Abstract
Tritium is a radioisotope that is extremely mobile in the biosphere and that can be transferred to the environment and to humans mainly via tritium oxide or tritiated water. Moreover, as is widely known, it is extremely difficult to detect in the environment. In the last decade, many studies and research activities have been performed to fill the knowledge gap on this radionuclide, the amount of which is expected to be increasingly released into the environment from nuclear installations in the near future. Considering this and the fact that the biological and environmental effects produced by tritium have been examined mainly from a medical and detection monitoring point of view, it is considered important to propose in this study a review of the critical aspects of tritium from the environmental, engineering, and waste management points of view. Identifying sources and effects of tritium, tritium materials and wastes containing tritium in the environment is also fundamental for planning the specific and necessary actions required for an effective waste management approach under, e.g., disposal conditions. The critical analysis of the published recent studies has allowed to evaluate, for example, that the expected rate of tritium generation in a fusion reactor is four orders of magnitude higher than that of LWRs, and the environmental release from a fusion reactor is 1.4–2.2‱, which is twice as much as from a heavy water reactor and more than two orders of magnitude higher than from a LWRs. Furthermore, with reference to the waste management strategy, it is emphasized, e.g., that the condensation of moisture inside vaults and the interaction of H2O with the disposal body determine the formation of tritiated water, which is filtered through the concrete and eventually released into the environment. Consequently, in the selection of engineered barrier materials for repositories/disposal facilities, the use of a mixture of a framework and layered silicates is proposed to improve its absorption and filtering properties.
1. Introduction
The use of nuclear energy is expected to play an important role in the near future in the transition towards clean energy (zero emissions goal).
Tritium, as is widely and globally known, is a radioactive isotope of hydrogen that can be transferred to the environment and to humans mainly via tritium oxide or tritiated water (HTO). It is also generated by the operation and decommissioning of nuclear power plants (including dismantling and spent fuel reprocessing), nuclear incidents, such as Fukushima, Japan, in 2011, radioisotope production and in medical and research applications [1,2,3].
In the future, tritium release is expected to increase through the operation of new nuclear power plants, whether they are fission or fusion reactors [4], because of its production by the neutron activation of 2H, 3He, 6Li, and 10B [5].
Tritium is a radioisotope that is extremely mobile in biological systems; it reacts with oxygen, and it rapidly integrates into the biosphere as HTO [1], which has chemical characteristics almost identical to water, from which it is technically extremely difficult to remove. As for its environmental effects, the largest impacts are likely, therefore, to occur only while relatively high activity concentrations of HTO persist.
Compared to other radionuclides, by taking advantage of these characteristics, tritium can be released into the environment, specifically diluted into water in huge quantities and at low concentrations per year, according to the laws and regulations of the respective countries where nuclear power plants are operated (for example, the Fukushima Daiichi plant will gradually release up to 22 trillion Bq or less of tritium per year over the next 20 to 30 years, a low level compared to the amounts released by nuclear power plants around the world, including those in Europe, North America and other parts of Asia outside of Japan).
Indeed, tritium is the only radioactive isotope in the triad of hydrogen isotopes that exists for a relatively long time. A soft beta emitter with an average energy of 5.7 keV and a half-life of 12.33 years, it is the most common natural light radioactive isotope. About 200 g of tritium is formed annually in the upper atmosphere under the influence of cosmic radiation, which maintains a natural tritium content in the biosphere at 1.3 × 1018 Bq. Along with its stable analogue, it can migrate quite easily into moisture in the air and water flows and can accumulate as a component of mineral and organic compounds. It is a potential hazard, since it, along with a stable hydrogen isotope, is a component of water and acts as a building block of cellular tissue in living organisms.
From what has been said, it appears clear that identifying the nature and extent of the problems of tritium or of the materials and/or wastes containing it is a fundamental aspect not only in terms of adequately evaluating the biological impact of tritium and other radionuclides of environmental relevance but also for planning the specific and the necessary actions required for an effective waste management approach under, e.g., disposal conditions. Moreover, the problem of tritium and tritium waste has become particularly critical since the late 70s, primarily due to the development of civil and military nuclear technologies.
With most of the studies available in the literature referring to the understanding of tritium transport, transfer and dispersion modelling, e.g., during the hydrological cycle, or to the assessment of health impacts resulting from historical operations [6,7,8], the present review aims to critically analyze the available literature regarding the biological effects, sources, waste management challenges and environmental consequence of tritium. Existing knowledge gaps are also identified.
2. Artificial Sources
2.1. Nuclear Test
The most powerful source of man-made tritium was nuclear testing. Tritium is formed in significant amounts in nuclear and especially thermonuclear explosions (both aboveground and underground) because of fission and fusion reactions. Explosions based on nuclear fission produced 2.6 × 1015 Bq· per production of tritium due to eplosion per megaton, while fusion ones produced up to 7.6 × 1017 Bq· per megaton 3H [9,10]. Therefore, about 2.4 × 1020 Bq (665 kg) of tritium was released into the atmosphere between 1945 and 1975. Most of it has since decayed, but the global tritium amount is today an order of magnitude higher than its natural content (Figure 1), and it is mainly concentrated in the ocean (Figure 2).
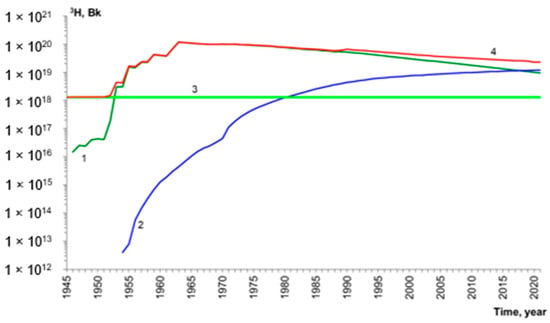
Figure 1.
Evolution of tritium inventory in the biosphere. Sources are as follows: 1—nuclear tests, 2—nuclear energy, 3—natural sources, 4—integrated value. Amounts were calculated by taking into account decay using a database of nuclear explosions [11,12] and the IAEA and WNA databases [13,14].
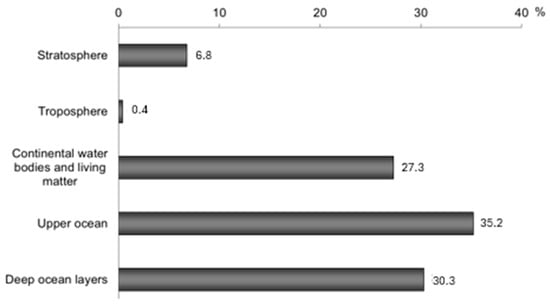
Figure 2.
Modern distribution of “global” tritium in the biosphere, plotted according to [15].
By 1990, the widespread use of phosphors based on tritium compounds led to the injection of about 7.4 × 1018 Bq into the biosphere [16]. However, scientific advances in the production of alternative sources of luminescence over the next two decades will lead to a gradual attenuation of this source of tritium. Instead, soon, we should expect a rapid development of the production of autonomous tritium-based power supplies.
2.2. Nuclear Power Engineering
With the cessation (restriction) of nuclear testing, the nuclear industry and nuclear power engineering, in particular, nuclear reactors and reprocessing plants, have become the most important sources of tritium in the environment.
In nuclear reactors, tritium is formed inside fission reactions of 235U and 239Pu nuclei, as well as in the interaction of neutrons with Li, B, and D. More than 96% of tritium in light-water reactors and gas-cooled reactors is produced by the ternary fission of uranium and plutonium [17,18]. When irradiating plutonium nuclei in the fuel elements, the tritium yield is three times more efficient than when irradiating uranium [19].
In water reactors, tritium is also produced by the activation of deuterium present in the coolant/moderator water. In light-water reactors, due to the small amount of deuterium in natural water (D2O:H2O = 1.5 × 10−4) and the low probability of neutron capture, this source of tritium is insignificant. In heavy-water reactors where D2O is used as a moderator/coolant, deuterium activation is the main source of tritium (Table 1). Tritium emissions to the environment during a reactor campaign vary from 0.36 (BWR) to 8.8% (HWR), depending on the process.
In a two-loop PWR, the intensity of the chain reaction (reactivity) is controlled by changing the concentration of boron in the water of the first circuit. The dissolution of boric acid in the primary cooling circuit of a PWR allows for the regulation of the amount of 10B in the reaction medium and the removal of heat from the reactor core. The concentration of 10B controls the rate of the nuclear reaction and, consequently, the energy it generates. Unlike PWRs, BWR cooling circuits do not use borates. In BWRs, boron is used in control rods fabricated from boron carbide, fuel pools, and the control system fluid reserve tank.
The normalized tritium generation amount due to ternary fission in PWRs and BWRs is of the same value. In addition, about 30% of the tritium in BWRs is generated by neutron capture reactions with 10B nuclei in the control rods, which is an order of magnitude more than in the primary cooling circuit of PWRs. Most of the tritium released from PWRs appears in the liquid effluent (about 85%), whereas 75% of the tritium released from BWRs is in the form of airborne effluents [20].
When a neutron is captured, the generated tritium atoms can be incorporated into the boron carbide structure, compensating for the ‘broken’ bonds and forming a stable B12C3(H, T)16 structure, which undoubtedly reduces tritium emissions. Due to the difference in boron control technology as well as the construction peculiarities of reactors and despite the fact that tritium generation in PWRs is 30% less than in BWRs, the amount of tritium released to the environment from PWRs is about six times greater than from BWRs.
The expected tritium generation in a fusion reactor is four orders of magnitude higher than in PWRs and BWRs, and the environmental release from a fusion reactor is estimated at 1.4–2.2‱, which, in absolute values, is two times higher than the release from a heavy-water reactor (Table 1).
The content of tritium generated by the global nuclear power complex in the biosphere was calculated using the WNA and IAEA reactor databases [13] and taking into account the type of reactor, the amount of electricity produced, and radioactive decay (Figure 3).
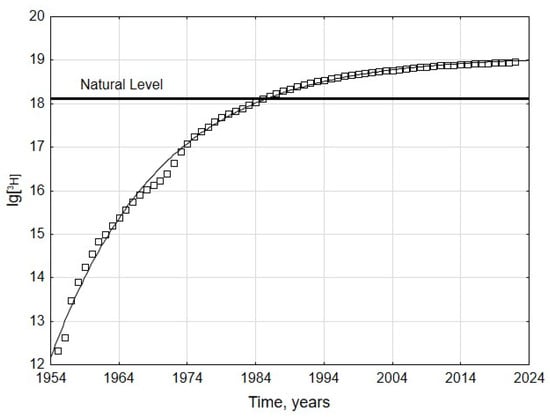
Figure 3.
Integrated Tritium Generation by nuclear power engineering.

Table 1.
Assessment of tritium generation and environmental emission in nuclear reactors, Bq·MWe·y−1 [21,22,23].
Table 1.
Assessment of tritium generation and environmental emission in nuclear reactors, Bq·MWe·y−1 [21,22,23].
| Tritium Source | PWR | BWR | HWR | GCR | ITER * |
| Fission | 7.5 × 1011 | 7.5 × 1011 | 5.5 × 1011 | 7.5 × 1011 | |
| Activation of: | |||||
| - Deuterium | 4.0 × 107 | 4.0 × 107 | 2.0 × 1013 | ||
| - Lithium | 7.0 × 108 | 2.0 × 1010 | |||
| - Boron | 2.6 × 1010 | 3.0 × 1011 | |||
| Totally | 8.0 × 1011 | 1.1 × 1012 | 2.0 × 1013 | 8.0 × 1011 | 2.0 × 1016 |
| Emission | 2.3 × 1010 | 3.8 × 109 | 1.8 × 1012 | 7.3 × 109 | 4.0 × 1012 |
Note: * expected generation and lost by fusion.
