Abstract
Background: Stability deficit is one of the most common and disabling signs of multiple sclerosis; therefore, balance training is essential for most patients. Intrinsic foot muscles are a key element in stability, but their influence in multiple sclerosis patients has not been assessed. Objective: The aim of the study was to assess the efficacy of intrinsic foot muscle training on stability in patients with multiple sclerosis. Methodology: A randomized single-blind clinical trial was conducted using a sample of multiple sclerosis patients divided into a control group (CG) and an intervention group (IG). Subjects in the intervention group completed an eight-week intrinsic foot muscles training programme. Static and dynamic stability were measured using the Activities-specific Balance Confidence questionnaire (AsBC), the Four Square Step Test (FSST), the Frailty and Injuries: Cooperative Studies of Intervention Techniques (FICSIT), and the Six Spot Step Test (SSST). Pre- and post-intervention outcomes and differences between groups were calculated. Results: The amount of change comparing pre- and post-intervention results was −0.94 (CG) and 5.59 (IG) in the AsBC questionnaire (p 0.17); −1.0 (CG) and −1.5 (IG) in the FSST (p 0.72); 0.0 for both groups in FICSIT (p 0.629); and −1.5 (CG) and −2.0 (IG) in SSST (p 0.692). Conclusions: Intrinsic foot muscle training produces positive changes in dynamic stability and self-perceived confidence in multiple sclerosis patients.
1. Introduction
Multiple sclerosis (MS) is a chronic and demyelinating disease of the central nervous system [1]. The reported prevalence in 2020 was 35.9/100,000 people. Furthermore, a comparison with 2013 data shows that the prevalence of this disease is increasing [2]. Moreover, this condition constitutes the most common cause of neurological disability in young adults after spinal cord injury [3]. MS is a chronic disease characterised by a wide variety of clinical manifestations that patients can suffer from. The most common symptoms in MS are a loss of stability and coordination, spasticity and muscle weakness, fatigue, somatosensory dysfunctions, and visual impairments [4,5]. Cognitive disturbances [6] and some movement disorders such as myoclonus, ballism, or parkinsonism [7] are other symptoms that MS patients may experience.
Stability deficit is one of the most debilitating problems for patients and is characterized by an increased sway in a standing position, delayed responses to postural perturbations, and a reduced capacity to move toward the limit of stability [8]. Static and dynamic stability impairment is associated with an increased risk of falls and leads to a reduction in the patient’s function [9] and quality of life [10]. Several studies have shown the benefits of stability training in MS patients. Some stability training programmes (e.g., conventional physiotherapy, virtual reality, core training, dual tasks, integration of sensory stimuli) have been shown to be effective in improving static and dynamic stability, reducing the number of falls, increasing the walking speed, and improving the self-perceived stability and quality of life [11,12,13,14,15,16].
One of the elements that can contribute to improved stability is the activation capacity of the intrinsic foot muscles (IFMs). Wallace et al. [17] showed that the abductor hallucis and abductor digiti minimi muscles play an important role in modulating static stability by increasing their activation in response to disturbances in the visual or vestibular systems. In addition, many authors have found that IFM training leads to a significant increase in static and dynamic stability in healthy subjects of different age groups, as well as in subjects with musculoskeletal disorders such as chronic ankle instability [18,19,20,21,22,23]. Finally, the training of IFMs leads to other physical benefits such as greater toe flexor strength [22,24], improvements in physical performance [24,25], proprioception and self-perceived stability [19], decreased medial longitudinal arch drop under load [18,24], and a reduction in the incidence of injury [26]. However, no studies have been found that have assessed the efficacy of an IFM training programme on the overall stability in patients diagnosed with MS. Considering that stability deficit is one of the most prevalent and limiting signs in MS patients and that IFMs have been shown to lead to improvements in stability, it seems important to study this association. The hypothesis of this study is that strengthening IFMs can lead to improvements in static and dynamic stability in MS patients.
Thus, the main objective of the study was to assess the efficacy of an 8-week IFM training programme on the stability in patients with MS.
2. Materials and Methods
2.1. Design
A randomized single-blind clinical trial was conducted in conformity with the Consolidated Standards of Reporting Trials (CONSORT) [27] requirements. The study was carried out following the Declaration of Helsinki (2013); all the participants agreed to participate and read and signed the informed consent by themselves. The study was approved by the ethics committee of the University of Salamanca (registration number 1233) and was registered in the United States Randomized Trials Register on clinicaltrial.gov (NCT06665607).
