Conventional and Innovative Methods for Reducing the Incidence of Listeria monocytogenes in Milk and Dairy Products
Abstract
1. Introduction
2. Thermal Methods
3. Nonthermal Methods
3.1. High-Pressure Processing (HPP)
3.2. Ultrasound Method
3.3. Pulsed Electric Field (PEF)
3.4. Ionizing Irradiation
3.5. Ultraviolet (UV) Radiation
3.6. Ozone Using Method
3.7. Sonodynamic Technology (SDT)
3.8. Intense Pulsed Light (IPL)
4. Biocontrol Methods
4.1. Lactic Acid Bacteria
4.2. Probiotics
4.3. Postbiotics
4.4. Bacteriophages
5. Natural Methods
5.1. Essentials Oils
5.2. Plant Extract
5.3. Organic Acids
5.4. Macroalgae
6. Use of Chemical Agents
7. Other Methods for Reducing L. monocytogenes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nema, P.K.; Sehrawat, R.; Ravichandran, C.; Kaur, B.P.; Kumar, A.; Tarafdar, A. Inactivating Food Microbes by High-Pressure Processing and Combined Nonthermal and Thermal Treatment: A Review. J. Food Qual. 2022, 2022, 5797843. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria Monocytogenes in Foods—From Culture Identification to Whole-Genome Characteristics. Food Sci. Nutr. 2022, 10, 2825–2854. [Google Scholar] [CrossRef]
- Imran, A.; Shehzadi, U.; Islam, F.; Afzaal, M.; Ali, R.; Ali, Y.A.; Chauhan, A.; Biswas, S.; Khurshid, S.; Usman, I.; et al. Bacteriophages and Food Safety: An Updated Overview. Food Sci. Nutr. 2023, 11, 3621–3630. [Google Scholar] [CrossRef]
- Duma, M.N.; Ciupescu, L.M.; Dan, S.D.; Crisan-Reget, O.L.; Tabaran, A. Virulence and Antimicrobial Resistance of Listeria monocytogenes Isolated from Ready-to-Eat Food Products in Romania. Microorganisms 2024, 12, 954. [Google Scholar] [CrossRef]
- Talari, G.; Nag, R.; O’Brien, J.; McNamara, C.; Cummins, E. A Data-Driven Approach for Prioritising Microbial and Chemical Hazards Associated with Dairy Products Using Open-Source Databases. Sci. Total Environ. 2024, 908, 168456. [Google Scholar] [CrossRef]
- Yan, Q.; Mei, J.; Li, D.; Xie, J. Application of Sonodynamic Technology and Sonosensitizers in Food Sterilization: A Review of Developments, Trends and Challenges. Crit. Rev. Food Sci. Nutr. 2024, 64, 740–759. [Google Scholar] [CrossRef]
- Petrova, P.; Arsov, A.; Tsvetanova, F.; Parvanova-mancheva, T.; Vasileva, E.; Tsigoriyna, L.; Petrov, K. The Complex Role of Lactic Acid Bacteria in Food Detoxification. Nutrients 2022, 14, 2038. [Google Scholar] [CrossRef]
- Brandelli, A.; Lopes, N.A.; Pinilla, C.M.B. Nanostructured Antimicrobials for Quality and Safety Improvement in Dairy Products. Foods 2023, 12, 2549. [Google Scholar] [CrossRef]
- Wu, M.; Ma, Y.; Dou, X.; Zohaib Aslam, M.; Liu, Y.; Xia, X.; Yang, S.; Wang, X.; Qin, X.; Hirata, T.; et al. A Review of Potential Antibacterial Activities of Nisin against Listeria monocytogenes: The Combined Use of Nisin Shows More Advantages than Single Use. Food Res. Int. 2023, 164, 112363. [Google Scholar] [CrossRef]
- Rudke, C.R.M.; Camelo-Silva, C.; Rudke, A.R.; Prudencio, E.S.; de Andrade, C.J. Trends in Dairy Products: New Ingredients and Ultrasound-Based Processing. Food Bioprocess Technol. 2024, 17, 811–827. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Cadavez, V.; De Oliveira Mota, J.; Guillier, L.; Sanaa, M. A Critical Review of Risk Assessment Models for Listeria monocytogenes in Produce. Foods 2024, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- Afloarei, C.-Ș.; Buculei, A.; Chetrariu, A.; Dabija, A. Listeria monocytogenes in Diary Products Occurrence, Monitoring and Surveillance. J. Appl. Life Sci. Environ. 2024, 57, 599–615. [Google Scholar]
- Rolon, M.L.; Chandross-cohen, T.; Kaylegian, K.E.; Roberts, R.F.; Kovac, J.; Rolon, M.L.; Chandross-cohen, T.; Kaylegian, K.E.; Roberts, R.F.; Kovac, J. Context matters: Environmental microbiota from ice cream processing facilities affected the inhibitory performance of two lactic acid bacteria strains against Listeria monocytogenes. Microbiol. Spectr. 2024, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Botondi, R.; Lembo, M.; Carboni, C.; Eramo, V. The Use of Ozone Technology: An Eco–Friendly Method for the Sanitization of the Dairy Supply Chain. Foods 2023, 12, 987. [Google Scholar] [CrossRef]
- Dincer, E. Detection of Listeria Species by Conventional Culture-Dependent and Alternative Rapid Detection Methods in Retail Ready-to-Eat Foods in Turkey. J. Microbiol. Biotechnol. 2024, 34, 349–357. [Google Scholar] [CrossRef]
- Wei, X.; Hassen, A.; McWilliams, K.; Pietrzen, K.; Chung, T.; Acevedo, M.M.; Chandross-Cohen, T.; Dudley, E.G.; Vipham, J.; Mamo, H.; et al. Genomic Characterization of Listeria monocytogenes and Listeria innocua Isolated from Milk and Dairy Samples in Ethiopia. BMC Genom. Data 2024, 25, 12. [Google Scholar] [CrossRef]
- Hameed, F.; Bandral, J.D.; Gupta, N.; Nayik, G.A.; Sood, M.; Rahman, R. Use of Bacteriophages as A Target Specific Therapy Against Food-Borne Pathogens in Food Industry- A Review. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e2949. [Google Scholar] [CrossRef]
- Sebastianski, M.; Bridger, N.A.; Featherstone, R.M.; Robinson, J.L. Disease Outbreaks Linked to Pasteurized and Unpasteurized Dairy Products in Canada and the United States: A Systematic Review. Can. J. Public Health 2022, 113, 569–578. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, J.; Luo, L.; Hong, Y.; Li, X.; Zhu, Y.; Wu, Y.; Bai, L. Thermal Inactivation Kinetics of Listeria monocytogenes in Milk under Isothermal and Dynamic Conditions. Food Res. Int. 2024, 179, 114010. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Q.; Liu, Y.; Liu, H.; Zhang, H.; Wang, X. Systematic Review of Listeria monocytogenes from Food and Clinical Samples in Chinese Mainland from 2010 to 2019. Food Qual. Saf. 2022, 6, 753–758. [Google Scholar] [CrossRef]
- Schneider, G.; Steinbach, A.; Putics, Á.; Solti-Hodován, Á.; Palkovics, T. Potential of Essential Oils in the Control of Listeria monocytogenes. Microorganisms 2023, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Adhikari, A. Novel Approaches to Environmental Monitoring and Control of Listeria monocytogenes in Food Production Facilities. Foods 2022, 11, 1760. [Google Scholar] [CrossRef]
- Abad, I.; Pemán, L.; Pérez, M.D.; Grasa, L.; Sánchez, L. Does Lactoferrin, Free, Encapsulated or in Dairy Matrices, Maintain Its Antibacterial Activity after in Vitro Digestion? J. Funct. Foods 2024, 112, 105936. [Google Scholar] [CrossRef]
- Eghbal, N.; Viton, C.; Gharsallaoui, A. Nano and Microencapsulation of Bacteriocins for Food Applications: A Review. Food Biosci. 2022, 50, 102173. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, L.; Wang, H.; Hu, Z.; Dong, H.; Yu, Z.; Yuan, Y. A Review of Curcumin in Food Preservation: Delivery System and Photosensitization. Food Chem. 2023, 424, 136464. [Google Scholar] [CrossRef]
- Sibanda, T.; Ntuli, V.; Neetoo, S.H.; Habib, I.; Njage, P.M.K.; Parry-Hanson Kunadu, A.; Andoh, A.H.; Coorey, R.; Buys, E.M. Listeria monocytogenes at the Food–Human Interface: A Review of Risk Factors Influencing Transmission and Consumer Exposure in Africa. Int. J. Food Sci. Technol. 2023, 58, 4114–4126. [Google Scholar] [CrossRef]
