Abstract
Psoriasis is a chronic inflammatory skin disease with a complex clinical picture that remains incurable and requires ongoing dermatological care. The purpose of this literature review is to identify the latest therapeutic strategies used to treat psoriasis, taking into account both the efficacy of modern approaches and their impact on patients’ quality of life. The cornerstone of psoriasis treatment remains regular skin care through the use of emollients, which support the effectiveness of other topical therapies used such as corticosteroids. In addition, the review presents current epidemiological data on the prevalence of psoriasis worldwide, with a focus on European countries and Poland. The article discusses innovative approaches in therapy, including targeted biologics, modern forms of topical therapy, and the role of emollients in comprehensive skin care, as well as various cosmetological treatments aimed at removing the excessively accumulated stratum corneum, and improving the degree of hydration of psoriatic skin. The review also included current expert recommendations and guidelines from scientific societies that guide the treatment and care of psoriatic skin. The results of the analyses underscore the need for targeted therapies and the importance of a holistic approach to patient care. As the collected data indicate, the possibilities for effective topical and systemic treatment of psoriasis are increasing all the time due to research into newer and newer active substances that are expected to improve the efficacy of treatment, the comfort of life for psoriasis patients and reduce the side effects of long-term therapy.
1. Introduction
Psoriasis is a chronic, autoimmune, relapsing skin disease of multifactorial etiology that affects about 2–3% of the general population [1,2,3]. According to the authors [4,5], the prevalence of psoriasis is known in only one-fifth of all countries and is unevenly distributed in different geographic regions. Epidemiological data show that the amount of incidence in East Asia is 0.14% up to 1.99% in Australia [4,6]. According to an epidemiological analysis [6], the incidence of psoriasis was highest in high-income countries such as Australia, Western and Central Europe, and North America. The authors [4] noted that the incidence of psoriasis is closely related to ethnicity. Psoriasis is diagnosed more frequently in Caucasians, people who live in Central Europe (1.84%) and Western Europe (1.92%), and people from North America (1.50%) [6].
The authors [1,4] report that psoriasis occurs with the same frequency in both sexes and its first symptoms can manifest at any age, but two peaks of incidence are noted. In men, this is 30–39 years or 60–69 years, while in women, psoriasis can appear at 18–29 years or 50–59 years. The average age of its onset is 33 years [5]. In the pediatric population, on the other hand, the authors [4] indicate that psoriasis is not common in this age group, as the prevalence is 0.13% in children aged 0–2 years to 0.67% in children aged 14–18 years.
The authors point out that the quality of life of patients with psoriasis is significantly impaired, which is associated not only with the presence of increased skin symptoms such as desquamation and pruritus, but also with significant psychosocial consequences, including social stigmatization and increased risk of psychiatric disorders. As studies [3,5] show, compared to the general population, patients with psoriasis are significantly more likely to be depressed (up to 20%) without excluding thoughts and attempts related to suicide.
Nowadays, psoriasis is not treated only as an inflammatory disease, but as a condition whose effects are systemic and increase the risk of coexisting metabolic syndrome, psoriatic arthritis, or depression [2,5]. Both genetic, immunological and environmental factors such as streptococcal infection, stress, smoking, obesity or alcohol consumption are involved in its etiopathogenesis [1]. Recent immunological studies conducted on the pathogenesis of psoriasis have identified interleukin-17 (IL-17) and interleukin-23 (IL-23) as a major pathogenetic factor, thereby highlighting the importance of the immune system in the course of psoriasis [5]. The interaction of IL-23 and IL-17 influences inflammation, which increases during the active phase of the disease, which triggers the body’s pro-inflammatory response, resulting in the activation and proliferation of keratinocytes [7]. The authors [8] identify IL-23 as the main cytokine in psoriatic disease. In addition to interleukins 17 and 23, other pro-inflammatory cytokines also play an important role in the etiopathogenesis of psoriasis, such as tumor necrosis factor alpha (TNF-α), interleukin 12 (IL-12), interleukin 36 (IL-36), and interleukin 6 (IL-6), which are important therapeutic targets in current biological therapies [5,7,8]. Targeted biological action on specific cytokines has revolutionized the treatment of psoriasis, surpassing traditional immunosuppressive therapies in terms of efficacy and safety. With this approach, a new standard in therapy has been achieved, based on precise immunomodulation [8]. The authors [2,5,7] point out that psoriasis is currently an incurable disease. The treatment of psoriasis is directed in two ways: through the use of topical preparations and the use of broad-spectrum systemic treatment. Topical treatment is a fundamental and essential part of psoriasis therapy, regardless of the severity or stage of the disease. Although it is primarily recommended for patients with mild or limited psoriasis, it also plays an important role in more severe cases and during systemic or biological treatment [9]. It involves the use of exfoliating drugs in the first stages of treatment, followed by anti-inflammatory and keratosis-regulating drugs. Systemic treatment, on the other hand, should be used for psoriasis of moderate to severe severity [9]. The authors [9,10] emphasize that systemic corticosteroids should not be used in the treatment of psoriasis because, despite their anti-inflammatory effect, they are associated with a high risk of relapse and exacerbation of psoriatic lesions when the dose is reduced or discontinued. Topical therapies, phototherapy, oral systemic therapies, and biologics are the standard of care for psoriasis [11]. According to the authors [9], an important factor in the treatment of psoriatic skin is skin care. Regardless of the severity of the disease and the therapeutic strategy taken, care is an important link in treatment. Regular care of psoriatic skin reduces the sensation of pruritus or exacerbation of lesions, and helps prolong the remission period. In particular, emollients are indicated by the authors as safe in the daily routine of patients with psoriasis, as they soothe skin lesions, reduce skin irritation and the risk of disease exacerbations [12].
In this review, the authors highlighted the role and effectiveness of emollients and other skin care products as primary therapy in the treatment of psoriasis, focusing primarily on new data and studies completed in this area. The authors pointed out that psoriatic skin care, in combination with other treatment strategies, is the cornerstone of the psoriasis treatment regimen.
2. Mechanisms of Changes Occurring in Psoriatic Skin—Characteristics of the Clinical Picture of Psoriasis
Psoriasis is a disease that occurs with increased epidermal proliferation [1,13]. The characteristic change in the structure of the skin that occurs in psoriasis is parakeratosis, or abnormal keratinization of the epidermis. It involves the presence of cell nuclei in the cells of the stratum corneum, which is a deviation from the norm, since in healthy skin these nuclei disappear during the process of keratinization. In the course of psoriasis, atrophy of the stratum granulosum of the epidermis is observed, with a concomitant hypertrophic increase in the stratum spinosum and stratum corneum, which is characteristic of the processes of acanthosis and hyperkeratosis. In addition, neutrophils accumulate in large numbers between epidermal cells, forming Munro’s microabscesses [13]. In addition to changes in the epidermis, psoriasis also disrupts the dermis—through elongation and hypertrophy of dermal papillae, and accumulation of lymphocytes and neutrophilic granulocytes. The authors [13] report that in the skin of psoriasis patients, capillaries are located shallowly and are tortuous and dilated, leading to Auspitz sign.
The primary psoriatic lesion is a reddish-brown colored papule that remains clearly demarcated from its surroundings [1]. Based on the size of the lesion, psoriasis is differentiated as punctate, droplet-like, plaque-like, and geographic. Psoriatic papules remain covered with silvery-white scales of varying layering [1]. In addition, patients in the course of psoriasis often report severe pruritus [13]. The most typical locations of psoriatic lesions are the knees, elbows, scalp, sacral region, and upright surfaces of the extremities [14]. The authors [13] point out that the severity of skin lesions appears in spring and autumn, which is determined by more frequent infections, decreased efficiency of the immune system and less sunshine. According to the authors [1,13], the characteristic clinical signs of psoriasis are the stearic candle sign, Auspitz sign and Koebner sign (Table 1).

Table 1.
Characteristic symptoms of psoriasis based on [1,13,15,16].
The authors [1] indicate that psoriasis can be divided into a juvenile type, the first symptoms of which appear between the ages of 20 and 40. This type is characterized by a severe course, high resistance to treatment, association with autosomal dominant inheritance and the presence of the HLA-Cw6 antigen. Adult psoriasis, on the other hand, occurs mainly in people over 40 years of age, is characterized by a milder course, despite a greater likelihood of joint and nail involvement, the absence of an increased family history, and the presence of the HLA-Cw2 antigen [1,14].
Clinically, several types of psoriasis are distinguished, but the most commonly diagnosed is plaque psoriasis vulgaris [17]. Other types of psoriasis include pustular, arthritic and nail psoriasis, as well as inverse and generalized psoriasis, (Table 2). In rare cases, psoriasis can involve the mucous membranes of the mouth and genitalia [14].

Table 2.
Clinical forms of psoriasis and their characteristics based on [13,14,17].
3. Ways to Assess Psoriasis Severity and Therapeutic Strategies for Psoriasis Treatment
Psoriasis, due to its genetic background, remains a chronic and incurable disease characterized by frequent relapses, which necessitates the use of therapy based on an empirical approach [18,19]. These authors emphasize that the goal of its treatment is primarily to reduce skin lesions and alleviate symptoms, which contributes to prolonged periods of remission. In addition, therapy aims to prevent the onset of complications that can lead to more severe forms of the disease and to improve the patient’s quality of life by restoring comfort and function [19].
Modern therapeutic approaches include a range of methods and regimens with varying efficacy to achieve control over the course of the disease. Primary treatment strategies for psoriasis include topical and systemic therapy, as well as physical therapy, which includes phototherapy and photochemotherapy. There are also complementary, alternative methods, which include dietary, nutritional changes, among others [19,20] (Table 3).

Table 3.
Methods used to treat psoriatic skin [19].
The choice of the appropriate treatment should be tailored to the presented clinical picture of the disease, taking into account the type of psoriasis and its severity [20]. Thus, the key factor determining the effectiveness of psoriasis therapy is the appropriate classification of the nature of the disease based on the proper physical evaluation of psoriatic lesions by the doctor and the subjective feelings of the patient. The only tools available to make the aforementioned assessment of skin lesions are measurement scales, among which the Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA) and Dermatology Life Quality Index (DLQI) scales are the most commonly used [21,22] (Table 4).

Table 4.
Scoring scheme according to the three clinically most important scales based on [22,23].
3.1. Methods for Determining the Severity of Psoriasis
According to the recommendations of the Polish Society of Dermatology [23] and the British Society of Dermatology [24], the so-called Rule of Tens is used to assess the severity of psoriasis, which is particularly applicable to psoriasis vulgaris. According to this rule, mild psoriasis is considered to be cases for which the skin lesions do not exceed 10% of the total body surface area (BSA < 10%—body surface area index), their severity index does not exceed 10 points (PASI < 10 points—Psoriasis Area and Severity Index), and the quality of life expressed by the DLQI is less than 10 points (DLQI < 10 points—Dermatology Life Quality Index). Other psoriasis cases, for which BSA ≥ 10% or PASI ≥ 10 points or DLQI ≥ 10 points, fall into the category of psoriasis of at least moderate severity (Figure 1). Currently, insufficient data have been collected to clearly delineate moderate from severe psoriasis [23]. Established delineation of psoriasis severity enables the selection of an appropriate and most beneficial treatment strategy for the patient [23].
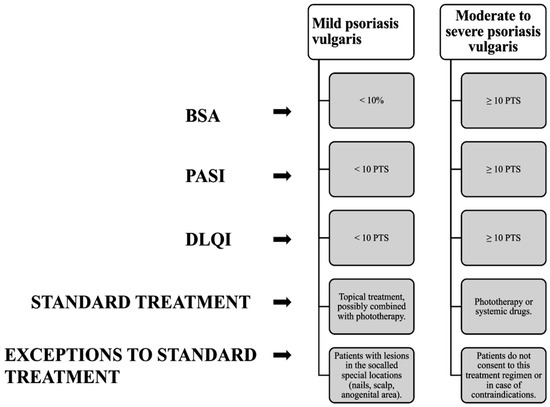
Figure 1.
