A Study of the Influence of Sodium Alginate Molecular Weight and Its Crosslinking on the Properties of Potato Peel Waste-Based Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Method
2.3. Characterization
2.3.1. Thermogravimetric Analysis (TGA)
2.3.2. Dynamical Mechanical Properties (DMA)
2.3.3. Tensile Characterization
2.3.4. Water Vapor Permeability (WVP)
2.3.5. Scanning Electron Microscopy (SEM)
2.4. Statistical Analysis
3. Results and Discussion
3.1. TGA Analysis
3.2. DMA Analysis
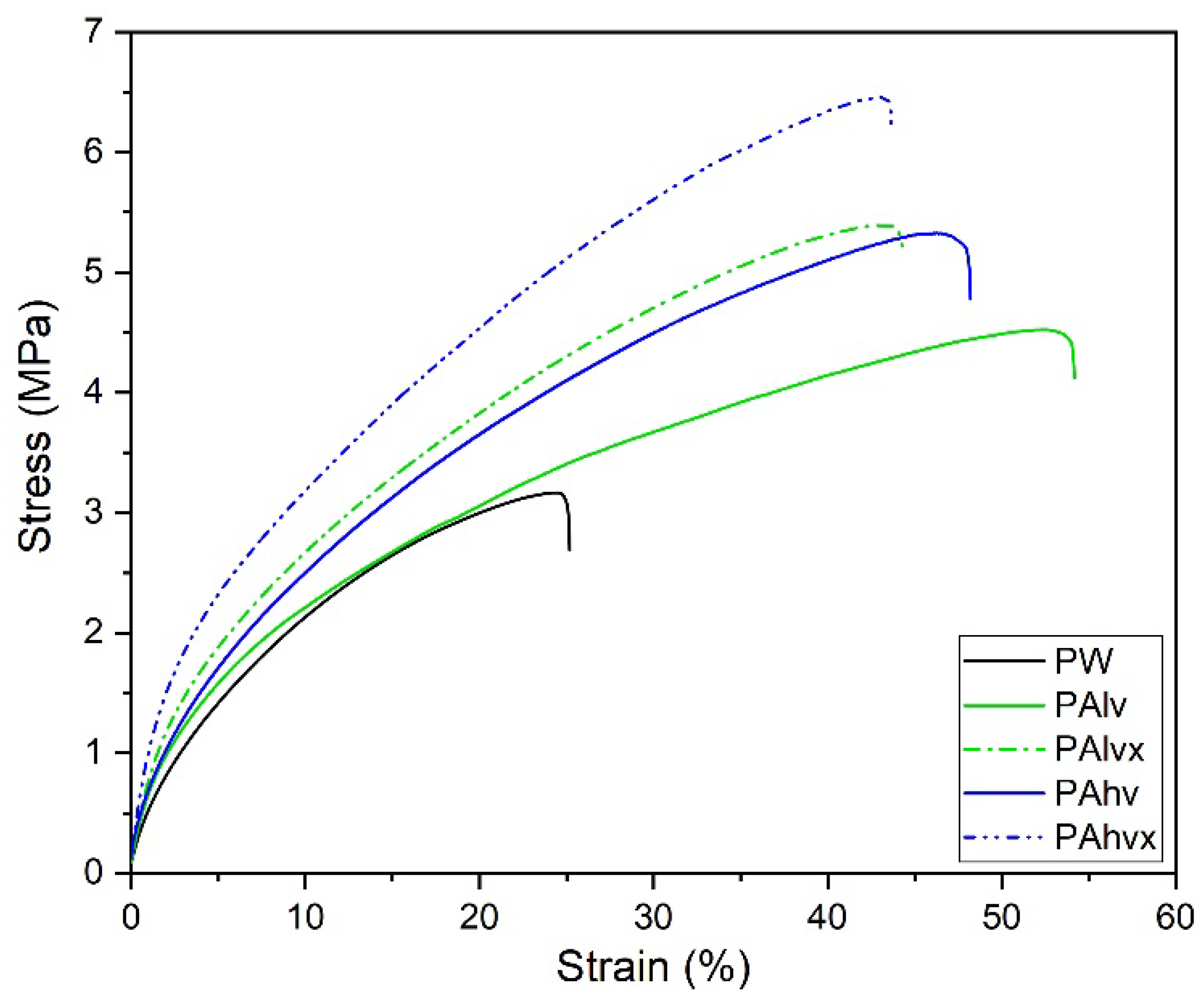
3.3. Mechanical Properties
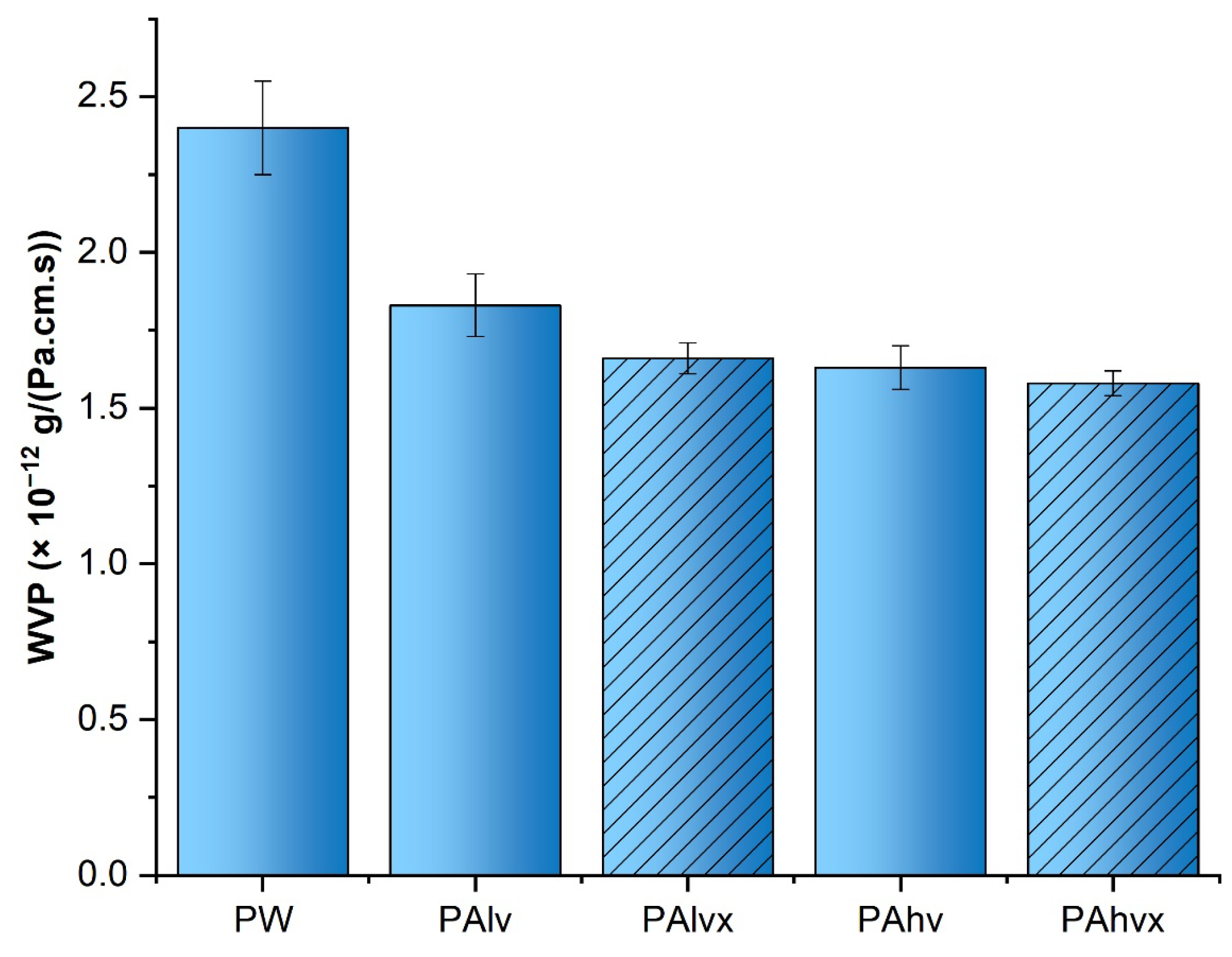
3.4. WVP Analysis
| Composites | Tensile Strength (MPa) | Elongation at Break (%) | WVP (×10−12 g/Pa·cm·s) | Ref. |
|---|---|---|---|---|
| Current study (PAhvx) | 6.47 | 43 | 1.41 | |
| PW + 5% bacterial cellulose (5–10) | 13.87 | 0.11 | 306 | Xie [22] |
| PW + sweet lime pomace (1:0.5) | 2.4 | 7.6 | 0.02 | Borah [59] |
| PW (3–0.3–0) | 9.48 | 5.33 | 8.31 | Kang [23] |
| PW starch/curcumin (CS-DES-Cur5) | 14.46 | 8.79 | 13.2 | Liu [60] |
| Starch/SA/ground coffee (TPS-Alg/SCG-10) | 67.6 | 5.2 | 9.6 | Nguyen [21] |
| Starch/SA/MMT (S-6%M) | 30 | 41 | 1.28 | Zhang [20] |
3.5. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PW | Potato peel waste |
| SA | Sodium alginate |
| lvSA | Low-viscosity sodium alginate |
| hvSA | High-viscosity sodium alginate |
| PAlv | Compound of PW/lvSA |
| PAhv | Compound of PW/hvSA |
| PAlvx | Crosslinked compound of PW/lvSA |
| PAhvx | Crosslinked compound of PW/hvSA |
References
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Mensah, R.A.; Försth, M.; Sas, G.; Restás, Á.; Addy, C.; Xu, Q.; Jiang, L.; Neisiany, R.E.; Singha, S. Circular economy in biocomposite development: State-of-the-art, challenges and emerging trends. Compos. Part C Open Access 2021, 5, 100138. [Google Scholar] [CrossRef]
