Unravelling the Potential of Seven Microalgae Species: Nutritional, Antioxidant, and Antimicrobial Properties and Application
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Biomass
2.2. Biomass Characterization
2.2.1. Color Analysis of the Microalgae Biomass
2.2.2. Determination of the Nutritional Composition of Microalgae Biomass
- Moisture content
- Ash
- Fat
- Protein
- Fiber
- Carbohydrates
- Energetic value
2.3. Evaluation of the Bioactive Potential
2.3.1. Extraction of Bioactive Compounds from the Microalgae Biomass
2.3.2. Determination of the Bioactivity of Microalgae Extracts
- Total Phenolic Content (TPC)
- Antioxidant Activity (AA)
- ABTS
- 2.
- DPPH
- 3.
- ORAC
- Antimicrobial activity
2.4. Evaluation of Application Potential
2.4.1. Extraction of Non-Polar Lipids from Nannochloropsis sp.
2.4.2. Film Production
2.4.3. Color Analysis of the Films
2.4.4. Film Thickness Determination
2.4.5. Determination of the Film Bioactivity
- Antioxidant activity
- Antimicrobial activity
2.5. Statistical Analysis
3. Results and Discussion
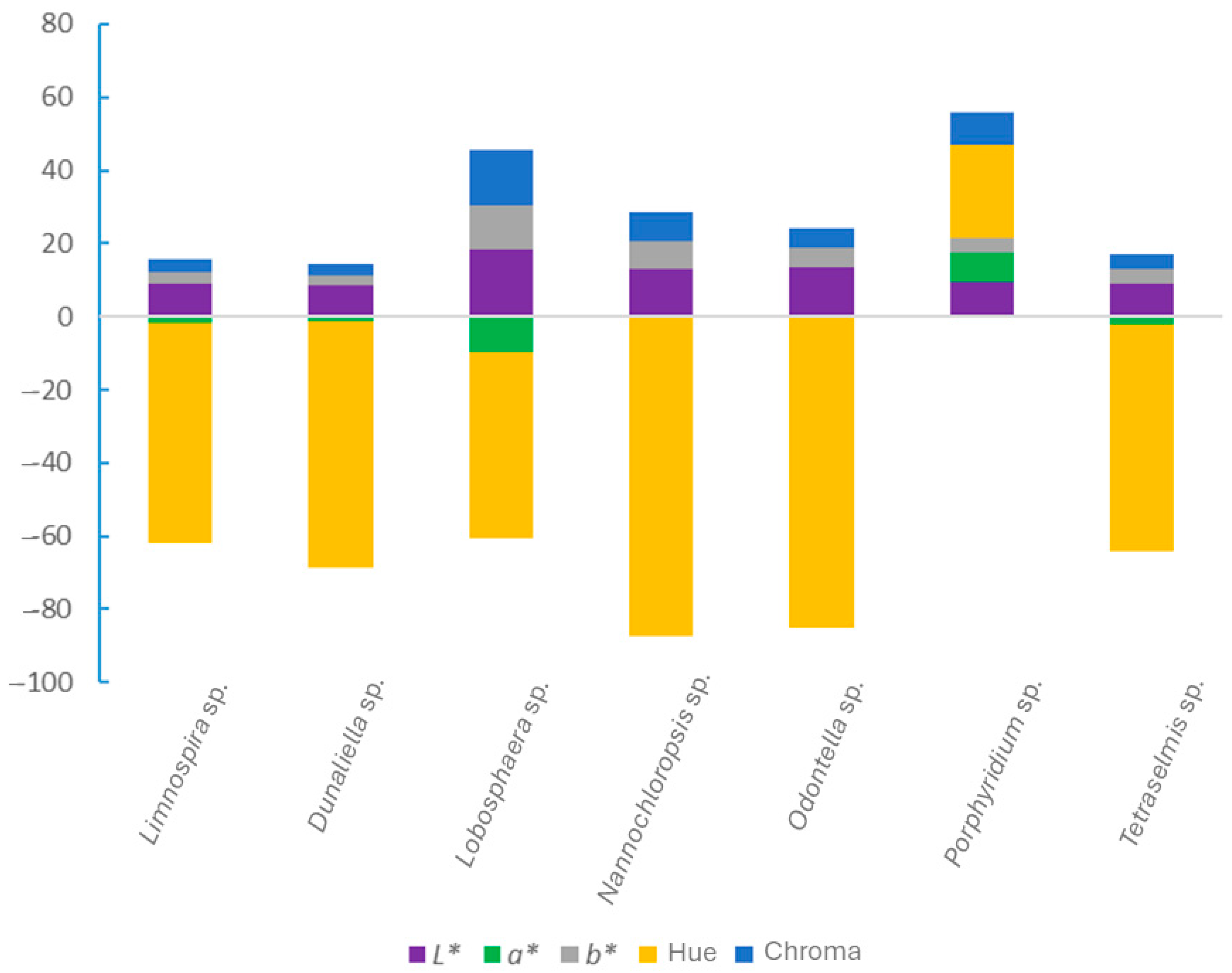
3.1. Color of the Microalgae Biomass
3.2. Nutritional Composition of the Microalgae Biomass
3.3. Yield of Extraction of Bioactive Compounds from the Microalgae Biomass
3.4. Bioactivity of the Microalgae Extracts
3.4.1. Total Phenolic Content (TPC) and Antioxidant Activity (AA) of the Microalgae Extracts
3.4.2. Antimicrobial Activity of Microalgae Extracts Against Bacteria
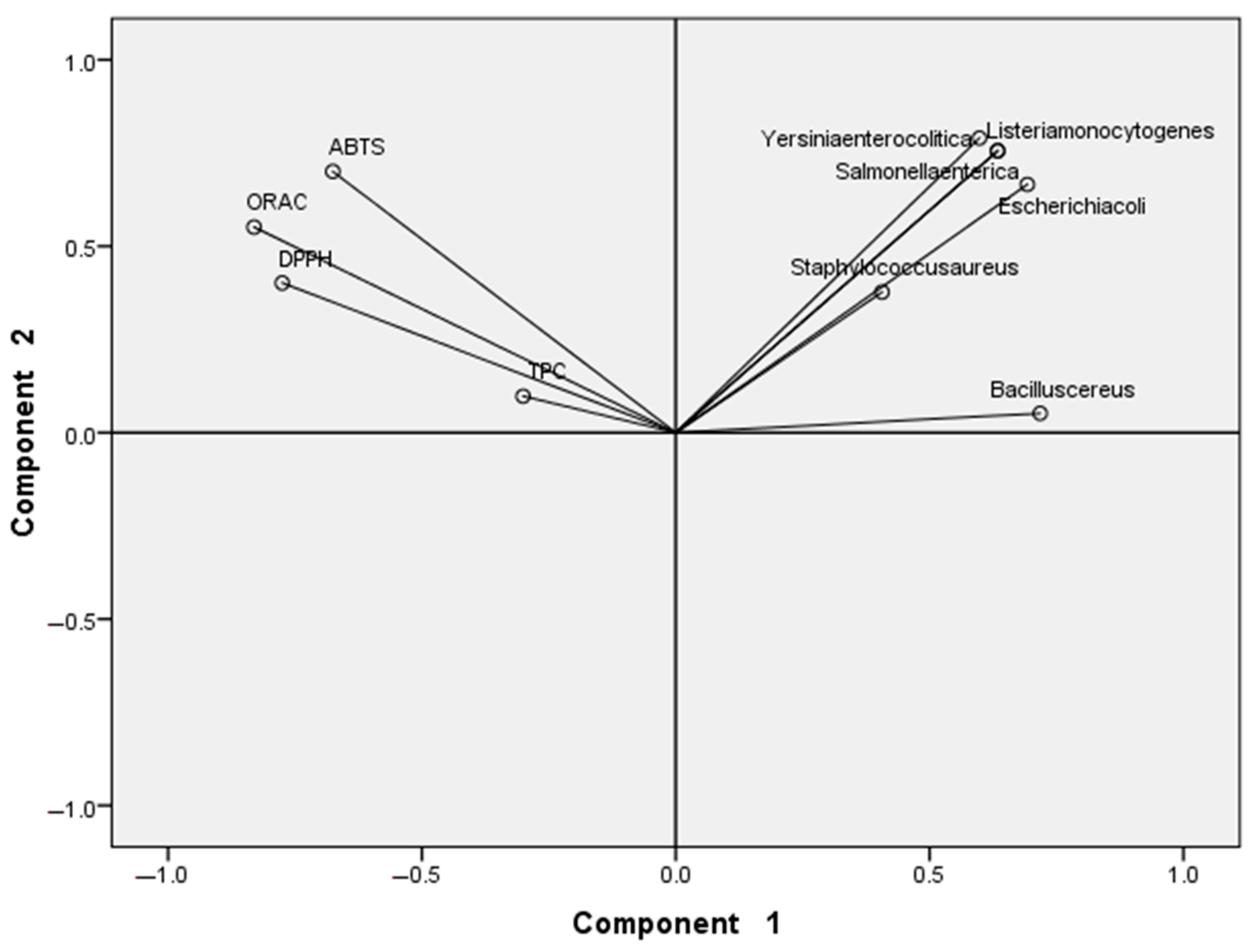
3.5. Principal Components Analysis (PCA)
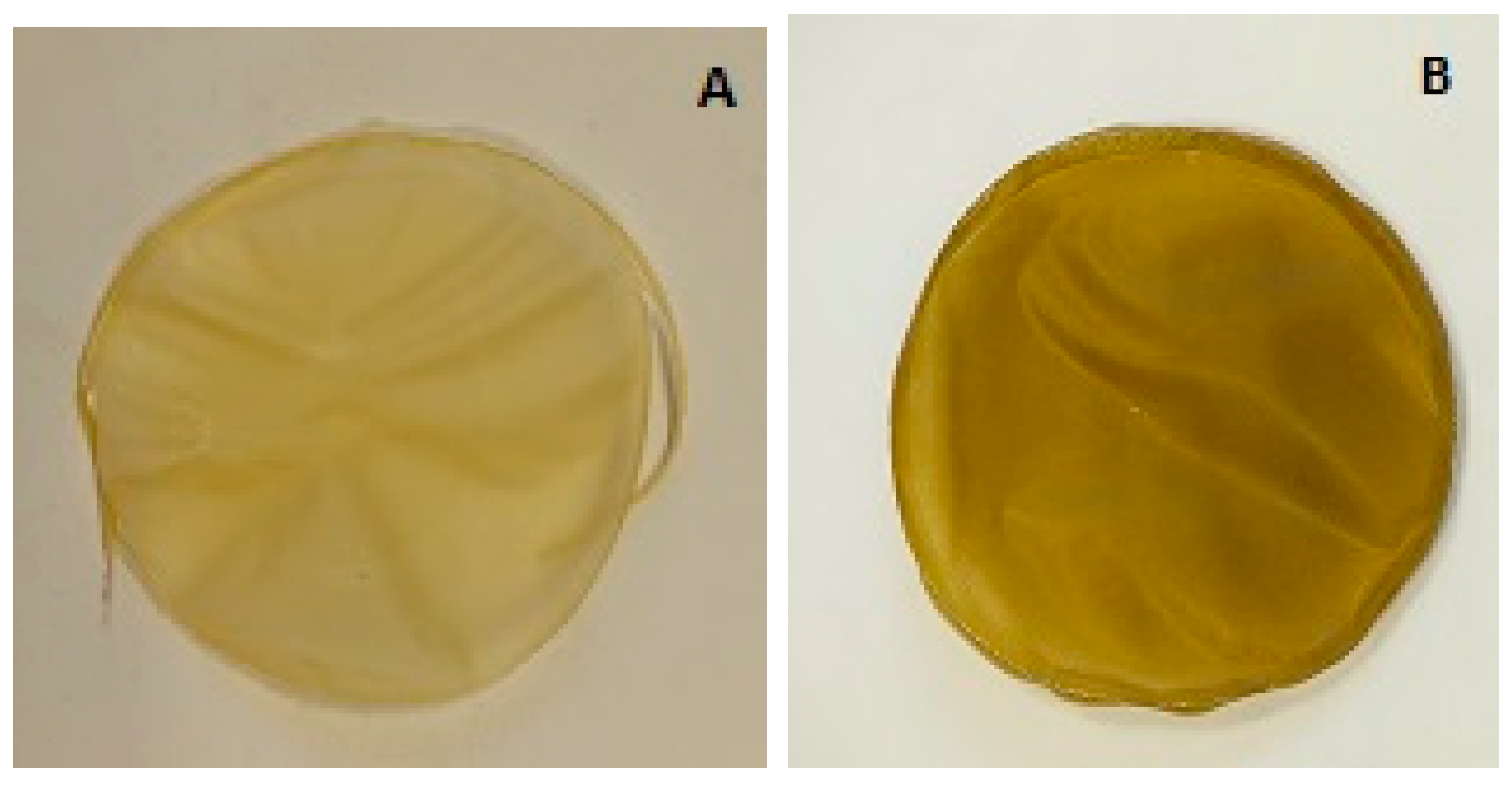
3.6. Films Produced
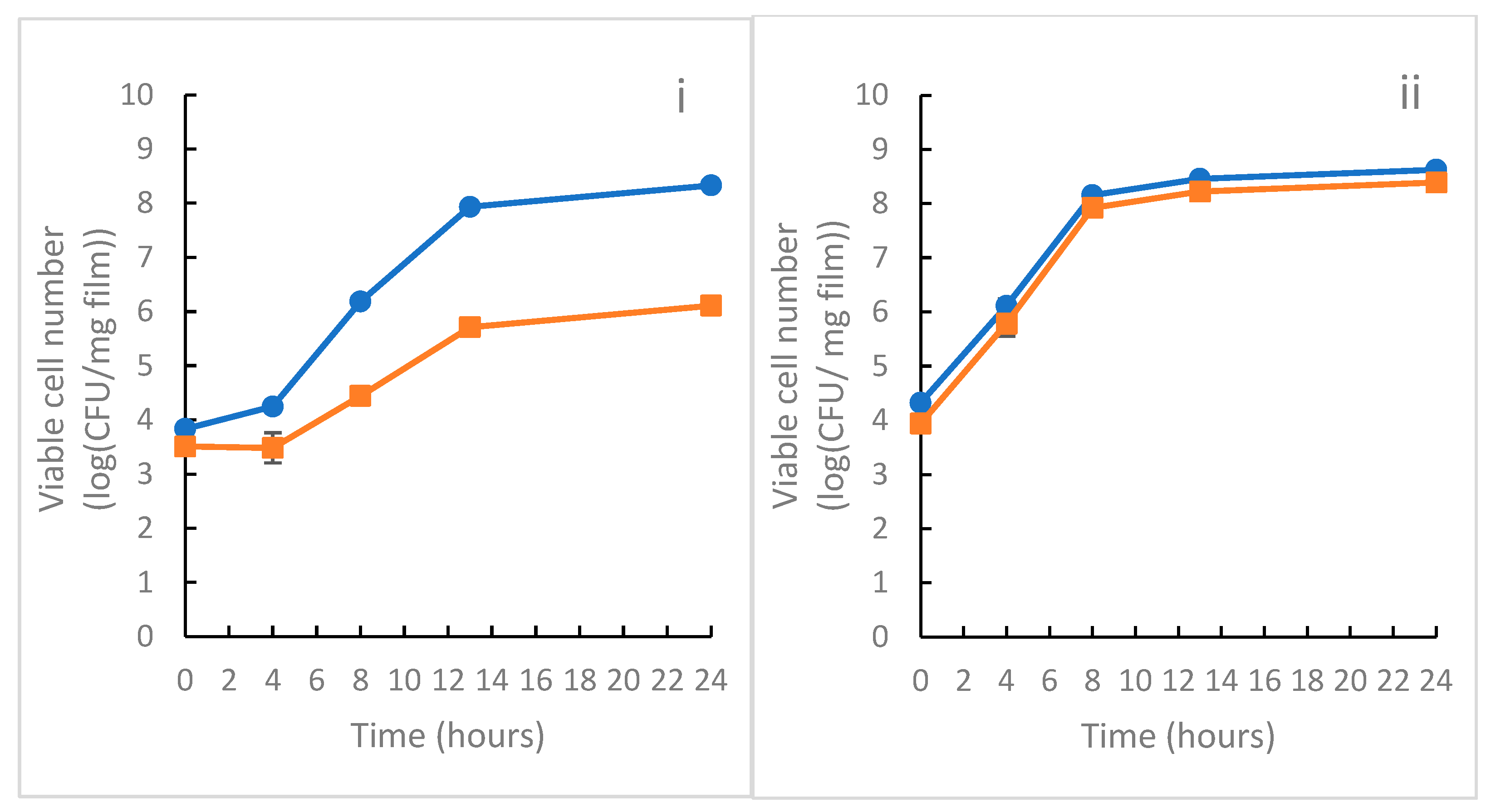
3.7. Antioxidant Activity of the Films
3.8. Antimicrobial Activity of the Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morais, A.M.M.B.; Morais, R.M.S.C.; Lackner, M. Biodegradable Bio-Based Plastics Toward Climate Change Mitigation. In Handbook of Climate Change Mitigation and Adaptation; Lackner, M., Sajjadi, B., Chen, W.Y., Eds.; Springer: New York, NY, USA, 2024; pp. 1–48. [Google Scholar] [CrossRef]
- Ilhami, S.; Rahmen, S.N.S.A.; Iqhrammullah, M.; Hamid, Z.; Chai, Y.O.; Lam, M.K. Polyhydroxyalkanoates production from microalgae for sustainable bioplastics: A review. Biotechnol. Adv. 2024, 79, 108529. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Marcet, I.; Rendueles, M.; Diaz, M. Edible Films from the Laboratory to Industry: A Review of the Different Production Methods. Food Bioprocess Technol. 2025, 18, 3245–3271. [Google Scholar] [CrossRef]