The Integrated Tritium Generation (ITG) values, which are indicated by the points on the graph in Figure 3, reflect the total amount of tritium generated from the commissioning of the first nuclear power reactor (1954) to the year corresponding to each point. The modelling results indicate that the ITG values are described with high confidence (R2 = 0.99) by the curve:
where A and B are the coefficients that determine the initial (A) and final (equilibrium) (A + B) conditions of the process, k is the rate constant, and t is the time.
The value of the rate constant determines the steepness of the curve (and reflects the rate of development of nuclear power engineering) and the rate of the asymptotic reaching of the equilibrium state. The long-term dynamics of the ITG is in good accordance with global trends in nuclear energy, which correspond to the laws of global development, kinetics, and thermodynamics [24].
Analysis of the parameters of Equation (1) determined by the iteration method (A = 12.16; B = 6.93; k = 0.0621 y−1) shows that given current technologies and global development trends, the tritium content in the biosphere generated by nuclear power plants will not exceed 1.23 × 1019 Bq, i.e., 10 times the natural level. Throughout the entire operation of the global nuclear power complex, almost 90% of the electricity has been generated by light-water PWRs and BWRs [24].
Meanwhile, the introduction of fusion technology can significantly disrupt the current natural and technogenic balance of tritium in the biosphere.
It is worth remembering, however, that although uranium-235 reserves can be used in thermal reactors for decades (according to the ratio of reserves to production in 2024), the use of plutonium-239 in fast neutron reactors, and its conversion into uranium-238, could ensure supplies even in the medium-to-long term. This will obviously depend on the level of technological readiness of nuclear energy; the same dependence characterizes fusion as well.
In the 1960s, nuclear testing injected an amount of tritium into the biosphere exceeding the natural level by more than 100 times. Importantly, the rate constant for establishing the current natural and anthropogenic balance of tritium in the biosphere is close in value to the radioactive decay constant (λ), which can be calculated as:
where T1/2 is the half-life. Considering that the decay rate was accepted in the calculations, it can be estimated that this rate is twice as high as the rate of tritium decay.
Since nuclear power is the main modern source of tritium in the biosphere and it has provided an order of magnitude exceeding the global natural level, the distribution and release of tritium at the different stages of the nuclear power cycle are of special interest. During the reactor campaign, tritium release into the environment does not exceed 3% for the most common PWRs (Table 1).
Tritium emissions during a reactor’s campaign depend significantly on the technological process (Figure 4). As of 1 January 2024, almost 90% of annual tritium emissions were due to the operation of heavy-water reactors. Canada leads the world, generating almost 50% of global tritium emissions.
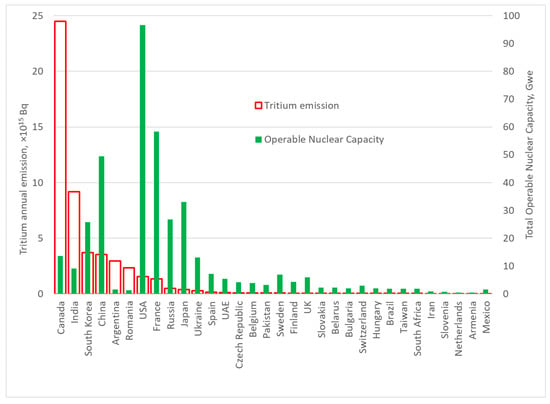
Figure 4.
Modern annual tritium emissions from nuclear power engineering.
A total of 97–99% of 3H is contained in spent fuel assemblies subjected to continuous water cooling inside the reactor or independent ponds for 5–30 years. During this period, the diffusion of tritium from the fuel rods into the coolant water environment can reach up to 45%, and the permanent tritium concentration in the pool may exceed 3 GBq·dm−3 [25].
After the wet storage period, the spent fuel is transported to concrete dry storage facilities and is subsequently sent to a reprocessing facility. Most of the tritium produced in the power reactors is released during reprocessing: about 20% of the tritium appears in the solvent off-gases and is emitted through the chimney in the gaseous phase. Most of the remaining tritium is transferred to the aqueous phase and released into the environment in low-level waste streams [26].
The utilization of water and nitric acid for reprocessing results in the generation of large volumes of tritiated wastewater, about 100 m3·t−1. At plants close to the coast, these effluents are discharged directly into the sea.
In India, tritium water is evaporated in the sun, while in the Russian Federation it is stored for a long time in open water reservoirs [5]. Thus, during the nuclear energy cycle, tritium generated in nuclear reactors is completely released into the environment (Figure 5).
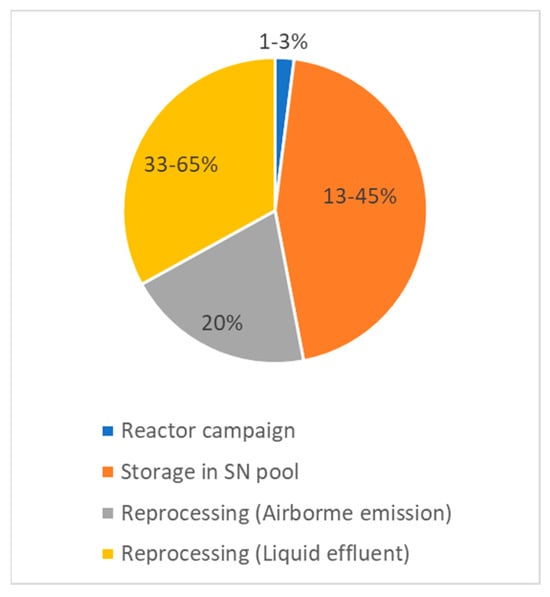
Figure 5.
Release of tritium into the environment during the nuclear power cycle.
Modern trends in the development of nuclear power demonstrate the depletion of heavy-element nuclear fission technology (Figure 6). This is determined by several objective reasons, such as the depletion of the raw material base, reaching the limits of increases in reactor capacity, safety issues, socio-economic problems, etc.
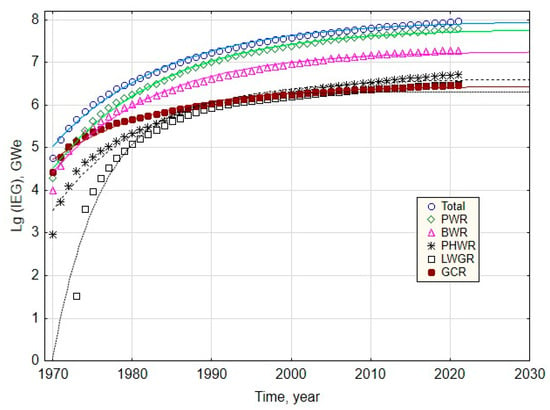
Figure 6.
Integrated Energy Generation (IEG) by different types of nuclear reactors. The shown trends were calculated based on the WNA and IAEA databases [13].
During 1954–1973, the unit capacity was increased 200 times: from 5 to 1000 MWe. Over the next 50 years, it was possible to increase the unit capacity by 40–60% (~1.5 times).
Currently, there are no technological developments that would allow for a significant (by an order of magnitude) increase in the unit capacity without compromising nuclear and radiation safety. If the current trend (Figure 7) continues, the share of nuclear energy (NEG, %) in global energy production will more than halve to 4% of global energy production by 2050, which is in line with the IAEA forecast (2.8–5.4%) [27], and to 1% by the end of this century [24].
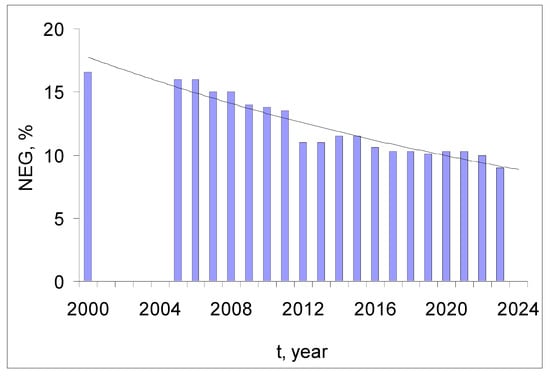
Figure 7.
The global share of Nuclear Electricity Generation (NEG). The trend interpolation function (NEG, % = 18.3e−0.029t, R2 = 0.91) was calculated according to the IAEA database [13].
The 28th United Nations Climate Change Conference (COP-28) in December 2023 was a historic event that identified nuclear energy as a possible way to address climate change by helping countries meet their net zero emission targets by 2050, achieving the goals of the Paris Agreement [28]. The main achievement was the signing of an agreement to triple nuclear power capacity by 2050 by 22 world leaders [29]. Contrary to the trend of decreasing the share of nuclear power in global energy production, this decision will make a significant contribution to maintaining energy security while reducing carbon dioxide emissions.
In anticipation of new fusion reactors in the coming decades, small modular reactors characterized by high construction rates, a higher level of safety, and acceptable technical and economic indicators can serve as a bridge between fission and fusion. The development of a hydrogen energy complex based on nuclear reactors also seems promising.
2.3. Fusion
The only technology known today that would allow for such a “technological breakthrough” is fusion. The International Thermonuclear Experimental Reactor (ITER) project has shown that such a machine can be built at the current level of technological development and will be able to conduct the physical and nuclear technological tests necessary to create the first experimental fusion power plant.
First-generation fusion reactors (TOKAMAK, STELLARATOR) will likely be powered by a mixture of deuterium and tritium. This is the most technically simple fusion reaction and is accompanied by the formation of a helium nucleus (alpha particle):
The disadvantage of this reaction is the production of a high-energy neutron. The high rate of this reaction and relatively high energy release (17.6 MeV) makes equal-component mixtures of deuterium and tritium the most promising for controlled fusion. The first of the two components involved in the D-T reaction, deuterium, is a stable, widespread isotope of hydrogen. Ordinary water contains approximately 0.015% heavy water, D2O. To ensure the operation of a fusion reactor that uses tritium as the nuclear fuel, it is necessary to provide for the possibility of its reproduction. For this purpose, the reactor core can be surrounded by a layer of light lithium isotopes, where the following reactions will take place:
For a 2 GWe fusion reactor, a tritium mass flow rate of 0.32 kg/day must be ensured. At the same time, its burnup rate is estimated at 0.3%, which means that the total tritium inventory in the reactor should reach several dozens of kilograms. Due to technological improvements, the total initial amount of tritium will be 3.0–3.3 kg and will double every 6.8–12.8 years [30]. Therefore, after 20 years of operation, the tritium inventory inside the reactor will be about 25 kg.
Assuming that current nuclear power capacity is replaced by fusion reactors within the next 20 years, the amount of tritium in the biosphere could reach 500 kg, which corresponds to the amount released into the atmosphere during the period of active nuclear testing and exceeds the natural background by more than two orders of magnitude.
Concerning fusion reactors, the main systems producing tritium are the tritium injection, collection, and purification systems, the blanket breeder, and the tritium extraction system from the blanket. This reactor uses about 1 × 1018 Bq of tritium [31]. According to [32], the total tritium content in a 517 MW nuclear fusion facility is 28.75 kg/y (1.0 × 1019 Bq). This corresponds to its modern content in the biosphere, both from natural and artificial sources, and annual tritium losses may reach 4.5–6.18 g (1.6–2.2) × 1015 Bq, which is 2–3 orders of magnitude exceeding the amount released from fission reactors. Expected losses to the environment are 1.6–2.2‱, which is in good agreement with several model assessments (between 0.0057 and 7.0; on average, 1.4‱ [23]). Meeting current global energy needs (161,000 TWh) through fusion alone would increase the tritium inventory in the biosphere to 1027 Bq and would involve annual tritium emissions of 1023 Bq. This evaluation could change if the production or breeder of tritium is for the continuous use of fusion energy.