- Basak, S.; Guillier, L.; Bect, J.; Christy, J.; Tenenhaus-Aziza, F.; Vazquez, E. Multipathogen Quantitative Risk Assessment in Raw Milk Soft Cheese. Microb. Risk Anal. 2024, 27–28, 100318. [Google Scholar] [CrossRef]
- Unger, P.; Sekhon, A.S.; Sharma, S.; Lampien, A.; Michael, M. Impact of Gas Ultrafine Bubbles on the Efficacy of Antimicrobials for Eliminating Fresh and Aged Listeria monocytogenes Biofilms on Dairy Processing Surfaces. J. Food Saf. 2023, 43, e13057. [Google Scholar] [CrossRef]
- Silva, B.N.; Teixeira, J.A.; Cadavez, V.; Gonzales-Barron, U. Mild Heat Treatment and Biopreservatives for Artisanal Raw Milk Cheeses: Reducing Microbial Spoilage and Extending Shelf-Life through Thermisation, Plant Extracts and Lactic Acid Bacteria. Foods 2023, 12, 3206. [Google Scholar] [CrossRef]
- Sung, H.J.; Kang, D.H. Effect of a 915 MHz Microwave System on Inactivation of Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes in Salsa. LWT 2014, 59, 754–759. [Google Scholar] [CrossRef]
- Awuah, G.B.; Ramaswamy, H.S.; Economides, A.; Mallikarjunan, K. Inactivation of Escherichia coli K-12 and Listeria innocua in Milk Using Radio Frequency (RF) Heating. Innov. Food Sci. Emerg. Technol. 2005, 6, 396–402. [Google Scholar] [CrossRef]
- Nefasa, A.N.; Christwardana, M.; Abdurrahman, Z.H.; Rohman, F.; Afif, A. A Mini Review on Technique of Milk Thermization. J. Bioresour. Environ. Sci. 2023, 2, 140–144. [Google Scholar] [CrossRef]
- Kelly, A.L.; Datta, N.; Deeth, H.C. Thermal Processing of Dairy Products. In Thermal Food Processing: New Technologies and Quality Issues; CRC Press: Boca Raton, FL, USA, 2012; p. 273. [Google Scholar]
- Muthuchamy, M.; Sheelamary, M.; Muthukumar, M. Effectiveness of Ozone in Inactivating Listeria monocytogenes from Milk Samples. J. Young Res. 2011, 1, 40–44. [Google Scholar]
- Bezie, A. The Effect of Different Heat Treatment on the Nutritional Value of Milk and Milk Products and Shelf-Life of Milk Products. A Review. J. Dairy Vet. Sci. 2019, 11, 555822. [Google Scholar] [CrossRef]
- Al-HilphyShirkole, A.R.S.; Ali, H.I.; Mohsin, G.F. Technology of Ohmic Heating for the Pasteurization of Milk for the Pasteurization of Milk; Apple Academic Press: Palm Bay, FL, USA, 2018. [Google Scholar]
- Rankin, S.A.; Bradley, R.L.; Miller, G.; Mildenhall, K.B. A 100-Year Review: A Century of Dairy Processing Advancements—Pasteurization, Cleaning and Sanitation, and Sanitary Equipment Design. J. Dairy Sci. 2017, 100, 9903–9915. [Google Scholar] [CrossRef]
- Lalwani, S.; Lewerentz, F.; Håkansson, A.; Löfgren, R.; Eriksson, J.; Paulsson, M.; Glantz, M. Impact of Thermal Processing on Micronutrients and Physical Stability of Milk and Cream at Dairy Production Scale. Int. Dairy J. 2024, 153, 105901. [Google Scholar] [CrossRef]
- Bhadania, A.G. Refrigeration Principles and Applications in the Dairy Industry; Apple Academic Press: Palm Bay, FL, USA, 2018. [Google Scholar]
- Marchi, B.; Bettoni, L.; Zanoni, S. Assessment of Energy Efficiency Measures in Food Cold Supply Chains: A Dairy Industry Case Study. Energies 2022, 15, 6901. [Google Scholar] [CrossRef]
- Guo, M.; Sheng, Z.; Wang, P.; Zhang, Y.; Zhang, X.; Zhang, Y.; Szeto, I.M.Y.; Wang, Y.; Ren, F.; Luo, J. Effects of Refrigerated Storage on the Functional Properties of Processed Cheese Analogue with Stretchability and Its Mechanisms. Int. Dairy J. 2023, 137, 105504. [Google Scholar] [CrossRef]
- Alinovi, M.; Mucchetti, G.; Wiking, L.; Corredig, M. Freezing as a Solution to Preserve the Quality of Dairy Products: The Case of Milk, Curds and Cheese. Crit. Rev. Food Sci. Nutr. 2021, 61, 3340–3360. [Google Scholar] [CrossRef]
- Prestes, A.A.; Helm, C.V.; Esmerino, E.A.; Silva, R.; da Cruz, A.G.; Prudencio, E.S. Freeze Concentration Techniques as Alternative Methods to Thermal Processing in Dairy Manufacturing: A Review. J. Food Sci. 2022, 87, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yu, Q.; Wu, W.; Dai, R. Inactivation of Microorganisms in Foods by Ohmic Heating: A Review. J. Food Prot. 2018, 81, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.O.; Guimarães, J.T.; Ramos, G.L.P.A.; do Prado-Silva, L.; Nascimento, J.S.; Sant’Ana, A.S.; Franco, R.M.; Cruz, A.G. Inactivation Kinetics of Listeria monocytogenes in Whey Dairy Beverage Processed with Ohmic Heating. LWT 2020, 127, 109420. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, S.; Samota, M.K. Lalremmawii Ohmic Heating Technology Systems, Factors Governing Efficiency and Its Application to Inactivation of Pathogenic Microbial, Enzyme Inactivation, and Extraction of Juice, Oil, and Bioactive Compounds in the Food Sector. Food Bioprocess Technol. 2024, 17, 299–324. [Google Scholar] [CrossRef]
- Roux, S.; Courel, M.; Birlouez-Aragon, I.; Municino, F.; Massa, M.; Pain, J.P. Comparative Thermal Impact of Two UHT Technologies, Continuous Ohmic Heating and Direct Steam Injection, on the Nutritional Properties of Liquid Infant Formula. J. Food Eng. 2016, 179, 36–43. [Google Scholar] [CrossRef]
- Obileke, K.C.; Onyeaka, H.; Miri, T.; Nwabor, O.F.; Hart, A.; Al-Sharify, Z.T.; Al-Najjar, S.; Anumudu, C. Recent Advances in Radio Frequency, Pulsed Light, and Cold Plasma Technologies for Food Safety. J. Food Process Eng. 2022, 45, e14138. [Google Scholar] [CrossRef]
- Tonti, M.; Verheyen, D.; Kozak, D.; Coombes, C.; Hossain, M.A.; Skåra, T.; Van Impe, J.F.M. Inactivation of Salmonella typhimurium and Listeria monocytogenes in Dairy Systems: Effect of Fat and Food Matrix Structure under Radio Frequency Heating. Innov. Food Sci. Emerg. Technol. 2024, 94, 103684. [Google Scholar] [CrossRef]
- Srisuma, C.; Santalunai, S.; Thosdeekoraphat, T.; Thongsopa, C. The Analysis and Design of Milk Pasteurization System by Using Radio Frequency Electric Fields. In Proceedings of the 2017 Asia-Pacific International Symposium on Electromagnetic Compatibility (APEMC), Seoul, Republic of Korea, 20–23 June 2017; pp. 158–160. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Bressan, F.; Leone, F.; Falqui, L.; Chiofalo, V. Radio Frequency Heating on Food of Animal Origin: A Review. Eur. Food Res. Technol. 2019, 245, 1787–1797. [Google Scholar] [CrossRef]
- Siefarth, C.; Tran, T.B.T.; Mittermaier, P.; Pfeiffer, T.; Buettner, A. Effect of Radio Frequency Heating on Yoghurt, i: Technological Applicability, Shelf-Life and Sensorial Quality. Foods 2014, 3, 318–335. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, W.; Jia, Y. Temperature-Dependent Dielectric Properties of Raw Cow’s and Goat’s Milk from 10 to 4500 MHz Relevant to Radio-Frequency and Microwave Pasteurization Process. Food Bioprocess Technol. 2014, 7, 1830–1839. [Google Scholar] [CrossRef]
- Chen, C.; Michael, M.; Phebus, R.K.; Thippareddi, H.; Subbiah, J.; Birla, S.L.; Schmidt, K.A. Short Communication: Radio Frequency Dielectric Heating of Nonfat Dry Milk Affects Solubility and Whey Protein Nitrogen Index. J. Dairy Sci. 2013, 96, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, Y.; Zheng, Y.; Zhu, H.; Liu, Z.; Jiao, S. Assessment of Radio Frequency Heating on Composition, Microstructure, Flowability and Rehydration Characteristics of Milk Powder. Food Sci. Technol. 2017, 37, 544–551. [Google Scholar] [CrossRef]