2.2. Sample Size Calculation
The sample size calculation analysis was performed using G*Power version 3.1.9.2 (G*Power©, Universidad de Dusseldorf, Düsseldorf, Alemania); considering a medium effect size (Cohen d = 0.501) for a one-tail hypothesis, a between-groups proportion of N/n = 1, and a power (1-β error probability) of 0.80 with an α level of 0.05, the minimum sample size required was 40 participants.
2.3. Participants
Patient recruitment was carried out between November 2024 and January 2025 in the Multiple Sclerosis Association of Valladolid following a sequential sampling method. Participants were randomly assigned to a control group or intervention group.
Inclusion and exclusion criteria:
The sample consisted of MS patients aged between 18 and 70 years, who suffered moderate neurological disability as scored by the Expanded Disability Status Scale (EDSS), ranging from 3.0 to 6.0. The EDSS is a clinical administered assessment scale that evaluates the functional system of CNS and is a suitable and valid tool to describe disease progression in patients with MS [28]. All study participants had to be receiving regular physiotherapy treatment.
The exclusion criteria were (1) cognitive impairments that may affect the results of any of the outcomes; (2) diagnosis of visual or vestibular disorders that may influence the patient’s stability; (3) change in MS-specific medication in the 2 months prior to study; (4) acute relapse in the 2 months prior to study; or (5) traumatologic or orthopaedic disorders that could negatively affect balance.
Socio-demographic and descriptive data were collected. Descriptive data comprised age, sex (male or female), body mass index (BMI-Kg/m2), years since diagnosis of the condition and phenotype of MS (relapsing–remitting MS, primary progressive MS or secondary progressive MS).
2.4. Outcome Measures
Static and dynamic stability and self-reported confidence were measured at baseline and after completing the eight-week IFM training programme. These variables were evaluated by one of the members of the research team (DGG) who was blinded to the group to which the participants had been assigned.
Dynamic stability was measured using the Four Square Step Test (FSST) and the Six Spot Step Test (SSST).
Dite et al. [29] described that FSST presents excellent inter- and intra-rater reliability (ICC 0.922) and very high sensitivity (89%) and specificity (85%) levels. The test consists of drawing a cross on the ground, delimiting four grids. The subject stands in square number 1 facing square number 2. The aim is to step as fast as possible into each square in the following sequence: square number 1, 2, 3, 4, 1, 4, 3, 2, and 1. This sequence requires the subject to step forward, backward, and sideways to the right and left. The score is recorded as the time taken to complete the sequence. The stopwatch starts when the first foot contacts the floor in square 2 and finishes when the last foot comes back to touch the floor in square 1. Two trials were completed with the best time taken as the score. A trial was repeated if the subject failed to complete the sequence successfully or lost balance.
The SSST assesses lower limb function, particularly dynamic balance and coordination [30]. Pavan et al. [31] described that the SSST is a reproducible and valid test for MS patients with excellent reliability (ICC 95%: 0.983). The SSST was superior to short- and long-distance walk tests (6MWT and T25FWT) in detecting walking capacity impairments in MS patients [32]. The test field measured one metre (width) by five metres (length). In the middle of the width line (0.50 m), a cone was placed in the proximal and distal areas of the line (start point and finish point). On each lateral line, two cones were placed at distances of one and three metres on the left. Another two cones were placed at two and four metres on the right. The subjects were instructed to walk on alternating sides and to kick the cones, alternating between the medial and lateral sides of the same foot. The subjects were instructed to complete the test as quickly as possible, without running. The test was timed from lifting the foot from the start point until kicking the last cone. Two trials with each lower extremity were completed, and the best results were registered.
Static balance was measured using the Frailty and Injuries: Cooperative Studies of Intervention Techniques test (FICSIT-4 test). This test presents good test–retest reliability (r = 0.66) [33]. Participants have to maintain four progressively difficult foot positions for 10 s each (side-by-side stance, semi-tandem stance, tandem stance, unipedal stance). Each standing position is scored as follows [34,35]: zero points (holding the position less than one second); one point (holding the position two to nine seconds); two points (holding the position more than ten seconds). The sum of the four components yields the maximum score, which can be nine points (unipedal stance scores out of three points). Two trials were completed with the best result taken as the score.