3. Waste Management
Unconditioned solid tritium wastes in the mixture of radioactive waste are usually stored in near-surface facilities designed to contain radioactive sources, radioactive material, spent fuel, or radioactive waste with the intention of retrieval [33], and they are arranged with a concrete biological barrier. Experimental studies [25], have shown that the condensation of moisture inside vaults (containers, packages, etc.) and the interaction of H2O with the body of storage due to isotopic exchange results in tritiated water formation that is filtered through the concrete, which is essentially a porous material, and eventually released into the environment (Figure 8). Additional water may enter the vaults by filtering through the concrete from the environment because of natural weakening or mechanical damage to the insulation barriers during long-term operation of the storage facility.
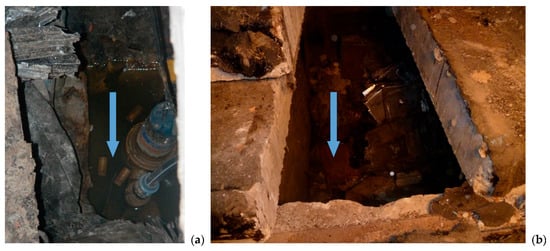
Figure 8.
Condensed tritiated water in the near-surface “RADON” RAW facility in Kyiv, Ukraine: (a) pumping and (b) opened concrete vault. The free water level is indicated by arrows.
It is interesting to note that extremal tritium contamination of a groundwater aquifer was eliminated in a few months after pumping the inventory of condensed tritiated water from the vaults (Figure 9). These data were confirmed during the operating of near-surface radioactive waste storage facilities in the United States, Canada, France, Hungary, Latvia, and other countries [34,35,36]. As radioactive waste storage facilities localized near the Earth’s surface usually contain condensed water, which may penetrate multiple engineered barriers, including the waste form, waste packaging, and other engineered barriers, including concrete, it is possible to conclude that a revision of the basic principles of tritium solid waste management could be necessary. Concrete does not provide a barrier function for the safe storage of tritium waste. It needs to be improved with a water-absorbing material that performs hydrogen isotopic fractionation.
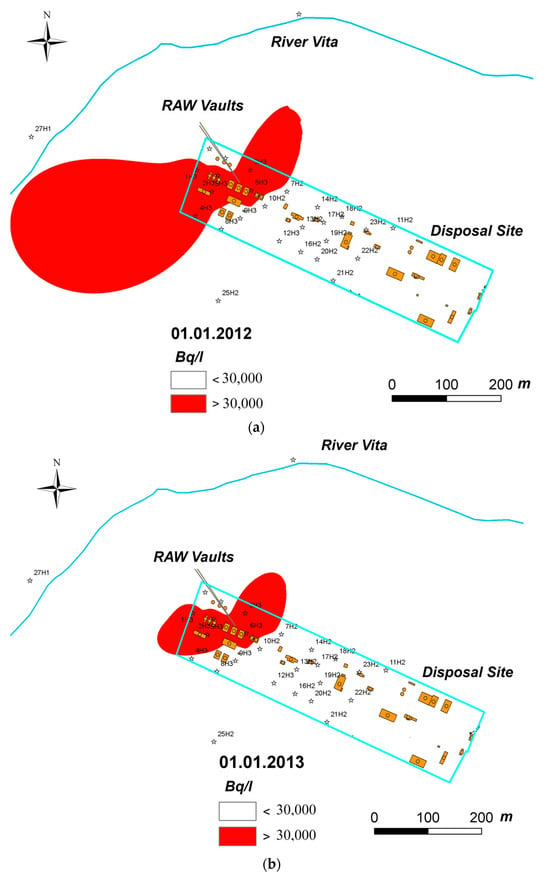
Figure 9.
The change in groundwater aquifer tritium contamination after pumping HTO from vaults during spring (a) and summer (b) in 2012. Near-surface “RADON” RAW facility in Kyiv, Ukraine; locations of the observation wells are indicated with asterisks.
Tritiated waste management is of great importance for the future of fusion. One of the most sensitive processes occurring in a nuclear fusion reactor is the interaction with hot plasma under temperatures higher than those found in the core of the Sun.
The interaction between the plasma and the first wall leads to extreme thermal stresses, repetitive intense thermal shocks, plasma ion and neutral particle (D, T, He) shocks, high-energy neutron shocks (energies up to 14 MeV), thermally induced defects (cracking, melting of plasma surface material), thermal fatigue damage, dusting, hydrogen-induced blisters, helium-induced nanoscale cluster formation, neutron degradation of the wall armor through thermal conductivity reduction, embrittlement, transmutation, and activation [37]. In these conditions, a great volume of solid tritiated materials will be produced, and the first metals will have a tritium inventory of up to 50 MBq·g−1.
The main method for detritization of solid waste is thermal desorption in an air atmosphere, which includes the desorption of tritium-containing substances from the metal surface and their isotopic exchange with water vapor in the air [38,39]. Schematically, this can be represented as follows:
However, this scheme seems to be imperfect, since heat treatment at about 1000 °C changes the physical state of the volatile contaminants, vaporizing them from a solid waste material to a (highly migratable) gas, determining a significant increase in the waste volume and, in turn, the risk of environmental contamination. Subsequently, the oxidation of tritium gas (HT, DT, T2) produces its aqueous vapor forms (HTO, DTO, T2O), the radiological hazard of which is 10,000 times higher [40]. During heating, most of the tritium will be released from the metal as tritium water vapor at relatively low temperatures at the beginning of the heating/melting process [38].
The content of water vapor in the air depends considerably on the temperature and can reach more than 300 kg·m−3 at a critical temperature of 647 K and 51 g·m−3 at a temperature of 313 K (40 °C). When decontaminating 1 ton of steel containing up to 50 MBq·g−1 of tritium, the heat treatment will release about 5 × 1016 Bq of tritium gas, which, when oxidized, will form 510 g of tritium water vapor that corresponds to the water content in 30 m3 of atmospheric air at room temperature (20 °C) and 100% humidity.
During breathing, only 0.04–0.10% of tritium gas is retained in the body, while the share of HTO is 86–100%. The radiological hazard of HTO when inhaled during the same activity is 10,000 times higher than two-atom tritium gas [40].
In general, the scheme of Equation (10) requires a significant revision in accordance with the basic principles of radioactive waste management, IAEA safety standards, and the ALARA principle [41,42].
Given that metal tritides are one of the most reliable ways to store tritium, the scheme of Equation (6) requires substantial economic and biomedical justification. For example, a method is known for producing titanium tritide with an atomic ratio of T/Ti = 1.7. In this case, the rate of tritium desorption does not exceed 4000 Bq·m−2·s−1 [43].
Given the half-life of tritium (12.33 years), significant decontamination occurs during the long-term storage of solid tritium waste: within 50 years, its content decreases by almost one order of magnitude; within 75 years, its content decreases by two orders of magnitude; and within 150 years, its content decreases by four orders of magnitude (Figure 10). Considering the physical state of tritium waste from fusion (anhydrous, absorbed in the solid phase in equilibrium and desorbed in the gaseous phase), spent salt mines that are completely isolated from the environment can be used to construct storage facilities.
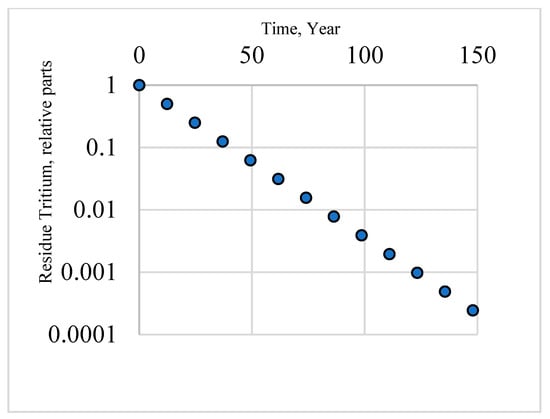
Figure 10.
Tritium decay during storage: the points on the graph correspond to successive half-decays; the scale of the ordinate axis is logarithmic.
Clay–Tritium Interactions
The storage of solid tritium waste is accompanied by a “fast” diffusion of tritium gas desorbed from the metal and a “slow” formation owing to oxidation, as well as the diffusion of aqueous forms of tritium, requiring an improvement to the design of the engineering barriers [44].
Experimental studies have shown that phyllosilicates (montmorillonite, sepiolite, palygorskite) can be such materials. The potential properties of layered silicates for the accumulation and retention of tritium are determined by the structural and chemical features of minerals [45].
The intensity and rate of isotopic exchange in the mineral–water system are determined by the accessibility of the reaction surfaces of the mineral particles for HTO molecules, the rate of exchange at the interface of these phases, and the rate of tritium diffusion in different parts of the system, including the pores, adsorbed layer, interlayer or zeolite water, and crystal structure. The main sorption positions for tritium in the order of exchangeability decrease are the surface, interlayer, and isotopic exchange of structural OH groups. The potential adsorption capacity of layered silicates (kaolinite, montmorillonite, palygorskite) for tritium is (1.06–1.81) × 1022 hydrogen atoms per gram of mineral [46,47].
Experimentally, we found that the most effective silicates were mixtures of bentonite and zeolite, with a potential tritium capacity of 1013 Bq·g−1. It was found that 63 ± 11% of the total tritium inventory in the mineral was accumulated in the surface-adsorbed state. In the interlayer space or channel structures, this amount was, on average, 24 ± 10%, and in structural OH groups, it was up to 13 ± 9%.
Since clays lose their filtration properties after swelling, a clay–sand–zeolite mixture can be used. Changes in the volumetric ratio of minerals allow for regulating the filtration and adsorption properties of composites for various technological tasks (creation of filtration reaction barriers, arrangement of beds of NPP cooling ponds and technological reservoirs of spent nuclear fuel reprocessing plants, storage of radioactive waste, etc.) The retention rate of tritium (including decay) in these mixtures reaches 90% [48,49].
Based on the filtration and sorption properties of silicates of various structures and their mixtures, several technological improvements for the arrangement of NPP technological reservoirs, radioactive waste storage facilities, and liquid waste purification from tritium have been developed at the SI “Institute of Environmental Geochemistry of NAS of Ukraine” jointly with the DICI, University of Pisa.
The current strategy in France to manage tritiated waste at 103–104 Bq·g−1 3H involves interim storage for up to 50 years without any treatment. Nevertheless, several options are under study that are aimed at reducing the temporary storage duration and minimizing out-gassing rates and tritium discharges into the environment. It is also worth mentioning that no strict out-gassing limit has been set due to the very low level of tritium [50,51]. An air emission assessment is required on a case-by-case basis before cementing large amounts of waste into disposal chambers. As for the disposal concept, it consists of two main biological barriers: an active one with a watertight geomembrane and a passive one that involves the use clay for its watertight properties [44].
4. Biological Effects
Being analogous to protium, except for in certain reactions where the difference in behavior is due to the mass of 3H, tritium is included in the hydrogen cycle in the biosphere, in the behavior of HTO and its vapor form, and in various organic and hydrogen compounds. The ecological interest in tritium is due to three main factors:
- (1)
- The rather high content of tritium in the biosphere due to the combined influence of natural and anthropogenic factors.
- (2)
- The extremely high rate of 3H’s incorporation into biochemical processes.
- (3)
- The potential radiation hazard to living organisms, primarily from the standpoint of genetic effects [1].