Diagram showing how to classify the severity of psoriatic lesions based on [23].
3.2. Therapeutic Strategies in the Treatment of Psoriasis
3.2.1. Topical Treatment
According to the recommendations of the Polish Society of Dermatology [23] and the standard way of classifying psoriatic lesions (Figure 1), patients presenting a picture of mild disease are qualified for topical treatment of psoriasis. The multitude of patients qualified for topical treatment [18] makes this method an extremely important element of therapeutic success in these patients.
In the first stage of topical treatment, the most important thing is proper skin preparation, which will allow drugs to penetrate deep into the emerging skin lesions and increase their effectiveness [23]. Removal of the skin scales covering the psoriatic lesions, which are an obstacle to the penetration of antipsoriatic drugs deep into the lesions, is a prerequisite for an effective course of topical treatment [23]. Preparations that soften the keratinized layer of the epidermis [19] and remove the crusts covering the scales include keratolytic preparations (drugs containing urea, salicylic acid, lactic acid, sulfur) [23]. A commonly used agent with proteolytic, keratolytic, penetration-enhancing and epidermal-thinning effects is urea. The results of the study presented by the authors [25] confirmed the above-mentioned actions of urea in monotherapy, as well as in combined therapy, but also proved that its use is safe and associated with mild side effects (mild irritation). Another preparation with a keratolytic effect is salicylic acid. It has the most effective effect on concentrations equal to 5% and higher. The mechanism of action of salicylic acid is to reduce intercellular cohesion and lower the pH of the stratum corneum, which increases the possibility of penetration of therapeutic preparations deep into the skin [25]. The effectiveness of this substance in the treatment of psoriatic skin was confirmed by the authors [26], who conducted a study on a group of patients with scalp psoriasis. The subject of the study was a foam containing 6% salicylic acid, the application of which resulted in complete removal of psoriatic lesions in 60% of the subjects. The same authors confirmed that salicylic acid has many adverse effects (systemic intoxication, central nervous system symptoms, tinnitus, hyperventilation). In their opinion, the validity of its use is still a controversial topic [25]. Emollients are also a group of topical preparations [23]. Their occlusive action minimizes the evaporation of water from the deeper layers of the skin and allows hydration of the stratum corneum [25]. On the other hand, the results of clinical studies presented by the authors [25], which included a total of 111 patients, allowed the thesis that the effectiveness of emollients in monotherapy is limited. However, the Polish Dermatological Society recommends the use of these substances for psoriatic skin [23]. Along with the duration of topical therapy, combination preparations (calcipotriol combined with betamethasone), topical corticosteroids, vitamin D3 analogs and calcineurin inhibitors, as indicated by the authors [18,23,27], are included in the treatment of psoriasis. The essential role of glucocorticosteroids is to neutralize inflammation, have antiproliferative, immunosuppressive, and vasoconstrictive effects [27,28]. The choice of the appropriate glucocorticosteroid preparation is related to the level of severity of the skin lesion, its location and the age of the patient [28]. The classifications presented by the authors [29] distinguish topical glucocorticosteroids by potency. The American classification distinguishes the division into VII groups, of which group I includes preparations with very high potency, and VII with the weakest potency [28,29]. The European classification lists IV groups of topical corticosteroids, but according to this division, class I includes preparations with the weakest potency, and group IV with the strongest potency [29]. Corticosteroids are effective drugs in monotherapy, as well as in polytherapy in combination with keratolytic agents, vitamin D analogs or retinoids [19,27]. Due to a large number of side effects, and because of their symptomatic effect, causing reappearance of symptoms [19], their method of use and dosage are still the subject of numerous clinical trials [28].
3.2.2. Use of Phototherapy in the Treatment of Psoriasis
Phototherapy is a method that uses light to treat psoriasis. It is a type of topical application of light in the 280–400 nm wavelength range to the affected skin [30]. It is available in the form of combination psoralen treatment with ultraviolet A radiation (PUVA), broad-band UVB (BB-UVB—280–315 nm) and narrow-band UVB (NB-UVB—311–313 nm). Narrow-band UVB is more effective than broad-band UVB and safer than PUVA [31]. NB-UVB is used as first-line therapy for psoriasis [30,31], while PUVA is being considered for more severe cases [30]. Accurate data on the course of narrow-band UVB therapy are provided by the authors [32]. Using NB-UVB therapy, selection of the appropriate dose is based on skin phototype or minimum erythema dose (MED). According to the recommendations of the American Academy of Dermatology and the National Psoriasis Foundation, the recommended and effective frequency of NB-UVB sessions is two or three times a week. A higher number is not associated with significant benefits for the patient and exposes him/her to side effects manifested as increased ocular toxicity (inflammation and corneal burns) and generalized photocarcinogenesis [32]. Another method used is PUVA, or UVA-based photochemotherapy, in which in addition, psoralens are used [30]. The value of the PASI-75 index obtained in the comparative studies conducted confirmed that monotherapy with PUVA shows greater efficacy compared to NB-UVB [32]. Despite the result obtained, the lower risk of adverse reactions and the lower cost of therapy determine that this narrow-band UVB is the treatment of choice used in psoriasis [32].
3.2.3. Systemic Treatment
In patients presenting psoriatic lesions classified as moderate to severe psoriasis, topical therapies, or phototherapy, prove ineffective [33]. In such patients, retinoids, immunosuppressive agents and biologic drugs are included in the therapy [19]. Many types of biologic drugs are used in treatment, and the basis of their mechanism of action is to block the activity of pro-inflammatory factors such as TNF-α, IL-17, IL-23, as well as neutralize lymphocytes [19,27]. An important agent in the fight against psoriasis is methotrexate, which has been used since 1972 and belongs to agents that exert an immunosuppressive effect [34]. Its mechanism of action is based on competitive inhibition of dihydrofolate reductase, which inhibits the synthesis of purine and pyrimidine nucleotides [34]. According to the authors [34], methotrexate given in low doses (<25 mg per week) reduces the proliferation of lymphoid cells and consequently alleviates the course of the disease. Other drugs in the same group, the use of which has been approved by the FDA, include the equally frequently used apremilast and cyclosporine [29,34]. A representative of retinoids effective in the treatment of psoriasis is azacitretin, a vitamin A derivative whose mechanism of action is not fully understood [34]. Its main function is modulation of epidermal differentiation and proliferation, as well as anti-inflammatory and immunomodulatory effects. According to studies presented by the authors [34], the use of aztrethin in combination with phototherapy is more effective than monotherapy.
3.3. New Therapeutic Approaches in the Treatment of Psoriasis
3.3.1. Topical Therapy
Despite the fact that topical agents for the treatment of mild to moderate psoriasis are the mainstay of treatment in these patients, progress in their development has remained limited over the years [35]. Defined as a cytosolic ligand-dependent transcription factor, the aromatic hydrocarbon receptor (AhR) is found in a variety of skin cell types, including (keratinocytes, fibroblasts and melanocytes) and plays an important role in the pathogenesis of psoriasis, and its modulator tapinarof, has become a topical therapeutic agent approved by the FDA in 2022 for the topical treatment of plaque psoriasis in adults [35,36]. The AhR receptor in healthy skin maintains homeostasis, regulates keratinocyte differentiation, maintains skin barrier function and gene expression [35]. Nogueira, M.R, R.V, T.T, highlighted the role of this receptor in the pathogenesis of psoriasis, in the course of which, within psoriatic plaques, AhR is overactivated and affects the induction of inflammation and the differentiation of keratinocytes and T lymphocytes. Animal studies described in [35] have shown that AhR deficiency leads to increased pro-inflammatory responses and increased production of cytokines such as IL-17 and IL-22, which contributes to the development of psoriasis-like inflammation. Tapinarof, as an AhR agonist, is a promising new therapeutic agent for the treatment of psoriasis [35]. Activation of AhR by tapinarof induces the production of antioxidant enzymes, reducing oxidative stress, and promotes the production of barrier proteins (loricrin, filaggrin), which strengthens the skin barrier [35]. In addition, it also affects the regulation of Th17 and Treg cells, reducing IL-17 expression and inflammation, and additionally exhibits antimicrobial activity, promoting beneficial changes in the skin microbiome and alleviating the course of the disease [35]. The validity of its use was confirmed by a randomized, double-blind Phase III study (PSOARING 1 and PSOARING 2), which evaluated the efficacy of 1% tapinarof cream applied once daily for 12 weeks [35]. The endpoint was PGA (Physician’s Global Assessment) response, defined as a score of 0 or 1 and an improvement of ≥2 degrees from baseline. In both studies, a significantly higher proportion of patients receiving tapinarof (35–40%) achieved a PGA response compared to the placebo group (6.0–6.3%). In addition, Tapinarof showed a favorable safety profile in both the short-term studies (PSOARING 1 and 2) and the long-term open-label study PSOARING 3. Adverse effects occurred in 50.3% (PSOARING 1) and 54.5% (PSOARING 2), respectively, but most were mild to moderate and resolved spontaneously. The most common conditions observed were folliculitis, contact dermatitis, pruritus and headaches. In the PSOARING 3 study, after 52 weeks of treatment, no new adverse reactions were observed. The frequency and severity of skin symptoms did not increase with the duration of therapy, and tolerability of the drug on the skin, including sensitive and flexural areas, was good. There was no accumulation of the drug in the body or increased risk of systemic effects such as cardiac arrhythmias or QT interval prolongation. Tapinarofem 1%-based formulation is an effective and safe agent for the treatment of patients with psoriasis. It is among the alternative therapies in the treatment of psoriasis, enriching the existing group of preparations with proven efficacy in the topical treatment of psoriasis [35].
3.3.2. Biologic Drugs as a New Era of Psoriasis Treatment
To date, psoriasis treatment approaches, which include ultraviolet radiation therapy and topical drug therapy, have shown the greatest efficacy in patients with mild to moderate psoriasis [23,34]. In moderate to severe cases, however, the efficacy of these therapeutic strategies may be limited. When used as monotherapy or as part of combination therapy, biologic drugs have high efficacy and a favorable safety profile, making them an important tool in the treatment of advanced forms of psoriasis [34,37,38]. The therapeutic success of biologic drugs, which are most often peptides, is due to their ability to mimic the function of human proteins. This allows them to modulate cellular receptor activity and intermolecular interactions [38]. Biological agents so far used in the treatment of moderate-to-severe plaque psoriasis include drugs with mechanisms of action based on inhibition of various inflammatory pathways, including tumor necrosis factor alpha (TNF-α), interleukins 17, 23 and 36 (IL-17, IL-23, IL-36), Janus kinase (JAK), nuclear receptors RORγt and protein kinase ROCK2 [27].
Interleukin-17 Inhibitors
The most recent biologic drugs registered by the European Medicines Agency (EMA) that interact with the IL-17 ligand or its receptor include those listed in (Figure 2). The interleukin-17 (IL-17) family of cytokines, which includes the IL-17A-F isoforms, plays an important role in the intercellular signaling network that is responsible for maintaining local tissue homeostasis [39]. These cytokines are involved in the recruitment of neutrophils within psoriatic lesions, contributing to the inflammation associated with psoriasis [40].
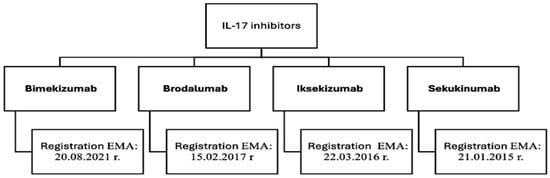
Figure 2.
Biologic drugs that act by inhibiting IL-17 [34,37,38,41].
