An Experimental Study of Glycerol Carbonate Synthesis over g-C3N4 Catalysts
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Catalysts Preparation
2.3. Catalyst Characterization Equipment
2.4. Procedure for Catalytic Test in the Glycerol Carbonylation Reaction
3. Results and Discussions
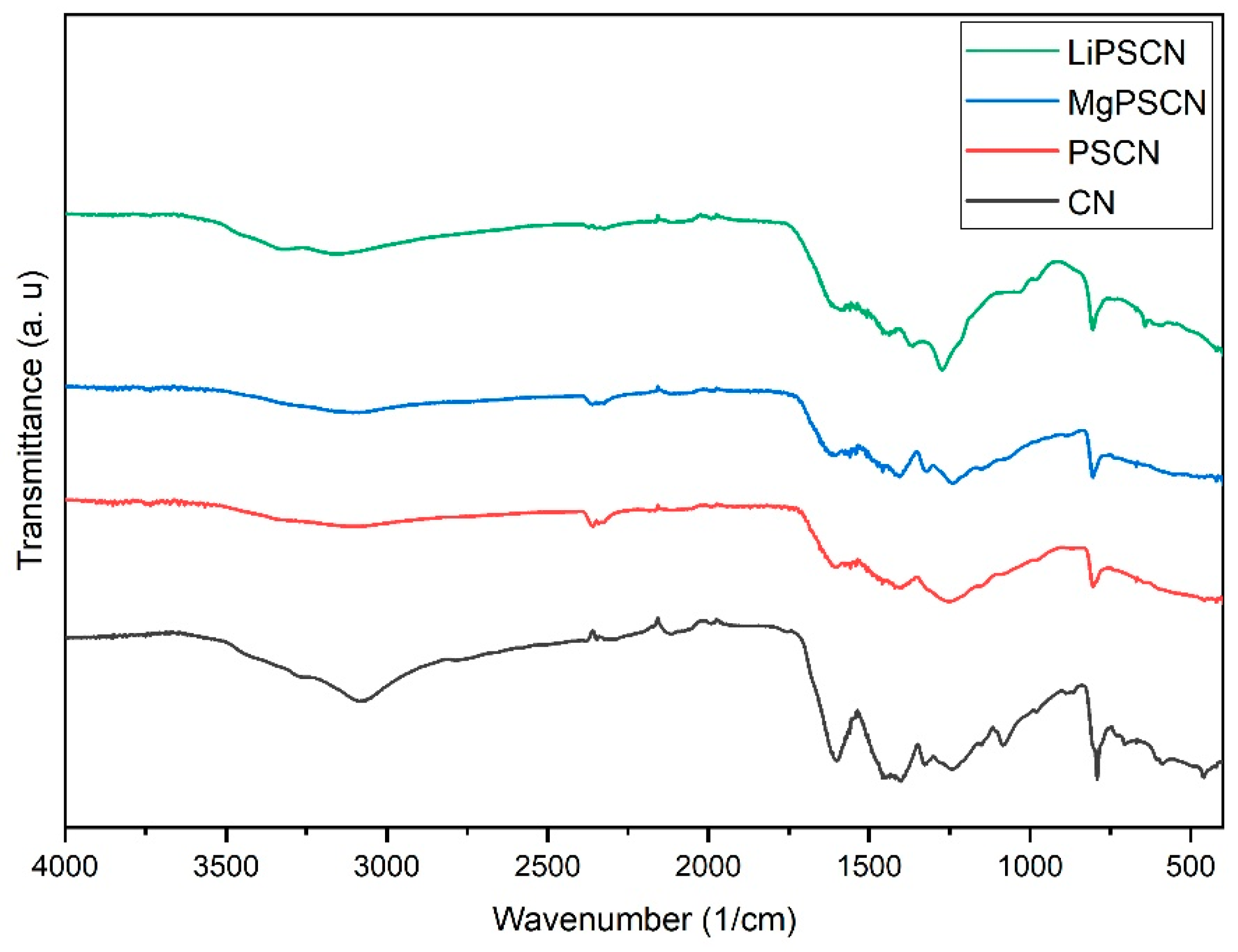
3.1. Catalyst Characterization
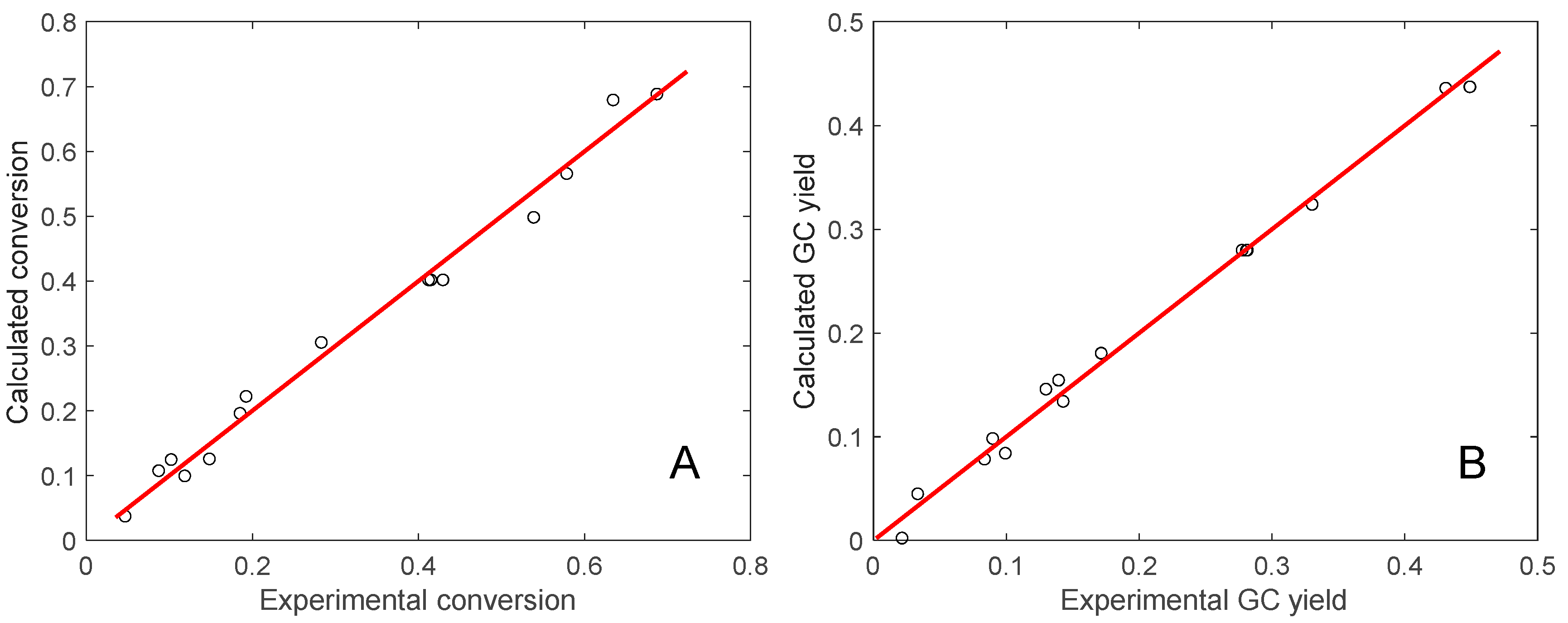
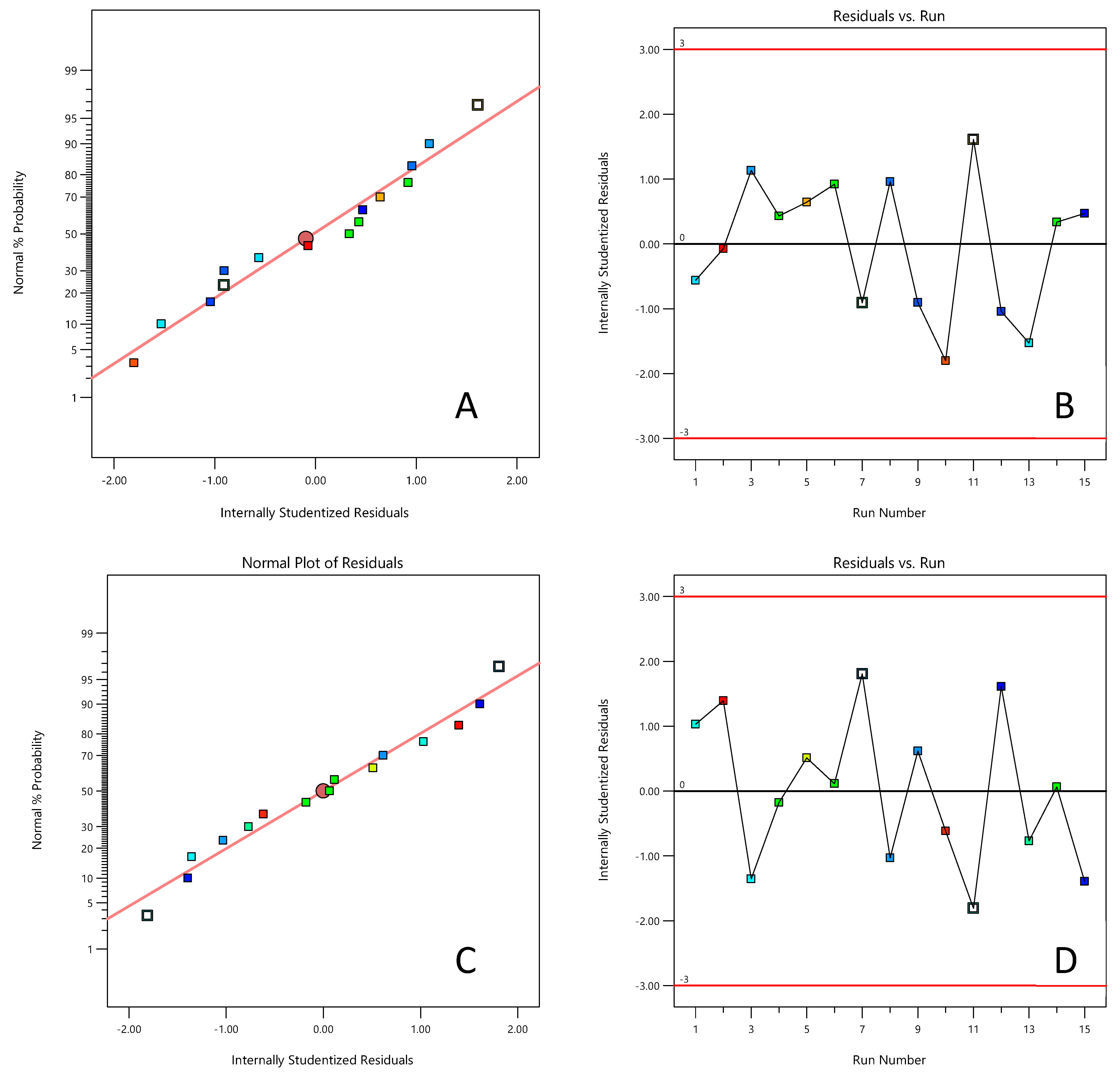
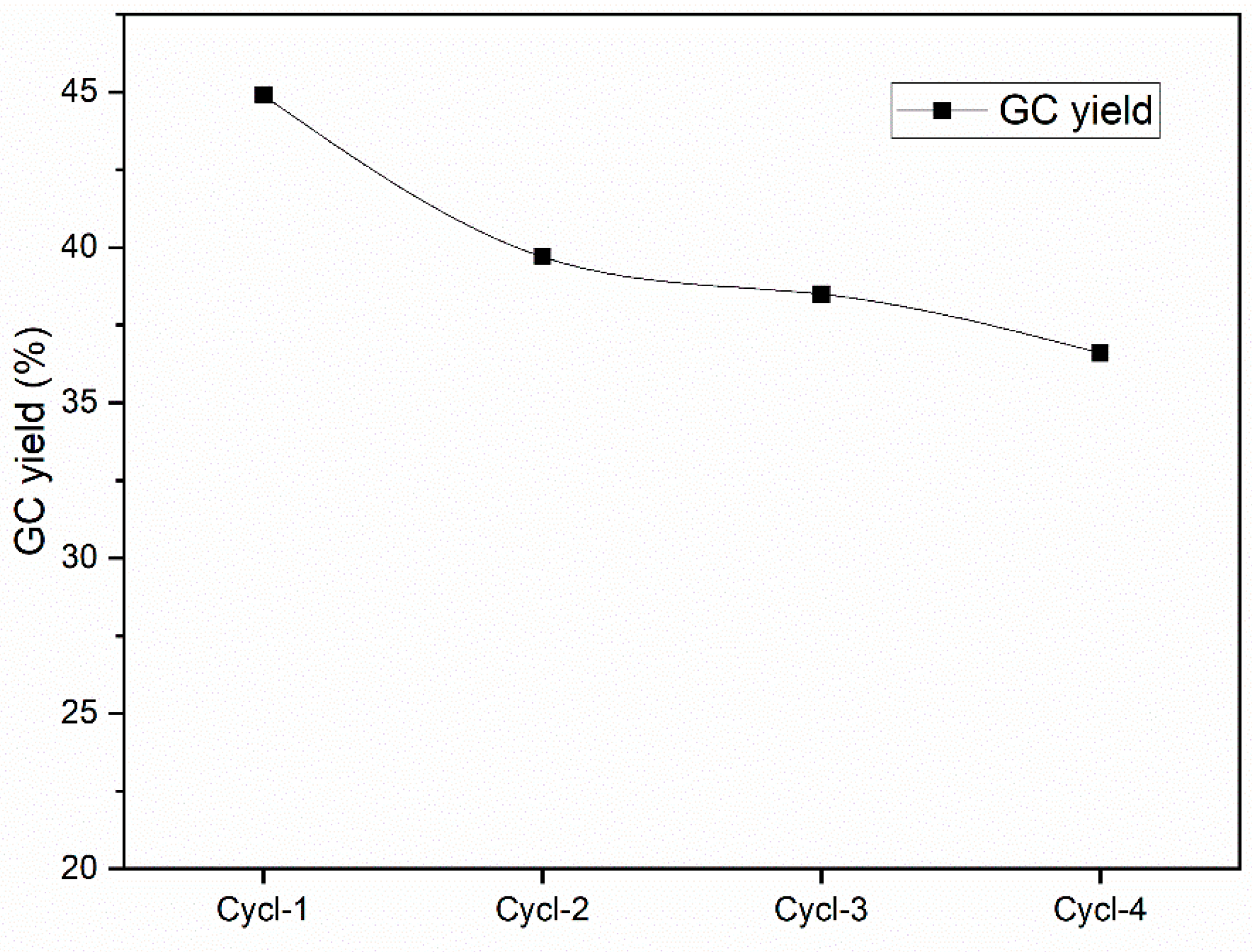
3.2. Optimization of Catalyst Activity Through Experimental Design Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. CO2 Emissions in 2023; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Usman, M.; Rehman, A.; Saleem, F.; Abbas, A.; Eze, V.C.; Harvey, A. Synthesis of cyclic carbonates from CO2 cycloaddition to bio-based epoxides and glycerol: An overview of recent development. RSC Adv. 2023, 13, 22717–22743. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yang, J.-W.; Ma, T.; Wang, J.; Xia, D.; Du, B.; Cui, Y.; Yang, C. Mechanism study on direct synthesis of glycerol carbonate from CO2 and glycerol over shaped CeO2 model catalysts. Chin. Chem. Lett. 2023, 34, 108395. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; He, D. Catalytic synthesis of glycerol carbonate from biomass-based glycerol and dimethyl carbonate over Li-La2O3 catalysts. Appl. Catal. A Gen. 2018, 564, 234–242. [Google Scholar] [CrossRef]
- Fan, X.; Burton, R.; Zhou, Y. Glycerol (Byproduct of Biodiesel Production) as a Source for Fuels and Chemicals–Mini Review. Open Fuels Energy Sci. J. 2010, 3, 17–22. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Inrirai, P.; Keogh, J.; Centeno-Pedrazo, A.; Artioli, N.; Manyar, H. Recent advances in processes and catalysts for glycerol carbonate production via direct and indirect use of CO2. J. CO2 Util. 2024, 80, 102693. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An Overview of Recent Research in the Conversion of Glycerol into Biofuels, Fuel Additives and other Bio-Based Chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Amigoni, S.; Taffin de Givenchy, E.P.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol carbonate as a versatile building block for tomorrow: Synthesis, reactivity, properties and applications. Green Chem. 2013, 15, 283–306. [Google Scholar] [CrossRef]
