degeneration and Osteoarthritis, Repair, Regeneration, and Transplantation. Instr. Course Lect. 1998, 47, 487–504. [Google Scholar]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.; Wong, D.D.; Chao, P.-H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional Tissue Engineering of Articular Cartilage through Dynamic Loading of Chondrocyte-Seeded Agarose Gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef]
- Khan, K.M.; Scott, A. Mechanotherapy: How Physical Therapists’ Prescription of Exercise Promotes Tissue Repair. Br. J. Sports Med. 2009, 43, 247–252. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage Diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Todros, S.; Pozzuoli, A.; Ruggieri, P.; Carniel, E.L.; Berardo, A. Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes 2023, 11, 1014. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Lohmander, S.L. The Role of the Meniscus in Knee Osteoarthritis: A Cause or Consequence? Radiol. Clin. N. Am. 2009, 47, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Favero, M.; El-Hadi, H.; Belluzzi, E.; Granzotto, M.; Porzionato, A.; Sarasin, G.; Rambaldo, A.; Iacobellis, C.; Cigolotti, A.; Fontanella, C.G.; et al. Infrapatellar Fat Pad Features in Osteoarthritis: A Histopathological and Molecular Study. Rheumatology 2017, 56, 1784–1793. [Google Scholar] [CrossRef]
- Belluzzi, E.; Macchi, V.; Fontanella, C.; Carniel, E.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6016. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial Inflammation in Osteoarthritis Progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Sowers, M. Epidemiology of Risk Factors for Osteoarthritis: Systemic Factors. Curr. Opin. Rheumatol. 2001, 13, 447–451. [Google Scholar] [CrossRef]
- Belluzzi, E.; El Hadi, H.; Granzotto, M.; Rossato, M.; Ramonda, R.; Macchi, V.; De Caro, R.; Vettor, R.; Favero, M. Systemic and Local Adipose Tissue in Knee Osteoarthritis. J. Cell. Physiol. 2017, 232, 1971–1978. [Google Scholar] [CrossRef]
- Olivotto, E.; Belluzzi, E.; Pozzuoli, A.; Cigolotti, A.; Scioni, M.; Goldring, S.R.; Goldring, M.B.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study. Int. J. Mol. Sci. 2022, 23, 3903. [Google Scholar] [CrossRef]
- Ghouri, A.; Conaghan, P.G. Update on Novel Pharmacological Therapies for Osteoarthritis. Ther. Adv. Musculoskelet. 2019, 11, 1759720X19864492. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. BioMed Res. Int. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. Int. J. Mol. Sci. 2022, 23, 4642. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Xiao, Z.; Su, G.; Hou, Y.; Chen, S.; Lin, D. Cartilage Degradation in Osteoarthritis: A Process of Osteochondral Remodeling Resembles the Endochondral Ossification in Growth Plate? Med. Hypotheses 2018, 121, 183–187. [Google Scholar] [CrossRef]
- Torzilli, P.A.; Allen, S.N. Effect of Articular Surface Compression on Cartilage Extracellular Matrix Deformation. J. Biomech. Eng. 2022, 144, 091007. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Aging, Articular Cartilage Chondrocyte Senescence and Osteoarthritis. Biogerontology 2002, 3, 257–264. [Google Scholar] [CrossRef]
- Goldring, M.B. Articular Cartilage Degradation in Osteoarthritis. HSS J.® Musculoskelet. J. Hosp. Spec. Surg. 2012, 8, 7–9. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Singh, P.; Marcu, K.B.; Goldring, M.B.; Otero, M. Phenotypic Instability of Chondrocytes in Osteoarthritis: On a Path to Hypertrophy. Ann. N. Y. Acad. Sci. 2019, 1442, 17–34. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Stanish, W.D.; Rosier, R.N.; Schenck, R.C.; Dennis, D.A.; Coutts, R.D. The Increasing Need for Nonoperative Treatment of Patients with Osteoarthritis. Clin. Orthop. Relat. Res. 2001, 385, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Ali, M.H.; Wydra, F.; Li, X.; Hamilton, J.L.; An, H.S.; Cs-Szabo, G.; Andrews, S.; Moric, M.; Xiao, G.; et al. Characterization of Degenerative Human Facet Joints and Facet Joint Capsular Tissues. Osteoarthr. Cartil. 2015, 23, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Statham, P.; Jones, E.; Jennings, L.M.; Fermor, H.L. Reproducing the Biomechanical Environment of the Chondrocyte for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 405–420. [Google Scholar] [CrossRef]
- Kempson, G.E. The Mechanical Properties of Articular Cartilage. In The Joints and Synovial Fluid; Elsevier: Amsterdam, The Netherlands, 1980; pp. 177–238. ISBN 978-0-12-655102-0. [Google Scholar]
- Kempson, G.E. Relationship between the Tensile Properties of Articular Cartilage from the Human Knee and Age. Ann. Rheum. Dis. 1982, 41, 508–511. [Google Scholar] [CrossRef]
- Kempson, G.E. Age-Related Changes in the Tensile Properties of Human Articular Cartilage: A Comparative Study between the Femoral Head of the Hip Joint and the Talus of the Ankle Joint. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1991, 1075, 223–230. [Google Scholar] [CrossRef]
- Mow, V. Mechanical Factors in Articular Cartilage and Their Role in Osteoarthritis. Osteoarthr. Disord. 1995. [Google Scholar]
- Buckwalter, J.A.; Rosenberg, L.C. Electron Microscopic Studies of Cartilage Proteoglycans. Direct Evidence for the Variable Length of the Chondroitin Sulfate-Rich Region of Proteoglycan Subunit Core Protein. J. Biol. Chem. 1982, 257, 9830–9839. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Kuettner, K.E.; Thonar, E.J. Age-related Changes in Articular Cartilage Proteoglycans: Electron Microscopic Studies. J. Orthop. Res. 1985, 3, 251–257. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Roughley, P.J.; Rosenberg, L.C. Age-Related Changes in Cartilage Proteoglycans: Quantitative Electron Microscopic Studies. Microsc. Res. Tech. 1994, 28, 398–408. [Google Scholar] [CrossRef]
- Thonar, E.J.; Buckwalter, J.A.; Kuettner, K.E. Maturation-Related Differences in the Structure and Composition of Proteoglycans Synthesized by Chondrocytes from Bovine Articular Cartilage. J. Biol. Chem. 1986, 261, 2467–2474. [Google Scholar] [CrossRef]
- Lee, H.-S.; Salter, D.M. Biomechanics of Cartilage and Osteoarthritis. In Osteoarthritis-Progress in Basic Research and Treatment; Chen, Q., Ed.; InTech: Hong Kong, China, 2015 ISBN 978-953-51-2136-7.