- Levasseur, W.; Perre, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Guihéneuf, F.; Stengel, D.B. Impact of Temperature on Fatty Acid Composition and Nutritional Value in Eight Species of Microalgae. Appl. Microbiol. Biotechnol. 2018, 102, 5279–5297. [Google Scholar]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical Composition and Nutritional Properties of Freshwater and Marine Microalgal Biomass Cultured in Photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Paterson, S.; Gómez-Cortés, P.; de la Fuente, M.A.; Hernández-Ledesma, B. Bioactivity and Digestibility of Microalgae Tetraselmis Sp. and Nannochloropsis Sp. as Basis of Their Potential as Novel Functional Foods. Nutrients 2023, 15, 477. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Yusoff, F.M.D.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging Applications of Chlorella Sp. and Spirulina (Arthrospira) Sp. Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef]
- Silva, M.R.O.B.; Moura, Y.A.S.; Converti, A.; Porto, A.L.F.; Viana Marques, D.A.; Bezerra, R.P. Assessment of the Potential of Dunaliella Microalgae for Different Biotechnological Applications: A Systematic Review. Algal. Res. 2021, 58, 102396. [Google Scholar] [CrossRef]
- Lee, S.; Lim, S.R.; Jeong, D.G.; Kim, J.H. Characterization of an Oleaginous Unicellular Green Microalga, Lobosphaera Incisa (Reisigl, 1964) Strain K-1, Isolated from a Tidal Flat in the Yellow Sea, Republic of Korea. Front. Microbiol. 2018, 9, 2159. [Google Scholar] [CrossRef]
- Vasilieva, S.; Lukyanov, A.; Antipova, C.; Grigoriev, T.; Lobakova, E.; Chivkunova, O.; Scherbakov, P.; Zaytsev, P.; Gorelova, O.; Fedorenko, T.; et al. Interactive Effects of Ceftriaxone and Chitosan Immobilization on the Production of Arachidonic Acid by and the Microbiome of the Chlorophyte Lobosphaera Sp. IPPAS C-2047. Int. J. Mol. Sci. 2023, 24, 10988. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Coelho, A.; Hemaiswarya, S.; Kumar, P.; Carvalho, I.S.; Alagarsamy, A. Applications of Microalgal Paste and Powder as Food and Feed: An Update Using Text Mining Tool. Beni-Suef. Univ. J. Basic Appl. Sci. 2018, 7, 740–747. [Google Scholar] [CrossRef]
- Alami, A.H.; Tawalbeh, M.; Alasad, S.; Ali, M.; Alshamsi, M.; Aljaghoub, H. Cultivation of Nannochloropsis Algae for Simultaneous Biomass Applications and Carbon Dioxide Capture. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 47, 8471–8482. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kwon, Y.M.; Kim, K.W.; Kim, J.Y.H. Exploring the Potential of Nannochloropsis Sp. Extract for Cosmeceutical Applications. Mar. Drugs 2021, 19, 690. [Google Scholar] [CrossRef]
- Moreau, D.; Tomasoni, C.; Jacquot, C.; Kaas, R.; Le Guedes, R.; Cadoret, J.P.; Muller-Feuga, A.; Kontiza, I.; Vagias, C.; Roussis, V.; et al. Cultivated Microalgae and the Carotenoid Fucoxanthin from Odontella Aurita as Potent Anti-Proliferative Agents in Bronchopulmonary and Epithelial Cell Lines. Environ. Toxicol. Pharmacol. 2006, 22, 97–103. [Google Scholar] [CrossRef]