- Christy, S.; Noschese, A.; Lomelí-Rodriguez, M.; Greeves, N.; Lopez-Sanchez, J.A. Recent progress in the synthesis and applications of glycerol carbonate. Curr. Opin. Green Sustain. Chem. 2018, 14, 99–107. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. Chemical equilibrium of glycerol carbonate synthesis from glycerol. J. Chem. Thermodyn. 2011, 43, 731–736. [Google Scholar] [CrossRef]
- Razali, N.; McGregor, J. Improving Product Yield in the Direct Carboxylation of Glycerol with CO2 through the Tailored Selection of Dehydrating Agents. Catalysts 2021, 11, 138. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakao, K.; Suzuki, K.; Nakagawa, Y.; Tomishige, K. Direct Cyclic Carbonate Synthesis from CO2 and Diol over Carboxylation/Hydration Cascade Catalyst of CeO2 with 2-Cyanopyridine. ACS Catal. 2014, 4, 1893–1896. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl. Catal. A Gen. 2016, 513, 9–18. [Google Scholar] [CrossRef]
- Hu, C.; Chang, C.-W.; Yoshida, M.; Wang, K.-H. Lanthanum nanocluster/ZIF-8 for boosting catalytic CO2/glycerol conversion using MgCO3 as a dehydrating agent. J. Mater. Chem. A 2021, 9, 7048–7058. [Google Scholar] [CrossRef]
- Takeuchi, K.; Matsumoto, K.; Fukaya, N.; Sato, K.; Choi, J.C. Synthesis of Glycerol Carbonate from Glycerol and CO2 Using CaO as a Dehydrating Agent. Asian J. Org. Chem. 2022, 11, e202200212. [Google Scholar] [CrossRef]
- Rozulan, N.; Halim, S.A.; Razali, N.; Lam, S.S. A review on direct carboxylation of glycerol waste to glycerol carbonate and its applications. Biomass Convers. Biorefin. 2022, 12, 4665–4682. [Google Scholar] [CrossRef]
- Gao, Z.; Xiang, M.; He, M.; Zhou, W.; Chen, J.; Lu, J.; Wu, Z.; Su, Y. Transformation of CO2 with Glycerol to Glycerol Carbonate over ETS-10 Zeolite-Based Catalyst. Molecules 2023, 28, 2272. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shu, X.; Zhu, Y.; Zhang, J.; Chai, Z.; Song, H.; An, Z.; He, J. Highly dispersed MgInCe-mixed metal oxides catalyzed direct carbonylation of glycerol and CO2 into glycerol carbonate. Chin. J. Chem. Eng. 2024, 72, 153–163. [Google Scholar] [CrossRef]
- Xu, H.; Ke, Y. Synthesis of glycerol carbonate from glycerol and CO2 over Cu-Zr complex oxide. J. Fuel Chem. Technol. 2024, 52, 171–182. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, G.; Wang, X.; Deng, L.; Wan, J.; Liu, Y.; Kan, J.; Shang, C.; Guo, Z. Resourcification of CO2 to high-value-added glycerol carbonate by ZnAlCe composite oxides with frustrated Lewis pairs. Mol. Catal. 2024, 564, 114348. [Google Scholar] [CrossRef]