- Altemimi, A.; Aziz, S.N.; Al-Hilphy, A.R.S.; Lakhssassi, N.; Watson, D.G.; Ibrahim, S.A. Critical Review of Radio-Frequency (RF) Heating Applications in Food Processing. Food Qual. Saf. 2019, 3, 81–91. [Google Scholar] [CrossRef]
- Dag, D.; Singh, R.K.; Chen, J.; Mishra, A.; Kong, F. Radio Frequency Assisted Thermal Processing for Pasteurization of Packaged Whole Milk Powder Surrounded by Oil. Food Control 2022, 135, 108762. [Google Scholar] [CrossRef]
- Lin, Y.; Subbiah, J.; Chen, L.; Verma, T.; Liu, Y. Validation of Radio Frequency Assisted Traditional Thermal Processing for Pasteurization of Powdered Infant Formula Milk. Food Control 2020, 109, 106897. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, G.; Xie, Y.; Liu, Y. Effects of Radio Frequency on Physicochemical Properties of Powdered Infant Formula Milk as Compared with Conventional Thermal Treatment. LWT 2020, 134, 110194. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, P.; Zhu, L.; Liu, Y.; Mao, Y.; Luo, X.; Han, G.; Hao, J.; Zhang, Y. A Systematic Review and Meta-Analysis of the Prevalence of Listeria monocytogenes in Livestock and Poultry in China. Chin. J. Food Hyg. 2023, 35, 1120–1128. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Impact of High-Pressure Processing (HPP) on Listeria monocytogenes—An Overview of Challenges and Responses. Foods 2024, 13, 14. [Google Scholar] [CrossRef]
- Silva, F.V.M. Evelyn Pasteurization of Food and Beverages by High Pressure Processing (HPP) at Room Temperature: Inactivation of Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Salmonella, and Other Microbial Pathogens. Appl. Sci. 2023, 13, 1193. [Google Scholar] [CrossRef]
- Evert-Arriagada, K.; Trujillo, A.J.; Amador-Espejo, G.G.; Hernández-Herrero, M.M. High Pressure Processing Effect on Different Listeria Spp. in a Commercial Starter-Free Fresh Cheese. Food Microbiol. 2018, 76, 481–486. [Google Scholar] [CrossRef]
- Shahein, M.H.; Amr, A.S.; Sadder, M.; Al-Qadiri, H.M.; Albawarshi, Y.; Al-khamaiseh, A.M.; Kanaan, O. Lethality of High Hydrostatic Pressure Processing on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli in Low Salt White Brined Cheese: D-Value. Int. Dairy J. 2023, 143, 105675. [Google Scholar] [CrossRef]
- Ozaybi, N. High-Pressure Processing of Milk and Dairy Products: Latest Update. Processes 2024, 12, 2073. [Google Scholar] [CrossRef]
- Machado, F.; Duarte, R.V.; Pinto, C.A.; Casal, S.; Lopes-da-Silva, J.A.; Saraiva, J.A. High Pressure and Pasteurization Effects on Dairy Cream †. Foods 2023, 12, 3640. [Google Scholar] [CrossRef]
- Lauteri, C.; Ferri, G.; Piccinini, A.; Pennisi, L.; Vergara, A. Ultrasound Technology as Inactivation Method for Foodborne Pathogens: A Review. Foods 2023, 12, 1212. [Google Scholar] [CrossRef]
- Alves de Aguiar Bernardo, Y.; Kaic Alves do Rosario, D.; Adam Conte-Junior, C. Ultrasound on Milk Decontamination: Potential and Limitations Against Foodborne Pathogens and Spoilage Bacteria. Food Rev. Int. 2023, 39, 320–333. [Google Scholar] [CrossRef]
- Cho, E.R.; Kang, D.H. Development and Investigation of Ultrasound-Assisted Pulsed Ohmic Heating for Inactivation of Foodborne Pathogens in Milk with Different Fat Content. Food Res. Int. 2024, 179, 113978. [Google Scholar] [CrossRef]
- Bansal, V.; Veena, N. Understanding the Role of PH in Cheese Manufacturing: General Aspects of Cheese Quality and Safety. J. Food Sci. Technol. 2024, 61, 16–26. [Google Scholar] [CrossRef]
- Moatsou, G. Emerging Technologies for Improving Properties, Shelf Life, and Analysis of Dairy Products. Foods 2024, 13, 1078. [Google Scholar] [CrossRef]
- Vashisht, P.; Singh, L.; Mahanta, S.; Verma, D.; Sharma, S.; Saini, G.S.; Sharma, A.; Chowdhury, B.; Awasti, N.; Gaurav; et al. Pulsed Electric Field Processing in the Dairy Sector: A Review of Applications, Quality Impact and Implementation Challenges. Int. J. Food Sci. Technol. 2024, 59, 2122–2135. [Google Scholar] [CrossRef]
- Vidovic, S.; Paturi, G.; Gupta, S.; Fletcher, G.C. Lifestyle of Listeria monocytogenes and Food Safety: Emerging Listericidal Technologies in the Food Industry. Crit. Rev. Food Sci. Nutr. 2024, 64, 1817–1835. [Google Scholar] [CrossRef]
- Kaavya, R.; Rajasekaran, B.; Shah, K.; Nickhil, C.; Palanisamy, S.; Palamae, S.; Chandra Khanashyam, A.; Pandiselvam, R.; Benjakul, S.; Thorakattu, P.; et al. Radical Species Generating Technologies for Decontamination of Listeria Species in Food: A Recent Review Report. Crit. Rev. Food Sci. Nutr. 2025, 65, 1974–1998. [Google Scholar] [CrossRef] [PubMed]
- Santiesteban-López, N.A.; Gómez-Salazar, J.A.; Santos, E.M.; Campagnol, P.C.B.; Teixeira, A.; Lorenzo, J.M.; Sosa-Morales, M.E.; Domínguez, R. Natural Antimicrobials: A Clean Label Strategy to Improve the Shelf Life and Safety of Reformulated Meat Products. Foods 2022, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, P.; Verma, D.; Singh, L.; Saini, G.S.; Sharma, S.; Charles, A.P.R.; Mahanta, S.; Mahanta, S.; Singh, K.; Gaurav, G.; et al. Ozone Processing of Milk and Milk Products: A Review of Applications, Quality Effect and Implementation Challenges. Int. J. Food Eng. 2024, 20, 669–680. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria Monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Xue, W.; Macleod, J.; Blaxland, J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods 2023, 12, 814. [Google Scholar] [CrossRef]
- Cavalcante, M.A.; Leite Júnior, B.R.C.; Tribst, A.A.L.; Cristianini, M. Improvement of the Raw Milk Microbiological Quality by Ozone Treatment. Int. Food Res. J. 2013, 20, 2017. [Google Scholar]
- Morandi, S.; Silvetti, T.; Vezzini, V.; Morozzo, E.; Brasca, M. How We Can Improve the Antimicrobial Performances of Lactic Acid Bacteria? A New Strategy to Control Listeria monocytogenes in Gorgonzola Cheese. Food Microbiol. 2020, 90, 103488. [Google Scholar] [CrossRef]
- Lee, G.M.; Shin, J.K. Nonthermal Sterilization of Animal-Based Foods by Intense Pulsed Light Treatment. Food Sci. Anim. Resour. 2024, 44, 309–325. [Google Scholar] [CrossRef]
- Reena; Kumar, A. Thermal and Non- Thermal Treatment of Milk—A Review. Int. Res. J. Eng. Technol. 2021, 8, 2330–2332. [Google Scholar]
- de Miranda, N.M.Z.; de Souza, A.C.; de Souza Costa Sobrinho, P.; Dias, D.R.; Schwan, R.F.; Ramos, C.L. Novel Yeasts with Potential Probiotic Characteristics Isolated from the Endogenous Ferment of Artisanal Minas Cheese. Braz. J. Microbiol. 2023, 54, 1021–1033. [Google Scholar] [CrossRef]
- Yang, X.; Peng, Z.; He, M.; Li, Z.; Fu, G.; Li, S.; Zhang, J. Screening, Probiotic Properties, and Inhibition Mechanism of a Lactobacillus antagonistic to Listeria monocytogenes. Sci. Total Environ. 2024, 906, 167587. [Google Scholar] [CrossRef]
- Rangel-Ortega, S.d.C.; Campos-Múzquiz, L.G.; Charles-Rodriguez, A.V.; Chávez-Gonzaléz, M.L.; Palomo-Ligas, L.; Contreras-Esquivel, J.C.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Biological Control of Pathogens in Artisanal Cheeses. Int. Dairy J. 2023, 140, 105612. [Google Scholar] [CrossRef]
- Anjana; Tiwari, S.K. Bacteriocin-Producing Probiotic Lactic Acid Bacteria in Controlling Dysbiosis of the Gut Microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 851140. [Google Scholar] [CrossRef]