- Heo, J.; Cho, D.-H.; Kim, U.; Kim, H.-S. Isolation and Characterization of Indigenous Diatom, Odontella Sp. BS-003 as Potential Fucoxanthin and Omega-3 Fatty Acid Producer. J. Mar. Biosci. Biotechnol. 2018, 10, 26–33. [Google Scholar]
- Tannin-Spitz, T.; Bergman, M.; Van-Moppes, D.; Grossman, S.; Arad, S. Antioxidant Activity of the Polysaccharide of the Red Microalga Porphyridium Sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Elhadef, K.; Hentati, O.; Blavignac, C.; Fendri, I.; Smaoui, S.; Michaud, P.; Abdelkafi, S. B-Phycoerythrin of Porphyridium Cruentum UTEX 161: A Multifunctional Active Molecule for the Development of Biodegradable Films. Eur. Polym. J. 2024, 208, 112851. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Mehariya, S.; Annamalai, S.N.; Thaher, M.I.; Quadir, M.A.; Khan, S.; Rahmanpoor, A.; Abdurahman, k.; Faisal, M.; Sayadi, S.; Al Hawari, A.; et al. A Comprehensive Review on Versatile Microalga Tetraselmis: Potentials Applications in Wastewater Remediation and Bulk Chemical Production. J. Environ. Manage. 2024, 365, 121520. [Google Scholar] [CrossRef] [PubMed]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and Characterization of Soluble Protein from the Green Microalgae Tetraselmis Sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar]
- Amna Kashif, S.; Hwang, Y.J.; Park, J.K. Potent Biomedical Applications of Isolated Polysaccharides from Marine Microalgae Tetraselmis Species. Bioprocess. Biosyst. Eng. 2018, 41, 1611–1620. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: Mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. (Ed.) Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Science: Oxford, UK; Ames, IA, USA, 2004. [Google Scholar]
- Commission Internationale de L’Eclairage (C.I.E.). Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; Supplement No. 2 to C.I.E. Publication No. 15, (E-1.3.1) 1971 (TC-1-3); C.I.E.: Paris, France, 1971. [Google Scholar]
- A.O.A.C. Official Methods of Analysis, 18th ed.; Method A.O.A.C. 930.15; Association of the Official Analytical Chemists: Arlington, VA, USA, 2005.
- Slack, P.T. Analytical Methods Manual, 3rd ed.; Leatherhead Food R.A.: London, UK, 1997; Issue 2. [Google Scholar]
- ISO 1871:2009; Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2009.
- A.O.A.C. Official Methods of Analysis; Method A.O.A.C. 991.43; Association of the Official Analytical Chemists: Arlington, VA, USA, 2003.
- A.O.A.C. Official Methods of Analysis; Method A.O.A.C. 985.29; Association of the Official Analytical Chemists: Arlington, VA, USA, 2000.
- Regulation (EC) No 1169/2011 of the European Parliament and of the council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 304.