- Lukato, S.; Wójcik, M.; Krogul-Sobczak, A.; Litwinienko, G. Enhancing the Green Synthesis of Glycerol Carbonate: Carboxylation of Glycerol with CO2 Catalyzed by Metal Nanoparticles Encapsulated in Cerium Metal–Organic Frameworks. Nanomaterials 2024, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-y.; Nguyen-Phu, H.; Shin, E.W. Glycerol carbonation with CO2 and La2O2CO3/ZnO catalysts prepared by two different methods: Preferred reaction route depending on crystalline structure. Mol. Catal. 2017, 435, 99–109. [Google Scholar] [CrossRef]
- Koranian, P.; Kumar Dalai, A.; Sammynaiken, R. Production of glycerol carbonate from glycerol and carbon dioxide using metal oxide catalysts. Chem. Eng. Sci. 2024, 286, 119687. [Google Scholar] [CrossRef]
- Fao, G.D.; Catherine, H.N.; Huang, C.-H.; Lee, Y.-L.; Jiang, J.-C.; Hu, C. Unraveling the effects of P and S doping over g-C3N4 in strengthening Lewis basicity for CO2/glycerol conversion: A theoretical and experimental study. Carbon 2023, 201, 129–140. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Arumugam, M.; Tahir, M.; Praserthdam, P. Effect of nonmetals (B, O, P, and S) doped with porous g-C3N4 for improved electron transfer towards photocatalytic CO2 reduction with water into CH4. Chemosphere 2022, 286, 131765. [Google Scholar] [CrossRef]
- Niu, H.; Wang, X.; Shao, C.; Liu, Y.; Zhang, Z.; Guo, Y. Revealing the oxygen reduction reaction activity origin of single atoms supported on g-C3N4 monolayers: A first-principles study. J. Mater. Chem. A 2020, 8, 6555–6563. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Kadi, M.W. Generation of Hydrogen Gas Using CuCr2O4-g-C3N4 Nanocomposites under Illumination by Visible Light. ACS Omega 2021, 6, 4485–4494. [Google Scholar] [CrossRef]
- Rajput, Y.; Kumar, P.; Zhang, T.C.; Kumar, D.; Nemiwal, M. Recent advances in g-C3N4-based photocatalysts for hydrogen evolution reactions. Int. J. Hydrogen Energy 2022, 47, 38533–38555. [Google Scholar] [CrossRef]
- Aboubakr, A.E.A.; El Rouby, W.M.A.; Khan, M.D.; Revaprasadu, N.; Millet, P. Effect of morphology and non-metal doping (P and S) on the activity of graphitic carbon nitride toward photoelectrochemical water oxidation. Sol. Energy Mater. Sol. Cells 2021, 232, 111326. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Lin, T.-J.; Lin, Y.-R.; Chiu, W.-L.; Nguyen, B.