Abstract
Sodium nitrite is an additive commonly used in meat processing to provide technological effects. However, the presence of nitrates in food can lead to the formation of carcinogenic N-nitrosamines; so, its use should be limited. This study concerns the possibility of reducing sodium nitrite (III) addition in the production of canned meat to 50 mg/kg by enriching the product with tomato peels and seeds powder (TPSP). The aim of this study was to evaluate the effect of TPSP on the physicochemical, chemical and microbiological quality of canned pork. Four different products were tested in this study: a control sample and samples with the addition of 0.5%, 1.5%, and 2.5% tomato peels and seeds powder. The addition of TPSP decreased the pH values of meat products and increased yellowness (b*) and redness (a*) values. The influence of TPSP on the increase in antioxidant activity of canned meat was also observed. However, the plant-based additive did not affect the chemical composition or water activity of the tested product. The control samples were characterized by a lower TBARS compared to the other samples of meat products. Microbiological analysis results indicate that the canned pork samples meet the product requirements. The most commonly isolated species from the samples were Enterobacter cloacae, Serratia liquefaciens, and Enterococcus faecalis.
1. Introduction
Nitrite added to meat products has many functions, including influencing the characteristic color, taste and aroma, as well as having antioxidant and antimicrobial properties. However, despite the benefits of nitrite use, its action may be associated with side effects due to the potential formation of carcinogenic N-nitrosamines [1]. Therefore, many modern studies recommend reducing the amount of added nitrite or completely eliminating it from meat products [2]. In recent years, increasing emphasis has been placed on enhancing the oxidative stability of meat and meat products, particularly through the use of natural antioxidants [3,4,5]. The meat industry needs effective and safe antioxidant solutions, and plant antioxidants can be an excellent alternative. However, it is essential to find the optimal combination of the target meat product and the appropriate source of plant bioactive compounds. Meat and meat products are particularly exposed to oxidation of proteins and lipids, which may necessitate enriching them with plant antioxidants. Incorporating plant-based antioxidants into processed meat products can also help create functional foods. In this context, meat products could act as carriers of antioxidants, bringing health benefits to consumers. The use of plant-based antioxidants in minimally processed foods may also enable the production of safe, clean-label meat products. One of the innovative additions to canned pork may be tomato pomace. Tomatoes are widely used to produce various products such as purees, powders, juices and sauces. However, the peel and seeds, which are byproducts of these processes, are often overlooked. This not only increases waste but also poses environmental risks [6]. Valuable components of tomato peel are carotenoids (mainly lycopene and β-carotene), polyphenols such as flavonols (quercetin, kaempferol, myricetin), flavonol glycosides (rutin), flavanones (naringenin) and hydroxycinnamic acids (chlorogenic acid, caffeic acid), p-coumaric and ferulic), and minerals (potassium, calcium, sodium, magnesium). It is peels that are the richest source of phenols compared to seeds and pulp of tomatoes [7,8]. As shown by [9], carotenoids have a beneficial effect on human health due to their strong antioxidant properties [9]. Tomatoes are considered functional foods due to minerals and nutrients, which means that regular consumption of them can support health and prevent certain diseases [10]. The possibility of reducing the use of nitrite is the subject of research in the context of various meat products, including fermented products [11,12,13], cooked meat products [14] and canned pork [15,16]. However, it should be emphasized that although tomato pomace has a high antioxidant potential and can be used as an antioxidant in meat products, research on this topic is still limited.
Animal products and meat can carry several micro-organisms that can cause food poisoning or spoilage. Microbial deterioration of food can result from the failure or inability to control organisms at various stages of food production [17]. One of the key stages in ensuring microbiological safety and the shelf life of meat preserves is the thermal sterilization process. High temperatures effectively eliminate pathogenic microorganisms, including Clostridium botulinum [18]. Although the process ensures high microbiological effectiveness, it can also lead to undesirable changes, affecting the content of biologically active compounds in meat products. Long-term exposure to high temperatures can result in the degradation of vitamins, particularly those from the B group (B1–thiamine), vitamin C, and natural antioxidants, as well as some phenolic compounds and carotenoids, if present in plant-based additives. Additionally, this process can cause lipid oxidation and protein denaturation, which not only impacts nutritional value but also the bioavailability of certain components and the sensory quality of the product [19]. Despite these negative effects, optimizing sterilization parameters can help minimize the loss of valuable compounds while maintaining microbiological effectiveness. As a result, there is growing interest in developing meat preserves enriched with natural plant-based additives, which can reduce antioxidant losses and the same time enhance the health-promoting properties of the final product [20].
This research was conducted in order to assess the possibility of producing canned pork with a reduced amount of sodium nitrite by enriching the product with tomato peels and seeds powder. The goals were achieved by assessing the color parameters (L*, a*, b*), water activity, pH, antioxidant properties (ABTS+, DPPH), fat oxidation index and microbiological quality of canned pork produced with the addition of sodium nitrite at the level of 50 mg/kg (i.e., reduced by half in relation to the limits specified in Regulation No. 1333/2008 [21]).
