Abstract
Background: Radiotherapy for brain tumors can induce cognitive decline, yet most studies examine white matter (WM) damage six months post-treatment, overlooking early microstructural changes. This study investigated whether early WM changes, as measured by diffusion tensor imaging (DTI) histogram and texture features, can predict later cognitive deficits. Methods: Nineteen adults with brain metastases underwent DTI before and immediately after radiotherapy. Ten features—eight histogram-based and two texture-based—were extracted from normal-appearing WM of major DTI indices. Changes (Δ) in these features, if any, were analyzed via multiple linear regression, correlating them with cognitive performance at four months after therapy. Results: Out of 40 features, four exhibited significant post-radiotherapy changes. These were the mean (ADmean) and skewness (ADskewness) of axial diffusivity and the kurtosis of mean diffusivity (MDkurtosis) and radial diffusivity (RDkurtosis). Regression identified ΔADmean (β = −3.303 × 104, p = 0.002) as negatively and ΔADskewness (β = 4.642, p = 0.006) and ΔRDkurtosis (β = −1.505, p = 0.027) as positively associated with semantic fluency. Conclusions: Early WM microstructural disruptions—particularly axonal damage and heterogeneous injury—correlate with declines in semantic fluency. DTI histogram and texture features may be promising as early non-invasive biomarkers for cognitive risk following radiotherapy.
Keywords:
brain; radiotherapy; cognitive function; diffusion tensor imaging; white matter; histogram; texture 1. Introduction
Brain tumors, including high-grade gliomas and brain metastases, frequently require whole-brain radiation therapy (WBRT) or stereotactic irradiation (STI) as primary treatment modalities [1,2]. While these interventions effectively control tumor growth and extend patient survival, they are frequently associated with neurotoxic effects. Notably, studies report that approximately 50% to 90% of patients experience varying degrees of cognitive dysfunction, including impairments in memory, attention, processing speed, and executive function, within 3 to 6 months following radiation therapy [3,4,5,6].
The pathophysiological mechanisms underlying radiation-induced cognitive impairment are multifaceted. Radiation disrupts the integrity of cerebral white matter (WM) and gray matter through several biological processes, including vascular damage, demyelination, neuroinflammation, and, ultimately, necrosis of brain tissues [7,8]. Current assessment methods for post-radiation cognitive impairment primarily include neurocognitive function tests such as the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) [9,10].
WM, which consists of myelinated axonal tracts, is particularly susceptible to radiation-induced injury. Damage to WM disrupts signal transmission across critical neural pathways, affecting various cognitive domains. Studies have demonstrated that specific WM tracts, such as the corpus callosum, cingulum bundle, and superior longitudinal fasciculus, are particularly susceptible to radiation damage, correlating with deficits in semantic fluency, executive function, and processing speed [11,12,13]. Damage to the WM can be assessed using diffusion tensor imaging (DTI) [14], a magnetic resonance imaging (MRI) technique that quantifies the microstructural integrity of WM tracts by measuring the diffusion of water molecules around axons [15]. DTI provides four indices: fractional anisotropy (FA), which reflects fiber density and coherence and typically decreases in cases of WM damage; mean diffusivity (MD), which represents the average apparent diffusion coefficient across fiber directions and is negatively correlated with cell density and positively correlated with edema and WM degeneration; radial diffusivity (RD), which measures water diffusion perpendicular to axonal fibers and is particularly sensitive to demyelination processes; and axial diffusivity (AD), which quantifies water diffusion parallel to the principal fiber direction and often reflects axonal integrity. These DTI indices have been demonstrated to be effective in evaluating WM changes, offering insights into the extent and distribution of WM injury [16,17]. Importantly, studies have shown that FA reduction and RD increase in major WM tracts, including the posterior thalamic radiation, sagittal stratum, and corpus callosum, are closely linked to impairments in cognitive processing speed, memory, and executive function [18].
Moreover, advanced imaging analysis, such as histogram analysis and gray level co-occurrence matrix (GLCM)-based texture analysis, extends the diagnostic capabilities of DTI. Histogram analysis evaluates the distribution and frequency of voxel intensity values within tissue regions. In contrast, GLCM-based texture analysis captures the spatial relationships between neighboring voxels with similar intensities, providing information about tissue heterogeneity and structural disorganization [19,20]. Park et al. effectively utilized these methods to characterize tissue heterogeneity and microstructural organization in gliomas and successfully predicted genetic profiles and clinical outcomes with high accuracy [21].
Despite extensive research on radiation-induced WM changes, significant gaps remain regarding immediate post-treatment microstructural alterations and their cognitive correlates. Most prior research has focused on late radiation effects on WM, occurring more than six months post-treatment, leaving a critical gap in understanding the immediate microstructural changes that could serve as early predictive biomarkers of cognitive decline. Studies on immediate post-treatment microstructural changes remain critically limited despite existing evidence that central nervous system alterations develop much earlier than 6 months and may contribute to the pathogenesis of long-term cognitive dysfunction [22]. Early detection of such microstructural WM alterations could enable the identification of patients at heightened risk and trigger timely, targeted interventions before permanent damage ensues. Compounding this, most studies rely on conventional DTI indices (e.g., FA, MD). However, advanced histogram and texture analysis may reveal subtle spatial damage patterns characteristic of the acute phase, which are overlooked by standard analyses.
Preclinical studies have demonstrated that microstructural changes in WM can occur within days to weeks following radiation exposure, even in areas that appear normal on conventional imaging. These early alterations include axonal swelling, oligodendrocyte damage, and acute inflammatory responses that precede clinically evident radiation-induced cognitive impairment. Studies on rodent models have shown that dividing oligodendrocyte precursor cells are particularly vulnerable to radiation damage, resulting in heterogeneous injury patterns across WM regions [23,24]. Furthermore, acute microglial activation has been documented immediately following radiation exposure, potentially explaining the early microstructural changes that occur before visible macrostructural damage becomes apparent [25,26]. These early microstructural changes likely disrupt neural connectivity and information processing efficiency, directly affecting cognitive function even before gross anatomical changes become apparent on conventional imaging post-radiation.