-S.; Hu, C. Influence of P,S,O-Doping on g-C3N4 for hydrogel formation and photocatalysis: An experimental and theoretical study. Carbon 2020, 169, 338–348. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A Facile One-Step Synthesis of Fe-Doped g-C3N4 Nanosheets and Their Improved Visible-Light Photocatalytic Performance. ChemCatChem 2017, 9, 1708–1715. [Google Scholar] [CrossRef]
- Wang, J.-C.; Cui, C.-X.; Li, Y.; Liu, L.; Zhang, Y.-P.; Shi, W. Porous Mn doped g-C3N4 photocatalysts for enhanced synergetic degradation under visible-light illumination. J. Hazard. Mater. 2017, 339, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.W.; Sembodo, B.S.T.; Alviansyah, A.; Putri, I.B.S. Application of titanium doped g-C3N4 for the degradation of toxic dyes in textiles wastewater. AIP Conf. Proc. 2020, 2217, 030127. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, S.; Zhang, X.; Jiang, Z.; Zheng, J.; Guo, C. Combination of experimental and theoretical investigation on Ti-doped g-C3N4 with improved photo-catalytic activity. Appl. Surf. Sci. 2019, 489, 427–434. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, F.; Chen, Z.; Huang, S.; Wei, W.; Xie, M.; Xu, H.; Li, H. One-step synthesis of Fe-doped surface-alkalinized g-C3N4 and their improved visible-light photocatalytic performance. Appl. Surf. Sci. 2019, 469, 739–746. [Google Scholar] [CrossRef]
- Deng, P.; Xiong, J.; Lei, S.; Wang, W.; Ou, X.; Xu, Y.; Xiao, Y.; Cheng, B. Nickel formate induced high-level in situ Ni-doping of g-C3N4 for a tunable band structure and enhanced photocatalytic performance. J. Mater. Chem. A 2019, 7, 22385–22397. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Singh, S.; Singh, P.; Panchal, P.; Sharma, A.; Devi, P.; Nehra, S.P. Mg/Li Co-doped g-C3N4: An excellent photocatalyst for wastewater remediation and hydrogen production applications towards sustainable development. Int. J. Hydrogen Energy 2023, 48, 37746–37761. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Choi, S.H.; Yang, W. Facile enhancement of photocatalytic efficiency of g-C3N4 by Li-intercalation. Catal. Today 2019, 321–322, 67–73. [Google Scholar] [CrossRef]
- Vuggili, S.B.; Khanth, S.K.; Kadiya, K.; Gaur, U.K.; Sharma, M. Improvement in visible light stimulated photocatalysis by the inducement of magnesium dopant inside graphitic carbon nitride frameworks. J. Environ. Chem. Eng. 2019, 7, 103440. [Google Scholar] [CrossRef]