The beta energy of tritium is significantly lower than the gamma-ray energy of major gamma-emitting radionuclides. Tritium does not pose a significant hazard in the case of external human exposure, since the maximum energy of the beta particle formed during its decay is insufficient to penetrate the skin. This beta particle’s calculated and measured maximum travelling distance in water does not exceed 6 μm (Table 2). At the same time, the outer stratum corneum of the skin is 10 to 100 μm thick, the epidermis is 100–200 μm thick, and the next layer is 1000–3000 μm thick [52]. Therefore, the main ways of 3H entering the human body include the consumption of drinking water, food containing the aqueous form of or organically bound tritium (OBT), inhalation, and sorption through the skin [53]. The contributions from the inhalation and the dermal absorption of HTO are equal.

Table 2.
Penetration range, m, of a beta particle into materials depending on its energy [54,55].
An analysis of the literature sources shows that the radiobiological properties of tritium have not been studied sufficiently. Uncertainty of different scientifically and experimentally grounded assessments considerably exceeds a factor of 100,000. Estimates of safe concentrations of tritium in drinking water, according to official WHO [56], EURATOM [57], ICRP [58], IAEA, and EPA [59] documents and scientific publications [46], differ by a factor of almost 600,000 [60,61,62,63,64].
According to the US EPA, cancer risk coefficients (CRCs) for tritium are 9.44 × 10−13 Bq−1 (mortality CRC) and 1.37 × 10−12 Bq−1 (morbidity CRC) [65]. The recommended health criterion established in the USA for water and food consumption is a 10−6 de minimis cancer risk level (CRL) [66]. According to [67], the recommended EC of a 1 × 10−6 lifetime risk of cancer for use in risk assessments means that in a population of one million people, one additional case of cancer will occur during their lifetime.
The calculation of the safe concentration (C1) of tritium for health is as follows:
where EP is the exposure period (70 years during a lifetime), and WIR is the water ingestion rate equal to 2 dm3∙day−1 or 730 dm3 annually for adults.
Calculations show that the “safe” concentration of tritium in drinking water (C1) should be between 14 and 21 Bq∙dm−3 to avoid exceeding the minimum risk of cancer morbidity and mortality, respectively.
Numerous experiments on experimental animals (mice, rats) have made it possible to establish a No Observed Adverse Effect Level (NOAEL) of tritium in the range of (0.63–3.7) × 107 Bq∙kg−1 of body weight (BW) [60,68,69,70,71]. The calculation of the “safe” concentration (C2) of tritium using the NOAEL is as follows:
where the NOAEL is 3.7 × 107 Bq∙kg−1, which is the highest experimental value, as previously indicated, and BW is 70 kg for adults and 10 kg for infants (i.e., children under the age of 1 year). RSC (relative source contribution) is the relative contribution of water consumption to the degree of exposure, estimated at 0.6. UF (uncertainty factor) is a complex uncertainty factor (assumed to be 100) that includes intra- and interspecies extrapolations of human variability, resistance to subchronic and chronic exposure, and other aspects of uncertainty, while IR (ingestion rate) is the level of drinking water consumption, which is 1 dm3∙day−1 for children and 2 dm3∙day−1 for adults.
The calculated value of the “safe” concentration of tritium (C2) is 7.8 × 106 and 2.2 × 106 Bq∙dm−3 for adults and children, respectively.
Radiation protection assumes that any exposure to radiation is associated with a certain level of risk. The WHO recommendations are based on a linear relationship between exposure and risk, with no threshold below which there is no risk. An individual dose criterion (IDC) of 0.1 mSv/year reflects a very low level of risk that is not expected to result in any detectable adverse health effects. The nominal risk factor used to assess the lifetime risk of fatal cancer arising from radiation exposure to the public is 0.05 per Sv (5 × 10−8 per µSv).
It was found that the risk of fatal and severe non-fatal cases from a dose of 0.1 mSv (100 µSv) per year is 10−5 to10−4 per year, or about 6 × 10−4 over a lifetime (70 years). If the dose of radioactivity from drinking water is below the WHO-recommended level of 0.1 mSv/year, the water is considered safe for human consumption from a radiological point of view [71].
To calculate the equivalent and effective dose from different radionuclides depending on the way they enter the body, the ICRP periodically publishes updated dose coefficients (dose per unit of exposure) (Table 3) [71]. These data also allow for solving the opposite issue, i.e., calculating the limits of radionuclide incorporation from the atmospheric air by inhalation and water and food by oral ingestion. As already mentioned, due to the low energy of beta particles, unlike, for example, gamma-emitters, tritium does not cause external irradiation.

Table 3.
Dose factors recommended by the ICRP [58].
The limit for the tritium content in water can be calculated similarly to the previous Equation (7):
where IDC is the WHO-recommended individual dose criterion, which is equal to 1.0 × 10−4 Sv·y−1, and DF is the dose factor of 1.8 × 10−11 Sv·Bq−1, which was determined in the official ICRP publication 119 according to the modern model of radionuclide metabolism based on the energy characteristics of beta particles emitted by tritium, radiobiological parameters, radiation weighting, and other factors.
The calculated value of the tritium limit in drinking water (C3) is 7610 and 4280 Bq·dm−3 for adults and children, respectively (Table 3).
The wide difference in experimentally and scientifically grounded assessments, which reach more than five orders of magnitude, as well as different individual dose criteria (40 to 1000 μSv∙y−1) is due to the fact that tritium drinking water limits around the world vary by a factor of more than 700, i.e., from 100 Bq·dm−3, as recommended by the EU Council Directive [57], to 76,103 (according to the concept of 1 mSv·y−1) in Australia [72] (Table 4).

Table 4.
Tritium water regulatory limits (TRLs) around the world.
Recent radiobiological studies [8] showed that the dangers of tritium ingestion have been previously underestimated; it is probably several times more dangerous than β exposure to 137Cs, and it is considerably more dangerous when ingested in amniotic fluid. The relative biological effectiveness (RBE) for tritium was determined in the range of 1–3 [62,63], and it was two orders of magnitude higher for 1–3% tritiated liquid, which can reach DNA. This means that the degree of health damage due to β radiation from tritium is assumed to be quantitatively equal to that from γ-rays; β-rays may even contribute more to the exposure per unit energy than γ-rays. The latter is especially effective for low doses [84].
A study of the effect of tritium water on peripheral blood lymphocytes allowed for determining the relative biological effectiveness (RBE) in the range of 2.12–2.8 [85,86,87]. The term RBE was introduced for the integrated assessment/comparison of the absorbed dose from different types of radioactivity and can be determined as the ratio of absorbed doses required to produce the same biological end point, using a reference radiation level and a comparison radiation level for given end point [88,89,90]:
The basis for comparing the RBE from low-energy beta radiation with a short travel time and high-energy gamma radiation is the degree of damage caused by radioactive exposure to a body organ or the body as a whole. However, at the microscopic level, exposure occurs in two main ways: by gamma radiation and by beta radiation.
For gamma radioactivity, the released high energy of the photoelectron is distributed over a large distance with a relatively low density. That is, absorbing many photons, the organ is irradiated evenly at the macroscopic level. The energy of tritium beta particles is distributed over a shorter distance with a higher intensity, leaving a significant space between individual tracks. However, since the HTO is distributed evenly in the organ, uniform irradiation also occurs at the macroscopic level. However, the uniform exposure is due to the propagation of the tritium nuclide itself and not to the overlapping of beta particle tracks in a wide range. Therefore, the uniform distribution characteristic of tritium water is not typical for organically bound tritium and can lead to uneven exposure of certain cells and organs (cells) of the body [52].
The RBE is calculated (with a linear dependence of the effect under study on the dose of both compared types of ionizing radiation) as the ratio of the angle of the slope of the dose line of the test radiation to the angle of the slope of the similar line of the standard radiation, with the different dose dependence being the ratio of isoeffective (causing the same effect) doses of the standard and test radiation. The dependence of the RBE on the dose may be different. The RBE of radiation depends mainly on differences in the spatial distribution of the absorbed energy in the irradiated biosubstrate, as measured by linear energy losses per unit length of the ionizing particle’s travel. The dependence of the RBE on the linear energy transfer factor (LET) varies for different objects and in different biological responses to irradiation. The efficiency of radiation with a low LET is usually similar. As the LET increases, the RBE usually increases as well.
The RBE coefficient for electron, positron, X-ray, and gamma radiation, as well as for fast protons, is usually close to 1; for alpha particles and fast neutrons, it increases to 10; and for heavy multi-charge ions and recoil nuclei, it increases to 20 [91,92].
Compared to other β-emitters, such as 137Cs, the energy of the β particle produced by tritium decay is low, which leads to the comparative safety of direct tritium exposure. However, the LET factor for the passage of a β particle produced by tritium decay through living tissues is 23 times higher than for a β particle produced by 137Cs decay. Accordingly, the number of ionic pairs formed during its passage through living tissue is almost 25 times higher, which leads to a much greater destructive effect of tritium exposure on living tissues. For comparison, the energy of α radiation from 239Pu is almost 1000 times higher, and the energy-to-length ratio and, accordingly, the ionization density are only 10 times higher than for a β tritium particle [52].
Studies in cellular radiobiology have shown that the RBE of high-energy radiation with a low LET (gamma radiation 2–5 MeV, electrons, 60Co) is 3–4 times lower than that of low-energy radiation with a low LET (X-rays, tritium beta rays) [93]. The RBE of 137Cs relative to that of 6 MV X-rays was experimentally determined to be ~1.00 [94].
Given the significant dependence of tritium’s RBE on the radiation dose, the ICRP recommends using a value of at least 1 (Table 5). For different age groups, when incorporated into different body tissues, experimentally obtained average values of tritium’s RBE exceed those of X-rays by 2.3–9.8 times.

Table 5.
Integrated values of relative biological effectiveness (RBE) and dose coefficients for tritium water (HTO) and organically bound tritium (OBT).
Even though the most common and hazardous form of tritium is its aqueous form, organically bound tritium contained in food of plant and animal origins is radiologically more dangerous [100].
In plant organisms, OBT can be formed both through isotopic exchange and photosynthesis. The organic matter of plants consists mainly of the polymer cellulose, which, in turn, consists of D-glucose links [101].
Theoretically, tritium can replace 10 protium atoms: 3 of them are bound to oxygen, and 7 atoms are bound to carbon. The cell membrane of some species of aquatic plants (e.g., kelp) is built mainly of the polysaccharide alginic acid. The alginic acid chain contains three hydroxyl and five alkyl protons. The mobile hydrogen from the hydroxyl groups is easily exchanged for tritium in the tritiated intercellular medium of the sap that washes over plant tissues. Instead, the isotopic exchange of alkyl protons for tritium is much slower.
In addition to isotopic exchange, tritium is incorporated into organic matter during photosynthesis. During biological evolution, a mechanism for primarily assimilating the light isotope was formed, as it requires less energy. The interaction between CO2 and H2O under the influence of sunlight in plants leads to the depletion of plant organic matter by deuterium relative to water [102]. It is likely that the isotopic exchange of protium for tritium in the -O-H groups of plant organic matter, depending on the content of the superheavy isotope in the environment, may occur in the opposite direction to the isotopic fractionation in the -C≡H groups due to photosynthesis.
When tritium and HTO enter the body, an isotopic exchange occurs between the radionuclide and hydrogen contained in organic structures. The proportion of tritium involved in this exchange is 0.5–4.0%, increasing to 10% with chronic intake of tritium [103,104].
The incorporation of tritium into organic molecules leads to an increase in the radiation dose by 1.4–1.5 times compared to the dose caused by tritium in the liquid phase of the body [105]. The metabolism of tritium entering the human body in the organically bound form with food is significantly different. Significantly higher rates of incorporation (approximately 10 times) and distribution of OBT in certain body structures are observed when these forms of 3H are consumed in food compared to the intake of HTO with drinking water. Usually, direct intake and metabolism of OBT is not considered in tritium dosimetry models. At the same time, the dose from the consumption of OBT with food may exceed the dose from an equivalent amount of tritium incorporated in the aqueous form through oral or inhalation intake.