Based on these observations, we hypothesized that DTI histogram and texture analysis would detect subtle microstructural changes in whole-brain normal-appearing WM (NAWM) immediately after radiotherapy, even in the absence of visible lesions on conventional MRI. We anticipated that these changes would be reflected in the heterogeneity of diffusion indices across the entire WM and would correlate with domain-specific cognitive functions. This study aimed to establish whether immediate post-radiotherapy DTI measures can serve as early biomarkers of cognitive vulnerability, potentially enabling earlier intervention strategies to preserve cognitive function in patients undergoing brain radiotherapy.
2. Materials and Methods
2.1. Study Population
This prospective study was approved by the Institutional Review Board of Hokkaido University Hospital (009-0001), and written informed consent was obtained from all participants. Patient recruitment took place over 22 months (March 2009 to December 2010).
Inclusion criteria were (1) patients diagnosed with brain metastases from active systemic disease (predominantly lung cancer and breast cancer); (2) age between 20 and 80 years; (3) Karnofsky Performance Status score ≥70%; (4) presence of clinical signs and symptoms associated with brain metastases; (5) expected survival exceeding 6 months; and (6) scheduled to undergo WBRT or STI. For patients with three or more brain metastases and active systemic disease, WBRT was delivered at a total dose of 35 Gy in 14 fractions over 3 weeks (35 Gy/14Fr/3 weeks). Patients with fewer metastatic lesions underwent STI, which included either single-session stereotactic radiosurgery or fractionated stereotactic radiotherapy. Lesion size determined the STI dosing protocol: smaller tumors measuring 1.5 cm in diameter or less received 25 Gy in a single fraction, whereas larger lesions were treated with 28–35 Gy divided into four fractions.
Exclusion criteria comprised (1) absolute contraindications for MRI; (2) history of prior WBRT or STI; and (3) unavailability of follow-up neuropsychological data up to 4 months post-treatment.
2.2. Neurocognitive Assessment
The neurocognitive assessment was performed using standardized tests from the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and the Trail Making Test (TMT). Five distinct cognitive domains, i.e., immediate memory (list learning in RBANS), delayed memory (list recall and list recognition in RBANS), word fluency (semantic fluency in RBANS), attention (TMT-Part A), and executive function (TMT-Part B), were evaluated. The Mini-Mental State Examination (MMSE) was also administered as a measure of global cognitive function. All cognitive assessments were conducted four months after the completion of radiotherapy to evaluate potential radiation-induced cognitive changes, as the 4-month neurocognitive status was suggested as a predictor of neurocognitive status at 12 months [27].
2.3. MRI Acquisition
All imaging was performed on a 1.5T MRI scanner (Magnetom Symphony, Siemens Healthcare, Erlangen, Germany) using a standard head coil. The imaging protocol consisted of DTI and conventional MRI sequences (T1-weighted imaging (T1WI), gadolinium-enhanced T1-weighted imaging (Gd-T1WI), and fluid-attenuated inversion recovery (FLAIR) imaging). The scan parameters are detailed in Table 1.

Table 1.
MRI scan parameters.
Images were acquired at two time points: pre-treatment (baseline) and immediately after treatment completion. The former included DTI and the complete conventional MRI protocol, whereas the latter included DTI and T1WI. All images were reviewed by an experienced neuroradiologist (20 years of neuroimaging experience) to exclude artifacts (e.g., motion artifacts) or coincidental findings that could potentially confound the study results.
2.4. DTI Data Processing
The diffusion tensor estimation was performed using least-squares fitting, yielding three eigenvalues (λ1, λ2, and λ3) that characterize diffusion along the principal axes. Four DTI indices were calculated: FA = √(3/2 × √[(λ1 − MD)2 + (λ2 − MD)2 + (λ3 − MD)2]/√(λ12 + λ22 + λ32)); MD = (λ1 + λ2 + λ3)/3; AD = λ1; and RD = (λ2 + λ3)/2. For image reconstruction and visualization, FA, MD, and AD maps were generated using Dr. View/LINUX R2.5 software (Asahi Kasei Corporation, Tokyo, Japan). RD maps were generated using Python with the SimpleITK library (version 2.5.0, Insight Software Consortium, Austin, TX, USA, https://simpleitk.org/ (accessed on 2 May 2025)).
2.5. Image Processing and Region of Interest Definition
A semi-automated image co-registration process aligned maps of DTI indices and images of conventional MRI sequences to the baseline dataset using Statistical Parametric Mapping (SPM12, Functional Imaging Laboratory, UCL Queen Square Institute of Neurology, London, UK). All images were spatially normalized to the Montreal Neurological Institute (MNI) standard space using a cost-function masking approach, where lesions were masked to prevent distortion during the normalization process. The spatial normalization parameters included affine regularization optimized for East Asian brains, with 4th-degree B-spline interpolation to maintain image quality.
Brain parenchyma (BP) was segmented from normalized FLAIR or echo planar images with no diffusion weighting (b0) images using a signal intensity threshold set-up in MRICron (version 1.0.2, Chris Rorden’s Neuropsychology Lab, University of South Carolina, Columbia, SC, USA, https://www.nitrc.org/projects/mricron (accessed on 5 January 2022)) [28]. To determine the appropriate segmentation thresholds, MRI signal intensities were measured across 14 normal-appearing brain regions, including the gray matter of the bilateral frontal, parietal, temporal, and occipital lobes, as well as the pons, lentiform nucleus, and cerebellar hemispheres. The mean and standard deviation (SD) of the measured signal intensities were calculated for each time point.
