Abstract
Pediatric dentistry continually seeks effective and efficient treatments for young patients, especially within pediatric endodontics, where cooperation can often be challenging. This in vivo study aimed to evaluate the effectiveness of a novel photodynamic therapy (PDT) protocol using a 5-aminolevulinic acid gel (Aladent, ALAD) combined with light irradiation during the endodontic treatment of primary teeth. This study included primary teeth requiring root canal therapy due to carious lesions or trauma, with clinical symptoms suggesting irreversible pulpitis or acute apical periodontitis. Following local anesthesia and isolation with a rubber dam, carious lesions were excavated, and access to the pulp chamber was established. Canal preparation included determining the working length and using a sequence of k-files. Afterward, ALAD gel was applied, and the patients were divided into two groups based on their visit duration (Group A with a single visit, Group B returning after one week). Microbiological analysis was conducted on the samples taken before and after treatment. The findings demonstrated significant antibacterial efficacy of the PDT protocol in reducing root canal bacterial load, suggesting ALAD-based PDT may serve as an alternative to traditional endodontic treatment in cases where retaining primary teeth is essential for orthodontic or strategic reasons. Clinically, improvement in symptoms and fistula resolution were observed. Treatment time, patient compliance, and protocol safety in pediatric applications are also discussed, highlighting the protocol’s potential to enhance clinical outcomes in pediatric endodontics.
1. Introduction
Pediatric dentistry is a specialized branch that addresses the oral health needs of children, from infancy through to adolescence. One of the most prevalent issues in this age group is dental caries, which affects approximately 2.3 billion people worldwide, with over 530 million school-age children experiencing caries in their primary teeth [1]. If left untreated, caries can progress to more severe conditions, such as irreversible pulpitis and pulp necrosis, which is especially concerning in primary teeth, as inflammation can evolve into necrosis more rapidly and with fewer symptoms than in permanent teeth [2,3].
Within pediatric dentistry, pediatric endodontics plays a critical role in managing infections in the pulp and root canals of primary teeth, requiring precise and efficient therapeutic strategies to prevent complications and support long-term oral health [4]. Treating young patients presents unique challenges due to limited cooperation, heightened anxiety, and smaller anatomical structures, necessitating tailored, minimally invasive protocols to enhance comfort and ensure success. These procedures often require local anesthesia and rubber dam isolation, making it essential to implement swift protocols that reduce discomfort and increase efficiency [5]. Ensuring cooperation is also vital for meeting therapeutic goals and fostering positive dental experiences that help reduce anxiety in future appointments [6,7].
In some cases, retaining a compromised primary tooth is crucial for space maintenance and preventing malocclusion. Palliative therapies may help manage symptoms and delay extraction, preserving occlusal integrity and supporting oral development [8].
Despite advances, achieving the complete disinfection of primary root canals remains a challenge. Conventional treatments may not fully control microbial infections, which can compromise treatment success [9]. In response, photodynamic therapy (PDT) has emerged as a promising adjunct to traditional endodontic protocols [10,11,12,13].
PDT uses a photosensitizing agent that, when activated by light, produces reactive oxygen species capable of destroying bacterial cells [14]. Various photosensitizers have been tested, with phenothiazine derivatives like methylene blue and toluidine blue being widely recognized for their bactericidal activity, including against Enterococcus faecalis, a common endodontic pathogen [15,16,17,18,19,20,21,22]. Light sources commonly used in PDT include lasers, halogen lamps, and LEDs, with LEDs being particularly advantageous due to their cost-effectiveness and ease of use across different tissues [18,23,24,25,26].
A recently explored protocol used 5-aminolevulinic acid (ALA) gel combined with red LED light, showing significant antibacterial effects against endodontic pathogens, including E. faecalis [27]. ALA stands out as an intrinsic photosensitizer, as it is metabolized in situ into protoporphyrin IX (PpIX), a key compound in heme synthesis and a potent photosensitizer [28]. Aladent (ALAD), a commercial gel with 5% ALA developed by Alphastrumenti Srl (Italy), has demonstrated broad-spectrum antibacterial activity against Gram-negative and Gram-positive bacteria, such as Staphylococcus aureus, Escherichia coli, Veillonella parvula, Porphyromonas gingivalis, and Pseudomonas aeruginosa [29,30,31,32,33,34]. ALA-based PDT thus represents a promising adjunctive treatment, especially beneficial in pediatric cases.
Building upon promising ex vivo studies demonstrating the antimicrobial efficacy of ALA-based PDT [17,27], this in vivo study evaluated the clinical effectiveness of this protocol in the endodontic treatment of primary teeth. Specifically, it investigated the application of ALAD with LED irradiation in clinical settings, aiming to optimize pediatric endodontic care and expand available therapeutic options.