The two-component model of tritium metabolism describes the retention of tritium in the bodies of adults and children. The main component is the body’s water compartment, which contains 97% of the total amount of tritium consumed with water. The second source is tritium in organic compounds in the body (3%).
Studies conducted on animals have shown that 1–5% of tritium water entering the bloodstream is converted into an organically bound form [58].
Studies of people irradiated due to oral incorporation of the aqueous form of tritium have shown that tritium metabolism is subject to a three-component exponential dependence [106]. These three components, i.e., (1) metabolism of the aqueous phase of the body, (2) hydrocarbon forms, and (3) tritium incorporated into organic molecules, have biological half-lives of (1) 6–12 days, with an average of 9 days, (2) 10–34 (22) days, and (3) 130–550 (340) days, respectively. According to the four-component metabolism model [107], the main component corresponds to the retention of tritium in the aqueous phase of the body, and the other three are in different types of organic molecules.
Available theoretically and experimentally substantiated studies and large number of publications show that the radiological impact of OBT on different age groups and tissues at (3.9–20) × 10−11 Sv/Bq is more than two times higher than that of HTO at (1.8–6.6) × 10−11 Sv/Bq (see Table 5).
5. Isotopic Effects of Hydrogen in Living and Inanimate Matter
Isotopic effects, i.e., differences in the properties of isotopes of a chemical element or its compounds containing different isotopes, are most often caused by differences in the mass of the isotope’s nuclei (isotopic effects of the first kind), but they can also be caused by differences in nuclear magnetic properties (isotopic effects of the second kind). The isotopic effects of the first kind are stronger the greater the difference in isotope masses, i.e., with increasing atomic mass, the isotopic effect decreases, and the largest is observed for isotopic hydrogen compounds. The isotopic effects of the second kind are instead caused by differences in properties of nuclei such as spin, the energy of γ-quanta emitted after neutron capture, the presence of isomeric properties, etc.
Hydrogen isotope fractionation in inanimate matter has been studied in some detail. It is known for certain that in living matter, isotope effects are associated with photosynthesis processes, which manifest mass-dependent (primarily diffusion and kinetic) effects of isotope fractionation.
For H-D and D-T pairs, both mass-dependent and nuclear spin (magnetic) isotopic effects can coexist simultaneously, and their magnitudes can even be comparable. For the H-T pair, the nuclear spin effect is insignificant, since the spins of hydrogen and tritium are the same. However, the masses of tritium and protium differ by a factor of three (300%), which causes significant mass-dependent isotopic effects in gas molecules and isotopic compounds.
Thus, in living and inanimate matter, the most significant mass-dependent effects in the isotopic fractionation of protium and tritium are for protium/deuterium and, likely, tritium/deuterium, and along with mass-dependent effects, the nuclear spin (magnetic) effect plays a significant role.
The increase in the binding energy in isotopic hydrogen compounds from protium to tritium largely explains several isotopic effects. In an isotopic water molecule (HTO), the T-O covalent bond is stronger than the H-O bond, so it is expected that protium is more attracted to the proton form (hydroxonium H3O+), and tritium is mainly attracted to the hydroxyl (TO−) groups. This substantiates the possibility of isotopic separation of hydrogen by electrodialysis methods, as well as by isotopic substitution in structural OH- groups of clay minerals (see Section 3).
Significantly stronger bonds of the superheavy hydrogen isotope in the structure of organic molecules may also explain the effects of tritium enrichment in the organic matter of living organisms observed by many authors.
During biological evolution, a mechanism of preferential assimilation of the light isotope was created, which has been confirmed by numerous studies of H/D and 12C/13C in living organisms. Therefore, there are reasonable grounds to believe that the same pattern is observed for the H/T ratio. However, due to stronger bonds with carbon and oxygen, tritium is removed from an organic molecule much more slowly than protium. At the same time, in its aqueous form, the concentrations equalize several orders of magnitude faster. Therefore, after the assimilation and formation of organically bound tritium (primarily because of photosynthesis), tritium is excreted from intracellular water into the external environment (atmospheric moisture, soil solution, water body) much faster than from the organically bound form.
In this regard, the content of organically bound tritium may exceed its concentration in the water of a dynamic environment for a certain period. This is especially true for studies in accident zones accompanied by short-term releases of tritiated water, routine releases of NPPs, and radioactive waste storage facilities that are metastable with respect to releases of the aqueous form of tritium. The experimentally estimated kinetic rate constant for tritium isotopic exchange in an inanimate aqueous medium is (1.5 ± 0.50) × 10−4 s−1, between the water of living organisms and inanimate aqueous medium, it is (8.44 ± 0.03) × 10−7, in H-O- groups of organic substances of living matter, it is (5.07 ± 0.06) × 10−7, and in HC≡ groups, it is (5.00 ± 0.10) × 10−8.
The latter value can be considered as an estimation of the photosynthesis rate. At the same time, white willow seedlings growing in tritium water transported water into the atmosphere like a pump, with a fractionation factor of 1.35 ± 0.085 [101].
Studies on the interactions of plant organisms with tritiated feeding solutions have shown the availability of a complex mechanism of the biological response of living matter to radioactive contamination of the environment and the existence of physiological barriers that significantly limit the biological assimilation of the heavy isotope. The fractionation of the superheavy hydrogen isotope in plant organs during vegetation occurs due to photosynthetic activity and is accompanied by dose-depended effects.
With an increase in the tritium content in the aqueous feeding solution, the rate of tritium excretion from the system increased; the degree of assimilation of this isotope by intracellular sap decreased alongside its share in the organic matter of wood and the relative proportion of transpired tritium [108].
The Phenomenon of Isotope Osmosis
Osmosis is the phenomenon of the spontaneous movement of a solvent towards equalization of concentrations across a semipermeable membrane separating solutions of different concentrations. The normal functioning of the cells of living organisms occurs thanks to osmotic processes. For the first time, the phenomenon of isotope osmosis was observed through a clay membrane that separated light (protium) and super-heavy (tritiated) water (Figure 11). Even though the mass difference between the molecules of protonated (H2O) and tritiated (HTO) water is small (mH2O/mHTO = 0.9), an equilibrium distribution of tritium was established between the liquid and gaseous phases of water, which is different from uniform. This distribution was temperature-dependent and was determined by the difference in vapor pressure between the tritium and protium water.
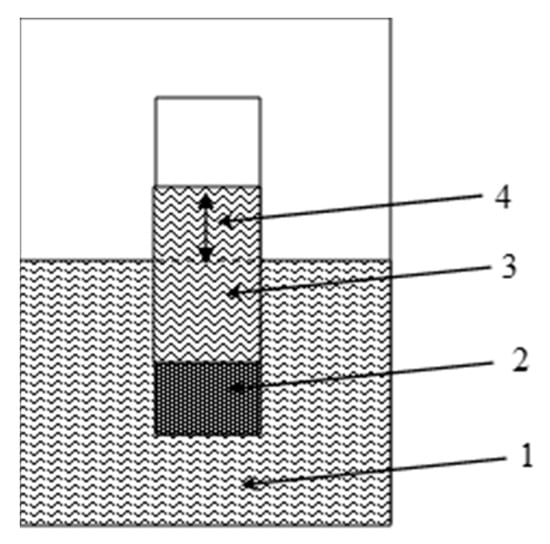
Figure 11.
Visualization of isotopic osmosis effect: 1—protium water, 2—bentonite membrane, 3—tritiated water, 4—level of the isotope osmosis pressure.
Subsequently, an isotope-osmotic effect was found for other membranes [109]. The isotope osmosis processes studied in greenhouse experiments on plants growing in tritiated water led to a significant (up to 40%) fractionation of hydrogen isotopes in solutions separated by wildlife membranes (Figure 12). At the same time, the heavy isotope was removed from the system, i.e., the processes of “isotope osmosis” through plant membranes contributed to the purification of the water environment from tritium. The isotope-osmotic effects of the separation of protium and tritium through membranes made from living matter open perspectives for the development of biotechnology for the phytoremediation of semi-natural reservoirs from tritium contamination.
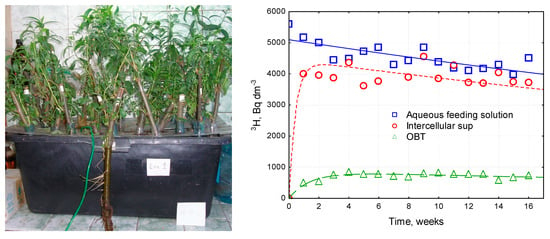
Figure 12.
Fractionation of tritium during the growth of white willow.
The phytoremediation technique uses plants to remediate contaminated environments, including soil and water, by utilizing biochemical processes that can transform or decompose toxic compounds (rhizo-degradation, phytodegradation), accumulate chemical elements (phytoextraction, rhizo-filtration), disperse compounds (phyto-vaporization), and immobilize compounds (hydraulic control and phyto-stabilization) [110].
Given that the aqueous form of tritium (HTO, with an absolute content of the superheavy isotope orders of magnitude less than nano concentrations) is the most abundant and migrating substance on the planet in both the gaseous and liquid phases, effective, environmentally safe, and economical methods of tritium remediation are still unknown.
A lot of literature data show that the content of tritium in living matter and the surrounding liquid and gaseous aquatic environment is strongly correlated [89,90]. Most sources claim that the main specie of tritium in living matter (75–95%) is HTO. However, in areas affected with a direct impact of tritiated water vapor emissions into the atmosphere and discharges into the hydrosphere, the effect of tritium accumulation in an organically bound form can often be observed. For the first time, such an anomaly was observed in the biotic components of an aquatic ecosystem receiving liquid wastewater from complex nuclear facilities located in Mol (BE). The specific activity of tritium incorporated in organic matter was 20 times that observed in river water. This unexpected observation contradicts the results of experiments in laboratory aquariums, in which tritium was present in the form of HTO [89].
The study of the tritium content in biotic levels of a wetland ecosystem near the “Radon” radioactive waste storage facility in Kiev (UKR) showed a similar effect: the 3H/1H fractionation ratio reached 18.3 [46]. Subsequently, in a greenhouse experiment, it was demonstrated this discrepancy was explained by the kinetics of tritium assimilation and dissimilation in the tissues of living organisms. The fastest isotopic exchange occurred in the external water environment, with the rate constant of 2–3 orders of magnitude higher than the “biogenic assimilation” of tritium and isotopic exchange in the organic matter of plants. So, after a “fast” decontamination of the external water environment (feeding solution), one can observe a “pseudo-accumulation” of tritium in plants due to “slow” decontamination in the organic matter of living organisms after an accidental release of tritiated water into the environment.
This was caused by the considerable difference between the rates of 3H/1H isotopic exchange in the water, the OH- and CH≡ groups of living organic matter, and the diffusion of tritiated water through pores, cell membranes, and the root system [101]. Thus, currently, the most effective technique for phytoremediation from tritium contamination is phyto-vaporization by plant species that are able to fractionate hydrogen isotopes, such as white willow (see Figure 12).
The electrochemical mobility of hydrated protons is almost twice that of hydroxyl anions. At the same time, the strength of the bond between the oxygen and hydrogen of the hydroxyl anion increases significantly in the triad of protium, deuterium, and tritium. When an external electric field is applied in the capillaries of clay membranes, ionic movement occurs uniformly in both directions at speeds corresponding to their mobility and the gradient of the applied electric field voltage. Therefore, the electro-osmotic method has a higher efficiency of hydrogen isotope fractionation compared to hydraulic filtration methods due to the electrocapillary effect.