The most recently registered and approved (Figure 2) biologic in the therapeutic area of psoriasis is bimekizumab. It is a humanized, monoclonal antibody of the IgG1 subclass [38] that binds to interleukins IL-17A, IL-17F and IL-17AF, which are transmitter molecules responsible for the body’s natural defense mechanisms [41,42]. This combination prevents the interaction of the aforementioned cytokines with specific receptors, which reduces inflammation and alleviates disease symptoms. According to studies, the dual potent and selective neutralization of IL-17A and IL-17F shows greater efficacy compared to previously registered secukinumab [39,42]. Bimekizumab has emerged as a new and promising form of therapy for psoriatic lesions [42].
Brodalumab has shown high therapeutic efficacy in patients for whom previous treatment with other IL-17 inhibitors has been ineffective [38]. Its unique mechanism of action involves blocking the IL-17 receptor and neutralizing IL-17A, IL-17F, IL-17A/F, IL-17C and IL-17E isoforms, resulting in reduced pro-inflammatory activity and improved therapeutic outcomes [34,37]. Brodalumab shows comparable antipsoriatic effects to ixekizumab, a slightly earlier registered IL-17 inhibitor [38]. Ixekizumab is a humanized IgG4 monoclonal antibody with high affinity for IL-17A and IL-17A/F [34,37,38]. It has been singled out by the Food and Drug Administration (FDA) as the only drug recommended for the treatment of genital psoriasis [34].
Interleukin 23 Inhibitors
Guselkumab, risankizumab and tildrakizumab are the most recent and effective interleukin 23 (IL-23) inhibitors used in the treatment of psoriasis (Figure 3). Interleukin 23 plays a key role in the pathomechanism of psoriasis, primarily through the activation and maintenance of the Th17 lymphocyte population [38]. It stimulates the proliferation and survival of these cells, leading to the release of pro-inflammatory mediators, such as IL-17 and IL-22, which increase inflammation and promote keratinocyte proliferation [38,40].
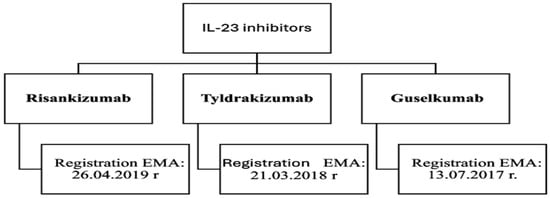
Figure 3.
Biologic drugs—interleukin 23 inhibitors [34,37,38].
Risankizumab is a humanized IgG1 monoclonal antibody that selectively blocks IL-23 by binding to the p19 subunit [34,37]. The drug, acting by inhibiting IL-23, suppresses IL-23-dependent intercellular signaling and the release of pro-inflammatory cytokines [43]. The efficacy of risankizumab is often compared to ustekinumab-a human IgG monoclonal antibody that binds to the p40 subunit common to both IL-12 and IL-23 [27]. Risankizumab does not affect IL-12, which promotes the development of Th1 lymphocytes responsible for the immune response and suppression of inflammatory lesions [40]. Its selectivity for IL-23 makes it more advantageous for long-term therapy of psoriasis [43]. Studies have shown that this drug has greater efficacy compared to ustekinumab, adalimumab and secukinumab [38]. Another relatively new human monoclonal antibody showing an identical mechanism of action to risankizumab is tildrakizumab. This drug has high efficacy, a low relapse rate, and is safe for the treatment of moderate to severe psoriasis [44].
4. Emollients and Their Role in the Treatment of Psoriasis
4.1. Lipid Emollients
Emollients are substances and preparations for topical application in the form of a gel, cream, lotion, or ointment. The most important goal of using emollients is to restore the disrupted epidermal barrier and maintain a normal structure with an appropriate composition for as long as possible [45]. Emollients should not contain allergens and irritants. The application of a formulation with such a composition to damaged skin can cause additional breach of the skin barrier, which can manifest as inflammation, swelling, or irritation [46].
In patients with mild psoriasis, the use of topical emollients is the mainstay of therapy [46]. Regular use of emollients maintains the tightness of the stratum corneum and increases the water-binding capacity of the stratum corneum, resulting in reduced desquamation [47]. The moisturizers in emollients also help normalize hyperproliferation, differentiation and apoptosis [48]. With the use of a moisturizer, damaged skin returns to its normal structure in a process in which lipids play a major role [49]. Skin that contains adequate amounts of lipids, ceramides, fatty acids, and cholesterol forms an airtight lipid barrier. When deficiencies in skin-building components occur, microbial ingress, water loss, and skin cracking occur, ultimately exacerbating psoriatic lesions [50]. Physiological lipids accumulate and are transported between keratinocytes, thus causing the skin barrier to seal [49]. In moderate and severe psoriasis, the use of emollients alone in therapy is insufficient. They can act as an adjunct to phototherapy or glucocorticosteroid treatment [46]. In 2021, Daniel Maroto-Morales et al. [51] conducted a study on the impact of emollients on epidermal barrier function in patients with psoriasis. The study involved patients aged 18 to 65 years. Skin changes were assessed before and after the application of two emollients: one being petrolatum and the other a water-based formulation without added lipids, containing the following ingredients: emulsifier base NEO PCL O/W, distilled water, Phenonip, and glycerol. Measurements of transepidermal water loss (TEWL) and stratum corneum hydration (SCH) were performed. The results demonstrated a decrease in TEWL following the application of petrolatum, while SCH increased after the application of both the moisturizing agent and petrolatum. Based on the findings, it can be concluded that the simultaneous use of moisturizing emollients and lipid-based agents is justified in order to achieve optimal therapeutic outcomes [51].
4.2. Occlusive Emollients
The reduction in water loss through the epidermis is mediated by the occlusive layer formed by emollients [25]. This occlusive layer is created by emollients containing occlusive agents such as mineral oils, petrolatum, lanolin, and waxes [45]. One such naturally occurring substance is beeswax. The study demonstrated a decrease in TEWL, indicating the preservation of skin barrier integrity. This study confirmed the ability of the mentioned substance to form a protective layer on the skin’s surface, which can protect the skin from irritating factors [52]. In 2019, M.-Q. Man et al. [53] conducted a study in which they demonstrated that emollients improve skin functionality while alleviating symptoms and reducing the recurrence of skin lesions. During the study, patients underwent local treatment, with an emollient or barrier cream possessing properties that support the restoration of epidermal barrier integrity applied to one arm. The mechanism of action of the product is based on improving the structure of the stratum corneum to reduce the clinical symptoms of psoriasis. The other arm remained untreated and served as a control. The study showed that the application of emollients to psoriatic skin lesions delayed the onset of relapses, with this effect observed in more than 50% of the participants [53]. An important argument for using emollients is that they soften scales, which enhances the absorption of locally applied medications for psoriatic lesions [52]. Furthermore, Xia Li et al. [54] demonstrated that emollients containing moisturizing agents, when combined with corticosteroids, increase the effectiveness of treatment. One group of patients in the aforementioned study used only corticosteroids (CS), while the other applied a moisturizing agent after the corticosteroid treatment. The results showed that the use of an emollient enriched with moisturizing substances reduced the relapse rate of psoriatic skin lesions. Additionally, patients who used the emollient showed improvement in skin dryness and scale shedding parameters [54].
4.3. Emollients “Plus”
It is common to enrich emollients with additional natural substances. Such action is aimed at enhancing the properties of the applied substance with, for example, anti-inflammatory, antipruritic or immunomodulatory effects [55]. The enrichment of emollients with other specific substances shows a synergistic effect in the treatment of psoriatic lesions compared to basic emollients such as petroleum jelly or paraffin wax [56]. The enrichment of emollients with additional substances and the positive effects resulting from their use have been identified by the authors of papers [54,57,58]. Studies have shown that emollients occurring with substances such as ceramides, dexpanthenol, celastor or polidocanol have shown an enhanced therapeutic effect in the treatment of psoriasis skin. One of the studied ingredients with positive effects on psoriatic skin is ceramides, which belong to the sphingolipid group [59]. A reduction in the amount of sphingolipids in the skin is associated with epidermal barrier dysfunction, and consequently causes excessive water loss. In addition, ceramides are the main lipid component of the intercellular spaces in the stratum corneum, playing a significant role in differentiation, proliferation and cell cycle inhibition. Their main role is to limit excessive water loss and prevent the penetration of pathogenic microorganisms into the skin [60,61]. Reduced levels of ceramides are observed in patients with psoriasis. The result is an impaired anti-apoptotic effect, resulting in characteristic skin lesions [62,63]. In addition, in a study conducted by the authors [54], it was observed that the decrease in ceramide levels was proportional to the area of skin affected by psoriasis. In contrast, Li et al. [54] studied the effect of an emollient containing ceramides and linoleic acid. The authors showed that the use of emollients before and after the application of glucocorticosteroids resulted in a prolonged state of skin improvement, while preventing relapse after treatment.
Dexpanthenol, due to its properties in restoring the skin’s protective barrier and alleviating symptoms of dry skin, has become the subject of intensive scientific research regarding its use in emollients [64]. It forms, along with other components, coenzyme A, which participates in the metabolism of fatty acids and sphingolipids. The resulting lipid compounds create a lipid layer in the stratum corneum of the epidermis. A tight stratum corneum ensures proper skin function by protecting the skin from external factors and excessive water loss [65]. In a study conducted by Araviiskaia et al. in 2022, it was found that after applying an emollient with dexpanthenol, the skin remained hydrated, making it less prone to damage and preventing the formation of scales [64]. In 2024, Augustin and others demonstrated the effectiveness of dexpanthenol in treating dry skin [66]. In another study by Marquardt Y. et al. in 2015, the effects of petrolatum and a 5% dexpanthenol ointment on the healing process of damaged skin were compared [57]. After a few days of using the ointment containing 5% dexpanthenol, clinicians observed a significant reduction in skin lesions compared to those treated with petrolatum. Furthermore, after 7 days of using both substances, it was observed that the ointment enriched with dexpanthenol, compared to petrolatum, improved epidermal regeneration through faster reepithelialization and more quickly restored the normal function of the skin barrier.
M.D. Thouvenin et al. in 2020 [58] conducted a study comparing the effectiveness of a standalone emollient to an emollient enriched with celastrol and polidocanol in patients with psoriasis [58]. Celastor is an immunomodulator whose properties inhibit interleukin-17A. One of the main cytokines involved in the pathogenesis of psoriasis is IL-17A, whose values are elevated in psoriasis patients [67]. The emollient also contained polidocanol, whose antipsoriatic effect is based on its antipruritic action. The use of an antipruritic agent avoids additional skin irritation that could occur as a result of the patient’s scratching. The study assessed the intensity of pruritus, erythema size, skin thickness, scaling, and dryness after the application of the emollient. Two groups of patients were analyzed: one group consisted of patients for whom the emollient was the only form of therapy, and the other group included patients who, in addition to the emollient, used corticosteroids, topical vitamin D3, received systemic treatment, or underwent phototherapy. The effects were evaluated after 29 days of using the emollient. The results confirmed a reduction in the dryness of psoriatic plaques, decreased scaling, and a reduction in pruritus in patients who used the emollient alone. In contrast, in patients for whom the emollient was part of the supplementary therapy, there was a reduction in the intensity of adverse effects such as skin dryness, which occurred after the use of acitretin and phototherapy. The effects were evaluated in comparison to the baseline, when the therapy began. The study demonstrated a beneficial effect of the emollient enriched with celastrol and polidocanol when applied to the skin of psoriasis patients [58]. In general, the categories of emollients, their role, importance, and action are summarized in Table 5.

Table 5.
Classification of emollients based on [25,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68].