The objective of this in vivo study was to assess the effectiveness of a novel PDT protocol using 5-aminolevulinic acid gel (Aladent, ALAD) combined with light irradiation during the endodontic treatment of primary teeth. By evaluating its antimicrobial efficacy and clinical outcomes, this study explored ALAD-PDT as an adjunctive approach in pediatric endodontics, supporting dental arch preservation and evidence-based treatment strategies. The tested hypothesis was that ALAD-PDT would enhance antimicrobial action and improve clinical outcomes compared to conventional treatment, contributing to the overall success of pediatric endodontic care.
2. Materials and Methods
2.1. Study Design
The experimental diagram in Figure 1 illustrates the sequential steps of the research process.
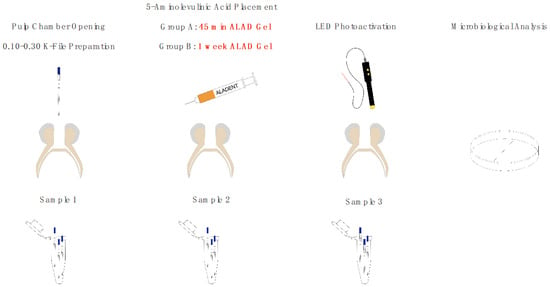
Figure 1.
Experimental diagram.
Thirty pediatric patients requiring root canal treatment in primary teeth were selected for this study. All participants underwent the initial protocol steps, including baseline microbiological analysis. Following these preliminary procedures, patients were randomly divided into two groups. In Group A, the full protocol was completed in a single appointment. In Group B, after the initial steps, a temporary filling was placed, and the protocol was resumed and completed one week later. All patients were selected from the Department of Pediatric Dentistry at the University “G. D’Annunzio” in Chieti. The custodial parent or legal guardian was informed of the tooth’s prognosis, the potential alternative treatment of extraction, and the benefits of retaining the tooth in the arch for an extended period. Consent was documented with a signed informed consent form, completed during the first visit after the anamnesis.
The protocol used in this study is already standard practice within the Department of Pediatric Dentistry and adheres to the Good Clinical Practice (GCP) guidelines. The Research Ethics Committee of the Department of Medical, Oral, and Biotechnological Sciences (University “G. d’Annunzio” of Chieti—Italy) approved this study (Protocol No. 599), in accordance with the ethical and human principles defined in the Declaration of Helsinki.
2.2. Aladent
The Aladent gel (ALAD) (Alphastrumenti Srl, Melzo, Italy) used in this study contains 5% 5-aminolevulinic acid (ALA) along with other proprietary ingredients protected under patent PCT/IB2018/060368 (filed 19 December 2018) [33] and is in a liquid state below 28 °C but transitions to a gel-like consistency at temperatures above 30 °C.
2.3. Light Source and Fiber TIP
A diode laser, AlGaInP TL-07 (Alphastrumenti Srl, Melzo, Italy) with a 635 nm wavelength, served as the light source for this study. Irradiation was conducted for 7 min in continuous emission mode at 50 mW intensity, delivered through an OM1 silica multimode fiber with a core diameter of 62.5 μm and an outer diameter of 125 μm (Alphastrumenti, Italy). The fiber’s distal end was stripped of its outer coating for 30 mm, exposing the bare fiber for optimal application
2.4. Inclusion Criteria
The inclusion criteria for this study required patients to be in the primary dentition stage, with selected teeth being either single-rooted or multi-rooted. Eligible teeth required root canal treatment due to either carious lesions or trauma. Key indicators for inclusion included clinical signs such as sinus tract presence, swelling, periapical radiolucency on radiographs, a history of antibiotic therapy, and tooth mobility not associated with natural exfoliation. Symptoms included pain consistent with irreversible pulpitis or acute apical periodontitis. All clinical procedures were conducted at the Department of Pediatric Dentistry, University “G. D’Annunzio”, in Chieti.
2.5. Microbiological Analysis
The effects of two different treatments on the total microbial cells in infected primary teeth were evaluated by quantifying the colony-forming units (CFUs) in each sample. Each sample, collected by placing three sterile ISO 0.30 paper tips at working length inside the root canal for 30 s, was vortexed for 1 min, serially diluted, and then plated on Trypticase Soy Agar (TSA) plates (Oxoid, Milan, Italy) (Figure 2). Following incubation at 37 °C for 48–72 h, the number of viable bacteria (CFUs) was counted and converted to actual bacterial counts according to the dilution factors used.
2.6. Statistical Analysis
All data were recorded in a Microsoft Excel data sheet (Washington, DC, USA) as total microbial counts (colony forming units-CFU/mL).
Then, SPSS Statistics for Windows, version 21 (IBM SPSS Inc., Chicago, IL, USA), was used to calculate the Fisher’s least significant difference (LSD). Statistically significant differences were considered to be a p-value < 0.05.
2.7. Endodontic Treatment
Once the clinical protocol of photodynamic therapy with Aladent gel was completed, a complete endodontic therapy was performed. In particular, the root canals were prepared with a sequence of K-files 0.10, 0.15, 0.20, 0.25 (Colorinox ReadySteel, Dentsply Sirona, Charlotte, NC, USA), intracanal washings were performed with 1% sodium hypochlorite, and then a final washing was performed with saline solution. Finally, the canals were dried with sterile paper points and were obturated using a resinous endodontic cement based on methacrylates (Endorez, Ultradent Italia Srl, Corsico, Milano, Italy).