- Lee, K.-T.; Chen, W.-H.; Sarles, P.; Park, Y.-K.; Ok, Y.S. Recover energy and materials from agricultural waste via thermochemical conversion. One Earth 2022, 5, 1200–1204. [Google Scholar] [CrossRef]
- Yevich, R.; Logan, J.A. An assessment of biofuel use and burning of agricultural waste in the developing world. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Khanal, S.; Karimi, K.; Majumdar, S.; Kumar, V.; Verma, R.; Bhatia, S.K.; Kuca, K.; Esteban, J.; Kumar, D. Sustainable utilization and valorization of potato waste: State of the art, challenges, and perspectives. Biomass Convers. Biorefinery 2023, 14, 23335–23360. [Google Scholar] [CrossRef]
- Rogols, S.; Sirovatka, D.M.; Widmaier, R.G. Non-Edible Composite Material Comprising Potato Peel Product. US20030034129A1, 20 February 2003. [Google Scholar]
- Chaffa, T.Y.; Meshesha, B.T.; Mohammed, S.A.; Jabasingh, S.A. Production, characterization, and optimization of starch-based biodegradable bioplastic from waste potato (Solanum tuberosum) peel with the reinforcement of false banana (Ensete ventricosum) fiber. Biomass Convers. Biorefinery 2022, 14, 27365–27377. [Google Scholar] [CrossRef]
- Zeleke, T.S.; Yihun, F.A.; Ayana, M.T.; Kassa, M.T.; Alemante, M.F. Enhancing the thermo-mechanical properties of thermoplastic starch films using rice straw fibers as reinforcement. Chem. Afr. 2023, 6, 2321–2329. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Gullón, B.; Yáñez, R. Identification and recovery of valuable bioactive compounds from potato peels: A comprehensive review. Antioxidants 2021, 10, 1630. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, S.; Milliron, H.; Han, Q. The efficacy of phenolic compound extraction from potato peel waste. Processes 2022, 10, 2326. [Google Scholar] [CrossRef]
- Hasanin, M.S. Simple, economic, ecofriendly method to extract starch nanoparticles from potato peel waste for biological applications. Starch-Starke 2021, 73, 2100055. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Shapourabadi, M.; Elkoun, S.; Robert, M. Microwave-Assisted Chemical Purification and Ultrasonication for Extraction of Nano-Fibrillated Cellulose from Potato Peel Waste. Macromol 2023, 3, 766–781. [Google Scholar] [CrossRef]
