Apoptotic Potential of Polyphenol Extract of Mexican Oregano Lippia graveolens Kunth on Breast Cancer Cells MDA-MB-231
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of the Polyphenol Extract
2.3. Identification of Phenolic Compounds by LC-ESI-QTOF-MS/MS
2.4. Cell Culture
2.5. Cytotoxicity Assay
2.6. Western Blot
2.7. Statistical Analysis
3. Results and Discussion
3.1. Identification and Quantification of Flavonoids with Antiproliferative Potential in the Polyphenol Extract of Mexican Oregano L. graveolens
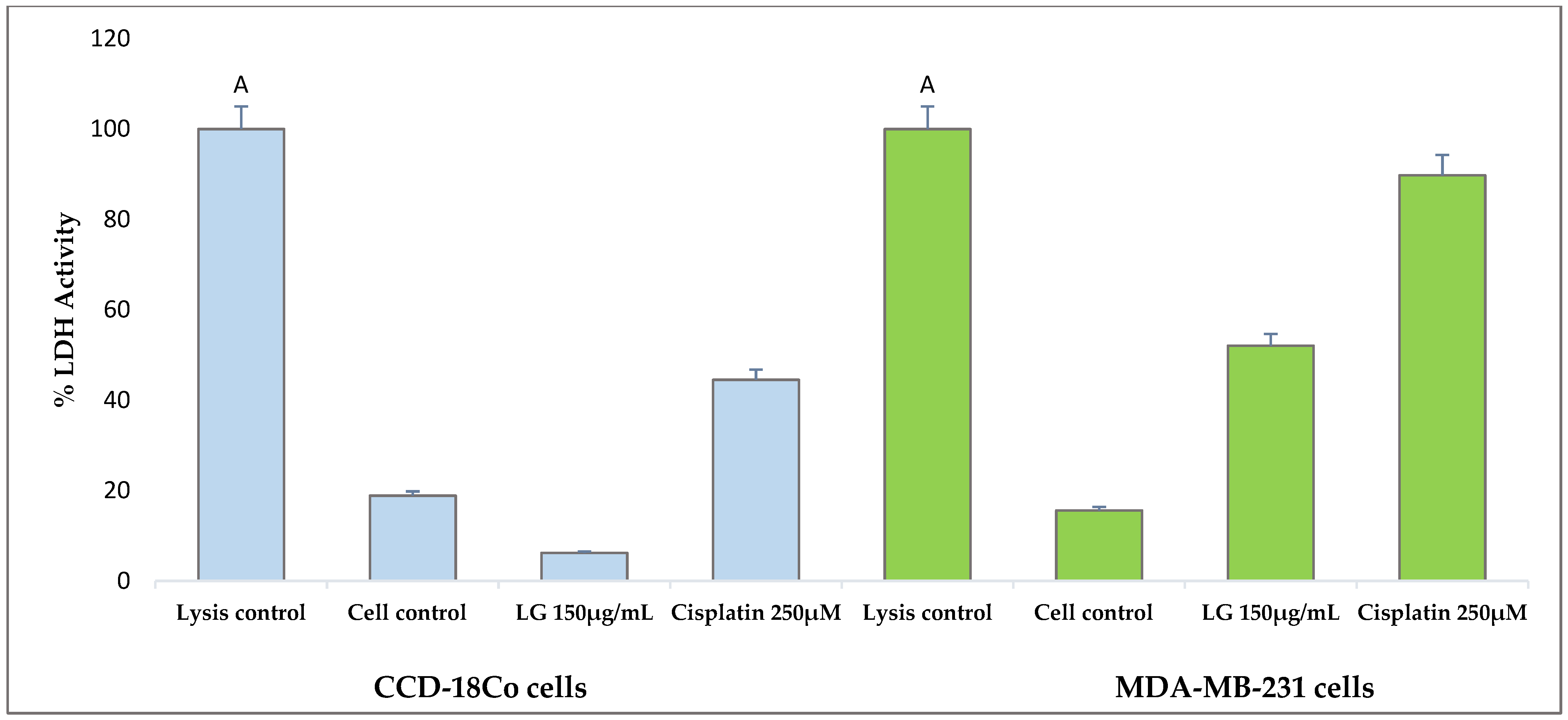
3.2. Non-Cytotoxic Effect of Polyphenol Extract of Mexican Oregano L. graveolens on Normal Human Cells CCD-18Co
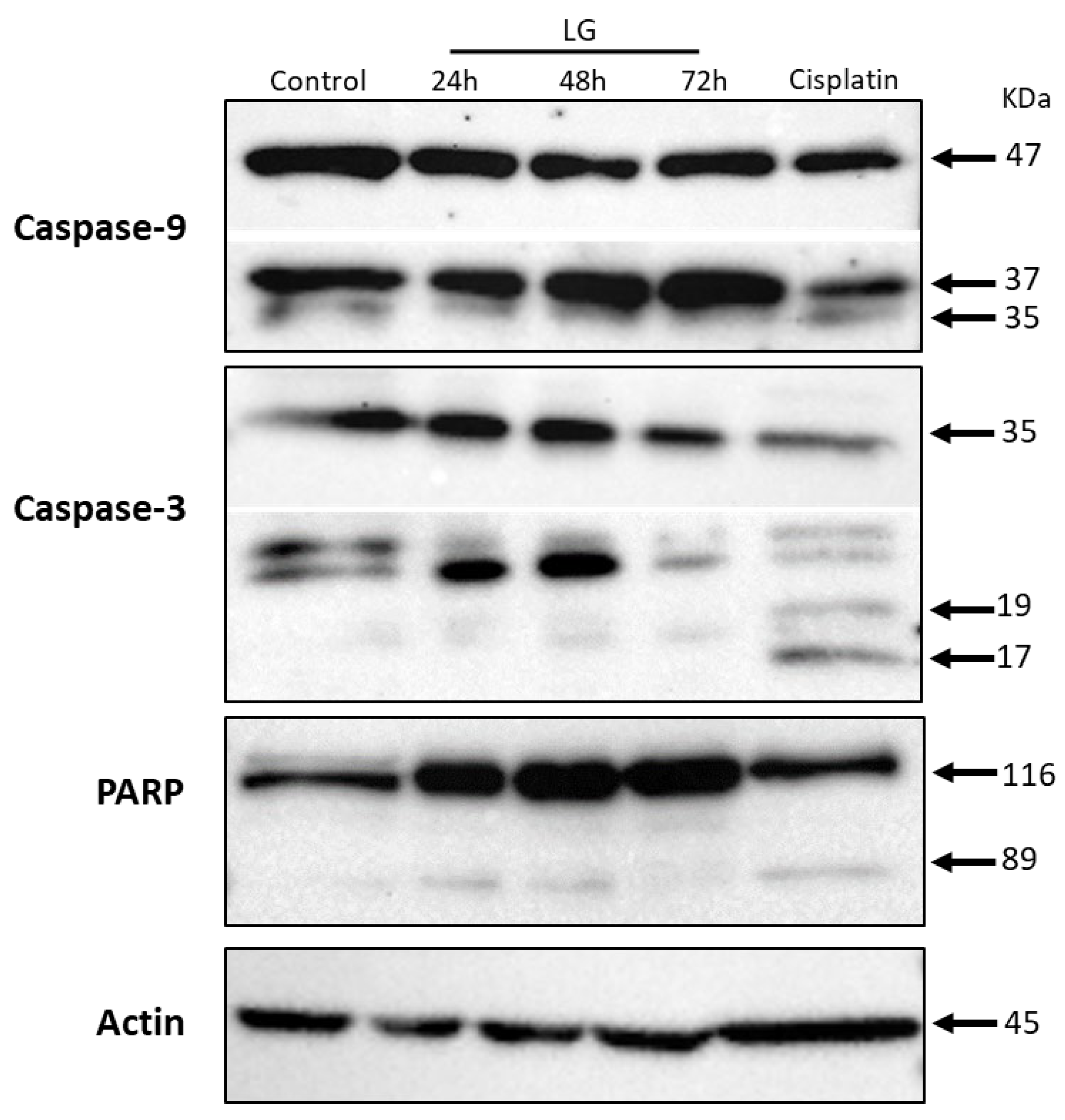
3.3. Polyphenolic Extract of L. graveolens Has Potential to Induce Apoptosis in Breast Cancer Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kintzios, S.E. 21–Oregano. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 417–436. [Google Scholar]
- Arcila, C.; Loarca, G.; Lecona, S.; González, E. El orégano: Propiedades, composición y actividad biológica de sus componentes. Arch. Latinoam. Nutr. 2004, 54, 100–111. [Google Scholar]
- Baser, K. The Turkish Origanum species. J. Med. Aromat. Plants 2002, 109, 109–126. [Google Scholar]
- Gutiérrez-Grijalva, E.; Picos-Salas, M.; Leyva-López, N.; Criollo-Mendoza, M.; Vazquez-Olivo, G.; Heredia, J. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef]
- Berdowska, I.; Zielinski, B.; Fecka, I.; Kulbacka, J.; Saczko, J.; Gamian, A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013, 141, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Chaouki, W.; Leger, D.Y.; Eljastimi, J.; Beneytout, J.L.; Hmamouchi, M. Antiproliferative effect of extracts from Aristolochia baetica and Origanum compactum on human breast cancer cell line MCF-7. Pharm. Biol. 2010, 48, 269–274. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kogiannou, D.A.; Kefalas, P.; Papassideri, I.S.; Kalogeropoulos, N. Phenolic profiles and antioxidant and anticarcinogenic activities of Greek herbal infusions; Balancing delight and chemoprevention? Food Chem. 2014, 142, 233–241. [Google Scholar] [CrossRef]