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.U.; Krocker, D.; Cedraro, A.; Tuischer, J.; Duda, G.N. Altered Cartilage Mechanics and Histology in Knee Osteoarthritis: Relation to Clinical Assessment (ICRS Grade). Osteoarthr. Cartil. 2005, 13, 958–963. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ojanen, S.; Mohammadi, A.; Finnilä, M.A.; Joukainen, A.; Kröger, H.; Saarakkala, S.; Korhonen, R.K.; Tanska, P. Elastic, Viscoelastic and Fibril-Reinforced Poroelastic Material Properties of Healthy and Osteoarthritic Human Tibial Cartilage. Ann. Biomed. Eng. 2019, 47, 953–966. [Google Scholar] [CrossRef]
- Hardingham, T.; Bayliss, M. Proteoglycans of Articular Cartilage: Changes in Aging and in Joint Disease. Semin. Arthritis Rheum. 1990, 20, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.C.; Dudhia, J.; Bayliss, M.T. Age-Related Changes in the Synthesis of Link Protein and Aggrecan in Human Articular Cartilage: Implications for Aggregate Stability. Biochem. J. 1999, 337, 77–82. [Google Scholar] [CrossRef]
- Martin, J.A.; Ellerbroek, S.M.; Buckwalter, J.A. Age-related Decline in Chondrocyte Response to Insulin-like Growth factor-I: The Role of Growth Factor Binding Proteins. J. Orthop. Res. 1997, 15, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Buckwalter, J.A. The Role of Chondrocyte–Matrix Interactions in Maintaining and Repairing Articular Cartilage. Biorheol. Off. J. Int. Soc. Biorheol. 2000, 37, 129–140. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral Bone in Osteoarthritis: Insight into Risk Factors and Microstructural Changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Volpin, G.; Dowd, G.; Stein, H.; Bentley, G. Degenerative Arthritis after Intra-Articular Fractures of the Knee. Long-Term Results. J. Bone Jt. Surgery. Br. Vol. 1990, 72-B, 634–638. [Google Scholar] [CrossRef]
- Link, T.M.; Stahl, R.; Woertler, K. Cartilage Imaging: Motivation, Techniques, Current and Future Significance. Eur. Radiol. 2007, 17, 1135–1146. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Tiderius, C.J.; Olsson, L.E.; Leander, P.; Ekberg, O.; Dahlberg, L. Delayed Gadolinium-enhanced MRI of Cartilage (dGEMRIC) in Early Knee Osteoarthritis. Magn. Reson. Med. 2003, 49, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Burstein, D.; Bashir, A.; Gray, M.L. MRI Techniques in Early Stages of Cartilage Disease. Investig. Radiol. 2000, 35, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Regatte, R.R.; Akella, S.V.S.; Borthakur, A.; Kneeland, J.B.; Reddy, R. In Vivo Proton MR Three-Dimensional T1ρ Mapping of Human Articular Cartilage: Initial Experience. Radiology 2003, 229, 269–274. [Google Scholar] [CrossRef] [PubMed]
- David-Vaudey, E.; Ghosh, S.; Ries, M.; Majumdar, S. T2 Relaxation Time Measurements in Osteoarthritis. Magn. Reson. Imaging 2004, 22, 673–682. [Google Scholar] [CrossRef]
- Mariappan, Y.K.; Glaser, K.J.; Ehman, R.L. Magnetic Resonance Elastography: A Review. Clin. Anat. 2010, 23, 497–511. [Google Scholar] [CrossRef]
- Lopez, O.; Amrami, K.K.; Manduca, A.; Rossman, P.J.; Ehman, R.L. Developments in Dynamic MR Elastography for in Vitro Biomechanical Assessment of Hyaline Cartilage under High-frequency Cyclical Shear. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2007, 25, 310–320. [Google Scholar] [CrossRef]