2. Materials and Methods
2.1. Preparation of Canned Pork
For the production of canned pork, pork jowl and shoulder from a slaughterhouse (Meat Plant Wasąg) were used and processed 48 h after slaughter. The meat was first minced manually and then finely ground using a KU2-3E machine (Mesko-AGD, Skarżysko-Kamienna, Poland) with a mesh with a hole diameter of 5 mm. The obtained meat was divided into four groups: control and three groups with the addition of various amounts of tomato peels and seeds powder (TPSP). The research material consisted of a single variety of tomato (Solanum lycopersicum L.) purchased from a local supermarket. The tomatoes were washed and then pressed using a kitchen press to obtain tomato pomace (tomato peels and seeds). The pomace of this vegetable was freeze-dried using a freeze dryer (Labconco Free-Zone, Kansas City, MO, USA) at the temperature −50 °C and a vacuum pressure of 0.04 mbar for 48 h. The dried material was then ground in a laboratory mill with a diameter of less than 0.3 mm. Freeze-dried TPSP was characterized by antioxidant properties ranging from 0.112 mg Trolox eqv. g−1 in studies with the ABTS•+ radical to 0.120 mg Trolox eqv. g−1 in studies with the DPPH• radical. The average total phenolic content (TPC) of pomace was 4.080 mg gallic acid eqv. g−1 [22]. The content of bioactive components, including lycopene and beta-carotene, determined in freeze-dried tomato pomace was 0.74 mg/100 g and 0.68 mg/100 g, respectively. The recipe of each variant included 20% jowl, 80% shoulder, 2% salt, 5% water and sodium(III) nitrite in the amount of 50 mg/kg. The meat and additives were thoroughly mixed in a KU2-3E machine (Mesko-AGD, Skarżysko-Kamienna, Poland). Then, the meat stuffing was then placed in aluminum cans with a capacity of 300 mL and sterilized on a vertical steam sterilizer (AS2, SMS, Góra Kalwaria, Poland). The experimental samples were heated at 121 °C, assuming that their degree of heating was achieved as measured with the sterilization value (F ≈ 4 min) determined using the formula:
where F is the sterilization value, L is the lethality degree, T is temperature at a given moment in the process inside the can, in the zone critical, T0 is the reference temperature (121 °C), and z is the sterilization effect factor (10 °C).
F = ∫ Ldt
L = 10 (T − T0)/z
L = 10 (T − T0)/z
The sterilization values were calculated by determining the degree of lethality by measuring temperature every minute. The limits of integration were assumed from 90 °C during the growth phase to 90 °C during the decrease (i.e., cooling). The degree of heating was determined for the cans in their critical zone using an electric thermometer equipped with a thermoelectric sensor. After sterilization, the cans were cooled in water and stored at 4 °C in the refrigerator [16]. Four groups of canned pork were produced: control (DFS C), DFS 0.5% (sample with 0.5% addition of TPSP), DFS 1.5% (sample with 1.5% addition of TPSP), DFS 2.5% (sample with 2.5% addition of TPSP). The experiment involved a preparation of 24 canned pork samples (two batches of canned pork—6 cans from each test variant). Each variant was tested 1 day after production. Figure 1 presents the cross-sectional appearance of canned pork.

Figure 1.
Cross-sectional appearance of canned pork: (a) DFS C; (b) DFS 0.5%; (c) DFS 1.5%; (d) DFS 2.5%.
2.2. Basic Chemical Composition
The content of protein, collagen, moisture and fat in canned pork was determined using the Food Scan Lab analyzer type 78810 (Foss Tecator Co., Ltd., Hillerod, Denmark). Approximately 200 g of each homogenized sample was placed into the instrument’s round sample dish and then loaded into the instrument’s sample chamber.
2.3. Physicochemical Parameters (pH, Water Activity)
A digital pH meter with temperature compensation (CPC-501, Elmetron, Zabrze, Poland) and a pH electrode (ERH-111, Hydromet, Gliwice, Poland) calibrated using buffer solutions with pH 4.0, 7.0 and 9.0 were used to perform pH measurements of canned pork. To this end, a 5 g ground canned food sample was homogenized with 25 mL of distilled water using a homogenizer (IKA® T25, IKA-Werke GmbH & CO. KG, Staufen, Germany). The water activity (aw) of ground canned meat samples was measured at 20 °C using a water activity analyzer (Novasina AG, Lachen, Switzerland).
2.4. Color Attributes (CIE L* a* b*, ΔΕ)
The color of canned pork was determined according to the CIE color convention [23] using an X-Rite 8200 colorimeter (X-Rite, Inc., Grand Rapids, MI, USA). After opening the cans, the jelly was removed from the meat, and a 3 × 5.5 cm cuboid was then cut out, which was used to measure the color. Each sample was measured 10 min after opening the can to allow for color stabilization. Before measurements, the colorimeter was calibrated using the provided black and white plates. The measurement was made on the cross-section immediately after cutting. The instrumental settings included using a 12 mm diameter aperture, a D65 light source, and a 10° standard colorimetric observer. The measurement was performed in the range from 360 to 740 nm. For each sample, measurements were taken at three cross-sectional locations. The color difference (∆E) between the control sample and the sample with the addition of TPSP was calculated according to AMSA [24] and interpreted according to Mokrzycki and Tatol [25].
2.5. Antioxidant Capacity and Lipid Oxidation Analysis (TBARS)
The DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) tests were carried out based on the method described by Jung et al. [26], Ferysiuk et al. [27] and Eler [28], with some modifications. The TBARS value was determined according to the procedure developed by Pikul et al. [29].
2.6. Microbiological Analysis
In order to perform microbiological analysis, 5 g of the canned pork was mixed with 45 mL of 0.1% sterile saline. Mixing was performed in a shaker incubator (GFL 3031, Burgwedel, Germany). Various microorganisms were examined as part of the assessment. Coliform bacteria (CB) were found after 24 to 48 h of incubation at 37 °C on Violet Red Bile Lactose Agar (VRBL, Oxoid, Basingstoke, UK). Plate count agar (PCA; Oxoid, Basingstoke, UK) was used to calculate the total viable count (TVC). The cells were grown at 30 °C for 48–72 h. De Man Rogosa and Sharpe agar (MRS; Oxoid, Basingstoke, UK) was used for the cultivation of lactic acid bacteria (LAB) at 37 °C for 48 to 72 h. Furthermore, eight colonies per Petri dish were cultured on tryptone soya agar (TSA; Oxoid, Basingstoke, UK) as part of a quick reinoculation at 30 °C.
2.7. Identification of Microorganisms Using Mass Spectrometry
Bacteria isolated from canned pork were identified using the MALDI-TOF MS Biotyper (Bruker, Daltonics, Bremen, Germany) and reference libraries.
2.7.1. Preparing the MALDI-TOF Matrix Solution
The MALDI-TOF matrix solution was initially created as a stock solution and eventually evolved into an organic formulation. The stock solution was made up of 50% acetonitrile, 47.5% water and 2.5% trifluoroacetic acid. In addition, 1 mL of the stock solution was made by mixing 500 mL of pure 100% acetonitrile, 475 mL of filtered water, and 25 mL of pure 10% trifluoroacetic acid. The organic solvent was combined with the “HCCA matrix portioned” that had been made in a 250 mL Eppendorf flask. Aloqence in Vrable, Slovakia, composed of all of the matrix components.
