Organic Acid-Assisted Hydrothermal Leaching of Silver from End-of-Life Photovoltaic Panels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment Procedures
2.2.1. Thermal Treatment of EoL Si PV Panels
2.2.2. Toluene Treatment of EoL Si PV Panels
2.3. Characterization of EoL Si PV Panels
2.4. Hydrothermal Leaching Experiments
2.5. Experimental Design: Response Surface Methodology
3. Results and Discussion
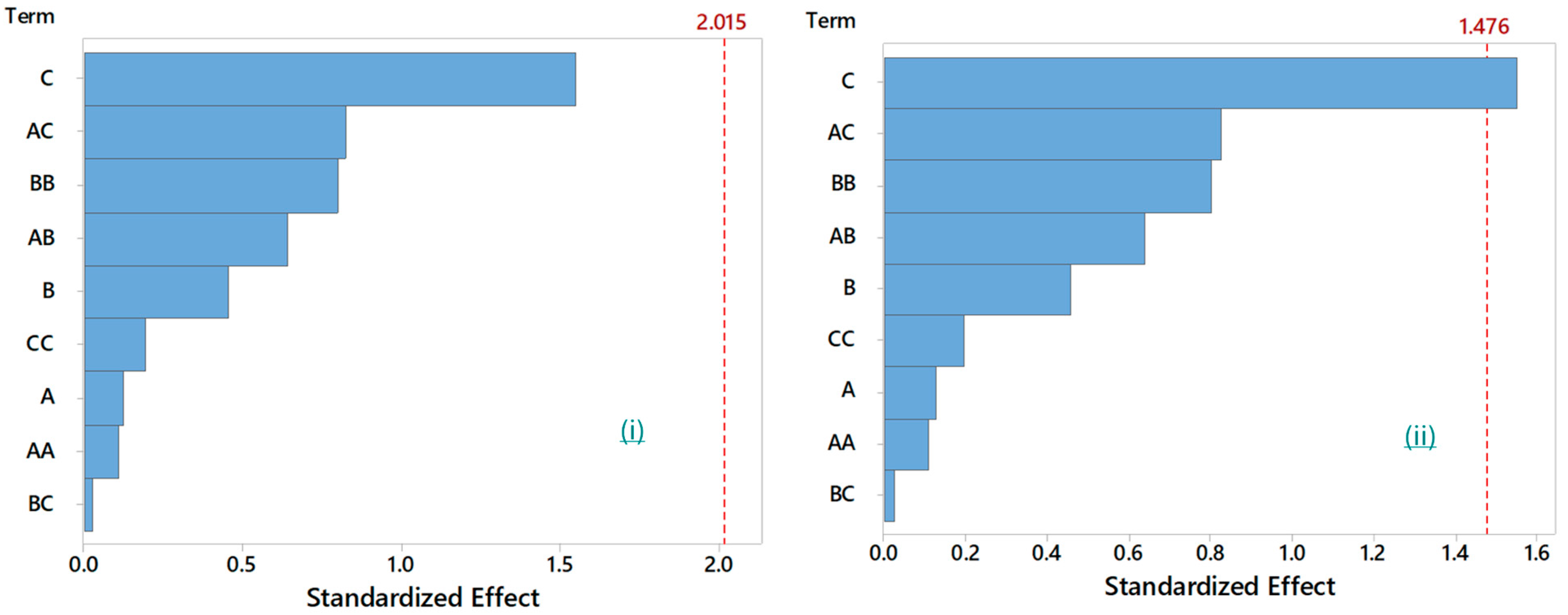
3.1. Hydrothermal Leaching with Citric Acid
3.2. Hydrothermal Leaching with Oxalic Acid
3.3. Comparison to HNO3 Leaching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kastanaki, E. Dynamic Assessment of Photovoltaic Waste Streams in the EU-27 Countries under the Circular Economy Principles of ‘Reduce, Reuse and Recycle. Resour. Conserv. Recycl. 2025, 214, 108033. [Google Scholar] [CrossRef]
- Kastanaki, E.; Lagoudakis, E.; Kalogerakis, G.; Giannis, A. Hydrothermal Leaching of Silver and Aluminum from Waste Monocrystalline and Polycrystalline Photovoltaic Panels. Appl. Sci. 2023, 13, 3602. [Google Scholar] [CrossRef]
- Farrell, C.C.; Osman, A.I.; Doherty, R.; Saad, M.; Zhang, X.; Murphy, A.; Harrison, J.; Vennard, A.S.M.; Kumaravel, V.; Al-Muhtaseb, A.H.; et al. Technical Challenges and Opportunities in Realising a Circular Economy for Waste Photovoltaic Modules. Renew. Sustain. Energy Rev. 2020, 128, 109911. [Google Scholar] [CrossRef]
- IRENA End-of-Life Management Solar Photovoltaic Panels. 2016. Available online: https://www.irena.org/publications/2016/Jun/End-of-life-management-Solar-Photovoltaic-Panels (accessed on 12 March 2025).