2.8. Case Report
A 5-year-old male patient was presented to the Department of Pediatric Dentistry at the University ‘G. D’Annunzio’ with swelling in the upper anterior arch and a history of trauma. A cold thermal test on tooth 61 indicated no response (Figure 2a). The preoperative radiograph revealed periapical radiolucency (Figure 2b). After administering local anesthesia and isolating the tooth with a rubber dam, carious lesions were excavated (for teeth affected by dental caries), and the pulp chamber was accessed. At this point, the working length was determined using the Apex Locator Dentaport Root ZX (J. Morita Corp., Osaka, Japan) with a 21 mm 0.10 K-file, and the canals were prepared to the working length with sequential K-files in the following sizes: 0.10, 0.15, 0.20, 0.25, and 0.30.
As a part of the study protocol, this patient was included in Group B, which followed a two-session treatment protocol. After root canal preparation, irrigation was performed with 1 mL of sterile saline solution (sodium hypochlorite was intentionally avoided to preserve the antimicrobial assessment of the ALAD protocol). The first sample (T0) was collected using sterile ISO 0.30 paper points. ALAD gel was then introduced into the canals using a fine syringe, and a temporary filling was placed. The patient was dismissed and returned one week later for the second session. At this second appointment, the temporary restoration was removed without irrigation, and a second sample (T1) was collected. Then, photoactivation with red LED light for 7 min was performed inside the root canal (Figure 2c), followed by collection of the third sample (T2). All paper point samples were transferred immediately to the microbiology laboratory for analysis (Figure 2d). The one-month follow-up radiograph (Figure 2e) demonstrated bone healing in the periapical area of tooth 61, and the clinical examination confirmed the absence of symptoms, with complete resolution of the fistula (Figure 2f).
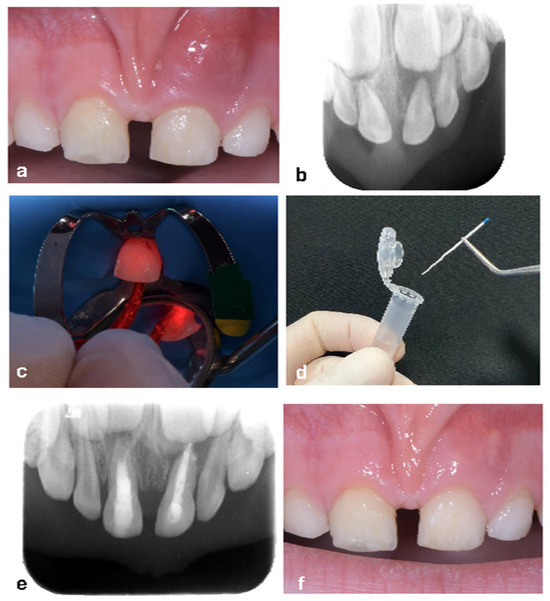
Figure 2.
Treatment of a primary superior central incisor after trauma—Group B. (a) Edema present in tooth 61. (b) Preoperative X-ray showing periapical radiolucency. (c) Photoactivation after 1 week placement of the 5-amilovelunic gel inside the root canal. (d) Sample collection using a sterile 0.30. (e) The one-month follow-up radiograph demonstrated bone healing in the periapical area of tooth 61. (f) Clinical examination confirmed the absence of symptoms, with complete resolution of the fistula.
3. Results
The following tables show the results for the determination of total microbial counts (colony-forming units—CFU/mL) for Group A (Table 1) and Group B (Table 2), respectively.

Table 1.
Total microbial count (colony-forming units/mL) for Group A (protocol performed in a single session). T0-A: pre-ALAD. T1-A: sample collection after 45 min of ALAD gel application. T2-A: sample collection after photoactivation using a red LED light with a dedicated optical fiber for 7 min. * p < 0.05.

Table 2.
Total microbial count (colony-forming units/mL) for Group B (protocol completed after one week). T0-B: sample collection after pulp chamber opening. T1-B: sample collection one week after ALAD gel application. T2-B: sample collection after photoactivation using a red LED light with a dedicated optical fiber for 7 min. * p < 0.05.
The results from determining the total microbial counts aligned with the current literature, confirming that photodynamic therapy (PDT) exerts potent antibacterial effects on bacteria colonizing root canals in teeth requiring endodontic treatment.
For both group, the LSD test showed that the treatments significantly decreased (p < 0.05) the CFUs, with respect to the sample collection after pulp chamber opening.