2.7.2. Identification of Microorganisms
Eight different colonies were selected on the Petri plate as part of the sample preparation. This was carried out according to previous guidelines. The biological material was transferred from the Petri plate to an Eppendorf flask containing 300 mL of distilled water. Then, 900 mL of ethanol was added and mixed thoroughly. The obtained mixture was centrifuged at 10,000× g for 2 min using a ROTOFIX 32A (Ites, Vranov, Slovakia). The precipitate was separated from the Eppendorf flask and allowed to air dry at room temperature (20 °C) after removal of the supernatant. The particles were then mixed with 30 µL acetonitrile and 30 µL 70% formic acid. After centrifugation at 10,000× g for 2 min, 1 µL of the liquid was added to a MALDI plate, followed by 1 µL of the MALDI matrix solution. The mass spectrometry evaluation procedure for the MALDI MS Biotyper was published in [30].
2.8. Statistical Analysis
The canned pork meat treatments were replicated twice by producing two different batches. Each sample was analyzed in triplicate. Statistical analysis of the results was performed using Statistica 9.1 software (StatSoft, Kraków, Poland). The results were presented as mean ± standard deviation. The normality of the distribution of variables in the studied groups was checked using the Shapiro–Wilk test. The differences between the groups were assessed using the ANOVA (together with Tukey’s post hoc RIR test), and in the case of failure to meet the conditions for its application, the Kruskal–Wallis test. A significance level of p < 0.05 was adopted, indicating statistically significant differences.
3. Results
3.1. Chemical Composition
The chemical composition (fat, protein, water, collagen, and salt content) is presented in Table 1. A statistical analysis did not show statistically significant differences in fat, protein, collagen and salt content (p ≤ 0.05) between the tested canned meat samples. Regarding moisture, the control sample (DFS C) was characterized by a significantly higher moisture compared to the other samples.

Table 1.
Proximate chemical composition of canned pork. Values are means ± standard deviation (n = 3).
3.2. Physicochemical Parameters (pH, aw, CIE L*a*b*)
The effect of freeze-dried tomato pomace on changes in pH and water activity (aw) in canned pork is presented in Table 2. The plant additive used in amounts of 0.5%, 1.5% and 2.5% caused a decrease in the pH of the tested meat products compared to the control sample. A significant decrease in the pH of the samples was observed with an increase in the level of TPSP additive. No significant effect of TPSP on water activity in canned pork was found. Significant differences were found in color parameters (L*, a*, b*) between the samples. A significant decrease in color lightness in the cross-section was observed, along with an increase in the level of TPSP additive, and a simultaneous increase in redness (a*) and yellowness (b*) was noted. ∆E values for canned pork containing TPSP indicate a noticeable color change compared to DFS C.

Table 2.
pH and water activity of canned pork. Values are means ± standard deviation (n = 3).
3.3. Antioxidant Properties and Secondary Lipid Oxidation Products
The effect of the addition powdered tomato peels and seeds on the antiradical activity (ABTS+ and DPPH) and lipid oxidative stability of meat product samples is presented in Table 3. Significant differences were found in the ability to neutralize radicals (ABTS+, DPPH) between samples. Samples with TPSP were characterized by higher antioxidant activity. Antioxidant activity increased with the increase in the TPSP content. The addition of the tomato peels and seeds’ powder affected TBARS associated with lipid oxidation. Compared to other samples, the DFS C sample had a significantly (p < 0.05) lower MDA content. The sample with 2.5% TPSP was characterized by an approximately five-times-higher TBARS value compared to the control sample.

Table 3.
Antioxidant activity and oxidative stability of canned pork. Values are means ± standard deviation (n = 3).
3.4. Results of Microbiological Analysis
The number of CB varied from 2.283 in DFS C to 2.536 log CFU/g in DFS 0.5%. The TVC varied from 2.615 in DFS 0.5% to 2.728 log CFU/g in DFS C, while the count of LAB in the samples ranged from 1.405 in DFS 0.5% to 1.528 log CFU/g in DFS 2.5% (Table 4).

Table 4.
Microbiological quality of canned pork samples. Values are means ± standard deviation (n = 3).
A total of 81 isolates were identified using mass spectrometry (Figure 2). Four species, three genera, and three families were identified in the control samples of canned pork. The Yersiniaceae and Enterobacteriaceae families were the most commonly isolated. Enterobacter cloacae and Serratia liquefaciens were the most abundant species (35%), followed by Enterococcus faecalis (18%) and Enterobacter hormaechei (12%).
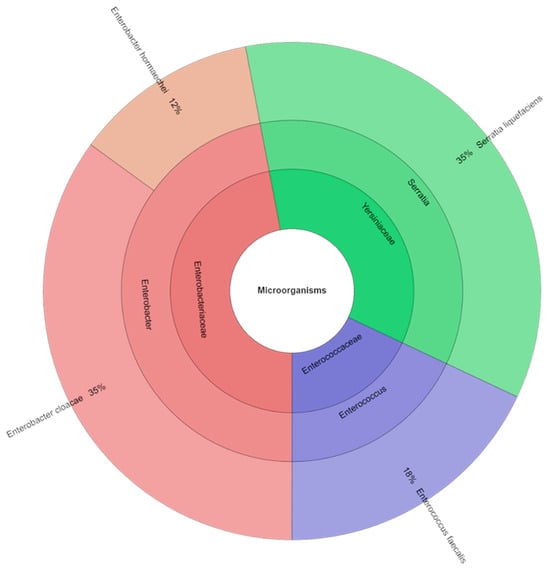
Figure 2.
Krona chart: isolated species, genera and family from control samples (DFS C).