The normal-appearing BP (NABP) was then delineated using the formula: BPsignal ≤ Mean + (0.5 × SD), where BPsignal represents the calculated MRI signal intensity for BP. Here, NABP refers to regions that are structurally intact on conventional MRI but potentially exhibit microstructural abnormalities detectable with DTI.
Subsequently, WM was segmented from T1-weighted images using SPM12’s segmentation algorithm with affine regularization optimized for East Asian brains, and the deformation field was set forward. In this analysis, we focused on NAWM, defined as WM within NABP. An experienced neuroradiologist visually assessed registration accuracy and spatial normalization procedures to ensure accuracy. The complete image processing workflow from DTI map reconstruction to NAWM extraction is illustrated in Figure 1.
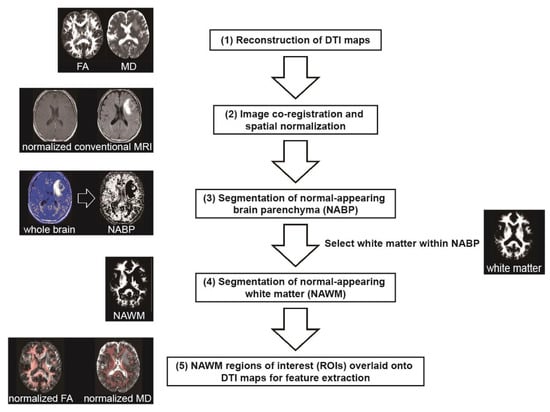
Figure 1.
Workflow for extracting diffusion tensor imaging (DTI) features from normal-appearing white matter (NAWM). The processing pipeline demonstrates sequential steps: (1) reconstruction of DTI maps (FA and MD shown as examples); (2) image co-registration and spatial normalization to the MNI standard space; (3) segmentation of normal-appearing brain parenchyma (NABP) from spatially normalized fluid-attenuated inversion recovery (FLAIR) or echo-planar images with no diffusion weighting (b0); (4) segmentation of white matter (WM) from spatially normalized T1-weighted images, followed by selection of regions of interest (ROIs) within NABP to define NAWM; (5) Final overlay of NAWM ROIs onto spatially normalized DTI maps (FA and MD shown as examples) for subsequent histogram and texture feature extraction.
2.6. Histogram and Texture Feature Extraction
A customized radiomic feature extraction workflow [29], implemented in Python (version 3.9.7, Python Software Foundation, Wilmington, DE, USA, https://www.python.org/ (accessed on 23 November 2024)), extracted quantitative features from NAWM regions of interest (ROIs). For each DTI index (FA, MD, AD, or RD), ten features were analyzed, which included eight histogram features (10th percentile, 90th percentile, image energy (∑I2, where I represents voxel intensity), entropy, kurtosis, mean, skewness, and uniformity) and two GLCM-based texture features (contrast and correlation). Thus, in total, 40 DTI features were analyzed (10 features × 4 DTI indices). The feature selection was based on several methodological considerations, including sample size constraints (extracting many features will inevitably lead to severe multiple comparison issues and risk overfitting) and interpretability of results (we focused on well-established, clinically interpretable features that have demonstrated utility in medical imaging). To evaluate treatment-induced changes specifically, we calculated the change (Δ) in each feature by subtracting the baseline (before radiotherapy) value from the immediate post-radiotherapy value.
2.7. Statistical Analysis
All statistical analyses were performed using R software (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria). To identify significant changes in DTI upon radiotherapy, each DTI feature was initially compared between the two time points (baseline and immediately after radiotherapy) using paired Wilcoxon signed-rank tests. Non-parametric statistics (paired Wilcoxon signed-rank tests in this study) were chosen due to the small sample size (n = 19). Non-parametric tests do not require distributional assumptions, and are generally preferred for small samples [30,31].
Multiple linear regression models were reconstructed for each cognitive domain using those Δ features that showed significant changes (p < 0.05) on the Wilcoxon signed-rank tests as predictors. Given the mathematical relationship among interdependent DTI indices, where MD can be derived from AD and RD as MD = (AD + 2RD)/3, we implemented separate analytical approaches between MD and AD or RD to avoid issues of collinearity. Specifically, we developed two distinct model configurations; models containing ΔMD features were analyzed separately from models containing ΔAD and ΔRD features. Regression diagnostics included examining residual plots to verify the assumptions of normality, homoscedasticity, and independence. Both unstandardized (β) and standardized (Std. β) coefficients, as well as the correlation coefficient (r), were calculated to facilitate comparisons among predictors and evaluate the strength of association with cognitive outcome. Relationships between significant features and cognitive outcomes were visualized using scatterplots with regression lines and 95% confidence intervals.
To control for multiple comparisons, we applied the Benjamini–Hochberg false discovery rate (FDR) correction procedure. p values were ordered from the smallest to the largest, with critical values calculated as (i/m) × α (where i = rank, m = total comparisons, and α = 0.05). Values below their corresponding critical threshold were considered significant after correction.
3. Results
3.1. Study Population
Nineteen patients (six men) were included in the final analysis. Fourteen patients received WBRT, and five patients received STI. Table 2 summarizes the neurocognitive status of the patients 4 months after radiotherapy.

Table 2.
4-month neurocognitive status of the patients.
3.2. DTI Feature Changes After Radiotherapy
Four features showed significant changes (p < 0.05), which are shown in Table 3: mean (ADmean) (ΔADmean = −4.30 ± 8.81 (×10−5 mm2 s−1), p = 0.023) and skewness (ADskewness) (ΔADskewness = −0.31 ± 0.59, p = 0.032) of AD, and kurtosis of MD (MDkurtosis) (ΔMDkurtosis = −1.04 ± 1.89, p = 0.049) and RD (RDkurtosis) (ΔRDkurtosis = −0.68 ± 1.12, p = 0.016). Δ of these features was selected for subsequent correlation analyses with cognitive outcome.