- Kogiannou, D.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal infusions; their phenolic profile, antioxidant and anti-inflammatory effects in HT29 and PC3 cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J. Food Sci. 2011, 76, C512–C518. [Google Scholar] [CrossRef]
- Marrelli, M.; Cristaldi, B.; Menichini, F.; Conforti, F. Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem. Toxicol. 2015, 86, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Kim, Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Kim, G.S. Function and Application of Flavonoids in the Breast Cancer. Int. J. Mol. Sci. 2022, 23, 7732. [Google Scholar] [CrossRef] [PubMed]
- Petroni, G.; Buqué, A.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Immunomodulation by targeted anticancer agents. Cancer Cell 2021, 39, 310–345. [Google Scholar] [CrossRef]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef]
- Li, Z.H.; Hu, P.H.; Tu, J.H.; Yu, N.S. Luminal B breast cancer: Patterns of recurrence and clinical outcome. Oncotarget 2016, 7, 65024–65033. [Google Scholar] [CrossRef]
- Berger, E.R.; Park, T.; Saridakis, A.; Golshan, M.; Greenup, R.A.; Ahuja, N. Immunotherapy Treatment for Triple Negative Breast Cancer. Pharmaceuticals 2021, 14, 763. [Google Scholar] [CrossRef]
- Van Swearingen, A.E.D.; Sambade, M.J.; Siegel, M.B.; Sud, S.; McNeill, R.S.; Bevill, S.M.; Chen, X.; Bash, R.E.; Mounsey, L.; Golitz, B.T.; et al. Combined kinase inhibitors of MEK1/2 and either PI3K or PDGFR are efficacious in intracranial triple-negative breast cancer. Neuro-Oncology 2017, 19, 1481–1493. [Google Scholar] [CrossRef]
- Telang, N.T. Natural products as drug candidates for breast cancer (Review). Oncol. Lett. 2023, 26, 349. [Google Scholar] [CrossRef]
- Fasoulakis, Z.; Koutras, A.; Syllaios, A.; Schizas, D.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; Ntounis, T.; et al. Breast Cancer Apoptosis and the Therapeutic Role of Luteolin. Chirurgia 2021, 116, 170–177. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, K.; Shen, H.; Yao, X.; Sun, Q.; Chen, G. Necroptosis: A novel manner of cell death, associated with stroke (Review). Int. J. Mol. Med. 2018, 41, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, R.; Tang, D. The STING1 network regulates autophagy and cell death. Signal Transduct. Target. Ther. 2021, 6, 208. [Google Scholar] [CrossRef]
