Investigation of the Effects and Mechanisms of Biomass-Derived Alternative Fuels on Cement Clinker Formation and Hydration Processes
Abstract
1. Introduction
2. Materials and Methods
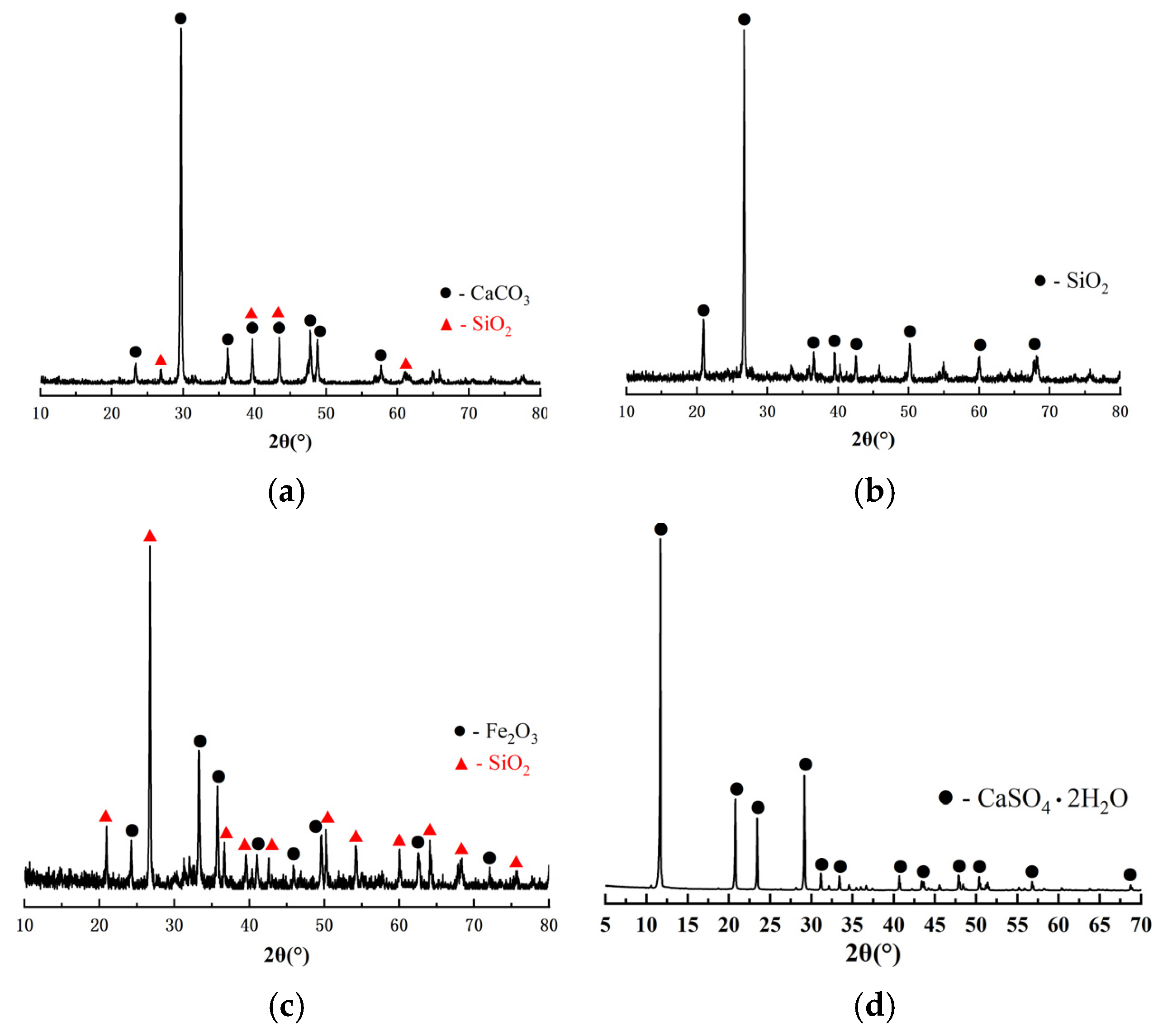
2.1. Raw Materials
2.2. Method
3. Results and Discussion
3.1. Combustion Analyzing
3.2. Clinker Characterization
3.2.1. f-CaO Content Analysis of Clinkers
3.2.2. XRD Analysis of Clinker
3.2.3. Cl− Contents Analysis of Clinkers
3.2.4. Effect of High Alkali Content on Mineral Phase of Clinker
3.2.5. Cement Physical Property
- (1)
- As shown in Table 6, within the same curing period, the addition of biomass alternative fuels led to a decrease in the 3-day flexural and compressive strength of the cement, Notably, this effect is particularly pronounced in the cement samples mixed with corncob alternative fuel. As the substitution ratio increases, the flexural strength after three days decreases by approximately 5%. However, after 28 days of curing, the flexural and compressive strengths were comparable to those of the cement without biomass fuel ash. Based on the conclusions drawn in the previous chapter of this study, on the one hand, high alkali content hinders the formation of C3S. On the other hand, when biomass alternative fuels are incorporated, some intermediate products, such as CaSiO3 formed during calcination, cannot be fully converted into C3S or C2S. This observation aligns well with the results obtained from the aforementioned clinker XRD analysis. These minerals, which are crucial for providing cement strength in the middle and late stages, exhibit a slower hydration rate in the early stage. However, after 28 days of continuous hydration, the primary hydration products (e.g., C-S-H gel and Ca(OH)2) stabilize, compensating for the lack of early strength. This further demonstrates that the ash deposited by biomass fuels at lower substitution ratios has a negligible impact on the long-term performance of clinker;
- (2)
- The setting time, standard consistency, specific surface area, and stability are key indicators for assessing the physical properties of cement [58]. As shown in Table 7, the incorporation of biofuel ash reduces the initial setting time of cement, the maximum reduction observed is 13 min. The effect on the final setting time is relatively minor. Furthermore, as the blending ratio of biomass alternative fuels increases, the standard consistency of the cement paste also increases. This is attributed to the rapid dissolution of alkali ions in water, which raises the ion concentration in the paste, thereby altering the hydration reaction rate and necessitating additional water to maintain the paste’s fluidity. Regarding volume stability, certain biomass fuels, such as HDG samples, exhibit slight expansion, primarily attributed to the presence of higher free lime (f-CaO), a finding consistent with the previously discussed XRD analysis of clinker. No significant abnormalities were observed in other aspects.
3.3. Characterization of Hydration Products
3.3.1. XRD Results
3.3.2. TG-DTG Analysis
3.3.3. SEM Analysis
4. Conclusions
- (1)