- Arapoglou, D.; Varzakas, T.; Vlyssides, A.; Israilides, C. Ethanol production from potato peel waste (PPW). Waste Manag. 2010, 30, 1898–1902. [Google Scholar] [CrossRef] [PubMed]
- Avérous, L.; Halley, P.J. Biocomposites based on plasticized starch. Biofuels Bioprod. Biorefining 2009, 3, 329–343. [Google Scholar] [CrossRef]
- Müller, C.M.; Laurindo, J.B.; Yamashita, F. Effect of cellulose fibers addition on the mechanical properties and water vapor barrier of starch-based films. Food Hydrocoll. 2009, 23, 1328–1333. [Google Scholar] [CrossRef]
- Wilhelm, H.-M.; Sierakowski, M.-R.; Souza, G.; Wypych, F. Starch films reinforced with mineral clay. Carbohydr. Polym. 2003, 52, 101–110. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Fazilah, A.; Maizura, M.; Abd Karim, A.; Bhupinder, K.; Rajeev, B. Physical and mechanical properties of sago starch—Alginate films incorporated with calcium chloride. Int. Food Res. J. 2011, 18, 1027–1033. [Google Scholar]
- Zhang, M.; Chen, H. Development and characterization of starch-sodium alginate-montmorillonite biodegradable antibacterial films. Int. J. Biol. Macromol. 2023, 233, 123462. [Google Scholar] [CrossRef]
- Nguyen, V.H.; MN, P.; Lee, D.-W.; Lee, I.C.; Song, J.-I. Films derived from thermoplastic starch/alginate/spent coffee grounds for food packaging applications. J. Polym. Res. 2023, 30, 191. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Min, S.C. Potato peel-based biopolymer film development using high-pressure homogenization, irradiation, and ultrasound. LWT-Food Sci. Technol. 2010, 43, 903–909. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; de Claville Christiansen, J. Mechanical properties of alginate hydrogels cross-linked with multivalent cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, D.; Lebedev, V.; Lebedeva, K.; Cherkashina, A.; Petrushenko, S.; Bogoyavlenska, O.; Olkhovska, A.; Hrubnyk, I.; Maloshtan, L.; Klochko, N. Thermosensitive and Wound-Healing Gelatin-Alginate Biopolymer Hydrogels Modified with Humic Acids. J. Renew. Mater. 2024, 12, 1691–1713. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Wang, H.; Song, Z.; Yu, D.; Li, G.; Liu, X.; Liu, W. Progress in Research on Metal Ion Crosslinking Alginate-Based Gels. Gels 2024, 11, 16. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Khair, T.; Kazantseva, M.A.; Zuev, Y.F.; Zueva, O.S. Ion-induced polysaccharide gelation: Peculiarities of alginate egg-box association with different divalent cations. Polymers 2023, 15, 1243. [Google Scholar] [CrossRef]
- Giz, A.S.; Berberoglu, M.; Bener, S.; Aydelik-Ayazoglu, S.; Bayraktar, H.; Alaca, B.E.; Catalgil-Giz, H. A detailed investigation of the effect of calcium crosslinking and glycerol plasticizing on the physical properties of alginate films. Int. J. Biol. Macromol. 2020, 148, 49–55. [Google Scholar] [CrossRef]
- Choi, I.; Lee, Y.; Lyu, J.S.; Lee, J.-S.; Han, J. Characterization of ionically crosslinked alginate films: Effect of different anion-based metal cations on the improvement of water-resistant properties. Food Hydrocoll. 2022, 131, 107785. [Google Scholar] [CrossRef]
- Vicini, S.; Castellano, M.; Mauri, M.; Marsano, E. Gelling process for sodium alginate: New technical approach by using calcium rich micro-spheres. Carbohydr. Polym. 2015, 134, 767–774. [Google Scholar] [CrossRef]
- ASTM D882. The Definitive Guide to ASTM D882—Tensile Testing of Thin Plastic Film. Available online: https://www.instron.com/en/testing-solutions/astm-standards/astm-d882/ (accessed on 27 May 2025).
- ASTM F1249; Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://store.astm.org/f1249-20.html (accessed on 27 May 2025).