- Assari, F.; Mojgani, N.; Sanjabi, M.; Mirdamadi, S.; Jahandar, H. Technological Assessment of Autochthonous Lactic Acid Bacteria and Their Antibacterial Activities Against Foodborne Pathogens in Goat Milk Lactic Cheese. Appl. Food Biotechnol. 2023, 10, 61–71. [Google Scholar] [CrossRef]
- Webb, L.; Ma, L.; Lu, X. Impact of Lactic Acid Bacteria on the Control of Listeria monocytogenes in Ready-to-Eat Foods. Food Qual. Saf. 2022, 6, fyac045. [Google Scholar] [CrossRef]
- Ahansaz, N.; Tarrah, A.; Pakroo, S.; Corich, V.; Giacomini, A. Lactic Acid Bacteria in Dairy Foods: Prime Sources of Antimicrobial Compounds. Fermentation 2023, 9, 1–21. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Viale, S.; Deplano, M.; Mereu, F.; Blažić, M.; Cosentino, S. Inhibitory Effect of Lactiplantibacillus plantarum and Lactococcus lactis Autochtonous Strains against Listeria monocytogenes in a Laboratory Cheese Model. Foods 2022, 11, 715. [Google Scholar] [CrossRef]
- Panebianco, F.; Rubiola, S.; Buttieri, C.; Di Ciccio, P.A.; Chiesa, F.; Civera, T. Understanding the Effect of Ozone on Listeria monocytogenes and Resident Microbiota of Gorgonzola Cheese Surface: A Culturomic Approach. Foods 2022, 11, 2640. [Google Scholar] [CrossRef]
- Benkirane, G.; Ananou, S.; Dumas, E.; Ghnimi, S.; Gharsallaoui, A. Moroccan Traditional Fermented Dairy Products: Current Processing Practices and Physicochemical and Microbiological Properties—A Review. J. Microbiol. Biotechnol. Food Sci. 2022, 12, 5636. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Córdoba, J.J. Application of Selected Lactic-Acid Bacteria to Control Listeria monocytogenes in Soft-Ripened “Torta Del Casar” Cheese. LWT 2022, 168, 113873. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Bucur, F.I.; Mihalache, O.A.; Nicolau, A.I. Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products. Foods 2024, 13, 734. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Doyle, M.P.; Zhao, P. Control of Listeria monocytogenes in a Biofilm by Competitive-Exclusion Microorganisms. Appl. Environ. Microbiol. 2004, 70, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.M.; Song, J.H.; Vasquez, R.; Hwang, I.C.; Lee, J.S.; Kang, D.K. Characterization of Novel Amylase-Sensitive, Anti-Listerial Class IId Bacteriocin, Agilicin C7 Produced by Ligilactobacillus Agilis C7. Food Sci. Anim. Resour. 2023, 43, 625–638. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Noshad, M.; Vasiee, A.; Brück, W.M. Probiotic Bacillus Strains Inhibit Growth, Biofilm Formation, and Virulence Gene Expression of Listeria monocytogenes. LWT 2024, 191, 115596. [Google Scholar] [CrossRef]
- Wu, M.; Dong, Q.; Ma, Y.; Yang, S.; Zohaib Aslam, M.; Liu, Y.; Li, Z. Potential Antimicrobial Activities of Probiotics and Their Derivatives against Listeria monocytogenes in Food Field: A Review. Food Res. Int. 2022, 160, 111733. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Rahmati-Joneidabad, M.; Taki, M. Examining the Impact of Probiotic Lactiplantibacillus pentosus 6MMI on Inhibiting Biofilm Formation, Adhesion, and Virulence Gene Expression in Listeria monocytogenes ATCC 19115. Biofilm 2025, 9, 100255. [Google Scholar] [CrossRef]
- Ewida, R.M.; Hasan, W.S.; Elfaruk, M.S.; Alayouni, R.R.; Hammam, A.R.A.; Kamel, D.G. Occurrence of Listeria Spp. in Soft Cheese and Ice Cream: Effect of Probiotic Bifidobacterium Spp. on Survival of Listeria monocytogenes in Soft Cheese. Foods 2022, 11, 3443. [Google Scholar] [CrossRef]
- Zavišić, G.; Ristić, S.; Petričević, S.; Janković, D.; Petković, B. Microbial Contamination of Food: Probiotics and Postbiotics as Potential Biopreservatives. Foods 2024, 13, 2487. [Google Scholar] [CrossRef]
- Rouhi, A.; Falah, F.; Azghandi, M.; Alizadeh Behbahani, B.; Mortazavi, S.A.; Tabatabaei-Yazdi, F.; Vasiee, A. Investigating the Effect of Lactiplantibacillus plantarum TW57-4 in Preventing Biofilm Formation and Expression of Virulence Genes in Listeria monocytogenes ATCC 19115. LWT 2024, 191, 115669. [Google Scholar] [CrossRef]
- Abou Elez, R.M.M.; Elsohaby, I.; Al-Mohammadi, A.R.; Seliem, M.; Tahoun, A.B.M.B.; Abousaty, A.I.; Algendy, R.M.; Mohamed, E.A.A.; El-Gazzar, N. Antibacterial and Anti-Biofilm Activities of Probiotic Lactobacillus plantarum against Listeria monocytogenes Isolated from Milk, Chicken and Pregnant Women. Front. Microbiol. 2023, 14, 1201201. [Google Scholar] [CrossRef]
- Prezzi, L.E.; Lee, S.H.I.; Nunes, V.M.R.; Corassin, C.H.; Pimentel, T.C.; Rocha, R.S.; Ramos, G.L.P.A.; Guimarães, J.T.; Balthazar, C.F.; Duarte, M.C.K.H.; et al. Effect of Lactobacillus rhamnosus on Growth of Listeria monocytogenes and Staphylococcus aureus in a Probiotic Minas Frescal Cheese. Food Microbiol. 2020, 92, 103557. [Google Scholar] [CrossRef] [PubMed]
- Van Gijtenbeek, L.A.; Singer, Q.; Steffensen, L.E.; Neuens, S.; Guldager, H.S.; Bidstrup, S.; Høgholm, T.; Madsen, M.G.; Glass, K.; Siedler, S. Lacticaseibacillus rhamnosus Impedes Growth of Listeria spp. in Cottage Cheese through Manganese Limitation. Foods 2021, 10, 1353. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Maktabdar, M. Lactic Acid Bacteria as Biopreservation Against Spoilage Molds in Dairy Products—A Review. Front. Microbiol. 2022, 12, 819684. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; de Almeida, F.A.; Medeiros, M.M.; Miranda, B.R.; Pinto, U.M.; Alves, V.F. Listeria monocytogenes: An Inconvenient Hurdle for the Dairy Industry. Dairy 2023, 4, 316–344. [Google Scholar] [CrossRef]
- Nikravan, L.; Zamanpour, S.; Noori, S.M.A. Postbiotics: An Innovative Approach to Increase Shelf Life and Quality of Foods. Nutr. Food Sci. 2024, 54, 192–206. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Sarkar, T.; Pati, S.; Basu, D.; Abdul Kari, Z.; Wei, L.S.; Smaoui, S.; Wen Goh, K.; et al. Bacteriocin: A Natural Approach for Food Safety and Food Security. Front. Bioeng. Biotechnol. 2022, 10, 1005918. [Google Scholar] [CrossRef]
- Divsalar, E.; Tajik, H.; Moradi, M.; Forough, M.; Lotfi, M.; Kuswandi, B. Characterization of Cellulosic Paper Coated with Chitosan-Zinc Oxide Nanocomposite Containing Nisin and Its Application in Packaging of UF Cheese. Int. J. Biol. Macromol. 2018, 109, 1311–1318. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Abbasi, A.; Sabahi, S.; Khani, N. Application of Postbiotics Produced by Lactic Acid Bacteria in the Development of Active Food Packaging. Biointerface Res. Appl. Chem. 2022, 12, 6164–6183. [Google Scholar] [CrossRef]
- Lenaerts, L.; Passos, T.F.; Gayán, E.; Michiels, C.W.; Nitschke, M. Hurdle Technology Approach to Control Listeria monocytogenes Using Rhamnolipid Biosurfactant. Foods 2023, 12, 570. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Yang, L.Y.; Ying, J.P.; Fu, C.M.; Wu, G.; Li, X.R.; Zhang, Q.L. A Novel Bacteriocin RSQ01 with Antibacterial Activity and Its Application and Metabolomic Mechanism in Milk Preservation. Food Control 2023, 151, 109823. [Google Scholar] [CrossRef]