- The co-combustion of biomass fuel and coal can significantly reduce the ignition point of the mixed fuel by up to 54 °C, initiating the combustion reaction earlier and lowering the combustion temperature by approximately 62~93 °C. This facilitates efficient combustion and enhances the overall combustion rate;
- (2)
- High alkaline conditions, introduced by K2CO3 and Na2CO3, stabilized C2S and inhibited its conversion to C₃S. Alkalis also reacted with SiO2, Al2O₃, and Fe2O₃ to form alkali silicates and sulfates, consuming SiO2 and CaO needed for C₃S, thus reducing its content and increasing f-CaO in clinker. However, alkalis do not always have adverse effects. In this study, at a 20 wt% substitution level, the high-alkali ash from corn cobs promoted free CaO dissolution and reaction, likely due to improved combustion uniformity induced by moderate alkali content;
- (3)
- At the studied substitution ratios, all biomass fuels affected clinker mineral phases but had little impact on mechanical strength and hydration products. High-silicon fuels like rice straw promoted calcium silicate intermediate formation. Although 3-day flexural strength was slightly lower (within 5% fluctuation), longer hydration led to formation of C2S, Ca(OH)2, and C-S-H, compensating early strength loss.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amran, M.; Makul, N.; Fediuk, R.; Lee, Y.H.; Vatin, N.I.; Lee, Y.Y.; Mohammed, K. Global carbon recoverability experiences from the cement industry. Case Stud. Constr. Mater. 2022, 17, e01439. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Wang, H.; Gao, J.; Zhang, R.; Xu, G.; Wang, Z. Research on the improvement of carbon neutrality by utilizing agricultural waste: Based on a life cycle assessment of biomass briquette fuel heating system. J. Clean. Prod. 2024, 434, 140365. [Google Scholar] [CrossRef]
- Gebreslassie, M.G.; Bahta, S.T.; Mihrete, A.S. Development of alternative fuel for cement industries: The case of Messebo cement factory in Ethiopia. Waste Manag. Bull. 2023, 1, 58–70. [Google Scholar] [CrossRef]
- Rasheed, R.; Tahir, F.; Afzaal, M.; Ahmad, S.R. Decomposition analytics of carbon emissions by cement manufacturing—A way forward towards carbon neutrality in a developing country. Environ. Sci. Pollut. Res. 2022, 29, 49429–49438. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.E.T.; Dragunski, D.C.; Neckel, R.M.; Silvestro, L.; Savaris, G.; Schneider, R. Fresh Properties of Low-Carbon Cement Pastes Incorporating Industrial By-Products. J. Mater. Civ. Eng. 2025, 37, 04024443. [Google Scholar] [CrossRef]
- Bramantiyo, R.; Lestianingrum, E.; Cahyono, R.B. Industrial Application of Rice Husk as an Alternative Fuel in Cement Production for CO2 Reduction. ASEAN J. Chem. Eng. 2022, 22, 364–372. [Google Scholar] [CrossRef]
- Bahnasawy, N.; Al Anany, S.; Allam, N.K. Electrochemical catalysis for the production of green cement: Towards decarbonizing the cement industry. Catal. Sci. Technol. 2024, 14, 4087–4105. [Google Scholar] [CrossRef]