- Tyuftin, A.A.; Pecorini, F.; Zanardi, E.; Kerry, J.P. Parameters affecting the water vapour permeability of gelatin films as evaluated by the infrared detecting method ASTM F1249. Sustainability 2022, 14, 9018. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Castillo, L.A.; Barbosa, S.E.; De Angelis, M.G. Barrier properties and mechanical strength of bio-renewable, heat-sealable films based on gelatin, glycerol and soybean oil for sustainable food packaging. React. Funct. Polym. 2018, 125, 29–36. [Google Scholar] [CrossRef]
- Yi, J.; Kim, Y.; Bae, H.; Whiteside, W.; Park, H. Influence of transglutaminase-induced cross-linking on properties of fish gelatin films. J. Food Sci. 2006, 71, E376–E383. [Google Scholar] [CrossRef]
- Fu, S.; Buckner, I.S.; Block, L.H. Inter-grade and inter-batch variability of sodium alginate used in alginate-based matrix tablets. AAPS PharmSciTech 2014, 15, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Montes, L.; Gisbert, M.; Moreira, R. Water sorption isotherms of different sodium alginates: Thermodynamic evaluation and influence of mannuronate-guluronate copolymers. J. Food Process. Preserv. 2022, 46, e17179. [Google Scholar] [CrossRef]
- Liang, S.; McDonald, A.G. Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J. Agric. Food Chem. 2014, 62, 8421–8429. [Google Scholar] [CrossRef]
- Jiang, Y.; Pang, X.; Deng, Y.; Sun, X.; Zhao, X.; Xu, P.; Shao, P.; Zhang, L.; Li, Q.; Li, Z. An alginate hybrid sponge with high thermal stability: Its flame retardant properties and mechanism. Polymers 2019, 11, 1973. [Google Scholar] [CrossRef]
- Pigłowska, M.; Kurc, B.; Rymaniak, Ł.; Lijewski, P.; Fuć, P. Kinetics and thermodynamics of thermal degradation of different starches and estimation the OH group and H2O content on the surface by TG/DTG-DTA. Polymers 2020, 12, 357. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Thermal behavior of green cellulose-filled thermoplastic elastomer polymer blends. Molecules 2020, 25, 1279. [Google Scholar] [CrossRef]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Physico-mechanical properties of biodegradable starch nanocomposites. Macromol. Mater. Eng. 2009, 294, 169–177. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A review of the effect of plasticizers on the physical and mechanical properties of alginate-based films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Siddaramaiah; Swamy, T.M.; Ramaraj, B.; Lee, J.H. Sodium alginate and its blends with starch: Thermal and morphological properties. J. Appl. Polym. Sci. 2008, 109, 4075–4081. [Google Scholar] [CrossRef]
- Russo, R.; Abbate, M.; Malinconico, M.; Santagata, G. Effect of polyglycerol and the crosslinking on the physical properties of a blend alginate-hydroxyethylcellulose. Carbohydr. Polym. 2010, 82, 1061–1067. [Google Scholar] [CrossRef]
- Urquiza, T.K.V.; Pérez, O.P.; Saldaña, M.G. Effect of the cross-linking with calcium ions on the structural and thermo-mechanical properties of alginate films. MRS Online Proc. Libr. (OPL) 2011, 1355, 1113550616. [Google Scholar] [CrossRef]
- Mileti, O.; Baldino, N.; Marchio, V.; Lupi, F.R.; Gabriele, D. Rheological and Textural Investigation to Design Film for Packaging from Potato Peel Waste. Gels 2024, 10, 681. [Google Scholar] [CrossRef]
- Luan, Q.Y.; Wang, Y.S.; Chen, Y.; Chen, H.H. Review on improvement of physicochemical properties of sodium alginate-based edible films. J. Food Sci. 2025, 90, e70016. [Google Scholar] [CrossRef]
- Gao, X.; Fu, C.; Li, M.; Qi, X.; Jia, X. Effects of biodegradation of corn-starch–sodium-alginate-based liquid mulch film on soil microbial functions. Int. J. Environ. Res. Public Health 2022, 19, 8631. [Google Scholar] [CrossRef]
- Qosim, N.; Dai, Y.; Williams, G.R.; Edirisinghe, M. Structure, properties, forming, and applications of alginate fibers: A review. Int. Mater. Rev. 2024, 69, 309–333. [Google Scholar] [CrossRef]