Participants were instructed to wear comfortable shoes to complete these three balance tests. Before the patient performed the test, the evaluator explained and demonstrated it to the subjects. Moreover, one practice trial was completed before the assessment trial.
Self-perceived confidence regarding stability was measured using the Activities-specific Balance Confidence scale (AsBC) questionnaire. This is a validated and reliable self-reported instrument (ICC = 0.92) [36] that evaluates the patient’s perceived of balance confidence during 16 different daily activities. Each item is scored from 0 to 100% (0% means not at all and 100% means fully confident). The final score is the mean value of all items. This questionnaire has been used in other studies to assess functionality in MS patients [37].
2.5. Intervention
Intervention group participants completed an IFM strengthening training for eight weeks. In the present study, a period of eight weeks was selected, as most authors use IFM training protocols of four to 12 weeks [18,19,20,22,23,24]. The IFM training consisted of daily practice of the following exercises:
- Short Foot Exercise (SFE): SFE is the most widely used and evidenced exercise in IFM training [18,19,22,23]. To perform the exercise, subjects were placed in a seated position, with both feet on the floor and with the hips, knees, and ankles at 90° of flexion. Participants were instructed to shorten their foot in the anterior–posterior direction, while actively attempting to bring the head of the first metatarsal toward the heel without curling the toes and without tibialis anterior activation. Participants maintained the position for 20 s with five repetitions completed. The exercise is performed first with one foot and then with the other.
- Toes-Spread Out (TSO): Gooding et al. [38] and Kim et al. [39] showed that TSO is the exercise that generates the greatest activation of the IFMs, compared with other exercises such as SFE, hallux extension, and lesser toes extension. The TSO exercise was performed by extending the toes and then simultaneously abducting all five toes while also flexing the first and fifth toes to the ground, keeping toes two to four extended. The middle toes were finally relaxed. Participants were asked to complete 10–15 repetitions. The exercise is performed first with one foot and then with the other.
- Vele’s forward lean: this exercise has been less commonly used in the literature, although there is also evidence to support its use for the improvement of IFM strength [25]. This exercise consists of performing a maximal forward lean from a standing position with arms alongside the body and with the feet shoulder-width apart, with body in line and without lifting the heels off the floor. Participants were instructed to maintain this position for 15–20 s if possible, with five repetitions completed.
These exercises were demonstrated to the participants by a member of the research group (JJAO). In addition to the specific IFM training, the subjects in the intervention group continued with their usual physiotherapy treatment, based on strength, mobility, stability, coordination, and gait exercises. Participants in the control group maintained their regular physiotherapy treatment, but no additional intervention was added.
2.6. Statistical Analysis
Statistical Package for the Social Sciences (SPSS) version 25.0 for Windows (Armonk, NY, USA: IBM Corporation) was used for statistical analysis.
Before between-groups comparisons, the normality and variances homogeneity were evaluated using the Kolmogorov–Smirnov and Levene test, respectively. For descriptive data, qualitative variables were presented as frequencies and percentages, while quantitative variables were presented as mean and standard deviation. Pre- and post-intervention within group differences were analysed using the paired-sample t-test. The U Mann–Whitney test for independent groups was used to identify significant differences between-groups in terms of the amount of change in the outcome variables. A p value < 0.05 was considered statistically significant.
3. Results
Forty participants were initially recruited for the study. After the eligibility criteria screening, six participants were excluded. Five presented visual or vestibular disorders, and one had suffered an acute relapse in the two months prior to study. Finally, 34 participants met all the eligibility criteria (control group, N = 17; intervention group, N = 17). Three patients did not complete the follow-up; thus, the final sample was 31 subjects (control group, N = 16; intervention group, N = 15). The detailed description of the demographic and clinical variables is shown in Table 1.

Table 1.
Demographic and clinical characteristics of participants at baseline (N = 31).
In the intervention group, post-intervention improvements were found in the outcomes of the AsBC, 4SST, 6SST right foot, 6SST left foot, and 6SST mean. On the other hand, in the control group, post-intervention improvements were found in the outcomes of the 4SST, 6SST right foot, 6SST left foot, and 6SST mean. Neither group had an improvement in static stability, as measured by the FICSIT. The detailed results of the pre–post-intervention comparison within each group is shown in Table 2 and Figure 1.

Table 2.
Mean of the scores obtained in the pre–post-intervention of each scale and within each group (paired t-test).