- Parton, M.; Dowsett, M.; Smith, I. Studies of apoptosis in breast cancer. BMJ 2001, 322, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Chaudhry, G.-e.-S.; Md Akim, A.; Sung, Y.Y.; Sifzizul, T.M.T. Cancer and apoptosis: The apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol. 2022, 13, 842376. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Cai, Y.; Zhang, L.; Liu, S.; Li, P.; Li, X. Promoting Apoptosis, a Promising Way to Treat Breast Cancer with Natural Products: A Comprehensive Review. Front. Pharmacol. 2022, 12, 801662. [Google Scholar] [CrossRef]
- Sankari, S.L.; Masthan, K.M.; Babu, N.A.; Bhattacharjee, T.; Elumalai, M. Apoptosis in cancer–An update. Asian Pac. J. Cancer Prev. APJCP 2012, 13, 4873–4878. [Google Scholar] [CrossRef]
- Criollo-Mendoza, M.S.; Ramos-Payán, R.; Contreras-Angulo, L.A.; Gutiérrez-Grijalva, E.P.; León-Félix, J.; Villicaña, C.; Angulo-Escalante, M.A.; Heredia, J.B. Cytotoxic Activity of Polyphenol Extracts from Three Oregano Species: Hedeoma patens, Lippia graveolens and Lippia palmeri, and Antiproliferative Potential of Lippia graveolens against Two Types of Breast Cancer Cell Lines (MDA-MB-231 and MCF-7). Molecules 2022, 27, 5240. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Xu, S. Natural Bioactive Compounds Promote Cell Apoptosis in Gastric Cancer Treatment: Evidence from Network Pharmacological Study and Experimental Analysis. J. Chem. 2023, 2023, 6316589. [Google Scholar] [CrossRef]
- Kaushik, N.; Yang, H.; Jeong, S.; Kaushik, N.K.; Bhartiya, P.; Nhat Nguyen, L.; Choi, E.H.; Kim, J.H. Antiproliferative Activity of Pyracantha and Paullinia Plant Extracts on Aggressive Breast and Hepatocellular Carcinoma Cells. Appl. Sci. 2020, 10, 7543. [Google Scholar] [CrossRef]
- Cavalcante, F.P.; Millen, E.C.; Zerwes, F.P.; Novita, G.G. Progress in Local Treatment of Breast Cancer: A Narrative Review. Rev. Bras. Ginecol. E Obstet. 2020, 42, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Suraweera, C.D.; Hinds, M.G.; Kvansakul, M. Poxviral Strategies to Overcome Host Cell Apoptosis. Pathogens 2020, 10, 6. [Google Scholar] [CrossRef]