- Silva, L.D.; Naves, E.A.A.; Gelamo, R.V.; Rubens, G.; Coutinho Filho, U. Clean in Place (CIP) Process: Effects of Geometry, Microorganism, Fluid Dynamic and Cold Plasma. J. Food Eng. 2024, 377, 112081. [Google Scholar] [CrossRef]
- El-Sayed, A.I.; El-Sayed, I.M.; Awad, S. Bacteriocins: Nisin as an Alternative Source to Chemical Preservatives. In Natural Food Preservatives; Jenny Stanford Publishing: Singapore, 2023; p. 35. [Google Scholar]
- Amenu, D.; Bacha, K. Bio-Preservation Potential and Antimicrobial Activity of Bacteriocin-Producing Lactic Acid Bacteria Isolated from Ethiopian Traditional Fermented Dairy Products. Probiotics Antimicrob. Proteins 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, A.R.; Ibarra-Sánchez, L.A.; Amaya-Llano, S.L.; Miller, M.J. Evaluation of Combinations of Nisin, Lauric Arginate, and ε-Polylysine to Control Listeria monocytogenes in Queso Fresco. J. Dairy Sci. 2020, 103, 11152–11162. [Google Scholar] [CrossRef] [PubMed]
- Charest, A.M.; Reed, E.; Bozorgzadeh, S.; Hernandez, L.; Getsey, N.V.; Smith, L.; Galperina, A.; Beauregard, H.E.; Charest, H.A.; Mitchell, M.; et al. Nisin Inhibition of Gram-Negative Bacteria. Microorganisms 2024, 12, 1230. [Google Scholar] [CrossRef]
- Tang, D.S.; Yin, G.M.; He, Y.Z.; Hu, S.Q.; Li, B.; Li, L.; Liang, H.L.; Borthakur, D. Recovery of Protein from Brewer’s Spent Grain by Ultrafiltration. Biochem. Eng. J. 2009, 48, 1–5. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Langa, S.; Landete, J.M.; Medina, M. Antimicrobial Activity of Lactic Acid Bacteria in Dairy Products and Gut: Effect on Pathogens. Biomed Res. Int. 2015, 2015, 584183. [Google Scholar] [CrossRef]
- Angelidis, A.S.; Grammenou, A.S.; Kotzamanidis, C.; Giadinis, N.D.; Zdragas, A.G.; Sergelidis, D. Prevalence, Serotypes, Antimicrobial Resistance and Biofilm-Forming Ability of Listeria monocytogenes Isolated from Bulk-Tank Bovine Milk in Northern Greece. Pathogens 2023, 12, 837. [Google Scholar] [CrossRef]
- Rendueles, C.; Duarte, A.C.; Escobedo, S.; Fernández, L.; Rodríguez, A.; García, P.; Martínez, B. Combined Use of Bacteriocins and Bacteriophages as Food Biopreservatives. A Review. Int. J. Food Microbiol. 2022, 368, 109611. [Google Scholar] [CrossRef]
- Shafique, B.; Ranjha, M.M.A.N.; Murtaza, M.A.; Walayat, N.; Nawaz, A.; Khalid, W.; Mahmood, S.; Nadeem, M.; Manzoor, M.F.; Ameer, K.; et al. Recent Trends and Applications of Nanoencapsulated Bacteriocins against Microbes in Food Quality and Safety. Microorganisms 2023, 11, 85. [Google Scholar] [CrossRef]
- Todorov, S.D.; Popov, I.; Weeks, R.; Chikindas, M.L. Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns. Foods 2022, 11, 3145. [Google Scholar] [CrossRef]
- Garmasheva, I.L.; Oleschenko, L.T. Screening of Bacteriocin-Producing Dairy Enterococcus Strains Using Low-Cost Culture Media. Front. Microbiol. 2023, 14, 1168835. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Xu, M.; Xia, R.; Zhou, Z.; Han, Y. Antimicrobial Mechanism of Recombinant Enterocin CHQS on Listeria monocytogenes and Its Application on Pasteurized Milk. Food Control 2024, 159, 110271. [Google Scholar] [CrossRef]
- Moghimani, M.; Salari, A.; Hashemi, M.; Soleimanpour, S.; Ranjbar, G.; Afshari, A. Iranian Traditional Kefir Beverage: Isolation and Identification of Beneficial Microorganisms and Evaluation of Antimicrobial Activity against Food-Borne Pathogens. Nutr. Food Sci. 2023, 53, 1257–1267. [Google Scholar] [CrossRef]
- Romero-Calle, D.X.; de Santana, V.P.; Benevides, R.G.; Aliaga, M.T.A.; Billington, C.; Góes-Neto, A. Systematic Review and Meta-Analysis: The Efficiency of Bacteriophages Previously Patented against Pathogenic Bacteria on Food. Syst. Rev. 2023, 12, 201. [Google Scholar] [CrossRef]
- Vikram, A.; Callahan, M.T.; Woolston, J.W.; Sharma, M.; Sulakvelidze, A. Phage Biocontrol for Reducing Bacterial Foodborne Pathogens in Produce and Other Foods. Curr. Opin. Biotechnol. 2022, 78, 102805. [Google Scholar] [CrossRef]
- Osek, J.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Uses Its Virulence Mechanisms to Infect the Hosts. Pathogens 2022, 11, 1491. [Google Scholar] [CrossRef]
- Fernandes, A.; Jobby, R. Bacteriocins from Lactic Acid Bacteria and Their Potential Clinical Applications. Appl. Biochem. Biotechnol. 2022, 194, 4377–4399. [Google Scholar] [CrossRef]
- Zia, S.; Alkheraije, K.A. Recent Trends in the Use of Bacteriophages as Replacement of Antimicrobials against Food-Animal Pathogens. Front. Vet. Sci. 2023, 10, 1162465. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Choi, D.; Bedale, W.; Chetty, S.; Yu, J.H. Comprehensive Review of Clean-Label Antimicrobials Used in Dairy Products. Compr. Rev. Food Sci. Food Saf. 2024, 23, 13263. [Google Scholar] [CrossRef]
- Li, Y.-x.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial Mechanisms of Spice Essential Oils and Application in Food Industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef] [PubMed]
- Salanță, L.C.; Cropotova, J. An Update on Effectiveness and Practicability of Plant Essential Oils in the Food Industry. Plants 2022, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.N.R.; Ferreira, C.D. Essential Oil for the Control of Fungi, Bacteria, Yeasts and Viruses in Food: An Overview. Crit. Rev. Food Sci. Nutr. 2023, 63, 8960–8974. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, J.B.; Garner, C.M. A Review of Essential Oils as Antimicrobials in Foods with Special Emphasis on Fresh Produce. J. Food Prot. 2022, 85, 1300–1319. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.E.; Gharsallaoui, A.; Dumas, E.; Ismail, A.; Karam, L. Essential Oils and Their Active Components Applied as: Free, Encapsulated and in Hurdle Technology to Fight Microbial Contaminations. A Review. Heliyon 2022, 8, e12472. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, F.; Ge, J.; Li, H.; Xia, F.; Bai, H.; Piao, X.; Shi, L. Potential of Aromatic Plant-Derived Essential Oils for the Control of Foodborne Bacteria and Antibiotic Resistance in Animal Production: A Review. Antibiotics 2022, 11, 1673. [Google Scholar] [CrossRef]
- El-Aziz, M.A.; Salama, H.H.; Sayed, R.S. Plant Extracts and Essential Oils in the Dairy Industry: A Review. Foods Raw Mater. 2023, 11, 321–337. [Google Scholar] [CrossRef]
- Jannat, B.; Mirza Alizadeh, A.; Farshi, P.; Dadgarnejad, M.; Hosseini, H.; Hashempour-Baltork, F.; Jafari, S.M. Anti-Biofilm Activity of Essential Oils in Fruit and Vegetable: A Systematic Review. Food Control 2023, 152, 109875. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 26, 100025. [Google Scholar] [CrossRef]
- Coimbra, A.; Ferreira, S.; Duarte, A.P. Biological Properties of Thymus zygis Essential Oil with Emphasis on Antimicrobial Activity and Food Application. Food Chem. 2022, 393, 133370. [Google Scholar] [CrossRef]
- Manju, G.; Grover, C.R. Assessment of Components of Essential Oils for Antimicrobial Activity in the Dairy Food Matrix. Indian J. Dairy. Sci. 2023, 76, 252–258. [Google Scholar]
- Maggio, F.; Serio, A.; Rossi, C.; Purgatorio, C.; Buccioni, F.; Chaves-López, C.; Paparella, A. Effectiveness of Essential Oils against Dual-Species Biofilm of Listeria monocytogenes and Pseudomonas fluorescens in a Ricotta-Based Model System. Ital. J. Food Saf. 2023, 12, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Pasha, I.; Jahangir Chughtai, M.F.; Ali, Z.; Bukhari, H.; Zuhair, M. Application of Essential Oils in Food Industry: Challenges and Innovation. J. Essent. Oil Res. 2022, 34, 97–110. [Google Scholar] [CrossRef]