In particular, a statistically significant reduction in bacterial count was observed from the initial T0 sample, collected before ALAD gel application during the first session in both groups, to the T1 sample. In Group A, this reduction occurred within 45 min of the same session; in Group B, it was observed one week later, following a temporary filling placement. These results highlight the inherent antibacterial properties of ALAD gel, even before photoactivation. This downward trend in bacterial count persisted at the T2 sampling after photoactivation, resulting in a statistically significant reduction in both groups, nearly eliminating the bacterial count.
However, in one Group B case, the temporary filling failed between sessions, leading to an increased bacterial count at T1, underscoring the necessity of a secure hermetic seal for effective endodontic healing. Thus, for cases where professional endodontic treatment is not feasible, but the protocol is still applied to preserve the tooth temporarily in the arch, a restoration technique ensuring a durable hermetic seal is essential to maintain the therapeutic effects.
4. Discussion
Analyzing the findings from our in vivo study within the context of pediatric dentistry, we underscored the potential of 5-aminolevulinic acid (ALA)-based photodynamic therapy (PDT) as an innovative adjunctive approach for managing endodontic infections in primary teeth. Our results confirmed the tested hypothesis, reinforcing this therapy’s effectiveness. While the literature has extensively explored PDT in permanent dentition—particularly its capacity to reduce Enterococcus faecalis counts [35,36]—our study provides novel insights into its application in primary teeth. Given its non-invasive nature and pediatric suitability, ALA-based PDT emerges as a promising tool for bacterial reduction and improved clinical outcomes in endodontics.
Hsieh et al. 2014 [37] showed that higher ALAD concentrations incubated for 1 h significantly reduced CFUs, even without LED irradiation, due to ALA’s “dark toxicity”. This effect, coupled with preservatives like potassium sorbate and sodium benzoate [38,39], may enhance its bactericidal action. Meanwhile, in vivo studies by Petrini [32] and D’Ercole [40] demonstrated that 7 min LED exposure after ALA incubation (50% concentration) nearly eliminated E. faecalis. LED’s sustained antimicrobial effect contrasts with chemical agents that pathogens may resist over time [32,40].
Photosensitizing agents such as methylene blue [41], methylene blue with nanoparticles [42], toluidine blue [41], and 5-aminolevulinic acid (ALA) [33,36]—each paired with various light sources have shown efficacy against E. faecalis. Some studies have compared their effects with sodium hypochlorite and conventional chemo-mechanical methods, with PDT showing comparable results [36,43,44]. In a clinical trial on primary teeth, standard treatment was compared to one combining PDT with 0.005% methylene blue, also showing similar efficacy [45]. Our findings support these results, reinforcing ALAD’s antimicrobial potential when combined with light irradiation.
The substantial reduction in bacterial load following ALA-based PDT highlights the potential of this treatment to eradicate pathogens within infected root canals [27]. ALA-based PDT uses light-activated photosensitizers to deliver targeted antimicrobial action, reducing the likelihood of failure and contributing to positive clinical outcomes. A key advantage lies in its selective targeting of pathogenic bacteria, preserving healthy host tissue. Unlike broad-spectrum antimicrobials that may disrupt beneficial microbiota, ALA-based PDT offers a focused intervention with fewer collateral effects [32,33,34,46]. For example, an ex vivo study on permanent teeth showed bacterial reduction comparable to 2.5% sodium hypochlorite in eliminating E. faecalis [27]. By leveraging the selective susceptibility of bacterial cells to photodynamic effects, ALA-based PDT not only eliminates pathogens but also promotes tissue healing and regeneration—especially valuable in pediatric care. In pediatric dentistry, this selective approach is crucial. While sodium hypochlorite remains a common irrigant, it has drawbacks: unpleasant taste and odor, inconsistent disinfection, and potential toxicity if extruded into periradicular tissues. It can also harm structures like permanent tooth follicles, peripheral tissues, and oral mucosa if not carefully managed [47]. ALA-based PDT offers a safer, targeted alternative that preserves the surrounding tissue while ensuring effective microbial decontamination—an ideal solution for pediatric endodontic infections.
In addition to its antimicrobial properties, ALA-based PDT presents promising applications for pediatric dentistry, especially in supporting patient-centered care and optimized treatment strategies [48,49,50]. Given the non-invasive nature of PDT and its low risk of adverse effects, it offers a compelling therapeutic option for young patients who may experience anxiety with conventional endodontic treatments. By introducing a less invasive and more comfortable alternative to standard antimicrobial therapies, ALA-based PDT can potentially improve patient compliance, decrease treatment-related stress, and make dental visits more positive for children. Notably, future research should explore how laser-assisted PDT might influence pediatric behavioral management, as this could further enhance its viability in clinical practice [51].
However, ALA-based PDT does come with certain limitations that require careful consideration. One critical factor is the effective delivery and penetration of light within the root canal system to ensure thorough microbial eradication. The complex morphology of root canals, especially in primary teeth with underdeveloped roots, can hinder uniform light distribution and tissue penetration [10]. This anatomical complexity may reduce the treatment’s efficacy unless these challenges are addressed. Future innovations in light delivery systems, potentially tailored to the specific anatomical features of pediatric patients, may help overcome these issues and improve clinical outcomes [27]. Custom treatment protocols that account for unique pediatric dental anatomy may be essential to fully realize the benefits of ALA-based PDT in young patients.