Table 3.
Features showing significant changes immediately after radiotherapy.
3.3. Correlation Between Changes in DTI Features and Cognitive Performance 4 Months Post-Radiation
Multiple linear regression analysis (Table 4) revealed that ΔADmean was significantly associated with list learning performance (β = −2.404 × 104, r = −0.330, p = 0.027). This negative association is illustrated in Figure 2A, which shows that a greater increase in ΔADmean corresponds to poorer list learning scores. There was a trend toward significance for ΔADskewness (β = 3.188, r = 0.154, p = 0.072), whereas ΔRDkurtosis showed no significant association (p = 0.683). The overall model explained approximately 31% of the variance in list learning scores (R2 = 0.308, adjusted R2 = 0.169, F(3,15) = 2.223, p = 0.128).

Table 4.
Multiple linear regression analysis of Δ DTI features predicting cognitive outcome.
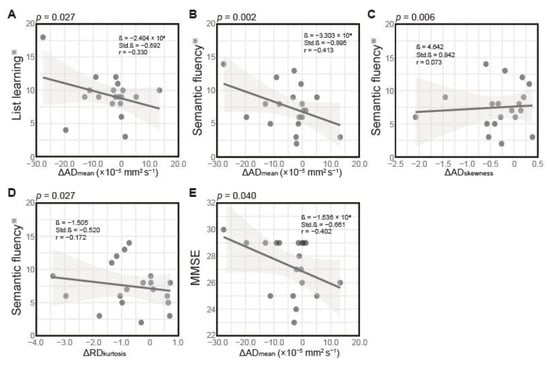
Figure 2.
Scatterplots showing associations between change (Δ) in diffusion tensor imaging (DTI) features and neurocognitive function test scores. (A) Association between Δ in the mean of axial diffusivity (ΔADmean) and list learning performance. Association between (B) ΔADmean, (C) Δ in skewness of AD (ΔADskewnes), and (D) Δ in kurtosis of radial diffusivity (ΔRDkurtosis), and semantic fluency. (E) Association between ΔADmean and MMSE score. Solid lines represent linear regression fits, and gray shading indicates 95% confidence intervals. Each dot represents one patient. Statistical parameters, including β coefficient, standardized coefficient (Std. β), correlation coefficient (r), and p-values, are displayed for each association. ※, age-adjusted.
As shown in Table 4, all three Δ DTI features significantly predicted semantic fluency performance. ΔADmean demonstrated the strongest effect (β = −3.303 × 104, r = −0.413, p = 0.002), whereas ΔADskewness (β = 4.642, r = 0.073, p = 0.006) and ΔRDkurtosis (β = −1.505, r = −0.172, p = 0.027) showed more minor but significant associations with semantic fluency scores. This model demonstrated the strongest predictive power, explaining approximately 52% of the variance (R2 = 0.521, adjusted R2 = 0.425, F(3,15) = 5.428, p = 0.010). Figure 2B–D illustrate these relationships, showing that greater increases in ΔADmean and ΔRDkurtosis were associated with poorer semantic fluency performance. In contrast, changes in ΔADskewness showed a weak positive association.
As for MMSE (Table 4), only ΔADmean significantly predicted the performance (β = −1.536 × 104, r = −0.402, p = 0.040). Figure 2E illustrates this negative association, showing that greater increases in ΔADmean were associated with lower MMSE scores. Neither ΔADskewness (p = 0.194) nor ΔRDkurtosis (p = 0.438) showed a significant association. The overall model explained approximately 25% of the variance in MMSE scores (R2 = 0.254, adjusted R2 = 0.105, F(3,15) = 1.703, p = 0.209).
Regression analysis also revealed a trend toward a negative correlation between ΔMDkurtosis and list recognition performance (β = −0.524, r = −0.451, p = 0.053), suggesting that increased heterogeneity in diffusion patterns may be associated with poorer delayed memory.
3.4. FDR Correction
After applying FDR correction for multiple comparisons, only the relationship between ΔADmean and semantic fluency remained statistically significant (p = 0.002, corrected p = 0.036). The association between ΔADskewness and semantic fluency approached significance after correction (p = 0.006, corrected p = 0.064). All other previously significant relationships did not survive FDR correction.
4. Discussion
This study investigated radiotherapy-induced microstructural WM changes and their relationship with cognitive function using DTI histogram and texture analysis. The findings revealed significant associations between the Δ of DTI features and cognitive performance, primarily semantic fluency. It is noteworthy that only the relationship between ΔADmean and semantic fluency remained statistically significant after FDR correction for multiple comparisons, highlighting its robust and reliable association.
The observed DTI changes likely reflect early, transient radiation-induced microstructural alterations in whole-brain WM rather than permanent damage. Our analysis revealed that ΔADmean was predominantly negative, indicating a decrease in AD following radiotherapy. This reduction in AD aligns with acute-phase reactions to radiation, potentially reflecting transient axonal swelling from inflammatory responses, cytotoxic edema, temporary disruption of axonal transport mechanisms, and early metabolic changes—all stemming from radiation effects on neurons and glial cells [32,33]. Since imaging was performed immediately after treatment completion, these changes likely represent acute radiation reactions rather than chronic degeneration. These early alterations may resolve, persist, or evolve into more permanent changes, such as demyelination over time [34].
ΔADskewness reflects alterations in the distribution pattern of AD values following radiotherapy. This suggests radiation effects on WM are not uniformly distributed, with some regions potentially showing differential vulnerability to radiation [33]. This heterogeneity in response patterns may explain why certain cognitive networks show greater functional changes despite similar radiation exposure [35]. Similarly, ΔRDkurtosis indicates alterations in the peakedness of the RD distribution, potentially affecting functions requiring coordination among multiple brain regions [34]. Furthermore, pathological studies on laboratory animals have provided concrete evidence of these microstructural changes occurring within hours to days post-radiotherapy. Acute responses observed in preclinical models have demonstrated early axonal swelling, microglial activation, and initial demyelination as immediate reactions to radiation exposure [32,36]. Notably, these alterations in WM integrity can begin within hours after exposure, as indicated by early-phase DTI changes in high-dose regions [37].