- Reisi, B.; Najafi Chermahini, A. Modification g-CN by MgO and its application for glycerol carbonate synthesis from glycerol and dimethyl carbonate. Environ. Prog. Sustain. Energy 2023, 42, e14007. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, X.; Gondal, M.A.; Liu, Y.; Shen, K.; Xu, Q. Comparative Study of Pure g-C3N4 and Sulfur-Doped g-C3N4 Catalyst Performance in Photo-Degradation of Persistent Pollutant Under Visible Light. J. Nanosci. Nanotechnol. 2018, 18, 4142–4154. [Google Scholar] [CrossRef] [PubMed]
- Lukato, S.; Kasozi, G.N.; Naziriwo, B.; Tebandeke, E. Glycerol carbonylation with CO2 to form glycerol carbonate: A review of recent developments and challenges. Curr. Res. Green Sustain. Chem. 2021, 4, 100199. [Google Scholar] [CrossRef]
- Charif, M.L.; Doukeh, R.; Ciuparu, D.M. The Catalytic Performance of Metal-Oxide-Based Catalysts in the Synthesis of Glycerol Carbonate: Toward the Green Valorization of Glycerol. Catalysts 2025, 15, 534. [Google Scholar] [CrossRef]
- Liu, D.Y.; Dong, J.H.; Liu, F.M.; Gao, X.F.; Yu, Y.; Zhang, S.B.; Dong, L.M.; Guo, Y.K. Synthesis and photocatalytic performance of g-C3N4 composites. J. Ovonic Res. Vol. 2019, 15, 239–246. [Google Scholar]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, H.; Sun, G.; Zhang, Z. Solid-State Method Synthesis of SnO2-Decorated g-C3N4 Nanocomposites with Enhanced Gas-Sensing Property to Ethanol. Materials 2017, 10, 604. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, B.; Yu, W.; Wu, X.; Mi, R.; Huang, Z.; Liu, Y.-g.; Fang, M.; Min, X. Synthesis and photocatalytic performance of composite g-C3N4 with functionalized multi-walled carbon nanotubes. J. Alloys Compd. 2023, 968, 171707. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Sun, C.; Wang, T.; Tao, K.; Gao, D. P dopants induced ferromagnetism in g-C3N4 nanosheets: Experiments and calculations. Appl. Phys. Lett. 2017, 110, 222403. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Chen, X.; Wu, Z.; Liang, J.; Zhang, J.; Wang, H.; Wang, H. Phosphorus- and Sulfur-Codoped g-C3N4: Facile Preparation, Mechanism Insight, and Application as Efficient Photocatalyst for Tetracycline and Methyl Orange Degradation under Visible Light Irradiation. ACS Sustain. Chem. Eng. 2017, 5, 5831–5841. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, S.; Wu, H.; Kang, Z.; Wang, L. Facile one-pot synthesis of Mg-doped g-C3N4 for photocatalytic reduction of CO2. RSC Adv. 2019, 9, 28894–28901. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Jia, D.; Yao, H.; Shang, X.; Li, C. One-step synthesis of Mg-doped g-C3N4 nanosheets for efficient photo-Fenton-like catalysis. Diam. Relat. Mater. 2022, 129, 109313. [Google Scholar] [CrossRef]