- Bekin, S.; Sarmad, S.; Gürkan, K.; Yenici, G.; Keçeli, G.; Gürdağ, G. Dielectric, thermal, and swelling properties of calcium ion-crosslinked sodium alginate film. Polym. Eng. Sci. 2014, 54, 1372–1382. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Mukhopadhyaya, P.; Kumaran, K.; Lackey, J.; van Reenen, D. Water Vapor Transmission Measurement and Significance of Corrections; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Yun, D.; Liu, J. Preparation, Characterization and Application of Active Food Packaging Films Based on Sodium Alginate and Twelve Varieties of Mandarin Peel Powder. Foods 2024, 13, 1174. [Google Scholar] [CrossRef]
- Li, W.; Zhao, M.; Xia, X.; Zhu, Y. Improving Structural, Physical, and Sensitive Properties of Sodium Alginate–Purple Sweet Potato Peel Extracts Indicator Films by Varying Drying Temperature. Foods 2024, 13, 2477. [Google Scholar] [CrossRef] [PubMed]
- Asiri, S.A.; Matar, A.; Ismail, A.M.; Farag, H.A. Sodium alginate edible films incorporating cactus pear extract: Antimicrobial, chemical, and mechanical properties. Ital. J. Food Sci. 2024, 36, 151–168. [Google Scholar] [CrossRef]
- bt Ibrahim, S.F.; Mohd Azam, N.A.N.; Mat Amin, K.A. Sodium alginate film: The effect of crosslinker on physical and mechanical properties. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012063. [Google Scholar] [CrossRef]
- Akshaya, S.; Nathanael, A.J. A Review on Hydrophobically Associated Alginates: Approaches and Applications. ACS Omega 2024, 9, 4246–4262. [Google Scholar] [CrossRef]
- Borah, P.P.; Das, P.; Badwaik, L.S. Ultrasound treated potato peel and sweet lime pomace based biopolymer film development. Ultrason. Sonochemistry 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Zhang, L.; Cao, W.; Wang, H.; Chen, G.; Wang, S. Preparation and properties of biodegradable films made of cationic potato-peel starch and loaded with curcumin. Food Hydrocoll. 2022, 130, 107690. [Google Scholar] [CrossRef]
- Mahmoudi, C.; Tahraoui Douma, N.; Mahmoudi, H.; Iurciuc, C.E.; Popa, M.; Hamcerencu, M.; Andrițoiu, C.V. Developing and Characterizing a Biocompatible Hydrogel Obtained by Cross-Linking Gelatin with Oxidized Sodium Alginate for Potential Biomedical Applications. Polymers 2024, 16, 3143. [Google Scholar] [CrossRef]