5. Substances, Standards, Methods—New Trends in Psoriatic Skin Care
As mentioned earlier, the skin of psoriasis patients requires properly selected care, which affects the treatment and helps accelerate the effect of therapy, as well as prolongs the time of remission [60]. The use of preparations that contain appropriate ingredients with moisturizing, lubricating and exfoliating properties leads to a reduction in transepidermal water loss, as well as enhances the process of exfoliation of the excessively accumulated stratum corneum [60,61,69]. The authors [60] emphasize that the selection of cosmetics for home care of psoriasis patients should be individualized to the needs of the patient. On the cosmetic market, product lines dedicated to people struggling with psoriasis are increasingly appearing, aimed at facilitating their proper skin care. The most common are shampoos, washing gels, moisturizing and lubricating creams, and body lotions, which should be used regularly—once or twice a day during or after bathing [2,13,61].
5.1. Keratolytics and Exfoliating Substances
According to the authors [25,69], in topical therapy of psoriatic skin, it is crucial to properly prepare the affected areas to increase the effectiveness of the preparations used. For this purpose, exfoliating substances such as salicylic acid, lactic acid or urea are used, which facilitate the removal of scales and improve the penetration of drugs, including glucocorticosteroids. The use of alpha-hydroxy acids (AHAs), beta-hydroxy acids (BHAs) and polyhydroxy acids (PHAs) facilitates the removal of keratinized epidermis by destroying protein bonds between corneocytes [69]. Salicylic acid, the best-known BHA, is widely used in the initial stages of psoriasis treatment for its exfoliating and anti-inflammatory properties. It also enhances the penetration of topical agents, such as corticosteroids, and reduces pruritus [25,69]. Its mechanism and risks have been discussed in Section 3.2.1. Clinical studies [70] confirm that salicylic acid is an effective treatment in the initial stages of plaque psoriasis therapy and as a regular adjunct to other methods used. Its efficacy is greatest in thick and scaly psoriatic plaques [25,70]. The authors [61] point out that extreme caution should be exercised when using salicylic acid, as its inappropriate extensive use leads to the salicylism mentioned earlier.
Glycolic and lactic acids, which belong to the AHA group of acids indicated by the authors [13,69] for psoriatic skin care, exhibit exfoliating properties, as well as moisturizing and stimulating collagen and glycosaminoglycan synthesis. AHA penetrate deep into the epidermis, inducing an increase in the frequency of replacement of the stratum corneum, resulting in exfoliation of its outer layers while maintaining the normal skin barrier [25,69]. Glycolic acid and lactic acid have the effect of reducing the cohesion of corneocytes by increasing the distance between them due to increased water retention in the stratum corneum [25]. PHA, on the other hand, have antioxidant and moisturizing properties, which have a protective effect on psoriatic skin whose skin barrier is disrupted. The use of AHA and PHA together with topical corticosteroids provides a synergistic effect and counteracts skin atrophy that can be caused by steroid therapy [25,69,71]. A double-blind clinical trial was conducted to evaluate the efficacy of AHA in patients with scalp psoriasis [71]. The effects of 5% glycolic acid and 5% lactic acid in combination with topical corticosteroids were analyzed. Three different preparations were used in 20 patients with scalp psoriasis: a lotion with 5% glycolic acid and 5% lactic acid at pH 3.5, a placebo lotion (containing 40% ethanol, 20% propylene glycol and 40% deionized water), and 0.1% betamethasone. Patients applied two different formulations to each side of the head twice a day without a bandage for 8 weeks. Clinical evaluation included monitoring of the treated skin areas, before treatment and at 2, 4, 6, and 8 weeks after treatment, depending on therapeutic progress [71]. The results of the above study [25,71] confirmed that the use of AHA along with the concomitant use of 0.1% betamethasone resulted in relief of specific skin areas. In addition, the concomitant use of AHA and betamethasone (a glucocorticosteroid) reduced the time required to relieve skin lesions to 4 weeks, half of the 8 weeks required when using each preparation separately. This confirms the synergistic effect of AHA and corticosteroids in scalp psoriasis [71].
5.2. Barrier-Restoring Substances
Another equally important group of preparations used in the care of psoriatic skin are fatty substances that supplement the deficiencies of naturally occurring lipids in human skin. Ingredients that support the restoration of the lipid barrier include waxes, cholesterol, ceramides and essential fatty acids (EFAs) [13,69]. Due to impaired ceramide synthesis in patients with psoriasis, their use improves skin barrier function by stimulating lipid synthesis [61]. On the other hand, EFAs, which are found in large amounts in, for example, hemp oil and oil or evening primrose seeds, facilitate the absorption of fat-soluble vitamins, restore the water-lipid balance and support the regenerative processes of the epidermis [13,69]. The authors [72] report that in a study of 25 patients with plaque psoriasis, a significant reduction in psoriasis plaque thickness was observed when fish oil (containing 15.8% eicosapentaenoic acid and 10.1% docosahexaenoic acid) was applied topically to the skin for 4 weeks. The moisturizers used in psoriatic skin care described elsewhere in this review are emollients, the regular use of which improves the comfort of psoriasis patients. Non-medicated moisturizers are available as cosmetic preparations and are available in drugstores in the form of both creams, ointments, liquids and gels [73]. In addition, there are also cosmetic preparations containing emollients and humectants in their formulation. Humectants are substances whose ability to moisturize the skin is due to the binding and retention of water in the epidermis [13,61,69]. Examples of humectants that are used in cosmetics aimed at psoriatic skin include glycerin, urea and hyaluronic acid [69]. The ability of humectant molecules to penetrate deep into the epidermal layers depends on their size. Smaller molecules, such as urea or glycerol, can penetrate the deeper layers of the epidermis, while larger molecules, such as hyaluronic acid, remain in the stratum corneum [69].
Urea belongs to the group of natural skin moisturizing factors (NMF). In low concentrations (5–10%) it has moisturizing and exfoliating properties, and in concentrations >10% it has a keratolytic effect and increases the penetration of other topical preparations [61]. The use of urea allows an increase in the penetration of corticosteroids, which significantly reduces the amount of corticosteroid delivered into the skin [2]. According to the authors [25], topical application of highly concentrated urea increases lipid biosynthesis. In addition, studies [2] conducted in this area confirmed that urea (10%) regulates the expression of genes involved in keratinocyte differentiation, increases the synthesis of antimicrobial peptides and lipids responsible for the normal skin barrier, thus confirming that proper skin care of psoriatic skin is important even during periods of remission. In vivo and in vitro studies [25,73] have shown that urea reduces epidermal hyperproliferation. Thinning of the epidermis by 20%, reduction in the number of cells in its layers and prolongation of the maturation time of cells after division were observed. According to the authors [73], urea’s mechanism of action involves breaking hydrogen bonds, which disrupts the quaternary structure of keratin, thereby denaturing keratin. Urea does not disrupt the skin’s hydrolipid barrier. The authors emphasize that the use of urea preparations enhances barrier function by, among other things, increasing lipid synthesis within the skin [73,74].
5.3. Modern Active Substances and Synergistic Combinations (Niacinamide, AHA + Steroids)
The authors [2,61,69] also mention niacinamide as one of the newer ingredients that is recommended for psoriatic skin care. It is a compound that is water-soluble and easily absorbed by the skin. In addition, it reduces the synthesis of pro-inflammatory cytokines (such as IL-6, IL-8, TNFα), which are involved in the pathogenesis of psoriasis. Studies conducted by the authors [2,75] showed that topical combination therapy, including 1.4% niacinamide in combination with 0.005% calcipotriol, was more effective than monotherapy. After 12 weeks of treatment, complete or nearly complete resolution of psoriatic lesions was observed in 50% of patients in the group using the combination of niacinamide and calcipotriol, 25% of patients in the group using 1.4% niacinamide alone, and 31.5% of patients in the calcipotriol monotherapy group. In addition to confirming the synergism of the use of niacinamide and calcipotriol, the authors of the aforementioned study additionally emphasize the possible use of niacinamide as a substitute for glucocorticosteroids [2]. According to the same authors, the appropriate preparations in psoriatic skin care are ingredients with exfoliating properties and those that help maintain the normal skin barrier. In addition, the care product should have good tolerance and not cause skin irritation. According to them, it is crucial to have a suitable pharmaceutical formulation that contains ingredients that help the active substances penetrate the skin, while minimizing the risk of irritation. Skin care formulations dedicated to psoriatic skin should be characterized by practicality—quickly absorbed, non-sticky and easily spread on the skin surface, so that the patient can easily integrate skin care into the daily routine [2].
5.4. Cosmetological Procedures Supporting Therapy (Sonophoresis, Carboxytherapy)
The ingredients described above are applicable to both home care and treatment of psoriatic skin [13,69]. The authors [69] emphasize that patients with psoriasis can benefit from in-office treatments from a cosmetologist, which have greater efficacy than preparations used by patients at home. In the case of psoriatic skin, treatments that allow the penetration of active substances such as needle-free mesotherapy are indicated, which allows the introduction of active ingredients into cells in the deep layers of the epidermis, thanks to the use of high-frequency electric current, which prevents the occurrence of Koebner’s symptoms, as it does not cause trauma to the skin [13,76,77]. In addition, it is possible to exfoliate the epidermis using cavitation or enzyme peels treating the skin with AHA or BHA [69]. Another method to help treat psoriasis is sonophoresis, which is based on the use of ultrasound, which increases the transdermal penetration of active substances [76]. More recently, experts point to carboxytherapy treatment, which involves the transdermal administration of carbon dioxide. This action leads to an improvement in blood supply to the tissues, dilation of blood vessels and an increase in oxygen pressure within the treated areas, which supports better perfusion and oxygenation of the tissues. The result of these changes is a reduction in the size, thickness and number of psoriatic lesions, as well as a reduction in the intensity of pruritus and skin erythema [69,76]. The authors [13,69] emphasize that the severity of the disease, location and size of the lesions should be assessed before performing the treatments. This is important because a procedure that will involve a break in the continuity of the epidermis may cause Koebner’s sign (lesions typical of the disease entity appear at the site of damage). For this reason, treatments such as waxing, sugar paste and laser hair removal, as well as mechanical scrubs (granular, etc.) and manual skin cleansing are contraindicated in psoriasis. On the other hand, if psoriasis is in the active phase of the disease then intradermal injections, subcutaneous injections, cryolipolysis, or treatments using light such as Intense Pulsed Light and lasers are inadvisable [13,69,76,77]. Among the new potential treatments offered to patients with psoriasis is the intradermal administration of botulinum toxin, which inhibits the release of acetylcholine and pro-inflammatory cytokines, thereby reducing inflammation and immune cell activity within psoriatic lesions [78]. Particularly satisfactory therapeutic effects were noted during a study evaluating the use of botulinum toxin A in the treatment of nail psoriasis. Patients received intradermal injections at two sites on the proximal nail shaft when lesions involved the nail matrix or at two sites around the nail bed for lesions occurring in the nail bed. The results presented here proved that performing a single intradermal injection of botulinum toxin had a long-lasting effect, yielding a reduction in the severity of psoriatic lesions for up to 24 weeks, a promising prospect for the treatment of nail psoriasis [79].
In summary, the appropriate selection of active ingredients and cosmetic treatments tailored to the needs of psoriatic skin not only complements pharmacological treatment, but also plays a preventive role by improving skin hydration, reducing inflammation, and supporting epidermal regeneration [13,25,69]
6. New Approaches in the Use of Natural Raw Materials in the Treatment of Psoriasis
Based on studies conducted by many researchers, it can be concluded that natural raw materials play a significant role in alleviating the skin symptoms of psoriasis by maintaining normal skin function and structure [80,81,82,83]. Therapy with natural products mainly plays an adjunctive role to primary treatment, and in special cases replaces traditional treatment. Elkhawaga O.Y. et al. in 2023 [82] in their review paper proved that the action of substances that have a positive effect during the treatment of psoriasis can be anti-inflammatory, keratolytic, moisturizing, regenerating, or reducing pruritus. The goal of using antipsoriatic preparations is to achieve the PASI (psoriasis severity index) score as low as possible. The PASI score refers to an assessment of the area of erythema, severity and type of skin lesions. This measure is aimed at reducing susceptibility to epidermal damage, which consequently reduces the development of psoriasis skin symptoms. In a study conducted by the authors [84], it was shown that the following substances have the most effective therapeutic effect among natural substances for psoriasis: Curcuma amada, Humulus lupulus and Hypericum perforatum.