- Martins, V.F.R.; Lopes, A.I.; Machado, M.; Costa, E.M.; Ribeiro, T.B.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Biodegradable Films with Polysaccharides, Proteins, and Bioactive Compounds from Lobosphaera sp.: Antioxidant and Antimicrobial Activities. Foods 2025, 14, 1327. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Yap, B.H.J.; Crawford, S.A.; Dumsday, G.J.; Scales, P.J.; Martin, G.J.O. A mechanistic study of algal cell disruption and its effect on lipid recovery by solvent extraction. Algal Res. 2014, 5, 112–120. [Google Scholar] [CrossRef]
- Lade, B.D.; Dhowlaghar, N.; Pillai, S.S.; Patil, B.S. Physicochemical, mechanical, and antimicrobial properties of sodium alginate films as carriers of zein emulsion with pelargonic acid and eugenol for active food packing. Food Packag. Shelf Life. 2023, 40, 101202. [Google Scholar] [CrossRef]
- Lopes, A.I.; Melo, A.; Caleja, C.; Pereira, E.; Finimundy, T.C.; Afonso, T.B.; Silva, S.; Ivanov, M.; Soković, M.; Tavaria, F.K.; et al. Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts. Coatings 2023, 13, 1487. [Google Scholar] [CrossRef]
- Gabr, G.A.; El-Sayed, S.M.; Hikal, M.S. Antioxidant Activities of Phycocyanin: A Bioactive Compound from Spirulina Platensis. J. Pharm. Res. Int. 2020, 32, 73–85. [Google Scholar] [CrossRef]
- Ambrico, A.; Trupo, M.; Magarelli, R.; Balducchi, R.; Ferraro, A.; Hristoforou, E.; Marino, T.; Musmarra, D.; Casella, P.; Molino, A. Effectiveness of Dunaliella Salina Extracts against Bacillus Subtilis and Bacterial Plant Pathogens. Pathogens 2020, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Ramos-Vega, A.; Angulo, C.; Monreal-Escalante, E.; Guardiola, F.A. Microalgae with Immunomodulatory Effects on Fish. Rev. Aquac. 2023, 15, 1522–1539. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Miranda, S.M.; Belo, I.; Spigno, G.; Teixeira, J.A.; Rocha, C.M.R. Sequential Multi-Stage Extraction of Biocompounds from Spirulina Platensis: Combined Effect of Ohmic Heating and Enzymatic Treatment. Innov. Food Sci. Emerg. Technol. 2021, 71, 102707. [Google Scholar] [CrossRef]
- Gnanakani, P.E.; Santhanam, P.; Kumar, K.E.; Dhanaraju, M.D. Chemical Composition, Antioxidant, and Cytotoxic Potential of Nannochloropsis Species Extracts. J. Nat. Sci. Biol. Med. 2019, 10, 167–177. [Google Scholar]
- Cuong, D.M.; Yang, S.H.; Kim, J.S.; Moon, J.Y.; Choi, J.; Go, G.M.; Cho, S.K. Evaluation of Antioxidant and Anti-Inflammatory Activity and Identification of Bioactive Compound from the Marine Diatom, Odontella Aurita Extract. Appl. Biol. Chem. 2024, 67, 46. [Google Scholar] [CrossRef]
- Bueno, M.; Gallego, R.; Chourio, A.M.; Ibáñez, E.; Herrero, M.; Saldaña, M.D.A. Green Ultra-High Pressure Extraction of Bioactive Compounds from Haematococcus Pluvialis and Porphyridium Cruentum Microalgae. Innov. Food Sci. Emerg. Technol. 2020, 66, 102532. [Google Scholar] [CrossRef]