- Dias, P.R.; Benevit, M.G.; Veit, H.M. Photovoltaic Solar Panels of Crystalline Silicon: Characterization and Separation. Waste Manag Res 2016, 34, 235–245. [Google Scholar] [CrossRef]
- de Oliveira, L.S.S.; Lima, M.T.W.D.C.; Yamane, L.; Siman, R.R. Silver Recovery from End-of-Life Photovoltaic Panels. Detritus 2020, 10, 62–74. [Google Scholar] [CrossRef]
- Click, N.; Teknetzi, I.; Adcock, R.; Tao, M.; Ebin, B. Silver Cementation Mechanism for Leaching Silicon Solar Cells in Nitric Acid. Sol. Energy 2024, 283, 113009. [Google Scholar] [CrossRef]
- Lisińska, M.; Gajda, B.; Saternus, M.; Brozová, S.; Wojtal, T.; Rzelewska-Piekut, M. The Effect of Organic Acids as Leaching Agents for Hydrometallurgical Recovery of Metals from PCBs. Metalurgija 2022, 61, 609–612. [Google Scholar]
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging Role of Organic Acids in Leaching of Valuable Metals from Refinery-Spent Hydroprocessing Catalysts, and Potential Techno-Economic Challenges: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1–43. [Google Scholar] [CrossRef]
- Yang, E.-H.; Lee, J.-K.; Lee, J.-S.; Ahn, Y.-S.; Kang, G.-H.; Cho, C.-H. Environmentally Friendly Recovery of Ag from End-of-Life c-Si Solar Cell Using Organic Acid and Its Electrochemical Purification. Hydrometallurgy 2017, 167, 129–133. [Google Scholar] [CrossRef]
- Chung, J.; Seo, B.; Lee, J.; Kim, J.Y. Comparative Analysis of I2-KI and HNO3 Leaching in a Life Cycle Perspective: Towards Sustainable Recycling of End-of-Life c-Si PV Panel. J. Hazard. Mater. 2021, 404, 123989. [Google Scholar] [CrossRef]
- Zheng, R.; Luo, M.; Li, B.; Jia, M.; Zhang, H.; Liu, S.; Lu, Y.; Jiang, L.; Zhang, Z.; Liu, F. Eco-Friendly Recovery and Preparation of High Purity Nano Silver Powders from Retired Photovoltaic Solar Cells. Sep. Purif. Technol. 2025, 359, 130343. [Google Scholar] [CrossRef]
- Fransson, R. Investigation of Silver Recovery from Thin Film CIGS Solar Cells by Selective Leaching. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2020. [Google Scholar]
- Tanong, K.; Coudert, L.; Chartier, M.; Mercier, G.; Blais, J.-F. Study of the Factors Influencing the Metals Solubilisation from a Mixture of Waste Batteries by Response Surface Methodology. Environ. Technol. 2017, 38, 3167–3179. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Q.; Nakajima, A.; Zhang, Z.; Watanabe, M. Organic Acid-Based Hydrothermal Leaching of LiFePO4 Cathode Materials. Adv. Sustain. Syst. 2024, 8, 2300421. [Google Scholar] [CrossRef]
- Zheng, Q.; Watanabe, M.; Iwatate, Y.; Azuma, D.; Shibazaki, K.; Hiraga, Y.; Kishita, A.; Nakayasu, Y. Hydrothermal Leaching of Ternary and Binary Lithium-Ion Battery Cathode Materials with Citric Acid and the Kinetic Study. J. Supercrit. Fluids 2020, 165, 104990. [Google Scholar] [CrossRef]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of Response Surface Methodology (RSM) for Optimization of Leaching Parameters for Ash Reduction from Low-Grade Coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Song, D.; Wang, T.; Liu, Z.; Zhao, S.; Quan, J.; Li, G.; Zhu, H.; Huang, J.; He, W. Characteristic Comparison of Leaching Valuable Metals from Spent Power Li-Ion Batteries for Vehicles Using the Inorganic and Organic Acid System. J. Environ. Chem. Eng. 2022, 10, 107102. [Google Scholar] [CrossRef]
- Savvilotidou, V.; Gidarakos, E. Pre-Concentration and Recovery of Silver and Indium from Crystalline Silicon and Copper Indium Selenide Photovoltaic Panels. J. Clean. Prod. 2020, 250, 119440. [Google Scholar] [CrossRef]
- Trivedi, H.K.; Meshram, A.; Gupta, R. Recycling of Photovoltaic Modules for Recovery and Repurposing of Materials. J. Environ. Chem. Eng. 2023, 11, 109501. [Google Scholar] [CrossRef]
- Kang, S.; Yoo, S.; Lee, J.; Boo, B.; Ryu, H. Experimental Investigations for Recycling of Silicon and Glass from Waste Photovoltaic Modules. Renew. Energy 2012, 47, 152–159. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef]