Another natural substance identified by subsequent authors [85] that shows probable effectiveness in the treatment of psoriasis is chamomile (Matricaria chamomilla). However, it should be noted that these studies are still in the developmental phase. Kolahdooz et al. in 2018 [85] studied the effectiveness of a chamomile-pumpkin oil gel (ChP) applied topically to psoriatic lesions. The oil gel demonstrated efficacy in mild and moderate plaque psoriasis. In the group of patients who applied the oil gel, after 4 weeks from the start of the study, erythema alleviation and skin softening were observed [85]. The anti-inflammatory activity of chamomile is primarily attributed to its flavonoids [86]. Other researchers have shown that chamomile extracts and apigenin, a pure compound derived from chamomile, reduce the cytotoxic activity and proliferation of human T cells, which play a crucial role in chronic wounds and inflammatory skin diseases [87]. Furthermore, chamomile consists of numerous secondary metabolites and active compounds (organic acids, terpenes, sesquiterpene lactones, essential oils, coumarin) that positively influence the skin [88]. Topical chamomile poultices are used to improve skin condition, repair the skin barrier in sores, burns, wounds, ulcers [88]. Chamomile supports granulation processes, which contributes to faster epidermal regeneration. In a study conducted in 2022, scientists analyzing the composition of essential oil derived from chamomile showed its repairing properties and ability to inhibit inflammation in keratinocytes. One of the substances in the composition of chamomile showing anti-inflammatory properties is azulene, which accounts for as much as 88.9% of the oil’s composition. When chamomile-derived essential oil was applied to the skin of mice with psoriatic lesions, the lesions were relieved [89]. In another study, researchers showed in a study conducted on rats that chamomile accelerates the healing of ulcers compared to the effects of corticosteroids [90]. Additionally, it exhibits antimicrobial activity, showing mainly fungicidal [91] and bactericidal [92] properties. Thus, it can be concluded that the use of chamomile extract can reduce the risk of infection of damaged skin tissue. Superinfection of psoriatic lesions can lead to the development of more serious dermatoses, so it is reasonable to consider the selection of new potential solutions that will reduce the possibility of such side effects of this disease [88]. A promising natural substance in the treatment of psoriasis is Aloe vera (Aloe vera) [93,94]. Aloe has been used for the treatment of chronic wounds for many years. It has been proven to be more effective in chronic wounds than in acute ones. Aloe consists of over 200 bioactive compounds, most of which are found in the gel and leaves [95,96]. Additionally, this plant contains up to 99% water, which gives it exceptionally strong moisturizing properties [96]. Scientific studies on the application of aloe gel to the skin show that, due to its high water content and bioactive compounds, the skin remains intensely hydrated and elastic [95,97]. At the same time, it becomes more resistant to damage, which effectively prevents the formation of wounds and ulcers [98,99]. The first studies on the effectiveness of aloe use were published in 1996, highlighting its potential in the care and treatment of psoriatic lesions [100]. Aloe vera, aloin and emodin are responsible for reducing inflammation. The anti-inflammatory mechanism of action of aloe vera and its substances involves inhibition of pro-inflammatory cytokines, reduction in inflammatory mediators: NO, COX-2, and suppression of signaling pathways [101]. In addition, aloe vera plays a significant role in skin regeneration, positively affecting each of the phases of wound healing, which ultimately translates into the process occurring faster [102,103]. According to a study, the application of aloe hydrogel led to a 29% reduction in the wound healing time compared to the control group, where no hydrogel was applied [102]. The acceleration of the healing process occurs through the stimulation of growth factor production, angiogenesis, fibroblast proliferation, collagen deposition, and growth factor production [104]. In 2022, authors conducted a study demonstrating the therapeutic properties of acemannan and glucomannan in the treatment of psoriasis. Acemannan belongs to the group of polysaccharides and has immunoregulatory properties on immune cells, while also stabilizing the pH level. In the study, the authors compared the local effects of two hydrogels: one containing aloe extract and the other containing a traditional psoriasis medication—clobetasol propionate. In the in vivo experiment, a 61% reduction in the stratum corneum thickness was observed with the use of the aloe hydrogel and a 66% reduction with clobetasol propionate. The difference between them was small, indicating that the aloe-based hydrogel also exhibits significant anti-psoriatic effects [105]. In the therapy of psoriasis using aloe vera, it is also worth noting the presence of aloe polysaccharide in it, responsible for a protective effect on the skin by inhibiting the level of TNF-α and the expression of IL-8 and IL-12 proteins. The mentioned cytokines play a key role in the pathogenesis of psoriasis, so the use of a substance that inhibits their expression can significantly affect the course of the disease and improve the condition of the skin [101,106].
Current studies report that nigella sativa can be effective in the treatment of skin problems, including psoriasis. As early as 2012. Dwarampudi et al. proved that Nigella sativa has anti-psoriasis effects [107]. The study compared the effects of a 95% extract of Nigella sativa (NS) seeds with the effects of tazarotene gel and the results obtained in the control group. It was shown that black cumin exhibits anti-proliferative effects on keratinocytes [107]. NS supports the wound healing process by reducing the number of white blood cells, limiting tissue damage, and inhibiting bacterial spread [108]. Black cumin oil exhibits antioxidant activity by capturing superoxide molecules, which are responsible for initiating the inflammatory process in psoriasis [109]. In addition, NS extract inhibits the activity of enzymes responsible for the production of free radicals, which slow down the wound healing process [110]. The main component of black cumin: thymoquinone, exhibits anti-inflammatory, antioxidant and antimicrobial properties [111]. The role of thymoquinone (TQ) in accelerating the wound healing process is based on reducing the risk of infection in the initial stages of tissue regeneration [108]. When using black cumin topically, the therapeutic effectiveness depends on the proper form of application. Thymoquinone is a lipophilic substance, which is why it is recommended to apply it locally in the form of oil, dispersion, or emulsion. Studies have shown that NS administered as a lipophilic substance achieves higher therapeutic effectiveness than when administered as an extract [112]. Negi et al. (2019) [113] compared the effectiveness of topical psoriasis treatment using substances derived from black cumin seeds. The study analyzed the use of vesicles filled with thymoquinone and compared the results to those of using black cumin extract, pure thymoquinone (TQ), and tazarotene gel. The study results showed that the use of an etosomal gel containing thymoquinone led to the greatest reduction in the parakeratotic layer compared to the thymoquinone suspension, black cumin extract, and tazarotene [113].
Research conducted by scientists between 1994 and 2023, confirmed the effectiveness of Hypericum perforatum (St. John’s wort) in the treatment of dermatological conditions. St. John’s wort exhibits antioxidant, antimicrobial, anti-inflammatory and anti-angiogenic activity [114]. It is responsible for wound healing by inhibiting fibroblast migration, increasing collagen deposition, or regenerating connective tissue [114]. Hyperforin has been shown to stimulate keratinocyte proliferation and differentiation. This phytochemical compound is the main component derived from the parts of the Hypericum perforatum plant. Promising local use of hyperforin for alleviating psoriatic lesions was confirmed by S. Zhang and others in a study conducted in 2021 on mice. The application of hyperforin in their study showed similar effects in inhibiting epidermal thickening, reducing the infiltration of inflammatory cells, and inhibiting the release of inflammatory cytokines compared to methotrexate. Summarizing the study results, the effectiveness of hyperforin in psoriasis therapy involves its action on the immune system and keratinocytes [68]. Another perspective on the effectiveness of Hypericum perforatum (HP) in psoriasis therapy was presented by Singh et al. (2024) [115]. The authors conducted a study aimed at verifying that a nanogel made from HP extract improves skin penetration and prolongs the action of topically applied medications for psoriasis. The study on psoriatic skin lesions in a rat model confirmed that the nanogel improved skin integrity and reduced the number of inflammatory mediators [115]. In another study, the authors highlighted that TNFa levels in psoriatic tissue samples decreased after topical application of St. John’s wort ointment. In addition, a significantly reduced PASI was noted on the skin of psoriasis patients [116].
Several studies have also been conducted on the efficacy of psoriasis treatment for curcumin [117,118,119]. Curcumin is a substance obtained from the herb long-stem (Curcuma longa). The legitimacy of curcumin (CUR) in the treatment of psoriasis is recommended by researchers around the world, mainly due to its anti-inflammatory, antioxidant and keratinocyte proliferation inhibitory properties [119]. The anti-inflammatory properties are confirmed by a study conducted on psoriatic lesions in mouse models [117]. Application of curcumin at a concentration of 10 μM resulted in the inhibition of inflammatory factor release, such as interleukins (IL-17, IL-22, IL-2, IL-8, IFN-γ) and TNF-α in T lymphocytes, by up to 60% [117]. Zhang et al. (2022) [119] conducted a review of previous studies to establish the rationale for using curcumin in psoriasis treatment. The review included 26 studies providing reliable information on the mechanism of action, effectiveness, and side effects of curcumin on psoriatic lesions. A meta-analysis of four studies demonstrated that the final effect of using curcumin in monotherapy did not significantly differ from combination therapy. However, the combination of curcumin and conventional treatment provided the most favorable results for patients. Based on the meta-analysis [119], it was confirmed that curcumin inhibits keratinocyte proliferation and the cell cycle, while showing no effect on apoptosis [119]. The detailed supportive action for psoriasis therapy has been proven for the substances listed in Table 6.

Table 6.
Selected natural substances; their mechanisms of action in the treatment of psoriatic skin.
7. Summary and Conclusions
The material cited in the paper was intended to summarize what possible and effective treatments exist for psoriasis. Various basic targeted treatments for it were summarized. New approaches in the treatment of psoriasis were indicated, focusing mainly on the characteristics of substances such as emollients. Also included in the study is a new approach to the use of natural resources in the treatment of psoriasis, confirming that it is possible. The constant search for new solutions for milder yet effective treatment is the goal of many researchers, doctors and specialists in the field. Organic substances or emollients do not pose a threat to patients, so it is important to look for solutions in this area of opportunity that guarantee progress in remission of the disease. Perhaps in the future it will be possible to find a group of organic—active substances, the synergism of which will allow the use of less aggressive, but equally effective action.