- Sedaghati-Jamalabad, G.; Bagheri-Mohagheghi, M.M. A study on the structural and optical properties of the SnFe2O4 spinel compound as anode electrode in Li ion-battery: The optical and dielectric parameters via synthesis methods. Opt. Quantum Electron. 2024, 56, 965. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, J.; Zhang, Y.; Xing, S.; Ran, J.; Ma, Y.; Li, X. S/P co-doped g-C3N4 with secondary calcination for excellent photocatalytic performance. Int. J. Hydrogen Energy 2024, 51, 962–974. [Google Scholar] [CrossRef]
- Cako, E.; Dudziak, S.; Głuchowski, P.; Trykowski, G.; Pisarek, M.; Fiszka Borzyszkowska, A.; Sikora, K.; Zielińska-Jurek, A. Heterojunction of (P, S) co-doped g-C3N4 and 2D TiO2 for improved carbamazepine and acetaminophen photocatalytic degradation. Sep. Purif. Technol. 2023, 311, 123320. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, T.; Chen, X.; He, Y.; Liang, X. Model construction of micro-pores in shale: A case study of Silurian Longmaxi Formation shale in Dianqianbei area, SW China. Pet. Explor. Dev. 2018, 45, 412–421. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Xu, L.; Li, P.; Liu, Y.; Dang, W. Pore Structure and Fractal Characteristics of Deep Shale: A Case Study from Permian Shanxi Formation Shale, from the Ordos Basin. ACS Omega 2022, 7, 9229–9243. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, Q.; Wang, S.; Dong, P.; Zhang, L. Regenerable g-C3N4–chitosan beads with enhanced photocatalytic activity and stability. RSC Adv. 2018, 8, 27516–27524. [Google Scholar] [CrossRef]
- Shirman, R.; Chakraborty, S.; Sasson, Y. Ru/GCN Nanocomposite as an Efficient Catalyst for Hydrogen Generation from Sodium Hypophosphite. Nanomaterials 2024, 14, 1187. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; Liu, Y.; Liu, H.; Jiang, Y.; Wang, N. Intumescent flame retardancy and smoke suppression of Eucommia ulmoides gum/natural rubber blends based on synergistic g-C3N4@Fe3O4 nanocomposites. RSC Adv. 2022, 12, 21704–21712. [Google Scholar] [CrossRef] [PubMed]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken experimental design for