An important aspect to consider is the timing and integration of PDT within the overall endodontic treatment protocol. While PDT provides rapid microbial decontamination and several adjunctive benefits, successful clinical application requires thoughtful alignment with existing treatment processes. Clinicians must carefully weigh factors such as session duration, pre-irradiation time, and post-treatment care to maximize the therapeutic potential of ALA-based PDT while avoiding unnecessary disruptions or delays. Though PDT is less invasive and simpler than full endodontic treatment [10], the 45 min gel application within the canal system may be challenging for pediatric patients with limited chair tolerance. By comparing two patient groups—one undergoing a single extended session (Group A) and another receiving a temporary filling with the option to leave immediately after gel placement (Group B)—we were able to evaluate the practical implications of each approach. This comparison highlights the flexibility of PDT protocols and the potential benefits of adapting treatment timing to patient needs, especially in pediatric dentistry where cooperation can vary significantly.
In Group A, clinicians had to actively engage the child throughout the entire treatment, often using video distractions to maintain cooperation. In contrast, with Group B, standard psychological techniques, such as tell-show-do and distraction, typically sufficed, making the session management smoother. Clinically, post-therapy follow-ups showed encouraging signs, such as the resolution of certain symptoms (e.g., fistula disappearance and reduced swelling) in some cases, and a stable, asymptomatic condition in others.
This article presents the detailed methodology, results, and discussion of our in vivo study, providing evidence on the potential of combining ALAD with PDT as a promising adjunct therapy in pediatric endodontics. Additionally, we explored the implications of this therapeutic approach in managing primary teeth, emphasizing the importance of preserving dental arch integrity and achieving favorable treatment outcomes for pediatric patients. Through a thorough analysis of our findings and a comprehensive review of the relevant literature, we aimed to elucidate the clinical utility of ALA-based PDT and its broader applications in enhancing pediatric dental care. By advancing our understanding of innovative treatment modalities, this research strives to improve treatment outcomes, optimize patient comfort, and reinforce the principles of evidence-based practice within pediatric dentistry.
Despite these promising observations, additional research is needed to further understand the long-term effectiveness and feasibility of ALA-based PDT in pediatric endodontic treatment. This is particularly important when PDT is considered as an alternative rather than a complement to conventional endodontic therapy—especially in situations where maintaining the tooth in the arch is therapeutically necessary but full endodontic treatment is not feasible. Longitudinal studies should evaluate the durability of PDT’s effects, the recurrence of endodontic infections, and implications for tooth survival and arch integrity to better establish PDT’s role and safety profile as an adjunct in pediatric endodontic care.
Moreover, comparative studies evaluating the cost-effectiveness and clinical outcomes of PDT against conventional therapies are crucial to guiding evidence-based treatment decisions and optimizing resource allocation in pediatric dental practice. In conclusion, our study highlights the potential of ALA-based PDT as a valuable adjunct in pediatric endodontics and explored its potential as an alternative therapy in specific cases. Through the targeted antimicrobial effects of light-activated photosensitizers, ALA-based PDT offers a promising approach for enhancing treatment outcomes, improving patient experiences, and advancing evidence-based pediatric dentistry. However, further research and clinical trials are needed to clarify the long-term efficacy, safety, and stability of PDT’s effects in pediatric endodontics. Additional studies with extended follow-ups (6–12 months) would be instrumental in establishing ALA-based PDT as a viable and effective alternative to traditional treatments.
5. Conclusions
Photodynamic therapy with 5-aminolevulinic acid gel appears to be a promising, minimally invasive alternative for managing bacterial infections in deciduous teeth, especially when conventional endodontic treatment is not feasible. Clinical improvement was observed, including fistula resolution, with easier management noted in Group B, despite requiring two sessions.