- Jo, W.S.; Choi, Y.J.; Kim, H.J.; Nam, B.H.; Hong, S.H.; Lee, G.A.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. Anti-Inflammatory Effect of Microalgal Extracts from Tetraselmis Suecica. Food. Sci. Biotechnol. 2010, 19, 1519–1528. [Google Scholar] [CrossRef]
- Khawli, F.A.; Marti-Quijal, F.J.; Pallares, N.; Barba, F.J.; Ferrer, E. Ultrasound Extraction Mediated Recovery of Nutrients and Antioxidant Bioactive Compounds from Phaeodactylum tricornutum Microalgae. Appl. Sci. 2021, 11, 1701. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Kumar, A.; Ramamoorthy, D.; Verma, D.K.; Kumar, A.; Kumar, N.; Kanak, K.R.; Marwein, B.M.; Mohan, K. Antioxidant and Phytonutrient Activities of Spirulina Platensis. Energy Nexus 2022, 6, 100070. [Google Scholar] [CrossRef]
- Guler, B.A.; Demirel, Z.; Imamoglu, E. Induction of Antioxidant Activities of Arthrospira Platensis and Chlorella Vulgaris by Modified Culture Conditions. Bioprocess. Biosyst. Eng. 2024, 47, 275–287. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharjee, S.K.; Mudenur, C.; Ghosh, T.; Goud, V.V.; Katiyar, V. Development of Antioxidant-Rich Edible Active Films and Coatings Incorporated with de-Oiled Ethanolic Green Algae Extract: A Candidate for Prolonging the Shelf Life of Fresh Produce. RSC. Adv. 2022, 12, 13295–13313. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, P.S.; Coimbra, R.S.T.; Caetano, N.S. Exploring the Antioxidant Potential of Lobosphaera Sp. and Odontella Sp. Biomasses under Different Extraction and Preservation Conditions. In Proceedings of the Seagriculture 2023-12th International Seaweed Conference EU, Trondheim, Norway, 21–22 June 2023. [Google Scholar]
- Wali, A.F.; Al Dhaheri, Y.; Pillai, J.R.; Mushtaq, A.; Rao, P.G.M.; Rabbani, S.A.; Firdous, A.; Elshikh, M.S.; Al Farraj, D.A. Lc-Ms Phytochemical Screening, in Vitro Antioxidant, Antimicrobial and Anticancer Activity of Microalgae Nannochloropsis Oculata Extract. Separations 2020, 7, 54. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Tounsi, L.; Hlima, H.B.; Duchez, D.; Gardarin, C.; Dubessay, P.; Drira, M.; Fendri, I.; Michaud, P.; Abdelkafi, S. Enhanced growth and metabolite production from a novel strain of Porphyridium sp. Bioengineered 2023, 15, 2294160. Bioengineered 2023, 15, 2294160. [Google Scholar]
- Assunção, M.F.G.; Amaral, R.; Martins, C.B.; Ferreira, J.D.; Ressurreição, S.; Santos, S.D.; Varejão, J.M.T.B.; Santos, M.L.A. Screening microalgae as potential sources of antioxidants. J. Appl. Phycol. 2017, 29, 865–877. [Google Scholar] [CrossRef]