- Oguntola, O.; Boakye, K.; Simske, S. Towards Leveraging Artificial Intelligence for Sustainable Cement Manufacturing: A Systematic Review of AI Applications in Electrical Energy Consumption Optimization. Sustainability 2024, 16, 4798. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Das, B.B.; Adak, D. Roadmap to a net-zero carbon cement sector: Strategies, innovations and policy imperatives. J. Environ. Manag. 2024, 359, 121052. [Google Scholar] [CrossRef]
- Liu, W.H.; Liu, Y.; Wang, S.Y.; Lei, F.; Liu, X.Y.; Wan, Y.F.; Zhou, Z.Y.; Li, H. Hazardous waste alternative fuels to novel ecological energy: Combustion characteristics and effects on clinker’s environmental safety. Ceram. Int. 2024, 50, 48736–48754. [Google Scholar] [CrossRef]
- Sambataro, L.; Bre, F.; Ukrainczyk, N.; Koenders, E.A.B. Environmental benchmarks for the European cement industry. Sustain. Prod. Consum. 2024, 45, 429–449. [Google Scholar] [CrossRef]
- Yu, B.; Fu, L.L.; Chen, T.B.; Zheng, G.D.; Yang, J.X.; Cheng, Y.; Liu, Y.; Huang, X. Environmental impacts of cement kiln co-incineration sewage sludge biodried products in a scale-up trial. Waste Manag. 2024, 177, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Boschiero, M.; Pierobon, F.; Cavalli, R.; Zanetti, M. Comparative Life Cycle Assessment of Bioenergy Production from Different Wood Pellet Supply Chains. Forests 2020, 11, 1127. [Google Scholar] [CrossRef]
- Hashem, F.S.; Razek, T.A.; Mashout, H.A. Rubber and plastic wastes as alternative refused fuel in cement industry. Constr. Build. Mater. 2019, 212, 275–282. [Google Scholar] [CrossRef]
- Huang, M.R.; Ying, X.B.; Shen, D.S.; Feng, H.J.; Li, N.; Zhou, Y.Y.; Long, Y.Y. Evaluation of oil sludge as an alternative fuel in the production of Portland cement clinker. Constr. Build. Mater. 2017, 152, 226–231. [Google Scholar] [CrossRef]
- Recko, K. Production of Alternative Fuels Based on Sewage Sludge. Energies 2024, 17, 48. [Google Scholar] [CrossRef]
- Luo, Z.F.; Song, H.T.; Huang, Y.J.; Jin, B.S. Recent Advances on the Uses of Biomass Alternative Fuels in Cement Manufacturing Process: A Review. Energy Fuels 2024, 38, 7454–7479. [Google Scholar] [CrossRef]
- Çankaya, S. Investigating the environmental impacts of alternative fuel usage in cement production: A life cycle approach. Environ. Dev. Sustain. 2020, 22, 7495–7514. [Google Scholar] [CrossRef]
- Mandal, S.; Adhikari, A.; Chaulagain, A.; Thapa, A.; Gautam, S.M.; Lohani, S.P.; Uprety, B. Techno-enviro-economic assessment of hydropower-driven decarbonization pathways for Nepalese cement industry. J. Environ. Chem. Eng. 2024, 12, 114729. [Google Scholar] [CrossRef]
- Li, T.; Li, W.; Lou, Z.Y.; Wang, L.C. Comprehensive Analysis of Industrial Solid-Waste-to-Energy by Refuse-Derived Fuel Technology: A Case Study in Shanghai. Sustainability 2024, 16, 4234. [Google Scholar] [CrossRef]
- Conversano, A.; Sogni, D.; Lombardelli, G.; Di Bona, D.; Viganò, F.; Consonni, S. Energy and environmental assessment of solid recovered fuels valorisation: Waste-to-Chemicals options vs co-combustion in cement plants. Waste Manag. 2024, 190, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.L.L.; Feng, Z.S.; Wu, J.; Liang, H.; Wang, Y.H.; Zeng, W.F.; Wang, Y.C.; Tian, J.P.; Liu, R.; Chen, L.Y.J. Cement kiln geared up to dispose industrial hazardous wastes of megacity under industrial symbiosis. Resour. Conserv. Recycl. 2024, 202, 107358. [Google Scholar] [CrossRef]