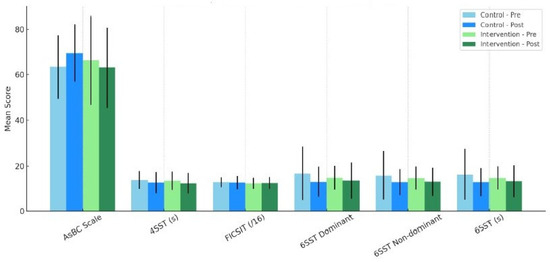
Figure 1.
Mean scores for pre- and post-intervention of each scale and within each group.
Finally, no significant differences were found between groups in the amount of change between pre- and post-intervention assessments (Table 3 and Figure 2).

Table 3.
Amount of change between-group comparison with the U Mann–Whitney test.
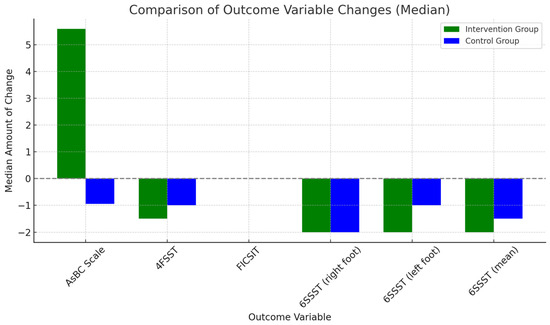
Figure 2.
Amount of change between-group comparison.
4. Discussion
The main objective of the present study was to determine the effectiveness of an IFM training programme in patients with MS. By adding an IFM training programme to their regular rehabilitation, subjects experienced a clinical improvement in dynamic stability and self-perceived confidence, compared to patients who did not undergo the intervention. In the present study, it was observed that there were improvements in dynamic stability but not in static stability. The hypothesis proposed is that dynamic stability may depends to a greater extent on the IFMs, whereas static stability may depend more on other elements such as vision, the vestibular system, or the somatosensory system. However, no statistically significant differences between-groups were observed in terms of the amount of change when comparing pre- and post-intervention outcomes. Wagner et al. [9] suggested that the minimal detectable change value for the FSST was 43%, which means that a 43% reduction in the time taken to complete the test is needed to demonstrate a real change in the MS patient’s performance. In the present study, subjects in the experimental group improved their performance on the FSST but did not reach the 43% difference between the pre- and post-intervention assessment. This statistical non-significance may be due to the small sample size. It is therefore proposed to increase the sample size in future studies. On the other hand, it should be taken into account that in order to improve stability it could be interesting to add training of the neural subsystem of the foot core.
To the authors’ knowledge this is the first study to evaluate the efficacy of an IFM training programme in subjects diagnosed with MS. However, several studies have shown the effectiveness of different modalities of stability training in MS patients. Most of these studies involved general stability or core-based exercises, in contrast to the present trial. Kalron et al. [13] showed that virtual reality stability training produced improvements in static (measured by posturography) and dynamic (assessed by functional reach test) balance in subjects with R-R MS; virtual reality training was superior to conventional treatment for dynamic stability. Monjezi et al. [14] compared the efficacy of single-task stability training with the same dual-task training, assessing self-perceived confidence using the AsBC questionnaire. The authors observed an improvement of 6.79 points in the single-task group and 8.0 points in the dual-task group; both results are slightly better than those observed in the present study (6.06 points for the IFM training group). Gandolfi et al. [15] performed a sensory integration balance training that improved the AsBC questionnaire by almost 10 points. This result improved on that reported by Monjezi et al. [14] and the present study.
Although statistically significant improvements have not been reported in static and dynamic balance in patients with MS, several studies have shown that IFM training leads to improved stability in subjects without neurological disorders. Kim et al. [18] showed a significant improvement (p < 0.05) in dynamic stability, measured by the Y balance test, in healthy subjects who performed a 5-week training protocol based on the SFE. In a sample of subjects with chronic ankle instability, Lee et al. [19] carried out an 8-week SFE training and reported an improvement in static stability of 49%, recording the medio-lateral, antero-posterior, and overall displacement of the centre of pressures on a force platform. The same authors also showed an increase in the Star Excursion Balance Test performance of 6.10% in subjects with CAI who had completed an IFM training protocol based on TSO, toe extension, and hallux extension exercises [20]. Mickle et al. [22] reported a significant improvement in static stability (p = 0.01–0.04) in healthy older people who performed a 12-week IFM training. Finally, Kao et al. [40] partially agree with the results shown in the present study; using a sample of 40 healthy older adults, a 4-week IFMs training programme was applied which produced improvements in static stability but no changes in dynamic stability. Evidence has shown that the three exercises used in this study are effective in strengthening IFMs; however, training was required to perform them correctly. Notably, the subjects in the present research sample reported difficulty in performing the TSO. Finally, in this study, daily but low-load IFM training was performed; in this way, a suitable stimulation for IFMs was achieved without producing muscle fatigue during the training sessions.