- Trentin, R.; Custódio, L.; Rodrigues, M.J.; Moschin, E.; Sciuto, K.; da Silva, J.P.; Moro, I. Total Phenolic Levels, In Vitro Antioxidant Properties, and Fatty Acid Profile of Two Microalgae, Tetraselmis Marina Strain IMA043 and Naviculoid Diatom Strain IMA053, Isolated from the North Adriatic Sea. Mar. Drugs 2022, 20, 207. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; Mernissi, N.E.; Ainane, T.; Amzazi, S.; Wahby, I.; Bakri, Y. Antimicrobial activity of marine microalgae isolated from Moroccan Coastlines. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1257–1260. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and Antifungal Activities of Selected Microalgae and Cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Pradhan, J.; Das, S.; Das, B.K. Antibacterial activity of freshwater microalgae. Afr. J. Pharm. Pharmacol. 2014, 8, 809–818. [Google Scholar]
- Ilieva, Y.; Zaharieva, M.M.; Najdensky, H.; Kroumov, A.D. Antimicrobial Activity of Arthrospira (Former Spirulina) and Dunaliella Related to Recognized Antimicrobial Bioactive Compounds. Int. J. Mol. Sci. 2024, 25, 5548. [Google Scholar] [CrossRef] [PubMed]
- Linares-Castañeda, A.; Franco-Hernández, M.O.; Gomez, Y.M.G.; Corzo-Rios, L.J. Physical properties of zein-alginate-glycerol edible films and their application in the preservation of chili peppers (Capsicum annuum L.). Food Sci. Biotech. 2024, 33, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.F.R.; Poças, F.; Pintado, M.; Morais, R.M.S.C.; Morais, A.M.M.B. Edible Films with Protein and Bioactive Compounds from Arthrospira Sp. Biol. Life Sci. Forum 2025, 40, 6. [Google Scholar]
- Kia, E.M.; Ghasempour, Z.; Alizadeh, M. Fabrication of an Eco-Friendly Antioxidant Biocomposite: Zedo Gum/Sodium Caseinate Film by Incorporating Microalga (Spirulina Platensis). J. Appl. Polym. Sci. 2017, 135, 46024. [Google Scholar]
- Golmakani, M.T.; Kiani, F.; Hajjari, M.M.; Sharif, N.; Fazaeli, M.; Hosseini, S.M.H. Electrospun zein incorporating phycocyanin and spirulina extract: Fabrication, characterization and potential application. LWT–Food Sci. Tech. 2023, 188, 115408. [Google Scholar] [CrossRef]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Antimicrobial activities of microalgae: An invited review. In Book Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 2, pp. 1272–1280. [Google Scholar]


| Microalga Species | pH | Temperature (°C) | Photosynthetically Active Radiation (PAR, mol m−2 d−1) | Industrial Medium (A4F) | Cultivation Technology |
|---|---|---|---|---|---|
| Limnospira sp. | 9–11 | <32 | 24.6 ± 8.9 | Alkaline | Open cascade raceway |
| Dunaliella sp. | 7–8 | <26 | 35.8 ± 13.1 | Hypersaline | Flat-panel photobioreactor (FP-PBR) |
| Lobosphaera sp. | 7–8 | <33 | 46.0 ± 6.89 | Freshwater | Multilayer horizontal tubular photobioreactor (MHT-PBR) |