chromium(VI) ions removal by bacterial cellulose-magnetite composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Perveen, R.; Latif, F.; Abbas, M.; Ayub, K.; Sarfaraz, S.; Saeed, M.; Rana, S.; Al-Rashida, M.; Nawaz, M.A.; Hameed, A. Optimization study and application of box-behnken model for probing eggshell supported transition metals based catalysts to synthesize hydrazone & dihydropyrimidinones. Sci. Rep. 2024, 14, 23270. [Google Scholar] [CrossRef]
- Matei, P.L.; Deleanu, I.; Brezoiu, A.M.; Chira, N.A.; Busuioc, C.; Isopencu, G.; Cîlțea-Udrescu, M.; Alexandrescu, E.; Stoica-Guzun, A. Ultrasound-Assisted Extraction of Blackberry Seed Oil: Optimization and Oil Characterization. Molecules 2023, 28, 2486. [Google Scholar] [CrossRef]
- Kamaroddin, M.F.; Rahaman, A.; Gilmour, D.J.; Zimmerman, W.B. Optimization and cost estimation of microalgal lipid extraction using ozone-rich microbubbles for biodiesel production. Biocatal. Agric. Biotechnol. 2020, 23, 101462. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
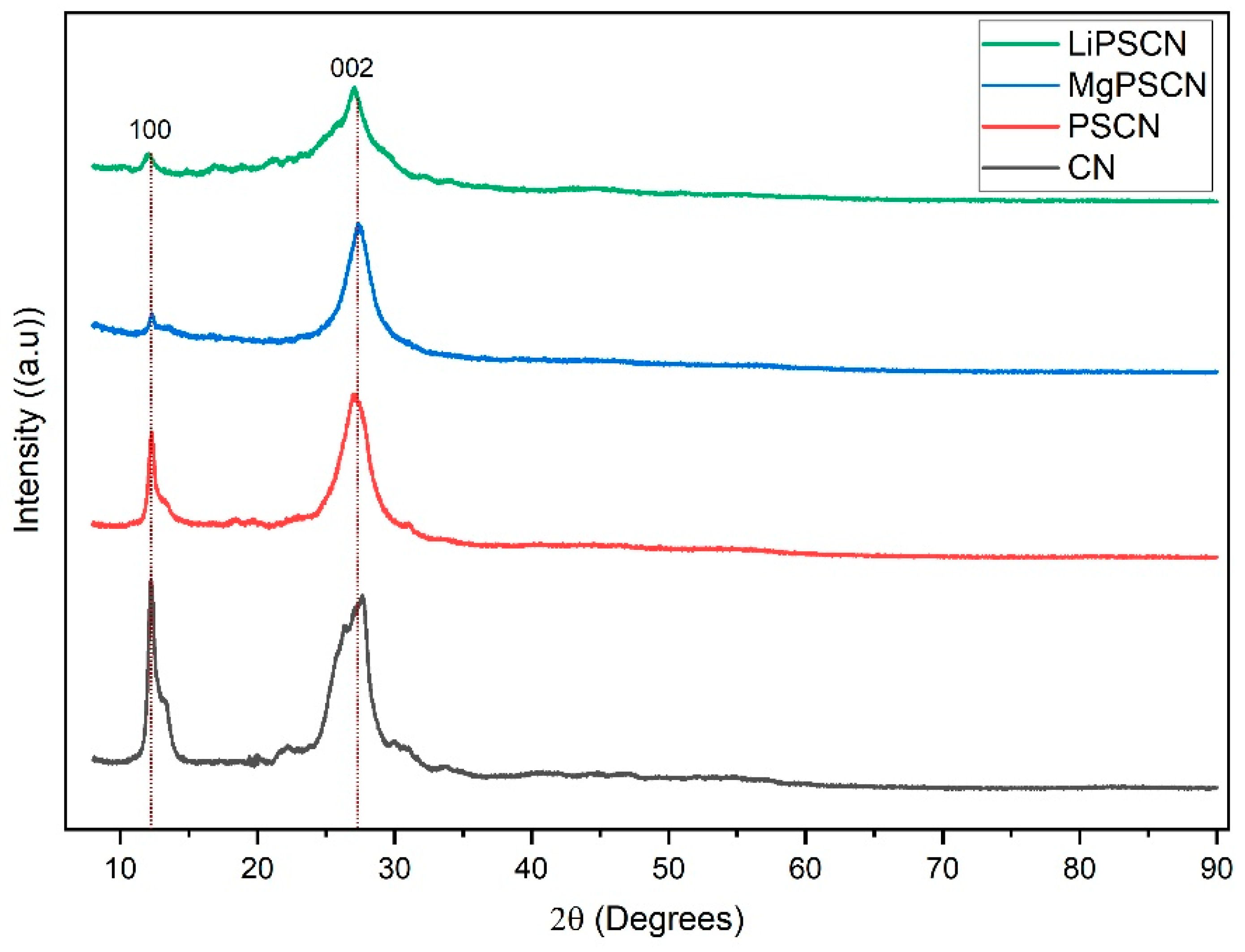
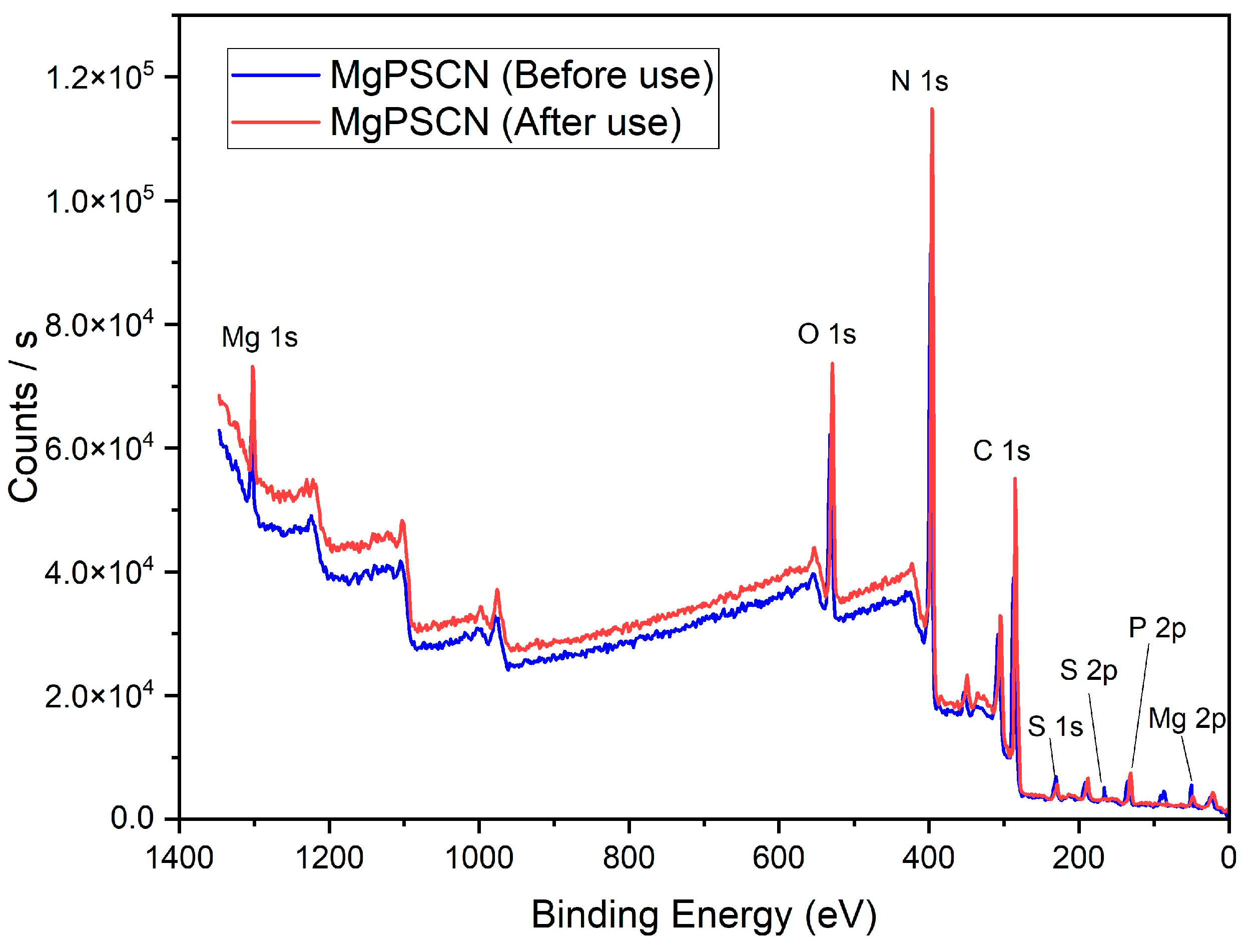

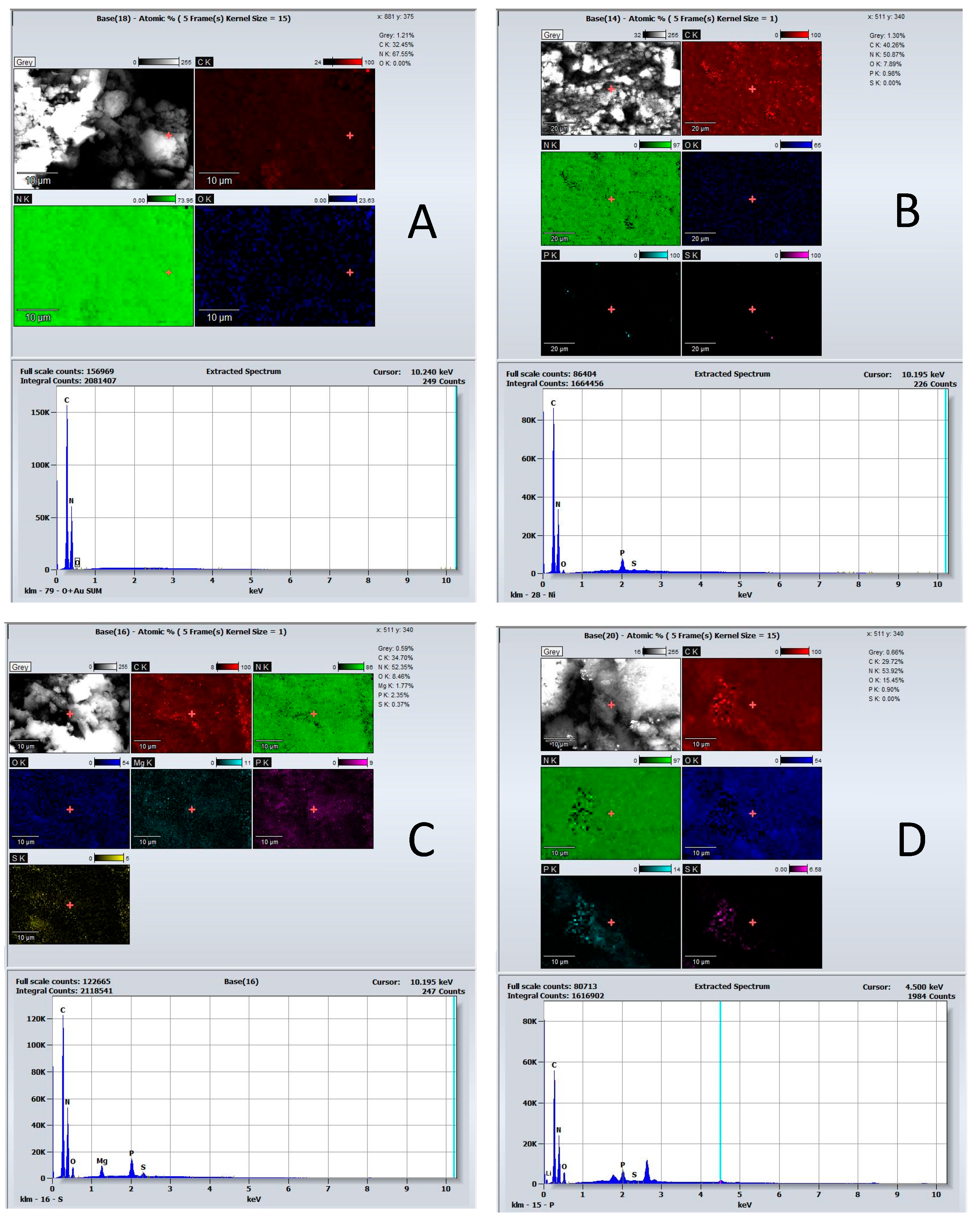

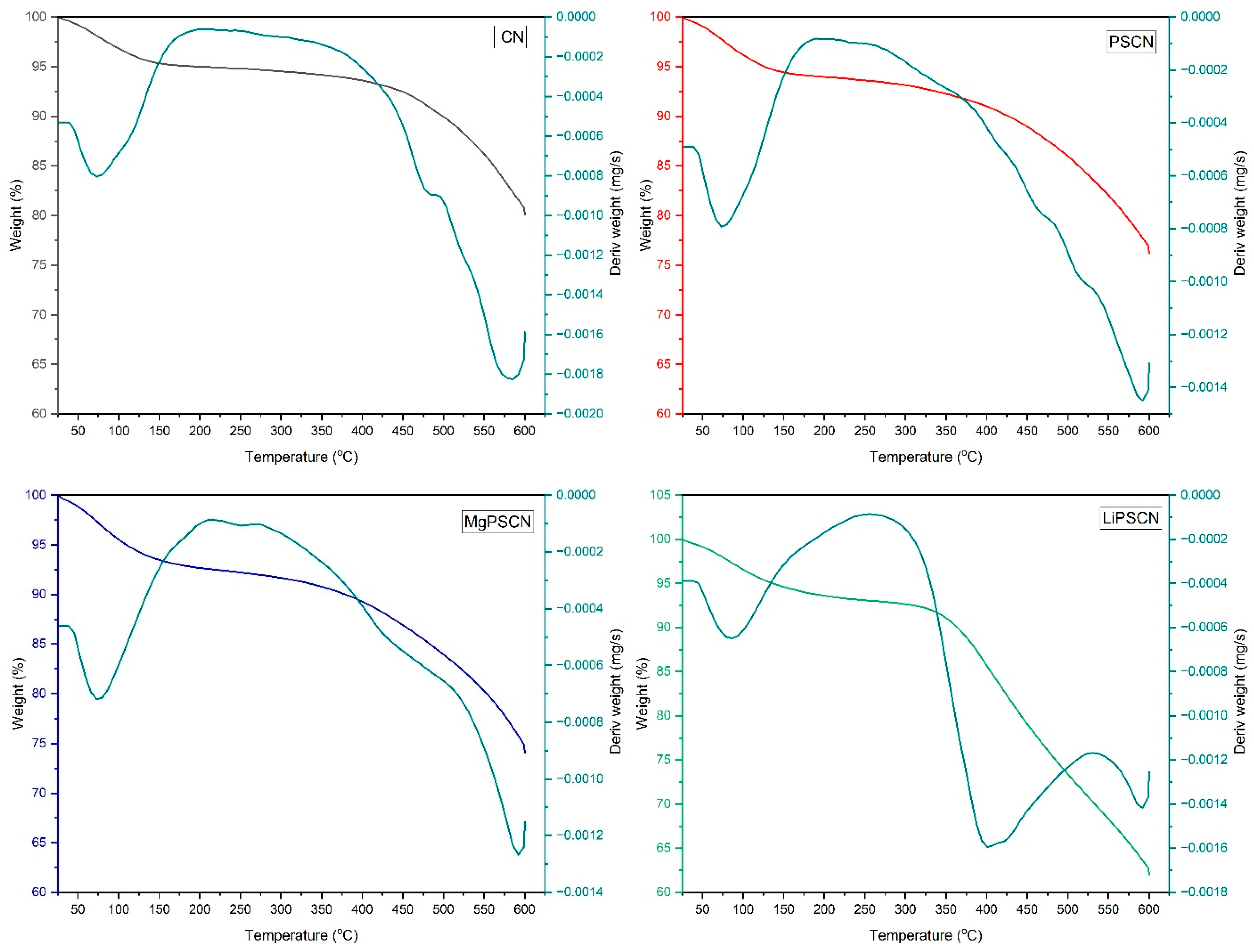

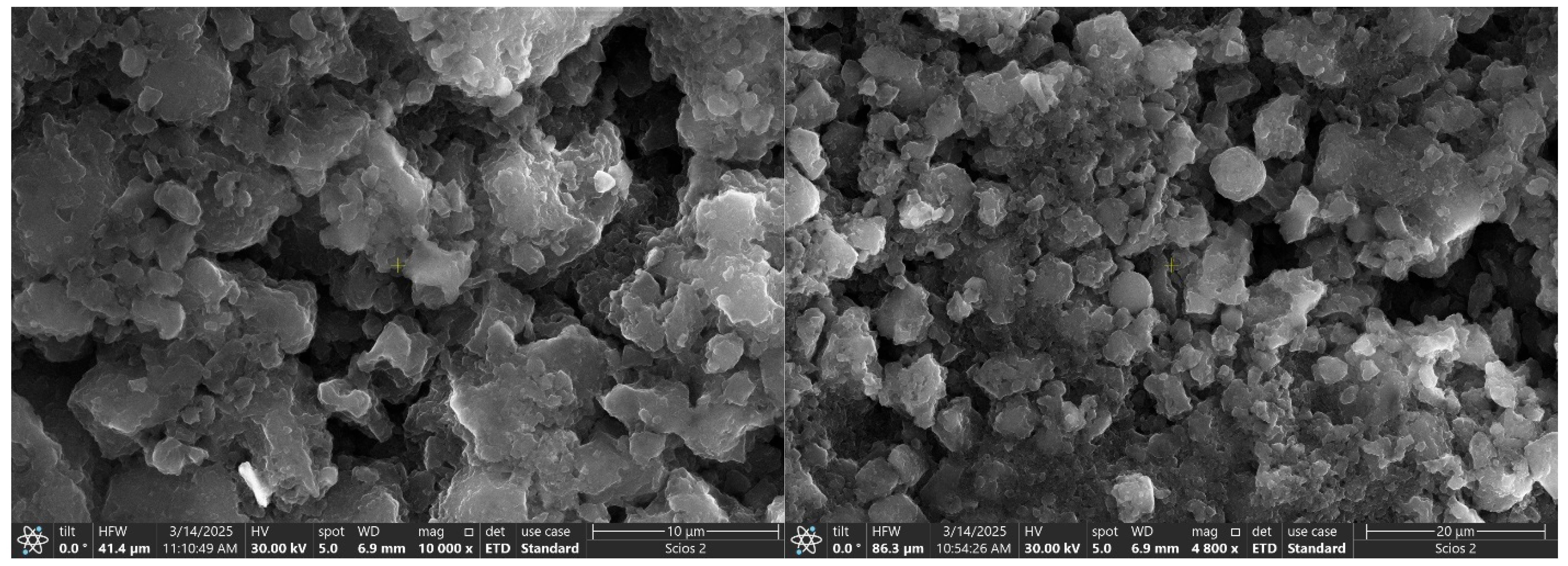
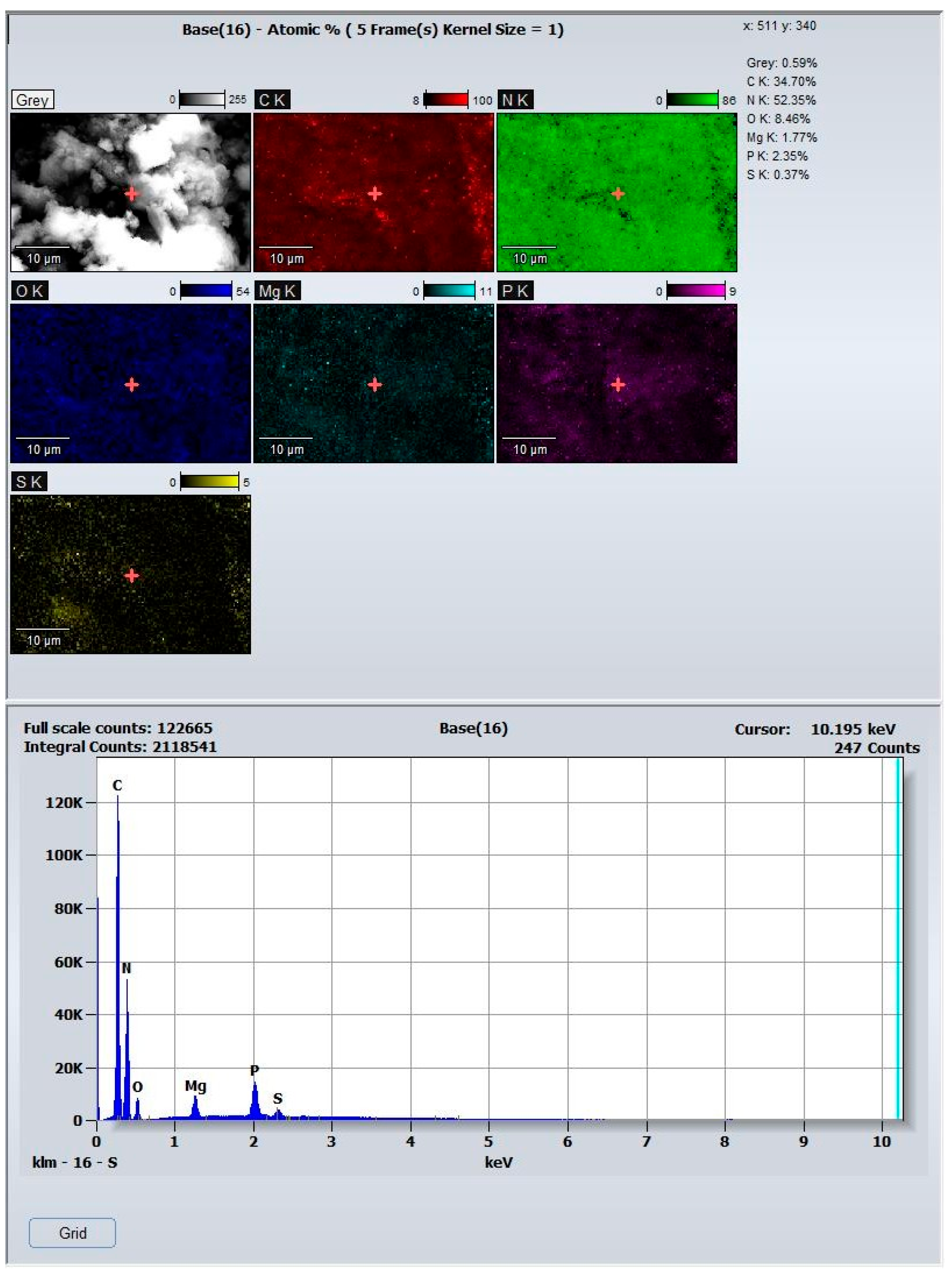
| Catalysts | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Width (nm) | Average Crystallite Size (nm) |
|---|---|---|---|---|
| CN | 10.85 | 0.062 | 5.20 | 10.94 |
| PSCN | 10.10 | 0.085 | 29.396 | 17.57 |
| MgPSCN | 9.19 | 0.063 | 16.09 | 10.71 |
| LiPSCN | 17.32 | 0.128 | 29.396 | 6.86 |
| Factor | Value/Level | |||||
|---|---|---|---|---|---|---|
| Low | Medium | High | ||||
| Natural (Z) | Coded (X) | Natural (Z) | Coded (X) | Natural (Z) | Coded (X) | |
| Reaction temperature (°C), X1 | 150 | −1 | 200 | 0 | 250 | +1 |
| Catalyst amount (%), X2 | 10 | −1 | 30 | 0 | 50 | +1 |
| Operating pressure (bar), X3 | 3 | −1 | 7 | 0 | 10 | +1 |
| Nr. Exp | X1 | X2 | X3 | Y1 | Y2 |
|---|---|---|---|---|---|
| 1 | −1 | 1 | 0 | 18.51 | 14.28 |
| 2 | 1 | 1 | 0 | 68.72 | 44.9 |
| 3 | −1 | 0 | 1 | 14.82 | 12.98 |
| 4 | 0 | 0 | 0 | 41.49 | 27.75 |
| 5 | 1 | 0 | 1 | 57.85 | 33.02 |
| 6 | 0 | 0 | 0 | 42.94 | 28.15 |
| 7 | 0 | −1 | 1 | 28.3 | 9.93 |
| 8 | 1 | −1 | 0 | 11.85 | 8.98 |
| 9 | 0 | −1 | −1 | 10.22 | 8.37 |
| 10 | 0 | 1 | 1 | 63.45 | 43.08 |
| 11 | 0 | 1 | −1 | 53.88 | 13.94 |
| 12 | −1 | 0 | −1 | 8.72 | 2.15 |