- Xu, X.; Lai, D.; Wang, G.; Wang, Y. Nondestructive Silicon Wafer Recovery by a Novel Method of Solvothermal Swelling Coupled with Thermal Decomposition. Chem. Eng. J. 2021, 418, 129457. [Google Scholar] [CrossRef]
- Kant, N.; Singh, P. Review of next Generation Photovoltaic Solar Cell Technology and Comparative Materialistic Development. Mater. Today Proc. 2022, 56, 3460–3470. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Y.; Zhou, M.; Huang, X.; Gao, Y.; Li, K.; Chen, Z.; Zhou, C.; Zhou, Z.; Zheng, C.; et al. Short Process Recovery of Silver and Purification Mechanism of Crystalline Silicon Deep Etching from End-of-Life Photovoltaic Cells. Chem. Eng. J. 2025, 510, 161651. [Google Scholar] [CrossRef]
- Yue, Y.; Zhuo, Y.; Li, Q.; Shen, Y. Experimental and Numerical Study of Extracting Silver from End-of-Life c-Si Photovoltaic Solar Cells in Rotating Systems. Resour. Conserv. Recycl. 2022, 186, 106548. [Google Scholar] [CrossRef]
- Jumari, A.; Yudha, C.S.; Nizam, M.; Dyartanti, E.R.; Suranto; Purwanto, A. An Environmentally Friendly Hydrometallurgy Process for the Recovery and Reuse of Metals from Spent Lithium-Ion Batteries, Using Organic Acid. Open Eng. 2022, 12, 485–494. [Google Scholar] [CrossRef]
- Jadhav, U.; Su, C.; Hocheng, H. Leaching of Metals from Printed Circuit Board Powder by an Aspergillus Niger Culture Supernatant and Hydrogen Peroxide. RSC Adv. 2016, 6, 43442–43452. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Somani, P.; Liang, H. Recycling of CIGS Flexible Solar Cells: Investigation of Organic Acids Leaching. 2023. Available online: http://hdl.handle.net/20.500.12380/307118 (accessed on 12 March 2025).
- Hamann, S.D. The Influence of Pressure on Ionization Equilibria in Aqueous Solutions. J Solut. Chem. 1982, 11, 63–68. [Google Scholar] [CrossRef]
- Klein, D. Organic Chemistry, 4th ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Luo, M.; Liu, F.; Zhou, Z.; Jiang, L.; Jia, M.; Lai, Y.; Li, J.; Zhang, Z. A Comprehensive Hydrometallurgical Recycling Approach for the Environmental Impact Mitigation of EoL Solar Cells. J. Environ. Chem. Eng. 2021, 9, 106830. [Google Scholar] [CrossRef]
- Deng, H.; Wang, B.; Xu, J.; Yang, G.; Shi, Z.; Zhu, H.; He, W.; Li, G. A Comprehensive Review of Whole Process Typical Hydrometallurgical Technologies for Recycling Waste Lithium-Ion Batteries. Sep. Purif. Technol. 2025, 363, 132234. [Google Scholar] [CrossRef]










| Element (% w/w) | p-Si Cell | m-Si Cell |
|---|---|---|
| Si | 64.46 | 65.18 |
| Al | 6.16 | 9.87 |
| Cu | 0.25 | 0.93 |
| Ag | 1.13 | 1.37 |
| Sn | 0.10 | 0.28 |
| Pb | 0.13 | 0.35 |
| Run Order | Concentration (M) | Time (min) | Temperature (°C) |
|---|---|---|---|
| 1 | 1.5 | 105 | 180 |
| 2 | 1 | 60 | 180 |
| 3 | 1.5 | 150 | 210 |
| 4 | 1 | 150 | 180 |
| 5 | 2 | 105 | 150 |
| 6 | 1.5 | 60 | 210 |
| 7 | 1 | 105 | 210 |
| 8 | 1.5 | 105 | 180 |
| 9 | 2 | 105 | 210 |
| 10 | 1.5 | 150 | 150 |
| 11 | 1 | 105 | 150 |
| 12 | 1.5 | 60 | 150 |
| 13 | 2 | 60 | 180 |
| 14 | 2 | 150 | 180 |
| 15 | 1.5 | 105 | 180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastanaki, E.; Athanasiadou, R.; Katsifou, A.; Giannis, A. Organic Acid-Assisted Hydrothermal Leaching of Silver from End-of-Life Photovoltaic Panels. Appl. Sci. 2025, 15, 6383. https://doi.org/10.3390/app15126383
Kastanaki E, Athanasiadou R, Katsifou A, Giannis A. Organic Acid-Assisted Hydrothermal Leaching of Silver from End-of-Life Photovoltaic Panels. Applied Sciences. 2025; 15(12):6383. https://doi.org/10.3390/app15126383
Chicago/Turabian StyleKastanaki, Eleni, Rafaela Athanasiadou, Anastasia Katsifou, and Apostolos Giannis. 2025. "Organic Acid-Assisted Hydrothermal Leaching of Silver from End-of-Life Photovoltaic Panels" Applied Sciences 15, no. 12: 6383. https://doi.org/10.3390/app15126383
APA StyleKastanaki, E., Athanasiadou, R., Katsifou, A., & Giannis, A. (2025). Organic Acid-Assisted Hydrothermal Leaching of Silver from End-of-Life Photovoltaic Panels. Applied Sciences, 15(12), 6383. https://doi.org/10.3390/app15126383