The results of a study on the electro-osmotic processes of hydrogen isotope separation in electrolyte solutions using clay membranes showed the high efficiency of this method for the purification of liquid radioactive waste from tritium contamination: up to 30% of the superheavy hydrogen isotope was removed from the solution in one purification cycle.
The accumulation of the superheavy isotope in a solution of protium water (HTO) occurred in the anolyte and the neutral chamber (Figure 13a); the degree of fractionation (α) reached 1.19. In a solution of deuterium water (DTO), tritium accumulated in the neutral chamber of the dialyzer (Figure 13b), and the degree of fractionation reached 1.25 [101].
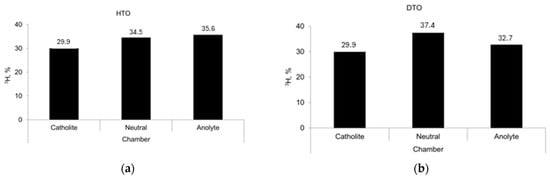
Figure 13.
Separation of hydrogen isotopes from water solution in 3-chamber dialyzer divided with bentonite membranes: (a) tritium–protium, (b) tritium–deuterium.
The application of isotope osmosis phenomena is especially important for HWRs. The accumulation of tritium in the moderator of heavy water reactors above 1011 Bq·kg−1 requires either the replacement of D2O (about USD 50 million/year for CANDU) or the isotopic purification of heavy water from tritium.
Current facilities are very energy intensive and use a combined method of isotope exchange in a water–hydrogen vapor system and cryoscopic hydrogen isotope rectification. Through a catalytic isotope exchange reaction between water vapor and deuterium (Equations (11) and (12)) at a temperature of 200 °C, tritium and protium are extracted from heavy water and transferred to the gas phase:
DTO + D2 = DT + D2O
HDO + D2 = HD + D2O
The degree of extraction of tritium from heavy water is limited by the equilibrium constant of the above reaction, and at the third stage, the purification is about 30%. The heavy water purified from tritium and protium is returned to the reactor. The mixture of hydrogen isotopes, i.e., D2, DT, and HD, after purification from impurities and cooling to a temperature of 25 K is fed to the low-temperature column. Due to the process of mass exchange between the gaseous and liquid mixture of hydrogen isotopes, tritium is concentrated in the lower part of the column, and protium is moved to the upper part. The tritium and protium-depleted deuterium stream return to the catalytic isotope exchange unit. The protium concentrate is taken from the upper part of the low-temperature column, and from the lower part, the concentrated or pure tritium is taken [111]. The improved catalytic scheme of isotope purification using a “hydrogen–palladium system”, which can concentrate tritium up to 98 at.%, is planned to be used in a thermonuclear reactor [112,113].
The application of cyclic electro-osmotic isotope separation with the use of proton-conducting membranes based on clay minerals for these purposes will enable achieving results at much lower costs.
6. Summary
Once released into the environment, tritium is distributed globally owing to its half-life and residence time; therefore, the consequences of its exposure should be considered to ensure the protection of the public and the environment from radioactive discharges.
Despite improvements in the operational practices of nuclear plants and in waste management and disposal, several problems remain. The main problems are as follows:
- -
- An increased content of tritium in the biosphere at about 20 times that at the beginning of the nuclear era.
- -
- Insufficient study of the radiobiological impact of tritium: the impact of incorporated tritium on living organisms is estimated to be three times higher than that of 137Cs, while the local impact (for DNA molecule) may be 300 times higher.
- -
- Uncertainty of safe concentrations: world standards for the tritium content in drinking water differ by more than 700 times (from 100 to 76,100 Bq·dm−3), and scientifically based estimates differ by 600,000 times.
- -
- Extremely high migration capacity of the aqueous form of tritium: the rate constant of the isotopic exchange of protium for tritium in water is estimated at (1.5 ± 0.5) × 10−4 s−1.
- -
- The crucial role of water for living organisms: there is no indication of the biomagnification of tritium in terrestrial systems.
- -
- The large amount of contradictory data on tritium accumulation in living organisms.
- -
- The fact that there are practically no ways to clean liquid discharges and gaseous emissions of tritium from nuclear facilities; existing methods of hydrogen isotope separation require significant energy costs.
- -
- The metastability of tritium emissions from nuclear reactors, which cause a paroxysmal nature of tritium emissions and create obstacles in predicting its distribution in the environment.
During the nuclear power cycle, tritium generated in nuclear units and contained in spent fuel may be released into the environment.
Present tritium emissions into the biosphere have reached equilibrium, reflecting the exhaustion of the current technological capabilities of nuclear power. Future development of thermonuclear synthesis could increase the tritium inventory by several orders of magnitude, and tritium would thus become the Achilles’ heel of fusion. However, despite widespread optimism (since the 1950s, when the first research began), significant obstacles between today’s understanding of nuclear fusion processes, technological capabilities, and practical applications of nuclear fusion have not yet been overcome.
The supply of nuclear fission energy mainly depends on the limitation of uranium resources. The reserves of uranium-235 used in thermal neutron reactors can be used by humans for decades (according to the 2024 reserve to production ratio), but the plutonium-239 used in fast neutron reactors is converted from uranium-238, and according to the reserves of uranium-238, it can provide humans with nuclear fission energy for thousands of years. This also applies to thorium fuel. So, the timetable for the use of nuclear fusion energy does not depend on changes in the existing energy structure but on the maturity of nuclear fusion technology, as well as economic, social, political, and other factors. Humans have enough time to obtain satisfactory answers in the utilization of nuclear fusion energy.
In nuclear fusion reactors, the production or breeder of tritium is used for the continuous use of fusion energy, that is, the production or breeder of tritium is used to consume tritium to produce continuous energy rather than for other products. In addition, the production capacity of tritium can be adjusted through the rational design of nuclear fusion reactors to ensure a reasonable allocation between continuous fusion energy production and minimum storage capacity.
Whilst waiting for this new type of nuclear reactor, the most promising projects for the transition period include the introduction of small modular reactors, which are characterized by high construction rates, a higher level of safety, and acceptable technical and economic indicators. The development of a hydrogen energy complex based on nuclear reactors also seems promising.
The basic principles of tritium waste management need improvement, and solid waste management should be the same as liquid radioactive waste management. It is shown that concrete (material that functions as a bioshield) can trap a large amount of tritium and release it into the environment; therefore, special attention must be paid to the selection of engineered barriers for repositories/disposal facilities. The use of a mixture of framework and layered silicates can improve the absorption and filtering properties of the biological shield.
The proposed management scheme of metal radioactive waste generated in a fusion reactor is accompanied by a change in the phase composition of the waste, an increase in mobility, and a radiological hazard, which does not comply with the basic principles of radioactive waste management. The alternative option of storage without treatment for 150 years reduces tritium’s activity by almost four orders of magnitude.
Natural membranes of living and inanimate matter are capable of significant hydrogen isotope fractionation owing to kinetic (mass-dependent) and nuclear magnetic effects.
The study of isotope-osmotic processes using membranes of a natural origin opens broad prospectives for their application in energy-saving technologies for the treatment of liquid radioactive waste and deactivation of technological reservoirs of nuclear facilities from radioactive contamination.
Author Contributions
Conceptualization, V.D.; methodology, V.D., Y.Y. and R.L.F.; software, S.A.C.; validation, V.D. and S.A.C.; formal analysis, V.D.; investigation, V.D. and R.L.F.; resources, R.L.F.; data curation, Y.Y., S.A.C. and V.D.; writing—original draft preparation, V.D. and R.L.F.; writing—review and editing, R.L.F. and V.D.; visualization, S.A.C.; supervision, R.L.F.; project administration, R.L.F.; funding acquisition, R.L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding by the European Union in the frame MSCA4Ukraine project, grant No. 1232235. The views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the MSCA4Ukraine Consortium as a whole nor any individual member institutions of the MSCA4Ukraine Consortium can be held responsible for them.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferreira, M.F.; Turner, A.; Vernon, E.L.; Grisolia, C.; Lebaron-Jacobs, L.; Malard, V.; Jha, A.N. Tritium: Its relevance, sources and impacts on non-human biota. Sci. Total Environ. 2023, 876, 162816. [Google Scholar] [CrossRef] [PubMed]
- Oms, P.E.; Du Bois, P.B.; Dumas, F.; Lazure, P.; Morillon, M.; Voiseux, C.; Le Corre, C.; Cossonnet, C.; Solier, L.; Morin, P. Inventory and distribution of Tritium in the oceans in 2016. Sci. Total Environ. 2019, 656, 1289–1303. [Google Scholar] [CrossRef]
- Nie, B.; Fang, S.; Jiang, M.; Wang, L.; Ni, M.; Zheng, J.; Yang, Z.; Li, F. Anthropogenic Tritium: Inventory, discharge, environmental behavior and health effects. Renew. Sustain. Energy Rev. 2021, 135, 110188. [Google Scholar] [CrossRef]
- Larsen, G.; Babineau, D. An Evaluation of the Global Effects of Tritium Emissions from Nuclear Fusion Power. Fusion Eng. Des. 2020, 158, 111690. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Management of Waste Containing Tritium and Carbon-14; Technical Reports Series No. 421; IAEA: Vienna, Austria, 2004. [Google Scholar]
- Killough, G.G.; Kocher, D.C. Global Environmental Transport Models for Tritium. Fusion Technol. 1985, 8, 2569–2574. [Google Scholar] [CrossRef]
- Murphy, C.E., Jr. The Transport; Dispersion, and Cycling of Tritium in the Environment; Westinghouse Savannah River Co.: Aiken, SC, USA, 1990. [Google Scholar] [CrossRef][Green Version]
- Eyrolle, F.; Ducros, L.; Le Dizès, S.; Beaugelin-Seiller, K.; Charmasson, S.; Boyer, P.; Cossonnet, C. An updated review on Tritium in the environment. J. Environ. Radioact. 2018, 181, 128–137. [Google Scholar] [CrossRef]
- Cochran, T.B.; Arkin, W.M.; Hoenig, M.M. Nuclear Weapons Databook: Vol. II: U.S. Nuclear Warhead Production; Ballinger: Pensacola, FL, USA, 1984. [Google Scholar]
- Glasstone, S.; Dolan, P.J. The Effects of Nuclear Weapons; U.S. GPO: Washington, DC, USA, 1977.
- Oklahoma Geological Survey Observatory Catalog of Nuclear Explosions: 2006. Available online: https://digitalprairie.ok.gov/digital/collection/stgovpub/id/9093/ (accessed on 18 January 2012).
- Roser, M.; Herre, B.; Hasell, J. Nuclear Weapons. 2013. Published online at OurWorldInData.org.. Available online: https://ourworldindata.org/nuclear-weapons (accessed on 10 May 2023).
- IAEA Power Reactor Information System. Available online: https://pris.iaea.org/ (accessed on 22 November 2024).
- World Nuclear Association. Available online: https://world-nuclear.org (accessed on 22 November 2024).