- Lee, D.; Yu, J.S.; Ryoo, R.; Kim, J.C.; Jang, T.S.; Kang, K.S.; Kim, K.H. Pulveraven A from the fruiting bodies of Pulveroboletus ravenelii induces apoptosis in breast cancer cell via extrinsic apoptotic signaling pathway. J. Antibiot. 2021, 74, 752–757. [Google Scholar] [CrossRef]
- Kushwaha, P.P.; Singh, A.K.; Prajapati, K.S.; Shuaib, M.; Fayez, S.; Bringmann, G.; Kumar, S. Induction of apoptosis in breast cancer cells by naphthylisoquinoline alkaloids. Toxicol. Appl. Pharmacol. 2020, 409, 115297. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Verma, R.; Salgotra, T.; Rahman, M.H.; Shah, M.; Akter, R.; Murad, W.; Mubin, S.; Bibi, P.; Qusti, S.; et al. Impacting the Remedial Potential of Nano Delivery-Based Flavonoids for Breast Cancer Treatment. Molecules 2021, 26, 5163. [Google Scholar] [CrossRef]
- Georgieva, A.; Sulikovska, I.; Toshkova-Yotova, T.; Djeliova, V.; Amiri, S.; Tsonevski, N.; Petkova-Kirova, P.; Tasheva, K. Antitumor Activity of Whole-Plant Extracts from In Vitro Cultured and Wild-Growing Clinopodium vulgare Plants on a Panel of Human Tumor Cell Lines. Appl. Sci. 2025, 15, 925. [Google Scholar] [CrossRef]
- Khojasteh Poor, F.; Keivan, M.; Ramazii, M.; Ghaedrahmati, F.; Anbiyaiee, A.; Panahandeh, S.; Khoshnam, S.E.; Farzaneh, M. Mini review: The FDA-approved prescription drugs that target the MAPK signaling pathway in women with breast cancer. Breast Dis. 2021, 40, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prever, L.; Hirsch, E.; Gulluni, F. Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer. Cancers 2021, 13, 3517. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Grijalva, E.P.; Angulo-Escalante, M.A.; Leon-Felix, J.; Heredia, J.B. Effect of In Vitro Digestion on the Total Antioxidant Capacity and Phenolic Content of 3 Species of Oregano (Hedeoma patens, Lippia graveolens, Lippia palmeri). J. Food Sci. 2017, 82, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Castillo, M.; Bonilla-Moreno, R.; Aleman-Lazarini, L.; Meraz-Rios, M.A.; Orozco, L.; Cedillo-Barron, L.; Cordova, E.J.; Villegas-Sepulveda, N. A Subpopulation of the K562 Cells Are Killed by Curcumin Treatment after G2/M Arrest and Mitotic Catastrophe. PLoS ONE 2016, 11, e0165971. [Google Scholar] [CrossRef]