Using mass spectrometry, fifty-six isolates (Figure 3) comprising three families, three genera, and four species were identified from the canned pork samples with the addition of 0.5% TPSP. S. liquefaciens (35%) and E. cloacae (29%) were the most commonly isolated species from the canned pork samples with an addition of 0.5% TPSP.
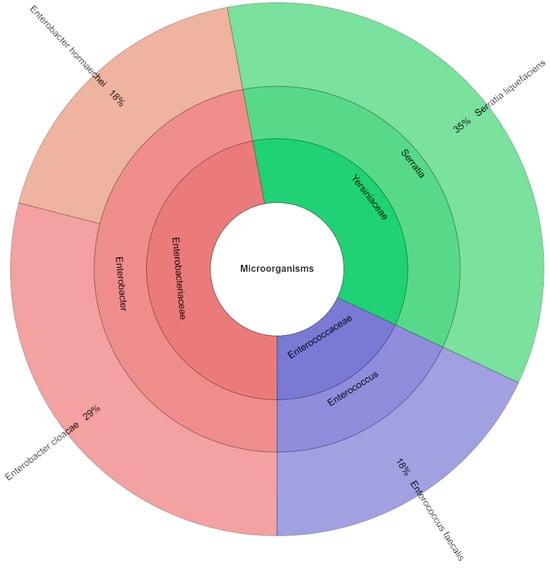
Figure 3.
Krona chart: isolated species, genera and family from samples with addition of 0.5% TPSP (DFS 0.5%).
Mass spectrometry was used to identify 63 isolates in total (Figure 4). In the canned pork samples with the addition of 1.5% of TPSP, a total of three species, three genera, and three families were isolated. The most frequently isolated families were Enterobacteriaceae and Yersiniaceae. E. cloacae was the most abundant species (44%), followed by S. liquefaciens (40%) and E. faecalis (16%).
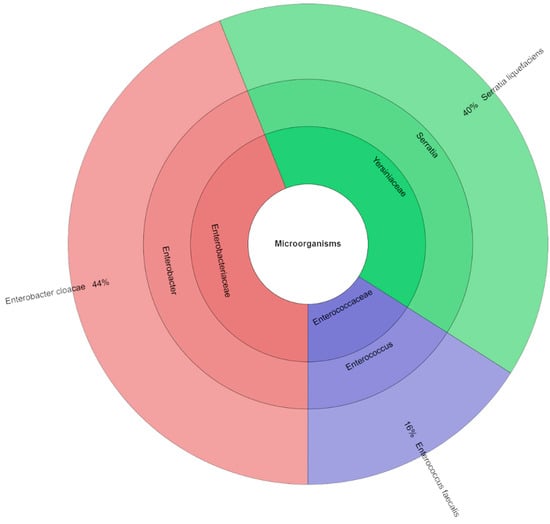
Figure 4.
Krona chart: isolated species, genera and family from samples with addition of 1.5% TPSP (DFS 1.5%).
Using mass spectrometry, seventy isolates with high scores (Figure 5) and three families, three genera, and three species were identified from the canned pork samples with an addition of 2.5% TPSP. S. liquefaciens (43%) and E. cloacae (36%) were the most commonly isolated species from the canned pork samples with an addition of 2.5% TPSP.
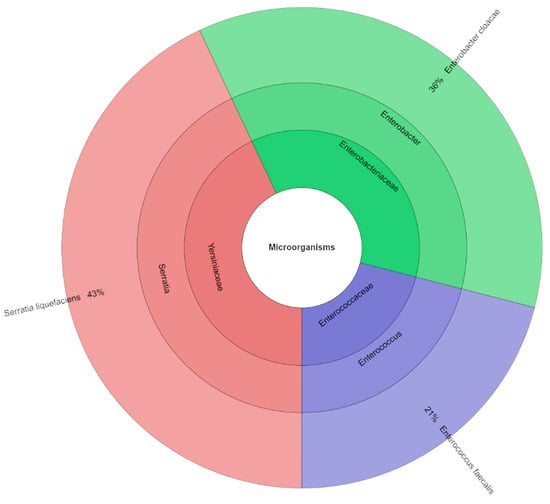
Figure 5.
Krona chart: isolated species, genera and family from samples with addition of 2.5% TPSP (DFS 2.5%).
4. Discussion
Recently, there has been growing interest among scientists in the topic of by-products of the fruit and vegetable industry. This phenomenon is associated with the development of innovative food products, including meat products. The meat industry is seeking new solutions to increase the nutritional value of products while at the same time striving to reduce the use of synthetic additives, such as nitrogen compounds [31]. Therefore, the use of tomato peels and seeds powder, which is rich in valuable bioactive compounds, in meat preserves with reduced nitrogen compound content may contribute to the development of functional meat products that have positive effects on human health.
When developing new products enriched with plant raw materials, it is essential to consider their impact on the physicochemical properties of the finished product. The addition of tomato peels and seeds’ powder in the amounts of 0.5%, 1.5% and 2.5% contributed to the lowering of the pH of the tested canned pork compared to the control sample. It was also observed that an increase in the concentration of the plant additive caused a decrease in the pH of the finished product. Similar observations were demonstrated by other researchers [16,27]. The trends observed may be due to the high acidity of tomato peels and seeds’ powder, as tomatoes are a good source of ascorbic acid [22]. They are also generally grouped as acidic foods (pH < 4.6), which is confirmed by the research of Adewale et al. [28], which showed that the pH of tomato powder from different tomato varieties was in the range of 4.19 to 4.29. Another important physicochemical indicator is water activity, which is important for the development of microorganisms in meat products. The additive used did not affect the value of this parameter in the tested products. The results ranged from 0.988 to 0.994.