- Sharafi, H.; Moradi, M.; Sharafi, K. A Systematic Review and Meta-Analysis of the Use of Plant Essential Oils and Extracts in the Development of Antimicrobial Edible Films for Dairy Application. Vet. Res. Forum 2023, 14, 179–194. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2022, 11, 20. [Google Scholar] [CrossRef]
- Moosavy, M.H.; Esmaeili, S.; Mostafavi, E. Antibacterial Effect of Mentha Spicata Essential Oil on Listeria monocytogenes in Traditional Lighvan Cheese. J. Food Saf. 2013, 33, 509–514. [Google Scholar] [CrossRef]
- Trajano, V.N.; Lima, E.d.O.; de Souza, E.L.; Travassos, A.E.R. Inhibitory Effect of the Essential Oil from Eugenia caryophyllata Thumb Leaves on Coalho Cheese Contaminating Microorganisms. Cienc. e Tecnol. Aliment. 2010, 30, 1001–1006. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Li, C.; Abdel-Samie, M.A.; Lin, L. Inhibition Effect of Moringa Oil on the Cheese Preservation and Its Impact on the Viability, Virulence and Genes Expression of Listeria monocytogenes. LWT 2020, 134, 110163. [Google Scholar] [CrossRef]
- Sharma, K.; Babaei, A.; Oberoi, K.; Aayush, K.; Sharma, R.; Sharma, S. Essential Oil Nanoemulsion Edible Coating in Food Industry: A Review. Food Bioprocess Technol. 2022, 15, 2375–2395. [Google Scholar] [CrossRef]
- Bukvicki, D.; D’Alessandro, M.; Rossi, S.; Siroli, L.; Gottardi, D.; Braschi, G.; Patrignani, F.; Lanciotti, R. Essential Oils and Their Combination with Lactic Acid Bacteria and Bacteriocins to Improve the Safety and Shelf Life of Foods: A Review. Foods 2023, 12, 3288. [Google Scholar] [CrossRef]
- Rubiño, S.; Aymerich, T.; Peteiro, C.; Bover-Cid, S.; Hortós, M. Antimicrobial Potential of Ericaria selaginoides Extracts against Listeria monocytogenes in “Mató”, a Catalan Fresh Cheese. J. Appl. Phycol. 2023, 35, 949–959. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Nieto, G.; Kumar, M.; Dhama, K.; Lorenzo, J.M. Bioactive Compounds from Fruits as Preservatives. Foods 2023, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Pintado, C.M.B.S.; Ferreira, M.A.S.S.; Sousa, I. Properties of Whey Protein-Based Films Containing Organic Acids and Nisin to Control Listeria monocytogenes. J. Food Prot. 2009, 72, 1891–1896. [Google Scholar] [CrossRef]
- Delgado, J.; Álvarez, M.; Cebrián, E.; Martín, I.; Roncero, E.; Rodríguez, M. Biocontrol of Pathogen Microorganisms in Ripened Foods of Animal Origin. Microorganisms 2023, 11, 1578. [Google Scholar] [CrossRef]
- van Nassau, T.J.; Lenz, C.A.; Scherzinger, A.S.; Vogel, R.F. Combination of Endolysins and High Pressure to Inactivate Listeria monocytogenes. Food Microbiol. 2017, 68, 81–88. [Google Scholar] [CrossRef]
- Louhichi, M. Effet de L’irradiation L’irradiatio n Sur La Texture d’un Fromage à Pâte Molle de Type Camembert; ECOLE SUPE; Tunisienne, Repub. Aliment. E N. Ind.: Tunis, Tunisia, 2008. [Google Scholar]
- Velasco, R.; Ordóñez, J.A.; Cabeza, M.C.; de la Hoz, L.; Cambero, M.I. Use of the E-Beam Radiation to Diminish the Late Blowing of Cheese. Int. Dairy J. 2011, 21, 493–500. [Google Scholar] [CrossRef]
- Velasco, R.; Cambero, M.I.; Ordóñez, J.A.; Cabeza, M.C. The Impact of E-Beam Treatment on the Microbial Population and Sensory Quality of Hard Annatto-Coloured Cheese. LWT 2019, 101, 315–322. [Google Scholar] [CrossRef]
- Velasco, R.; Ordóñez, J.A.; Cabeza, M.C.; Cambero, M.I. Effect of E-Beam Sanitation of Surface Mould Cheese on Texture and Sensory Attributes. LWT 2016, 70, 1–8. [Google Scholar] [CrossRef]
- Hosken, B.d.O.; Melo Pereira, G.V.; Lima, T.T.M.; Ribeiro, J.B.; de Magalhães Júnior, W.C.P.; Martin, J.G.P. Underexplored Potential of Lactic Acid Bacteria Associated with Artisanal Cheese Making in Brazil: Challenges and Opportunities. Fermentation 2023, 9, 409. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, X.; Franklin, T.; Yang, R.; Moraru, C.I. Mechano-Bactericidal Surfaces: Mechanisms, Nanofabrication, and Prospects for Food Applications. Annu. Rev. Food Sci. Technol. 2023, 14, 449–472. [Google Scholar] [CrossRef]
- Grujović, M.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and Disadvantages of Non-Starter Lactic Acid Bacteria from Traditional Fermented Foods: Potential Use as Starters or Probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, N.; Liao, W.; Dumas, E.; Azabou, S.; Dantigny, P.; Gharsallaoui, A. Microencapsulation of Natural Food Antimicrobials: Methods and Applications. Appl. Sci. 2022, 12, 3837. [Google Scholar] [CrossRef]
- Aleksic, B.; Udovicki, B.; Kovacevic, J.; Miloradovic, Z.; Djekic, I.; Miocinovic, J.; Tomic, N.; Smigic, N. Microbiological Assessment of Dairy Products Produced by Small-Scale Dairy Producers in Serbia. Foods 2024, 13, 1456. [Google Scholar] [CrossRef]
- Hu, M.; Dong, Q.; Liu, Y.; Sun, T.; Gu, M.; Zhu, H.; Xia, X.; Li, Z.; Wang, X.; Ma, Y.; et al. A Meta-Analysis and Systematic Review of Listeria monocytogenes Response to Sanitizer Treatments. Foods 2023, 12, 154. [Google Scholar] [CrossRef]
- Bashiry, M.; Javanmardi, F.; Taslikh, M.; Sheidaei, Z.; Sadeghi, E.; Abedi, A.S.; Alizadeh, A.M.; Hashempour-Baltork, F.; Beikzadeh, S.; Riahi, S.M.; et al. Listeria monocytogenes in Dairy Products of the Middle East Region: A Systematic Review, Meta-Analysis, and Meta-Regression Study. Iran. J. Public Health 2022, 51, 292–305. [Google Scholar] [CrossRef]
- Bevilacqua, A.; De Santis, A.; Sollazzo, G.; Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R. Microbiological Risk Assessment in Foods: Background and Tools, with a Focus on Risk Ranger. Foods 2023, 12, 1483. [Google Scholar] [CrossRef]
- Bolten, S.; Lott, T.T.; Ralyea, R.D.; Gianforte, A.; Trmcic, A.; Orsi, R.H.; Martin, N.H.; Wiedmann, M. Intensive Environmental Sampling and Whole Genome Sequence-Based Characterization of Listeria in Small- and Medium-Sized Dairy Facilities Reveal Opportunities for Simplified and Size-Appropriate Environmental Monitoring Strategies. J. Food Prot. 2024, 87, 100254. [Google Scholar] [CrossRef]
- Aleksic, B.; Djekic, I.; Miocinovic, J.; Miloradovic, Z.; Savic-Radovanovic, R.; Zdravkovic, N.; Smigic, N. The Hygienic Assessment of Dairy Products’ Selling Places at Open Markets. Food Control 2023, 148, 109628. [Google Scholar] [CrossRef]
- Bland, R.; Brown, S.R.B.; Waite-Cusic, J.; Kovacevic, J. Probing Antimicrobial Resistance and Sanitizer Tolerance Themes and Their Implications for the Food Industry through the Listeria monocytogenes Lens. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1777–1802. [Google Scholar] [CrossRef]
- Trinetta, V.; Linton, R.H.; Morgan, M.T. The Application of High-Concentration Short-Time Chlorine Dioxide Treatment for Selected Specialty Crops Including Roma Tomatoes (Lycopersicon esculentum), Cantaloupes (Cucumis melo Ssp. Melo Var. Cantaloupensis) and Strawberries (Fragaria ananassa). Food Microbiol. 2013, 34, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Chhetri, V.S.; Janes, M.E.; King, J.M.; Adhikari, A. Efficacy of Gaseous Chlorine Dioxide in Reducing Salmonella enterica, E. coli O157:H7, and Listeria monocytogenes on Strawberries and Blueberries. LWT 2021, 141, 110906. [Google Scholar] [CrossRef]