- Khalilzad-Sharghi, V.; Han, Z.; Xu, H.; Othman, S.F. MR Elastography for Evaluating Regeneration of Tissue-engineered Cartilage in an Ectopic Mouse Model. Magn. Reson. Med. 2016, 75, 1209–1217. [Google Scholar] [CrossRef]
- Karpiński, R. Knee joint osteoarthritis diagnosis based on selected acoustic signal discriminants using machine learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P. Application of eemd-dfa algorithms and ann classification for detection of knee osteoarthritis using vibroarthrography. Appl. Comput. Sci. 2024, 20, 90–108. [Google Scholar] [CrossRef]
- Choi, D.; Ahn, S.; Ryu, J.; Nagao, M.; Kim, Y. Knee Acoustic Emission Characteristics of the Healthy and the Patients with Osteoarthritis Using Piezoelectric Sensor. Sens. Mater. 2018, 30, 1629. [Google Scholar] [CrossRef]
- Vatolik, I.; Everington, M.; Hunter, G.; Swann, N.; Augousti, A.T. Development of a Multi-Modal Sensor Network to Detect and Monitor Knee Joint Condition. Meas. Sens. 2022, 24, 100483. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Maciejewski, M.; Jonak, J.; Krakowski, P.; Syta, A. Multi-Scale Analysis of Knee Joint Acoustic Signals for Cartilage Degeneration Assessment. Sensors 2025, 25, 706. [Google Scholar] [CrossRef] [PubMed]
- Georgas, E.; Rayes, A.; Zhang, J.; Zhou, Q.; Qin, Y.-X. Shear Wave Ultrasound Elastography for Estimating Cartilage Stiffness: Implications for Early Detection of Osteoarthritis. Med-X 2024, 2, 4. [Google Scholar] [CrossRef]
- Zhou, X.; Eltit, F.; Yang, X.; Maloufi, S.; Alousaimi, H.; Liu, Q.; Huang, L.; Wang, R.; Tang, S. Detecting Human Articular Cartilage Degeneration in Its Early Stage with Polarization-Sensitive Optical Coherence Tomography. Biomed. Opt. Express 2020, 11, 2745. [Google Scholar] [CrossRef]
- Chu, C.R.; Williams, A.; Tolliver, D.; Kwoh, C.K.; Bruno, S.; Irrgang, J.J. Clinical Optical Coherence Tomography of Early Articular Cartilage Degeneration in Patients with Degenerative Meniscal Tears. Arthritis Rheum. 2010, 62, 1412–1420. [Google Scholar] [CrossRef]
- Li, X.; Martin, S.; Pitris, C.; Ghanta, R.; Stamper, D.L.; Harman, M.; Fujimoto, J.G.; Brezinski, M.E. High-Resolution Optical Coherence Tomographic Imaging of Osteoarthritic Cartilage during Open Knee Surgery. Arthritis Res. Ther. 2005, 7, R318. [Google Scholar] [CrossRef]
- Korhonen, R.; Laasanen, M.; Töyräs, J.; Rieppo, J.; Hirvonen, J.; Helminen, H.; Jurvelin, J. Comparison of the Equilibrium Response of Articular Cartilage in Unconfined Compression, Confined Compression and Indentation. J. Biomech. 2002, 35, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lin, Q.; Wang, X.; Liu, Y.; Yu, X.; Ren, Z.; Zhang, Y.; Guo, L.; Wu, X.; Zhang, X. Biomechanical Properties of Articular Cartilage in Different Regions and Sites of the Knee Joint: Acquisition of Osteochondral Allografts. Cell Tissue Bank. 2024, 25, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Kabir, W.; Di Bella, C.; Choong, P.F.; O’Connell, C.D. Assessment of Native Human Articular Cartilage: A Biomechanical Protocol. Cartilage 2021, 13, 427S–437S. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Huang, Y.-P.; Saarakkala, S.; Zheng, Y.-P. Quantitative Assessment of Articular Cartilage with Morphologic, Acoustic and Mechanical Properties Obtained Using High-Frequency Ultrasound. Ultrasound Med. Biol. 2010, 36, 512–527. [Google Scholar] [CrossRef]