Due to the content of natural pigment (carotenoid and polyphenolic) in tomatoes, especially in their peel, the main effect of using tomato pomace in meat products is its impact on color parameters. It was indicated that the addition of tomato pomace to food products causes a decrease in the L* parameter as well as an increase in the a* and b* parameters [32,33]. This phenomenon is also confirmed in the current study. The findings of other authors [34,35,36,37,38], who studied different types of meat products enriched with tomato processing by-products, were also in accordance with the indicated relationship. The reason for the increase in the a* value in the meat preserves can be attributed to the high content of lycopene in the chemical composition of tomato pomace [39]. Although lycopene is generally a red pigment, which additionally contributes to the increase in the b* parameter, its addition to meat can also cause a color change to more orange [37]. When analyzing the color differences (∆E) between the samples of the preserves, it should be noted that the greatest color changes occurred in the preserves with the highest content of tomato peels and seeds’ powder. Therefore, as soon as color is concerned, tomato peels and seeds’ powder can be used as a natural ingredient that can improve the overall appearance of canned pork.
By-products of vegetable processing, including tomato peels and seeds, can be used to obtain valuable bioactive ingredients and as a source of antioxidants in the food industry, including meat products [40]. The most popular methods used to study various relationships between the mechanisms of antioxidant properties are based on the mechanism of inhibition of free radicals [41,42]. In contrast to the DPPH method, ABTS·+ is soluble in both organic and aqueous environments, and therefore enables the detection of both lipophilic and hydrophilic compounds [43]. In the current study, samples containing the highest concentration of tomato pomace showed the highest antioxidant properties. These observations were also confirmed by other authors [44], who studied the effect of grape pomace addition in the production of meat products with reduced nitrite content. Similar results were obtained by other researchers [34] on the antioxidant properties of fermented sausages with the addition of tomato by-products. A similar trend was also observed by Ramli et al. [45] in the experiments on the effect of powdered passion fruit extract on the antioxidant activity of preserved meat products. Based on the current study, it can be concluded that tomato peels and seeds powder can be used as a valuable natural preservative in processed meat. Despite these promising findings, it is important to consider the role of traditional antioxidants widely used in the meat industry, such as ascorbic acid and rosemary extract. Although tomato peels and seeds exhibit promising antioxidant properties, their effectiveness as standalone antioxidants may be somewhat lower compared to traditionally used additives. Nevertheless, due to their natural origin, potential health benefits, and the possibility of being used as secondary raw materials, they represent an attractive alternative or complement to conventional preservatives. Therefore, it appears justified to conduct further comparative studies aimed at a comprehensive evaluation of the effectiveness of tomato-derived components in relation to conventional antioxidants used in the meat industry [18].
Lipid oxidation can be considered one of the main causes of meat product quality degradation. This phenomenon can lead to the formation of many products that contribute to the deterioration of the smell and taste of meat products [40]. Tomato peels and seeds provide many valuable bioactive compounds with antioxidant properties. The most representative antioxidant compounds found in both tomato peel and seeds include carotenoids, polyphenols and phytosterols [33]. The dominant flavonoids are rutin, naringenin and their derivatives, and quercetin [46]. Additionally, tomato pomace, especially tomato seeds, contains large amounts of vitamin C. Recently, polyphenols and secondary plant metabolites, such as flavonoids, have attracted much attention due to their use in the food industry [47]. Bioactive compounds present in plant raw materials used as natural additives in meat products caused an antioxidant effect and inhibited oxidation, thus reducing the TBARS values of meat products [38,48,49,50]. However, the results obtained in the current study were not consistent with the observations of other authors. In the presented studies, the addition of tomato peels and seeds powder accelerated the oxidation reaction of fats in meat products. Compared to other samples, the control sample had a significantly (P < 0.05) lower MDA content. The reason for this is likely that the flavonoids and vitamin C present in TPSP are sensitive to environmental conditions (temperature, light, oxygen and pH) [47]. Therefore, the heating processes and high temperatures applied to the experimental canned meat may have altered the structure of flavonoids. As a result, this change could have shifted their role from acting as antioxidants to exhibiting pro-oxidant activity. Pro-oxidant activity is usually catalyzed by metals, especially transition metals such as Fe and Cu [51]. Moreover, during thermal processing, polyphenols degrade according to the first-order kinetics, leading to the formation of degradation products such as quinones and phenoxy radicals, which can increase the generation of reactive oxygen species (ROS) and enhance prooxidant activity [52,53]. Studies on fruit extracts subjected to sterilization (110–130 °C) showed a decrease in the total content of polyphenols by 30–60% with the course of the first-order kinetics, which indicates a significant loss of their original antioxidant properties [54]. The activation energy of the decomposition of these compounds is in the range of 40–85 kJ/mol, which emphasizes their sensitivity to thermal conditions [55].
Tomatoes are a source of many vitamins and minerals, including iron and copper [56]. Additionally, [57] demonstrated that rutin, naringenin and quercetin are equally sensitive to high temperatures. They also found that the degradation of flavonoids increases with the intensity and duration of heating. In their studies, it was indicated that at temperatures above 100 °C, rutin underwent rapid degradation, becoming undetectable after 45 min at 130 °C. Similar findings were noted for narigin, which degraded by 20% at 130 °C. All these phenomena confirm the trend observed in this study. Similar conclusions were also reached by [16], whose meat preserves with the addition of red pepper extract were characterized by higher TBARS values. However, the results obtained in [58] for pork preserves with the addition of tomato pomace were in the range of 2.0 to 2.5 mg MDA/kg. This range is considered high, as exceeding these levels can lead to the development of an unpleasant aroma and aftertaste in meat and its products. Thus, it can be concluded that the addition of tomato peels and seeds’ powder reduces the amount of synthetic preservative used to 50 mg/kg, thereby not exceeding the rancidity threshold limit of meat products [59].
The analyses performed in the present study indicated that certain bacterial groups, such as CB, LAB, and TVC, were found in every sample. The counts of evaluated groups of microorganisms show that proper sterilization procedures were followed, raw meat was not contaminated, and good hygiene practices were maintained throughout the production process. According to [60], the addition of powders or juices, primarily from celery, did not prevent bacteria growth in “natural-cured” products over a ten-day storage period. These products do not receive adequate protection if the appropriate temperature for storing meat is not maintained or if “natural-cured” or uncured items become contaminated by microorganisms. Furthermore, [61] noted that after 35 days of storage, the addition of solely pre-converted celery juice powder to uncured ham that had been inoculated with strains of L. monocytogenes did not offer any inhibiting properties. The inhibiting process was similar to that of conventionally cured ham only when natural antibacterial and nitrate-reducing starting cultures were added. Nitrite inhibits the growth of various pathogenic agents, including Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus, and Clostridium perfringens [1], as well as the outgrowth of C. botulinum spores in cured pork products, according to [62]. None of the dangerous bacteria mentioned were found in the present study.