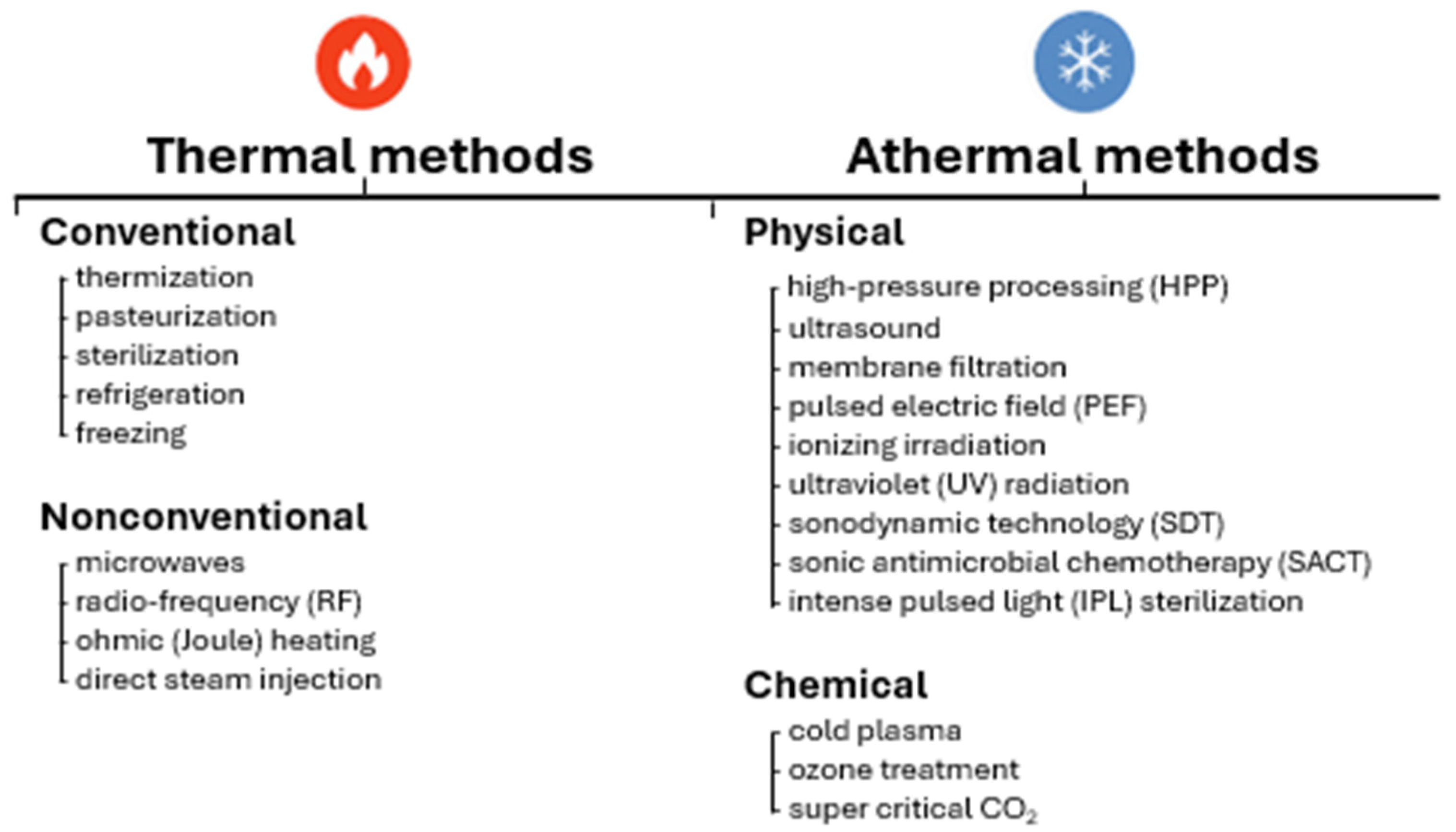
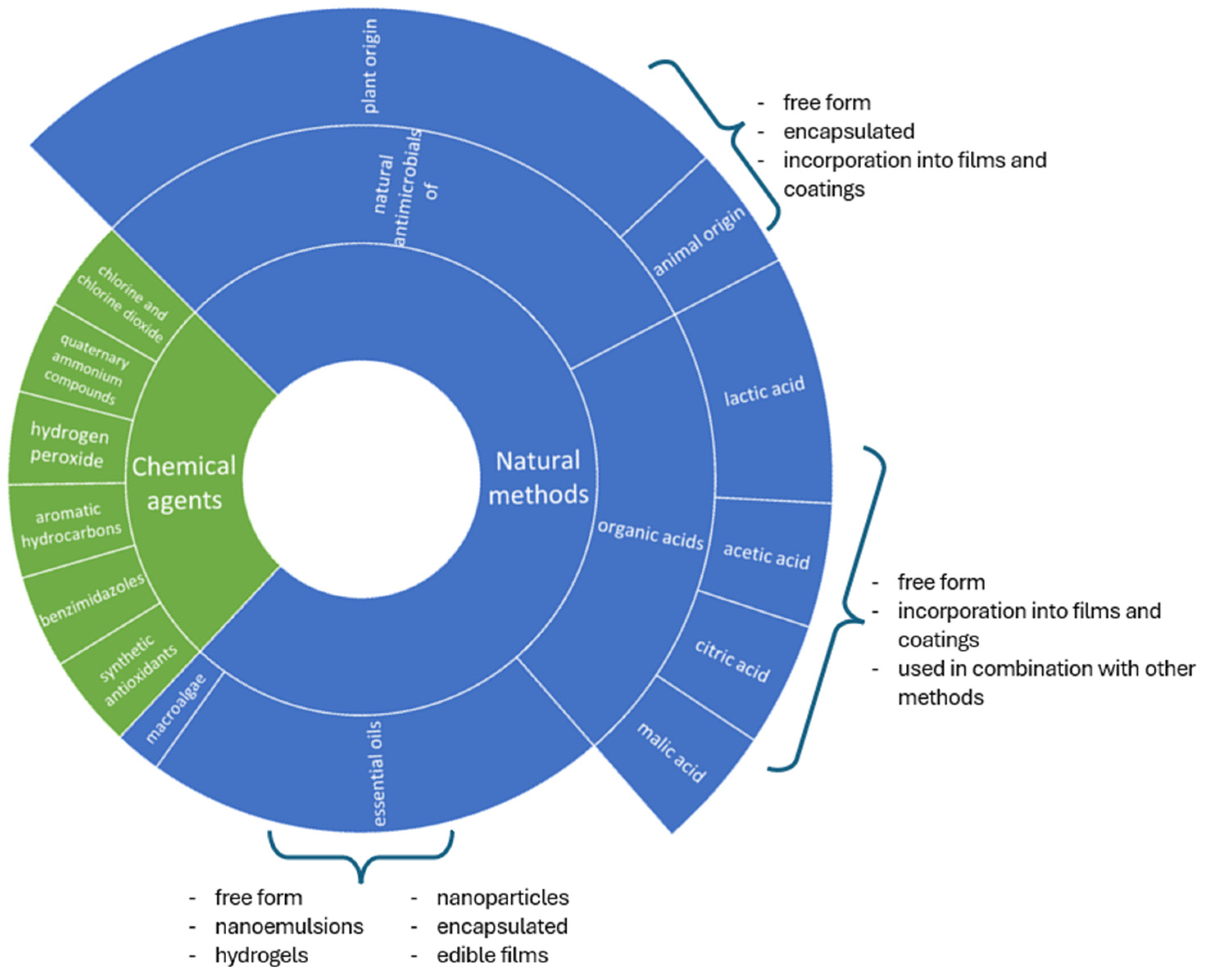
| Method | Description | Product | Reference |
|---|---|---|---|
| Thermalization | Preheating procedure in which milk is heated at low temperatures for a brief time prior to further processing: 57–68 °C /10–20 s | milk | [33] |
| Heat treatment of milk at 57–68 °C /15 s | cheese | [34] | |
| Heat treatment of sheep milk at 62 °C /20 s or 68 °C/20 s | cheese | [34] | |
| Pasteurization | Pasteurization LTLT (low temperature, long time)—the process of heating every particle of milk to at least 63 °C/30 min. Pasteurization HTST (high temperature, short time)—the process of heating every particle of milk to at least 72 °C/15 s Vacuum pasteurization—90.5–96.1 °C under vacuum pressure 62.8 °C/30 min. or 71.7 °C/15 s | milk | [35,36,37,38] |
| 65.6 °C/30 min. or 74.4 °C/15 s | cream | [38] | |
| 68.3 °C/30 min. or 79.4 °C/25 s | ice cream mix | [38] | |
| Milk before fermentation: 90–95 °C/ 5–10 min. Milk before fermentation: 85–95 °C/15 min. | yogurt | [36,39] | |
| Heat treatment of milk at 63 °C /30 min. | soft cheese | [36] | |
| Heat treatment of milk at 72 °C, no hold | semi-hard cheese | [36] | |
| Heat treatment of milk at 64–68 °C/10 s | hard cheese | [36] | |
| Heat treatment of cream at 65 °C /10 min. | butter | [36] | |
| Sterilization | Conventional method: Packaging is undertaken before heat treatment. The processing is usually carried out at 105–110 °C /30–45 min. UHT or aseptic method: Packaging is undertaken after heat treatment. For the ultra-high temperature and short time (UHTST) and very high temperature and short time (VHTST), the processing is at 135–150 °C/1–20 s | milk | [35,36] |
| Refrigeration | Artificial cooling of foods to temperatures below their freezing point Basic requirement for the processing and storage of milk and milk products is low temperature in the cold storage depending on the type of product to be stored. For example, milk is stored at 3–4 °C | milk | [40,41] |
| 2–8 °C | cheese | [42] | |
| Freezing | Artificial cooling of food to temperatures at which a sufficient amount of water solidifies, stopping the activity of microorganisms Ice cream is stored at −30 °C. | ice cream | [43] |
| Freeze concentration | skimmed milk skimmed powder milk fermented dairy beverages probiotic fresh cheese whey protein ice cream | [44] |
| Treatment Method | Treatment Conditions | Inactivation Efficiency | Food Matrix | Reference |
|---|---|---|---|---|
| Use of LAB | General application in food; produces lactic acid, antimicrobial peptides, diacetyl, etc. | Inhibits L. monocytogenes and other pathogens | Dairy products (e.g., cheeses) and fermented foods | [86,87,88] |
| 0.5% lactic acid | Applied for 2 h | 6 log10 cfu/g reduction of L. monocytogenes | Not specified | [90] |
| LAB (Lactococcus, Lactobacillus) strains | Isolated from Sardinian dairy; used in fresh cheeses | 4 log10 cfu/g reduction (bactericidal); others are bacteriostatic | Fresh cheeses | [91] |
| L. sakei, L. plantarum | Native strains from Calabria; applied in cheeses | 0.5–1.0 log10 cfu/g reduction | Cheeses | [92] |
| L. lactis + lactic acid/sodium lactate | Gorgonzola cheese, stored at 4 °C for 60 days | Pathogen load reduced below detection limit | Gorgonzola cheese | [81] |
| L. lactis | Added to Moroccan fermented milk, stored at 7 °C | Complete inhibition of L. monocytogenes in 24 h | Fermented milk | [93] |
| L. plantarum + nisin producers | Applied in cheese; monitored for 4 weeks | Pathogen load reduced below detection level | Cheese | [93] |
| L. brevis, E. faecalis | Used in soft cheeses; monitored during cold storage | 4 log10 cfu reduction in few weeks | Soft cheeses | [94] |
| L. brevis, L. plantarum, E. faecalis | Soft vs. semi-hard cheeses, over 20 days | Bacteriostatic (soft); bactericidal (semi-hard) | Soft and semi-hard cheeses | [95] |
| LAB + lactic acid | Applied to ripened cheeses | Long-term inhibition of L. monocytogenes | Ripened cheeses | [95] |
| L. lactis + E. durans | Used in biofilms over a wide temperature range | Significant reduction in L. monocytogenes in biofilms | Food processing environments (biofilms) | [96,97] |
| Probiotics (Bifidobacterium, probiotic yeasts) | General application; used in dairy and meat products | Inhibits L. monocytogenes; improves shelf life | Dairy (milk, cheese), meat | [98,99] |
| L. plantarum, L. sakei, L. rhamnosus | Various food products | Effective against L. monocytogenes | Dairy products | [104] |
| LAB in food packaging | Innovative packaging strategy | Prevents pathogen growth | General food applications | [109] |
| Probiotic metabolites (organic acids, EPS) | Under real processing conditions (to be evaluated) | Significant antimicrobial effects (potential) | General food products | [110] |
| Postbiotics (peptides, vitamins, fatty acids) | Used in films; encapsulated for better solubility | Demonstrated significant antibacterial activity | General food packaging | [110] |
| Nisin in processed cheese | Widely used; approved food additive (E234); GRAS; more effective at low pH | Effective against L. monocytogenes and S. aureus | Processed cheese | [112] |
| Nisin in queso fresco/fresh cheese | Neutral pH (above 6); high fat and calcium content reduce effectiveness | Ineffective inhibition of L. monocytogenes | Queso fresco, fresh cheese, whole milk | [113] |
| Nisin + hurdle technology | Combined with multiple mild treatments (e.g., low pH, refrigeration, salt) | Enhanced safety; reduced treatment intensity | General foods | [113] |
| Nisin (produced by L. lactis) | Nisin formation; thermal stability; resistant to digestion | Antimicrobial against Gram-positive bacteria | Meat, dairy, and aquatic products | [115] |
| Combined treatments (e.g., probiotics + UV or H2O2) | Requires screening to avoid probiotic inactivation | Potential synergistic antibacterial effects | General food matrices | [9] |
| Nisin + high-intensity ultrasound/UV-A light | Not specified | Enhanced antibacterial protection | General food | [118] |
| Encapsulated nisin | Long-term application | Altered sensory properties; efficacy under evaluation | Dairy (e.g., cheese) | [118] |
| Nisin + sesamol/carvacrol | Not specified | Synergistic effect; increased efficacy | Not specified | [118] |
| Nisin + lauric alginate + ε-polysine | 28 days of cold storage | Effectively controlled L. monocytogenes | Fresh cheese | [118] |
| Nisin + phytic acid | Not specified | Increased efficacy against E. coli (Gram-negative bacteria) | Not specified | [119] |
| Bacteriocins (general, including nisin and pediocin) | Refrigerated storage for up to 12 weeks at 4 °C | Prevented L. monocytogenes growth | Meat, fish, dairy, salads, and juices | [120] |
| Liposome-encapsulated nisin | 14 days at 7 °C | Reduced L. monocytogenes from 4.5 log CFU/mL to undetectable level | Whole and skim milk | [8] |