Author Contributions
Conceptualization, M.K.K., S.M.O. and Ł.B. formal analysis, M.K.K.; data curation, Ł.B., S.M.O., J.E.M. and W.H.; writing—original draft preparation, Ł.B., S.M.O., J.E.M., W.H. and M.K.K.; writing—review and editing, Ł.B., S.M.O., J.E.M., W.H. and M.K.K.; supervision, M.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nowak, A.; Popko, M.; Klimowicz, A. Preparaty pochodzenia roślinnego w pielęgnacji skóry u chorych z łuszczycą. Postep. Fitoter. 2016, 17, 332–337. [Google Scholar]
- Thaçi, D.; Augustin, M.; Krutmann, J.; Luger, T. Importance of Basic Therapy in Psoriasis. J. Dtsch. Dermatol. Ges. 2015, 13, 415–418. [Google Scholar] [CrossRef]
- Jurel, P.; Bahadur, S.; Bajpai, M. Treatment of Chronic Plaque Psoriasis: An Overview on Current Update. Pharmacol. Res. Rep. 2024, 2, 100004. [Google Scholar] [CrossRef]
- Man, A.-M.; Orăsan, M.S.; Hoteiuc, O.-A.; Olănescu-Vaida-Voevod, M.-C.; Mocan, T. Inflammation and Psoriasis: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16095. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. Global Psoriasis Atlas. National, Regional, and Worldwide Epidemiology of Psoriasis: Systematic Analysis and Modelling Study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Dascălu, R.C.; Bărbulescu, A.L.; Stoica, L.E.; Dinescu, Ș.C.; Biță, C.E.; Popoviciu, H.V.; Ionescu, R.A.; Vreju, F.A. A Contemporary, Multifaced Insight into Psoriasis Pathogenesis. J. Pers. Med. 2024, 14, 535. [Google Scholar] [CrossRef] [PubMed]
- Brownstone, N. Psoriasis Therapies in 2024 and Beyond. Dermatol. Times 2024, 45, 1–34. [Google Scholar]
- Jakubowska, W.; Pisera, P.; Kiełkowicz, A.; Pactwa, F.; Popińska, Z.; Ślusarczyk, D.; Żmuda, B.; Żuberek, M. From Pathogenesis to Current Treatment of the Cutaneous Psoriasis—A Literature Review. J. Educ. Health Sport 2023, 49, 56–70. [Google Scholar] [CrossRef]
- Mrowietz, U.; Domm, S. Systemic steroids in the treatment of psoriasis: What is fact, what is fiction? J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1022–1025. [Google Scholar] [CrossRef]
- Sugumaran, D.; Yong, A.C.H.; Stanslas, J. Advances in psoriasis research: From pathogenesis to therapeutics. Life Sci. 2024, 355, 122991. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Verzì, A.E.; Guglielmi, G.; Zappulla, S.; Micali, G. A New Prescription Emollient Device (PED) for Psoriasis of Sensitive Areas and Folds: A Randomized Prospective Open Trial. Psoriasis 2024, 14, 135–142. [Google Scholar] [CrossRef]
- Nowicka, D. Łuszczyca—Pielęgnacja i postępowanie w gabinecie kosmetycznym. Kosmetol. Estet. 2018, 5, 529–532. [Google Scholar]
- Narbutt, J.; Woźniacka, A.; Lesiak, A. Efficacy and tolerability of Cerkobaza and Cerkoderm 15 emollients in combination with narrow band UVB phototherapy in psoriatic patients. Forum Dermatol. 2016, 2, 68–72. [Google Scholar]
- Schreve, B.S.; Boehncke, W.H. Psoriasis. In Psoriatic Arthritis and Psoriasis; Adebajo, A., Boehncke, W.H., Gladman, D., Mease, P., Eds.; Springer: Cham, Switzerland, 2016; pp. 129–137. [Google Scholar] [CrossRef]
- Kangle, S.; Amladi, S.; Sawant, S. Scaly signs in dermatology. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Romańska-Gocka, K. Farmakoterapia łuszczycy. Pol. Tow. Farm. 2009, 65, 647–654. [Google Scholar]
- Krzaczyński, J.; Strzałka-Mrozik, B. Farmakologiczne i niefarmakologiczne metody terapii łuszczycy ze szczególnym uwzględnieniem leków biologicznych. Pol. Tow. Farm. 2020, 76, 333–343. [Google Scholar]
- Kiełtyka, K.; Romańska-Kistela, A.; Rymarczyk-Kapuścik, A. Metody leczenia łuszczycy w oparciu o informacje uzyskane od pacjentów dotkniętych tą chorobą. Medsportpress 2019, 4, 185–190. [Google Scholar] [CrossRef]
- Szepietowski, J.; Adamski, Z.; Chodorowska, G.; Gliński, W.; Kaszuba, A.; Placek, W.; Rudnicka, L.; Reich, A. Leczenie łuszczycy zwyczajnej—Rekomendacje ekspertów Polskiego Towarzystwa Dermatologicznego. Część I: Łuszczyca łagodna, łuszczyca wieku dziecięcego. Prz. Dermatol. 2012, 99, 83–96. [Google Scholar]
- Bożek, A.; Reich, A. W jaki sposób miarodajnie oceniać nasilenie łuszczycy? Forum Dermatol. 2016, 2, 6–11. [Google Scholar]
- Reich, A.; Adamski, Z.; Chodorowska, G.; Kaszuba, A.; Krasowska, D.; Lesiak, A.; Maj, J.; Narbutt, J.; Osmola-Mańkowska, A.; Owczarczyk-Saczonek, A.; et al. Łuszczyca. rekomendacje diagnostyczno-terapeutyczne Polskiego towarzystwa Dermatologicznego. część I: Łuszczyca łagodna. Prz. Dermatol. 2018, 105, 225–243. [Google Scholar] [CrossRef]
- Finlay, A.Y. Current Severe Psoriasis and the Rule of Tens. Br. J. Dermatol. 2005, 152, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, A.; Mayer, A.; Augustin, M. Keratolytics and Emollients and Their Role of Psoriasis a Systematic Review. Dermatol. Ther. 2015, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L. Salicylic Acid 6% in an ammonium lactate emollient foam vehicle in the treatment of mild-to-moderate scalp psoriasis. J. Drugs Dermatol. 2011, 10, 270–273. [Google Scholar]
- Li, H.J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef]
- Elmets, C.A.; Korman, N.J.; Prater, E.F.; Wong, E.S.; Rupani, R.N.; Kivelevitch, D.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Wspólne wytyczne AAD–NPF dotyczące opieki nad pacjentem i leczeniem łuszczycy za pomocą terapii miejscowej i alternatywnych metod leczenia w celu zmniejszenia nasilenia objawów łuszczycy. Am. Acad. Dermatol. 2020, 84, 432–470. [Google Scholar] [CrossRef]
- Baran, A.; Flisiak, I. Jaka Jest Klasyfikacja Miejscowych Glikokortykosteroidów ze Względu na Siłę Działania i Jakie Jest Znaczenie Praktyczne Podziału? Medycyna Praktyczna. 2018. Available online: https://www.mp.pl/pytania/pediatria/chapter/B25.QA.7.1.40 (accessed on 15 June 2025).
- Sadowska, M.; Lesiak, A.; Narbutt, J. Zastosowanie fototerapii w leczeniu łuszczycy zwyczajnej. Prz. Dermatol. 2019, 106, 198–209. [Google Scholar] [CrossRef]
- Lapolla, W.; Brad, A.; Yentzer; Bagel, J.; Halvorson, C.R.; Feldman, S.R. Przegląd metod fototerapii w leczeniu łuszczycy. Dermatol. Dyplomie 2012, 3, 8–23. [Google Scholar] [CrossRef]
- Elmets, C.A.; Lim, H.W.; Stoff, B.; Connor, C.; Cordoro, K.M.; Lebwohl, M.; Armstrong, A.W.; Davis, D.M.; Elewski, B.E.; Gelfand, J.M. Wspólne wytyczne Amerykańskiej Akademii Dermatologii i Narodowej Fundacji Łuszczycy dotyczące opieki nad leczeniem łuszczycy za pomocą fototerapii. Am. Acad. Dermatol. 2019, 81, 775–804. [Google Scholar] [CrossRef]
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.; et al. Wspólne wytyczne AAD-NPF dotyczące postępowania i leczenia łuszczycy lekami biologicznymi. Am. Acad. Dermatol. 2018, 80, 1029–1072. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Gelfand, J.M.; Connor, C.; Armstrong, A.W.; Cordoro, K.M.; Davis, D.M.; Elewski, B.E.; Gordon, K.B.; Gottlieb, A.B.; Kaplan, D.H.; et al. Wspólne wytyczne Amerykańskiej Akademii Dermatologii i Narodowej Fundacji Łuszczycy dotyczące leczenia łuszczycy za pomocą ogólnoustrojowych terapii niebiologicznych. Am. Acad. Dermatol. 2020, 82, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.; Rodrigues, M.A.; Vender, R.; Torres, T. Tapinarof w leczeniu łuszczycy. Dermatol. Ther. 2022, 35, e15931. [Google Scholar] [CrossRef]
- Konarska, I. FDA Zatwierdziła Miejscowy Tapinarof w Leczeniu Łuszczycy Plackowatej. Medscape. 2022. Available online: https://www.termedia.pl/dermatologia/FDA-zatwierdzila-miejscowy-tapinarof-w-leczeniu-luszczycy-plackowatej,47231.html (accessed on 15 June 2025).
- Megna, M.; Camela, E.; Ruggiero, A.; Battista, T.; Martora, F.; Cacciapuoti, S.; Potestio, L. Use of biological therapies for the management of pustular psoriasis: A new era? Clin. Cosmet. Investig. Dermatol. 2023, 16, 1677–1690. [Google Scholar] [CrossRef]
- Pietrzak, B.; Zwierzyńska, E.; Hytroś, E. Łuszczyca cz. 1—Obraz kliniczny, patogeneza, leczenie systemowe. Farm. Pol. 2023, 79, 91–100. [Google Scholar] [CrossRef]
- Strober, B.; Pau, C.; Blauvelt, A.; Deherder, D.; Nunez Gomez, N.; Eyerich, K. Skuteczność i bezpieczeństwo bimekizumabu u pacjentów z umiarkowaną do ciężkiej łuszczycą plackowatą: Dwuletnie wyniki pośrednie otwartego rozszerzenia randomizowanego badania fazy 3b BE RADIANT. Am. Acad. Dermatol. 2023, 89, 486–495. [Google Scholar] [CrossRef]
- Dubois Declercq, S.; Pouliot, R. Promising New Treatments for Psoriasis. Sci. World J. 2013, 2013, 980419. [Google Scholar] [CrossRef] [PubMed]
- Bimekizumab—Medicine Overview; European Medicines Agency: Amsterdam, The Netherlands. 2024. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/bimzelx (accessed on 15 June 2025).
- Adams, R.; MarooF, A.; Baker, T.; DG Lawson, A.; Oliver, R.; Paveley, R.; Rapecki, S.; Shaw, S.; Vajjah, P.; Zachód, S.; et al. Bimekizumab, a Novel Humanized IgG1 Antibody That Neutralizes Both IL-17A and IL-17F. Front. Immunol. 2020, 11, 1894. [Google Scholar] [CrossRef]
- Lesiak, A.; Ciążyńska, M. Risankizumab Jako Skuteczna i Długodziałająca Opcja Terapeutyczna w Leczeniu Łuszczycy; Wiadomości dermatologiczne; Forum Media Polska: Poznań, Poland, 2020; Available online: https://www.wiadomoscidermatologiczne.pl/artykul/risankizumab-jako-skuteczna-i-dlugodzialajaca-opcja-terapeutyczna-w-leczeniu-luszczycy (accessed on 15 June 2025).