- Goodwin, M.; Workman, J.; Thambyah, A.; Vanholsbeeck, F. Impact-Induced Cartilage Damage Assessed Using Polarisation-Sensitive Optical Coherence Tomography. J. Mech. Behav. Biomed. Mater. 2021, 117, 104326. [Google Scholar] [CrossRef] [PubMed]
- Catalano, E. Biophysical and Biomechanical Properties of Cartilage. arXiv 2023, arXiv:2305.01529. [Google Scholar]
- Pavlou, E.; Zhang, X.; Wang, J.; Kourkoumelis, N. Raman Spectroscopy for the Assessment of Osteoarthritis. Ann. Jt. 2018, 3, 83. [Google Scholar] [CrossRef]
- Casal-Beiroa, P.; Balboa-Barreiro, V.; Oreiro, N.; Pértega-Díaz, S.; Blanco, F.J.; Magalhães, J. Optical Biomarkers for the Diagnosis of Osteoarthritis through Raman Spectroscopy: Radiological and Biochemical Validation Using Ex Vivo Human Cartilage Samples. Diagnostics 2021, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.; Murugkar, S.; Speirs, A.D. Measurement of Cartilage Sub-component Distributions through the Surface by Raman Spectroscopy-based Multivariate Analysis. J. Biophotonics 2021, 14, e202000289. [Google Scholar] [CrossRef]
- Jensen, M.; Horgan, C.C.; Vercauteren, T.; Albro, M.B.; Bergholt, M.S. Multiplexed Polarized Hypodermic Raman Needle Probe for Biostructural Analysis of Articular Cartilage. Opt. Lett. 2020, 45, 2890–2893. [Google Scholar] [CrossRef]
- Li, X.; Majumdar, S. Quantitative MRI of Articular Cartilage and Its Clinical Applications. Magn. Reson. Imaging 2013, 38, 991–1008. [Google Scholar] [CrossRef]
- Park, E.H.; Fritz, J. The Role of Imaging in Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101866. [Google Scholar] [CrossRef]
- Mononen, M.E.; Jurvelin, J.S.; Korhonen, R.K. Implementation of a Gait Cycle Loading into Healthy and Meniscectomised Knee Joint Models with Fibril-Reinforced Articular Cartilage. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 141–152. [Google Scholar] [CrossRef]
- Peña, E.; Calvo, B.; Martínez, M.A.; Doblaré, M. A Three-Dimensional Finite Element Analysis of the Combined Behavior of Ligaments and Menisci in the Healthy Human Knee Joint. J. Biomech. 2006, 39, 1686–1701. [Google Scholar] [CrossRef]
- Mononen, M.E.; Tanska, P.; Isaksson, H.; Korhonen, R.K. A Novel Method to Simulate the Progression of Collagen Degeneration of Cartilage in the Knee: Data from the Osteoarthritis Initiative. Sci. Rep. 2016, 6, 21415. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Mequanint, K.; Herzog, W. Computational Aspects in Mechanical Modeling of the Articular Cartilage Tissue. Proc. Inst. Mech. Eng. H 2013, 227, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Gardiner, B.S.; Davidson, J.B.; Grodzinsky, A.J. Computational Model for the Analysis of Cartilage and Cartilage Tissue Constructs: Computational Model for Cartilage and Cartilage Tissue Constructs Analysis. J. Tissue Eng. Regen. Med. 2016, 10, 334–347. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M. Comparison of selected classification methods based on machine learning as a diagnostic tool for knee joint cartilage damage based on generated vibroacoustic processes. Appl. Comput. Sci. 2023, 19, 136–150. [Google Scholar] [CrossRef]