- Frias-Zepeda, M.E.; Ibarra-Berumen, J.; Ordaz-Pichardo, C.; Rosales-Castro, M. Cytotoxic activity of ethanolic extracts of Lippia graveolens HBK leaves and stem against lung cancer cell line SK-LU-1. Bol. Latinoam. Caribe Plantas Med. Aromat. 2022, 21, 646–653. [Google Scholar] [CrossRef]
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3265–3271. [Google Scholar] [CrossRef]
- Huang, C.; Wei, Y.-X.; Shen, M.-C.; Tu, Y.-H.; Wang, C.-C.; Huang, H.-C. Chrysin, Abundant in Morinda citrifolia Fruit Water–EtOAc Extracts, Combined with Apigenin Synergistically Induced Apoptosis and Inhibited Migration in Human Breast and Liver Cancer Cells. J. Agric. Food Chem. 2016, 64, 4235–4245. [Google Scholar] [CrossRef]
- Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef]
- Gird, C.E.; Ostea, T.; Itran, V. Evaluation of cytotoxic activity and anticancer potential of indigenous Rosemary (Rosmarinus officinalis L.) and Oregano (Origanum vulgare L.) dry extracts on MG-63 bone osteosarcoma human cell line. Rom. J. Morphol. Embryol. 2021, 62, 525–535. [Google Scholar] [CrossRef]
- Nanni, V.; Di Marco, G.; Sacchetti, G.; Canini, A.; Gismondi, A. Oregano Phytocomplex Induces Programmed Cell Death in Melanoma Lines via Mitochondria and DNA Damage. Foods 2020, 9, 1486. [Google Scholar] [CrossRef]
- Criollo-Mendoza, S.M.; Heredia, B.J.; Vazquez-Olivo, G.; Avilés-Gaxiola, S.; Gutiérrez-Grijalva, P.E.; Garcia-Carrasco, M. Antiproliferative Activity and Mechanisms of Action of Plant-derived Flavonoids on Breast Cancer. Curr. Top. Med. Chem. 2023, 23, 1937–1951. [Google Scholar] [CrossRef] [PubMed]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Guo, S.; Rajaram, P.; Lee, M.; Chen, G.; Fong, R.; Gonzalez, A.; Zhang, Q.; Zheng, S.; et al. Structure-activity relationship and pharmacokinetic studies of 3-O-substitutedflavonols as anti-prostate cancer agents. Eur. J. Med. Chem. 2018, 157, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, H.; Yuan, M.; Zhou, J.; Tu, Q.; Liu, J.-J.; Wang, J. Synthesis and Biological Evaluation of Apigenin Derivatives as Antibacterial and Antiproliferative Agents. Molecules 2013, 18, 11496–11511. [Google Scholar] [CrossRef]