- Ruggiero, A.; Fabbrocicni, G.; Cacciapuoti, S.; Potestio, L.; Gallo, Ł.; Megna, M. Tildrakizumab for the Treatment of Moderate-to-Severe Psoriasis: Results from 52 Weeks Real-Life Retrospective Study. Clin. Cosmet. Investig. Dermatol. 2023, 16, 529–536. [Google Scholar] [CrossRef]
- Szepietowski, J.; Kaszuba, A.; Adamski, Z.; Placek, W.; Salomon, J. Emollients in dermatological treatment: Position paper of the experts’ group. Dermatol. Klin. 2011, 13, 3–15. [Google Scholar]
- Mijaljica, D.; Spada, F.; Harrison, I.P. Topical moisturisers for the management of psoriasis vulgaris. In Psoriasis-New Research; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Almeida, C.; Madeira, A.; Marto, J.; Graça, A.; Pinto, P.; Ribeiro, H. Monfortinho Thermal Water-Based Creams: Effects on Skin Hydration, Psoriasis, and Eczema in Adults. Cosmetics 2019, 6, 56. [Google Scholar] [CrossRef]
- Torsekar, R.; Gautam, M.M. Topical Therapies in Psoriasis. Indian Dermatol. Online J. 2017, 8, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Lee, S.-Y.; Park, J.-S.; Kim, J.-C.; Park, C.-W.; Kim, H.-O. Moisturizer in Patients with Inflammatory Skin Diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef]
- August, S.; Granier, S.; Tighe, M.P.; Tbaily, L.W.; Chowdhury, S.; Ahlbom, H. A Clinical Investigation of the Performance and Safety of Epaderm®, an Emollient Cream. Clin. Cosmet. Investig. Dermatol. 2021, 14, 909–920. [Google Scholar] [CrossRef]
- Maroto-Morales, D.; Montero-Vilchez, T.; Arias-Santiago, S. Study of Skin Barrier Function in Psoriasis: The Impact of Emollients. Life 2021, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef]
- Man, M.Q.; Ye, L.; Hu, L.; Jeong, S.; Elias, P.M.; Lv, C. Improvements in epidermal function prevent relapse of psoriasis: A self-controlled study. Clin. Exp. Dermatol. 2019, 44, 654–657. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Zheng, J.; Gu, H.; Chen, K.; Jin, H.; He, C.; Xu, A.E.; Xu, J.; Zhang, J.; et al. Efficacy and safety of a topical moisturizer containing linoleic acid and ceramide for mild-to-moderate psoriasis vulgaris: A multicenter randomized controlled trial. Dermatol. Ther. 2020, 33, e14263. [Google Scholar] [CrossRef]
- Kowalska, M.K.; Orłowska, S.M.; Bednarczyk, Ł. Applied Research on Atopic Dermatitis with Special Emphasis on the Role of Emollients in This Disorder: A Review. Appl. Sci. 2024, 14, 8315. [Google Scholar] [CrossRef]
- Gorski, J.; Proksch, E.; Baron, J.M.; Schmid, D.; Zhang, L. Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing). Pharmaceuticals 2020, 13, 138. [Google Scholar] [CrossRef]
- Marquardt, Y.; Amann, P.M.; Heise, R.; Czaja, K.; Steiner, T.; Merk, H.F.; Skazik-Voogt, C.; Baron, J.M. Characterization of a novel standardized human three-dimensional skin wound healing model using non-sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg. Med. 2015, 47, 257–265. [Google Scholar] [CrossRef]
- Thouvenin, M.D.; Dalmon, S.; Theunis, J.; Lauze, C.; Coubetergues, H.; Mengeaud, V.; Calvet, B. Tolerance and efficacy of a new celastrol-containing balm as adjunct care in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2020, 34 (Suppl. S6), 10–16. [Google Scholar] [CrossRef]
- Uchida, Y.; Park, K. Ceramides in Skin Health and Disease: An Update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef]
- Zwierzyńska, E.; Hytroś, E.; Pietrzak, B. Łuszczyca cz. 2—Leczenie miejscowe, fototerapia oraz pielęgnacja skóry. Farm. Pol. 2023, 79, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Ramic, L.; Sator, P. Topical Treatment of Psoriasis Vulgaris. J. Dtsch. Dermatol. Ges. 2023, 21, 631–642. [Google Scholar] [CrossRef]
- Matwiejuk, M.; Mysliwiec, H.; Chabowski, A.; Flisiak, I. The Role of Sphingolipids in the Pathogenesis of Psoriasis. Metabolites 2020, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, D.; Harasim-Symbor, E.; Myśliwiec, H.; Milewska, A.J.; Chabowski, A.; Flisiak, I. Serum sphingolipid level in psoriatic patients with obesity. Postep. Dermatol. Alergol. 2019, 36, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Araviiskaia, E.; Pincelli, C.; Sparavigna, A.; Luger, T. The Role of a Novel Generation of Emollients, ‘Emollients Plus’, in Atopic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2705–2719. [Google Scholar] [CrossRef]
- Proksch, E.; de Bony, R.; Trapp, S.; Boudon, S. Topical use of dexpanthenol: A 70th anniversary article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef]
- Augustin, M.; Berardesca, E.; Blume-Peytavi, U.; Elsner, P.; Scafa, D.; Schmeel, L.C.; Proksch, E. Managing dry skin in patients with comorbidities or with advanced age: Unmet needs and roles for products containing potential emollient-plus ingredients. J. Dermatol. Treat. 2024, 35, 2326171. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, J.; Yu, J.; Chen, X.; Zhang, F.; Wei, W.; Zhang, L.; Chen, W.; Lin, N.; Wu, Y. Hyperforin Ameliorates Imiquimod-Induced Psoriasis-Like Murine Skin Inflammation by Modulating IL-17A-Producing γδ T Cells. Front. Immunol. 2021, 12, 635076. [Google Scholar] [CrossRef]
- Wielowieyska-Szybińska, D.; Wojas-Pelc, A. Przebieg i postępowanie w łuszczycy zwykłej. In Przebieg i Postępowanie w Łuszczycy Zwykłej; Katedra i Klinika Dermatologii Collegium Medicum Uniwersytetu Jagiellońskiego: Kraków, Poland, 2012. [Google Scholar]
- Naldi, L.; Rzany, B. Psoriasis (Chronic Plaque). Clin. Evid. 2009, 2009, 1706. [Google Scholar]
- Kostarelos, K.; Teknetzis, A.; Lefaki, I.; Ioannides, D.; Minas, A. Double-Blind Clinical Study Reveals Synergistic Action Between Alpha-Hydroxy Acid and Betamethasone Lotions Towards Topical Treatment of Scalp Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 5–9. [Google Scholar] [CrossRef]
- Mari, N.L.; Simão, A.N.C.; Dichi, I. n-3 Polyunsaturated Fatty Acids Supplementation in Psoriasis: A Review. Nutrire 2017, 42, 5. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Cavallotti, C.; Berardesca, E. Emollients, Moisturizers and Keratolytic Agents in Psoriasis. Clin. Dermatol. 2008, 26, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Granger, C.; Trullàs, C.; Jesús-Silva, A.; Krutmann, J. Urea in Dermatology: A Review of its Emollient, Moisturizing, Keratolytic, Skin Barrier Enhancing and Antimicrobial Properties. Dermatol. Ther. 2021, 11, 1905–1915. [Google Scholar] [CrossRef]
- Levine, D.; Even-Chen, Z.; Lipets, I.; Pritulo, O.A.; Svyatenko, T.V.; Andrashko, Y.; Lebwohl, M.; Gottlieb, A. Pilot, Multicenter, Double-Blind, Randomized Placebo-Controlled Bilateral Comparative Study of a Combination of Calcipotriene and Nicotinamide for the Treatment of Psoriasis. J. Am. Acad. Dermatol. 2010, 63, 775–781. [Google Scholar] [CrossRef]
- Majchrzycka, M.; Adamska, K.; Rachwalska, K.; Adamski, Z. The Role of the Adjuvant Aesthetic Therapy in the Lives of Patients with Psoriatic Disease. Adv. Dermatol. Allergol. 2022, 39, 1106–1109. [Google Scholar] [CrossRef]
- Michalska, A.D. Łuszczyca—Etiopatogeneza i Leczenie. In Problemy Nauk Medycznych i Nauk o Zdrowiu; Tom 11; Pujer, K., Ed.; Exante Wydawnictwo Naukowe: Wrocław, Poland, 2020; pp. 44–53. [Google Scholar]
- Ghaseminejad-Bandpey, A.; Etemadmoghadam, S.; Jabbari, B. Botulinum Toxin Treatment of Psoriasis—A Comprehensive Review. Toxins 2024, 16, 449. [Google Scholar] [CrossRef]
- Juntongjin, P.; Srisinlapakig, S.; Nitayavardhana, S. Botulinum Toxin Injection Shows Promise in Nail Psoriasis: A Comparative Randomized Controlled Trial. JAAD Int. 2024, 16, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Le, S.; Wu, X.; Dou, Y.; Song, T.; Fu, H.; Luo, H.; Zhang, F.; Cao, Y. Promising strategies in natural products treatments of psoriasis-update. Front. Med. 2024, 11, 1386783. [Google Scholar] [CrossRef]
- Neema, S.; Sandhu, S.; Gupta, A.; Jagadeesan, S.; Vasudevan, B. Unconventional treatment options in psoriasis: A review. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 137–143. [Google Scholar] [CrossRef]
- Elkhawaga, O.Y.; Ellety, M.M.; Mofty, S.O.; Ghanem, M.S.; Mohamed, A.O. Review of natural compounds for potential psoriasis treatment. Inflammopharmacology 2023, 31, 1183–1198. [Google Scholar] [CrossRef]
- Dhanabal, S.P.; Priyanka Dwarampudi, L.; Muruganantham, N.; Vadivelan, R. Evaluation of the antipsoriatic activity of Aloe vera leaf extract using a mouse tail model of psoriasis. Phytother. Res. 2012, 26, 617–619. [Google Scholar] [CrossRef]
- Gendrisch, F.; Haarhaus, B.; Krieger, N.; Quirin, K.W.; Schempp, C.M.; Wölfle, U. The Effect of Herbal Medicinal Products on Psoriasis-Like Keratinocytes. Biomolecules 2021, 11, 371. [Google Scholar] [CrossRef]
- Kolahdooz, S.; Karimi, M.; Esmaili, N.; Zargaran, A.; Kordafshari, G.; Mozafari, N.; Ayati, M.H. Evaluation of the efficacy of a topical chamomile-pumpkin oleogel for the treatment of plaque psoriasis: An intra-patient, double-blind, randomized clinical trial. Biomed. Res. Ther. 2018, 5, 2811–2819. [Google Scholar] [CrossRef]
- Asadi, Z.; Ghazanfari, T.; Hatami, H. Anti-inflammatory Effects of Matricaria chamomilla Extracts on BALB/c Mice Macrophages and Lymphocytes. Iran. J. Allergy Asthma Immunol. 2020, 19, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lairikyengbam, D.; Wetterauer, B.; Schmiech, M.; Jahraus, B.; Kirchgessner, H.; Wetterauer, P.; Berschneider, K.; Beier, V.; Niesler, B.; Balta, E.; et al. Comparative analysis of whole plant, flower and root extracts of Chamomilla recutita L. and characteristic pure compounds reveals differential anti-inflammatory effects on human T cells. Front. Immunol. 2024, 15, 1388962. [Google Scholar] [CrossRef]
- Szeleszczuk, Ł.; Zielińska-Pisklak, M. Matricaria chamomilla—Why is it worth to keep it in home first aid kit? Med. Pol. 2013, 23, 72–79. [Google Scholar]
- Chen, G.; Lv, C.; Nie, Q.; Li, X.; Lv, Y.; Liao, G.; Liu, S.; Ge, W.; Chen, J.; Du, Y. Essential Oil of Matricaria chamomilla Alleviate Psoriatic-Like Skin Inflammation by Inhibiting PI3K/Akt/mTOR and p38MAPK Signaling Pathway. Clin. Cosmet. Investig. Dermatol. 2024, 17, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.D.; Marques, M.M.; Bussadori, S.K.; Martins, M.A.; Pavesi, V.C.; Mesquita-Ferrari, R.A.; Fernandes, K.P. Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother. Res. 2009, 23, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Seyedjavadi, S.S.; Khani, S.; Eslamifar, A.; Ajdary, S.; Goudarzi, M.; Halabian, R.; Akbari, R.; Zare-Zardini, H.; Imani Fooladi, A.A.; Amani, J.; et al. The Antifungal Peptide MCh-AMP1 Derived From Matricaria chamomilla Inhibits Candida albicans Growth via Inducing ROS Generation and Altering Fungal Cell Membrane Permeability. Front. Microbiol. 2020, 10, 3150. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Alesci, A.; Lauriano, E.R.; Fumia, A.; Irrera, N.; Mastrantonio, E.; Vaccaro, M.; Gangemi, S.; Santini, A.; Cicero, N.; Pergolizzi, S. Relationship between Immune Cells, Depression, Stress, and Psoriasis: Could the Use of Natural Products Be Helpful? Molecules 2022, 27, 1953. [Google Scholar] [CrossRef]