| Nannochloropsis sp. | 7–8 | <30 | 44.0 ± 4.64 | Artificial saltwater | Unilateral horizontal tubular photobioreactor (UHT-PBR) |
| Odontella sp. | 7–8 | <20 | 10.8 ± 3.15 | Artificial saltwater | FP-PBR |
| Porphyridium sp. | 7–8 | <25 | 37.8 ± 7.91 | Artificial saltwater | FP-PBR |
| Tetraselmis sp. | 7–8 | <30 | 36.7 ± 10.9 | Artificial saltwater | FP-PBR |
| Microalgae Species | L* | a* | b* | Hue (°) | C* |
|---|---|---|---|---|---|
| Limnospira sp. | 8.78 ± 0.47 c | −1.88 ± 0.26 d | 3.20 ± 0.21 e | −59.8 ± 2.5 c | 3.71 ± 0.31 f |
| Dunaliella sp. | 8.55 ± 1.34 c | −1.15 ± 0.18 c | 2.78 ± 0.48 f | −67.4 ± 1.7 e | 3.01 ± 0.50 g |
| Lobosphaera sp. | 18.27 ± 0.63 a | −9.63 ± 0.19 e | 11.96 ± 0.25 a | −51.17 ± 0.10 b | 15.36 ± 0.31 a |
| Nannochloropsis sp. | 13.07 ± 2.37 b | −0.43 ± 0.11 b | 7.73 ± 0.72 b | −86.8 ± 0.9 f | 7.74 ± 0.72 c |
| Odontella sp. | 13.51 ± 1.17 b | −0.47 ± 0.06 b | 5.23 ± 0.18 c | −84.9 ± 0.8 f | 5.25 ± 0.18 d |
| Porphyridium sp. | 9.35 ± 0.87 c | 8.09 ± 0.40 a | 3.85 ± 0.24 d | 25.48 ± 2.36 a | 8.97 ± 0.29 b |
| Tetraselmis sp. | 9.14 ± 1.66 c | −2.01 ± 0.11 d | 3.78 ± 0.18 d | −62.0 ± 1.7 d | 4.28 ± 0.16 e |
| Microalgae Species | Ash | Fat | Protein | Fiber | Carbohydrates | Energetic Value |
|---|---|---|---|---|---|---|
| (g/100 g DW) | (kcal/100 g DW) | |||||
| Limnospira sp. | 8.8 ± 0.1 c | 7.4 ± 1.5 cd | 59.8 ± 1.6 a | 8.2 ± 0.0 c | 24.0 ± 3.0 cd | 401.6 ± 7.9 b |
| Dunaliella sp. | 24.7 ± 0.4 a | 11.7 ± 0.5 b | 31.7 ± 1.2 d | 3.6 ± 1.1 c | 32.1 ± 1.5 bc | 359.7 ± 6.3 c |
| Lobosphaera sp. | 6.9 ± 0.0 d | 7.5 ± 1.7 cd | 39.2 ± 0.7 bc | 24.6 ± 1.0 a | 46.1 ± 1.0 a | 409.0 ± 8.5 b |
| Nannochloropsis sp. | 7.2 ± 0.0 c | 36.6 ± 0.0 a | 22.9 ± 0.1 e | 25.9 ± 0.0 a | 31.4 ± 0.1 bcd | 546.3 ± 0.0 a |
| Odontella sp. | 24.6 ± 0.5 a | 6.8 ± 0.1 cd | 38.9 ± 3.2 bc | 9.7 ± 1.8 c | 29.7 ± 3.6 cd | 335.5 ± 2.4 d |
| Porphyridium sp. | 25.8 ± 0.3 a | 5.3 ± 0.0 d | 42.5 ± 1.3 b | 22.0 ± 1.3 ab | 26.3 ± 1.7 cd | 322.9 ± 1.7 d |
| Tetraselmis sp. | 18.4 ± 1.0 b | 9.4 ± 0.0 bc | 34.2 ± 0.3 cd | 24.0 ± 2.1 a | 38.0 ± 0.7 b | 373.4 ± 4.1 c |
| Microalgae Species | Yield of Extraction (%) |
|---|---|
| Limnospira sp. | 12.67 ± 0.88 d |
| Dunaliella sp. | 52.96 ± 3.25 a |
| Lobosphaera sp | 15.48 ± 1.35 d |
| Nannochloropsis sp. | 27.72 ± 0.76 bc |
| Odontella sp. | 31.01 ± 0.57 b |
| Porphyridium sp. | 26.12 ± 0.86 c |
| Tetraselmis sp. | 11.64 ± 0.28 d |
| Microalgae Species | TPC | ABTS | DPPH | ORAC |
|---|---|---|---|---|
| (mg GAE/100 mg DW) | (µmol TE/100 mg DW) | |||
| Limnospira sp. | 0.97 ± 0.06 bc | 2.85 ± 0.45 a | 2.28 ± 0.06 a | 18.38 ± 1.00 a |
| Dunaliella sp. | 1.52 ± 0.09 b | 2.75 ± 0.36 ab | 1.14 ± 0.06 c | 13.77 ± 1.38 b |
| Lobosphaera sp | 1.07 ± 0.05 bc | 2.44 ± 0.27 ab | 1.67 ± 0.15 b | 11.90 ± 1.22 bc |