- Hennebelle, T.; Sahpaz, S.; Gressier, B.; Joseph, H.; Bailleul, F. Antioxidant and neurosedative properties of polyphenols and iridoids from Lippia alba. Phytother. Res. PTR 2008, 22, 256–258. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation. Foods 2013, 2, 90–99. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. PCA 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Zgorka, G.; Glowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Al-Kalaldeh, J.Z.; Abu-Dahab, R.; Afifi, F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010, 30, 271–278. [Google Scholar] [CrossRef]
- Savini, I.; Arnone, R.; Catani, M.V.; Avigliano, L. Origanum Vulgare Induces Apoptosis in Human Colon Cancer Caco2 Cells. Nutr. Cancer 2009, 61, 381–389. [Google Scholar] [CrossRef]
- Son, Y.-O.; Lee, K.-Y.; Lee, J.-C.; Jang, H.-S.; Kim, J.-G.; Jeon, Y.-M.; Jang, Y.-S. Selective antiproliferative and apoptotic effects of flavonoids purified from Rhus verniciflua Stokes on normal versus transformed hepatic cell lines. Toxicol. Lett. 2005, 155, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Lin, Y.; He, L.; Ou, R.X.; Chen, T.; Zhang, X.; Li, Q.R.; Zeng, Z.; Long, Q.D. Two New Isoprenoid Flavonoids from Sophora flavescens with Antioxidant and Cytotoxic Activities. Molecules 2021, 26, 7228. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Jo, J.K.; Ku, J.M.; Choi, H.S.; Choi, Y.K.; Woo, J.K.; Kim, H.I.; Kang, S.Y.; Lee, K.M.; Nam, K.W.; et al. Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci. Rep. 2015, 35, e00276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Q.; Huang, L.; Yang, F.; Liu, A.; Zhang, J. Combination of lapatinib and luteolin enhances the therapeutic efficacy of lapatinib on human breast cancer through the FOXO3a/NQO1 pathway. Biochem. Biophys. Res. Commun. 2020, 531, 364–371. [Google Scholar] [CrossRef]