Author Contributions
Conceptualization, S.D. and M.P.; methodology, S.D., S.D.L. and L.D.G.; validation, S.D. and D.T.; formal analysis, S.D. and M.P.; investigation, S.D. and L.D.G.; resources, S.D., D.T. and M.P.; data curation, T.C.D., S.D.L., L.D. and M.P.; writing—original draft preparation, S.D. and M.P.; writing—review and editing, T.C.D. and L.D.; visualization, S.D. and M.P.; supervision, S.D., D.T. and M.P.; project administration, S.D. and M.P.; funding acquisition, S.D. and D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding provided to Simonetta D’Ercole and Domenico Tripodi by the FAR GRANT University of the Chieti–Pescara Fund.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki from 1975, as revised in 2013, and ethical review and approval were waived for this study due to the retrospective study setting, in which existing data from a specific cohort of previously treated patients were collected and analyzed, using biomaterials that are not experimental but CE approved and on the market for several years with procedures that fall within established therapeutic guidelines and are therefore also nonexperimental.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Authors would like to express their gratitude to Alessandro Recchia e Fabio Ricapito for the technical support. Aladent gel was provided by Alphastrumenti Srl (Melzo, MI), Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of Deep Caries and the Exposed Pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Kritikou, K.; Imre, M.; Tanase, M.; Vinereanu, A.; Totan, A.R.; Spinu, T.C.; Miricescu, D.; Stanescu-Spinu, I.I.; Bordea, M.; Greabu, M. Assessment of Mineralization, Oxidative Stress, and Inflammation Mechanisms in the Pulp of Primary Teeth. Appl. Sci. 2022, 12, 1554. [Google Scholar] [CrossRef]
- Oztek, M.A.; Noda, S.; Beauchemin, E.A.; Otto, R.K. Gentle Touch: Noninvasive Approaches to Improve Patient Comfort and Cooperation for Pediatric Imaging. Top. Magn. Reson. Imaging 2020, 29, 187–195. [Google Scholar] [CrossRef]
- Afshari, E.; Sabbagh, S.; Khorakian, F.; Sarraf Shirazi, A.; Akbarzadeh Baghban, A. Reducing Pain and Discomfort Associated with Rubber Dam Clamp Placement in Children and Adolescents: A Systematic Review and Meta-Analysis of Effectiveness. BMC Oral Health 2023, 23, 398. [Google Scholar] [CrossRef] [PubMed]
- Carrotte, P. Endodontic Treatment for Children. Br. Dent. J. 2005, 198, 9–15. [Google Scholar] [CrossRef]
- Ahmed, H.M.A. Pulpectomy Procedures in Primary Molar Teeth. Eur. J. Gen. Dent. 2014, 3, 3–10. [Google Scholar] [CrossRef]
- da Silva Barbosa, P.; Duarte, D.A.; Leite, M.F.; de Sant’ Anna, G.R. Photodynamic Therapy in Pediatric Dentistry. Case Rep. Dent. 2014, 2014, 1–5. [Google Scholar] [CrossRef][Green Version]
- Rossi, R.; Rispoli, L.; Lopez, M.A.; Netti, A.; Petrini, M.; Piattelli, A. Photodynamic Therapy by Mean of 5-Aminolevulinic Acid for the Management of Periodontitis and Peri-Implantitis: A Retrospective Analysis of 20 Patients. Antibiotics 2022, 11, 1267. [Google Scholar] [CrossRef]
- Kattan, H.F. The Efficacy of Antimicrobial Photodynamic Therapy in the Disinfection of Coronal and Radicular Dentine of Primary Teeth: A Systematic Review and Meta-Analysis. Photodiagnosis Photodyn. Ther. 2023, 44, 103697. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alsahhaf, A.; Alofi, R.S.; Al-Aali, K.A.; Abduljabbar, T.; Vohra, F. Efficacy of Photodynamic Therapy versus Local Nystatin in the Treatment of Denture Stomatitis: A Randomized Clinical Study. Photodiagnosis Photodyn. Ther. 2019, 28, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Varela Kellesarian, S.; Abduljabbar, T.; Vohra, F.; Malmstrom, H.; Yunker, M.; Varela Kellesarian, T.; Romanos, G.E.; Javed, F. Efficacy of Antimicrobial Photodynamic Therapy in the Disinfection of Acrylic Denture Surfaces: A Systematic Review. Photodiagnosis Photodyn. Ther. 2017, 17, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Alhenaki, A.M.; Alqarawi, F.K.; Tanveer, S.A.; Alshahrani, F.A.; Alshahrani, A.; AlHamdan, E.M.; Alzahrani, K.M.; Aldahiyan, N.; Naseem, M.; Vohra, F.; et al. Disinfection of Acrylic Denture Resin Polymer with Rose Bengal, Methylene Blue and Porphyrin Derivative in Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2021, 35, 102362. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Asnaashari, M.; Homayuni, H.; Paymanpour, P. The Antibacterial Effect of Additional Photodynamic Therapy in Failed Endodontically Treated Teeth: A Pilot Study. J. Lasers Med. Sci. 2016, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.B.; Novaes, A.B.; De Oliveira, R.R.; Nelson-Filho, P.; Santamaria, M.; Silva, R.A.B. Antimicrobial Photodynamic Therapy for the Treatment of Teeth with Apical Periodontitis: A Histopathological Evaluation. J. Endod. 2012, 38, 360–366. [Google Scholar] [CrossRef]