| Liposome-encapsulated P34 peptide | 8 days | Controlled L. monocytogenes only in skim milk | Skim milk | [22] |
| Liposome-encapsulated sakacin + DOTAP | 5 days at 7 °C | ~5 log reduction in L. monocytogenes | Goat milk | [8] |
| Reuterin (8 AU/mL) | 24 h at 37 °C | Completely inactivated L. monocytogenes | Milk | [121] |
| Reuterin + nisin | Not specified | Synergistic effect | Milk | [97] |
| Agilicin C7 | Stable in various pH and solvents | Destroyed L. monocytogenes via membrane damage | Dairy and meat products | [97] |
| Bacteriocins + heat treatments | Lower temperature and duration | Preserved nutrients; improved efficacy | General food | [24,91] |
| Nisin-loaded zein microcapsules | Not specified | Reduced L. monocytogenes | Milk | [5] |
| Nisin in edible polymer films/coatings | Surface application | Prevented aggregation; maintained activity | Food surfaces (general) | [24,123] |
| Nanoencapsulated bacteriocins | Not specified | Improved antimicrobial activity | Food matrices (general) | [117,124,126,127] |
| Bacteriocins + probiotics/tea polyphenols/essential oils | Not specified | Effective in reducing L. monocytogenes | General food | [134] |
| Pediocin PA-1 | Used in fermentation | Prevented contamination | Cheese (animal-derived foods) | [122] |
| Nisin + propolis | Not specified | Synergistic effect; preserved quality | Ice cream | [132] |
| LAB + grapefruit seed extract | Not specified | Effective in reducing bacterial growth | Fresh soft cheese | [132] |
| ListShield™ phage product | Applied on frozen foods | 2.2 log reduction in L. monocytogenes | Prepackaged frozen foods | [130] |
| Listex™ P100 phage product | Applied on food and stainless steel | Reduced biofilms and contamination | Cheese, smoked fish, food surfaces | [130] |
| Phage endolysin PlyP100 | Applied to queso fresco | Significant bacterial load reduction | Queso fresco | [132] |
| Identified Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Conventional thermal methods | Destroys contaminating microorganisms and inactivates enzymes | Reduce the sensory and nutritional qualities of the finished product | [13,30] |
| Direct steam injection | It is considered one of the best technologies to prevent thermal deterioration of milk | High cost and increased complexity | [48] |
| High-pressure processing (HPP) | More effective method in liquid foods than in solid foods Negligible effects on health and organoleptic and nutritional properties of food products | May cause incomplete microbial inactivation | [1,63,160] |
| Ionizing radiation | Cheese decontamination Use for treating packaged foods to reduce the risk of post-processing contamination | May cause incomplete microbial inactivation A high dose of irradiation may cause product discoloration and unpleasant odors | [161,162,163,164] |
| Ultrasounds | Efficient, cost-effective, and environmentally friendly process Extending the shelf life of finished products | Prolonged exposure can develop a metallic, burnt taste and rubbery appearance in the milk | [10,68] |
| LAB | Produce bacteriocins Minimally processed food products and better nutritional and sensory value Shortens the ripening process of cheeses Extends the shelf life of finished products Resistance to acids | In vitro antilisterial activity must be demonstrated in food applications Only two genera of LAB (Lactobacillus and Lactococcus) are considered GRAS More strains of LAB should be used for more effective control | [7,22,81,89,91,92,98,107,165] |
| Probiotics | Maintaining the initial physicochemical properties of food products Improving organoleptic properties Extending the shelf life of finished products | Antilisterial activity must be demonstrated when obtaining food products May cause some metabolic disorders or the generation of biogenic amines | [166,167] |
| Bacteriocins Bacteriolysins | Directly disrupts the integrity of the bacterial membrane, producing inactivation of the bacterial cell High efficiency and convenience Active over a wide pH range, resistant to high temperatures Extends the shelf life of finished products Provides additional protection in high-temperature processes Prevents loss of organoleptic and nutritional properties In situ production (protective cultures) | Poor solubility, uneven distribution High cost Limited antilisterial activity Inactivation by other food additives Efficacy may be affected by various environmental factors, e.g., storage temperature Sensitivity to the presence of proteolytic enzymes | [9,10,24,87,90,124,125,127,132,168] |
| Bacteriophages | High specificity towards the pathogen Does not affect the beneficial natural microflora, organoleptic qualities, or nutritional value Easy to isolate and propagate Destroys biofilms Safe for humans and the environment | Multiple bacteriophages may be required if food is contaminated with multiple bacterial pathogens Sensitive to commonly used disinfectant Negative consumer perception of the use of “viruses” in food | [3,17,129,130,133] |
| Natural antimicrobials | They enhance the flavor of food, improve nutritional value, and bring health benefits Maintaining the original physicochemical properties of food products | Undesirable organoleptic properties at high concentrations Chemical instability Limited availability Limited dispersibility | [8,142,157,169] |
| Essential oils | Extending the shelf life of finished products Natural flavoring agents Improving the functional and sensory properties of dairy products | Production of strong flavors that may be undesirable flavors in some food products Less effective in controlling L. monocytogenes | [136,138,140,143,144,148,149,155] |
| Chemical agents | Significantly reduce the adhesion of L. monocytogenes to the surface of food products | Negative health effects (allergic or carcinogenic) | [26,99] |
| Mechanical–bactericidal (MB) surfaces | High biofilm control potential | Challenges related to scalability, cost-effectiveness, mechanical and chemical durability, and complex composition of food products | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabija, A.; Afloarei, C.Ș.; Dabija, D.; Chetrariu, A. Conventional and Innovative Methods for Reducing the Incidence of Listeria monocytogenes in Milk and Dairy Products. Appl. Sci. 2025, 15, 6580. https://doi.org/10.3390/app15126580
Dabija A, Afloarei CȘ, Dabija D, Chetrariu A. Conventional and Innovative Methods for Reducing the Incidence of Listeria monocytogenes in Milk and Dairy Products. Applied Sciences. 2025; 15(12):6580. https://doi.org/10.3390/app15126580
Chicago/Turabian StyleDabija, Adriana, Cristina Ștefania Afloarei, Dadiana Dabija, and Ancuța Chetrariu. 2025. "Conventional and Innovative Methods for Reducing the Incidence of Listeria monocytogenes in Milk and Dairy Products" Applied Sciences 15, no. 12: 6580. https://doi.org/10.3390/app15126580
APA StyleDabija, A., Afloarei, C. Ș., Dabija, D., & Chetrariu, A. (2025). Conventional and Innovative Methods for Reducing the Incidence of Listeria monocytogenes in Milk and Dairy Products. Applied Sciences, 15(12), 6580. https://doi.org/10.3390/app15126580