- Niculet, E.; Radaschin, D.S.; Nastase, F.; Draganescu, M.; Baroiu, L.; Miulescu, M.; Arbune, M.; Tatu, A.L. Influence of phytochemicals in induced psoriasis (Review). Exp. Ther. Med. 2020, 20, 3421–3424. [Google Scholar] [CrossRef]
- Kaur, S.; Bains, K. Aloe Barbadensis Miller (Aloe Vera). Int. J. Vitam. Nutr. Res. 2024, 94, 308–321. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef]
- Adlakha, K.; Koul, B.; Kumar, A. Value-added products of Aloe species: Panacea to several maladies. S. Afr. J. Bot. 2022, 147, 1124–1135. [Google Scholar] [CrossRef]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The Effect of Aloe Vera Clinical Trials on Prevention and Healing of Skin Wound: A Systematic Review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Marra, M.; Conforti, F.; Lupi, F.R.; Gabriele, D.; Borges, F.; Sinicropi, M.S. Aloe vera-An Extensive Review Focused on Recent Studies. Foods 2024, 13, 2155. [Google Scholar] [CrossRef] [PubMed]
- Syed, T.A.; Ahmad, S.A.; Holt, A.H.; Ahmad, S.A.; Ahmad, S.H.; Afzal, M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: A placebo-controlled, double-blind study. Trop. Med. Int. Health 1996, 1, 505–509. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef]
- Meza-Valle, K.Z.; Saucedo-Acuña, R.A.; Tovar-Carrillo, K.L.; Cuevas-González, J.C.; Zaragoza-Contreras, E.A.; Melgoza-Lozano, J. Characterization and Topical Study of Aloe Vera Hydrogel on Wound-Healing Process. Polymers 2021, 13, 3958. [Google Scholar] [CrossRef] [PubMed]
- Wahedi, H.M.; Jeong, M.; Chae, J.K.; Do, S.G.; Yoon, H.; Kim, S.Y. Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomed. Int. J. Phytother. Phytopharm. 2017, 28, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hormozi, M.; Assaei, R.; Boroujeni, M.B. The effect of aloe vera on the expression of wound healing factors (TGFβ1 and bFGF) in mouse embryonic fibroblast cell: In vitro study. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 88, 610–616. [Google Scholar] [CrossRef]
- Jales, S.T.L.; Barbosa, R.M.; de Albuquerque, A.C.; Duarte, L.H.V.; da Silva, G.R.; Meirelles, L.M.A.; da Silva, T.M.S.; Alves, A.F.; Viseras, C.; Raffin, F.N.; et al. Development and characterization of aloe vera mucus-based hydrogels for the treatment of psoriasis. J. Compos. Sci. 2022, 6, 231. [Google Scholar] [CrossRef]
- Leng, H.; Pu, L.; Xu, L.; Shi, X.; Ji, J.; Chen, K. Effects of aloe polysaccharide, a polysaccharide extracted from Aloe vera, on TNF-α-induced HaCaT cell proliferation and the underlying mechanism in psoriasis. Mol. Med. Rep. 2018, 18, 3537–3543. [Google Scholar] [CrossRef]
- Dwarampudi, L.P.; Palaniswamy, D.; Nithyanantham, M.; Raghu, P.S. Antipsoriatic activity and cytotoxicity of ethanolic extract of Nigella sativa seeds. Pharmacogn. Mag. 2012, 8, 268–272. [Google Scholar] [CrossRef]
- Sallehuddin, N.; Nordin, A.; Bt Hj Idrus, R.; Fauzi, M.B. Nigella sativa and Its Active Compound, Thymoquinone, Accelerate Wound Healing in an In Vivo Animal Model: A Comprehensive Review. Int. J. Environ. Res. Public Health 2020, 17, 4160. [Google Scholar] [CrossRef]
- Ashraf, M.; El-Sawy, H.S.; El Zaafarany, G.M.; Abdel-Mottaleb, M.M.A. Can Essential Oils/Botanicals Smart-Nanoformulations Be Winning Cards in the Fight Against Psoriasis? Pharmaceutics 2023, 15, 750. [Google Scholar] [CrossRef] [PubMed]
- Kmail, A.; Said, O.; Saad, B. How Thymoquinone from Nigella sativa Accelerates Wound Healing through Multiple Mechanisms and Targets. Curr. Issues Mol. Biol. 2023, 45, 9039–9059. [Google Scholar] [CrossRef]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef]
- Nasiri, N.; Ilaghi Nezhad, M.; Sharififar, F.; Khazaneha, M.; Najafzadeh, M.J.; Mohamadi, N. The Therapeutic Effects of Nigella sativa on Skin Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2022, 2022, 7993579. [Google Scholar] [CrossRef]
- Negi, P.; Sharma, I.; Hemrajani, C.; Rathore, C.; Bisht, A.; Raza, K.; Katare, O.P. Thymoquinone-loaded lipid vesicles: A promising nanomedicine for psoriasis. BMC Complement. Altern. Med. 2019, 19, 334. [Google Scholar] [CrossRef] [PubMed]
- Farasati Far, B.; Gouranmohit, G.; Naimi-Jamal, M.R.; Neysani, E.; El-Nashar, H.A.S.; El-Shazly, M.; Khoshnevisan, K. The potential role of Hypericum perforatum in wound healing: A literature review on the phytochemicals, pharmacological approaches, and mechanistic perspectives. Phytother. Res. 2024, 38, 3271–3295. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yadav, S.D.; Gupta, P.; Ali, F.; Arora, S. Dermal Delivery of Hypericum perforatum (L.) Loaded Nanogel: Formulation to Preclinical Psoriasis Assessment. Recent Adv. Drug Deliv. Formul. 2024, 18, 138–154. [Google Scholar] [CrossRef]
- Mansouri, P.; Mirafzal, S.; Najafizadeh, P.; Safaei-Naraghi, Z.; Salehi-Surmaghi, M.H.; Hashemian, F. The impact of topical Saint John’s Wort (Hypericum perforatum) treatment on tissue tumor necrosis factor-alpha levels in plaque-type psoriasis: A pilot study. J. Postgrad. Med. 2017, 63, 215–220. [Google Scholar] [CrossRef]
- Kang, D.; Li, B.; Luo, L.; Jiang, W.; Lu, Q.; Rong, M.; Lai, R. Curcumin shows excellent therapeutic effect on psoriasis in mouse model. Biochimie 2016, 123, 73–80. [Google Scholar] [CrossRef]
- Antiga, E.; Bonciolini, V.; Volpi, W.; Del Bianco, E.; Caproni, M. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. BioMed Res. Int. 2015, 2015, 283634. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Liu, L.; Sun, X.; Zhou, Y.; Chen, S.; Lu, Y.; Cai, X.; Hu, M.; Yan, G.; et al. Efficacy and safety of curcumin in psoriasis: Preclinical and clinical evidence and possible mechanisms. Front. Pharmacol. 2022, 13, 903160. [Google Scholar] [CrossRef]
- Janeczek, M.; Moy, L.; Lake, E.P.; Swan, J. Review of the Efficacy and Safety of Topical Mahonia aquifolium for the Treatment of Psoriasis and Atopic Dermatitis. J. Clin. Aesthetic Dermatol. 2018, 11, 42–47. [Google Scholar]
- Hurkul Muhammed, M.; Sarialtin, S.Y.; Koroglu, A.; Coban, T. Potencial inhibidor in vitro de frutos de aguacate, Persea americana (Lauraceae) contra la oxidacion, inflamacion y enzimas clave vinculadas a enfermedades de la piel. Rev. Biol. Trop. 2021, 69, 472–482. [Google Scholar]
- de Oliveira, A.P.; Franco, E.S.; Rodrigues Barreto, R.; Cordeiro, D.P.; de Melo, R.G.; de Aquino, C.M.; Silva, A.A.r.E.; de Medeiros, P.L.; da Silva, T.G.; Góes, A.J.D.S.; et al. Effect of semisolid formulation of Persea americana mill (avocado) oil on wound healing in rats. Evid. Based Complement. Altern. Med. 2013, 2013, 472382. [Google Scholar] [CrossRef] [PubMed]
- Stücker, M.; Memmel, U.; Hoffmann, M.; Hartung, J.; Altmeyer, P. Vitamin B(12) cream containing avocado oil in the therapy of plaque psoriasis. Dermatology 2001, 203, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ashwani, T. Natural Compounds Used in the Treatment and Management of Psoriasis: A Review. Int. J. Pharmacogn. 2023, 10, 489–510. [Google Scholar]
- Michalsen, A.; Eddin, O.; Salama, A. A case series of the effects of a novel composition of a traditional natural preparation for the treatment of psoriasis. J. Tradit. Complement. Med. 2016, 6, 395–398. [Google Scholar] [CrossRef]
- Basharat, S.; Gilani, S.A.; Iftikhar, F.; Murtaza, M.A.; Basharat, A.; Sattar, A.; Qamar, M.M.; Ali, M. Capsaicin: Plants of the Genus Capsicum and Positive Effect of Oriental Spice on Skin Health. Ski. Pharmacol. Physiol. 2020, 33, 331–341. [Google Scholar] [CrossRef]
- Chan, T.C.; Lee, M.S.; Huang, W.C.; Chang, W.Y.; Krueger, J.G.; Tsai, T.F. Capsaicin attenuates imiquimod-induced epidermal hyperplasia and cutaneous inflammation in a murine model of psoriasis. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 141, 111950. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, M.; Mangal, S.; Agrawal, U.; Vyas, S.P. Capsaicin-loaded vesicular systems designed for enhancing localized delivery for psoriasis therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 825–834. [Google Scholar] [CrossRef]
- Anzar, C.A.; Joseph, M.V.; Vadiraj, G.; Shariq Afsar Thandu, B.; Anjaneya Reddy Lebaka, P. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar]
- Chadha, H.; Chandra, P.; Meher, B.; Sachan, N. Molecular Docking Analysis of Psoriasis Specific Mediator IL-17 with Active Phytoconstituents from Cocos nucifera, Carica papaya, Ichnocarpus frutescens. Lett. Appl. NanoBioScience 2024, 14, 18. [Google Scholar] [CrossRef]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid. Based Complement. Altern. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef]
- Rai, V.K.; Sinha, P.; Yadav, K.S.; Shukla, A.; Saxena, A.; Bawankule, D.U.; Tandon, S.; Khan, F.; Chanotiya, C.S.; Yadav, N.P. Anti-psoriatic effect of Lavandula angustifolia essential oil and its major components linalool and linalyl acetate. J. Ethnopharmacol. 2020, 261, 113127. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical Composition and Effect against Skin Alterations of Bioactive Extracts Obtained by the Hydrodistillation of Eucalyptus globulus Leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; El-Sawy, H.S.; El Zaafarany, G.M.; Abdel-Mottaleb, M.M.A. Eucalyptus oil nanoemulsion for enhanced skin deposition of fluticasone propionate in psoriatic plaques: A combinatorial anti-inflammatory effect to suppress implicated cytokines. Arch. Pharm. 2025, 358, e2400557. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, M.-Y. Aktywność przeciwłuszczycowa ekstraktów z owoców Ficus carica in vitro i in vivo poprzez modulację JAK-STAT. Life 2023, 13, 1671. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Shi, R.; Zhang, L.; Zhao, R.; Duan, R.; Qin, Y.; Gao, S.; Li, X.; Duan, J.; et al. Allicin ameliorates imiquimod-induced psoriasis-like skin inflammation via disturbing the interaction of keratinocytes with IL-17A. Br. J. Pharmacol. 2023, 180, 628–646. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).