- Tihin, G.L.; Mo, K.H.; Onn, C.C.; Ong, H.C.; Taufiq-Yap, Y.H.; Lee, H.V. Overview of municipal solid wastes-derived refuse-derived fuels for cement co-processing. Alex. Eng. J. 2023, 84, 153–174. [Google Scholar] [CrossRef]
- Uliasz-Bochenczyk, A.; Deja, J.; Mokrzycki, E. The use of alternative fuels in the cement industry as part of circular economy. Arch. Environ. Prot. 2021, 47, 109–117. [Google Scholar]
- de Oliveira, A.D.; Ducom, G.; de Castilhos, A.B., Jr.; Peres, S.; Juca, J.F.T.; Perier-Camby, H.; Bayard, R. Refuse derived fuel produced in Brazil: Physico-chemical analysis, standards and opportunities for energy recovery. J. Mater. Cycles Waste Manag. 2025, 27, 666–683. [Google Scholar] [CrossRef]
- Kusuma, R.T.; Hiremath, R.B.; Rajesh, P.; Kumar, B.; Renukappa, S. Sustainable transition towards biomass-based cement industry: A review. Renew. Sustain. Energy Rev. 2022, 163, 112503. [Google Scholar] [CrossRef]
- Park, S.; Ki, D.; Kang, S.Y. Standard operating procedures for utilizing household combustible waste as an alternative fuel for the cement industry. J. Mater. Cycles Waste Manag. 2024, 26, 2853–2863. [Google Scholar] [CrossRef]
- Turakulov, Z.; Kamolov, A.; Norkobilov, A.; Variny, M.; Fallanza, M. Techno-economic and environmental analysis of decarbonization pathways for cement plants in Uzbekistan. Chem. Eng. Res. Des. 2024, 210, 625–637. [Google Scholar] [CrossRef]
- Deng, J.J.; Wang, X.C.; Wei, Z.L.; Wang, L.; Wang, C.Y.; Chen, Z.B. A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fuelled vessels. Sci. Total Environ. 2021, 766, 144319. [Google Scholar] [CrossRef]
- Shah, I.A.; Gou, X.; Zhang, Q.; Wu, J.; Wang, E.; Liu, Y. Experimental study on NOx emission characteristics of oxy-biomass combustion. J. Clean. Prod. 2018, 199, 400–410. [Google Scholar] [CrossRef]
- Tosti, L.; van Zomeren, A.; Pels, J.R.; Comans, R.N.J. Evaluating Biomass Ash Properties as Influenced by Feedstock and Thermal Conversion Technology towards Cement Clinker Production with a Lower Carbon Footprint. Waste Biomass Valorization 2021, 12, 4703–4719. [Google Scholar] [CrossRef]
- Hossain, M.U.; Poon, C.S.; Wong, M.Y.K.; Khine, A. Techno-environmental feasibility of wood waste derived fuel for cement production. J. Clean. Prod. 2019, 230, 663–671. [Google Scholar] [CrossRef]
- Pitre, V.; La, H.; Bergerson, J.A. Impacts of alternative fuel combustion in cement manufacturing: Life cycle greenhouse gas, biogenic carbon, and criteria air contaminant emissions. J. Clean. Prod. 2024, 475, 143717. [Google Scholar] [CrossRef]
- Pargar, F.; Talukdar, S.; Pal, K.; Zanotti, C. Hemp Waste Valorization as Biofuel and Cement Replacement in Cement and Concrete Production. Waste Biomass Valorization 2021, 12, 913–923. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Samouhos, M.; Perraki, M. Valorization of Dried Olive Pomace as an alternative fuel resource in cement clinkerization. Constr. Build. Mater. 2017, 153, 202–210. [Google Scholar] [CrossRef]
- GB/T 28731-2012; Proximate Analysis of Solid Biofuels. Standardization Administration of China: Beijing, China, 2012.