- Ebrahimkhani, S.; Jaward, M.H.; Cicuttini, F.M.; Dharmaratne, A.; Wang, Y.; De Herrera, A.G.S. A Review on Segmentation of Knee Articular Cartilage: From Conventional Methods towards Deep Learning. Artif. Intell. Med. 2020, 106, 101851. [Google Scholar] [CrossRef]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A Self-Configuring Method for Deep Learning-Based Biomedical Image Segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhong, J.; Zhang, L.; Khan, S.; Chen, W. CartiMorph: A Framework for Automated Knee Articular Cartilage Morphometrics. Med. Image Anal. 2023, 91, 103035. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, Z.; Samsonov, A.; Blankenbaker, D.; Larison, W.; Kanarek, A.; Lian, K.; Kambhampati, S.; Kijowski, R. Deep Learning Approach for Evaluating Knee MR Images: Achieving High Diagnostic Performance for Cartilage Lesion Detection. Radiology 2018, 289, 160–169. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part I: Femoral-Tibial Joint. Sensors 2022, 22, 2176. [Google Scholar] [CrossRef]
- Tiulpin, A.; Klein, S.; Bierma-Zeinstra, S.M.A.; Thevenot, J.; Rahtu, E.; Meurs, J.V.; Oei, E.H.G.; Saarakkala, S. Multimodal Machine Learning-Based Knee Osteoarthritis Progression Prediction from Plain Radiographs and Clinical Data. Sci. Rep. 2019, 9, 20038. [Google Scholar] [CrossRef]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.E.; et al. Human Cartilage Repair with a Photoreactive Adhesive-Hydrogel Composite. Sci. Transl. Med. 2013, 5, 167ra6. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Nauman, E.A.; Pedersen, C.B.W.; Neu, C.P. Finite Deformation Elastography of Articular Cartilage and Biomaterials Based on Imaging and Topology Optimization. Sci. Rep. 2020, 10, 7980. [Google Scholar] [CrossRef]
- Albano, D.; Viglino, U.; Esposito, F.; Rizzo, A.; Messina, C.; Gitto, S.; Fusco, S.; Serpi, F.; Kamp, B.; Müller-Lutz, A.; et al. Quantitative and Compositional MRI of the Articular Cartilage: A Narrative Review. Tomography 2024, 10, 949–969. [Google Scholar] [CrossRef]
- Żylińska, B.; Sobczyńska-Rak, A.; Lisiecka, U.; Stodolak-Zych, E.; Jarosz, Ł.; Szponder, T. Structure and Pathologies of Articular Cartilage. In Vivo 2021, 35, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Solanki, K.; Shanmugasundaram, S.; Shetty, N.; Kim, S.-J. Articular Cartilage Repair & Joint Preservation: A Review of the Current Status of Biological Approach. J. Clin. Orthop. Trauma 2021, 22, 101602. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.U.; Aslam, H.M.; Iftakhar, T.; Abdullah, M. Pathophysiology of Cartilage Damage in Knee Osteoarthritis and Regenerative Approaches toward Recovery. J. Bone Jt. Dis. 2024, 39, 32–44. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.; Vaysbrot, E.; Arden, N.; Bennell, K.; Bierma-Zeinstra, S.; Kraus, V.; Lohmander, L.S.; Abbott, J.; Bhandari, M. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Mithoefer, K.; Williams III, R.J.; Warren, R.F.; Potter, H.G.; Spock, C.R.; Jones, E.C.; Wickiewicz, T.L.; Marx, R.G. The Microfracture Technique for the Treatment of Articular Cartilage Lesions in the Knee: A Prospective Cohort Study. J. Bone Jt. Surg. 2005, 87, 1911–1920. [Google Scholar] [CrossRef]
- Hangody, L.; Füles, P. Autologous Osteochondral Mosaicplasty for the Treatment of Full-Thickness Defects of Weight-Bearing Joints: Ten Years of Experimental and Clinical Experience. J. Bone Jt. Surg. 2003, 85, 25–32. [Google Scholar] [CrossRef]