| 13 | 1 | 0 | −1 | 19.25 | 17.15 |
| 14 | 0 | 0 | 0 | 41.21 | 28.08 |
| 15 | −1 | −1 | 0 | 4.67 | 3.34 |
| Independent Variable | Correlation Coefficient, R2 | Adjusted R2 | Calculated F-Value | p-Value |
|---|---|---|---|---|
| Y1 | 0.991 | 0.985 | 162.9 | <0.0001 |
| Y2 | 0.993 | 0.984 | 114.3 | <0.0001 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2577.19 | 8 | 322.15 | 114.30 | <0.0001 | significant |
| 635.46 | 1 | 635.46 | 225.47 | <0.0001 | significant | |
| 915.49 | 1 | 915.49 | 324.83 | <0.0001 | significant | |
| 411.85 | 1 | 411.85 | 146.13 | <0.0001 | significant | |
| 156.00 | 1 | 156.00 | 55.35 | 0.0003 | significant | |
| 190.16 | 1 | 190.16 | 67.47 | 0.0002 | significant | |
| 147.09 | 1 | 147.09 | 52.19 | 0.0004 | significant | |
| 53.50 | 1 | 53.50 | 18.98 | 0.0048 | significant | |
| 105.95 | 1 | 105.95 | 37.59 | 0.0009 | significant | |
| Cor Total | 2594.10 | 14 |
| Catalysts | Glycerol Conversion | GC Yield |
|---|---|---|
| CN | 47.24 | 11.91 |
| PSCN | 61.88 | 29.74 |
| MgPSCN | 70.05 | 45.85 |
| LiPSCN | 58.42 | 27.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charif, M.L.; Ciuparu, D.M.; Lixandru Matei, I.L.; Vasilievici, G.; Banu, I.; Băjan, M.; Bomboș, D.; Dușescu-Vasile, C.; Ghețiu, I.V.; Panaitescu, C.; et al. An Experimental Study of Glycerol Carbonate Synthesis over g-C3N4 Catalysts. Appl. Sci. 2025, 15, 6236. https://doi.org/10.3390/app15116236
Charif ML, Ciuparu DM, Lixandru Matei IL, Vasilievici G, Banu I, Băjan M, Bomboș D, Dușescu-Vasile C, Ghețiu IV, Panaitescu C, et al. An Experimental Study of Glycerol Carbonate Synthesis over g-C3N4 Catalysts. Applied Sciences. 2025; 15(11):6236. https://doi.org/10.3390/app15116236
Chicago/Turabian StyleCharif, Mirna Lea, Dragoș Mihael Ciuparu, Ioana Lavinia Lixandru Matei, Gabriel Vasilievici, Ionuț Banu, Marian Băjan, Dorin Bomboș, Cristina Dușescu-Vasile, Iuliana Veronica Ghețiu, Cașen Panaitescu, and et al. 2025. "An Experimental Study of Glycerol Carbonate Synthesis over g-C3N4 Catalysts" Applied Sciences 15, no. 11: 6236. https://doi.org/10.3390/app15116236
APA StyleCharif, M. L., Ciuparu, D. M., Lixandru Matei, I. L., Vasilievici, G., Banu, I., Băjan, M., Bomboș, D., Dușescu-Vasile, C., Ghețiu, I. V., Panaitescu, C., & Doukeh, R. (2025). An Experimental Study of Glycerol Carbonate Synthesis over g-C3N4 Catalysts. Applied Sciences, 15(11), 6236. https://doi.org/10.3390/app15116236