- GB/T 28732-2012; Determination of Total Sulfur in Solid Biofuels. Standardization Administration of China: Beijing, China, 2012.
- GB/T 28727-2014; Determination of Calorific Value for Solid Biofuels. Standardization Administration of China: Beijing, China, 2014.
- GB/T 33304-2016; Testing Method of Combustion Characteristics of Coal—Thermogravimetric Analysis. Standardization Administration of China: Beijing, China, 2016.
- Cao, H.H.; Liu, W.; Xu, J.C.; Liu, J.; Huang, J.W.; Huang, X.F.; Li, G.M. Utilization of lime-dried sludge for eco-cement clinker production: Effects of different feeding points. Water Sci. Technol. 2018, 77, 960–970. [Google Scholar] [CrossRef]
- Wu, Q.S.; Wu, Y.; Tong, W.H.; Ma, H. Utilization of nickel slag as raw material in the production of Portland cement for road construction. Constr. Build. Mater. 2018, 193, 426–434. [Google Scholar] [CrossRef]
- Huang, M.R.; Feng, H.J.; Li, N.; Shen, D.S.; Zhou, Y.Y.; Jia, Y.F. Addition of large amount of municipal sewage sludge as raw material in cement clinker production. Environ. Sci. Pollut. Res. 2017, 24, 27862–27869. [Google Scholar] [CrossRef]
- GB/T 176-2017; Methods for Chemical Analysis of Cement. Standardization Administration of China: Beijing, China, 2017.
- Pei, T.R.; Zheng, Y.; Wang, Y.L.; Zhang, D.J.; Zhang, P.; Cui, S.P.; Zheng, Y.C.; Zhao, S.X. Utilization of copper tailings in the preparation of low-calcium Portland cement clinker and carbonation-hardening mechanism. Constr. Build. Mater. 2024, 457, 139362. [Google Scholar] [CrossRef]
- GB/T 175-2023; Common Portland Cement. Standardization Administration of China: Beijing, China, 2023.
- Yao, G.; Wang, Q.; Wang, Z.M.; Wang, J.X.; Lyu, X.J. Activation of hydration properties of iron ore tailings and their application as supplementary cementitious materials in cement. Powder Technol. 2020, 360, 863–871. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, G.; Zhu, X.N.; Wang, J.X.; Wu, P.; Lyu, X.J. Preparation of Portland Cement with Gold Ore Tailings. Adv. Mater. Sci. Eng. 2019, 2019, 1324065. [Google Scholar] [CrossRef]
- Yao, G.; Wang, Q.; Su, Y.W.; Wang, J.X.; Qiu, J.; Lyu, X.J. Mechanical activation as an innovative approach for the preparation of pozzolan from iron ore tailings. Miner. Eng. 2020, 145, 106068. [Google Scholar] [CrossRef]
- Lv, T.; Zhang, J.R.; Hou, D.S.; Long, W.J.; Dong, B.Q. Mechanical properties and microstructural characteristics of seawater-mixed sintered sludge cement paste. Constr. Build. Mater. 2024, 414, 134996. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. Standardization Administration of China: Beijing, China, 2011.
- Liu, Y.P.; Yang, C.; Wang, F.Z.; Hu, S.G.; Zhu, M.; Hu, C.L.; Lu, L.N. Performance evaluation of regenerated clinker from completely recyclable mortar. Constr. Build. Mater. 2021, 309, 125184. [Google Scholar] [CrossRef]
- GB/T 21732-2024; Portland Cement Clinker. Standardization Administration of China: Beijing, China, 2024.