- Le, J.; Peng, Q.; Sperling, K. Biochemical Magnetic Resonance Imaging of Knee Articular Cartilage: T1rho and T2 Mapping as Cartilage Degeneration Biomarkers. Ann. N. Y. Acad. Sci. 2016, 1383, 34–42. [Google Scholar] [CrossRef]
- Nissi, M.J.; Rieppo, J.; Töyräs, J.; Laasanen, M.S.; Kiviranta, I.; Nieminen, M.T.; Jurvelin, J.S. Estimation of Mechanical Properties of Articular Cartilage with MRI–dGEMRIC, T2 and T1 Imaging in Different Species with Variable Stages of Maturation. Osteoarthr. Cartil. 2007, 15, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Bini, F.; D’Alessandro, S.; Pica, A.; Marinozzi, F.; Cidonio, G. Harnessing Biofabrication Strategies to Re-Surface Osteochondral Defects: Repair, Enhance, and Regenerate. Biomimetics 2023, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, M.; Yin, Z.; Magin, R.L. Magnetic Resonance in the Assessment of Tissue Engineered Cartilage. In Biophysics and Biochemistry of Cartilage by NMR and MRI; Xia, Y., Momot, K., Eds.; The Royal Society of Chemistry: London, UK, 2016; pp. 529–551. ISBN 978-1-78262-133-1. [Google Scholar]
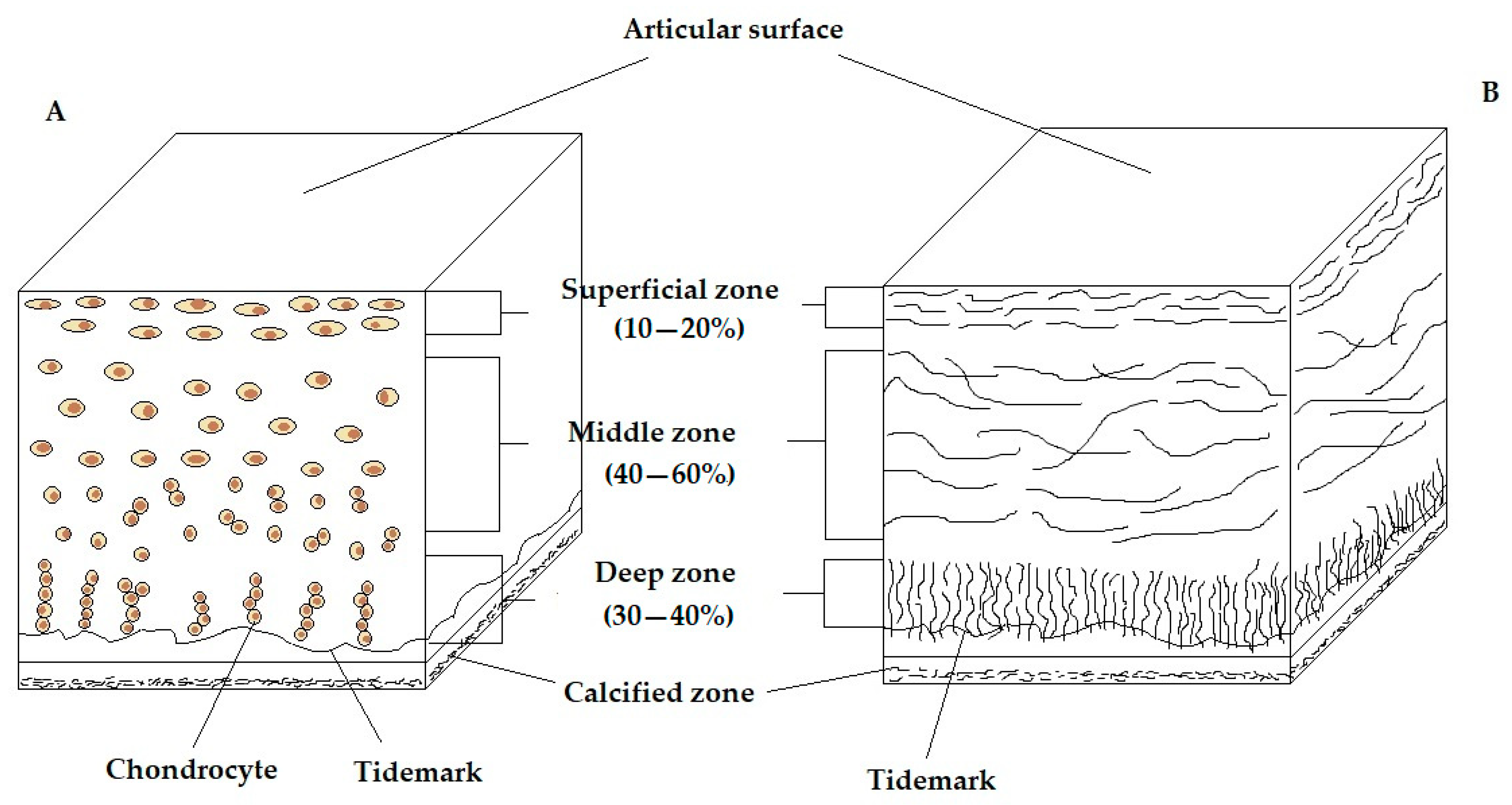

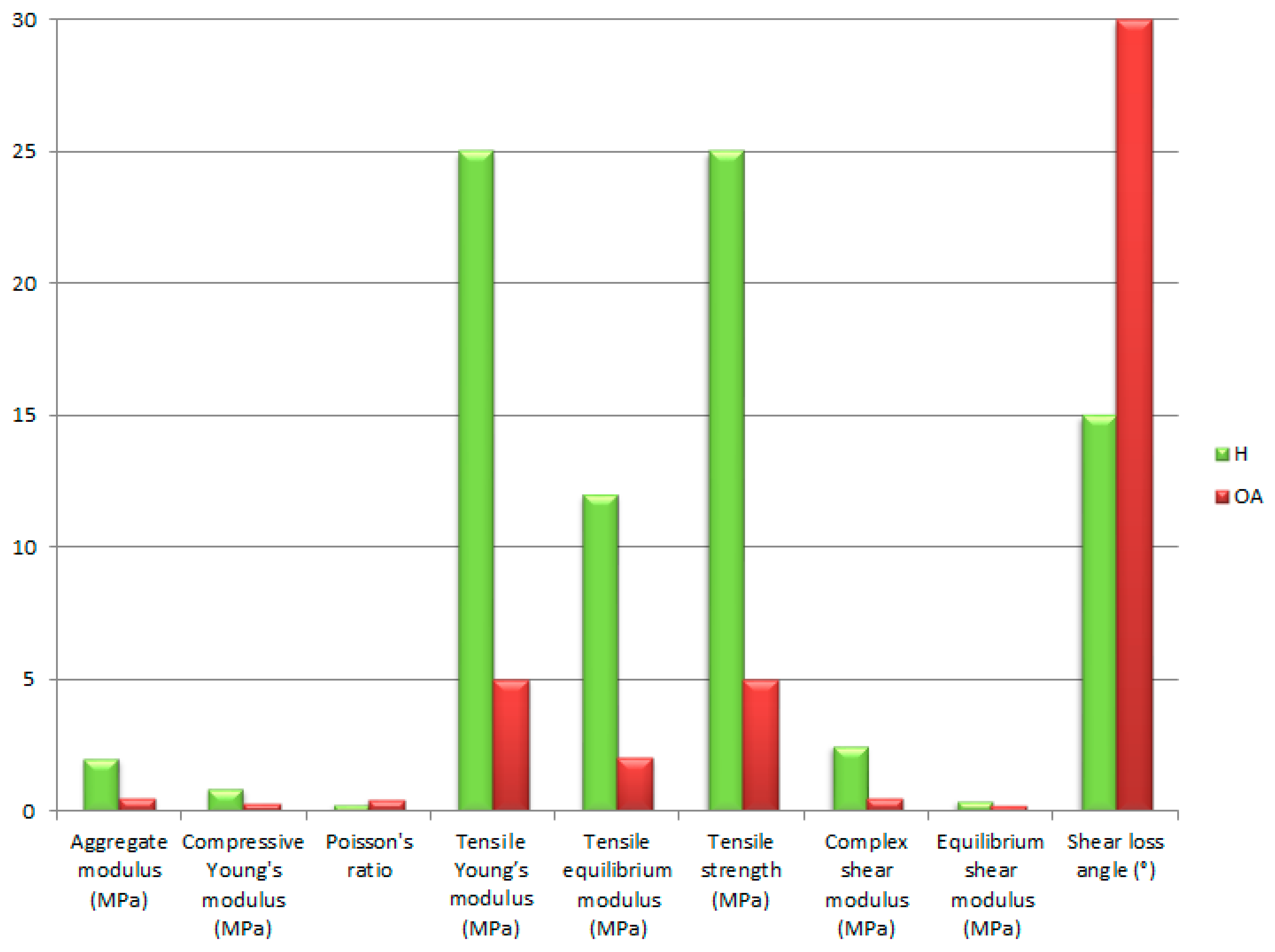
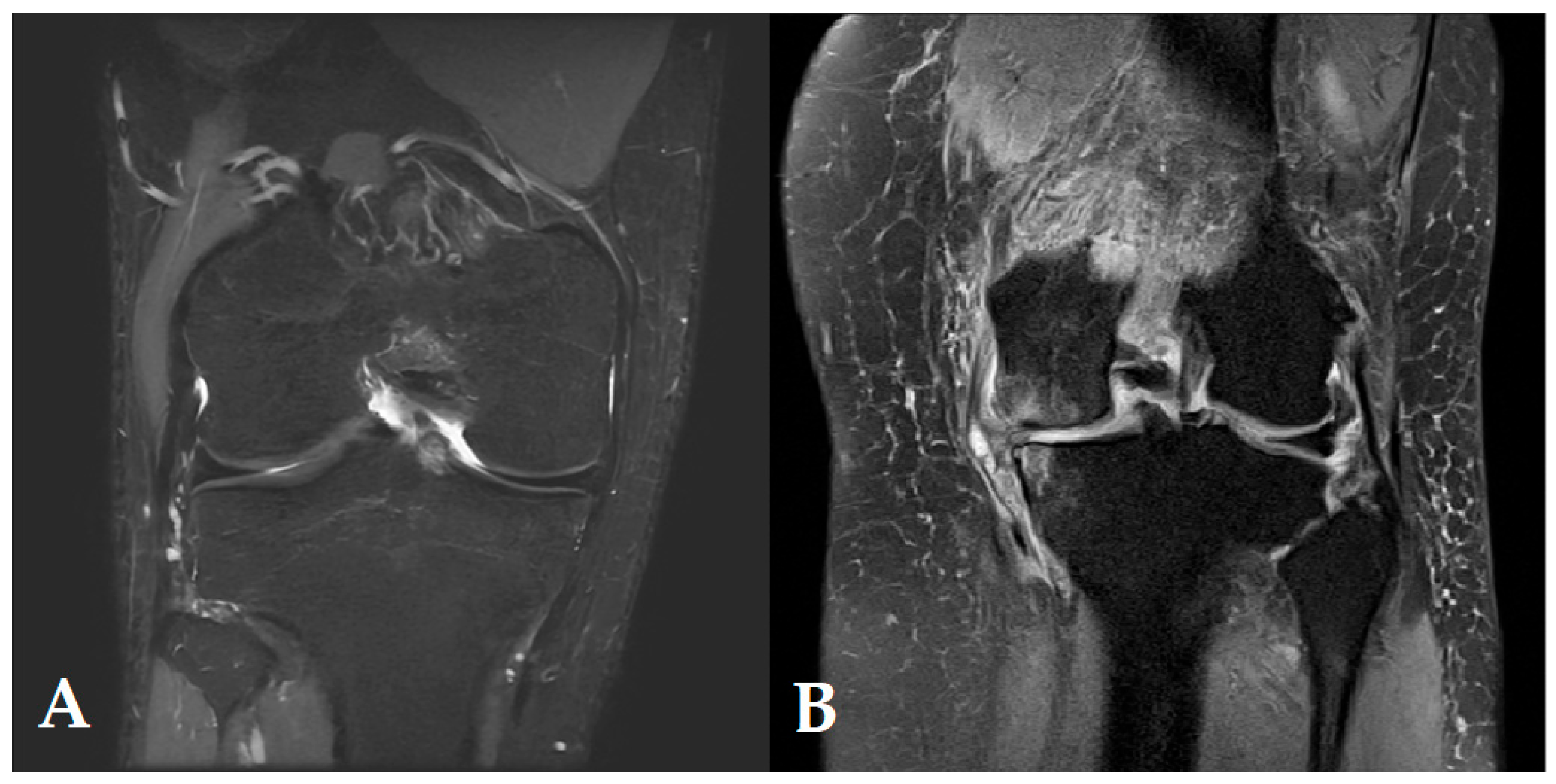
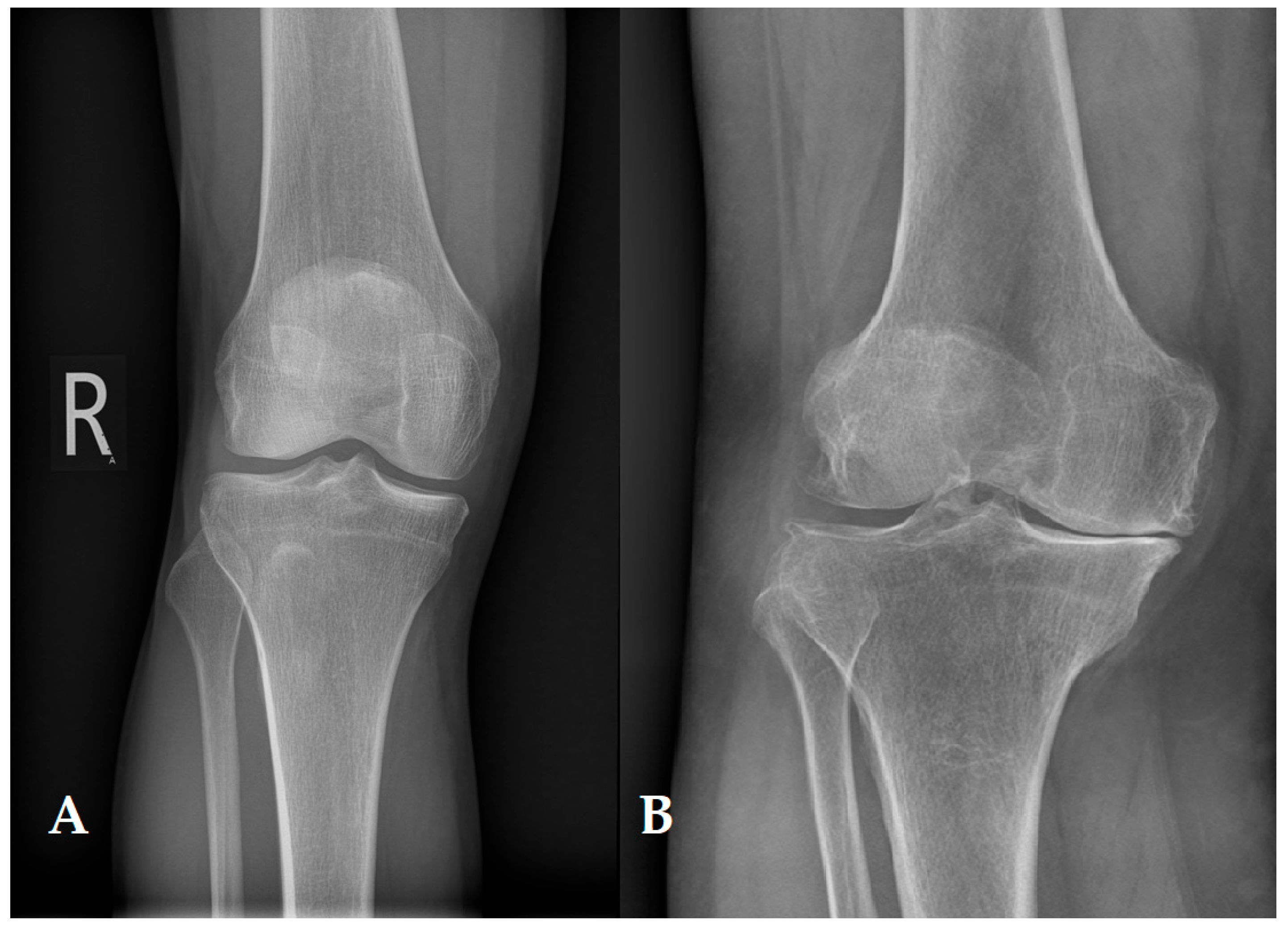

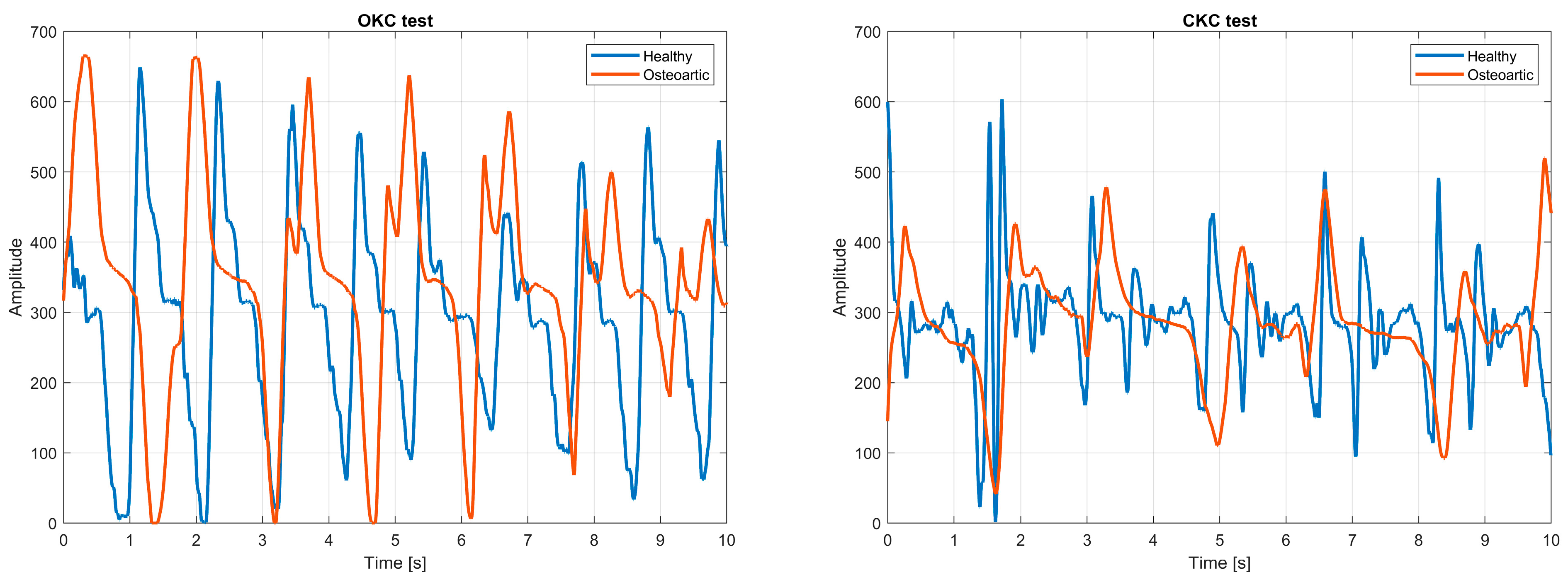
| Classification | Type | Molecules | Concentrations [%] |
|---|---|---|---|
| II | 3 × α1(II)—triple helix | 90–95 | |
| Fibrillar | XI | α1(XI), α2(XI), α3(XI) | 3–5 |
| V | α1(V), α2(V), α3(V) | <1 | |
| Fibrillar-associative (FACIT) | IX | 2 × α1(IX), α2(IX)—collagen side-bonding | 1–2 |
| Microfibrillar | VI | 3 × α1(VI)—chondrocyte envelope | 1–2 |
| Cross-linking | X | Homo-trimers α1(X)—calcified zone | <1 |
| Parameter | Typical Value | Measurement Method | Author |
|---|---|---|---|
| Aggregate modulus (MPa) | 0.1–2.0 | Confined compression tests, indentation | Mow et al. [51] Mow et al. [128] Jurvelin et al. [172] Mak et al. [178] |
| Compressive Young’s modulus (MPa) | 0.24–0.85 | Unconfined compression tests | Mow et al. [51] Mow et al. [173] Jurvelin et al. [172] |
| Hydraulic permeability (m4/Ns) | 10−16–10−15 | Confined compression tests, unconfined compression tests, indentation | Mansour [45] Mow et al. [173] |
| Poisson’s ratio | 0.06–0.3 | Unconfined compression tests, indentation | Mow et al. [51] Jurvelin et al. [172] Moroni et al. [179] Jurvelin et al. [180] |
| Tensile Young’s modulus (MPa) | 5.0–25.0 | Tensile constant strain rate | Mow et al. [51] Williamson et al. [174] Williamson et al. [181] |
| Tensile equilibrium modulus (MPa) | 5.0–12.0 | Tensile stress relaxation | Korhonen et al. [182] |
| Tensile strength (MPa) | 8–25.0 | Tensile constant strain rate | Roth et al. [154] |
| Complex shear modulus (MPa) | 0.2–2.5 | Dynamic shear | Setton et al. [156] LeRoux et al. [176] Zhu et al. [175] |
| Equilibrium shear modulus (MPa) | 0.05–0.4 | Equilibrium shear | Spirt et al. [183] LeRoux et al. [176] |
| Shear loss angle (°) | 10–15 | Dynamic shear | LeRoux et al. [176] Moroni et al. [179] Zhu et al. [175] |