5. Conclusions
This study addressed the problem of nitrogen compounds in meat products. The meat industry needs effective and safe solutions, and antioxidants derived from plants can be an excellent choice for the production of safe, clean-label meat products. Therefore, the focus of this experiment was on the possibility of reducing the amount of sodium nitrite (III) to 50 mg/kg and thus fortifying the produced canned pork with tomato peels and seeds powder. Analyses of the ability to scavenge free radicals (ABTS+, DPPH) confirmed the strong antioxidant properties of meat products with the addition of TPSP. The antioxidant activity of the tested meat products was closely related to the concentration of tomato peels and seeds powder. An increase in the antioxidant properties of the experimental meat products was noted, along with an increase in the concentration of added TPSP. However, TBARS (thiobarbituric acid reactive substances) analysis showed that the TPSP samples had higher values than the control samples, suggesting a higher degree of lipid oxidation. This may be due to the fact that despite the presence of high levels of antioxidant compounds in tomato peels and seeds, their effectiveness in inhibiting lipid oxidation under the influence of the sterilization process was limited. Additionally, the acidity and phenolic profile of TPSP could promote pro-oxidant reactions under certain conditions. Increased additions of TPSP also resulted in an increase in the yellowness (b*) and redness (a*) of the meat product, which may have a positive effect on consumer acceptability. The samples with tomato pomace were also characterized by lower pH values, which was related to the high acidity of the additive used. However, no effect of the TPSP on the chemical composition or water activity values was noted. The incorporation of tomato peels and seeds’ powder (TPSP), recognized as a source of bioactive compounds, into meat products with reduced nitrogen content may contribute to enhancing their nutritional profile. However, the potential degradation of these compounds during sterilization, along with the absence of clinical and bioavailability data, precludes definitive conclusions regarding any direct health benefits. Therefore, further research is necessary to assess the functional potential of TPSP in the final product. Additionally, microbiological analyses confirmed that the samples exhibited good microbiological quality, with no pathogenic bacteria detected that could pose a risk to consumer health.
From the perspective of industrial implementation, key factors include economic aspects, scalability, and regulatory compliance of using freeze-dried tomato peels and seeds (TPSP) in meat processing. TPSP, as a by-product of tomato processing, represents an easily accessible and potentially low-cost raw material. The scalability of the process depends on the application of technologies that enable obtaining a powder with consistent physicochemical and microbiological properties, as well as the implementation of quality control systems ensuring product safety. Compliance with food safety regulations and regulations concerning novel foods depends on the form of TPSP application and the applicable standards in each country. Therefore, further research is needed to optimize the process and assess the potential for industrial-scale implementation.
Research is also needed on the use of an additive on the quality of pork preservatives with a reduced addition of sodium nitrate (III), with detailed consideration of sensory evaluation, texture analysis and nitrite residues in meat products.
Author Contributions
Conceptualization, P.S.; methodology, P.S., M.K. (Miroslava Kačániová) and M.K. (Małgorzata Karwowska); investigation, P.S. and M.K. (Miroslava Kačániová); data curation, P.S.; writing—original draft preparation, P.S., M.K. (Miroslava Kačániová) and M.K. (Małgorzata Karwowska); writing—review and editing, M.K. (Miroslava Kačániová), M.K. (Małgorzata Karwowska) and K.M.W.; supervision, M.K. (Miroslava Kačániová) and M.K. (Małgorzata Karwowska); project administration, M.K. (Miroslava Kačániová). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C.H. Alternatives to Nitrite in Processed Meat: Up to Date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries Extracts as Natural Antioxidants in Meat Products: A Review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for the Meat Industry: A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural Antioxidants from Seeds and Their Application in Meat Products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Munekata, P.E.; Pateiro, M.; Maggiolino, A.; Bohrer, B.; Lorenzo, J.M. Red Beetroot: A Potential Source of Natural Additives for the Meat Industry. Appl. Sci. 2020, 10, 8340. [Google Scholar] [CrossRef]
- Shao, D.; Atungulu, G.G.; Pan, Z.; Yue, T.; Zhang, A.; Fan, Z. Characteristics of Isolation and Functionality of Protein from Tomato Pomace Produced with Different Industrial Processing Methods. Food Bioprocess Technol. 2014, 7, 532–541. [Google Scholar] [CrossRef]
- Nour, V.; Ionica, M.E.; Trandafir, I. Bread Enriched in Lycopene and Other Bioactive Compounds by Addition of Dry Tomato Waste. J. Food Sci. Technol. 2015, 52, 8260–8267. [Google Scholar] [CrossRef]
- Abbassi, N.; Ait Talhajt, S.; Fadel, S.; Ahra, M. Tomato Pomace Valorization by Oil and Bioactive Compounds Extraction: Case of Souss-Massa Region. Am. J. Innov. Res. Appl. Sci. 2021, 12, 211–216. [Google Scholar]