- Castillo, J.A.; Brostroem, M.; Eriksson, M. Phase evolution and burnability of cement raw meal. Adv. Cem. Res. 2023, 35, 577–587. [Google Scholar] [CrossRef]
- Huang, L.; Yan, P.Y. Effect of alkali content in cement on its hydration kinetics and mechanical properties. Constr. Build. Mater. 2019, 228, 116833. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.J.; Yao, G.; Zhu, X.N.; Hu, S.G.; Qiu, J.; Chen, P.; Lyu, X.J. Characterization of the mechanical properties and microcosmic mechanism of Portland cement prepared with soda residue. Constr. Build. Mater. 2020, 241, 117994. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J.; Fernández-Jiménez, A.; Palomo, A. Alkaline Hydration Of C2S and C3S. J. Am. Ceram. Soc. 2016, 99, 604–611. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J.; Fernández-Jiménez, A.; Palomo, A. C3S and C2S hydration in the presence of Na2CO3 and Na2SO4. J. Am. Ceram. Soc. 2017, 100, 3188–3198. [Google Scholar] [CrossRef]
- Cui, S.H.; Fan, K.J.; Yao, Y. Preparation and characterization of quaternary clinker-free cementitious materials containing phosphorus slag, calcium carbide slag, desulfurization gypsum, and metakaolin. Constr. Build. Mater. 2024, 411, 134602. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Douglas, B.; Arkless, L.; Cardinal, N.; Zajac, M. Mixed hydrogen and biofuels cement clinker: Characterisation, microstructure, and performance. Cem. Concr. Compos. 2025, 155, 105814. [Google Scholar] [CrossRef]
- Bhagat, T.S.; Pancharathi, R.K. Performance, microstructure and carbon sequestration potential of agro biochar based cement mortars. Cem. Concr. Compos. 2025, 156, 105867. [Google Scholar] [CrossRef]
- Xue, J.W.; Li, S.M.; Liu, S.H.; Guan, X.M. Effect of Ferrite Phase Content on Hydration Reaction, Mechanical Properties, and Chloride-Binding Behavior of Ordinary Portland Cement. J. Mater. Civ. Eng. 2025, 37, 04024527. [Google Scholar] [CrossRef]
- Si, W.; Ming, X.; Cao, M.L. Time-dependent rheological behavior of hydrating cement paste containing calcium carbonate whiskers. Cem. Concr. Compos. 2024, 154, 105775. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, H.; Zhu, L.; Hao, L.C.; Zhang, S.P.; Poon, C.S. Rapid CO2 catalytic activation of binary cementing system of CSA and Portland cement. Cem. Concr. Compos. 2024, 154, 105771. [Google Scholar] [CrossRef]
- Yao, J.W.; Song, H.; Ling, W.J.; Yang, Z.X. Effects of municipal solid waste incineration bottom ash on corrosion resistance of cement mortar. Constr. Build. Mater. 2024, 453, 139088. [Google Scholar] [CrossRef]
- Wang, D.Z.; Jin, K.R.; Wang, N.; Zhang, H.W.; Wang, J.H.; Zhou, X.M. Compressive strength and water resistance of magnesium oxysulfate (MOS) cement incorporating magnesium slag. Constr. Build. Mater. 2024, 453, 139053. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.Q.; Huang, W.J.; Chao, H.Y.; Yu, L.; Ma, X.; Ouyang, X.W. Multiscale study on the effect of Seashell powder on rheology, hydration and strength development of cement paste. Constr. Build. Mater. 2024, 456, 139257. [Google Scholar] [CrossRef]