Limitations and strengths: This study has several limitations. First, the small sample size may result in the difference in the results between the two groups not being statistically significant; a larger sample will be included in future studies to support these data. Second, the inclusion of MS patients with different disease phenotypes may make the results more variable. Third, no gender or age analysis of the data was carried out. Finally, although a weekly review was conducted, patients were not supervised in the daily performance of IFM exercises. The main strengths of this study are the use of easy and functional tests or questionnaires for the assessment of static and dynamic stability and the application of IFM exercises with strong scientific evidence.
Perspectives: Although the improvement in stability associated with IFMs training is not statistically significant, it may be clinically relevant. Further research using larger and more homogeneous samples is recommended. In the present research, visual and vestibular problems have been considered as exclusion criteria, with the aim of reducing biases in the stability tests. However, these alterations are common in patients with MS, so it would be interesting to perform the IFM training program in patients with visual or vestibular disturbances as well. It is also suggested to assess the efficacy of IFM training programmes in other neurological disorders with balance deficits, such as Parkinson’s disease.
5. Conclusions
An eight-week IFM training programme improves stability and self-perceived confidence in MS patients; although the results are not statistically significant, they could have clinical significance. A larger sample will be collected in future studies to support the results.
Author Contributions
Conceptualization, D.G.-G., J.J.A.-O., R.L.-R. and I.L.-R.; methodology, D.G.-G., J.J.A.-O., R.L.-R. and I.L.-R.; formal analysis, R.L.-R. and I.L.-R.; investigation, D.G.-G., J.J.A.-O., R.L.-R., M.M.-Q. and I.L.-R.; writing—original draft preparation, D.G.-G., J.J.A.-O., R.L.-R. and I.L.-R.; writing—review and editing, D.G.-G., J.J.A.-O., R.L.-R., M.M.-Q. and I.L.-R.; supervision, I.L.-R.; project administration, D.G.-G., R.L.-R. and I.L.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Salamanca (1233; 23 October 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We are grateful to all patients who have participated in the study and to the staff of the Multiple Sclerosis Association of Valladolid.
Conflicts of Interest
Author Jorge Juan Alvarado-Omenat was employed by the company FisioSport Salamanca S.L. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body Mass Index |
| CON | Consolidated Standards of Reporting Trials |
| EDSS | Expanded Disability Status Scale |
| IFMs | Intrinsic Foot Muscles |
| SFE | Short Foot Exercise |
| SPSS | Statistical Package for the Social Sciences |
| FICSIT-4 test | The Frailty and Injuries: Cooperative Studies of Intervention Techniques |
| TSO | Toes-Spread Out |
References
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insight from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Rivera, V.M. Editorial of Special Issue “Multiple Sclerosis: Diagnosis and Treatment II”. Biomedicines 2021, 9, 1605. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.O.; Keenan, A.; Kalau, O.; Worthington, E.; Cohen, L.; Singh, S. Prevalence and burden of multiple sclerosis-related fatigue: A systematic literature review. BMC Neurol. 2021, 21, 468. [Google Scholar] [CrossRef]
- Patti, F.; Vila, C. Symptoms, Prevalence and Impact of Multiple Sclerosis in Younger Patients: A Multinational Survey. Neuroepidemiology 2014, 42, 211–218. [Google Scholar] [CrossRef]
- Meca-Lallana, V.; Gascón-Giménez, F.; Ginestal-López, R.C.; Higueras, Y.; Téllez-Lara, N.; Carreres-Polo, J.; Eichau-Madueño, S.; Romero-Imbroda, J.; Vidal-Jordana, Á.; Pérez-Miralles, F. Cognitive impairment in multiple sclerosis: Diagnosis and monitoring. Neurol. Sci. 2021, 45, 5183–5193. [Google Scholar] [CrossRef]