Different cognitive domains exhibited varying sensitivity to WM changes. Semantic fluency demonstrated the strongest associations with all three ΔDTI features (ΔADmean, ΔADskewness, ΔRDkurtosis), possibly because it relies on distributed neural networks and long-range WM connections [16,38]. This aligns with prior studies showing that semantic fluency tasks engage frontotemporal and interhemispheric pathways, which are particularly vulnerable to radiotherapy-related WM injury [39]. In contrast, MMSE showed limited associations, emphasizing the importance of domain-specific assessments when evaluating radiation-induced cognitive changes. Compared to ΔADmean, which showed robust associations across multiple cognitive domains, the weak correlation between ΔADskewness and semantic fluency (r = 0.073) warrants caution. Although this association reached statistical significance in the regression model, the minimal correlation coefficient value suggests limited practical and likely clinical relevance.
The findings of this study have important clinical applications. DTI histogram and texture features may serve as early biomarkers of radiation-induced cognitive vulnerability, potentially identifying high-risk patients before symptom onset [36]. The differential sensitivity of cognitive domains suggests that targeted assessments focusing on semantic fluency and list learning may better detect early radiation effects than global measures like MMSE [16]. This is corroborated by findings that adding a brief semantic fluency test dramatically improves mild cognitive impairment detection over MMSE alone [40]. Understanding regional damage patterns could inform treatment planning strategies aimed at preserving critical cognitive networks.
This study presents several advances in understanding radiation-induced WM injury. First, it characterizes the immediate post-treatment microstructural alterations, revealing predominantly negative ΔADmean, which suggests an acute-phase response different from later-stage changes. Second, the histogram and texture analysis approach demonstrates that damage distribution patterns—not merely extent—largely impact cognitive outcomes. Third, this study identifies semantic fluency as particularly sensitive to early WM changes, potentially serving as a valuable early indicator of radiation-induced cognitive vulnerability. Finally, the Δ feature approach isolates treatment-specific effects by controlling for individual baseline differences.
Pathological studies on laboratory animals further contextualize our findings. Animal models have demonstrated microstructural WM changes beginning within days to weeks post-radiotherapy, with early axonal swelling and initial myelin alterations [41]. These models reveal both acute changes and potential progression over time, with our observations of increased ΔADmean potentially capturing early axonal responses to radiation [42]. Histological analyses confirmed the differential vulnerability of brain regions to radiation, with oligodendrocyte precursor cells critical for myelin maintenance and repair showing particular sensitivity [43], consistent with the observation of increased ΔADskewness. Their early disruption may contribute to the heterogeneous injury patterns detected in our DTI analysis. Additionally, acute inflammatory responses and microglial activation occur soon after radiation exposure, potentially explaining the early microstructural changes, likely explaining early microstructural changes, and suggesting mechanisms for longer-term effects beyond our study timeframe [44].
Human longitudinal studies also provide an important context for interpreting our early post-treatment findings. These suggest that WM changes observed immediately after radiotherapy may represent the initial phase of a dynamic process that evolves over time [45]. Research examining DTI changes at multiple time points—from 6 months to 5 years post-radiotherapy—indicates that frontal and temporal regions often show continued microstructural alterations, particularly in high-dose areas [46,47]. There is compelling evidence of reversible changes in some patients, especially during the early-delayed phase (1–6 months post-treatment), when physiological adaptations can occur without permanent structural damage [48,49,50]. The eventual outcome appears influenced by multiple factors, including radiation parameters (dose, volume, fractionation), patient characteristics (age, vascular risk factors), and concurrent treatments. Our findings capture an early snapshot in this continuum—a critical window where both recovery and progression remain possible pathways. This temporal perspective emphasizes the potential of early DTI markers in identifying individuals who are vulnerable to long-term neurocognitive effects. Additionally, these markers can help guide timely interventions that may lead to better outcomes and improved quality of life for patients undergoing radiotherapy.
Several limitations should be considered. First, our relatively small sample size limited statistical power, which may explain the modest number of significant associations observed. Second, this study included patients receiving different radiotherapy regimens, i.e., WBRT and STI. Because our primary objective was to identify the DTI correlates of radiotherapy-induced cognitive decline, and partly due to the small sample size within each regimen, we did not evaluate treatment-specific microstructural WM changes or cognitive outcomes. Third, data collection at only two time points prevented tracking the longitudinal evolution of microstructural changes. Fourth, our whole-brain WM analysis lacked regional specificity, which may have masked important localized effects in critical cognitive networks. Fifth, the follow-up period was limited to four months post-radiotherapy, although cognitive changes may continue to develop over longer periods. Sixth, although regression models showed significant associations, some correlations were weak, particularly the associations of ΔADskewness (r = 0.073) and ΔRDkurtosis (r = −0.172) to semantic fluency. This is thought to suggest that these individual features are yet to be clinically actionable on their own. Finally, the relatively large slice thickness of our DTI data may have reduced sensitivity to subtle changes and introduced partial volume effects.
To mitigate some of these challenges, we limited feature extraction to only 10 features. While the extraction of hundreds of features is technically possible, our approach prioritized statistical validity and clinical interpretability over sheer quantity. In small sample studies, focusing on a concise feature set reduces the risk of spurious associations and alleviates the penalties associated with multiple comparisons—an established concern in high-dimensional, low-sample-size research. We also employed the non-parametric Wilcoxon signed-rank test—well-suited for small samples and non-normal data—and applied FDR correction to control for multiple comparisons, further enhancing the robustness of our findings. While not limitations per se, these methodological choices strengthen the validity of our results. Moreover, although we did not conduct treatment-specific analyses, the cohort’s heterogeneity reflects real-world clinical diversity and underscores the need for future research to disentangle regimen-specific effects on WM and cognition.