- Ng, R.; Singh, F.; Papamanou, D.A.; Song, X.; Patel, C.; Holewa, C.; Patel, N.; Klepac-Ceraj, V.; Fontana, C.R.; Kent, R.; et al. Endodontic Photodynamic Therapy Ex Vivo. J. Endod. 2011, 37, 217–222. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Hioka, N.; Kimura, E.; Batistela, V.R.; Terada, R.S.S.; Graciano, A.X.; Baesso, M.L.; Hayacibara, M.F. Antibacterial Photodynamic Therapy for Dental Caries: Evaluation of the Photosensitizers Used and Light Source Properties. Photodiagnosis Photodyn. Ther. 2012, 9, 122–131. [Google Scholar] [CrossRef]
- Foschi, F.; Fontana, C.R.; Ruggiero, K.; Riahi, R.; Vera, A.; Doukas, A.G.; Pagonis, T.C.; Kent, R.; Stashenko, P.P.; Soukos, N.S. Photodynamic Inactivation of Enterococcus Faecalis in Dental Root Canals in Vitro. Lasers Surg. Med. 2007, 39, 782–787. [Google Scholar] [CrossRef]
- Fonseca, M.B.; Tessare, P.O.; Pallota, R.C.; Filho, H.F.; Denardin, O.V.P.; Rapoport, A.; Dedivitis, R.A.; Veronezi, J.F.; Genovese, W.J.; Ricardo, A.L.F. Photodynamic Therapy for Root Canals Infected with Enterococcus Faecalis. Photomed. Laser Surg. 2008, 26, 209–213. [Google Scholar] [CrossRef]
- Poggio, C.; Arciola, C.R.; Dagna, A.; Florindi, F.; Chiesa, M.; Saino, E.; Imbriani, M.; Visai, L. Photoactivated Disinfection (PAD) in Endodontics: An in Vitro Microbiological Evaluation. Int. J. Artif. Organs 2011, 34, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, S.; Kangarlou, A.; Shahbazi, R.; Nazari-Nasab, A.; Naseri, M. Comparison of the Bactericidal Effi Cacy of Photodynamic Therapy, 2.5% Sodium Hypochlorite, and 2% Chlorhexidine against Enterococcous Faecalis in Root Canals; an in Vitro Study. Dent. Res. J. 2012, 9, 613–618. [Google Scholar] [CrossRef]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Martin, G.M.; Munavalli, G.S. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, H.; Chen, K.; Yan, J.; Yu, B.; Wang, S.; Yin, R.; Nong, X.; Zou, X.; Chen, Z.; et al. Chinese Guidelines on the Clinical Application of 5-Aminolevulinic Acid-Based Photodynamic Therapy in Dermatology (2021 Edition). Photodiagnosis Photodyn. Ther. 2021, 35, 102340. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Moan, J. The History of PDT in Norway. Part II. Recent Advances in General PDT and ALA-PDT. Photodiagnosis Photodyn. Ther. 2007, 4, 80–87. [Google Scholar] [CrossRef]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yılmaz, S. Photodynamic Therapy in Dentistry: A Literature Review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef]
- Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Lepore, S.; Tripodi, D.; Piattelli, A.; D’Ercole, S.; Petrini, M. Efficacy of 5% Aminolaevulinic Acid and Red Light on Enterococcus Faecalis in Infected Root Canals. Gels 2023, 9, 125. [Google Scholar] [CrossRef]
- Howley, R.; Chandratre, S.; Chen, B. 5-Aminolevulinic Acid as a Theranostic Agent for Tumor Fluorescence Imaging and Photodynamic Therapy. Bioengineering 2023, 10, 496. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (Ala) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Collaud, S.; Juzeniene, A.; Moan, J.; Lange, N. On the Selectivity of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Formation. Curr. Med. Chem.-Anti-Cancer Agents 2004, 4, 301–316. [Google Scholar] [CrossRef]
- Juzeniene, A.; Juzenas, P.; Moan, J. Application of 5-Aminolevulinic Acid and Its Derivatives for Photodynamic Therapy In Vitro and In Vivo. Methods Mol. Biol. 2010, 635, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Spoto, G.; Scarano, A.; D’Arcangelo, C.; Tripodi, D.; Di Fermo, P.; D’Ercole, S. Near-Infrared LEDS Provide Persistent and Increasing Protection against E. Faecalis. J. Photochem. Photobiol. B 2019, 197, 111527. [Google Scholar] [CrossRef] [PubMed]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a Novel Gel Containing 5-Aminolevulinic Acid and Red LED against Bacteria Involved in Peri-Implantitis and Other Oral Infections. J. Photochem. Photobiol. B 2020, 205, 111826. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Pierfelice, T.V.; D’amico, E.; Carlesi, T.; Iezzi, G.; D’arcangelo, C.; Di Lodovico, S.; Piattelli, A.; D’ercole, S. Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus Faecalis and Human Gingival Fibroblasts. Int. J. Env. Res. Public Health 2022, 19, 3048. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Awan, K.H.; Javed, F. Bactericidal Efficacy of Photodynamic Therapy against Enterococcus Faecalis in Infected Root Canals: A Systematic Literature Review. Photodiagnosis Photodyn. Ther. 2013, 10, 632–643. [Google Scholar] [CrossRef]