| Nannochloropsis sp. | 0.57 ± 0.10 c | 1.35 ± 0.27 c | 0.25 ± 0.06 d | 7.53 ± 0.68 cd |
| Odontella sp. | 3.18 ± 0.53 a | 1.91 ± 0.37 abc | 0.40 ± 0.01 d | 9.62 ± 0.20 de |
| Porphyridium sp. | 0.53 ± 0.09 c | 1.61 ± 0.36 bc | 0.34 ± 0.06 d | 6.25 ± 0.16 e |
| Tetraselmis sp. | 0.57 ± 0.01 c | 1.09 ± 0.15 c | 0.29 ± 0.05 d | 2.37 ± 0.58 f |
| Microalgae Species | Gram− | Gram+ | ||||
|---|---|---|---|---|---|---|
| Escherichia coli | Yersinia enterocolitica | Salmonella enterica Serovar Enteritidis | Staphylococcus aureus | Bacillus cereus | Listeria monocytogenes | |
| Limnospira sp. | 2.5 | 1.25 | 1.25 | 2.5 | 2.5 | 1.25 |
| Dunaliella sp. | >5 | >5 | >5 | 2.5 | >5 | >5 |
| Lobosphaera sp | 2.5 | 2.5 | 1.25 | 5 | 5 | 1.25 |
| Nannochloropsis sp. | 1.25 | 0.63 | 1.25 | 1.25 | >5 | 1.25 |
| Odontella sp. | 2.5 | 2.5 | 2.5 | 2.5 | 1.25 | 2.5 |
| Porphyridium sp. | >5 | >5 | >5 | >5 | >5 | >5 |
| Tetraselmis sp. | 5 | 2.5 | 2.5 | 2.5 | >5 | 2.5 |
| Film | L* | a* | b* | Hue (°) | Chroma |
|---|---|---|---|---|---|
| A | 49.98 ± 5.55 a | −0.22 ± 0.11 b | 15.60 ± 0.96 b | −89.18 ± 0.36 b | 15.61 ± 0.96 b |
| B | 30.49 ± 2.26 b | 4.70 ± 0.32 a | 23.94 ± 1.12 a | 78.89 ± 0.63 a | 24.40 ± 1.14 a |
| Film | ABTS | DPPH |
|---|---|---|
| (µM TE/mg Film) | ||
| A | 128.81 ± 10.40 b | 104.98 ± 14.31 b |
| B | 316.80 ± 32.29 a | 240.53 ± 14.75 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, V.F.R.; Lopes, A.I.; Gomes, D.; Parreira, C.; Badenes, S.M.; Costa, L.; Pintado, M.; Morais, A.M.M.B.; Morais, R.M.S.C. Unravelling the Potential of Seven Microalgae Species: Nutritional, Antioxidant, and Antimicrobial Properties and Application. Appl. Sci. 2025, 15, 6691. https://doi.org/10.3390/app15126691
Martins VFR, Lopes AI, Gomes D, Parreira C, Badenes SM, Costa L, Pintado M, Morais AMMB, Morais RMSC. Unravelling the Potential of Seven Microalgae Species: Nutritional, Antioxidant, and Antimicrobial Properties and Application. Applied Sciences. 2025; 15(12):6691. https://doi.org/10.3390/app15126691
Chicago/Turabian StyleMartins, Valter F. R., Ana I. Lopes, Diana Gomes, Celina Parreira, Sara M. Badenes, Luís Costa, Manuela Pintado, Alcina M. M. B. Morais, and Rui M. S. C. Morais. 2025. "Unravelling the Potential of Seven Microalgae Species: Nutritional, Antioxidant, and Antimicrobial Properties and Application" Applied Sciences 15, no. 12: 6691. https://doi.org/10.3390/app15126691
APA StyleMartins, V. F. R., Lopes, A. I., Gomes, D., Parreira, C., Badenes, S. M., Costa, L., Pintado, M., Morais, A. M. M. B., & Morais, R. M. S. C. (2025). Unravelling the Potential of Seven Microalgae Species: Nutritional, Antioxidant, and Antimicrobial Properties and Application. Applied Sciences, 15(12), 6691. https://doi.org/10.3390/app15126691