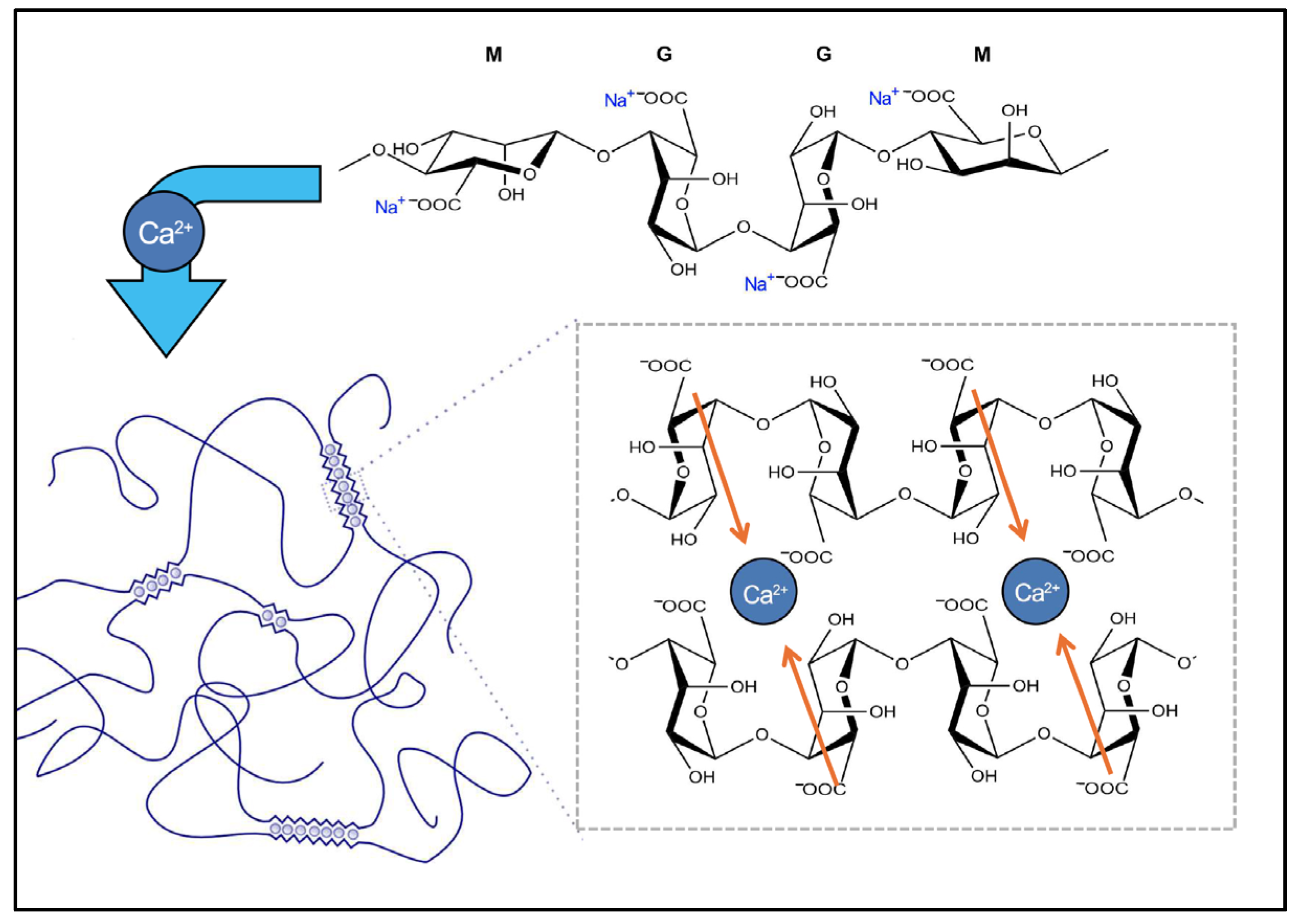
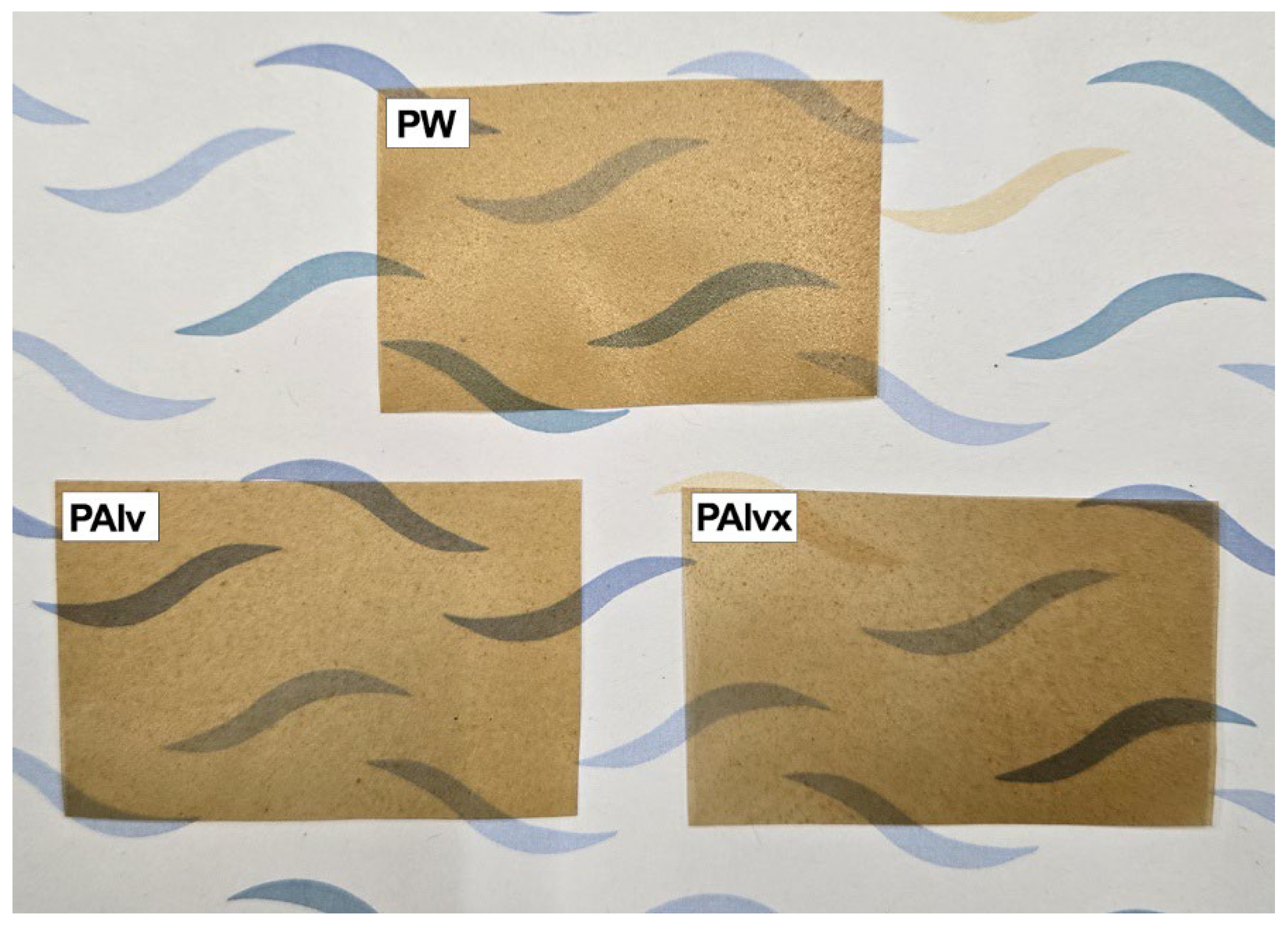



| Sample | Stage I (50–150 °C) | Stage II (200–350 °C) | ||
|---|---|---|---|---|
| Tg (°C) | Δm (%) | Decomposition Temperatures (°C) | Δm (%) | |
| Pure PW | 60 ± 2 | 11 ± 0.8 | 290 ± 1 | 62 ± 1.4 |
| PAlv | 48 ± 5 | 15 ± 1.1 | 225 ± 1; 275 ± 1 | 57 ± 0.5 |
| PAlvx | 45 ± 5 | 16 ± 1 | 230 ± 2; 280 ± 1 | 55 ± 0.3 |
| PAhv | 38 ± 4 | 8.5 ± 1.4 | 220 ± 2; 270 ± 1 | 60 ± 2.8 |
| PAhvx | 30 ± 3 | 8.5 ± 1.2 | 240 ± 1; 290 ± 1 | 60 ± 1.5 |
| Composition | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| Pure PW | 3.17 ± 0.42 | 25 ± 2.9 |
| PAlv | 4.55 ± 0.28 | 54 ± 2.3 |
| PAlvx | 5.4 ± 0.4 | 44 ± 5.6 |
| PAhv | 5.34 ± 0.74 | 48 ± 6.7 |
| PAhvx | 6.47 ± 0.16 | 43 ± 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghi-Shapourabadi, M.; Robert, M.; Elkoun, S. A Study of the Influence of Sodium Alginate Molecular Weight and Its Crosslinking on the Properties of Potato Peel Waste-Based Films. Appl. Sci. 2025, 15, 6385. https://doi.org/10.3390/app15126385
Sadeghi-Shapourabadi M, Robert M, Elkoun S. A Study of the Influence of Sodium Alginate Molecular Weight and Its Crosslinking on the Properties of Potato Peel Waste-Based Films. Applied Sciences. 2025; 15(12):6385. https://doi.org/10.3390/app15126385
Chicago/Turabian StyleSadeghi-Shapourabadi, Mohsen, Mathieu Robert, and Said Elkoun. 2025. "A Study of the Influence of Sodium Alginate Molecular Weight and Its Crosslinking on the Properties of Potato Peel Waste-Based Films" Applied Sciences 15, no. 12: 6385. https://doi.org/10.3390/app15126385
APA StyleSadeghi-Shapourabadi, M., Robert, M., & Elkoun, S. (2025). A Study of the Influence of Sodium Alginate Molecular Weight and Its Crosslinking on the Properties of Potato Peel Waste-Based Films. Applied Sciences, 15(12), 6385. https://doi.org/10.3390/app15126385