- D’Ercole, S.; Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Tripodi, D.; Iezzi, G.; Piattelli, A.; Petrini, M. 5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report. Gels 2022, 8, 697. [Google Scholar] [CrossRef]
- Hsieh, C.M.; Huang, Y.H.; Chen, C.P.; Hsieh, B.C.; Tsai, T. 5-Aminolevulinic Acid Induced Photodynamic Inactivation on Staphylococcus Aureus and Pseudomonas Aeruginosa. J. Food Drug Anal. 2014, 22, 350–355. [Google Scholar] [CrossRef]
- Santiesteban-Lopez, N.A.; Rosales, M.; Palou, E.; Lopez-Malo, A. Growth Response of Escherichia Coli ATCC 35218 Adapted to Several Concentrations of Sodium Benzoate and Potassium Sorbate. J. Food Prot. 2009, 72, 2301–2307. [Google Scholar] [CrossRef]
- Zare, M.A.; Rohani, S.M.R.; Raeisi, M.; Hosseini, S.J.; Hashemi, M. Antibacterial Effects of Monolaurin, Sorbic Acid and Potassium Sorbate on Staphylococcus Aureus and Escherichia Coli. J. Food Qual. Hazards Control 2014, 1, 52–55. [Google Scholar]
- D’Ercole, S.; Spoto, G.; Trentini, P.; Tripodi, D.; Petrini, M. In Vitro Inactivation of Enterococcus Faecalis with a Led Device. J. Photochem. Photobiol. B 2016, 160, 172–177. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Diagnostic Accuracy of Multiplex Real-Time PCR Approaches Compared with Cultivation -Based Detection Methods: Monitoring the Endopathogenic Microbiota Pre and Post Photo-Activated Disinfection. Photodiagnosis Photodyn. Ther. 2018, 22, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-Based Endodontic Antimicrobial Photodynamic Therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.; He, J.; Glickman, G.N.; Spears, R.; Schneiderman, E.D.; Honeyman, A.L. Evaluation of Photodynamic Therapy Using a Light-Emitting Diode Lamp against Enterococcus Faecalis in Extracted Human Teeth. J. Endod. 2011, 37, 856–859. [Google Scholar] [CrossRef]
- Strazzi-Sahyon, H.B.; Oliveira, A.K.L.; Carvalho, A.P.; Figueiredo, R.B.; Cintra, L.T.A.; Gomes-Filho, J.E.; dos Santos, P.H.; Sivieri-Araujo, G. Influence of Photodynamic Therapy and Intracanal Medication on Martens Hardness, Elastic Modulus and Bond Strength of Glass-Fiber Posts to Endodontically Treated Root Dentin. Photodiagnosis Photodyn. Ther. 2021, 36, 102571. [Google Scholar] [CrossRef]
- Okamoto, C.B.; Bussadori, S.K.; Prates, R.A.; da Mota, A.C.C.; Tempestini Horliana, A.C.R.; Fernandes, K.P.S.; Motta, L.J. Photodynamic Therapy for Endodontic Treatment of Primary Teeth: A Randomized Controlled Clinical Trial. Photodiagnosis Photodyn. Ther. 2020, 30, 101732. [Google Scholar] [CrossRef]
- Petrini, M.; Trentini, P.; Tripodi, D.; Spoto, G.; D’Ercole, S. In Vitro Antimicrobial Activity of LED Irradiation on Pseudomonas Aeruginosa. J. Photochem. Photobiol. B 2017, 168, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, J.A.; Nihal, N.K.; Nagarathna, C.; Vora, M.S. Root Canal Irrigants in Primary Teeth. World J. Dent. 2015, 6, 229–234. [Google Scholar] [CrossRef]
- Garcez, A.S.; Arantes-Neto, J.G.; Sellera, D.P.; Fregnani, E.R. Effects of Antimicrobial Photodynamic Therapy and Surgical Endodontic Treatment on the Bacterial Load Reduction and Periapical Lesion Healing. Three Years Follow Up. Photodiagnosis Photodyn. Ther. 2015, 12, 575–580. [Google Scholar] [CrossRef]
- Jurič, I.B.; Plečko, V.; Pandurić, D.G.; Anić, I. The Antimicrobial Effectiveness of Photodynamic Therapy Used as an Addition to the Conventional Endodontic Re-Treatment: A Clinical Study. Photodiagnosis Photodyn. Ther. 2014, 11, 549–555. [Google Scholar] [CrossRef]
- Garcez, A.S.; Nuñez, S.C.; Hamblim, M.R.; Suzuki, H.; Ribeiro, M.S. Photodynamic Therapy Associated with Conventional Endodontic Treatment in Patients with Antibiotic-Resistant Microflora: A Preliminary Report. J. Endod. 2010, 36, 1463–1466. [Google Scholar] [CrossRef]
- Araújo, L.P.; Gobbo, L.B.; Silva, T.A.; Rosa, W.L.d.O.; Almeida, J.F.A.; Gomes, B.P.F.A.; Ferraz, C.C.R. Photodynamic Therapy in the Root Canal Treatment of Primary Teeth: A Systematic Review of Clinical Trials. Int. J. Paediatr. Dent. 2024, 34, 114–124. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).