5. Conclusions
In conclusion, radiotherapy-induced microstructural WM changes, particularly in terms of axonal integrity and damage distribution patterns, are significantly correlated with cognitive performance. These findings enhance our understanding of radiation-induced cognitive decline and highlight the potential of DTI histogram and texture analysis for monitoring treatment effects and guiding cognitive interventions.
Author Contributions
Conceptualization, K.K.T.; methodology, all authors; validation, J.W. and K.K.T.; formal analysis, all authors; investigation, all authors; resources, H.A. and K.K.T.; data curation, J.W., M.K., S.O., S.T., and K.K.T.; writing—original draft preparation, J.W., P.K.J.O., and K.K.T.; writing—review and editing, J.W. and K.K.T.; visualization, K.K.T.; supervision, H.A., S.T., and K.K.T.; project administration, K.K.T.; funding acquisition, H.A. and K.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Hokkaido University Hospital (009-0001).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available because of ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WBRT | whole-brain radiation therapy |
| STI | stereotactic irradiation |
| WM | white matter |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| DTI | diffusion tensor imaging |
| MRI | magnetic resonance imaging |
| FA | fractional anisotropy |
| MD | mean diffusivity |
| AD | axial diffusivity |
| RD | radial diffusivity |
| GLCM | gray level co-occurrence matrix |
| NAWM | normal-appearing WM |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Status |
| TMT | Trail Making Test |
| TR | repetition time |
| TE | echo time |
| NEX | number of excitations |
| FOV | field of view |
| T1WI | T1-weighted imaging |
| Gd-T1WI | gadolinium-enhanced T1-weighted imaging |
| FLAIR | fluid-attenuated inversion recovery |
| TI | inversion time |
| SPM12 | Statistical Parametric Mapping |
| MNI | Montreal Neurological Institute |
| BP | brain parenchyma |
| b0 | echo planar images with no diffusion weighting |
| SD | standard deviation |
| NABP | normal-appearing BP |
| ROI | region of interest |
| Δ | change |
| β | unstandardized coefficient |
| Std.β | standardized coefficient |
| FDR | false discovery rate |
| MDkurtosis | kurtosis of MD |
| ADmean | mean of AD |
| ADskewness | skewness of AD |
| RDkurtosis | kurtosis of RD |
References
- Chi, A.; Komaki, R. Treatment of Brain Metastasis from Lung Cancer. Cancers 2010, 2, 2100–2137. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Arbiser, J.; Zelnak, A.; Shu, H.-K.G.; Shim, H.; Robin, A.M.; Kalkanis, S.N.; Whitsett, T.G.; Salhia, B.; Tran, N.L.; et al. Current Approaches to the Treatment of Metastatic Brain Tumours. Nat. Rev. Clin. Oncol. 2014, 11, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular Pathways: Radiation-Induced Cognitive Impairment. Clin. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef]
- McDuff, S.G.R.; Taich, Z.J.; Lawson, J.D.; Sanghvi, P.; Wong, E.T.; Barker, F.G.; Hochberg, F.H.; Loeffler, J.S.; Warnke, P.C.; Murphy, K.T.; et al. Neurocognitive Assessment Following Whole Brain Radiation Therapy and Radiosurgery for Patients with Cerebral Metastases. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1384–1391. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-Induced Brain Injury: A Review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Mesny, E.; Jacob, J.; Noël, G.; Bernier, M.-O.; Ricard, D. Specific Radiosensitivity of Brain Structures (Areas or Regions) and Cognitive Impairment after Focal or Whole Brain Radiotherapy: A Review. Cancer Radiother. 2025, 29, 104625. [Google Scholar] [CrossRef]
- Shamsesfandabadi, P.; Patel, A.; Liang, Y.; Shepard, M.J.; Wegner, R.E. Radiation-Induced Cognitive Decline: Challenges and Solutions. Cancer Manag. Res. 2024, 16, 1043–1052. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E. Radiation-Induced Cognitive Impairment--from Bench to Bedside. Neuro Oncol. 2012, 14 (Suppl. 4), iv37–iv44. [Google Scholar] [CrossRef]
- Corn, B.W.; Wang, M.; Fox, S.; Michalski, J.; Purdy, J.; Simpson, J.; Kresl, J.; Curran, W.J.; Diaz, A.; Mehta, M.; et al. Health Related Quality of Life and Cognitive Status in Patients with Glioblastoma Multiforme Receiving Escalating Doses of Conformal Three Dimensional Radiation on RTOG 98-03. J. Neurooncol. 2009, 95, 247–257. [Google Scholar] [CrossRef]
- Olson, R.A.; Chhanabhai, T.; McKenzie, M. Feasibility Study of the Montreal Cognitive Assessment (MoCA) in Patients with Brain Metastases. Support. Care Cancer 2008, 16, 1273–1278. [Google Scholar] [CrossRef]