- Ghosh, R.; Roy, D.; Dubey, S.; Das, S.; Benito-León, J. Movement disorders in multiple sclerosis: An update. Tremor Other Hyperkinetic Mov. 2022, 12, 14. [Google Scholar] [CrossRef]
- Cameron, M.H.; Lord, S. Postural control in multiple sclerosis: Implications for fall prevention. Curr. Neurol. Neurosci. Rep. 2010, 10, 407–412. [Google Scholar] [CrossRef]
- Wagner, J.M.; Norris, R.A.; Van Dillen, L.R.; Thomas, F.P.; Naismith, R.T. Four square step test in ambulant persons with multiple sclerosis: Validity, reliability and responsiveness. Int. J. Rehabil. Res. 2013, 36, 253–259. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Sung, J. Reducing falls and improving mobility in multiple sclerosis. Expert Rev. Neurother. 2015, 15, 655–666. [Google Scholar] [CrossRef]
- Abadi-Marand, L.; Noorizadeh Dehkordi, S.; Roohi-Azizi, M.; Dadgoo, M. Effect of dynamic neuromuscular stabilization on balance, trunk funtion, falling and spasticity in people with multiple sclerosis: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2023, 104, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Molhemi, F.; Monjezi, S.; Mehravar, M.; Shaterzadeh-Yazdi, M.; Salehi, R.; Hesam, S.; Mohammadianinejad, E. Effects of virtual reality vs conventional balance training on balance and falls in people with multiple sclerosis: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2021, 102, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Fonkatz, I.; Frid, L.; Baransi, H.; Achiron, A. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Monjezi, S.; Negahban, H.; Tajali, S.; Yadollahpour, N.; Majdinasab, N. Effects of dual-task balance training on postural performance in patients with multiple sclerosis: A double-blind, randomized controlled pilot trial. Clin. Rehabil. 2016, 31, 234–241. [Google Scholar] [CrossRef]
- Gandolfi, M.; Munari, D.; Geroin, C.; Gajofatto, A.; Benedetti, M.D.; Midiri, A.; Carla, F.; Picelli, A.; Waldner, A.; Smania, N. Sensory integration balance training in patients with multiple sclerosis: A randomized, controlled trial. Mult. Scler. J. 2015, 21, 1453–1462. [Google Scholar] [CrossRef]
- Kasser, S.L.; Jacobs, J.V.; Ford, M.; Tourville, T.W. Effects of balance-specific exercises on balance, physical activity and quality of life in adults with multiple sclerosis: A pilot investigation. Disabil. Rehabil. 2015, 37, 2238–2249. [Google Scholar] [CrossRef]
- Wallace, J.W.; Rasman, B.G.; Dalton, B.H. Vestibular-evoked responses indicate a functional role for intrinsic foot muscles during standing balance. Neuroscience 2018, 377, 150–160. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, J.S. The effects of short foot exercises and arch support insoles on improvement in the medial longitudinal arch and dynamic balance of flexible flatfoot patients. J. Phys. Ther. Sci. 2016, 28, 3136–3139. [Google Scholar] [CrossRef]
- Lee, E.; Cho, J.; Lee, S. Short-foot exercise promotes quantitative somatosensory function in ankle instability: A randomized controlled trial. Med. Sci. Monit. 2019, 25, 618–626. [Google Scholar] [CrossRef]
- Lee, D.R.; Choi, Y.E. Effects of a 6-week intrinsic foot muscle exercise program on the functions of intrinsic foot muscle and dynamic balance in patients with chronic ankle instability. J. Exerc. Rehabil. 2019, 15, 709–714. [Google Scholar] [CrossRef]
- Lynn, S.K.; Padilla, R.A.; Tsang, K.K.W. Differences in static- and dynamic-balance task performance after 4 weeks of intrinsic-foot-muscle training: The short-foot exercise versus the towel-curl exercise. J. Sport Rehabil. 2012, 21, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Mickle, K.J.; Caputi, P.; Potter, J.M.; Steele, J.R. Efficacy of a progressive resistance exercise program to increase toe flexor strength in older people. Clin. Biomech. 2016, 40, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, E.P.; Cook, P.G. Effect of plantar intrinsic muscle training on medial longitudinal arch morphology and dynamic function. Man. Ther. 2013, 18, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Sakuraba, K. Strength training for the intrinsic flexor muscles of the foot: Effects on muscle strength, the foot arch, and dynamic parameters before and after the training. J. Phys. Ther. Sci. 2014, 26, 373–376. [Google Scholar] [CrossRef]