- Tao, S.F.; He, H.F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Choi, Y.K.; Woo, J.K.; Kim, M.; Kim, I.; Na, C.H.; Hur, H.; Jang, B.H.; et al. Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in HER2-overexpressing BT-474 breast cancer cells. Oncol. Rep. 2016, 36, 31–42. [Google Scholar] [CrossRef]
- Abaza, M.S.I.; Orabi, K.Y.; Al-Quattan, E.; Al-Attiyah, R.a.J. Growth inhibitory and chemo-sensitization effects of naringenin, a natural flavanone purified from Thymus vulgaris, on human breast and colorectal cancer. Cancer Cell Int. 2015, 15, 46. [Google Scholar] [CrossRef]
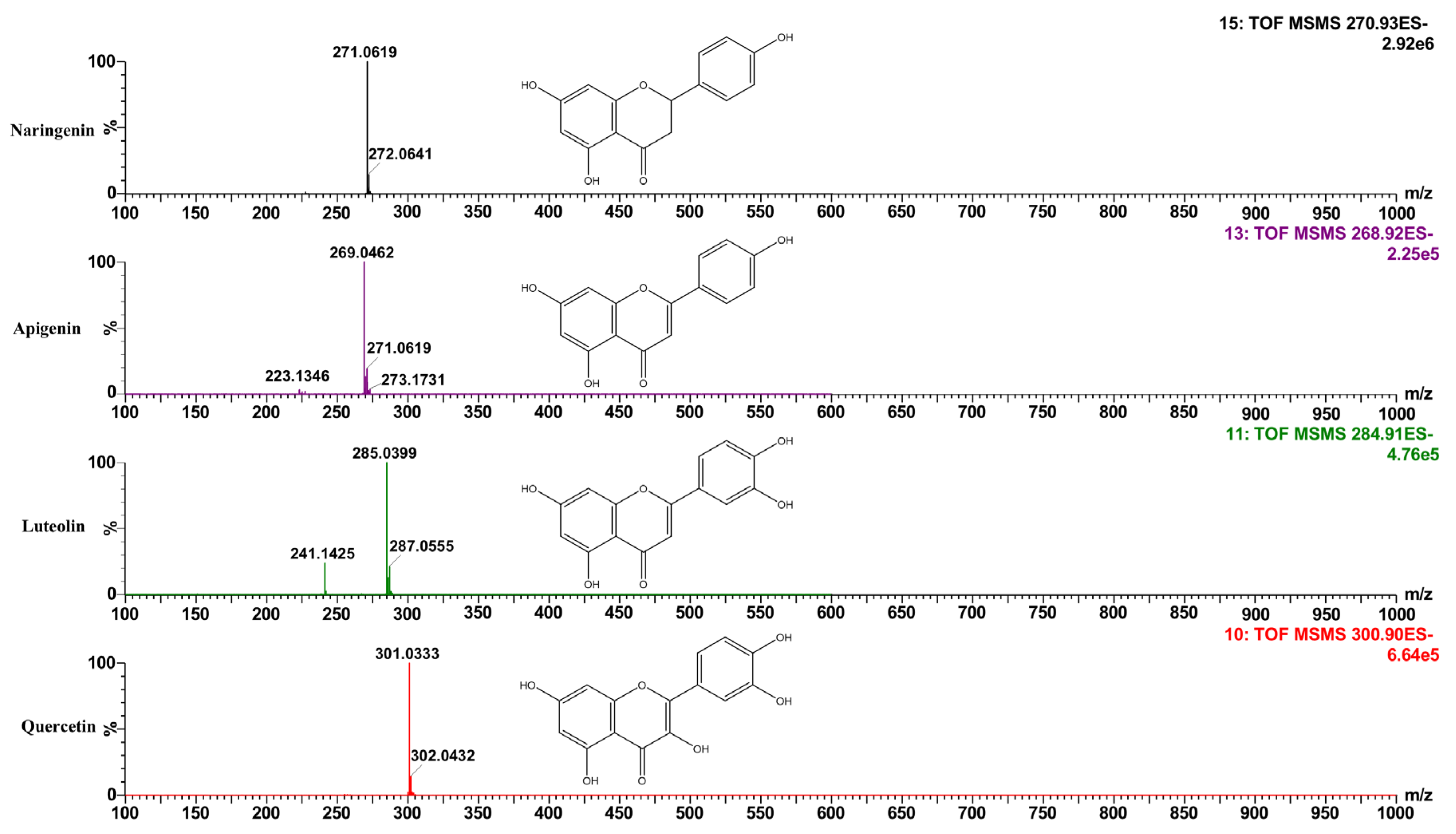

| MS | [M-H]- | Fragmentation Pattern | Flavonoid | Concentration (µg/g) |
|---|---|---|---|---|
| 302.04 | 301.03 | 302.03, 303.04 | Quercetin | 250.23 ± 18.15 |
| 286.04 | 285.04 | 151.00, 286.04 | Luteolin | 38.24 ± 1.12 |
| 272.06 | 271.06 | 151.00, 177.02, 269.04 | Naringenin | 7758.71 ± 32.15 |
| 270.05 | 269.04 | 151.00, 270.05 | Apigenin | 122.60 ± 10.65 (EQ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Criollo-Mendoza, M.S.; Heredia, J.B.; Contreras-Angulo, L.A.; García-Aguiar, I.; Gutiérrez-Grijalva, E.P. Apoptotic Potential of Polyphenol Extract of Mexican Oregano Lippia graveolens Kunth on Breast Cancer Cells MDA-MB-231. Appl. Sci. 2025, 15, 6393. https://doi.org/10.3390/app15126393
Criollo-Mendoza MS, Heredia JB, Contreras-Angulo LA, García-Aguiar I, Gutiérrez-Grijalva EP. Apoptotic Potential of Polyphenol Extract of Mexican Oregano Lippia graveolens Kunth on Breast Cancer Cells MDA-MB-231. Applied Sciences. 2025; 15(12):6393. https://doi.org/10.3390/app15126393
Chicago/Turabian StyleCriollo-Mendoza, Marilyn S., José Basilio Heredia, Laura A. Contreras-Angulo, Israel García-Aguiar, and Erick Paul Gutiérrez-Grijalva. 2025. "Apoptotic Potential of Polyphenol Extract of Mexican Oregano Lippia graveolens Kunth on Breast Cancer Cells MDA-MB-231" Applied Sciences 15, no. 12: 6393. https://doi.org/10.3390/app15126393
APA StyleCriollo-Mendoza, M. S., Heredia, J. B., Contreras-Angulo, L. A., García-Aguiar, I., & Gutiérrez-Grijalva, E. P. (2025). Apoptotic Potential of Polyphenol Extract of Mexican Oregano Lippia graveolens Kunth on Breast Cancer Cells MDA-MB-231. Applied Sciences, 15(12), 6393. https://doi.org/10.3390/app15126393