- Whitford, T.J.; Kubicki, M.; Pelavin, P.E.; Lucia, D.; Schneiderman, J.S.; Pantelis, C.; McCarley, R.W.; Shenton, M.E. Cingulum Bundle Integrity Associated with Delusions of Control in Schizophrenia: Preliminary Evidence from Diffusion-Tensor Tractography. Schizophr. Res. 2015, 161, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, F.; Khanmirzaei, M.H.; Hosseinzadeh, F.; Kolahchi, Z.; Jafarimehrabady, N.; Moghisseh, B.; Aarabi, M.H. Cingulum and Uncinate Fasciculus Microstructural Abnormalities in Parkinson’s Disease: A Systematic Review of Diffusion Tensor Imaging Studies. Biology 2023, 12, 475. [Google Scholar] [CrossRef]
- Raghavan, S.; Przybelski, S.A.; Reid, R.I.; Graff-Radford, J.; Lesnick, T.G.; Zuk, S.M.; Knopman, D.S.; Machulda, M.M.; Mielke, M.M.; Petersen, R.C.; et al. Reduced Fractional Anisotropy of the Genu of the Corpus Callosum as a Cerebrovascular Disease Marker and Predictor of Longitudinal Cognition in MCI. Neurobiol. Aging 2020, 96, 176–183. [Google Scholar] [CrossRef]
- Assaf, Y.; Pasternak, O. Diffusion Tensor Imaging (DTI)-Based White Matter Mapping in Brain Research: A Review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, C.; Jezzard, P.; Basser, P.J.; Barnett, A.; Di Chiro, G. Diffusion Tensor MR Imaging of the Human Brain. Radiology 1996, 201, 637–648. [Google Scholar] [CrossRef]
- Chapman, C.H.; Zhu, T.; Nazem-Zadeh, M.; Tao, Y.; Buchtel, H.A.; Tsien, C.I.; Lawrence, T.S.; Cao, Y. Diffusion Tensor Imaging Predicts Cognitive Function Change Following Partial Brain Radiotherapy for Low-Grade and Benign Tumors. Radiother. Oncol. 2016, 120, 234–240. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Lang, J.; Hu, Z.; Xu, X.; Zhang, Y.; Chen, Q.; Yang, L.; Wang, H.; Li, H. Dose-Dependent Early White Matter Alterations in Patients with Brain Metastases after Radiotherapy. Neuroradiology 2023, 65, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Christodoulou, C.; Bhise, V.; Greenblatt, D.; Patel, Y.; Serafin, D.; Maletic-Savatic, M.; Krupp, L.B.; Wagshul, M.E. Multiple White Matter Tract Abnormalities Underlie Cognitive Impairment in RRMS. Neuroimage 2012, 59, 3713–3722. [Google Scholar] [CrossRef]
- Kunimatsu, A.; Yasaka, K.; Akai, H.; Sugawara, H.; Kunimatsu, N.; Abe, O. Texture Analysis in Brain Tumor MR Imaging. Magn. Reson. Med. Sci. 2022, 21, 95–109. [Google Scholar] [CrossRef]
- Kim, J.P.; Kim, J.; Jang, H.; Kim, J.; Kang, S.H.; Kim, J.S.; Lee, J.; Na, D.L.; Kim, H.J.; Seo, S.W.; et al. Predicting Amyloid Positivity in Patients with Mild Cognitive Impairment Using a Radiomics Approach. Sci. Rep. 2021, 11, 6954. [Google Scholar] [CrossRef]
- Park, Y.W.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kim, S.H.; Kang, S.-G.; Kim, E.H.; Lee, S.-K. Whole-Tumor Histogram and Texture Analyses of DTI for Evaluation of IDH1-Mutation and 1p/19q-Codeletion Status in World Health Organization Grade II Gliomas. AJNR Am. J. Neuroradiol. 2018, 39, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of Radiotherapy-Associated Cognitive Disability in Patients with Brain Tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef]
- Piao, J.; Major, T.; Auyeung, G.; Policarpio, E.; Menon, J.; Droms, L.; Gutin, P.; Uryu, K.; Tchieu, J.; Soulet, D.; et al. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitors Remyelinate the Brain and Rescue Behavioral Deficits Following Radiation. Cell Stem Cell 2015, 16, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, G.; Alshamy, G.; Chan, B.; Abrams, R.; Greenberg, E.; Saxena, A.; Bradbury, M.; Edgar, M.; Gutin, P.; Tabar, V. Long-Term Impact of Radiation on the Stem Cell and Oligodendrocyte Precursors in the Brain. PLoS ONE 2007, 2, e588. [Google Scholar] [CrossRef]
- Zhou, K.; Boström, M.; Ek, C.J.; Li, T.; Xie, C.; Xu, Y.; Sun, Y.; Blomgren, K.; Zhu, C. Radiation Induces Progenitor Cell Death, Microglia Activation, and Blood-Brain Barrier Damage in the Juvenile Rat Cerebellum. Sci. Rep. 2017, 7, 46181. [Google Scholar] [CrossRef]
- Acharya, M.M.; Green, K.N.; Allen, B.D.; Najafi, A.R.; Syage, A.; Minasyan, H.; Le, M.T.; Kawashita, T.; Giedzinski, E.; Parihar, V.K.; et al. Elimination of Microglia Improves Cognitive Function Following Cranial Irradiation. Sci. Rep. 2016, 6, 31545. [Google Scholar] [CrossRef]
- Onodera, S.; Aoyama, H.; Tha, K.K.; Hashimoto, N.; Toyomaki, A.; Terae, S.; Shirato, H. The Value of 4-Month Neurocognitive Function as an Endpoint in Brain Metastases Trials. J. Neurooncol. 2014, 120, 311–319. [Google Scholar] [CrossRef]
- Rorden, C.; Karnath, H.-O.; Bonilha, L. Improving Lesion-Symptom Mapping. J. Cogn. Neurosci. 2007, 19, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Öztuna, D.; Elhan, A.; Tüccar, E. Investigation of Four Different Normality Tests in Terms of Type 1 Error Rate and Power under Different Distributions. Turk. J. Med. Sci. 2006, 36, 171–176. [Google Scholar]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Mesri, H.; Bodiut, V.; Nagtegaal, S.; Elhalawani, H.; de Luca, A.; Philippens, M.; Viergever, M.; Mohamed, A.; Ding, Y.; et al. Dose-Dependent Degeneration of Non-Cancerous Brain Tissue in Post-Radiotherapy Patients: A Diffusion Tensor Imaging Study. medRxiv 2019, 19005157. [Google Scholar] [CrossRef]