- Kun, Y.; Ssonko Lule, U.; Xiao-Lin, D. Lycopene: Its Properties and Relationship to Human Health. Food Rev. Int. 2006, 22, 309–333. [Google Scholar] [CrossRef]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Büyükünal, S.K.; Sakar, F.S.; Turhan, I.; Erginba, Ç.; Sandikçi Altunatmaz, S.; Yilmaz Aksu, F.; Yilmaz Eker, F.; Kahraman, T. Presence of Salmonella spp., Listeria monocytogenes, Escherichia coli O157 and Nitrate-Nitrite Residue Levels in Turkish Traditional Fermented Meat Products (Sucuk and Pastırma). Kafkas Univ. Vet. Fak. Derg. 2016, 22, 233–236. [Google Scholar]
- Hospital, X.F.; Hierro, E.; Stringer, S.; Fernández, M. A Study on the Toxigenesis by Clostridium botulinum in Nitrate- and Nitrite-Reduced Dry Fermented Sausages. Int. J. Food Microbiol. 2016, 218, 66–70. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Kęska, P.; Okoń, A.; Solska, E.; Libera, J.; Dolatowski, Z.J. The Influence of Acid Whey on the Antioxidant Peptides Generated to Reduce Oxidation and Improve Colour Stability in Uncured Roast Beef. J. Sci. Food Agric. 2018, 98, 3728–3734. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Karwowska, M.; Dolatowski, Z.J. Use of Acid Whey and Mustard Seed to Replace Nitrites during Cooked Sausage Production. Meat Sci. 2014, 96, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Ferysiuk, K.; Wójciak, K.M. The Possibility of Reduction of Synthetic Preservative E 250 in Canned Pork. Foods 2020, 9, 1869. [Google Scholar] [CrossRef]
- Ferysiuk, K.; Wójciak, K.M.; Trzaskowska, M. Fortification of Low-Nitrite Canned Pork with Willow Herb (Epilobium angustifolium L.). Int. J. Food Sci. Technol. 2022, 57, 4194–4210. [Google Scholar] [CrossRef]
- Singh, M.; Novoa Rama, E.; Kataria, J.; Leone, C.; Thippareddi, H. Emerging Meat Processing Technologies for Microbiological Safety of Meat and Meat Products. Meat Muscle Biol. 2020, 4, 1–18. [Google Scholar] [CrossRef]
- Gaze, J.E. Microbiological aspects of thermally processed foods. J. Appl. Microbiol. 2005, 98, 1381–1386. [Google Scholar] [CrossRef]
- Eneji, C.A. The Effect of heat treatment on the chemical composition of canned meat. Glob. J. Pure Appl. Sci. 2001, 7, 49–56. [Google Scholar] [CrossRef][Green Version]
- Olvera-Aguirre, G.; Piñeiro-Vázquez, Á.T.; Sanginés-García, J.R.; Sánchez Zárate, A.; Ochoa-Flores, A.A.; Segura-Campos, M.R.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Using plant-based compounds as preservatives for meat products: A review. Heliyon 2023, 9, e17071. [Google Scholar] [CrossRef]
- Regulation (EC) No 1333/2008 of the European Parliament of the Council of 16 December 2008 on Food Additives. Off. J. Eur. Union 2008, L354, 16–33.
- Adewale, O.; Jumoke, I.; Ifeoluwa, A. Influence of Drying Temperature and Storage Period on the Quality of Cherry and Plum Tomato Powder. Food Sci. Nutr. 2018, 6, 1146–1153. [Google Scholar] [CrossRef]
- Commission Internationale de L’Eclairage (CIE). Recommendations on Uniform Colour Spaces, Colour-Difference Equations, Psychometric Colour Terms; CIE: Paris, France, 1978. [Google Scholar]
- AMSA. Meat Color Measurements Guidelines; American Meat Science Association: Savoy, IL, USA, 2012. [Google Scholar]
- Mokrzycki, W.S.; Tatol, M. Color Difference ∆E—A Survey. In Proceedings of the Machine Graphic & Vision, Warsaw, Poland, 8 October 2012. [Google Scholar]
- Jung, S.; Choe, J.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. Effect of Dietary Mixture of Gallic Acid and Linoleic Acid on Antioxidative Potential and Quality of Breast Meat from Broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Ferysiuk, K.; Wójciak, K.M.; Materska, M.; Chilczuk, B.; Pabich, M. Modification of Lipid Oxidation and Antioxidant Capacity in Canned Refrigerated Pork with a Nitrite Content Reduced by Half and Addition of Sweet Pepper Extract. LWT-Food Sci. Technol. 2020, 118, 108738. [Google Scholar] [CrossRef]
- Erel, O. A Novel Automated Direct Measurement Method for Total Antioxidant Capacity Using a New Generation, More Stable ABTS Radical Cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of Three Modified TBA Methods for Measuring Lipid Oxidation in Chicken Meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Kęska, P.; Kačániová, M.; Čmiková, N.; Solska, E.; Ogórek, A. Evaluation of Quality of Nitrite-Free Fermented Roe Deer (Capreolus capreolus) Sausage with Addition of Ascorbic Acid and Reduced NaCl. Foods 2024, 13, 3823. [Google Scholar] [CrossRef]
- Estévez, M. Critical Overview of the Use of Plant Antioxidants in the Meat Industry: Opportunities, Innovative Applications, and Future Perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Yagci, S.; Caliskan, R.; Gunes, Z.S.; Capanoglu, E.; Tomas, M. Impact of Tomato Pomace Powder Added to Extruded Snacks on the In Vitro Gastrointestinal Behaviour and Stability of Bioactive Compounds. Food Chem. 2022, 368, 130847. [Google Scholar] [CrossRef]
- Chabi, I.B.; Zannou, O.; Emmanuelle, S.C.A.; Dedehou, B.; Ayegnon, P.; Oloudé, B.; Odouaro, O.; Maqsood, S.; Galanakis, C.H.M.; Kayodè, P. Tomato Pomace as a Source of Valuable Functional Ingredients for Improving Physicochemical and Sensory Properties and Extending the Shelf Life of Foods: A Review. Heliyon 2024, 10, 25261. [Google Scholar] [CrossRef]
- Skwarek, P.; Karwowska, M. Fatty Acids Profile and Antioxidant Properties of Raw Fermented Sausages with the Addition of Tomato Pomace. Biomolecules 2022, 12, 1695. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.M.; García, M.L.; Selgas, M.D. Dry Fermented Sausages Enriched with Lycopene from Tomato Peel. Meat Sci. 2008, 80, 167–172. [Google Scholar] [CrossRef]