- Sulowska, I.; Mika, A.; Oleksy, Ł.; Stolarczyk, A. The influence of plantar short foot muscle exercises on the lower extremity muscle strength and power in proximal segments of the kinematic chain in long-distance runners. BioMed Res. Int. 2019, 2019, 694273. [Google Scholar] [CrossRef]
- Taddei, U.T.; Matias, A.B.; Duarte, M.; Sacco, I.C.N. Foot core training to prevent running-related injuries: A survival analysis of a single-blind, randomized controlled trial. Am. J. Sports Med. 2020, 48, 3610–3619. [Google Scholar] [CrossRef]
- Schulz, K.; Atman, D.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 23, c332. [Google Scholar] [CrossRef]
- Meyer-Moock, S.; Feng, Y.S.; Maeurer, M.; Dippel, F.W.; Kohlmann, T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and de Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014, 14, 58. [Google Scholar] [CrossRef]
- Dite, W.; Temple, V.A. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch. Phys. Med. Rehabil. 2002, 83, 1566–1571. [Google Scholar] [CrossRef]
- Nieuwenhuis, M.M.; Van Tongeren, H.; Sørensen, P.S.; Ravnborg, M. The Six Spot Step Test: A new measurement for walking ability in multiple sclerosis. Mult. Scler. 2006, 12, 495–500. [Google Scholar] [CrossRef]
- Pavan, K.; Tilbery, C.P.; Lianza, S.; Matsuda Marangoni, B.E. Validation of the “Six Step Spot Test” for gait among patients with multiple sclerosis in Brazil. Arq. Neuropsiquiatr. 2010, 68, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Skjerbæk, A.G.; Dalgas, U.; Stenager, E.; Boesen, F.; Hvid, L.G. The six spot step test is superior in detecting walking capacity impairments compared to short- and long-distance walk tests in persons with multiple sclerosis. MSJ Exp. Transl. Clin. 2023, 9, 20552173231218127. [Google Scholar] [CrossRef] [PubMed]
- Rossiter-Fornoff, J.; Wolf, S.; Wolfson, L.; Buchner, D. A cross-sectional validation study of the FICSIT common data base static balance measures. Frailty and Injuries: Cooperative Studies of Intervention Techniques. J. Gerontol. Biol. Sci. Med. 1995, 50, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; Odonkor, C.; Griffith, L.; Holt, N.; Percac-Lima, S.; Leveille, S.; Ni, P.; Latham, N.K.; Jette, A.M.; Bean, J.F. Reconceptualizing balance: Attributes associated with balance performance. Exp. Gerontol. 2014, 57, 218–223. [Google Scholar] [CrossRef]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhances muscles mass, power output, and functional outcomes in instituzionalized frail nonagenarians. Age 2014, 36, 773–785. [Google Scholar] [CrossRef]
- Cattaneo, D.; Jonsdottir, J.; Repetti, S. Reliability of four scales on balance disorders in persons with multiple sclerosis. Disabil. Rehabil. 2007, 29, 1920–1925. [Google Scholar] [CrossRef]
- Garg, H.; Bush, S.; Gappmaier, E. Associations between fatigue and disability, functional mobility, depression, and quality of life in people with multiple sclerosis. Int. J. MS Care 2016, 18, 71–77. [Google Scholar] [CrossRef]
- Gooding, T.M.; Feger, M.A.; Hart, J.M.; Hertel, J. Intrinsic foot muscle activation during specific exercises: A T2 time magnetic resonance imaging study. J. Athl. Train. 2016, 51, 644–650. [Google Scholar] [CrossRef]
- Kim, M.; Kwon, O.; Kim, S.; Jung, D. Comparison of muscle activities of abductor hallucis and adductor hallucis between the short foot and toes-spread-out exercises in subjetcs with mild hallux valgus. J. Back Musculoskelet. Rehabil. 2013, 26, 163–168. [Google Scholar] [CrossRef]
- Kao, S.L.; Hsiao, M.L.; Wang, J.H.; Chen, C.S.; Chen, S.Y.; Shiau, Y.J.; Yang, C.H. Effects of integrated intrinsic foot muscle exercise with foot core training device on balance and body composition among community-dwelling adults aged 60 and above. BMC Geriatr. 2024, 24, 403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).