- Wang, Z.X.; Sang, G.C.; Yu, H.K.; Zhang, Y.K.; Guo, T.; Cui, X.L.; Cai, P.Y. Preparation of green and durable magnesium oxysulfate cement using sewage sludge ash: Physical properties, microstructure, and leaching behavior. Constr. Build. Mater. 2024, 452, 138951. [Google Scholar] [CrossRef]
- Savija, B.; Lukovic, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Medepalli, S.; Wang, T.; Dai, J.G.; Zheng, Y.Q.; Ishida, T. Effect of alkali-hydroxide on hydration kinetics and microstructure of high-volume fly ash blended cement pastes. Cem. Concr. Res. 2024, 185, 107641. [Google Scholar] [CrossRef]
- Li, K.X.; Yao, J.; Li, X.M.; Li, S.Q.; Li, Z.H.; Li, X.L.; Ling, H. All-solid-waste cementitious materials for grouting: Effects of alkali content and elemental ratios on performance and sustainability. J. Environ. Chem. Eng. 2025, 13, 115000. [Google Scholar] [CrossRef]
- Li, H.W.; Liu, F.; Zhen, H.; Xiong, Z.; Song, Y.Y.; Wang, J.H.; Li, L.J. Evaluating strength, hydration characteristics, microstructure evolution, and sustainability of seawater-sea sand cement-based materials containing iron ore tailings. Constr. Build. Mater. 2024, 457, 139163. [Google Scholar] [CrossRef]
- Gao, Q.W.; Jiu, S.; Chen, Y.X.; Zhao, S.J.; Chen, C.; Jia, R.Q. Modification of low-quality calcined coal gangue and its effect on mechanical properties and microstructure. Constr. Build. Mater. 2025, 458, 139433. [Google Scholar] [CrossRef]
- Ying, J.W.; Li, C.Y.; Tian, J.S.; Chen, B.X.; Tian, Z.Q.; Liang, L.Z.; Liu, Y.; Yan, Z.G. Experimental study on the effect of polycarboxylate-modified three-dimensional porous graphene on the microstructure and properties of ground granulated blast furnace slag-cement based materials. Constr. Build. Mater. 2024, 457, 139492. [Google Scholar] [CrossRef]











| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | MgO | K2O | Na2O | Cl− | LoI |
|---|---|---|---|---|---|---|---|---|---|---|
| Limestone | 6.77 | 2.37 | 0.74 | 49.16 | 0.05 | 0.61 | 0.40 | 0.05 | 0.02 | 39.57 |
| Sandstone | 74.23 | 11.81 | 4.63 | 1.27 | 0.16 | 0.54 | 1.82 | 0.13 | 0.03 | 4.18 |
| Iron tailings | 44.99 | 3.91 | 22.53 | 14.80 | 0.25 | 2.99 | 0.45 | 0.15 | 0.04 | 8.49 |
| Gypsum | - | - | - | 45.75 | 30.18 | - | - | - | - | 20.33 |
| Materials | Moisture | Ash Content | Volatile Matter | Fixed Carbon | Sulfur | Gross CV (KJ/kg) |
|---|---|---|---|---|---|---|
| Coal | 5.87 | 17.06 | 24.94 | 52.13 | 0.48 | 24,260 |
| DG | 10.78 | 16.86 | 58.00 | 14.36 | 0.10 | 13,030 |
| YMX | 6.61 | 2.16 | 75.02 | 16.21 | 0.07 | 15,330 |
| JM | 10.96 | 0.34 | 75.76 | 12.94 | 0.06 | 16,500 |
| SY | 10.36 | 8.30 | 63.34 | 18.00 | 0.14 | 15,770 |
| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | MgO | K2O | Na2O | Cl− | LoI |
|---|---|---|---|---|---|---|---|---|---|---|
| Coal | 42.26 | 30.55 | 6.41 | 9.45 | 5.29 | 0.73 | 1.14 | 0.39 | 0.02 | 1.64 |
| DG | 87.56 | 1.37 | 0.93 | 1.63 | 0.29 | 0.99 | 4.75 | 0.22 | 0.08 | 0 |
| YMX | 29.66 | 1.49 | 0.90 | 2.22 | 1.37 | 4.15 | 50.68 | 0.39 | 0.67 | 0 |
| JM | 8.23 | 1.85 | 1.81 | 56.98 | 2.50 | 7.20 | 8.34 | 1.98 | 0.08 | 0 |
| SY | 35.77 | 1.97 | 1.61 | 49.37 | 1.79 | 3.83 | 1.94 | 0.16 | 0.16 | 0 |
| Sample | Raw Materials | Coal Ash | KH a | SM b | IM c | |||
|---|---|---|---|---|---|---|---|---|
| Limestone | Sandstone | Iron Tailings | Alternative Fuel Ash | |||||