- Lal, D.; Peters, B. Cosmic Ray Produced Radioactivity on the Earth. In Kosmische Strahlung II/Cosmic Rays II; Handbuch der Physik/Encyclopedia of Physics Series Volumes 9/46/2; Sitte, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1967. [Google Scholar] [CrossRef]
- Okada, S.; Momoshima, N. Overview of Tritium: Characteristics, sources, and problems. Health Phys. 1993, 65, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Egorov, Y.A. Assessment of radiation hazard of Tritium produced at NPPs. Ecol. Ind. Russ. 2003, 2, 27–30. (In Russian) [Google Scholar]
- Stephen, E. Atkins Historical Encyclopedia of Atomic Energy; Bloomsbury Academic: London, UK, 2000; 491p. [Google Scholar]
- Serot, O.; Wagemans, C.; Heyse, J. New Results on Helium and Tritium Gas Production From Ternary Fission. AIP Conf. Proc. 2005, 769, 857–860. [Google Scholar] [CrossRef]
- Peterson, H.T.; Baker, D.A. Tritium Production, Releases and Population Doses at Nuclear Power Reactors. Fusion Technol. 1985, 8, 2544–2550. [Google Scholar] [CrossRef]
- Weaver, C.L.; Harward, E.D.; Peterson, H.T., Jr. Tritium in the environment from nuclear powerplants. Public Health Rep. 1969, 84, 363–371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Investigation of the Environmental Fate of Tritium in the Atmosphere; Report CC172-51/2009E-PDF Minister of Public Works and Government Services; Canadian Nuclear Safety Commission: Ottawa, ON, Canada, 2009; 110p.
- Kalinowski, M.B. Uncertainty and range of alternatives in estimating Tritium emissions from proposed fusion power reactors and their radiological impact. J. Fusion Energ. 1993, 12, 157–161. [Google Scholar] [CrossRef]
- Dolin, V.V.; Zabulonov, Y.L.; Kopylenko, O.L.; Shramenko, I.F. Global Tendencies in Nuclear Power Engineering. Geochem. Technog. 2023, 36, 5–13. [Google Scholar] [CrossRef]
- Frano, R.L.; Dolin, V.; Cancemi, S.A. The influence of Tritium behaviour on spent fuel pool concrete. Prog. Nucl. Energy 2024, 169, 105053. [Google Scholar] [CrossRef]
- Jacobs, D.G. Sources of Tritium and Its Behavior upon Release to the Environment; AEC Critical Review Series; USAEC Division of Technical Information Exension: Oak Ridge, TN, USA, 1968. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Energy, Electricity and Nuclear Power Estimates for the Period up to 2050; Reference Data Series No. 1; IAEA: Vienna, Austria, 2023. [Google Scholar]
- European Parliamentary Research Service. Briefing. Nuclear Energy in the European Union. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2023/751456/EPRS_BRI(2023)751456_EN.pdf (accessed on 22 November 2024).
- UNFCCC. Outcome of the First Global Stocktake. Draft Decision. FCCC/PA/CMA/2023/L.17. Available online: https://unfccc.int/sites/default/files/resource/cma2023_L17E.pdf (accessed on 22 November 2024).
- Daya, C.; Battes, K.; Butler, B.; Davies, S.; Farina, L.; Frattolillo, A.; George, R.; Giegerich, T.; Hanke, S.; Härtl, T.; et al. The pre-concept design of the DEMO Tritium, matter injection and vacuum systems. Fusion Eng. Des. 2022, 179, 113139. [Google Scholar] [CrossRef]
- Fomin, G.V. Problem of the Tritium for the International Fusion Reactor (IFR). At. Ehnergiya 1995, 78, 290–293. [Google Scholar]
- Humrickhouse, P.W.; Merrill, B.J. Tritium aspects of the fusion nuclear science facility. Fusion Eng. Des. 2018, 135, 302–313. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. IAEA Safety Glossary, 2018 ed.; Non-serial Publications; IAEA: Vienna, Austria, 2019. [Google Scholar]
- Kobisk, E.H.; Ramey, D.W.; Aaron, W.S.; Tompkins, J.A.; Haff, K.W.; Devore, J.R.; Adair, H.L. Tritium-Processing Operations at the Oak Ridge National Laboratory with Emphasis on Safe-Handling Practices; The Netherlands, 1989. Available online: https://www.osti.gov/etdeweb/biblio/5368299#fullrecord (accessed on 22 November 2024).
- Centre de Stockage de la Manche: Rapport Annuel 2008, ANDRA, 2009. Available online: https://manche.andra.fr/sites/manche/files/2018-04/ra-csm-2008.pdf (accessed on 22 November 2024).
- U.S. DOE. Tritium Handling and Safe Storage. DOE-STD-1129-2015, September 2015. Available online: https://www.standards.doe.gov/standards-documents/1100/1129-AStd-2015/@@images/file (accessed on 22 November 2024).
- Alba, R.; Iglesias, R.; Cerdeira, M.Á. Materials to Be Used in Future Magnetic Confinement Fusion Reactors: A Review. Materials 2022, 15, 6591. [Google Scholar] [CrossRef]
- Perevezentsev, A.N.; Bell, A.C.; Rivkis, L.A.; Filin, V.M.; Gushin, V.V.; Belyakov, M.I.; Bulkin, V.I.; Prykina, I.G.; Kravchenko, I.M.; Semenov, A.A.; et al. Experimental trials of methods for metal detritiation for JET. Fusion Sci. Technol. 2007, 52, 84–99. [Google Scholar] [CrossRef]
- Lassoued, A.; Chêne, J.; Brass, A.M.; Gastaldi, O.; Trabuc, P.; Marbach, G. Influence of heat treatments on residual Tritium amount in tritiated stainless steel waste. Fusion Eng. Des. 2005, 75–79, 731–735. [Google Scholar] [CrossRef]
- ICRP; Eckerman, K.; Harrison, J.; Menzel, H.-G.; Clement, C.H. ICRP Publication 119: Compendium of Dose Coefficients based on ICRP Publication 60. Ann. ICRP 2012, 41 (Suppl. 1), 1–130. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Predisposal Management of Radioactive Waste from Nuclear Power Plants and Research Reactors; IAEA Safety Standards Series No. SSG-40; IAEA: Vienna, Austria, 2016. [Google Scholar]
- European Atomic Energy Community; Food and Agriculture Organization of the United Nations; International Atomic Energy Agency; International Labour Organization; International Maritime Organization; OECD Nuclear Energy Agency; Pan American Health Organization; United Nations Environment Programme; World Health Organization. Fundamental Safety Principles; IAEA Safety Standards Series No. SF-1; IAEA: Vienna, Austria, 2006. [Google Scholar] [CrossRef]
- Vladimirovich, A.; Nikolaevna, S.V.; Nikolay, G.A.; Kazakovsky, T.; Korotkova, G.P.; Erokhin, A.V. Production Method of Metal Tritium Target. Russian Patent no. RU2529399C1, 27 September 2013. Available online: https://patents.google.com/patent/RU2529399C1/en (accessed on 22 November 2024).
- Mandoki, R.; Wasselin, V.; Maillard, J.L. Tritiated Radwaste Management in France: Status and Perspectives. In Proceedings of the Tritium School Book of Abstracts, Marseille, France, 18–22 March 2024. [Google Scholar]
- Maiti, G.C.; Freund, F. Dehydration-related proton conductivity in kaolinite. Clay Miner. 1981, 16, 395–413. [Google Scholar] [CrossRef]
- Dolin, V.; Pushkarev, O.V.; Shramenko, I.F.; Bobkov, V.M. Tritium in the Biosphere; Sobotovych, E., Dolin, V., Eds.; Naukova Dumka: Kiev, Ukraine, 2012. [Google Scholar] [CrossRef]
- Dolin, V.; Yakovlev, Y.; Shcherbak, O.; Kutska, Y. Evolution of profile of radiohydrogeochemical anomaly of Tritium contamination within affected zone of surface radioactive waste repository. In Proceedings of the Geoinformatics 2015 XIVth International Conference—Geoinformatics: Theoretical and Applied Aspects, Kiev, Ukraine, 11–14 May 2015. [Google Scholar] [CrossRef]
- Pushkarov, O.; Sevruk, I.; Dolin, V. Influence of the structure of a mineral adsorbent on the detritization of aqueous solutions. In Visnyk of V.N. Karazin Kharkiv National University, Series «Geology. Geography. Ecology»; Katazin University: Kharkiv, Ukraine, 2021; No. 55. [Google Scholar] [CrossRef]
- Pushkariov, O.V.; Pryimachenko, V.M.; Zolkin, I.O. Bentonite-Ceolite Composites’ properties with respect to Tritium extraction from Tritium water. Geochem. Technog. 2012, 20, 98–108. Available online: https://journals.igns.kyiv.ua/index.php/geotech/article/view/250 (accessed on 22 November 2024).
- Pamela, J.; Bottereau, J.M.; Canas, D.; Decanis, C.; Liger, K.; Gaune, F. ITER tritiated waste management by the Host state and first lessons learned for fusion development. Fusion Eng. Des. 2014, 89, 2001–2007. [Google Scholar] [CrossRef]
- Decanis, C.; Kresina, M.; Canas, D. Methodology to identify appropriate options to manage tritiated waste. Fusion Eng. Des. 2018, 136, 276–281. [Google Scholar] [CrossRef]
- Mathur-De Vré, R.; Binet, J. Molecular aspects of tritiated water and natural water in radiation biology. Prog. Biophys. Mol. Biol. 1984, 43, 161–193. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Biological Implications of Radionuclides Released from Nuclear Industries; Vienna, 26–30 March 1979, Proceedings Series; IAEA: Vienna, Austria, 1980. [Google Scholar]
- Martin, J.E. Physics for Radiation Protection; Wiley: Hoboken, NJ, USA, 2013; ISBN 9783527667062. [Google Scholar] [CrossRef]
- DOE-HDBK-1079-94; Primer on Tritium Safe Handling Practices. Department of Energy: Washington, DC, USA, 1995. Available online: https://www.standards.doe.gov/standards-documents/1000/1079-bhdbk-1994 (accessed on 11 February 2025).
- Environmental Health Criteria 25: Selected Radionuclides; WHO: Geneva, Switzerland, 1983; Available online: https://iris.who.int/bitstream/handle/10665/37191/9241540850-eng.pdf?sequence=1 (accessed on 11 February 2025).
- European Union. COUNCIL DIRECTIVE 2013/51/EURATOM of 22 October 2013 Laying Down Requirements for the Protection of the Health of the General Public with Regard to Radioactive Substances in Water Intended for Human Consumption. Off. J. Eur. Union 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013L0051 (accessed on 11 February 2025).
- ICRP. Age-dependent Doses to Members of the Public from Intake of Radionuclides—Part 1. Ann. ICRP 1990, 20, 56. [Google Scholar]
- EPA 402-R-99-001 Federal Guidance Report No. 13. 1999. Cancer Risk Coefficients for Environmental Exposure to Radionuclides. Available online: https://www.epa.gov/system/files/documents/2025-03/402-r-99-001_508-d_2.pdf (accessed on 27 March 2025).
- Balonov, M.I.; Bruk, Y.G.; Zhesko, T.V. Dosimetry and Standardization of Tritium in Russia. Fusion Technol. 1995, 28, 809–813. [Google Scholar] [CrossRef]
- Balonov, M.I.; Muksinova, K.N.; Mushkacheva, G.S. Tritium radiobiological effects in mammals: Review of experiments of the last decade in Russia. Health Phys. 1993, 65, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Straume, T.; Carsten, A.L. Tritium radiobiology and relative biological effectiveness. Health Phys. 1993, 65, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Bannister, L.; Serran, M.; Bertrand, L.; Klokov, D.; Wyatt, H.; Blimkie, M.; Gueguen, Y.; Priest, N.; Jourdain, J.-R.; Sykes, P. Environmentally Relevant Chronic Low-Dose Tritium and Gamma Exposures do not Increase Somatic Intrachromosomal Recombination in pKZ1 Mouse Spleen. Radiat. Res. 2016, 186, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Advisory Committee on Environmental Standards; Comite Consultatif sur les Normes Environmentales. A Standard for Tritium: A Recommendation to the Minister of the Environment and Energy; Queen’s Printer for Ontario: Toronto, ON, Canada, 1994; Available online: https://archive.org/details/standardfortriti00adviuoft (accessed on 27 March 2025).