- Ghafouri-Oskuei, H.; Javadi, A.; Reza Saeidi Asl, M.; Azadmard-Damirchi, S.; Armin, M. Quality Properties of Sausage Incorporated with Flaxseed and Tomato Powders. Meat Sci. 2020, 161, 107957. [Google Scholar] [CrossRef]
- García, M.L.; Calvo, M.M.; Selgas, M.D. Beef Hamburgers Enriched in Lycopene Using Dry Tomato Peel as an Ingredient. Meat Sci. 2009, 83, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Jin, S.K.; Yang, M.R.; Chu, G.M.; Park, J.H.; Rashid, R.H.I.; Kim, Y.Y.; Kang, S.N. Efficacy of Tomato Powder as Antioxidant in Cooked Pork Patties. Asian-Australas. J. Anim. Sci. 2013, 26, 1339–1346. [Google Scholar] [CrossRef]
- Østerlie, M.; Lerfall, J. Lycopene from Tomato Products Added Minced Meat: Effect on Storage Quality and Colour. Int. Food Res. 2005, 38, 925–929. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s New in Biopotential of Fruit and Vegetable By-Products Applied in the Food Processing Industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total Antioxidant Capacities of Raw and Cooked Meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods. 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Arnao, M.B. Some Methodological Problems in the Determination of Antioxidant Activity Using Chromogen Radicals: A Practical Case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Riazi, F.; Zeynali, F.; Hoseini, E.; Behmadi, H.; Savadkoohi, S. Oxidation Phenomena and Color Properties of Grape Pomace on Nitrite-Reduced Meat Emulsion Systems. Meat Sci. 2016, 121, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Ramli, A.N.M.; Manap, N.W.A.; Bhuyar, P.; Azelee, N.I.W. Passion Fruit (Passiflora edulis) Peel Powder Extract and Its Application Towards Antibacterial and Antioxidant Activity on the Preserved Meat Products. Appl. Sci. 2020, 2, 1748. [Google Scholar] [CrossRef]
- Farinon, B.; Felli, M.; Sulli, M.; Diretto, G.; Savatin, D.V.; Mazzucato, A.; Merendino, N.; Costantini, L. Tomato Pomace Food Waste from Different Variants as a High Antioxidant Potential Resource. Food Chem. 2024, 452, 139509. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; Chekir, L.; Mohamed, G. Effect of Heat Treatment and Light Exposure on the Antioxidant Activity of Flavonoids. Processes 2020, 8, 1078. [Google Scholar] [CrossRef]
- Candogan, K. The Effect of Tomato Paste on Some Quality Characteristics of Beef Patties During Refrigerated Storage. Eur. Food Res. Technol. 2002, 215, 305–309. [Google Scholar] [CrossRef]
- Kęska, P.; Wójciak, K.; Stadnik, J.; Kluz, M.J.; Kačániová, M.; Čmiková, N.; Solska, E.; Mazurek, K. Influence of Apple Pomace on the Oxidation Status, Fatty Acid Content, Colour Stability and Microbiological Profile of Baked Meat Products. Int. J. Food Sci. Technol. 2024, 59, 1591–1604. [Google Scholar] [CrossRef]
- Babaoğlu, A.S.; Unal, K.; Dilek, N.M.; Poçan, H.B.; Karakaya, M. Antioxidant and Antimicrobial Effects of Blackberry, Black Chokeberry, Blueberry, and Red Currant Pomace Extracts on Beef Patties Subject to Refrigerated Storage. Meat Sci. 2022, 187, 108765. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins, and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic Voltammetry Method Suitable for Characterizing Antioxidant Properties of Wine and Wine Phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Ramos, A.; Arboleda, L.; Ramos, S.; Mejia, E. Effect of Heat Treatment on the Antioxidant Capacity of Fruits and Vegetables—A Review Study. ESPOCH Congr. Ecuadorian J. STEAM 2024, 3, 87–102. [Google Scholar] [CrossRef]
- Zapata, J.E.; Sepúlveda, C.T.; Álvarez, A.C. Kinetics of the Thermal degradation of phenolic compounds from achiote leaves (Bixa orellana L.) and its effect on the antioxidant activity. Food Sci. Technol. 2022, v42, e30920. [Google Scholar] [CrossRef]
- Fuentes, E.; Carle, R.; Astudillo, L.; Guzman, L.; Gutierrez, M.; Carrasco, G. Antioxidant and Antiplatelet Activities in Extracts from Green and Fully Ripe Tomato Fruits (Solanum lycopersicum) and Pomace from Industrial Tomato Processing. Evid.-Based Complement. Altern. Med. 2013, 2013, 867578. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of Heat Processing on Thermal Stability and Antioxidant Activity of Six Flavonoids. J. Food Process. Preserv. 2017, 41, 13203. [Google Scholar] [CrossRef]
- Zhang, Y.; Holman, B.W.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.L. Understanding Beef Flavour and Overall Liking Traits Using Two Different Methods for Determination of Thiobarbituric Acid Reactive Substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef]
- Jin, S.K.; Choi, J.S.; Yang, H.S.; Park, T.S.; Yim, D.G. Natural Curing Agents as Nitrite Alternatives and Their Effects on the Physicochemical, Microbiological Properties and Sensory Evaluation of Sausages During Storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Sullivan, G.A.; Kulchaiyawat, C.; Sebranek, J.G.; Dickson, J.M. Survival and Growth of Clostridium perfringens in Commercial No-Nitrate-or-Nitrite-Added (Natural and Organic) Frankfurters, Hams, and Bacon. J. Food Prot. 2011, 3, 410–416. [Google Scholar] [CrossRef]
- Sullivan, G.A.; Jackson-Davis, A.L.; Niebuhr, S.E.; Xi, Y.; Schrader, K.D.; Sebranek, J.G.; Dickson, J.S. Inhibition of Listeria monocytogenes Using Natural Antimicrobials in No-Nitrate-or-Nitrite-Added Ham. J. Food Prot. 2012, 75, 1071–1076. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Cordray, J.C.; Sebranek, J.G.; Love, J.A.; Ahn, D.U. Effects of Varying Levels of Vegetable Juice Powder and Incubation Time on Color, Residual Nitrate and Nitrite, Pigment, pH, and Trained Sensory Attributes of Ready-to-Eat Uncured Ham. J. Food Sci. 2007, 72, 388–395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).