| Measurement Method | Measured Parameters | Test Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Confined compression test | Aggregate modulus, Hydraulic permeability | Cartilage compressed in a cylindrical chamber, without lateral expansion | Measurement of liquid-solid properties (biphasic model) | No mapping of natural anatomical conditions | Patel et al. [184] Mow et al. [128] |
| Unconfined compression test | Compressive Young’s modulus, Hydraulic permeability, Poisson’s ratio | Cartilage compressed freely vertically, with possible outflow of fluid and lateral expansion | Simple to carry out, well modelled | Strong dependence of the results on the boundary conditions and sample geometry | Patel et al. [184] Mow et al. [128] |
| Indentation test | Aggregate modulus, Hydraulic permeability, Poisson’s ratio | Local indentation pressure on the cartilage surface | Possibility of in situ and in vivo testing | High sensitivity to tissue heterogeneity and alignment | Patel et al. [184] Jurvelin et al. [172] |
| Tensile constant strain rate | Tensile Young’s modulus, Tensile strength | Cartilage stretching at a constant speed | Mapping of collagen fibre behaviour | Difficulties with sample preparation, risk of damage | Little et al. [177] |
| Tensile stress relaxation | Tensile equilibrium modulus | Maintaining constant strain and observing stress reduction | Analysis of viscoelastic properties | Long measuring time | Little et al. [177] |
| Dynamic shear test | Complex shear modulus, Shear loss angle | Cyclic shear load application | Dynamic response analysis, testing of viscoelastic properties | Complex apparatus, model-dependent interpretation | Zhu et al. [175] |
| Equilibrium shear test | Equilibrium shear modulus | Slow deformation until equilibrium is reached | Measurement of elasticity after stabilisation of fluid flow | Long testing time, requires precision | Zhu et al. [175] |
| Modality | Main Strengths | Key Limitations |
|---|---|---|
| MRI | High-resolution soft tissue imaging; good for cartilage morphology; non-invasive | Limited sensitivity to early biochemical changes; long scan times; expensive |
| CT-Arthrography | Excellent surface detail; useful in patients with MRI contraindications | Radiation exposure; requires contrast agent; not suitable for repeated use |
| Ultrasound | Low cost; widely available; real-time assessment | Low spatial resolution; operator dependent; limited to surface evaluation |
| MRE | Quantitative stiffness mapping; non-invasive biomechanical insight | Specialised sequences required; limited availability; long acquisition times |
| T2/T1ρ/dGEMRIC MRI | Sensitive to biochemical changes; useful for early OA detection | Requires special protocols or contrast agents; expensive; time-consuming |
| Ultrasound Elastography | Provides mechanical property estimates; fast | Surface-limited; low penetration; operator-dependent |
| Vibroarthrography (VAG) | Functional, real-time mechanical assessment; simple setup | Low spatial specificity; experimental; signal variability |
| OCT | Micron-level resolution; near-histological visualisation | Shallow penetration depth; limited to intra-articular or open procedures |
| Raman Spectroscopy | Molecular composition profiling; label-free biochemical insights | Low depth penetration; motion sensitive; experimental |