- Hope, T.R.; Vardal, J.; Bjørnerud, A.; Larsson, C.; Arnesen, M.R.; Salo, R.A.; Groote, I.R. Serial Diffusion Tensor Imaging for Early Detection of Radiation-Induced Injuries to Normal-Appearing White Matter in High-Grade Glioma Patients. J. Magn. Reson. Imaging 2015, 41, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Liyan, L.; Si, W.; Qian, W.; Yuhui, S.; Xiaoer, W.; Yuehua, L.; Wenbin, L. Diffusion Kurtosis as an in Vivo Imaging Marker of Early Radiation-Induced Changes in Radiation-Induced Temporal Lobe Necrosis in Nasopharyngeal Carcinoma Patients. Clin. Neuroradiol. 2018, 28, 413–420. [Google Scholar] [CrossRef]
- Xie, X.; Feng, M.; Rong, Y.; Hu, J.; Zhou, W.; Li, Y.; Liao, H.; Shi, L.; He, H.; Tong, Q.; et al. Whole Brain Atlas-Based Diffusion Kurtosis Imaging Parameters for the Evaluation of Multiple Cognitive-Related Brain Microstructure Injuries after Radiotherapy in Lung Cancer Patients with Brain Metastasis. Quant. Imaging Med. Surg. 2023, 13, 5321–5332. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Diffusion Tensor Imaging Indices to Predict Cognitive Changes Following Adult Radiotherapy. Eur. J. Cancer Care 2021, 30, e13329. [Google Scholar] [CrossRef]
- Mahmoud, B.E.; Mohammad, M.E.; Serour, D.K. What Can DTI Add in Acute Ischemic Stroke Patients? Egypt. J. Radiol. Nucl. Med. 2019, 50, 67. [Google Scholar] [CrossRef]
- Xie, Y.; Xie, L.; Kang, F.; Jiang, J.; Yao, T.; Mao, G.; Fang, R.; Fan, J.; Wu, D. Association between White Matter Alterations and Domain-Specific Cognitive Impairment in Cerebral Small Vessel Disease: A Meta-Analysis of Diffusion Tensor Imaging. Front. Aging Neurosci. 2022, 14, 1019088. [Google Scholar] [CrossRef]
- Peres, M.; Castro, B.; Messas, C.; Martucci, C.; Chaim, K.; Pastorello, B.; Valerio, R.; Jorge, C.; Lyra, K.; Otaduy, M.; et al. Semantic Fluency Impairment in Unilateral Mesial Temporal Sclerosis Related Epilepsy Is Associated with Extensive White Matter Involvement: A Diffusion Tensor Imaging Study. (P2.239). Neurology 2017, 88, P2.239. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Lee, G.J. Cognitive Screening for Early Detection of Mild Cognitive Impairment. Int. Psychogeriatr. 2020, 32, 1015–1017. [Google Scholar] [CrossRef]
- Armstrong, R.C.; Mierzwa, A.J.; Sullivan, G.M.; Sanchez, M.A. Myelin and Oligodendrocyte Lineage Cells in White Matter Pathology and Plasticity after Traumatic Brain Injury. Neuropharmacology 2016, 110, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Nellessen, A.; Nyamoya, S.; Zendedel, A.; Slowik, A.; Wruck, C.; Beyer, C.; Fragoulis, A.; Clarner, T. Nrf2 Deficiency Increases Oligodendrocyte Loss, Demyelination, Neuroinflammation and Axonal Damage in an MS Animal Model. Metab. Brain Dis. 2020, 35, 353–362. [Google Scholar] [CrossRef]
- Seo, J.H.; Miyamoto, N.; Hayakawa, K.; Pham, L.-D.D.; Maki, T.; Ayata, C.; Kim, K.-W.; Lo, E.H.; Arai, K. Oligodendrocyte Precursors Induce Early Blood-Brain Barrier Opening after White Matter Injury. J. Clin. Investig. 2013, 123, 782–786. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E.H. Experimental Models for Analysis of Oligodendrocyte Pathophysiology in Stroke. Exp. Transl. Stroke Med. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Rao, J.-H.; Lan, X.-Y.; Li, X.; Chu, C.-Y.; Liang, Y.; Janowski, M.; Zhang, H.-T.; Walczak, P. White Matter Demyelination Predates Axonal Injury after Ischemic Stroke in Cynomolgus Monkeys. Exp. Neurol. 2021, 340, 113655. [Google Scholar] [CrossRef]
- Dinkel, J.G.; Lahmer, G.; Mennecke, A.; Hock, S.W.; Richter-Schmidinger, T.; Fietkau, R.; Distel, L.; Putz, F.; Dörfler, A.; Schmidt, M.A. Effects of Hippocampal Sparing Radiotherapy on Brain Microstructure-A Diffusion Tensor Imaging Analysis. Brain Sci. 2022, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Uh, J.; Merchant, T.E.; Li, Y.; Feng, T.; Gajjar, A.; Ogg, R.J.; Hua, C. Differences in Brainstem Fiber Tract Response to Radiation: A Longitudinal Diffusion Tensor Imaging Study. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 292–297. [Google Scholar] [CrossRef]
- Kerklaan, J.P.; Lycklama á Nijeholt, G.J.; Wiggenraad, R.G.J.; Berghuis, B.; Postma, T.J.; Taphoorn, M.J.B. SMART Syndrome: A Late Reversible Complication after Radiation Therapy for Brain Tumours. J. Neurol. 2011, 258, 1098–1104. [Google Scholar] [CrossRef]
- Kumar, M.; Haridas, S.; Trivedi, R.; Khushu, S.; Manda, K. Early Cognitive Changes Due to Whole Body γ-Irradiation: A Behavioral and Diffusion Tensor Imaging Study in Mice. Exp. Neurol. 2013, 248, 360–368. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Shera, D.M.; Lustig, R.A.; Phillips, P.C. Phase Measurement of Cognitive Impairment Specific to Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e319–e324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).