| H0 | 86.91 | 7.95 | 5.14 | 0 | 2.06 | 0.90 | 2.50 | 1.60 |
| HDG1 | 87.00 | 7.83 | 5.17 | 0.213 | 1.942 | 0.90 | 2.52 | 1.59 |
| HDG2 | 87.09 | 7.70 | 5.21 | 0.448 | 1.814 | 0.90 | 2.54 | 1.57 |
| HYMX1 | 86.84 | 8.01 | 5.15 | 0.270 | 1.921 | 0.90 | 2.52 | 1.58 |
| HYMX2 | 86.77 | 8.07 | 5.16 | 0.056 | 1.774 | 0.90 | 2.55 | 1.57 |
| HJM1 | 86.77 | 8.08 | 5.15 | 0.004 | 1.913 | 0.90 | 2.53 | 1.58 |
| HJM2 | 86.64 | 8.21 | 5.15 | 0.009 | 1.758 | 0.90 | 2.52 | 1.57 |
| HSY1 | 86.85 | 8.00 | 5.16 | 0.104 | 1.919 | 0.90 | 2.52 | 1.59 |
| HSY2 | 86.78 | 8.05 | 5.17 | 0.215 | 1.770 | 0.90 | 2.54 | 1.57 |
| Sample | Ti | Tf | Tp | (dw/dt)max | Ṽ |
|---|---|---|---|---|---|
| °C | °C | °C | %/min | %/min | |
| Coal | 420 | 724 | 534 | 17.8 | 4.93 |
| C+DG1 | 397 | 640 | 535 | 13.3 | 5.73 |
| C+DG2 | 386 | 631 | 534 | 13.2 | 6.31 |
| C+YMX1 | 416 | 642 | 544 | 13.2 | 7.21 |
| C+YMX2 | 389 | 647 | 543 | 13 | 6.40 |
| C+JM1 | 417 | 638 | 539 | 13.4 | 7.36 |
| C+JM2 | 366 | 662 | 526 | 13.8 | 5.45 |
| C+SY1 | 390 | 656 | 534 | 13.2 | 5.96 |
| C+SY2 | 415 | 643 | 545 | 12.6 | 6.92 |
| Sample | Flexural Strength (MPa) | Compressive Strength (MPa) | ||
|---|---|---|---|---|
| 3 d | 28 d | 3 d | 28 d | |
| H0 | 6.73 | 8.58 | 35.17 | 60.88 |
| HDG1 | 6.62 | 8.55 | 34.63 | 60.86 |
| HDG2 | 6.53 | 8.53 | 34.51 | 60.84 |
| HYMX1 | 6.47 | 8.54 | 34.23 | 61.09 |
| HYMX2 | 6.41 | 8.54 | 34.11 | 60.90 |
| HJM1 | 6.70 | 8.55 | 34.70 | 60.85 |
| HJM2 | 6.68 | 8.55 | 34.55 | 60.79 |
| HSY1 | 6.64 | 8.55 | 34.50 | 60.85 |
| HSY2 | 6.62 | 8.55 | 34.48 | 60.86 |
| Sample | Setting Time (min) | Consistency (%) | Fineness (%%) | Soundness (%mm) | |
|---|---|---|---|---|---|
| Inital | Final | ||||
| H0 | 197 | 305 | 23.8 | 14.0 | 1.2 |
| HDG1 | 193 | 303 | 23.8 | 14.2 | 1.3 |
| HDG2 | 189 | 302 | 23.9 | 14.2 | 1.4 |
| HYMX1 | 189 | 301 | 24.1 | 14.1 | 1.0 |
| HYMX2 | 184 | 299 | 24.5 | 14.2 | 1.2 |
| HJM1 | 193 | 303 | 23.8 | 14.3 | 1.1 |
| HJM2 | 191 | 303 | 23.8 | 14.5 | 1.1 |
| HSY1 | 195 | 302 | 23.8 | 13.8 | 1.2 |
| HSY2 | 191 | 301 | 23.9 | 14.0 | 1.2 |
| Reference | ≥45 | ≤390 | - | ≥5.0 | ≤5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, Y.; Hua, S.; Zhang, D. Investigation of the Effects and Mechanisms of Biomass-Derived Alternative Fuels on Cement Clinker Formation and Hydration Processes. Appl. Sci. 2025, 15, 6294. https://doi.org/10.3390/app15116294
Wang Z, Zhou Y, Hua S, Zhang D. Investigation of the Effects and Mechanisms of Biomass-Derived Alternative Fuels on Cement Clinker Formation and Hydration Processes. Applied Sciences. 2025; 15(11):6294. https://doi.org/10.3390/app15116294
Chicago/Turabian StyleWang, Zhengquan, Yongmin Zhou, Sudong Hua, and Dongrui Zhang. 2025. "Investigation of the Effects and Mechanisms of Biomass-Derived Alternative Fuels on Cement Clinker Formation and Hydration Processes" Applied Sciences 15, no. 11: 6294. https://doi.org/10.3390/app15116294
APA StyleWang, Z., Zhou, Y., Hua, S., & Zhang, D. (2025). Investigation of the Effects and Mechanisms of Biomass-Derived Alternative Fuels on Cement Clinker Formation and Hydration Processes. Applied Sciences, 15(11), 6294. https://doi.org/10.3390/app15116294