| Modal Analysis | Provides dynamic mechanical parameters; sensitive to tissue degradation | High experimental complexity; limited to research applications |
| Modality | Diagnostic Accuracy | Invasiveness | Operator Dependence | Clinical Readiness | Ability to Assess Biomechanics | Other Notes |
|---|---|---|---|---|---|---|
| MRI | High for structure, moderate for early OA | Non-invasive | Low | Widely available | Indirect (via advanced protocols) | High cost; long exam duration; sensitive to motion artifacts |
| CT-Arthrography | High for surface integrity | Minimally invasive (contrast + radiation) | Low | Available, especially when MRI is contraindicated | No | Risk of infection, allergic reaction; repeated use limited due to radiation |
| Ultrasound | Low-moderate (surface only) | Non-invasive | High | Widely available | No | Inexpensive; real-time imaging; limited depth penetration |
| MRE | Emerging; promising for stiffness maps | Non-invasive | Moderate | Limited clinical use | Yes (shear stiffness mapping) | Requires advanced MRI hardware and postprocessing |
| T2/T1ρ/dGEMRIC MRI | High (biochemical properties) | Non-invasive | Low | Research settings | Indirect (composition- based) | Enables early detection of degeneration; requires contrast (dGEMRIC) |
| Ultrasound Elastography | Moderate (surface stiffness) | Non-invasive | Moderate | Limited clinical use | Yes (local stiffness) | Operator-dependent; resolution limitations |
| Vibroarthrography (VAG) | Moderate (early degeneration detection) | Non-invasive | Moderate | Experimental | Yes (mechanical friction detection) | Simple to use; promising for dynamic functional assessment |
| OCT | High (microscopic structure) | Minimally invasive (intra-articular) | High | Experimental | No | High-resolution imaging; limited to accessible joints (e.g., during arthroscopy) |
| Raman Spectroscopy | High (biochemical markers) | Minimally invasive | Moderate | Experimental | Indirect (molecular composition) | Promising for collagen/proteoglycan content analysis |
| Modal Analysis | Moderate (mechanical dynamics) | Non-invasive | High | Experimental | Yes (dynamic stiffness) | Requires controlled loading; research-stage tool |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, R.; Prus, A.; Baj, J.; Radej, S.; Prządka, M.; Krakowski, P.; Jonak, K. Articular Cartilage: Structure, Biomechanics, and the Potential of Conventional and Advanced Diagnostics. Appl. Sci. 2025, 15, 6896. https://doi.org/10.3390/app15126896
Karpiński R, Prus A, Baj J, Radej S, Prządka M, Krakowski P, Jonak K. Articular Cartilage: Structure, Biomechanics, and the Potential of Conventional and Advanced Diagnostics. Applied Sciences. 2025; 15(12):6896. https://doi.org/10.3390/app15126896
Chicago/Turabian StyleKarpiński, Robert, Aleksandra Prus, Jacek Baj, Sebastian Radej, Marcin Prządka, Przemysław Krakowski, and Kamil Jonak. 2025. "Articular Cartilage: Structure, Biomechanics, and the Potential of Conventional and Advanced Diagnostics" Applied Sciences 15, no. 12: 6896. https://doi.org/10.3390/app15126896
APA StyleKarpiński, R., Prus, A., Baj, J., Radej, S., Prządka, M., Krakowski, P., & Jonak, K. (2025). Articular Cartilage: Structure, Biomechanics, and the Potential of Conventional and Advanced Diagnostics. Applied Sciences, 15(12), 6896. https://doi.org/10.3390/app15126896