- Cancer Risk Coefficients Environmental Exposure to Radionuclides; Report No. 13 [A-98-11-V-B-1]; USEPA: Washington, DC, USA, 15 September 2021.
- Water Quality Standards Regulatory Revisions To Protect Tribal Reserved Rights. US EPA, 05/02/2024. Available online: https://www.federalregister.gov/documents/2024/05/02/2024-09427/water-quality-standards-regulatory-revisions-to-protect-tribal-reserved-rights (accessed on 27 March 2025).
- Introduction to Risk Assessment—European Commission. Available online: https://ec.europa.eu/health/ph_projects/2003/action3/docs/2003_3_09_a23_en.pdf (accessed on 7 February 2025).
- Carsten, A.L.; Benz, R.D.; Hughes, W.P.; Ichimasa, Y.; Ikushima, T.; Tezuka, H. Summary Update of the Brookhaven Tritium Toxicity Program with Emphasis on Recent Cytogenetic and Life-Shortening Studies; Upton: New York, NY, USA, 1988; Available online: https://digital.library.unt.edu/ark:/67531/metadc1188844/ (accessed on 7 February 2025).
- Wang, B.; Takeda, H.; Gao, W.M.; Zhou, X.Y.; Odaka, T.; Ohyama, H.; Yamada, T.; Hayata, I. Induction of Apoptosis By Beta Radiation From Tritium Compounds In Mouse Embryonic Brain Cells. Health Phys. 1999, 77, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Toeroek, P.; Schmahl, W.; Meyer, I.; Kistner, G. Effects of a Single Injection of Tritiated Water During Organogeny on the Prenatal and Postnatal Development of Mice; IAEA: Vienna, Austria, 1979; Volume 1, pp. 241–252. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 27 March 2025).
- National Health and Medical Research Council. Australian Drinking Water Guidelines. Version 3.8 Updated September 2022; National Health and Medical Research Council: Canberra, Australia, 2011. [Google Scholar]
- Schweizerische Eidgenossenschaft.Verordnung des EDI über Trinkwasser sowie Wasser in öffentlich zugänglichen Bädern und Duschanlagen (TBDV). AS 2017 1023, 21. März 2017. Available online: https://www.fedlex.admin.ch/eli/oc/2017/153/de (accessed on 27 March 2025).
- Departement Helse- og Omsorgsdepartementet (Norge). Forskrift om Endring i Forskrift om visse Forurensende Stoffer i Næringsmidler, 2016. Available online: https://lovdata.no/dokument/LTI/forskrift/2016-12-22-1869 (accessed on 7 February 2025).
- U.S. EPA. National Primary Drinking Water Regulation (NPDWRs). Available online: https://www.gvsu.edu/cms4/asset/E1327343-09F0-03FF-AA9032F47AD1EB9C/standards.pdf (accessed on 7 February 2025).
- Canadian Nuclear Safety Commission. Standards and Guidelines for Tritium in Drinking. INFO-0766. January 2008. Available online: https://www.nrc.gov/docs/ml1029/ml102990104.pdf (accessed on 7 February 2025).
- Canadian Nuclear Safety Commission. Radiation Protection. REGDOC-2.7.1. July 2021. Available online: https://api.cnsc-ccsn.gc.ca/dms/digital-medias/REGDOC-2_7_1__Radiation_Protection_2021.pdf/object (accessed on 7 February 2025).
- NRB-99/2009; Sanitary and Epidemiological Rules and Regulations. Federal Center of Hygiene and Epidemiology of Rospotrebnadzor: Moscow, Russia, 2009; ISBN 978-5-7508-0805-2.
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; Available online: https://iris.who.int/bitstream/handle/10665/352532/9789240045064-eng.pdf?sequence=1 (accessed on 7 February 2025).
- STUK. Radiation and Nuclear Safety Authority. Available online: https://stuk.fi/en/radiation-safety-of-nuclear-facilities (accessed on 7 February 2025).
- Norms of Radiation Safety of Ukraine. Supplement: Radiation Protection from the Sources of Potential Exposure (NRBU-97/D-2000); State Hygienic Standards (DGN 6.6.1.–6.5.061-2000). Available online: https://zakon.rada.gov.ua/rada/show/v0062282-97#Text (accessed on 22 November 2024).
- Nuclear Regulation Authority (NRA) Japan. Available online: https://www.nra.go.jp/english/ (accessed on 22 November 2024).
- Hashimoto, S.; Komatsu, M.; Miura, S. Radiation Protection and Criteria. In Forest Radioecology in Fukushima; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Tokyo Electric Power Company Holdings, Inc. (TEPCO). Available online: https://www.tepco.co.jp/en/decommission/progress/watertreatment/faq/index-e.html (accessed on 7 February 2025).
- Straume, T. Tritium risk assessment. Health Phys. 1993, 65, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Kase, Y.; Yamashita, W.; Matsufuji, N.; Takada, K.; Sakae, T.; Furusawa, Y.; Yamashita, H.; Murayama, S. Microdosimetric calculation of relative biological effectiveness for design of therapeutic proton beams. J. Radiat. Res. 2013, 54, 485–493, Erratum in J. Radiat. Res. 2013, 54, 971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balakrishnan, S.; Rao, B.S. Cytogenetic Effects of Tritiated Water (HTO) in Human Peripheral Blood Lymphocytes in vitro. Int. J. Hum. Genet. 2004, 4, 237–242. [Google Scholar] [CrossRef]
- Available online: https://www.andra.fr/les-dechets-radioactifs/les-solutions-de-gestion (accessed on 22 November 2024).
- Pivetz, B.E. Phytoremediation of Contaminated Soil and Ground Water at Hazardous Waste Sites, EPA/540/S-01/500 February 2001. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa_540_s01_500.pdf (accessed on 22 November 2024).
- International Atomic Energy Agency. Tritium in Some Typical Ecosystems; Technical Reports Series No. 207; IAEA: Vienna, Austria, 1981. [Google Scholar]
- Alpen, E.L. Radiation Biophysics, 2nd ed.; Academic Press: Cambridge, MA, USA, 1998; ISBN 978-0-12-053085-4. [Google Scholar] [CrossRef]
- Review of Risks of Tritium: UK. Health Protection Agency, November 2007. Available online: https://www.gov.uk/government/publications/tritium-review-of-risks (accessed on 27 March 2025).
- Hunter, N.; Muirhead, C.R. Review of relative biological effectiveness dependence on linear energy transfer for low-LET radiations. J. Radiol. Prot. 2009, 29, 5. [Google Scholar] [CrossRef]
- Howard, M.; Beltran, C.; Sarkaria, J.; Herman, M.G. Characterization of relative biological effectiveness for conventional radiation therapy: A comparison of clinical 6 MV X-rays and 137Cs. J. Radiat. Res. 2017, 58, 608–613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrison, J.D.; Khursheed, A.; Lambert, B.E. Uncertainties in Dose Coefficients for Intakes of Tritiated Water and Organically Bound Forms of Tritium by Members of the Public. Radiat. Prot. Dosim. 2002, 98, 299–311. [Google Scholar] [CrossRef]
- Dobson, R.L.; Kwan, T.C. The tritium RBE at low-level exposure—Variation with dose, dose rate, and exposure duration. Curr. Top. Radiat. Res. Q. 1978, 12, 44–62. [Google Scholar] [PubMed]
- Snigireva, G.P.; Khaimovich, T.I.; Nagiba, V.I. Estimation of relative biological effectiveness of tritium according to chromosome aberration frequency in human blood lymphocytes. Biophysics 2011, 56, 364–370. [Google Scholar] [CrossRef]
- Authors on behalf of ICRP; Higley, K.; Real, A.; Chambers, D. Annals of the ICRP Publication 148: Radiation Weighting for Reference Animals and Plants; SAGE: Thousand Oaks, CA, USA, 2021; Volume 50, Available online: https://journals.sagepub.com/doi/pdf/10.1177/ANIB_50_2 (accessed on 27 March 2025).
- Melintescu, A.; Galeriu, D.; Takeda, H. Reassessment of tritium dose coefficients for the general public. Radiat. Prot. Dosim. 2007, 127, 153–157. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Age-dependent Doses to Members of the Public from Intake of Radionuclides—Part 2 Ingestion Dose Coefficients. Ann. ICRP 1993, 23, 67. [Google Scholar]
- Kirchmann, R.; Bittel, R.; Fagniart, E.; van Gelder-Bonnijns, G.; Koch, G. Transfer of Tritium from Radioactive Waste to Aquatic Organisms, Under Natural Conditions; Working Group on Environmental Pollution, ESNA Meeting; IAEA: Leuven, Belgium, 1973; p. 31. [Google Scholar]
- Bennett, B.G. Radiation Dose Due to Acute Intake of Tritium by Man; Health and Safety Laboratory, U.S. Atomic Energy Comission: New York, NY, USA, 1972. [Google Scholar] [CrossRef][Green Version]
- Schiegl, W.E.; Vogel, J.C. Deuterium content of organic matter. Earth Planet. Sci. Lett. 1970, 7, 307–313. [Google Scholar] [CrossRef]
- Smith, B.N.; Epstein, S. Biogeochemistry of the Stable Isotopes of Hydrogen and Carbon in Salt Marsh Biota. Plant Physiol. 1970, 46, 738–742. [Google Scholar] [CrossRef]
- Slini, G.; Metalli, P.; Vulpis, G. Radiotoxicity of Tritium in Mammals Critical Analysis of the Extrapolation to Man of the Results of Tritium Incorporation into Animal Tissues; EURATOM; Commission of the European Communities: Luxembourg, 1973. [Google Scholar]
- Evans, A. New dose estimates from chronic Tritium exposures. Health Phys. 1970, 16, 57–63. [Google Scholar] [CrossRef]
- Hill, R.L.; Johnson, J.R. Metabolism and dosimetry of tritium. Health Phys. 1993, 65, 628–647. [Google Scholar] [CrossRef]
- Dolin, V.V.; Bobkov, V.M.; Pushkarev, O.V.; Koshliakova, T.O. Isotopic Effects of Tritium during the Growth of White Willow. Univers. J. Geosci. 2018, 6, 175–183. [Google Scholar] [CrossRef]
- Etnier, E.L.; Travis, C.C.; Hetrick, D.M. Metabolism of Organically Bound Tritium in Man. Radiat. Res. 1984, 100, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, V.V.; Burdeinaya, T.N.; Romanyukina, I.Y.; Skil’skaya, M.D.; Demchenko, V.Y.; Kavitskaya, A.A. Isotope osmosis—Osmotic transfer of water isotopologues through the polymer membrane in the course of direct osmosis. J. Water Chem. Technol. 2014, 36, 103–109. [Google Scholar] [CrossRef]
- Pushkarov, O.V.; Zubko, O.V.; Sevruk, I.M.; Dolin, V.V. Membrane Properties of Montmorillonite, Saponite and Clinoptilolite During Electroosmotic Fractionation of Hydrogen Isotopes. Mineral. J. 2020, 42, 23–32. [Google Scholar] [CrossRef]
- Andreev, B.M.; Perevezentsev, A.N.; Selivanenko, I.L.; Tenyaev, B.N.; Vedeneev, A.I.; Golubkov, A.N. Hydrogen Isotope Separation Installation for Tritium Facility. Fusion Sci. Technol. 1995, 28, 505–510. [Google Scholar] [CrossRef]
- Andreev, B.M.; Sakharovsky, Y.A.; Rozenkevich, M.B.; Magomedbekov, E.P.; Park, Y.S.; Uborskiy, V.V. A New Concept of ISS for Fusion Reactor. Fusion Technol. 1995, 28, 511–514. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).